Abstract
Solitary bees are usually considered superior pollinators to the honeybee and with so many different species, there are ample options to choose from. Regardless, there is still a lack of knowledge when it comes to their communication and especially the use of vibrational signals. Osmia bicornis shows a complex mating behavior including thorax vibrations, which we have intensely studied. We were able to show that these vibrational signals actually play a vital role in the mating by encoding not only a signal for the male’s physical fitness but also a signal associated with their region of origin—both of which are being used by the female to choose a suitable male. Furthermore, we have found that the vibrations produced by a male are influenced by changes in temperature, which leads to different males having an advantage. In view of climate change, this could lead to important population changes and should be considered as a factor also when looking at different bee species. Overall we show that vibrational signals can be very important in solitary bees and should not be neglected in future studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The “buzzing” of a bee is certainly widely recognized, so much so that it is not only known to scientists and non-scientists alike, but it even has found its place in songs and literature. However, while there are estimated to be more than 30,000 bee species worldwide (Michener 2000), vibrational communication has only been studied in very few bee species to date.
So far most evidence of vibrational communication in Apoidea has been found in the eusocial species such as the honeybee, bumblebees, and stingless bees, though this is probably due to lack of research rather than the non-occurrence of this type of communication (Hill 2008).
One of the main reasons for this lack of research may be the focus on chemical communication in the Hymenoptera over the past decades, which has been very fruitful and does play a major role in a lot of bee species (Ayasse et al. 2001). However, as the field of biotremology emerges and more and more work is being done, we have come to realize that more species use vibrations as a means of communication than previously thought and that thoracic oscillations are actually widespread among bees. Thanks to the work of Michelsen, Tautz, Kirchner, and others over the past four decades, we now know about the importance of substrate vibrations in the waggle dance of the honeybee; although, a lot of questions still remain unanswered (Michelsen 2014). Additionally, our knowledge of the use of biotremology in the foraging of stingless bees has greatly increased through the work of Hrncnir and Barth over the last 20 years. Unlike in the waggle dance in honeybees the vibrational signals in stingless bees do not convey information about the direction of the food source but rather quality and net gain of the food source. So the informational content of these signals can be quite diverse (Hrncir and Barth 2014). Buzz pollination is a third area that has been intensely studied and has led to some exciting new insights concerning the frequency and acceleration used by bumblebees during foraging. We are looking at an exciting co-evolution between plants and bees driven by vibrational signals (Vallejo-Marín 2018).
As early as 1986, Ole Larsen mentioned that vibrations are widespread and might convey important messages between the sexes during mating (Larsen et al. 1986). It is therefore remarkable that almost no research on the role of biotremology during mating and/or sexual selection has been done since then. Considering the substantial number of solitary bees displaying complex mating behaviors that include “buzzes” in one way or another, this is an area that promises to be rewarding if explored and will allow us to better understand the purpose of these signals. While bees are the most important insect pollinators, we have come to realize that honeybees are actually not the best choice in a lot of cases. Wild bees are generally considered superior pollinators (Valido et al. 2019), so it is of vital importance to increase our knowledge in this area. Life history information, especially knowledge of communication, mating, and speciation, will improve our ability to use these bees as efficient pollinators and, of course, protect them and their habitats.
2 Mating Behavior of the Red Mason Bee
The reproductive biology of the red mason bee, Osmia bicornis, has been studied already in the past (Seidelmann 1991, 1995). Osmia bicornis usually lays its eggs into holes it finds in dead wood. However, anything of the right size may be used, even old bullet caps (O’Toole 2000). It is one of the first bees to occur in spring and normally emerges in late March or early April, which leads to its use as a pollinator for agricultural plants like apple trees (Westrich 1989). Osmia bicornis is a strongly proterandric species in which the males emerge first to be present in great numbers all through the emergence of the females during spring time (Seidelmann 1995). The males either wait in front of the nests or around flowers for the appearance of potential females for mating (Ayasse et al. 2001). Since the gender ratio is shifted in favor of the males (approx. 1.2 ♂:1♀), the pre-condition for female choice is met (Andersson 1994).
Contrary to the males, females only mate once and the mating can be described in three phases: precopulation, copulation, and postcopulation (Seidelmann 1995). During the precopulatory courtship, the male embraces the female by sitting on her back, holding the female’s mesothorax with its first and second pairs of legs. The male’s antennae are pointing toward the upper-front, while the female’s antennae point toward the side (Seidelmann 1995). The male then engages in a series of behaviors in order to persuade the female to mate. He vibrates his thorax, rubs himself against the female, and passes his antennae repeatedly over those of the female. Meanwhile, he also moves his forelegs over the female’s compound eyes (Seidelmann 1995). After this complex mating behavior, which can last from only a few seconds to up to an hour, the male moves back on the female’s back and tries to insert his genitalia into the genital chamber, while using his antennae for a tremolo on the female’s face (Seidelmann 1995). The female may then reject the male by physically pushing him off her back or bending her abdomen away from him (Conrad et al. 2010). In the former case, the male will fly off in search of another female, while in the latter case the male will go back to his mating behavior and try again at a later time.
3 Female Choice in Osmia bicornis
Charles Darwin noticed the strong sexual dimorphism we find in many species (Darwin 1871). The males can actually be so different from females that on many occasions they have been thought to be two entirely different species until their actual mating was observed. Looking at the magnificent tail of the peacock, it is not hard to understand that something other than natural selection is at play here. Obviously, the peacock is truly handicapped by his tail, especially if he is attacked by a predator. The explanation for this can of course be found in sexual selection (Dimijian 2005).
In many cases, sexual selection is based on a male’s fitness, its freedom from parasites, or its genetic relatedness to the female, all of which are evaluated through various different male traits (Clarke and Faulkes 1999; Kose and Møller 1999). In the barn swallow, for example, the white spots on the tail are directly correlated with parasite infestation and thus are used by females as an honest signal for male health (Kose and Møller 1999). All of these male traits can be of various different types, and sometimes even hard to spot or to distinguish from traits resulting from selection by other natural selection (Brown 1975; Endler 1986). In the case of the peacock, it is due to female choice that the male’s tail developed in such a way. Female choice is just one kind of sexual selection in which the female chooses its mate according to a set of traits the male possesses. Although male signals like color, size, or odor are probably more commonly known in regards to sexual selection, in many cases, female choice is based on vibrational signals, for example in blood-sucking bugs (Roces and Manrique 1996) and treehoppers (Rodríguez et al. 2004). Various parts of the male signal may be responsible for female choice, such as base frequency, length of the whine section, pulse rate, and number of pulses as is seen in the case in the treehopper Enchenopa binotata (Rodríguez et al. 2004).
Considering the aforementioned gender ratio and the complex mating behavior in O. bicornis one would expect that female choice is at play in this species. We, therefore, set out to find the male characteristics a female might use to evaluate the male’s suitability as a mate. In a series of behavioral experiments, coupled with chemical and molecular analyses, we tested if size, odor, relatedness, and vibrations differed between those males that were accepted by the females and those that were rejected. Size was determined by interocular distance, as that correlates with overall size in bees. Odor was determined by gas chromatography and relatedness between a mating pair was estimated using microsatellite analyses. We recorded the vibrations using a laser vibrometer aimed at the thorax of a male during pre-copulation. Pairs were established by introducing one female at a time into a flight cage with about 40–50 males. After a bit of scramble competition one male gained the position on the female’s back and started his mating behavior. Usually, the other males would then fly off and leave the pair alone. All males were marked with a white dot on their thorax to better reflect the laser used to record vibrational behavior.
Interestingly there was no significant difference in size between accepted and rejected males (Mann–Whitney U test, P > 0.05). However, we observed a significant difference in the variances of accepted and rejected males, with the variance of rejected males being much higher than that of the accepted males (Ansari–Bradley Test, P < 0.05). This indicates a more consistent choice by females for males of an intermediate size (Conrad et al. 2010). Since in most species larger males are preferred, this points toward a disadvantage for males that are too large in this species. One such disadvantage could be that larger males have trouble with temperature regulation, as is the case in the sphecid wasp Bembix rostrata (Larsson 1991).
Our other results also showed clear differences between accepted and rejected males for odor and relatedness, showing that certain odors are preferred, which are possibly used to gain information about relatedness. We also found that females follow optimal outbreeding, in that they avoid mating with brothers but also males too distantly related to them.
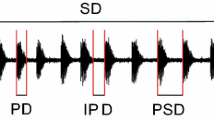
Our vibrational recordings showed that male vibrations occur in a series of trains (periods of vibrations) with longer breaks in between. Each train, in turn, consists of a series of bursts or pulses, which can either occur with short breaks between them or without any distinguishable breaks at all. We measured pulse length, break length, and dominant frequency and found that only the pulse length differed significantly between accepted and rejected males (Mann–Whitney U test, P < 0.05; Fig. 9.1), leading us to the conclusion that males who were able to vibrate longer, which of course is energetically costly, were the ones preferred by the females—presumably as they are the stronger individuals. Since often vibrational signals, especially frequency, are directly related to size with bigger males producing lower frequencies, we also ran a correlation between size and vibrations but found no link between the two (Spearman’s rho, P > 0.05; Fig. 9.2). Overall our results confirmed for us that female choice is present in O. bicornis (Conrad et al. 2010). Numerous traits of the males are used in this choice and are therefore under sexual selection.
4 The Role of Vibrations as Isolation Barriers in Osmia bicornis Populations in Europe
Traits under sexual selection can be a driving force of speciation if female preference differs between different forms of trait development (Lande 1981; Boake 2002; Coyne and Orr 2004; Andersson and Simmons 2006) and a Fisherian runaway process may consequently divide the two populations. It is possible that in certain populations one male in dozens could actually mate with the majority of females if by chance he develops a trait preferred by the females. In consequence, very few males would be fathering the next generation of offspring and passing on their genes and phenotypic traits. That could explain why we find sexual selection to act so fast in changing populations (Turner and Burrows 1995; Gavrilets and Boake 1998). A Fisherian runaway process is especially likely in situations where there is already a pre-existing bias in the females, like in the swordtail fish group. Here, females from species that do not have swords still prefer them when confronted with a choice (Basolo 1990).
There are various examples of sexual selection in insects playing a potential role in speciation, like in the well-known Hawaiian Drosophila (Hoy et al. 1988), where different songs have led to the evolution of over 600 species, or the Australian scorpionflies of the genus Harpobittacus. Two species of scorpionflies, both found sympatrically in Australia, fail to mate in the lab due to different male sex pheromones known to have evolved under sexual selection (Bornemissza 1966).
There are also many reports of intraspecific differences in vibrational signals between populations and their influence in mate choice (Gillham 1992; Claridge and DeVrijer 1993; Ryan et al. 1996; Čokl et al. 2000). In the southern green stink bug, Nezara viridula, for example, there are distinct differences in female calls between populations and males are able to recognize not only conspecific calls but also females from their own population, which are preferred (Miklas et al. 2003).
It is clear today that vibrational signals are very often species-specific and can be used for species recognition, which may lead to discrimination of individuals of different populations. Slight signal differences involved in the mating process have already been recognized as a possible first-step toward reproductive isolation and can play a role in speciation (Darwin 1871; West-Eberhard 1983; Andersson 1994; Panhuis et al. 2001; Coyne and Orr 2004), especially where female choice leads to the selection of male traits. It is therefore highly likely that vibrational communication plays a part in speciation by sexual selection.
In bees, prezygotic isolation barriers are predominantly found to prevent interspecific mating. There are many reports of species-specific odors in bees, especially in the female sex pheromone, which function as isolating barriers, since they only attract con-specific males (Bergman and Bergström 1997; Ayasse et al. 2001). In other bees, however, heterospecific mating is limited by behavioral differences or diverging flight times (Ayasse et al. 2001), like in the cave-dwelling Apis nuluensis. Here, isolation from other Apis species is achieved by a different flight period of the males (Koeniger and Koeniger 2000). Surprisingly, research into vibrational signals being used for species recognition or isolation barriers in bees was lacking entirely until our research.
According to Peters (1978), O. bicornis is found throughout Europe mainly in two subspecies, O. bicornis rufa and O. bicornis cornigera, which are distinguishable only by the difference in coloration at the tip of the abdomen. Only on the balearic islands a third subspecies, O. bicornis fracticornis, can be found. The subspecies O. bicornis cornigera can be found allopatrically mainly in central Europe, whereas O. bicornis rufa is found allopatrically around the edges of Europe. However, there are two overlapping regions in which both subspecies are found sympatrically—one in northern Spain and one in Denmark, where hybrids are supposed to occur (Peters 1978) (Fig. 9.3).
Map showing the distribution of the two main subspecies of O. bicornis (red = O. bicornis rufa, yellow = O. bicornis cornigera) in Europe as suggested by Peters (1978). The location of the populations used for our analyses are marked in England (Kent, Hereford, and Tonbridge), Germany (Halle, Regensburg, and Constance), and Denmark (Copenhagen, Vejle, and Møn)
Since the classification into these subspecies is solely based on one morphological trait, it is worthwhile to take a closer look at their behavior. The aim of our following research was, therefore, to first ascertain if the subspecies described by Peters (1978) do still mate with each other, and to then investigate the role of the male’s vibrational signals in the female’s choice. In order to establish if O. bicornis from different populations in Europe did indeed still mate with each other, or if some sort of isolation barriers were already in place, we first conducted cross-matings with bees from the allopatrically occurring Osmia from England and Germany, as well as bees from Denmark, where both subspecies are supposed to occur sympatrically.
Our results clearly showed that there is no pre-copulatory isolation in place as all of the different male/female combinations had at least some successful matings. However, the females clearly preferred to mate with males from their own country, i.e., the region of origin (Fig. 9.4). We, therefore, concluded that there is a female preference for the “own” population and we are potentially witnessing the beginning of an ongoing separation process (Conrad and Ayasse 2015).
The next question was obviously which characteristics of a male the female uses to recognize their region of origin. Although odor has been shown to be used by various bees in the past (Ayasse et al. 2001), we were interested to see if vibrations might be used in Osmia and how much they might factor into the female’s decision. However, to test whether or not vibrations are the key, we had to design a set-up in which it was possible to change the vibrations a male produces without influencing any other parameters, such as odor or visual cues, e.g., the movement of the antennae or front legs. Because males are mounted on top of the female and the only way to establish rejection or acceptance is during a copulation attempt, this also had to happen on a live male without it being too invasive to hinder him in his usual mating behavior. We were able to develop an innovative new bioassay to test this by using small strong magnets as mini-vibrators and gluing them onto the males’ thoraxes. We began by establishing mating pairs and then placed the pair on top of an inductor that was connected to a frequency generator equipped with a pre-recorded signal of a male. The signal was then transferred to the magnet through the electromagnetic field of the inductor, leading to vibrations in the desired frequency and modulation (Fig. 9.5). Fortunately, most males stopped emitting their own signals during this process but continued their usual mating behavior, including copulation attempts. This enabled us to impose the signal of successful English males onto German males and vice versa. We used this combination as it was least successful in the previous cross-mating experiments (Conrad and Ayasse 2015).
When we changed the signal of a German bee to that of an English bee, while coupling it with an English female, the success rate of the males increased until it was not significantly different from that of the natural English males (Fig. 9.6). The same was true for English bees that had a German signal imposed on them, as they coupled with a German female. Since this could be a positive effect of the inductor set-up itself we also tried a control during which we changed a German male into one with an English signal while mating with a German female. As expected, the success rate decreased significantly (2 × 2 contingency table, df = 1, P < 0.05), confirming that the vibrational signal is used by the females to make their choices in this case (Conrad and Ayasse 2015). These experiments showed that females in O. bicornis are indeed able to use the vibrational signals emitted by the males to choose a male from their own country/region of origin, meaning that the signal does not only encode fitness, as we showed before, but also information about their origin.
Mating success of O. bicornis males with English females. Comparison of mating success of O. bicornis males of different origin (shown by flags) with a natural or an imposed artificial signal (E, English; G, German), which tried to mate with English O. bicornis females. The sample sizes are shown beneath each flag. Significant differences are marked by different letters (Waldchi-square, χ2 = 13.804, df = 2, P < 0.05; q-values <0.05)
However, our attempts to identify which part of the signal encodes this information have so far been inconclusive, as there are no discernible differences between frequency, pulse length, modulation range, or pulse pattern between English and German populations in Europe. Surprisingly, there is a difference in frequency and modulation range between German and Danish bees (Conrad and Ayasse 2019). We currently suspect that another aspect of the signal, which we have not been able to determine yet, may be responsible for this remarkable behavior. Another explanation is that the differences are extremely subtle and a much higher sample size is needed to identify them.
It is important to keep in mind that animals may be able to perceive differences in signals that researchers are currently unable to detect. There are many examples of this, yet only a few of them are published so far (pers. comm.). In experiments with the elm leaf beetle, for example, the beetle can distinguish between the odors of two differently treated elms. However, the difference was undetectable for the researchers (Büchel et al. 2013).
5 The Influence of Temperature on Vibrations
While there is still a, thankfully dwindling, minority of climate change deniers, it has become a sad reality that we are facing today (Parmesan and Yohe 2003). Fortunately, temperature changes and their effect on natural systems have moved into the focus of research to increase our knowledge of the challenges we might be facing. Many plant and animal species from various different taxonomical groups are already known to be affected in one way or another (Root et al. 2003; Pörtner and Knust 2007; Kearney et al. 2009; Sentis et al. 2013). Unfortunately, a disproportionate amount of research in insects has focused on more popular species from the Lepidoptera, Diptera, or Orthoptera, while orders with much higher species richness have been neglected (Andrew et al. 2013). Among the abiotic factors influencing an animal’s life, temperature changes are of particular importance, not only because of climate change but also because they universally affect almost every organism to some degree during their life span (Chapman 1982). With insects being poikilotherms, they end up especially affected. More importantly, we know that in addition to the influence on bodily functions, temperature changes also affect communication systems, as has been shown in electric fish (Feng 1976), fireflies (Carlson et al. 1976), anurans (Zweifel 1968; Gayou 1984), and orthoptera (Walker 1975; Pires and Hoy 1992). However, studies on the effect of temperature on communication signals in animals and their consequence are scarce and often solely based on field work, as opposed to lab experiments under controlled conditions.
Research on the consequences temperature changes can have on acoustic signals in insects so far focuses on the Orthoptera, which have specialized sound-producing organs. In anurans and crickets, the studies show that temporal parameters of the song are particularly altered by temperature (Gayou 1984; Pires and Hoy 1992), leading to an increase in pulse rate with raising temperatures (Gayou 1984).
When female choice is at play, there are usually two ways a female can deal with a change in temperature when it comes to the male. One option is to take the change in temperature into account when evaluating the male’s signal and then interpret it accordingly. As a result, female preference actually changes with temperature. We find that strategy, for example, in odor signaling in moths (Linn et al. 1988) and in acoustic signaling in crickets (Pires and Hoy 1992). The second possibility is that female preference stays the same regardless of temperature. In this case, those males whose signals are not affected by temperature would be chosen over those that cannot keep up their usual signal and thus have the advantage (Fig. 9.7).
Possible changes in male signals and female preference due to changes in temperature. There are two possible outcomes for a male signal subjected to temperature changes: either the signal stays the same (1) or the signal changes (2). Female preference can either stay the same (a) or females can adapt to a change in temperature with a change in preference for a, now changed, male signal (b)
There have always been high temperature fluctuations during the flight time of Osmia bees in spring. Osmia bicornis need temperatures around 15 °C to emerge from their nest. In recent years, temperature fluctuations during March, April, and June have led to temperatures of 25 °C regularly, and sometimes even more (Seidelmann 1991; Conrad personal observation). We, therefore, investigated the possible variations in male vibrational signals and female preference at different temperatures. For this, we once again recorded mating pairs in a controlled environment in a climate chamber, at either 17 °C–21 °C as a low-temperature setting, or 22 °C–26 °C as a high-temperature setting. Looking at the results we found a significant difference in pulse duration and dominant frequency for the rejected males but surprisingly not for the accepted males (Mann–Whitney U-Test, P < 0.05; Figs. 9.8 and 9.9). We, therefore, concluded that female preference for male vibratory signals does not change with temperature. However, at least some males are obviously unable to produce the desired signal, particularly at low temperatures. This means that males, who are able to produce the appropriate vibrations, may be able to mate much earlier during the day when temperatures are still low, which is actually a common situation in solitary bees. In my experience, the bees start flying as soon as the temperature reaches 15 °C and then stop activity when conditions become too hot (Conrad et al. 2017). Nevertheless, one can easily imagine that this clear advantage for “early risers” in males will diminish more and more as global temperatures rise. The bees will then either face a disruption in their mate choice, leading presumably to a loss of their temperature adaptations, or they might have to move to higher latitudes or elevations to avoid higher temperatures. With habitat loss already being a global issue, however, this might not be an option. This might ultimately lead to unforeseeable evolutionary changes in this species (Conrad et al. 2017).
Comparison of the pulse duration during different temperature settings between accepted and rejected males. The medians, quartiles, outliers (circles) and sample sizes (numbers) are shown. Significant differences are marked by an asterisk (* Mann–Whitney U-Test, P < 0.05). Rejected males showed significantly longer pulse durations at lower temperatures
Comparison of the dominant frequency during different temperature settings between accepted and rejected males. The medians, quartiles, outliers (circles) and sample sizes (numbers) are shown. Significant differences are marked by an asterisk (* t-Test, P < 0.05). Rejected males showed significantly lower dominant frequencies at lower temperatures
6 Conclusion
Vibrational signals are obviously a vital part of the communication between male and female in O. bicornis. Not only is a representation of the male’s physical fitness encoded in the signal but we have shown that information on the region of origin is also contained in these vibrations. This suggests once again the high potential vibrational signals have for encoding information, even if they look “simple” at first glance. With our studies on the red mason bee, we have barely scratched the surface when it comes to potential vibrational communication in solitary bees. With 30,000 bee species worldwide, I strongly believe it is worth taking a closer look at how different species use biotremology not only during their mating behavior but also in other behavioral contexts. The more we know about these important pollinators, the better we will undoubtedly understand how to protect and use them in regard to the challenges we face in the future. Rising temperature, an increasing world population, and insufficient sustainable agriculture mean that this kind of research goes far beyond the purely intellectual search for knowledge. Finally, I hope that our methodology of using magnets and inductors proves useful in other experiments, in which vibrating the ground an animal stands on is not sufficient, but where the animal itself is actually the substrate.
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, NJ
Andersson M, Simmons LW (2006) Sexual selection and mate choice. Trends Ecol Evol 21:296–302
Andrew NR, Hill SJ, Binns M, Bahar MH, Ridley EV, Jung M-P, Fyfe C, Yates M, Khusro M (2013) Assessing insect responses to climate change: what are we testing for? Where should we be heading? PeerJ 1:e11. https://doi.org/10.7717/peerj.11
Ayasse M, Paxton RJ, Tengö J (2001) Mating behavior and chemical communication in the order hymenoptera. Annu Rev Entomol 46:31–78. https://doi.org/10.1146/annurev.ento.46.1.31
Basolo AL (1990) Female preference predates the evolution of the sword in swordtail fish. Science 250:808–810. https://doi.org/10.1126/science.250.4982.808
Bergman P, Bergström G (1997) Scent marking, scent origin, and species specificity in male premating behavior of two Scandinavian bumblebees. J Chem Ecol 23:1235–1251. https://doi.org/10.1023/B:JOEC.0000006461.69512.33
Boake CRB (2002) Sexual signaling and speciation, a microevolutionary perspective. Genetica 116:205–214. https://doi.org/10.1023/A:1021224207727
Bornemissza GF (1966) Observations on the hunting and mating behaviours of two species of scorpion flies (Bittacidae Mecoptera). Aust J Zool 14:371. https://doi.org/10.1071/ZO9660371
Brown JL (1975) The evolution of behavior. Norton, New York
Büchel K, Austel N, Mayer M, Gershenzon J, Fenning T, Meiners T (2013) Smelling the tree and the forest: Elm background odours affect egg parasitoid orientation to herbivore induced terpenoids. BioControl 59:1–15. https://doi.org/10.1007/s10526-013-9544-9
Carlson AD, Copeland J, Raderman R, Bulloch AGM (1976) Role of interflash intervals in a firefly courtship (Photinus macdermotti). Anim Behav 24:786–792. https://doi.org/10.1016/S0003-3472(76)80009-7
Chapman RF (1982) The insects: structure and function. Harvard University Press, Cambridge, MA
Claridge MF, DeVrijer PWF (1993) Reproductive behavior: the role of acoustic signals in species recognition and speciation. In: Denno RF, Perfect TJ (eds) Planthoppers, their ecology and management. Chapman & Hall, New York
Clarke FM, Faulkes CG (1999) Kin discrimination and female mate choice in the naked mole-rat Heterocephalus glaber. Proc R Soc B Lond 266:1995–2002
Čokl A, Virant-Doberlet M, Stritih N (2000) The structure and function of songs emitted by southern green stink bugs from Brazil, Florida, Italy and Slovenia. Physiol Entomol 25:196–205. https://doi.org/10.1046/j.1365-3032.2000.00187.x
Conrad T, Ayasse M (2015) The role of vibrations in population divergence in the red mason bee, Osmia bicornis. Curr Biol 25:2819–2822. https://doi.org/10.1016/j.cub.2015.08.059
Conrad T, Ayasse M (2019) The differences in the vibrational signals between male O. bicornis from three countries in Europe. J Low Freq Noise Vibr Act Control 38:871–878. https://doi.org/10.1177/1461348418816263
Conrad T, Paxton RJ, Barth FG, Francke W, Ayasse M (2010) Female choice in the red mason bee, Osmia rufa (L.) (Megachilidae). J Exp Biol 213:4065–4073. https://doi.org/10.1242/jeb.038174
Conrad T, Stöcker C, Ayasse M (2017) The effect of temperature on male mating signals and female choice in the red mason bee, Osmia bicornis (L.). Ecol Evol 1:8966–8975. https://doi.org/10.1002/ece3.3331
Coyne JA, Orr HA (2004) Speciation. Sinauer Associates, Sunderland, MA
Darwin C (1871) The descent of man and selection in relation to sex. Murray, London
Dimijian GG (2005) Evolution of sexuality: biology and behavior. Baylor Univ Med Center Proc 18:244–258
Endler JA (1986) Natural selection in the wild. Princeton University Press, Princeton, NJ
Feng AS (1976) The effect of temperature on a social behavior of weakly electric fish Eigenmannia virescens. Comp Biochem Phys A 55:99–102. https://doi.org/10.1016/0300-9629(76)90074-8
Gavrilets S, Boake CRB (1998) On the evolution of premating isolation after a founder event. Am Nat 152:706–716. https://doi.org/10.1086/286201
Gayou DC (1984) Effects of temperature on the mating call of Hyla versicolor. Copeia 1984:733–738. https://doi.org/10.2307/1445157
Gillham MC (1992) Variation in acoustic signals within and among leafhopper species of the genus Alebra (Homoptera, Cicadellidae). Biol J Linn Soc 45:1–15. https://doi.org/10.1111/j.1095-8312.1992.tb00628.x
Hill PSM (2008) Vibrational communication in animals. Harvard University Press, London
Hoy RR, Hoikkala A, Kaneshiro K (1988) Hawaiian courtship songs: evolutionary innovation in communication signals of Drosophila. Science 240:217–219. https://doi.org/10.1126/science.3127882
Hrncir M, Barth FG (2014) Vibratory communication in stingless bees (Meliponini). In: Cocroft RB, Gogala M, Hill PSM, Wessel A (eds) Studying vibrational communication. Springer, Berlin, pp 349–374
Kearney M, Shineb R, Porter WP (2009) The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc Natl Acad Sci USA 106:3835–3840
Koeniger N, Koeniger G (2000) Reproductive isolation among species of the genus Apis. Apidologie 31(2):313–339
Kose M, Møller AP (1999) Sexual selection, feather breakage and parasites: the importance of white spots in the tail of the barn swallow (Hirundo rustica). Behav Ecol Sociobiol 45:430–436
Lande R (1981) Models of speciation by sexual selection on polygenic traits. Proc Natl Acad Sci USA 78:3721–3725
Larsen ON, Gleffe G, Tengö J (1986) Vibration and sound communication in solitary bees and wasps. Physiol Entomol 11:287–296. https://doi.org/10.1111/j.1365-3032.1986.tb00416.x
Larsson FK (1991) Some take it cool, some like it hot: a comparative study of male mate searching tactics in two species of Hymenoptera (Colletidae and Sphecidae). J Therm Biol 16:45–51
Linn CE, Campbell MG, Roelofs WL (1988) Temperature modulation of behavioural thresholds controlling male moth sex pheromone response specificity. Physiol Entomol 13:59–67
Michelsen A (2014) Mechanical signals in honeybee communication. In: Cocroft RB, Gogala M, Hill PSM, Wessel A (eds) Studying vibrational communication. Springer, Berlin, pp 333–347
Michener CD (2000) The bees of the world. John Hopkins University Press, Baltimore, MD
Miklas N, Čokl A, Renou M, Virant-Doberlet M (2003) Variability of vibratory signals and mate choice selectivity in the southern green stink bug. Behav Process 61:131–142. https://doi.org/10.1016/s0376-6357(02)00186-9
O’Toole C (2000) The red mason bee: taking the sting out of bee-keeping: a practical guide to managing Osmia rufa as a pollinator in gardens, allotments, and orchards. Osmia Publications, Banbury, UK
Panhuis TM, Butlin R, Zuk M, Tregenza T (2001) Sexual selection and speciation. Trends Ecol Evol 16:364–371. https://doi.org/10.1016/s0169-5347(01)02160-7
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42. https://doi.org/10.1038/nature01286
Peters DS (1978) Systematik und Zoogeographie der west-paläarktischen Arten von Osmia sstr, Monosmia und Orientosmia. Senckenberg Biol 58:287–346
Pires A, Hoy RR (1992) Temperature coupling in cricket acoustic communication. J Comp Physiol A 171:69–78
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97. https://doi.org/10.1126/science.1135471
Roces F, Manrique G (1996) Different stridulatory vibrations during sexual behaviour and disturbance in the blood-sucking bug Triatoma infestans (Hemiptera: Reduviidae). J Insect Physiol 42:231–238
Rodríguez RL, Sullivan LE, Cocroft RB (2004) Vibrational comunication and reproductive isolation in the Enchenopa binotata species complex of treehoppers (Hemiptera: Membracidae). Evolution 58:571–578
Root TL, Price JT, Hall KR, Schneider SH (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60. https://doi.org/10.1038/nature01309
Ryan MA, Cokl A, Walter GH (1996) Differences in vibratory sound communication between a Slovenian and an Australian population of Nezara viridula (L.) (Heteroptera: Pentatomidae). Behav Process 36:183–193. https://doi.org/10.1016/0376-6357(95)00026-7
Seidelmann K (1991) Ausgewählte Aspekte der Populationsökologie der Roten Mauerbiene, Osmia rufa (L.), untersucht in Stammzuchten. Diploma thesis, Martin-Luther University, Halle-Wittenberg, Germany
Seidelmann K (1995) Untersuchungen zur Reproduktionsbiologie der Roten Mauerbiene, Osmia rufa (L., 1758). PhD thesis, Martin-Luther University Halle-Wittenberg, Germany
Sentis A, Hemptinne J-L, Brodeur J (2013) Effects of simulated heat waves on an experimental plant-herbivore-predator food chain. Glob Chang Biol 19:833–842. https://doi.org/10.1111/gcb.12094
Turner GF, Burrows MT (1995) A model of sympatric speciation by sexual selection. Proc R Soc B Lond 260:287–292. https://doi.org/10.1098/rspb.1995.0093
Valido A, Rodríguez-Rodríguez MC, Jordano P (2019) Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci Rep 9:4711. https://doi.org/10.1038/s41598-019-41271-5
Vallejo-Marín M (2018) Buzz pollination: studying bee vibrations on flowers. New Phytol. https://doi.org/10.1111/nph.15666
Walker T (1975) Effects of temperature on rates in poikilotherm nervous systems: evidence from the calling songs of meadow katydids (Orthoptera: Tettigoniidae:Orchelimum) and reanalysis of published data. J Comp Physiol A 101:57–69. https://doi.org/10.1007/BF00660119
West-Eberhard MJ (1983) Sexual selection, social competition, and speciation. Q Rev Biol 58:155–183
Westrich P (1989) Die Wildbienen Baden-Württembergs I, II. Eugen Ulmer Verlag, Stuttgart
Zweifel RG (1968) Effects of temperature, body size, and hybridization on mating calls of toads, Bufo americanus and Bufo woodhousii fowleri. Copeia 1968:269–285
Acknowledgments
I am grateful to my husband Markus Conrad for comments on the manuscript. I would also like to thank my PhD supervisor Prof. Manfred Ayasse for making this work possible and for introducing me to the bees. Big thanks to everyone who has provided me with bees over the years: Robin Dean, Dr. Karsten Seidelmann, Dr. Erhard Strohm, Sabine Rademacher, Mike Hermann, Henrik F Brødsgard, Anja Amtoft Wynns, and Adam Bates. Valerie Littlewood and Iris Thiede-Conrad I would like to thank for permission for the use of their artwork (Figs. 9.4 and 9.5) Special thanks to Dr. Axel Schmid for the initial idea of using magnets in the bioassay, Dr. Sebastian Breckerbohm for help with the physics, and Carina Stöker and Melanie Marquardt for their help collecting data. This research was funded by the German Federal Environmental Foundation (DBU).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Conrad, T. (2022). Sexual Selection in the Red Mason Bee: Vibrations, Population Divergence, and the Impact of Temperature. In: Hill, P.S.M., Mazzoni, V., Stritih-Peljhan, N., Virant-Doberlet, M., Wessel, A. (eds) Biotremology: Physiology, Ecology, and Evolution. Animal Signals and Communication, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-030-97419-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-97419-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-97418-3
Online ISBN: 978-3-030-97419-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)