Abstract
Proteoglycan 4 (PRG4) is a mucin-like glycoprotein present in synovial fluid and at the surface of articular cartilage. The objectives of this study were to (1) assess the articular cartilage surface adsorption and in vitro cartilage boundary lubricating ability of full-length recombinant human PRG4 (rhPRG4), and (2) cartilage boundary lubricating ability of purified rhPRG4, both alone and in combination with hyaluronan (HA). rhPRG4 adsorption onto articular cartilage explants was assessed by immunohistochemistry and dot blot. An in vitro cartilage–cartilage friction test was used to assess rhPRG4’s cartilage boundary lubricating ability compared to bovine PRG4, and that of purified rhPRG4 both alone and in combination with HA. rhPRG4 was able to adsorb to the articular surface, as well as the cut surface, of cartilage explants. The kinetic coefficient of friction of rhPRG4 was similar to that of PRG4 (p = 0.16) and lower than phosphate-buffered saline (p < 0.05), while that of purified rhPRG4 + HA was significantly lower than rhPRG4 alone (p < 0.05). This study demonstrates that rhPRG4 can adsorb to an intact articular cartilage surface and functions as an effective boundary lubricant, both alone and with HA, and provides the foundation for in vivo evaluation of this clinically relevant full-length rhPRG4 for treatment of osteoarthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteoglycan 4 (PRG4) is a mucin-like glycoprotein present in synovial fluid (SF) and at the surface of articular cartilage. It is synthesised and secreted by cells at the surface of articular cartilage, meniscus, synovial lining and tendons.31 PRG4 is a boundary lubricant22 that has several biophysical properties that contribute to joint health.21 , 27 These include functioning as a critical cartilage boundary lubricant, both alone in a dose-dependent manner and in combination with hyaluronan (HA),24 , 30 in addition to inhibiting apoptosis of chondrocytes.39 PRG4 contains a central mucin-like domain that is heavily glycosylated with O-linked oligosaccharides, which are necessary for PRG4’s cartilage boundary lubricating ability.22 Terminal globular protein domains, rich in cysteine, facilitate the inter- and intra-molecular disulfide bonding of PRG431 that may also be functionally determinant in terms of cartilage lubrication.
HA is a glycosaminoglycan present in native SF that has been used clinically as a viscosupplement in attempt to restore lubricating function. Although HA alone reduces friction at a cartilage–cartilage interface in vitro,30 the functional utility of intra-articular HA injections (as a lubricant) has been questioned in preclinical models of post-traumatic osteoarthritis (OA) (e.g., Teeple et al. 38). Nevertheless, cartilage friction is significantly reduced when HA is combined with PRG4 in vitro through an undefined mechanism,24 , 30 and intra-articular injection of HA + PRG4 reduces cartilage damage in preclinical post-traumatic OA compared to HA alone.38
Although the precise cause of OA is unknown, one possible biomechanical mechanism involves the failure of joint lubrication.22 Indeed, knee injury can result in decreased PRG4 concentration in SF, thus reducing the cartilage boundary lubricating ability of SF and contributing to the initiation of cartilage damage. Decreased PRG4 concentrations have also been reported in SF of patients soon after an anterior cruciate ligament (ACL) injury.10 In patients with acute knee injuries or progressive chronic inflammatory arthritis, cartilage damage has been associated with decreased boundary lubricating ability of SF.11 This association has also been observed in animal models following ACL transections, where a reduction in PRG4 concentration in SF was observed.11 Studies in rat models with ACL transections found an intra-articular injection of purified human PRG4 reduced cartilage degeneration,18 slowed cartilage damage and preserved the superficial zone chondrocytes’ viability.13 Collectively these studies suggest improving cartilage lubrication and preserving chondrocyte viability via intra-articular administration of PRG4 may slow or halt the initiation or progression of OA.39
Recombinant human versions of PRG4 have also been studied, and demonstrated to be effective in preserving joint function and reducing cartilage degeneration when administered intra-articularly. LUB:1, a truncated version of recombinant human PRG4 where the mucin-like domain was approximately a third of the size of full-length PRG4, was able to reduce cartilage degradation and structural damage in a rat OA meniscus model.14 Full-length recombinant human lubricin (rhLub) also demonstrated boundary lubricating ability in an in vitro cartilage-glass friction system16 and the ability to bind to a depleted cartilage surface.23 In another study, full-length recombinant human PRG4 (rhPRG4) effectively reduced cartilage damage after an ACL transection in a rat model.19 However, it was unclear if this version of rhPRG4 was able to multimerise into the functionally important multimeric form of PRG4.31 Furthermore, neither of these previously studied full-length forms was able to be expressed at high levels necessary for future (clinical) evaluation.
Recently, a full-length rhPRG4 has been successfully expressed at high (>1 g/L) levels28 using Chinese Hamster Ovary (CHO) cells. This rhPRG4 has been demonstrated to have appropriate higher order structure and O-linked glycosylations, and an enriched preparation of rhPRG4 demonstrated in vitro ocular surface boundary lubricating ability similar to that of bovine PRG4.28 However, it remains unclear if this rhPRG4 is able to adsorb to the surface of articular cartilage and function as an effective in vitro friction-reducing boundary lubricant at a cartilage–cartilage interface. Furthermore, the ability of purified rhPRG4 to synergistically function with HA as an in vitro cartilage boundary lubricant remains to be determined.
Hence, the objectives of this study were to (1) assess the articular cartilage surface adsorption and in vitro cartilage boundary lubricating ability of full-length rhPRG4, and (2) cartilage boundary lubricating ability of purified rhPRG4, both alone and in combination with HA. It was expected that rhPRG4 would function in a similar manner to that of bovine PRG4 in terms of cartilage adsorption and friction reducing boundary lubricating ability.
Materials and Methods
(rh)PRG4 Preparations
PRG4
PRG4 was prepared from fresh skeletally mature bovine stifle joints (Calgary, AB, Canada), as described previously.30 Cartilage discs were cultured in Dulbecco’s Modified Eagle’s Medium (Life Technologies, Carlsbad, CA) with 0.01% bovine serum albumin, with the addition of 25 μg/mL of ascorbic acid and 10 ng/mL of recombinant human transforming growth factor-β.29 Purification of the media was then performed using salt gradient diethylaminoethanol anion exchange chromatography (DEAE) (GE Healthcare Life Sciences, Baie d’Urfe, QC, Canada).29 , 31 Total concentration of the 0.615 M NaCl eluent was determined by bicinchoninic acid assay (BCA) (Sigma-Aldrich, St. Louis, MO). The purity of the concentrated and filtered solution was confirmed using 3–8% Tris–Acetate SDS-PAGE (Life Technologies, Carlsbad, CA) followed by Simply Blue protein stain and densitometry analysis (ImageJ, Bethesda, MD).31 , 36
rhPRG4
rhPRG4 was generated as described previously.28 Culture media conditioned by a CHO line transfected with the PRG4 gene was provided by Lµbris, LLC (Framingham, MA) in collaboration with Selexis SA (Geneva, Switzerland). Briefly, the gene encoding the full length 1404 amino acid human PRG4 was inserted into plasmid vectors commercially available at Selexis SA, for enhanced gene expression in mammalian CHO cells. rhPRG4 rich media was obtained from a shake flask culture with fed-batch cultivation (SFM4CHO medium (Hyclone, Logan, UT) supplemented with 8 mM l-Glutamine, hypoxanthine and thymidine (1× HT, Life Technologies, Carlsbad, CA) of a high expressing rhPRG4 clonal cell line.
Enriched rhPRG4 preparations were prepared for immunohistochemistry (IHC) and friction testing by concentrating the rhPRG4 rich media using a 100 kDa MW cut-off centrifugal filter. The purity of high MW rhPRG4 species was assessed to be 50% by SDS-PAGE and densitometry, with concentration being determined by BCA and adjusted to take into account the level of purity. SDS-PAGE western blotting was also used to characterise the size distributions of immunoreactive species of non-reduced (NR) and reduced (R) (NuPAGE Sample Reducing Agent, Life Technologies) rhPRG4 samples, essentially as described previously, with anti-PRG4 monoclonal antibody (mAb) 9G3 (EMD Millipore, MA, USA).36
Purified rhPRG4 was prepared for subsequent friction testing, alone and in combination with HA, via DEAE. Media was concentrated 10× and buffer exchanged using a 100 kDa MW cut-off filter into 20 mM Tris–HCl buffer at pH 8.0, left to equilibrate for 2 h. PRG4-enriched samples were then subject to Sepharose Q anion exchange chromatography (HiTrap Q, GE Healthcare Life Sciences) eluted with a step to 400 mM NaCl followed by a linear gradient to 1 M NaCl. Fractions from 400 to 1000 mM NaCl were collected and pooled together. Total concentration was determined by BCA and the purity of the concentrated and filtered solution was determined to be 90% using SDS-PAGE and densitometry.
Immunohistochemistry (IHC)
Immunohistochemistry (IHC) Samples
Cartilage discs with the intact articular surface (n = 15, diameter = 6 mm) were harvested from bovine stifle joints. Three intact cartilage discs were embedded in media (Tissue Tek OCT, Sakura, Torrance, CA) and snap frozen in isopentane cooled by liquid nitrogen, and served as positive control samples (labelled as “fresh”).
Specimen Processing
All other samples were placed in phosphate-buffered saline (PBS) and shaken vigorously overnight at 4 °C to rid the articular surface of residual PRG4, and subsequently frozen at −80 °C to prevent further production of PRG4 from viable chondrocytes. Samples were then thawed, and again shaken overnight at 4 °C, before incubation with lubricants of interest. Samples were incubated over night at room temperature in a 48-well plate; PBS (negative control), rhPRG4 and PRG4 at a physiological concentration of 450 μg/mL, and bovine SF (Animal Technologies, Tyler, TX) (positive control) and left to incubate at room temperature for 24 h.26 Samples were then fixed in OCT and stored at −80 °C.
Five-micron thick sections were cut using a cryostat microtome (Microm HM550, Thermo Scientific, Waltham, MA) and placed on positively charged glass slides (Superfrost Plus Adhesion Slides, Thermo Scientific). Sections were then fixed in 4% paraformaldehyde in PBS and washed in PBS to remove OCT.26 Samples were blocked with 10% hydrogen peroxide in methanol, followed by 10% goat serum with 1% BSA in PBS in a humidity chamber. Samples were then incubated overnight in mAb 9G3; in 1.5% normal goat serum at a ratio of 1:200.18 Samples were then washed with PBS and incubated with secondary antibody Alexa Fluor-594 rhodamine-conjugated goat-anti mouse IgG (Life Technologies, Carlsbad, CA) in 1.5% normal goat serum at a ratio of 1:100. These were then washed with PBS, mounted with mounting medium containing the nuclear counterstain DAPI (Vectashield, Vector Laboratories, Inc., Burlingame, CA), and sealed with microscope cover slips (VWR Scientific Products, PA). Results were imaged using Zeiss LSM 780 microscope (Carl Zeiss, Oberkochen, Germany) at a magnification of ×20 objective (dry, 0.8 NA). Fluorescence images were obtained for both red (Alexa Fluor-594 rhodamine detected PRG4; excitation/emission of 590/617 nm) and blue (DAPI detected cell staining; excitation/emission of 358/461 nm) fluorescence.
Dot Blot Enzyme-Linked Immunosorbent Assay (ELISA)
Sample Preparation
The amount of PRG4 adsorbed at the cartilage surfaces (articular and cut) was quantified by extraction using 4 M guanidine hydrochloride (GuHCl) and enumerated using densitometry analysis of dot blot ELISA, essentially as described previously.29 , 33 Cartilage discs (n = 25, diameter = 6 mm) were harvested from five skeletally mature bovine stifle joints. Five “fresh” samples underwent the extraction process as a positive control. All other samples were incubated in lubricants of interest as described above. Following lubricant incubation, cartilage samples were rinsed then incubated for 24 h in 400 μL of 4 M GuHCl, 0.02 M Tris, pH 8.2 containing protease inhibitors (PIs)2 then stored at −80 °C.
Dot blot ELISA
100 μL of extract solution and rhPRG4 control samples were applied to a nitrocellulose membrane in a Bio-Dot apparatus (Bio-Rad, Hercules, CA). The membrane was blocked with 5% non-fat dry milk, incubated in mAb 9G3, and then exposed to Cy3 goat-anti mouse (Life Technologies, Carlsbad, CA). Membranes were imaged using GE Typhoon FLA 9500 (GE Healthcare Life Sciences, Baie d’Urfe, QC, Canada), and densitometry analysis was performed using ImageQuant TL Software (GE Healthcare Life Sciences).
Statistical Analysis
Data was log-transformed and tested for normality using Shapiro-Wilks test. ANOVA was used to assess the effect of PRG4 adsorption followed by Dunnett post hoc testing, with the “fresh” sample as control. Statistical analysis was performed with SPSS 22.0 (IBM SPSS software, New York, NY).
Boundary Lubrication Tests
Sample Preparation
Fresh osteochondral samples (n = 13) were prepared for friction testing from the patellofemoral groove of four skeletally mature bovine stifle joints.30 , 32 Cores (radius = 6 mm) and annuli (R O = 3.2 mm and R i = 1.5 mm) were harvested from osteochondral blocks.32 Osteochondral samples were then rinsed vigorously overnight in PBS at 4 °C to rid the articular surface of residual SF (a procedure confirmed previously by lubrication testing30 , 32) and were then frozen in PBS with PIs at −80 °C.2 Samples were re-shaken overnight in PBS to further deplete the surface of any residual PRG4 at the surface upon thawing before friction testing. After the first PBS test, samples were bathed in ~ 0.3 ml of the subsequent test lubricants, completely immersing the cartilage, at 4 °C overnight prior to the next day’s lubrication test.
Lubrication Test
The Bose ELF 3200 was used to analyse the boundary lubrication ability of each of the PRG4 forms, using the previously described cartilage-on-cartilage friction test.30 , 32 Samples were compressed to 18% of the total thickness and were allowed to stress-relax for 40 min to enable fluid depressurisation of the interstitial fluid. The samples were then rotated at an effective velocity of 0.3 mm/s (shown to maintain boundary mode lubrication at a depressurised cartilage–cartilage interface)32 at ±2 revolutions. Samples were then left in a pre-sliding duration (T ps) of 1200, 120, 12 and 1.2 s (s). Samples were then rotated after each subsequent stationary period, ±2 revolutions and repeated in the ∓2 revolutions.
Lubrication Test Sequences
Two test sequences were employed to assess the rhPRG4 preparations. In both test sequences, PBS served as the negative control and bovine SF served as a positive control. The first sequence tested the enriched PRG4 preparation alone, compared to bovine PRG4, and the second sequence tested purified PRG4, alone and in combination with HA. Both rhPRG4 and PRG4 were prepared in PBS at a concentration of 450 μg/mL. HA at 1.5 MDa (Lifecore Biomedical, Chaska, MN) was prepared in PBS at a physiological concentration of 3.33 mg/mL. Lubricants were tested in presumed increasing order of lubricating function.30
-
Test Sequence 1: PBS, rhPRG4, PRG4, SF (n = 7).
-
Test Sequence 2: PBS, rhPRG4, rhPRG4 + HA, SF (n = 6).
Statistical Analysis
The two coefficients of friction; static (μ static, Neq, resistance of start-up motion from static condition) and kinetic (〈μ kinetic, Neq〉, resistance of steady sliding motion) were calculated for each lubricant as described previously,30 and unless otherwise indicated data is presented as mean ± SEM. 〈μ kinetic,Neq〉 increased only slightly with T ps, therefore for brevity and clarity, average 〈μ kinetic,Neq〉 values across T ps for each lubricant are presented, as done previously.2 Data was tested for normality using Shapiro–Wilks test. Repeated measures ANOVA was used to assess the effect of lubricant and T ps on μ static,Neq data, as well as to assess the effect of lubricant on 〈μ kinetic,Neq〉 values followed by Bonferonni post hoc testing. In the event of non-normality, Friedman test was used to assess the effect of lubricant and T ps on μ static,Neq data, as well as to assess the effect of lubricant on 〈μ kinetic,Neq〉 values. Followed by Wilcoxon-signed rank test for pairwise comparisons. Statistical analysis was performed with SPSS 22.0.
Results
SDS-PAGE
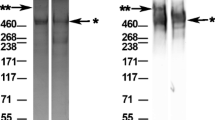
rhPRG4 demonstrated high MW bands and immunoreactivity as assessed by SDS-PAGE (Fig. 1). Protein staining (Fig. 1a) of the NR rhPRG4 preparation, both enriched and purified, showed two prominent high MW bands with apparent MW of ~1 MDa and ~460 kDa, and a single high MW ~460 kDa band for the R rhPRG4. Western blotting (Fig. 1b) indicated high MW 9G3-immunoreactive bands in the NR and R rhPRG4 preparations with similar apparent MW to those observed in the protein staining.
Immunohistochemistry
IHC (Fig. 2) of whole cartilage discs indicated a discrete 9G3-immunoreactive layer of PRG4 at the articular surface of fresh explants, the majority of which could be removed with vigorous shaking in PBS. This immunoreactivity was localised to the articular surface of fresh explants, with none observed at the cut edges, and was specific as no immunoreactivity was observed in the non-immune serum control. Shaken articular discs incubated in PRG4 containing solutions showed 9G3 immunoreactivity (red) at the articular surface and cut edge, with the immunoreactivity of rhPRG4 being similar to that observed for PRG4 as well as SF. The 9G3 immunoreactive layer at the articular surface appeared to have a greater intensity to that observed at the cut edge of the explant incubated in PRG4 containing solutions (i.e., PRG4, rhPRG4, and SF). Again, there was no immunoreactivity observed for the non-immune control. Immunoreactivity was also observed at the cartilage surface with cartilage discs incubated in PBS, though not to the same intensity as those incubated in PRG4 containing solutions.
Immunohistochemistry results indicating immunolocalisation of PRG4 at the articular cartilage surface and cut edge of samples shaken overnight again in PBS at 4 °C then incubated in PRG4 containing solutions. Specifically, rhPRG4 was able to bind to the surface of articular cartilage depleted of PRG4, similar to that of fresh samples as well as those samples incubated in other PRG4 containing solutions (PRG4 and SF). Signal to anti-PRG4 mAb 9G3 is depicted by the red staining and was specific as no immunoreactivity was detected with negative samples (−), chondrocyte cells are depicted by the blue DAPI staining.
Dot Blot ELISA
Dot blot ELISA (Fig. 3) indicated vigorous shaking of cartilage samples significantly depleted explant PRG4 (PBS: 0.007 ± 0.005 μg/cm2) compared to fresh samples (Fresh: 0.026 ± 0.007 μg/cm2) (p < 0.05). Furthermore, PRG4 from all three PRG4-containing solutions was able to re-adsorb to depleted cartilage explants to levels similar to that of fresh samples (PRG4: 0.190 ± 0.006 μg/cm2; rhPRG4: 0.011 ± 0.005 μg/cm2; SF: 0.014 ± 0.005 μg/cm2) (p > 0.05).
Concentration analysis of dot blot enzyme-linked immunosorbent assay of PRG4 indicated vigorous shaking of cartilage samples significantly depleted explant PRG4 compared to fresh samples (p < 0.05). Furthermore, PRG4 from all three PRG4-containing solutions was able to re-adsorb to depleted cartilage explants to levels similar to that of fresh samples. Anti-PRG4 mAb 9G3 was used to probe the nitrocellulose membrane. * indicates p < 0.05 compared to Fresh.
Boundary Lubrication Tests
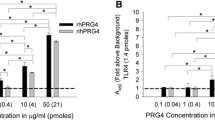
rhPRG4 functioned similarly to PRG4 as an effective friction-reducing cartilage boundary lubricant. μ static,Neq data varied with T ps and test lubricant (both p < 0.001) with no interaction (p = 0.45). Values increased with T ps and were consistently highest in PBS and lowest in SF, rhPRG4 and PRG4 were intermediate (Fig. 4a). Friedman test indicated the 〈μ kinetic,Neq〉 data varied at the lubricant level (p < 0.001). Values for rhPRG4 (0.106 ± 0.007) and PRG4 (0.087 ± 0.010) were again intermediate and not significantly different from each other (p < 0.05). 〈μ kinetic,Neq〉 in PBS was greater than PRG4 and rhPRG4 (p < 0.05), and that in SF was lower than PRG4 and rhPRG4 (p < 0.05) (Fig. 4b).
rhPRG4 functioned similarly to PRG4 as an effective friction-reducing cartilage boundary lubricant. Static (μ static,Neq) (a) and kinetic 〈μ kinetic,Neq〉 (b) friction coefficients in PBS, rhPRG4 and PRG4 preparations both at 450 µg/mL, and SF. Different letters signify statistically significant differences (p < 0.05), n = 7.
Purified rhPRG4 functioned as a more effective friction-reducing cartilage boundary lubricant in the presence of HA. Friedman test indicated the μ static,Neq data varied with T ps and test lubricant (both p < 0.001). Values increased with T ps and were consistently highest in PBS and lowest in SF. rhPRG4 and rhPRG4 + HA were intermediate (Fig. 5a). 〈μ kinetic,Neq〉 values exhibited similar trends, varying with lubricant [F(3,15) = 53.06, p < 0.001]. 〈μ kinetic,Neq〉 values were greatest in PBS and lowest in SF. Values for rhPRG4 (0.104 ± 0.016) and rhPRG4 + HA (0.063 ± 0.006) were again intermediate but significantly different (p < 0.05). 〈μ kinetic,Neq〉 in PBS was significantly greater than rhPRG4 (p < 0.05) and rhPRG4 + HA (p < 0.01), and 〈μ kinetic,Neq〉 values in SF was significantly lower than rhPRG4 (p < 0.01) and rhPRG4 + HA (p < 0.05) (Fig. 5b).
Purified rhPRG4 functioned as an effective friction-reducing cartilage boundary lubricant, both alone and in combination with hyaluronan (HA). Static (μ static,Neq) (a) and kinetic 〈μ kinetic,Neq〉 (b) friction coefficients in PBS, purified rhPRG4 and purified rhPRG4 + HA with rhPRG4 at 450 µg/mL and HA (1.5 MDa) at 3.33 mg/mL, and SF. Different letters signify statistically significant differences (p < 0.05), n = 6.
Discussion
The objective of this study was to analyse the ability of the recently available full-length rhPRG4 to adsorb to the surface of articular cartilage and function as an effective in vitro friction-reducing boundary lubricant at a cartilage–cartilage interface. The rhPRG4 examined here demonstrated immunoreactivity, cartilage adsorption and boundary lubricating function equivalent to that of native PRG4. SDS-PAGE showed high MW immunoreactivity of rhPRG4 with the anti-PRG4 mAb 9G3, suggesting the presence of O-linked glycosylations consistent with those of native PRG4. The NR rhPRG4 also demonstrated a higher apparent MW species of ~1 MDa in addition to ~460 KDa, consistent with previous descriptions as a putative dimer and monomer respectively.36 Finally, purified rhPRG4 functioned synergistically with HA to further reduce friction at the cartilage–cartilage biointerface to levels near that of SF.
The results of this study agree with and extend a previous study that used a similar enriched rhPRG4 preparation, whose protein identity was confirmed by tandem mass spectrometry.28 The present study extended the characterisation of the high MW immunoreactivity to mAb 9G3, presenting as a broad distribution typical of glycosylation-dependent epitopes, and demonstrated cartilage adsorption and boundary lubrication. Furthermore, purified rhPRG4 demonstrated similar cartilage boundary lubricating ability to the enriched preparation used (〈μ kinetic,Neq〉 = 0.104 ± 0.016 compared to 0.106 ± 0.007, respectively), suggests any impurities observed in the enriched rhPRG4 did not significantly inhibit function in these tests. Unfortunately the purification scheme employed here resulted in significant (>90%) losses of protein, making it unacceptable for any potential future scaled up use. Though, this preliminary study still provided a framework for future development of an industrial purification scheme (and quantification by extinction coefficient є 0.1% = 0.50, where mg/mL = A 280/є 0.1%, may be useful). Collectively, the results of this study extend upon the initial characterisation of the rhPRG4 and reported ocular surface boundary lubricating ability28 by demonstrating rhPRG4’s efficacy as a cartilage boundary lubricant, alone and in combination with HA.
rhPRG4 was able to bind to the surface of articular cartilage depleted of PRG4, a property required for function as a boundary lubricant. The method employed to remove the PRG4 from the articular surface, vigorous shaking in PBS, was chosen over other enzymatic, ionic, or mechanical methods employed as to avoid any potential alterations to the articular cartilage surface.23 , 26 For example, treating cartilage samples with hyaluronidase has been shown to significantly deplete the surface of PRG4, yet also result in significantly reduced repleted levels of PRG4 compared to initial levels26 suggesting an alteration to the native articular surface structure and/or PRG4 adsorption mechanism thus introducing a confounding factor. While some residual PRG4 was visualised and detected on the articular surface by IHC and dot blot ELISA, respectively, it demonstrated substantially less intensity compared to fresh or repleted samples. Calculated mass of rhPRG4 per articular surface area were an order of magnitude less than previously reported for immature bovine cartilage using bovine PRG4 and mAb 3A4 (which does not react with human PRG4).26 Such differences could be due to the different mAb, age of joints and controls used, though are consistent with the amount of surface bound PRG4 being an extremely small fraction of that present in solution. Future work, potentially with I-125 radiolabeling of rhPRG4,37 is required to extend these results to further characterise and quantify the kinetics of rhPRG4 cartilage surface adsorption.
The IHC data presented here demonstrates the ability of rhPRG4 to not only adsorb to the surface of articular cartilage, a critical property for a potential biotherapeutic treatment aimed at improving surface lubrication, but also interestingly to the non-articular (cut) surfaces in a manner similar to that of native PRG4 and whole SF. Although somewhat unexpected, it was observed in all PRG4 containing solutions, including SF, and was not visualised in the non-immune control. Future studies could consider incubation of osteochondral samples23 instead of cut cartilage explants to prevent adsorption to lower cut surfaces as well as examining the potential effect of other molecules (such as HA) on rhPRG4’s adsorption to the cartilage surface. A gene level injury response has been reported in excised cartilage explants,40 as well as an upregulation in matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), which are known to compromise the cartilage structure and function.5 , 25 Thus, there is a possibility of further cartilage degradation during the experimental procedure that could affect cartilage surface structure and friction. However, these potential effects are limited here since no tissue degradation, indicative of either aggrecanase nor collagenase activity, was observed (and both catabolic and anabolic activities have been observed for fibroblast growth factor (FGF)-2).40
This study also agrees with and extends previous studies examining the lubricating ability of other rhPRG4. The studies by Jones et al. 23 and Gleghorn et al. 16 demonstrated rhLub bound to articular surfaces,23 and reduced friction in a boundary mode at a cartilage-glass interface.16 Consistent with these previous studies, this study demonstrated the ability of newly available full-length rhPRG4 to bind to the articular cartilage surface and reduce friction in a boundary mode at a cartilage–cartilage biointerface. PRG4 has consistently demonstrated friction reducing ability in a boundary mode at macro scale interfaces such as latex-glass,22 cartilage-glass,16 and cartilage–cartilage,24 , 30 as well as the ability reduce friction on various synthetic hydrophilic and hydrophobic surfaces at a micro scale.7 , 9 , 41 In all cases, the ability of PRG4 to bind to the articulating surfaces is crucial for its lubrication function. The absolute value of friction coefficients obtained from various in vitro test setups can vary due to different interfaces (materials, surface properties), test parameters (load, velocity, geometry, scale), and operative modes of lubrication. Indeed, the value of friction coefficients measured here are potentially larger than those estimated in vivo due to the diminished effect of fluid pressurisation within the cartilage samples, which is a critical contributor to the low friction in fluid mediated lubrication.3 , 15 While each test system has their advantages and disadvantages in terms of studying PRG4’s biological boundary lubrication function, in vivo PRG4 alone is a critical chondroprotective boundary lubricant as demonstrated by lack of PRG4 resulting in early precocious joint damage and failure20 , 27 despite high levels of HA.
The ability and role of HA as a cartilage boundary lubricant, either alone or in combination with PRG4, is an ongoing area of research and discussion. At a cartilage–cartilage interface, HA (of various MW) has been shown to reduce friction alone,8 , 24 with PRG4 removed from the surface, and more importantly function synergistically with PRG4 to reduce friction levels close to that of SF.24 , 30 Recently, peptide-mediated surface bound HA reduced friction at a cartilage–cartilage interface in a saline bath, even with PRG4 removed from the surface.35 Conversely, at cartilage-glass6 and mica–mica4 interfaces, HA in solution does not function as friction-reducing boundary lubricant alone, and on hydrophobic/hydrophilic self-assembled monolayer7 shows no synergism with PRG4. However, if HA is chemically bound to a mica surface it is able to provide wear protection9 and has been proposed to function synergistically with PRG4 at the surface of cartilage through adaptive mechanically controlled lubrication mechanism.17 More recently, Seror et al. 34 demonstrated surface bound HA functions synergistically with phosphatidylcholine lipids (PCs) at a mica–mica interface, providing physiological levels of lubrication (with a coefficient of friction of ~10−3 at pressures to the order of 100 atm), and proposed that HA thus complexed with PCs is anchored to the surface of articular cartilage through PRG4. Combined efforts using sophisticated molecular level mica–mica friction measurements along with cartilage–cartilage macroscale tissue friction measurements will be useful, and perhaps necessary, in ongoing studies to elucidate the individual and combined role(s) of SF constituents PRG4, HA, and lipids in providing efficient boundary lubrication of cartilage.
Collectively, these results demonstrate the ability of rhPRG4 ability to function as an effective in vitro cartilage boundary lubricant, alone and in combination with HA, and provide the basis for future in vivo evaluation of a highly purified version of this clinically relevant full-length rhPRG4. The high expression levels will enable the potential for clinical evaluation and translation as a cartilage lubricant and OA biotherapeutic. Indeed, given the abundance of data on PRG4’s efficacy in preventing cartilage degradation in animal models of post traumatic OA,13 , 14 , 18 , 19 , 38 the potential use of this recently available rhPRG4 to preserve joint function is of great promise. Recently, based on the findings of this study, a highly (industrially) purified form of the full-length rhPRG4 administered intra-articularly was shown to function synergistically with interleukin-1 receptor antagonist in reducing caspase 3 positive chondrocytes in an ACL-transection rat model of OA, suggesting a combined treatment may act synergistically to reduce cartilage catabolism.12 Pre-clinical studies and future in vivo large animal combined with continued efforts towards industrial production and scale up, will provide the foundation and motivation for clinical evaluation of this full-length rhPRG4 as a biotherapeutic treatment to prevent or slow the progression of OA potentially through both mechanical and biological mechanisms,1 , 12 and therefore improve the quality of life of those that suffer from the disease.
References
Al-Sharif, A., M. Jamal, L. Zhang, K. Larson, T. Schmidt, G. Jay, and K. Elsaid. Lubricin/proteoglycan 4 binding to CD44 receptor: a mechanism of lubricin’s suppression of pro-inflammatory cytokine induced synoviocyte proliferation. Arthritis Rheum. 67(6):1503–1513, 2015.
Antonacci, J. M., T. A. Schmidt, L. A. Serventi, M. Z. Cai, Y. L. Shu, B. L. Schumacher, C. W. McIlwraith, and R. L. Sah. Effects of equine joint injury on boundary lubrication of articular cartilage by synovial fluid: role of hyaluronan. Arthritis Rheum. 64:2917–2926, 2012.
Ateshian, G. A. The role of interstitial fluid pressurization in articular cartilage lubrication. J. Biomech. 42:1163–1176, 2009.
Benz, M., N. Chen, G. Jay, and J. Israelachvili. Static forces, structure and flow properties of complex fluids in highly confined geometries. Ann. Biomed. Eng. 33:39–51, 2005.
Blain, E. J., S. J. Gilbert, R. J. Wardale, S. J. Capper, D. J. Mason, and V. C. Duance. Up-regulation of matrix metalloproteinase expression and activation following cyclical compressive loading of articular cartilage in vitro. Arch. Biochem. Biophys. 396:49–55, 2001.
Bonnevie, E. D., C. Secchieri, D. Galesso and L. J. Bonassar. Stribeck analysis of synovial lubricants: lubricating mechanisms and interaction of hyaluronic acid and lubricin. Trans. Orthop. Res. Soc. Poster No: 0437, 2014.
Chang, D. P., N. I. Abu-Lail, J. M. Coles, F. Guilak, G. D. Jay, and S. Zauscher. Friction force microscopy of lubricin and hyaluronic acid between hydrophobic and hydrophilic surfaces. Soft Matter 5:3438–3445, 2009.
Corvelli M., B. Che, C. Saeui, A. Singh, and J. Elisseeff. Biodynamic performance of hyaluronic acid versus synovial fluid of the knee in osteoarthritis. Methods 2015. doi:10.1016/j.ymeth.2015.03.019.
Das, S., X. Banquy, B. Zappone, G. W. Greene, G. D. Jay, and J. N. Israelachvili. Synergistic interactions between grafted hyaluronic acid and lubricin provide enhanced wear protection and lubrication. Biomacromolecules 14:1669–1677, 2013.
Elsaid, K. A., B. C. Fleming, H. L. Oksendahl, J. T. Machan, P. D. Fadale, M. J. Hulstyn, R. Shalvoy, and G. D. Jay. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 58:1707–1715, 2008.
Elsaid, K. A., G. D. Jay, M. L. Warman, D. K. Rhee, and C. O. Chichester. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 52:1746–1755, 2005.
Elsaid, K. A., L. Zhang, Z. Shaman, C. Patel, T. A. Schmidt, and G. D. Jay. The impact of early intra-articular administration of interleukin-1 receptor antagonist on lubricin metabolism and cartilage degeneration in an anterior cruciate ligament transection model. Osteoarthr. Cartil. 23:114–121, 2015.
Elsaid, K. A., L. Zhang, K. Waller, J. Tofte, E. Teeple, B. C. Fleming, and G. D. Jay. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin’s effect in exercised joints following ACL transection. Osteoarthr. Cartil. 20:940–948, 2012.
Flannery, C. R., R. Zollner, C. Corcoran, A. R. Jones, A. Root, M. A. Rivera-Bermudez, T. Blanchet, J. P. Gleghorn, L. J. Bonassar, A. M. Bendele, E. A. Morris, and S. S. Glasson. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 60:840–847, 2009.
Forster, H., and J. Fisher. The influence of loading time and lubricant on the friction of articular cartilage. Proc. Inst. Mech. Eng. H 210:109–119, 1996.
Gleghorn, J. P., A. R. Jones, C. R. Flannery, and L. J. Bonassar. Boundary mode lubrication of articular cartilage by recombinant human lubricin. J. Orthop. Res. 27:771–777, 2009.
Greene, G. W., X. Banquy, D. W. Lee, D. D. Lowrey, J. Yu, and J. N. Israelachvili. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proc Natl Acad Sci USA 108:5255–5259, 2011.
Jay, G. D., K. A. Elsaid, K. A. Kelly, S. C. Anderson, L. Zhang, E. Teeple, K. Waller, and B. C. Fleming. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 64:1162–1171, 2012.
Jay, G. D., B. C. Fleming, B. A. Watkins, K. A. McHugh, S. C. Anderson, L. X. Zhang, E. Teeple, K. A. Waller, and K. A. Elsaid. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 62:2382–2391, 2010.
Jay, G. D., J. R. Torres, D. K. Rhee, H. J. Helminen, M. M. Hytinnen, C. J. Cha, K. Elsaid, K. S. Kim, Y. Cui, and M. L. Warman. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 56:3662–3669, 2007.
Jay, G. D., J. R. Torres, M. L. Warman, M. C. Laderer, and K. S. Breuer. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci USA 104:6194–6199, 2007.
Jay, G. D., and K. A. Waller. The biology of Lubricin: near frictionless joint motion. Matrix Biol. 39:17–24, 2014.
Jones, A. R., J. P. Gleghorn, C. E. Hughes, L. J. Fitz, R. Zollner, S. D. Wainwright, B. Caterson, E. A. Morris, L. J. Bonassar, and C. R. Flannery. Binding and localization of recombinant lubricin to articular cartilage surfaces. J. Orthop. Res. 25:283–292, 2007.
Kwiecinski, J. J., S. G. Dorosz, T. E. Ludwig, S. Abubacker, M. K. Cowman, and T. A. Schmidt. The effect of molecular weight on hyaluronan’s cartilage boundary lubricating ability—alone and in combination with proteoglycan 4. Osteoarthr. Cartil. 19:1356–1362, 2011.
Lee, J. H., J. B. Fitzgerald, M. A. Dimicco, and A. J. Grodzinsky. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 52:2386–2395, 2005.
Nugent-Derfus, G. E., A. H. Chan, B. L. Schumacher, and R. L. Sah. PRG4 exchange between the articular cartilage surface and synovial fluid. J. Orthop. Res. 25:1269–1276, 2007.
Rhee, D. K., J. Marcelino, M. Baker, Y. Gong, P. Smits, V. Lefebvre, G. D. Jay, M. Stewart, H. Wang, M. L. Warman, and J. D. Carpten. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Invest. 115:622–631, 2005.
Samsom, M. L., S. Morrison, N. Masala, B. D. Sullivan, D. A. Sullivan, H. Sheardown, and T. A. Schmidt. Characterization of full-length recombinant human proteoglycan 4 as an ocular surface boundary lubricant. Exp. Eye Res. 127:14–19, 2014.
Schmidt, T. A., N. S. Gastelum, E. H. Han, G. E. Nugent-Derfus, B. L. Schumacher, and R. L. Sah. Differential regulation of proteoglycan 4 metabolism in cartilage by IL-1alpha, IGF-I, and TGF-beta1. Osteoarthr. Cartil. 16:90–97, 2008.
Schmidt, T. A., N. S. Gastelum, Q. T. Nguyen, B. L. Schumacher, and R. L. Sah. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 56:882–891, 2007.
Schmidt, T. A., A. H. Plaas, and J. D. Sandy. Disulfide-bonded multimers of proteoglycan 4 (PRG4) are present in normal synovial fluids. Biochim. Biophys. Acta 1790:375–384, 2009.
Schmidt, T. A., and R. L. Sah. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthr. Cartil. 15:35–47, 2007.
Schumacher, B. L., T. A. Schmidt, M. S. Voegtline, A. C. Chen, and R. L. Sah. Proteoglycan 4 (PRG4) synthesis and immunolocalization in bovine meniscus. J. Orthop. Res. 23:562–568, 2005.
Seror, J., L. Zhu, R. Goldberg, A. J. Day, and J. Klein. Supramolecular synergy in the boundary lubrication of synovial joints. Nat. Commun. 6:6497, 2014.
Singh, A., M. Corvelli, S. A. Unterman, K. A. Wepasnick, P. McDonnell, and J. H. Elisseeff. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater. 13:988–995, 2014.
Steele, B. L., M. C. Alvarez-Veronesi, and T. A. Schmidt. Molecular weight characterization of PRG4 proteins using multi-angle laser light scattering (MALLS). Osteoarthr. Cartil. 21:498–504, 2013.
Swann, D. A., R. B. Hendren, E. L. Radin, S. L. Sotman, and E. A. Duda. The lubricating activity of synovial fluid glycoproteins. Arthritis Rheum. 24:22–30, 1981.
Teeple, E., K. A. Elsaid, G. D. Jay, L. Zhang, G. J. Badger, M. Akelman, T. F. Bliss, and B. C. Fleming. Effects of supplemental intra-articular lubricin and hyaluronic acid on the progression of posttraumatic arthritis in the anterior cruciate ligament-deficient rat knee. Am. J. Sports Med. 39:164–172, 2011.
Waller, K. A., L. X. Zhang, K. A. Elsaid, B. C. Fleming, M. L. Warman, and G. D. Jay. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc. Natl. Acad. Sci. USA 110:5852–5857, 2013.
Watt, F. E., H. M. Ismail, A. Didangelos, M. Peirce, T. L. Vincent, R. Wait, and J. Saklatvala. Src and fibroblast growth factor 2 independently regulate signaling and gene expression induced by experimental injury to intact articular cartilage. Arthritis Rheum. 65:397–407, 2013.
Zappone, B., M. Ruths, G. W. Greene, G. D. Jay, and J. N. Israelachvili. Adsorption, lubrication, and wear of lubricin on model surfaces: polymer brush-like behavior of a glycoprotein. Biophys. J. 92:1693–1708, 2007.
Acknowledgements
We would like to thank the Dr. Umberto Banderalli for his help with the confocal microscopy and Dr. Tak Fung for his assistance with statistical data analysis.
Funding Source
This work was supported by funding from the Natural Sciences and Engineering Research Council of Canada (355591), The Arthritis Society, the Alberta Innovates Health Solutions Team in Osteoarthritis.
Conflict of interest
TAS and GDJ have a financial interest in, and are named inventors on issued patents held by a commercial entity (Lubris) developing rhPRG4 for therapeutic uses. TAS is also a paid consultant for the same entity.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Eric Darling oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Abubacker, S., Dorosz, S.G., Ponjevic, D. et al. Full-Length Recombinant Human Proteoglycan 4 Interacts with Hyaluronan to Provide Cartilage Boundary Lubrication. Ann Biomed Eng 44, 1128–1137 (2016). https://doi.org/10.1007/s10439-015-1390-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1390-8