Abstract
The mining industry plays an important role in the industrial development of almost all countries in the world. The occupational risks associated with minerals mining are well-documented in mining structures to health problems associated with chronic exposure to mining dust. Constant intake of the occupational doses of mineral-rich dust is dangerous, often leading to diseases such as asthma, silicosis, asbestosis, and pneumoconiosis, which are the major contributors to chronic obstructive pulmonary diseases (COPD). It can be exemplified by incremental, weakly reversible airflow limitation frequently associated with prolonged inhalation of harmful dust in mines. The onset of pneumoconiosis is rooted in the inhalation of mineralized dust that is retained in the lung parenchyma. However, there are several variants of pneumoconiosis, namely fibrosis or asbestosis (inhalation of fibrous minerals such as asbestos), silicosis (mineralized silica), coal workers’ pneumoconiosis (CWP) or ‘black lung’ (coal dust), berylliosis (mineral beryl, though rare) and talcosis (inhalation of soft minerals talc or steatite) are some of the examples of fibrotic pneumoconiosis. Among all, CWP resulted in > 30,000 deaths globally from the beginning of the twenty-first century. Most cases of CWP occur due to poor occupational hygiene and negligible dust control.
Pyrite (FeS2) is the most common ore mineral of iron. It is commonly associated with coal seams, almost invariably associated with the Gondwana coal all over the world. The toxic element arsenic is often associated with pyrites, which on oxidation and hydration releases ionic As3+, As5+, Fe2+, Fe3+, S−, sometimes Co2+, Mn2+, and Mg2+, in water. Such highly toxic mineralized water is channelized as an ‘Acid Mine Drainage’, which often contaminates the groundwater and thereby poses a severe threat to miners as well as the local population. The CWP is caused due to mineralized carbon particles reaching the lungs, however, recent studies have suggested that the efficacy of coal-bearing dust multiplies due to the addition of iron released from pyrite associated with coal. Iron is a redox metal and participates in most of the reversible one-electron oxidation–reduction reactions by switching between the two oxidation states, i. e. ferrous (Fe2+) and ferric (Fe3+). Bioavailable iron (BAI) (both ferrous and ferric) released from coal dust after inhalation shows redox activity and produces free radicals (oxidants) involved in oxidative stress development, cell signaling processes and cellular damage. The presumed mechanism is through the ability of reactive oxygen species (ROS) to induce biochemical alterations in macromolecules such as DNA, lipids and proteins. Moreover, the prevalence of CWP among various coal mining regions positively correlates with average levels of BAI present in the coals.
BAI mainly originates from oxidation of pyrite (FeS2) present in coal as follows,
\(2{\text{FeS}}_2 + 7{\text{O}}_2 + 2{\text{H}}_2 {\text{O}} \to {{\varvec{2FeSO}}}_4 + 2{\text{H}}_2 {\text{SO}}_4\)
OR
Acid solubilization of siderite (FeCO3) as follows,
\({\text{FeCO}}_3 + {\text{H}}_2 {\text{SO}}_4 \to {{\varvec{FeS}}}{\bf{O}}_{{\varvec{4}}} + {\text{H}}_2 {\text{O}} + {\text{CO}}_2\)
Free iron radicals present in the inhaled coal dust, Fe2+ and Fe3+, are mainly associated with increased hydroxyl radical, ROS and DNA oxidation product production through the Fenton reaction:
\(\begin{gathered} {\text{Fe}}^{2 + } + {\text{O}}_2 \to {\text{Fe}}^{3 + } + {\text{O}}_2^* \hfill \\ {\text{Fe}}^{2 + } + {\text{O}}_2^* + 2{\text{H}}^+ \to {\text{Fe}}^{3 + } + {\text{H}}_2 {\text{O}}_2 \hfill \\ {\text{Fe}}^{2 + } + {\text{H}}_2 {\text{O}}_2 \to {\text{Fe}}^{3 + } +^* {\text{OH}} + {\text{OH}}^- \hfill \\ {\text{Fe}}_{3 + } + {\text{H}}_2 {\text{O}}_2 \to {\text{Fe}}^{2 + } +^* {\text{OOH}} + {\text{H}}^+ \hfill \\ \end{gathered}\)
The mining operations are inevitable; these cannot be stopped as long as mining is active and workers are supposed to work in the active mining areas, where dust is constantly generated. Hence, personnel safety, proper education and understanding about the potential health hazards caused by inhalation of dust, and preventive measures are the chief requirements for disease control. However, identification of significant risk factors associated with CWP prevalence, understanding of disease mechanisms and appropriate disease diagnosis using significant markers, are the key aspects to develop a reliable medical management plan to counter the problem. Also, constant health check-ups and medication to primarily affected people are key steps to mitigate this deadly occupational health hazard.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The mining industry plays an important role in the industrial development of almost all countries in the world. The occupational risks associated with coal minerals mining are well-documented in mining structures to health problems associated with chronic exposure to mining dust (Cohn et al. 2006). Constant intake of the occupational doses of mineral-rich dust is dangerous, often leading to diseases such as asthma, silicosis, asbestosis, and pneumoconiosis (Moitra et al. 2015). Pneumoconiosis refers to a group of fibrotic lung diseases includes; fibrosis or asbestosis (inhalation of fibrous minerals such as asbestos), silicosis (mineralized silica), coal workers’ pneumoconiosis (CWP) or ‘black lung’ (coal dust), berylliosis (mineral beryl, though rare) and talcosis (inhalation of soft minerals talc or steatite) (Moitra et al. 2015; Zosky et al. 2016). Coal is prime fossil fuel mined globally for energy production. Also, there are millions of workers from coal-producing countries that are associated with different coal mining operations and transportations (Mukherjee et al. 2005). These occupational activities are responsible to produce a high density of fine respirable coal dust in the working environment. Long term inhalation of coal dust by exposed workers and its retention in the lung parenchyma leads to diffuse fibrosis of lung tissue, which refers to a kind of chronic systemic disease known as CWP (Karkhanis and Joshi 2013; Han et al. 2017). The disease has been divided into two major forms; Simple form which is called Simple Coal Workers’ Pneumoconiosis (SCWP). Another form is complicated or Progressive Massive Fibrosis (PMF) (Zheng et al. 2017). The global prevalence of the disease varies with countries and responsible factors are discussed in the following sections.
1.1 Global Scenario of CWP Associated with Coal Industries
The CWP is the most common occupational disease encountered among coal miners (Cohn et al. 2006). As one of the oldest lung diseases, the first case of CWP was recognized in 1822 as ‘miner’s asthma’ and the first case report was reported by Gregory in 1831 (Moitra et al. 2015). After that several studies have been confirmed the cases of CWP and also reported its prevalence in different countries. In addition, significant variation in the prevalence of CWP has been found between countries over time.
The Coal Mine Safety Act (CMSA) of 1969 has been introduced in the United States (US) to compensate the miners and lessen the dust permissible limit. In consequence, the prevalence of CWP has been reduced to 2%; 1995–1999 from 11.2%; 1970–1974 (Moitra et al. 2015). Since CMSA was imposed in the US in 1969, a similar reduction of CWP prevalence from 6.5%; 1970 to 2.1%; 1990, was reported. However, the increased prevalence of CWP 3.2% in 2000 along with PMF 0.31%; 2000 from 0.14%; 1990 was reported in US (Zosky et al. 2016). In the same years, the study conducted by the Centers for Disease Control (CDC) showed 46 (2.0%) of 2257 miners had CWP (Moitra et al. 2015). Coal Industry Ministry of China conducted the national Chinese epidemiological cross-sectional survey in 1992 and reported a 6.49% overall prevalence of CWP among the total of 1,839,456 surveyed coal miners (Li et al. 2007). Systemic analysis of CWP prevalence in China has been estimated using data from 11 studies conducted from 2001 to 2011 on different geographical coal mining locations. The pulled number of dust exposed miners’ population was 173,646 from which 10,821 has been confirmed for CWP with 6.23% of prevalence (Mo et al. 2014). Moreover, the prevalence of CWP (60.28%) was shown by the China National Institute of Occupational Health and Poison Control (CNIOHPC) amongst a total of 23,152 diagnosed cases of pneumoconiosis in 2013 (Han et al. 2015). Another study was showed a comparative analysis of CWP prevalence in China, the USA and Australia. In detail, the data presented by the Chinese Center for Disease Control and Prevention (CCDC) and National Health and Family Planning Commission (NHFPC) was stated that 127,368 cases of CWP have been diagnosed against the total of 249,864 pneumoconiosis cases with a 50.98% prevalence from 2003 to 2016. National Institute for Occupational Safety and Health (NIOSH) was reported 37,965 confirmed cases of CWP of International Labour Office (ILO) category from 1968 to 2015. Whereas in Australia, a 24-year mortality surveillance study reported that <10% CWP fatalities accounted against 1000 cases of pneumoconiosis between 1979 and 2002, while, only 26 cases were reported by the Queensland government from 1986 to 2018 (Han et al. 2018). The overall average CWP prevalence in other countries including United Kingdom (UK) for the year 1959 to 2000 was 4.07%, India for the year 1986 to 1988 was 15.4%, Turkey for the year 1985 to 2004 was 4.01% and Brazil for the year 1988 was 5.6% has been reported (Mo et al. 2014).
Despite the variation in reported CWP cases throughout the globe, the prevalence of CWP is usually found higher in developing countries than in developed countries (Zheng et al. 2017). Coal rank, geographical locations of coal mines, major mining activities, rules and regulations regarding occupational hygiene and dust permissible limits, years of working, age, personnel safety measure and advancement in disease diagnostic parameters, etc., are major accountable as well variable factors associated with the global scenario of CWP.
1.2 Types of Coal and CWP
Coal is a mixture of heterogeneous substances made up of inorganic and organic materials. Coal rank is defined as the extent to which organic materials have matured during a geological time, which refers to the quality of the coal. Anthracite, bituminous, sub-bituminous and lignite are the four major coal types ranks based on fixed carbon, volatile matter, heating and gross-calorific value shown in Table 1 (Huang et al. 2005; Gamble et al. 2012; McCunney et al. 2009). Regional differences in CWP prevalence have historically been understood to be related to coal rank. The prevalence of CWP spans an order of magnitude from anthracite to low volatile coal (Gamble et al. 2012; Zhang et al. 2002; Antao et al. 2005).
A systematic meta-analysis study in china showed a 5.88%, 5.38% and 0.15% prevalence rate of CWP for coal workers exposed to bituminous, anthracite and lignite coals respectively (Mo et al. 2014). China has 75% bituminous, 13% lignite and 12% anthracite coal reserves and reported 6.23% CWP prevalence against total coal mining workers from 2003 to 2017. While the USA has 89.73% reserve coal including bituminous and sub-bituminous, 10.04% lignite and only 0.23% anthracite coal which can be associated with a lower CWP prevalence of 3.2% than China. Moreover, a negligible 0.5% CWP prevalence rate has been accounted for in Australia; the country has 48.56% black and 51.44% brown coal reserve (Han et al. 2018). Interestingly, a report from the UK mentioned the relationship between the period of exposure such as 8, 16 and 36 years and coal rank viz; highest, intermediate and lowest respectively were required for developing 20% prevalence of CWP. Moreover, a study in South Wales confirmed the highest number of radiological abnormalities in anthracite mines and intermediate in steam coal with the lowest in bituminous coal mines (Gamble et al. 2012).
1.2.1 Diagnosed Cases of CWP
Earlier reported global picture of CWP prevalence will simply alleviate the number of confirmed CWP cases from coal-producing countries. In brief, major coal-producing countries including China have 119,380 diagnosed cases up to the end of the year 1992 and 127,368 cases for the year 2003 to 2016. Whereas 37,965 confirmed CWP cases have been reported from the USA for the period of the year 1968 to 2015 and from the year 1986 to 2018, Queensland has reported 26 cases (Li et al. 2007; Han et al. 2018). In 1997, a study has been undertaken to find the CWP prevalence in 10 coal mines regions of Madhya Pradesh and Orissa states of India by re-reading of chest x-ray plates taken during the Periodical Medical Examination over 5 years and confirmed the total of 1,317 cases for CWP from 43,504 x-ray film read (Parihar et al. 1997). Although several studies have been commenced in different coal mining regions of various countries to elucidate the prevalence of CWP, the prevalence of CWP could not be identified precisely.
1.2.2 Death Rate
Chronic doses of respirable coal dust particles for the whole duration of service leads to the condition of CWP; which may transform into PMF and can be lethal (Zosky et al. 2016). A study on mortality probability from state-owned mines in the east of China from 1963 to 2014 explained the mortality rate of 19.19% amongst a total of 495 diagnosed cases of CWP including 12.1 and 57.4 years of average life span and death age respectively (Han et al. 2017). In 2003, NIOSH proposed an average 50% death rate for CWP attributed to the total death accounted for pneumoconiosis for the period of 10 years from 1990 to 1999 (Huang et al. 2005). Besides that, the Global Burden of Disease (GBD) Study 2013 on the cause of specific mortality demonstrated the global deaths for diseases encountered under chronic respiratory disease for years 1990 and 2013. The overall deaths accounted for chronic respiratory diseases in 1990 were 3,490,200 and in 2013 the number went to 4,267,500 with a median percent change of 21.9. Also, the reported deaths for CWP were 28,900 and 25,200 in 1990 and 2013 respectively showing –13.7% of median. From this data the global percent death rate for CWP was calculated at 0.82; 1990 and 0.59; 2013 with respect to total death accounted for chronic respiratory diseases along with 35.6% of margin for median percent change from 1990 to 2013 (GBD 2015).
1.2.3 Importance of Geographic Locations
The progressive geological alteration or coalification can be specifying the coal rank from lignite to anthracite (Gamble et al. 2012). The higher geological age of coal resulted in the coalification of high-rank coal. In West Germany, maximum pneumoconiosis occurrence was reported in high ranked coal mines which, eventually has a high mass-number index (MNI: the mass in mg/m3 per 1000 particles/cm3 of dust in respirable range) besides, the similar MNI and comparable mineral content associated with low-rank coal. Also, the cytotoxicity studies signified the more damage to cells exposed with respirable dust of coal had maximum geological age (Bennett et al. 2015). Despite the coal rank, coal mine type (underground and opencast), structural geology of mines, size diversity in the coal seam, the geographical distribution of coal with mineral contents, varieties in mining technique and dust controlling measures or enforcement of permissible exposure limits are the cumulative factors may also participate in the progression of CWP (Han et al. 2018; Huang et al. 2005; Antao et al. 2005). Moreover, the maximum CWP incident has been observed with underground coal miners than open-cast due to the denser dust concentration and confined ventilation circuits in underground environments (Han et al. 2018). Principal working locations and periods of underground exposure are the relative factors responsible for CWP prevalence. Coalface > Miscellaneous > Maintenance > Transportation > Surface are the working environment having higher to lower CWP prevalence rate with increasing years of exposure (Huang et al. 2006). Geographical locations having a large proportion of high-rank coal, the maximum number of underground coal mines and structural geology including complex fold and faults with thin-medium coal seam width and increased collision of rock during coal extraction activities have resulted in increased MNI which was further associated with prevalent CWP episodes.
1.2.4 Compensation Schemes
Many diseases are associated with occupational exposure and are clinically indistinguishable from non-occupational diseases. Evolution in diagnostic parameters for respiratory lung diseases facilitates the eligibility determination for compensation and benefits. Most of the coal-producing countries has evolved the disease diagnostic criteria and their compensation scheme for victims of CWP. Lung tissue examination from biopsy, autopsy specimen or by the chest x-ray film developed and interpreted according to an international convention developed under the auspices of the ILO has been accepted for the benefits. Based on x-ray film interpretations, the positive CWP miners have been allowed to transfer their job from dusty to least dusty workplaces (Weeks and Wagner 1986). The benefit has been provided to survivor miners (disabled), or dependants in case of death due to CWP by the U.S. Department of Labor, Division of Coal Mine Workers’ Compensation (DOL) operated, Title IV of Federal Black Lung Program and administered claims filed under the Black Lung Benefits Act of 1969 with time to time amendments (Whitaker 1981; Almberg et al. 2018). The Department of Natural Resources Mines and Energy (DNRME), Queensland offers the free respiratory assessment through the Coal Mine Workers’ Health Scheme. Depending upon the degree of permanent lung impairment, the victim has been entitled with lump-sum compensation of $330,240 and additionally he/she will be eligible for an amount of $123,700 in cases diagnosed with CWP (Queensland 2020). In India, a compensation policy for CWP and other occupational diseases has been established under Mines Act- 1952 & Employee Compensation Act–2009. Occupational disease board, constituted by Coal India Limited, Ministry of Coal examines the suspected CWP cases, detected during Periodic Medical Examination to confirm the disease and compensation to be paid to the employee and rehabilitation by change of job. Also, the disease progression has been reviewed by the board in retired coal workers (Fourteenth Report 2015).
This portion reveals the cover on circumstances of CWP and associated global fatalities. Geographic seating, coal rank and mining activities are key factors for the production of toxic dust which are found to be dealing with the worldwide variation in the prevalence of CWP. Even though, it is needed to locate the actual component associated with coal mine dust and what role it plays in the buildup of CWP.
2 Factors Contributing to the Development of CWP
In the past 3–5 decades, CWP; the most common disease encountered among coal miners (Cohn et al. 2006) has received more attention due to its clear occupational association than other respiratory diseases (Huang et al. 2002). Predominant exposure of airborne (Han et al. 2015) respirable mixed coal dust has a strong relationship with the prevalence of CWP (Mukherjee et al. 2005; Mo et al. 2014; Zhang et al. 2002; Huang et al. 2002) while its rate of progression is related to the mass of exposed dust during working lifetime (Antao et al. 2005). Initially, silica or quartz content was thought to be a major potential confounding risk factor until CWP was found to occur when there was minimal silica in the coal mine dust (Gamble et al. 2012; McCunney et al. 2009). Likewise, quartz composition does not emerge to account wholly for alteration in CWP prevalence. However, the contribution of coal rank in the development of CWP has been confirmed by epidemiological studies and also suggested that the carbon content of coal is a vital factor to assess CWP risk (McCunney et al. 2009). A different, modern epidemiological (Huang et al. 2005; McCunney et al. 2009) in vivo animal (Aladdin et al. 2013) in vitro cell line (Zhang et al. 2002; Huang et al. 2002; Aladdin et al. 2013) and experimental laboratory studies (Cohn et al. 2006; Dalal et al. 1995) investigated and proved the constructive role of iron which, released from pyrite fraction present in the coal as a strong contributing factor in the prevalence of CWP (McCunney et al. 2009).
2.1 Coal Dust
Though organic carbon is the predominant constituent, a wide range of inorganic minerals is also present in coal, including carbonates (calcite and siderite), sulfide ores (pyrite), clays (kaolinite and illite), oxide ores, quartz, phosphates and other elements including metals, nonmetals and metalloids shown in Table 3 (Cohn et al. 2006; Zosky et al. 2016; Huang et al. 2005; Gamble et al. 2012; Zhang et al. 2002; McCunney et al. 2009; IARC 1997). The proportion of minerals (general range; Table 2) in coal varies broadly along with the type of coal and from coal seam to seam (Gamble et al. 2012; IARC 1997). Numerous activities are involved in the process of coal excavation, primarily responsible for the production of heterogeneous fine particulate matter, known as coal dust. Along with coal cutting, roof bolting, fractured rock or distribution of rock dust (low-silica limestone dust) to prevent explosions and diesel exhausts (> 1 micron) are other major sources associated with an elevated level of mixed respirable (> 10-micron aerodynamic diameter) dust density in coal mines (IARC 1997).
2.1.1 Composition of Coal Dust
The complex and heterogeneous nature of coal mine dust acts as a main environmental factor in the development of simple CWP (McCunney et al. 2009; IARC 1997). More than 50 different elements, their oxides (Table 2) and minerals (Table 3) at variable concentrations are ancillary components involved in the formation of coal dust with carbon as the primary component (IARC 1997).
Most information on coal mine dust composition has been raised from industrial hygiene studies in coal mines of different countries. Additionally, coal dust sampling in coal mines focused on the components associated with lung diseases, which includes mixed coal dust, oxides, silicates and mineral metals and non-metals. Earlier studies eventually reported the composition of oxides (Quartz or silica) and silicates (Kaolinite and Sericite/Illite) in coal dust. In contrast, the composition of toxic elements has been started to report in several recent industrial hygiene studies. The average composition of these elements from different mines and countries is shown in Table 4 with the year of the study conducted (Mukherjee et al. 2005; IARC 1997; Aladdin et al. 2013; Dalal et al. 1995). Percent content of elements (Fe, Ca, S, Al) was represented amongst the total element composition in coal dust.
Knowing the amount of diversity in coal dust compositions and concentrations of various coal mines with periods of exposure throughout miners’ populations can be considered as collective measures to establish statistics of CWP prevalence. However, the identification of crucial components and bioavailability from coal with exposure doses through inhalation of respirable coal dust will provide strong evidence-based data on CWP.
2.1.2 Release of Toxic Elements from Coal
Excavation of coal, transportation, washing or purification and final industrial and other utilizations are the activities and occupations firmly involved in coal processing and use. These coal processing activities release several toxic pollutants along with heavy metals and harmful trace elements into water bodies (mineralized water) which channelize as “Mine drainage” and often elevate the mineral contents in groundwater and soil (Khan et al. 2017) causing environmental pollution. The ordinary route of emission for toxic trace pollutants, organic and inorganic compounds goes through the natural geochemical process of hydration and oxidation of coal. Oxidation reactions take place in iron pyrite (FeS2) present in coal seam eventually produce sulphuric acid and emit the ferrous (Fe2+) ions (Jaishankar et al. 2014). Another process of toxic components (As, Cd, Co, Cr, Cu, Hg, Fe, Mn, Ni, Pb, Se, and Zn) emission is discharging of fly ash via direct combustion of coal and fuel oil from coal-fired power generation plants (Reddy et al. 2005). Moreover, pyrolysis is a critical initial reaction stage of coal combustion also responsible for the release of heavy metal elements including Hg, Cd, As and Pb via increased volatility with increasing the temperature (Zhou et al. 2019).
Major inorganic constituents such as silicon, aluminium, sulfur and oxides of Fe, Ca, Mg, Na, K, Ti and other trace elements including Ni, Zn, Cd, Hg, Li, Cr etc. analyzed as ash are necessary to remove from coal to retain its calorific value. Various chemical processes including step-wise acid leaching (HCl, H2SO4), use of oxidizing and chelating agents (H2O2, K2Cr2O7, NaOCl, Fe2(SO4)3 and EDTA, citric acid), alkali-acid leaching treatments (NaOH, caustic-HCl–HNO3 and caustic-HCl–H2SO4, aq. KOH or aq. Ca (OH)2) (Dhawan and Sharma 2019; Praharaja et al. 2002) and bioleaching using Acidithiobacillus ferrooxidans (Hong et al. 2013) prove 80%–90% demineralization and desulfurization of coal varieties. Releasing of such toxic elemental pollutants from coal has been accumulated in the environment and prevalently acts as a health threat for workers along with local populations living near coal processing industries.
2.2 Pyrite Associated with Coals and Liberation Mechanism of Allied Trace Elements
Coal formation has taken place in a reduced environment with an irregular oxidation process (Zhao et al. 2020; Elsetinow et al. 2001). Various stages of coal development from peat to coal are associated with the formation of pyrite (FeS2) by enrichment of sulfur (S) and iron (Fe) content. In detail, disseminated sulfate from seawater into peat converted in hydrogen sulfide, polysulfides and elemental sulfur by the bacterial reduction process. The reaction between ferrous iron and hydrogen sulfide resulted in the generation of pyrite crystal and mackinawite (FeS0.9) which further reacts with elemental sulfur to form greigite (Fe3S4) and finally converted to framboidal pyrite (FeS2). Pyrite in coal commonly appears in microscopic or macroscopic form as a massive, disseminated and thin layer deposited in cleats/fractures, cell fillings and pyrite veins through circulating underground water or from basinal fluids after the solidification of coal (Hong et al. 2013; Elsetinow et al. 2001; Chou 2012). Megascopic and microscopic analysis of coal lumps of sub-bituminous coal samples of Padhrar coal mine region of Pakistan further clarifies the presence of pyrites in thick and thin bands and nodules forms. Quartz masses and clay materials were also observed closely associated with the organic coal matter shown in Fig. 1a and b (Shahzad et al. 2016).
Pyrite is the major iron sulfides mineral contributed as an inorganic part of coal with the association of some trace elements including As, Cd, Fe, Hg etc. Moreover, iron is present in the coal predominantly associated with sulfur in the form of iron disulfide or pyrite (Cohn et al. 2006; Zhou et al. 2019; Elsetinow et al. 2001; Rezaee and Honaker 2020). Some weathering products of pyrites including szomolnokite, rosenite, melanterite, coquimbite, roemerite, jarosite, and halotrichite, all are majorly composed of ferric sulfide. Oxidation of pyrite started through the adsorption of oxygen and water at the surface of pyrite resulting in the release of ferrous iron (Fe2+), sulfate (SO42−) and hydrogen (H+), leading to the development of acidic conditions in coal. Low pH (pH ~ 3) condition in medium further responsible to release the associated elements (Elsetinow et al. 2001; Rezaee and Honaker 2020). Also, the bioleaching process of coal by A. ferrooxidans has been involved in the oxidation of pyrite to release the sulfur (S) and Fe2+ associated with pyrite and released S can further oxidize to form SO42− (Hong et al. 2013). The earlier mentioned techniques of demineralization and desulfurization of coal are ultimately associated with the oxidation of pyrite and resulting in the release of allied trace elements (Dhawan and Sharma 2019; Praharaja et al. 2002; Hong et al. 2013). The oxidative condition of pyrite associated with coal has the potential to increase total dissolved solids (TDS) and promote the degradation of water quality (Rezaee and Honaker 2020).
Recent epidemiological and experimental studies have demonstrated the concrete role of bioavailable iron (both ferrous and ferric), an oxidation product of pyrite present in coal mine dust in the generation of reactive oxygen species (ROS), a key component in the inflammatory response associated with CWP. Additionally, the findings of these studies stated that the CWP can be correlated with the amount of pyrite present in the coals (Cohn et al. 2006; Zhang et al. 2002; Huang et al. 2002; McCunney et al. 2009; Dalal et al. 1995).
2.3 Bioavailable Iron (BAI)
Formerly, CWP was considered to be an alteration of silicosis, caused due to the inhalation of a crystalline form of silica or quartz associated with coal dust. Now, several evidence-based studies reverse the former understanding of silica exposure and CWP prevalence by validating the role of iron-containing coal dust in the associated evolutionary mechanism of CWP. In general, iron is well-known transition metal present in coal having redox potential, which participates in most of the reversible one-electron oxidation–reduction reactions by switching between the two oxidation states, i.e. ferrous (Fe2+) and ferric (Fe3+) which are further accountable for the production of oxidants (Huang et al. 2005). Free iron radicals present in the inhaled coal dust, Fe2+ and Fe3+, are mainly associated with increased hydroxyl radical, ROS and deoxyribonucleic acid (DNA) oxidation product production through the Fenton reaction (Cohn et al. 2006; Huang et al. 2005; Dalal et al. 1995; Elsetinow et al. 2001; Schins and Borm 1999) given as follows:
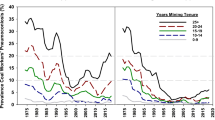
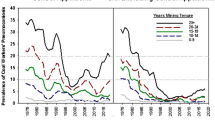
Fenton reaction-based evolution of such toxic radicals is found to be a chief component in inflammatory responses and biochemical as well as immunological alterations associated with CWP and positively correlated to the iron content of inhaled coal dust (Cohn et al. 2006; Huang et al. 2005, 2002; Zhang et al. 2002; Dalal et al. 1995; Schins and Borm 1999). Moreover, using electron spin resonance and the spin-trapping agent DMPO, scientists show that, Fe2+ can reduce oxygen molecule (O2) in an aqueous medium (O2 solubility in water: 3%, v/v) to produce oxidants. The abundance of O2 and hydrogen peroxide (H2O2) in the pulmonary medium makes smoother the progress of production of hydroxyl (*OH) radicals after inhalation of Fe2+−containing coal particles, ultimately resulting in lung injury (Huang et al. 1998). The potential to generate *OH radicals and induce lipid peroxidation by the bituminous coal samples from a different source of origin (Utah and West Virginia) were reported which was correlated with the available surface iron and further suggested its role in the development of CWP in different coal mining areas (Dalal et al. 1995). Effects of coal dust from Pennsylvania (PA) (high CWP prevalence) and Utah (UT) (low CWP prevalence) coal mine region on JB6 mouse epidermal cells were studied. Activator protein-1 (AP-1) and nuclear factor of activated T cells (NFAT) which are transcription factors associated with regulation of cytokines were activated by PA coal while UT coal did not show any activation in JB6 mouse epidermal cells. For comparison, cells were also treated with ferrous sulfate and found that iron transactivated both AP-1 and NFAT. The observed results showed that a high amount of bioavailable iron present in PA coal is responsible for the activation of AP-1 and NFAT which are the mediator for inflammation associated with CWP (Huang et al. 2002). Another study reported clear regional differences in the prevalence of CWP and correlated with the predicted amount of BAI present in coal samples of seven US state coal mining regions shown in Fig. 2. The highest CWP prevalence commonly observed in bituminous coal miners of PA (cumulated prevalence of 45.4%) and least common in miners of Colorado (CO) i.e. 4.6%, showed a positive correlation with predicted BAI 11.82 and 0.15 mmol/100 gm dry coal from studied coal samples of PA and CO respectively after adjusting for age and years spent in underground mining shown in Table 5 (Huang et al. 2005).
Correlations between pyrite content of coal and generation of H2O2 and *OH radicals were reported, which further led to degradation of RNA. The possible mechanism behind oxidation of biomolecules shows in (Fig. 3), dissolved O2 reacts with either Fe2+ at the pyrite surface or dissolved Fe2+ to form H2O2 through the Haber–Weiss reactions (1), which may further react with dissolved Fe2+ to form *OH through the Fenton reaction (2). The study concluded that the size, shape, exposed surface area, exposure period along with pyrite content of coal will be cumulative factors determining the prevalence of CWP among coal miners (Cohn et al. 2006).
Amount of BAI (both Fe2+ and Fe3+) released in medium (pH 4.5, 3 days incubation period; which mimicking lysosomes condition) from coal dust samples of three known CWP prevalence (Utah; UT, West Virginia; WV and Pennsylvania; PA) mining regions established good correlation (r = 0.92) between BAI and prevalence of CWP. Moreover, the released amount of Ca2+ associated with calcite (CaCO3); known as an inhibitory factor for iron bioavailability was found to inversely correlate with CWP prevalence shown in Table 6. The percent release of bioavailable metals (Fe and Ca) was calculated by dividing the amount of metal released under acidic conditions (10 mM phosphate, pH 4.5) with the total amounts of that metal present in the coal × 100 shown in Table 7. The results were further confirmed by measuring cellular iron and lipid peroxidation in human lung epithelial Type II A549 cells exposed with coal dust samples against control, which showed the active role of BAI in the generation of oxidative stress; promoting lipid peroxidation and promising inference in CWP prevalence (Zhang et al. 2002).
However, there was also noted that the accumulation of iron could result in part from its coordination by humic-like substances (HLS), which comprise up to 30% of dust weight in certain coals. Scientists suggested that HLS in coal dust with iron clad-ions subsequently catalyze oxidant generation and the accumulation of this metal in the lungs. The relevance of iron coordination by HLS in lung injury after exposure to coal dust can assist in the understanding of certain clinical features of CWP (Ghio and Quigley 1994). Hence, the efficacy of coal-bearing dust in CWP multiplies due to the addition of iron. Despite all, it is important to understand the mechanism of iron bioavailability from coal dust.
Iron released in 10 mM phosphate solution of pH 4.5, which mimics the phagolysosomes of cells are defined as bioavailable iron (BAI). The amount of BAI can be calculated according to the chemical interactions of pyrite, sulfuric acid, calcite, and total iron. BAI mainly consists of water-soluble iron including ferrous and ferric sulfate released from coal via oxidation of pyrite by the following equation:
One mole FeS2 will produce 1 mol BAI as ferrous sulfate (FeSO4) and 1 mol sulfuric acid (H2 SO4) i.e. iron present in coals can become bioavailable by pyrite oxidation, which produces ferrous sulfate and sulfuric acid. However, calcite (CaCO3) is the major component in coals that neutralizes the available acid and inhibits iron bioavailability. Therefore, levels of BAI in the coals are determined by the available amounts of acid after neutralization by calcite and the amount of total iron in the coals. If CaCO3 is present in the coal, it will consume the acid and neutralize the pH as follows:
The consumption of acid will accelerate the oxidation of bioavailable iron to non-bioavailable forms of iron. Therefore, no BAI will accumulate when CaCO3 is present (Huang et al. 2005, 1998; Zhang et al. 2002). If CaCO3 is absent in the coals, acid solubilization (H2SO4 earlier produced from oxidation reaction of pyrite) of another iron-containing compound, i.e. siderite (FeCO3) would take place and release more BAI as per the following reaction (Huang et al. 2005):
2.3.1 Physicochemical Factors Associated with Iron Bioavailability
Pyrite mineral oxidation is the central and well-known process for releasing iron from coal. Consequently, multiple physicochemical factors responsible for pyrite oxidation including oxygen partial pressure, temperature, and relative humidity along with the amount of calcite, pH and buffering capacity of coal are found to be associated with iron bioavailability (Cohn et al. 2006; Huang et al. 2005, 1998, 1994; Zhang et al. 2002; Elsetinow et al. 2001; Rezaee and Honaker 2020; Santos et al. 2016). However, the physicochemical conditions of coal are likely to vary in different coal mines due to variable amounts of sulfur and other minerals content in the coal (Huang et al. 1994). A limited amount of BAI released from the coal having higher CaCO3 and FeSO3 content was observed because the acidic condition of coal simply neutralized by available CaCO3 leading to less acid available for iron solubilization and cannot become bioavailable (Huang et al. 2005; Zhang et al. 2002; Aladdin et al. 2013). Also, it was observed that, released Fe2+ in acidic pH conditions, acted as the primary oxidant thus escalating the pyrite oxidation process (Huang et al. 1998) followed by maximum bioavailability of iron. Furthermore, higher pH (Huang et al. 2005) and low-temperature oxidation of coal (Elsetinow et al. 2001; Sen et al. 2009) would facilitate ferrous and ferric ion oxidation and leads to the formation of goethite (FeOOH), which is water-insoluble and thus limited the more iron bioavailability. Other factors such as humidity, ageing period and grinding duration of coal (pyrite particle size) and pH were also manipulating the formation and stability of BAI (hydrated FeSO4) which resulted in oxidation of pyrite. Briefly, 15- and 30-min air grinded pyrite powder divided and allowed to age (to be oxidized with oxygen) in saturated solution further these saturated solutions allowed to establish relative humidities of 5.7%, 57.6% and 89.8% respectively. Aged pyrite particles of 15 and 30 min grinding and respective humidities mixed with three coal samples and oxidizing activity of suspension filtrated of pyrite-coal mixtures (Gardanne suspension, Escarpelles suspension, La Mure suspension) were evaluated. Results showed the highest oxidizing activity measured through electron spin resonance (ESR) in Escarpelles and La Mure suspensions compared to Gardanne suspension containing equal aged pyrite. Moreover, the pyrite oxidation activity as a function of relative humidity and time were reported higher for 30 min grinding and 89.8% of relative humidity while it was decreased for 15 min grinding and decreasing humidity (57.6% and 5.7%). Whereas the maximum change in pH from 7.18 to 3.05 and 6.53 to 3.04 were observed in high oxidizing pyrite-coal suspensions (Elsetinow et al. 2001; Huang et al. 1994). All such physiochemical conditions of coal were reported as influencing factors for the bioavailability of iron from coal.
2.3.2 Environmental Stability of BAI
The stability of iron once released as a result of pyrite oxidation depends upon the pH and air exposure in the coal dust (Huang et al. 1994). Stability of BAI i.e. Fe2+ was also influenced by the rate of oxygenation (Singer and Stumm 1970) of Fe2+ to Fe3+ which was higher and immediate in the high pH condition where CaCO3 neutralizes the acid (Cohn et al. 2006; Huang et al. 2005; Zhang et al. 2002) available from the initial steps of Fe2S oxidation. Besides that, a half-life of 3 years was reported for BAI at pH 3 i.e. pH < 4.5 has been optimum for BAI stability while BAI gets precipitated at pH < 4.5 and 8 min of half-life was reported at neutral pH 7 (Aladdin et al. 2013; Huang et al. 1994). Again, the study showed maximum stability of Fe2+ in low buffering capacity coal which release Fe2+ from the exposed coal dust in the phagolysosomes of macrophages and subsequent phagocytosis due to the acidic environment of macrophages (Huang et al. 1998). The environmental, as well as biological stability of BAI, facilitates its involvement in the mechanism of coal dust induced lung impairment in the mining workers. This is one of the concerning health factors associated with coal mine dust and acid mine drainage which needs to be overcome at the mine level.
3 Pathophysiology of CWP
The CWP is a slowly progressive parenchymal lung disease comprising a variety of pulmonary radiological and pathological changes caused by the inhalation of toxic coal mine dust (Zosky et al. 2016; Lassalle et al. 1989). A strong relationship has been associated between inhaled doses of respirable toxic coal dust for working lifetime by miners’ and the risk of developing various forms of CWP with its rate of progression i.e. simple CWP and complicated CWP or PMF (Zosky et al. 2016; Zheng et al. 2017; Antao et al. 2005; Lassalle et al. 1989; Fujimura 2000). The mechanism of pathophysiology in the occurrence of CWP was well studied and dominated by the preceding oxidative potential of inhaled toxic coal dust and subsequent implications of the inflammatory processes (Zhang et al. 2002; Dalal et al. 1995; Lassalle et al. 1989; Fujimura 2000; Huang 2011; Lee et al. 2002). However, the characteristics and inhalation course of toxic dust are required to understand the scenery system of disease.
3.1 Characteristics of Toxic Dust and Inhalation Mechanism
In coal mining, especially in underground coal mines, emission of dust and density is much higher in coal working segments or front galleries and its level decrease to coal extraction galleries. Whereas, higher dust dispersion has been found in open-cut mines. The impact on human health (lung diseases) of coal dust varies with the size, morphology and mineralogical composition of inhaled particulate matter. Respirable dust fraction < 4 μm has been accepted widely, as finer dust particles easily entered the pulmonary system causing higher penetration at alveolar space (Trechera et al. 2020) and able to stimulate ROS which can create oxidative cellular damage and initiate pulmonary inflammation (Valavanidis et al. 2008). A review study on CWP and coal rank mentioned the maximum surface oxygen content, higher electrostatic charge and smaller particle size with larger surface area usually associated with fresh fractured high-rank coal. The charging characteristics of coal dust showed enhanced respiratory deposition and toxicity of airborne respirable particles and the increased incidence of CWP (Gamble et al. 2012). Maximum depletion of ascorbic acid (AA) and reduced glutathione (GSH); an important antioxidant found within respiratory tract lining fluid (RTLF) were recorded for the coal dust has a higher composition of particulate matter (PM) < 4 μm than the dust with more PM > 4. The amount of oxidants generated by coal dust or PM is associated with the size of particles; fine particles having a larger surface area for oxidation reactions compare to bigger PM (Trechera et al. 2020). An appropriate relationship between particle size and the toxicity mechanism of coal dust was proven by the in vitro study conducted on the A549 cell line. Size segregated coal fly ash (CFA) fractions (<1 μm, <2.5 μm, 2.5–10 μm and >10 μm) from three different mining regions (Utah, Illinois, and North Dakota) were tested on A549 cell line for induction and synthesis of inflammatory cytokine, interleukin-8 (IL-8) and amount of ferritin concentration in cells. In results, ferritin concentrations were recorded 1.4, 1.3 and 1.3 fold higher for the CFA fraction of <1 μm exposed cells than <2.5 μm fraction treated cells of Utah, Illinois, and North Dakota CFA, respectively. Moreover, the level of IL-8 in cell growth mediums exposed with CFA fraction of Utah, Illinois, and North Dakota were found to be 1.6, 1.9, 1.4 fold higher than the levels of IL-8 with the <2.5 μm fractions of the same coal type. Results have summarized that the amount of IL-8 secretion and ferritin concentration induced by CFA were reliant on the type of coal bioavailability of iron from the particles, with utmost response to smaller size fractions which released maximum amount of iron (Smith et al. 2000). The characteristics of coal mine dust samples including its ash content, the size distribution of particles, type of dust, clay mineral content, trace element content, wettability, surface carbon and oxygen content, explosion tendency, specific surface area were examined and showed its possible health impact. Two underground coal dust samples collected from return airways locations of mines namely JLS-3 and LTS from Jiulishan and Lutaishan coal mines located in China respectively contain high amounts of ash, whereas the relation between dust particles was agglomeration and adhesive type. Both the samples also had higher clay minerals and the greatest wettability. The surface carbon and oxygen element content of LTS was lowest, 53.76% and the highest 43.75% were recorded. The specific surface area for LTS was highest i.e. >400m3/kg and for JLS-3 was 350–400m3/kg which indicates both the dust samples had higher explosion tendency. Moreover, the particle size distribution proportions of PM10 were 58.95 and 60.09% respectively for JLS-3 and LTS samples which were fine types and highest among the all studied samples. These characteristic measures of dust particles are indicating their toxicity, however, the proportion of fine particles >10 μm in both samples were highest, indicating more harm by directly entering the respiratory tract and alveoli causing permanent lung injury (Su et al. 2020).
Miners exposed continuously to high-density dust concentrations with the maximum composition of respirable PM are prone to getting chronic inhalation doses and the greatest dust deposition in the lung parenchyma. The inhalation mechanism associated with this dust mostly depends upon the particle size and its density in the exposed environment (Mukherjee et al. 2005; Huang et al. 2005; Lee et al. 2002). The US Environmental Protection Agency and other agencies in the air pollution regulation proposed two main categories of PM such as PM2.5 which refer to particles with an aerodynamic diameter (a.d.) <2.5 µm and PM10. The human respiratory system is divided into an extrathoracic (oro- or nasopharyngeal) region where the PM10 has been deposited, the tracheobronchial tree (cylindrical airways) where the particle with a.d. between PM2.5–PM10 has been restricted while the last acinar (alveoli, gas-exchange section) region which is most important for PM2.5 and ultrafine (<0.05 µm) particle retention and disease progression (Trechera et al. 2020; Valavanidis et al. 2008; Su et al. 2020). Some important factors are associated with the inhalation mechanism and particle toxicity which includes the chemical and morphological properties of the dust, particle durability and leaching, particle deposition and translocation. Also, the host factors such as lung volume, breathing rate and depth, and particle clearance via mucociliary and interstitial “lymph nodes” clearance routes are deliberating with the severity status of toxicity induced with inhaled dust (Schins RPF and Borm PJA 1999). Such inhalation mechanism and amount of dust deposited at each pulmonary region can be correlated with the histopathological alteration or biopsy evidence of confirmed CWP cases. Moreover, the dust permissible limit in mines of coal-producing countries is varying from 1–3 mg/m3 and somewhere 4 mg/m3 which influenced the dust inhalation pattern and frequency of CWP cases in the globe (Fourteenth Report 2015; IARC 1997). However, the biochemical and molecular changes of CWP come into the picture at letter stages of exposure which strongly facilitate the understanding of definite pathophysiological mechanisms linked with CWP.
3.2 Biochemical and Immunological Aspects
The CWP is considered as one of the human lung pathologies related to oxidative stress (Gamble et al. 2012; Fujimura 2000; Huang 2011; Lee et al. 2002; Valavanidis et al. 2008). Oxidative stress and inflammation are reported to be the most important part of the development of fibrotic damage to the lung tissue in coal dust-induced CWP (Schins RPF and Borm PJA 1999; Lassalle et al. 1989; Fujimura 2000; Huang 2011; Valavanidis et al. 2008). There is an acceptance of the concept of the implication of the inflammatory process in the development of pneumoconiosis. The iron present in the coal reacts with the O2/H2O2 (Cohn et al. 2006; Gamble et al. 2012) when inhaled by the lung to form ROS. These ROS are the mediators which stimulate the activation of alveolar macrophage (AM) and other immune cells (Schins and Borm 1999; Fujimura 2000; Huang 2011). In turn, the activated macrophages are either clear by the lysosomal enzymes or tend to release cytokines. The secretion of these cytokines by the AM as well as lung epithelial cells exposed to coal dust mediate the pathogenesis of CWP. Moreover, oxygen radicals, lysosomal enzymes, inflammatory cytokines, and other pro-inflammatory and pro-fibrotic mediators initiate the process of alveolitis and followed by reparative and fibrotic phase, which stimulates the growth factor and overproduction of fibronectin and collagen, resulting in the development of fibrosis (Zheng et al. 2017; Huang et al. 2002; Aladdin et al. 2013; Schins and Borm 1999; Fujimura 2000; Huang 2011). Thus, oxidative stress and inflammation are the most important parts associated with the pathophysiology of CWP.
The oxidative degradation of RNA molecules was reported by the action of *OH molecules which were generated as a result of H2O2 oxidation through soluble Fe2+ released from the pyrite crystal associated with coal (Cohn et al. 2006). High BAI containing coal dust induced activation of transcription factors (AP-1 and NFA) which regulates inflammatory cytokines secretion were found to be associated with the high level of oxidative stress generation (Huang et al. 2002). The author reviewed the possible intracellular or biological mechanisms involved in the progression of CWP and PMF. They proposed that reaction of coal dust with macrophages cells eventually resulted in the membrane lipid peroxidation and releasing the intracellular enzymes which effectively obliterate the alveolar septa of lung tissue. Another step is the secretion of fibrogenic factors from AMs and epithelial cells exposed to coal dust. These fibrogenic factors are responsible for the development of lung fibrosis (PMF) via fibroblast proliferation and collagen deposition. Also, the pulmonary phagocytosis of coal dust by AMs stimulates cytokine secretion leading to the production of oxidant species, which destroy antioxidant defences mechanism resulting in lipid peroxidation and lung scarring (McCunney et al. 2009).
The biochemical and immunological changes and concerned mediators involved in the pathology of CWP and PMF induced by coal dust were studied by several scientists and proposed the mechanism of associated inflammation. In brief, inhaled coal dust particles stimulates the activation of phagocytes, leads to generation of O2*− and ROS (Huang et al. 2005; Schins and Borm 1999) which further shown to induce the proinflammatory cytokines, tumor necrosis factor-alpha (TNF-α) (Schins and Borm 1999, 1995; Smith et al. 2000; Zhai et al. 1998, 2002; Lassalle et al. 1990), interleukin-1beta (IL-1β) (Schins and Borm 1999; Lassalle et al. 1990), interleukin-6 (IL-6) (Schins and Borm 1999; Zhai et al. 2002), interleukin-8 (IL-8) (Schins and Borm 1999; Smith et al. 2000), monocyte chemotactic protein-1 (MCP-1), transforming growth factor-beta (TGF-β) and intercellular adhesion molecule-1 (ICAM-1) (Schins and Borm 1999) and finally resulted into fibrotic tissue. Specific roles of these molecules in the pathophysiology of disease are shown in Table 8 and the networking of cytokines in Fig. 4.
3.3 Lung Burden and Histopathological Alterations
The CWP is a major inflammatory disease caused by activation of challenged macrophages and effectors molecule in response to coal mining dust. There is experimental evidence on the correlation between silica content in coal and fibrogenic activity in the lungs. Irrespective of silica, pyrite content in coal releases BAI and induced ROS production, which worsens the condition of miners and epidemiologically defined the dose–response relationship between coal dust exposure and CWP prevalence (Cohn et al. 2006; Huang et al. 2005; Zhang et al. 2002). The proposed dust transport mechanism in the lung parenchyma is through erosion of the bronchiolar wall and rupture of the lymph node capsule. Laboratory in vivo studies (King et al. 1958; Kolling et al. 2011; Green et al. 2007; Pinho et al. 2004; Caballero-Gallardo and Olivero-Verbel 2016) was reported the pathological changes in animal lungs exposed to different doses of respirable coal dust.
An acute dose of pure saline (control) or coal dust (iron 2.48% and silica 27.3%) at 3 mg/0.5 ml saline directly exposed by intratracheal instillation in rats, were showed peribronchial tissue and interstitial septa without important alteration in a control group, Fig. 5a1 and a2. Peribronchial and perivascular inflammatory infiltration with macrophages in alveolar space at 7th day of (3 mg coal/ 0.5 saline) installation shown in Fig. 5b1 and b2, while at the 30th day, infiltration of alveolar giant cell and macrophage were observed shown in Fig. 5c1 and c2 (Pinho et al. 2004).
Scientists have different opinions in association to parenchymal changes and the magnitude of coal dust induced lung pathologies. However, exposure history and respiratory symptoms with lung imagining, computed tomography scan and pulmonary function testing could be clinical basics for the diagnosis of simple CWP and other coal dust-related fibrotic lung diseases (PMF and DDF: Dust-related diffuse fibrosis) (Petsonk et al. 2013; Gorman and Cagle 2018).
The inflammatory and fibrotic lesions with emphysema to be found in the lining of a respiratory bronchiole in biopsy spacemen of lung shown in Fig. 6a, which have been proposed specific histopathological alterations for CWP. Whereas, coal nodule associated scar and prominent fibrosis associated with collagen bundle and abundant infiltration of macrophages shown in Fig. 6b were noted for complicated CWP. The study also reported focal emphysema; enlarged air spaces adjacent to coal nodule in lung parenchyma shown in Fig. 6c which also found to be associated with all types of CWP pathologies (PMF and DDF) (Gorman and Cagle 2018). The possible reason behind emphysematous lesions in lung parenchyma is the uncontrolled proteolysis of lung tissue by the elevated level of trypsin. The inactivation of α1-antitrypsin (α1-AT) through oxidative stress generated as a result of pyrite containing coal dust exposure and generation of oxidant species was the basics associated with the proposed reason of lung emphysema in CWP suggested in an experimental research study (Huang et al. 1994).
4 Possible Diagnostic Biomarkers for CWP
Several incurable diseases are associated with older workers having a prolonged history of respirable air-borne dust exposure. Some diseases of coal miners such as CWP and PMF are non-reversible and prevention is supposed to be the best remedial step. Biomarkers play a great role to improve the early identification of risk assessment and disease prevention in the mining environment. The Committee on Biological Markers of the National Research Council (NRC) in the United States of America has defined biomarkers as “Indicators of variation in cellular or biochemical components or processes, structure or function that are measurable in biologic systems or samples” (NRC 1989). Several epidemiological human studies and in vivo animals as well as in vitro cell line experimental studies proposed the utilization of biomarkers for early diagnosis and validation of CWP and PMF. Moreover, biomarkers may indicate the principal steps between exposure of coal dust and resultant lung pathologies in miners (Gulumian et al. 2006; Ayaaba et al. 2017).
In detail, coal dust induces the oxidation process resulting in the production of varieties of oxidants. To scavenge these oxidants, the activity of several antioxidant enzymes such as glutathione peroxidase (GPx), superoxide dismutase (SOD), has been elevated. The level of lipid peroxidation has also increased with respect to increased oxidants. Estimation of these enzymes would act as a biomarker for early diagnosis of CWP in pyrite or bioavailable iron-containing coal dust exposed populations (Zhang et al. 2002; McCunney et al. 2009; Dalal et al. 1995; Gulumian et al. 2006). Bioavailable iron (FeSO4) induced activation of AP-1 and NFAT were reported in the coal dust exposure in vitro JB6 mouse epidermal cell line. Increased levels of these transcription factors in the cell growth medium can translate it as a human serum biomarker for CWP. Moreover, increased ferritin levels in serum can confirm the role of iron in CWP (Huang et al. 2002). TNF-α and IL-1β are proinflammatory cytokines, expressed in the early onset of lung inflammation induced by coal dust. Several studies confirmed the validity of both cytokines as a biomarker in coal miners having CWP (Schins and Borm 1995; Zhai et al. 2002; Lassalle et al. 1990; Gulumian et al. 2006). IL-8 and IL-6 act as a secondary mediators of lung inflammation. Increased level of IL-8 in human lung epithelial cells (A549) induced by high BAI containing coal dust was reported and positively correlated with the higher prevalence of CWP in the same coal mine region (Smith et al. 2000). Another case–control study showed a significantly elevated level of IL-6 in retired coal miners classified with CWP (Zhai et al. 2002). Both IL-8 and IL-6 were proposed as attractive additional serum markers for CWP especially when measured along with TNF-α and IL-1 (Gulumian et al. 2006). Other biomarkers such as platelet-derived growth factor (PDGF) and transforming growth factor (TGF) which showed their role in collagen deposition and fibrosis in the lung. The expression of these biomarkers has been associated with late inflammatory responses and can be utilized as a diagnostic marker for pulmonary fibrotic diseases (Schins and Borm 1999; Gulumian et al. 2006). These stage-specific biomarkers are found to be associated with the prognosis of CWP and PMF. Though several epidemiological and experimental results proved the possible role of such biomarkers in the early diagnosis of these diseases, more adequate data on human studies are necessary to confirm the use of these peripheral blood biomarkers in non-invasive routine medical examinations in dust exposed workers.
5 Preventive Measures
The mining operations are inevitable; these cannot be stopped as long as mining is active and workers are supposed to work in the active mining areas, where dust is constantly generated. Chronic exposure to mining dust often leads to untreatable pulmonary diseases amongst active mining workers and surrounding populations located near mining areas. Therefore, different preventive measures are needed to be taken at several mining stages and activities. However, identification of significant risk factors associated with coal workers lung diseases and their relevant medical understanding is a must to assist reduce the mining hazards. Following are the various preventive approaches proposed to counter the coal mine dust-related occupational health hazards.
Preventive measures at beginning of active mining
-
Geographic locations and related biodiversity should be taken into account before mine planning.
-
Identification of toxic components i.e. traces metals and their concentration in coal or associated with coal seams.
-
Grading of mine according to associated toxic components in coal. i.e. pyrite or BAI.
-
Involvement of innovative engineering approaches in mine design and progression which minimize mining hazards.
Preventive measures during active mining
-
Effective guidelines to use mechanical equipment as a replacement for human workers at mining activities associated with high levels of dust generation.
-
Dilution of dust density with proper circulating aeration and ventilation in underground mining.
-
Use of vigorous dust suppressing interventions in mining regions e.g. continuous water spraying over active coal harvesting phase, haulage road, coal transportation route, coal storage and other dusty areas.
-
Calcite present in certain coals can prevent acid solubilization of iron compounds. Spraying with an aqueous mixture of calcium (active element in calcite) and water may actively reduce iron bioavailability from coal dust before inhalation and thus, may play a protective role in CWP.
-
Chemical and biological leaching of toxic metal from coal before its industrial use and combustions, also reduce the emission of toxic pollutants in the environment.
-
Neutralizing the acidic mine water and leaching of toxic components before drainage into surrounding water bodies.
Preventive measures regarding occupational safety and hygiene
-
Organizing brainstorming sessions for mineworkers towards encouragement for personnel hygiene and safety with eliminations of poor hygienic practices.
-
Research innovations in the development of authentic and handy protective equipment/accessories against dust exposure.
Clinical measure to mitigate incidence and severity of respiratory diseases of coal workers’
-
Identifying the key risk factors, their role in disease mechanism, pathophysiological assessment of diseases, use of early responded diagnostic biomarkers and routines medical checkup of disease suspects are the whole cascade that may prevent the disease incidence and prevalence.
-
Mine rules and regulations associated with mining hazards should be improvised with results of several epidemiological and experimental researches conducted on mining populations.
-
Periodic medical examinations frequency should be increased in high CWP prevalence regions.
-
Identification of disease-prone workers using early diagnostic non-invasive biomarkers is one of the promising approaches toward the development of a reliable medical management plan.
-
Following the international rules and criteria for CWP diagnostic using reliable tools (e.g. lung spirometry, radiography, test in BAL fluids etc.) which assist the disease discrimination with other related diseases.
With the identification of the crucial role of pyrite in aggravating CWP, further research studies need to conduct a more reliable quantitative analysis of coal and associated components with their correlation in chronic lung diseases. Primary health measures and enactment of laws and regulations determined by various mine safety acts of different countries should be properly employed in coal mines. Also, disease surveillance, dust exposure control and compensations to miners are important aspects related to the control of CWP (Ayaaba et al. 2017). Moreover, the disease prevention program in the mining population should involve national and international co-operations, government bodies, research communities and social organizations along with health compensations and insurances under judiciary, healthcare and physicians.
6 Summary
Coal workers’ pneumoconiosis (CWP) is an untreatable but preventable lung disease commonly encountered in coal miners. It is one of the oldest occupational lung diseases where the first case of CWP was recognized in 1822 as ‘miner’s asthma’ and the first case report was reported by Gregory in 1831. Earlier epidemiological studies and surveys including active coal workers as well as retired workers as subjects concluded that the quartz or silica fractions present in coal seams is the key risk factor associated with CWP. These findings were also supported by several experimental laboratory and autopsy studies. Many scientists had studied the coal composition and associated hazardous components which were found to be responsible for dust-related diseases. These occupational health-related investigations have mainly focused on the quartz, clay mineral and some silicates concentrations in coal. The research published in the last 3–4 decades from different countries clarified the uncertainty associated with coal components responsible for the development of CWP. These studies reported the high prevalence of CWP in coal miners working in coal regions where negligible quartz concentration is present in coal. In recent decades, some experimental and epidemiological data on coal miners’ lung diseases and associated risk factors proposed the significant correlation between iron content in coal and the higher prevalence of CWP.
Chronic inhalation of coal dust is the utmost cause of CWP occurrence in coal miners. However, its pathological and biochemical mechanisms were not known earlier. At the end of the twentieth century, the reported evidence showed the bioavailable iron (BAI) present in coal dust and ash has been associated with the generation of oxidant radicals in an aqueous medium. Later, at the beginning of the twenty-first century, several epidemiological studies confirmed the high prevalence of CWP in coal mine regions having maximum bioavailability of iron from coal. Iron is a redox metal mostly combined with sulfur to form pyrite which is an iron sulfide mineral contributed as an inorganic part of coal. Oxidation of pyrite (FeS2) resulting in the release of water-soluble iron which participates in most of the reversible one-electron oxidation–reduction reactions by switching between the two oxidation states i.e. ferrous (Fe2+) and ferric (Fe3+), also known as BAI. Inhaled coal dust particles have been settled in alveolar spaces where its phagocytosis by macrophages further resulted in the release of BAI from pyrite in an acidic phagolysosomal environment. The redox potential of BAI contributed to the development of oxidative stress which eventually triggered the inflammatory processes in the lung. As a result of inflammation, the pulmonary structure got hampered and showed normal i.e. simple CWP to severe (complicated CWP or progressive massive fibrosis) pathological alterations in the lung. The severity of CWP has been also associated with the number of inhalable coal doses throughout the working professional, the composition of the inhaled coal dust and the lifestyle of coal workers.
The prevalence of CWP varies according to coal rank, geological locations of mines and working occupation of miners at mining workplace along with pyrite content in coal. The data published by various government and occupational health-related agencies of coal-producing countries can be accounted for in estimating the global burden of CWP and the death rate. Such global data on death, caused due to CWP has been published in one of the highly reputed medical journals; Lancet which showed more than 28,000 deaths up to 1990 and 25,000 up to 2013 reported for CWP. The top coal producing countries including China have reported 127,368, USA; 37,965, Australia (Queensland); 26 and India; 1,317 confirmed CWP cases of International Labor Organization (ILO) category from 1980 to 2015. Several studies and health surveys have been commenced in different coal mining regions of various countries to elucidate the prevalence of CWP, but the actual prevalence of CWP could not be obtained precisely, which is the drawback for health care programs and miners safety. The lack of implementation of diagnostic criteria and the burden of heavy compensation benefits might be the reasons behind this drawback.
The CWP is a worldwide concern for the occupational health of miners working in coal industries. Prevention is the primary means to tackle this incurable lung disease. Proper implementation of occupational safety rules and laws, dust management controls, utilization of respiratory equipment for minimizing dust inhalation, and removal of toxic components from coal before its final utilization are some preventive measures that can be taken at the mining workplace. Whereas, awareness about personal hygiene and skill improvement for safe mining along with consistent training for miners will also reduce the CWP scenario at the mine level. Despite these preventive approaches, conventional periodic health examinations which have been designed and conducted for mining populations should be modernized. It could be updated with the addition of early diagnostic biomarkers and trustworthy medical diagnostic parameters of ILO standard, which will advance the efforts taken for CWP delineation. However, identification of important risk factors allied with CWP prevalence and understanding the disease progression mechanism are key aspects to develop a reliable medical management plan to counter this problem. Further, the execution of compensation rules with appropriate regulations and free health care plans will improve the economic as well as the living status of coal miners.
References
Aladdin M, Jian J, Yang Q (2013) Laboratory studies of the impact of calcite on in vitro and in vivo effects of coal dust: a potential preventive agent for coal workers’ pneumoconiosis? Am J Ind Med 56:292–299
Almberg KS, Halldin CN, Blackley DJ (2018) Progressive massive fibrosis resurgence identified in U.S. Coal miners filing for black lung benefits, 1970–2016. Ann Am Thorac Soc 15(12):1420–1426
Antao VC, Petsonk EL, Sokolow LZ et al (2005) Rapidly progressive coal workers’ pneumoconiosis in the United States: geographic clustering and other factors. Occup Environ Med 62:670–674
Ayaaba E, Liu Y, Li Y (2017) Measures to control the prevalence of pneumoconiosis in coal mining: a review of the literature. Int J Trans Med Res Pub Health 1(1):4–13
Bennett JG, Dick JA, Kaplan YS et al (2015) The Relationship between coal rank and the prevalence of pneumoconiosis. Brit J Industr Med 36(3):206–210
Caballero-Gallardo K, Olivero-Verbel J (2016) Mice housed on coal dust-contaminated sand: a model to evaluate the impacts of coal mining on health. Toxicol Appl Pharm 294:11–20
Chou CL (2012) Sulfur in coals: a review of geochemistry and origins. Int J Coal Geo 100:1–13
Cohn CA, Laffers R, Simon SR, O’Riordan T, Schoonen MAA (2006) Role of pyrite in formation of hydroxyl radicals in coal: possible implications for human health. Part Fibre Toxicol 3:16
Dalal NS, Newman J, Pack D et al (1995) Hydroxyl radical generation by coal mine dust: possible implication to coal workers’ pneumoconiosis (CWP). Free Rad Bio Med 18(1):11–20
Dhawan H, Sharma DK (2019) Advances in the chemical leaching (inorgano-leaching), bioleaching and desulphurisation of coals. Int J Coal Sci Technol 6(2):169–183
Dos Santos EC, De Mendonça Silva JC, Duarte HA et al (2016) Pyrite oxidation mechanism by oxygen in aqueous medium. J Phys Chem C 120(5):2760–2768
Elsetinow AR, Schoonen MAA, Strongin DR (2001) Aqueous geochemical and surface science investigation of the effect of phosphate on pyrite oxidation. Environ Sci Technol 35:2252–2257
Fourteenth Report (2015) Safety, health and education facilities for inhabitants/workers in coal /lignite mining areas. Standing committee on coal and steel (2014–2015), Sixteenth Lok Sabha, Ministry of Coal. Lok Sabha Secretariat, New Delhi. (August, 2015)
Fujimura N (2000) Pathology and pathophysiology of pneumoconiosis. Curr Opin Pulm Med 6(2):140–144
Gamble JF, Reger RB, Glenn RE (2012) A critical review of coal workers pneumoconiosis (CWP) and coal rank for evaluation of safe exposure levels in coal mining. J Clinic Toxicol S 1:009
GBD (2013) (2015) Global, regional and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385:117–171
Ghio AJ, Quigley DR (1994) Complexation of iron by humic-like substances in lung tissue: role in coal workers’ pneumoconiosis. Am J Phy 267 (Lung Cellular and Molecular Physiology, 11):173–179
Gorman BK, Cagle PT (2018) Coal workers’ pneumoconiosis. Pulmonary pathology, encyclopedia of pathology book series
Green FH, Vallyathan V, Hahn FF (2007) Comparative pathology of environmental lung disease: an overview. Toxicol Pathol 35(1):136–147
Gulumian M, Borm PJA, Vallyathan V et al (2006) Mechanistically Identified suitable biomarkers of exposure, effect, and susceptibility for silicosis and coal-worker’s pneumoconiosis: a comprehensive review. J Toxicol Enviro Health, Part B 9:357–395
Han L, Han R, Ji X (2015) Prevalence characteristics of coal workers’ pneumoconiosis (CWP) in a state-owned mine in Eastern China. Int J Environ Res Public Health 12(7):7856–7867
Han L, Gao Q, Yang J et al (2017) Survival analysis of coal workers’ pneumoconiosis (CWP) patients in a state-owned mine in the east of China from 1963 to 2014. Int J Environ Res Public Health 14:489
Han S, Chen H, Harvey MA (2018) Focusing on coal workers’ lung diseases: a comparative analysis of China, Australia, and the United States. Int J Environ Res Public Health 15:2565
Hong FF, He H, Liu JY (2013) Comparison analysis of coal biodesulfurization and coal’s pyrite bioleaching with Acidithiobacillus ferrooxidans. Sci World J 9
Huang X (2011) Iron, oxidative stress, and cell signaling in the pathogeneses of coal workers’ pneumoconiosis, silicosis, and asbestosis. Am J Biomed Sci 3(2):95–106
Huang X, Fournier J, Koenig K, Chen LC (1998) Buffering capacity of coal and its acid-soluble Fe2+ content: possible role in coal workers’ pneumoconiosis. Chem Res Toxicol 11:722–729
Huang C, Li J, Zhang Q, Huang X (2002) Role of bioavailable iron in coal dust-induced activation of activator protein-1 and nuclear factor of activated t cells: difference between pennsylvania and utah coal dusts. Am J Respir Cell Mol Biol 27(5):568–574
Huang X, Li W, Attfield MD (2005) Mapping and prediction of coal workers’ pneumoconiosis with bioavailable iron content in the bituminous coals. Environ Health Perspect 113(8):964–968
Huang X, Zalma R, Pezerat H, Huang X (1994) Factors that influence the formation and stability of hydrated ferrous sulfate in coal dusts. Possible relation to the emphysema of coal miners. Chem Res Toxicol 7(3):451–457
Huang X, Gordon T, Rom WN (2006) Interaction of iron and calcium minerals in coals and their roles in coal dust-induced health and environmental problems. Rev Miner Geochem 64:153–178
IARC (1997) IARC monographs on the evolution of carcinogenic risks to humans: silica, some silicates, coal dust and para-armed fibrils. International Agency for Research on Cancer, vol 68, pp 41–242
Jaishankar M, Tseten T, Anbalagan N et al (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72
Karkhanis VS, Joshi JM (2013) Pneumoconiosis. Indian J Chest Dis Allied Sci 55
Khan MHR, Seddique AA, Rahman A, Shimizu Y (2017) Heavy metals contamination assessment of water and soils in and around barapukuria coal mine area, Bangladesh. Am J Enviro Prot 6(4):80–86
King KJ, Zaidi S, Harrison CV, Nagelschmidt G (1958) The tissue reaction in the lungs of rats after the inhalation of coal dust containing 2% of quartz. Brit J Ind Med 15:172–177
Kolling A, Ernst H, Rittinghausen S, Heinrich U (2011) Relationship of pulmonary toxicity and carcinogenicity of fine and ultrafine granular dusts in a rat bioassay. Inhal Toxicol 23(9–11):544–554
Lassalle P, Gosset P, Aerts C et al (1989) Alveolar macrophages secretory dysfunctions in coal workers’s pneumoconiosis. Comparison between simple pneumoconiosis and progressive massive fibrosis. NATO ASI Ser H30:1–2
Lassalle P, Gosset P, Aerts C et al (1990) Abnormal secretion of interleukin-1 and tumor necrosis factor α by alveolar macrophages in coal worker’s pneumoconiosis: comparison between simple pneumoconiosis and progressive massive fibrosis. Exp Lung Res 16(1):73–80
Lee CY, Lee SL, Sheehan CE, Wang Y (2002) Composition of coal dusts and their cytotoxicity on alveolar macrophages. Adv X-Ray Anal 39:561–569
Li BQ, Zhou YZ, Yang DC, Zeng QY (2007) The epidemiology of pneumoconiosis. Occup and Health 23:549–552
McCunney RJ, Morfeld P, Payne S (2009) What component of coal causes coal workers’ pneumoconiosis? JOEM 51(4):462–471
Mo J, Wang L, Au W, Su M (2014) Prevalence of coal workers’ pneumoconiosis in China: a systematic analysis of 2001–2011 studies. Int J Hyg Environ Health 217(1):46–51
Moitra S, Puri R, Paul D, Huang YCT (2015) Global perspectives of emerging occupational and environmental lung diseases. Curr Opin Pulm Med 21:114–120
Mukherjee AK, Bhattacharya SK, Saiyed HN (2005) Assessment of respirable dust and its free silica contents in different Indian coalmines. Ind Health 43(2):277–284
Parihar YS, Patnaik JP, Nema BK et al (1997) Coal workers’ pneumoconiosis: a study of prevalence in coal mines of Eastern Madhya Pradesh and Orissa States of India. Ind Health 35:467–473
Petsonk EL, Rose C, Cohen R (2013) Coal mine dust lung disease: new lessons from an old exposure. Am J Respir Crit Care Med 187(11):1178–1185
Pinho RA, Bonatto F, Andrades M et al (2004) Lung oxidative response after acute coal dust exposure. Environ Res 96(3):290–297
Praharaja T, Powellb MA, Hartb BR, Tripathya S (2002) Leachability of elements from sub-bituminous coal fly ash from India. Environ Int 27(8):609–615
Queensland W (2020) Coal Workers’ Pneumoconiosis (CWP) and other mine dust lung diseases (MDLD) Workers’ compensation information. Office of industrial relation. www.worksafe.qld.gov.au.
Reddy MS, Basha S, Joshi HV, Jha B (2005) Evaluation of the emission characteristics of trace metals from coal and fuel oil fired power plants and their fate during combustion. J Hazard Mater 123(1–3):242–249
Rezaee M, Honaker RQ (2020) Long-term leaching characteristic study of coal processing waste streams. Chemosphere 249:126081
Schins RPF, Borm PJA (1995) Epidemiological evaluation of release of monocyte TNF-a as an exposure and effect marker in pneumonoconiosis: a five year follow up study of coal workers. Occup Environ Med 52:441–450
Schins RPF, Borm PJA (1999) Mechanisms and mediators in coal dust induced toxicity: a review. Ann Occup Hyg 43(1):7–33
Sen R, Srivastava SK, Singh MM (2009) Aerial oxidation of coal-analytical methods, instrumental techniques and test methods: a survey. Ind J Chem Tech 16(2):103–135
Shahzad M, Ali Z, Majeed Y, Emad Z (2016) Liberation studies of padhrar coal by using fractionation method, XRD analysis and megascopic and microscopic techniques. Pak J Sci Ind Res Ser A Phys Sci 59(2):90–95
Singer PC, Stumm W (1970) Acidic mine drainage: the rate-determining step. Science 167(3921):1121–1123
Smith KR, Veranth JM, Hu AA (2000) Interleukin-8 levels in human lung epithelial cells are increased in response to coal fly ash and vary with the bioavailability of iron, as a function of particle size and source of coal. Chem Res Toxicol 13:118–125
Su X, Ding R, Zhuang X (2020) Characteristics of dust in coal mines in Central North China and its research significance. ACS Omega 5:9233–9250
Trechera P, Moreno T, Córdoba P et al (2020) Mineralogy, geochemistry and toxicity of size-segregated respirable deposited dust in underground coal mines. J Hazard Mater 399:122935
Valavanidis A, Fiotakis K, Vlachogianni T (2008) Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Enviro Sci Health, Part C 26(4):339–362
Weeks JL, Wagner GR (1986) Compensation for occupational disease with multiple causes: the case of coal miners’ respiratory diseases. AJPH 76(1):58–61
Whitaker PJ (1981) Coal workers’ pneumoconiosis and the compensation dilemma. J Occup Med 23(6):422–426
Zhai R, Jetten M, Schins RPF et al (1998) Polymorphisms in the promoter of the tumor necrosis factor-a gene in coal miners. Am J Ind Med 34:318–324
Zhai R, Liu G, Ge X (2002) Serum levels of tumor necrosis factor-a (TNF-a), interleukin 6 (IL-6), and their soluble receptors in coal workers’ pneumoconiosis. Rep Med 96:829–834
Zhang Q, Dai J, Ali A (2002) Roles of bioavailable iron and calcium in coal dust-induced oxidative stress: possible implications in coal workers’ lung disease. Free Radical Res 36(3):285–294
Zhao Q, Niu Y, Xie Z et al (2020) Geochemical characteristics of elements in coal seams 41 and 42 of Heshan Coalfield, South China. Energy Explor Exploit 38(1):137–157
Zheng Y, Liang L, Qin T (2017) Cross-section analysis of coal workers’ pneumoconiosis and higher brachial-ankle pulse wave velocity within Kailuan study. BMC Public Health 17(1):1–8
Zhou L, Guo H, Wang X et al (2019) Effect of occurrence mode of heavy metal elements in a low rank coal on volatility during pyrolysis. Int J Coal Sci Technol 6(2):235–246
Zosky GR, Hoy RF, Silverstone EJ et al (2016) Coal workers’ pneumoconiosis: an Australian perspective. MJA 11:414-418.e2
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sherekar, P., Jain, R., Pingle, S., Suke, S. (2022). Role of Pyrite in Aggravating Coal Worker’s Pneumoconiosis. In: Randive, K., Pingle, S., Agnihotri, A. (eds) Medical Geology in Mining. Springer Geology. Springer, Cham. https://doi.org/10.1007/978-3-030-99495-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-99495-2_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99494-5
Online ISBN: 978-3-030-99495-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)