Abstract
Spastic paresis due to neurologic injury results in both disruption of active force generation and increase in passive muscle forces that generate resistance to movement. However, the mechanisms of development of passive mechanical forces and their effect on active force generation are not well understood. This chapter considers the three-dimensional geometry of muscle, the structure and function of the extracellular matrix (ECM), and the role of intramuscular fluid in influencing the passive mechanical properties of the ECM via changes in intramuscular fluid volume, hyaluronan (HA) content, and ECM viscosity. These mechanisms form the basis of the hyaluronan hypothesis of muscle stiffness, where disruption of ECM homeostasis leads to the accumulation, biophysical alteration, and aggregation of HA in muscle. Both muscle unloading, due to paresis and immobility, and muscle overloading due to spastic muscle overactivity and compensatory overuse of specific muscles can trigger the accumulation of HA, increased viscoelasticity of the ECM, and muscle stiffness. The HA-rich matrix, in turn, controls the deposition of fibronectin and collagen and modulates the induction of myofibroblasts that are responsible for the excessive production of collagen leading to fibrosis and eventually to contracture.
…at each level of complexity entirely new properties appear, and the understanding of the new behaviors requires research which I think is as fundamental in its nature as any other.– Philip W. Anderson, More is Different, Science, 1972.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
-
Spastic paresis due to neurologic injury results in both disruption of active force generation and increase in passive muscle forces that generate resistance to movement. However, the mechanisms of development of passive mechanical forces and their effect on active force generation are not well understood.
-
This chapter considers the three-dimensional geometry of muscle, the structure and function of the extracellular matrix (ECM), and the role of intramuscular fluid in influencing the passive mechanical properties of the ECM via changes in intramuscular fluid volume, hyaluronan (HA) content, and ECM viscosity. These mechanisms form the basis of the hyaluronan hypothesis of muscle stiffness, where disruption of ECM homeostasis leads to the accumulation, biophysical alteration, and aggregation of HA in muscle.
-
Both muscle unloading, due to paresis and immobility, and muscle overloading due to spastic muscle overactivity and compensatory overuse of specific muscles can trigger the accumulation of HA, increased viscoelasticity of the ECM, and muscle stiffness.
-
The HA-rich matrix, in turn, controls the deposition of fibronectin and collagen and modulates the induction of myofibroblasts that are responsible for the excessive production of collagen leading to fibrosis and eventually to contracture.
Introduction
Spastic muscles are weaker, shorter, and stiffer – a key question is how do they get this way? Muscle weakness is explained by the paresis that invariably accompanies spasticity as part of the upper motor neuron syndrome. However, muscle shortening and stiffness have eluded a full explanation. The mechanisms of active muscle force generation are relatively well-described by excitation-contraction coupling, where the neural signal leads to the release of the neurotransmitter acetylcholine from the motor neuron terminal, which initiates the action potential and its spread along the sarcolemma of the sarcomeres (the basic functional unit of a muscle fiber) releasing calcium, which in turn facilitates the cross-bridge interactions between actin and myosin filaments that conclude with the release and reuptake of calcium by the sarcoplasmic reticulum, and the cycle repeats [1]. The active muscle force generated depends on the level of activation of each muscle fiber, as well as its length, and the velocity of contraction. The relationship between sarcomere length and active force generation is thought to reflect the degree of overlap between the actin and myosin filaments. At longer and shorter sarcomere lengths, the binding of actin and myosin is not complete leading to reduced force generation.
Even in the absence of any active force generation, the intracellular and extracellular noncontractile elastic elements in muscle generate passive forces. When a muscle is stretched, resistance from these noncontractile elements increases the passive force [2], and even small changes in muscle length have large implications for active force generation [3]. However, the precise mechanisms underlying passive mechanical properties of whole skeletal muscles are not well understood [4]. It was proposed that passive muscle stiffness may arise from a change in the configuration of the giant intracellular protein titin, also known as connectin, which forms elastic links between the ends of the thick filaments and the ends of the sarcomere (Z disks) [5], especially in shortened muscles. However, as reviewed in Chap. 5, subsequent studies found that the sarcomere is lengthened in patients with shortened whole muscle due to contracture [6, 7], and that the titin isoform and passive mechanics of single muscle fibers are not significantly different in spastic muscles, even though the muscle fascicles are stiffer [8]. This suggests that passive stiffness may arise from alterations in the extracellular matrix (ECM) of the muscle rather than from alterations in single muscle fibers. The structure and function of the ECM in muscle and its role in passive force development is complex. It has been found that the passive mechanical properties of muscle scale nonlinearly with size, i.e., small muscle fiber bundles are stiffer than single muscle cells, and larger fascicles and whole muscles are at least an order of magnitude stiffer [4, 9, 10], likely due to greater amounts of ECM. These findings are extremely relevant to individuals with spasticity and necessitate an understanding of the three-dimensional geometry of muscle and its effect on active and passive muscle force generation.
Three-dimensional Muscle Geometry and its Effect on Active and Passive Force Generation
The Architecture of Muscle Affects its Force Generating Capacity
Skeletal muscle is often thought of as a linear force generator, where the muscle fibers are connected end-to-end, i.e., between the origin and insertion of a muscle defining its line of action. However, there are several different muscle architecture types including parallel, pennate, and hydrostats. The vast majority of limb muscles have pennate architecture [11]. In pennate muscles, the fibers are oriented at an angle to the muscle’s line of action; the “pennation angle” is the angle between the fiber orientation and the muscle’s line of action. Hence, the direction in which the fibers generate force is not the same as the direction of the whole muscle’s action. The muscle fibers also rotate as they shorten, becoming more oblique such that the fraction of force directed along the muscle’s line of action decreases throughout a contraction. Fiber rotation decreases a muscle’s output force but increases output velocity by allowing the muscle to function at a higher gear ratio (muscle velocity/fiber velocity). The magnitude of fiber rotation, and therefore gear ratio, depends on how the muscle changes shape in the dimensions orthogonal to the muscle’s line of action. The gear ratio is not fixed for a given muscle but decreases significantly with the force of contraction, where dynamic muscle shape changes promote fiber rotation at low forces and resist fiber rotation at high forces [12,13,14]. Pennate architecture also allows muscle fibers to be packed such that there is a larger cross-sectional area of muscle for a given volume [15]. A muscle with a larger cross-sectional area can generate higher force per unit muscle compared to a muscle with parallel arrangement of fibers [16]. Thus, muscle force production varies depending on various parameters such as muscle length, fiber length, pennation angle, gearing, and its physiological cross-sectional area.
The ECM Transmits Force Laterally and Facilitates Muscle Shape Changes
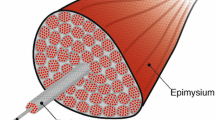
The broad organization of a muscle and its ECM is as follows: each muscle fiber is surrounded by endomysium, the muscle fiber bundles or fascicles are surrounded by perimysium, and the thick outer layer, the epimysium, surrounds the whole muscle and is continuous with the tendon. The endomysium, perimysium, and epimysium collectively form the ECM and are composed of collagens and elastic fibers embedded in a viscoelastic gel of proteoglycans, glycosaminoglycans (GAGs) such as hyaluronan, and assorted glycoproteins, which interact by entanglement, cross-linking, and charge-dependent interactions. When the myofibrillar proteins and proteoglycans are removed, the honeycombed three-dimensional network of the connective tissue can be seen (Fig. 6.1) [17,18,19]. Given that most muscle fibers do not span the entire length of the fascicle, or the whole muscle, the contractile forces generated by these muscle fibers can only be transmitted laterally via the endomysium by translaminar shear [20, 21]. In fact, force is transmitted laterally from contractile proteins to endomysial collagen fibrils across the fiber membranes via transverse filaments known as costameres, which mark sites of attachment between myofibrils and the sarcolemma [22,23,24]. The endomysium itself is a reticular fibrous network of quasi-randomly orientated wavy collagen fibers embedded in a proteoglycan matrix that forms a continuum between the basement membranes of two adjacent muscle fibers. When a muscle fiber contracts or is stretched, the preferred orientation of the collagen fibers in the endomysium changes but remains wavy and relatively compliant under tension at all sarcomere lengths. The change in collagen fibril orientation accounts for the nonlinear increase in passive resistance with increasing sarcomere length, and leads to translaminar shear through the endomysium and perimysium during lateral force transmission [19, 25]. The perimysium and epimysium define slip planes between muscle fascicles and whole muscles, and enable the fascicles and muscles to slide past each other allowing large shear displacements and shape changes in the whole muscle [26]. The shear forces across adjacent muscle fibers, muscle bundles, and whole muscles are strongly determined by the composition and viscoelastic properties of the proteoglycan matrix of the ECM (see below).
Structure of intramuscular connective tissue. (a) Scanning electron micrographs (SCM) of muscle after NaOH treatment to remove the myofibrillar proteins and proteoglycans at low magnification (×100) showing the honeycombed endomysial connective tissue within fascicles separated by the thick perimysial connective tissue. (b) Higher magnification (×3200) SCM view of the endomysial network separating (extracted) individual muscle cells. The planar feltwork of collagen fibrils in the endomysial reticular layer is clearly seen. (From Purslow PP (1994), with permission)
The Three-dimensional Nature of Muscle Contraction
Until recently, only the linear interaction of the actin and myosin filaments within sarcomeres was accounted for in force generation. However, the actin and myosin filaments are arranged in a hexagonal array, known as the filament lattice, within the sarcomere. It is now recognized that changes in the transverse or radial distance between the actin and myosin filaments (i.e., lattice spacing, Fig. 6.2) can also significantly influence the muscle force generated [27, 28]. The decline in muscle force or tension at longer and shorter lengths than optimal has previously been attributed solely to changes in actin-myosin overlap in the longitudinal or axial dimension [29]. However, as a muscle shortens longitudinally, it also swells radially, changing lattice spacing. It has been found that lattice spacing may actually explain 20–50% of the length-dependent change in force in the radial dimension [28]. Modeling muscle three-dimensionally to generate both axial and radial forces suggests that the radial forces generated are of the same order of magnitude as the axial forces, but that radial forces and axial forces vary differently with changes in sarcomere length – the magnitude of the radial force is 2.4 times greater than the axial force at extremely short sarcomere lengths, and 0.9 times that of the axial force at the longest sarcomere lengths [30].
A consideration of the three-dimensional nature of muscle contraction (see [31] for review) suggests that the structure and composition of the ECM [32, 33], and intracellular and extracellular fluids [34, 35], also influence active and passive forces in the muscle through their effects on muscle architecture, radial and axial force generation, and lateral force transmission (Fig. 6.2) [36, 37]. The ECM acts as a pathway for lateral force transmission across myofibrils via the endomysium [38], across multiple heads of the same muscle via the perimysium [39], and also across adjacent synergistic and antagonistic muscles via the epimysium and the intermuscular fascia [40,41,42]. The composition of the ECM is particularly important in the generation of passive muscle tension in mammalian muscles [43, 44]. As the proportion of ECM in muscle increases, the thickened ECM acts as a splint or sleeve and resists the radial expansion of the muscle, which in turn restricts muscle shortening in the axial dimension as well [45]. Transverse compression of a muscle with a load has a similar effect in limiting radial expansion and reducing the force generated by the muscle [46, 47]. Interestingly, release of fascial compartment boundaries also reduces force output [48], suggesting that restoring the health of the ECM may be critically important to preserve force output.
Effect of Fluid-ECM Interactions on Muscle Mechanics
Role of Intramuscular Fluid on the Properties of the ECM
Forces transmitted by fluid within muscle have been shown to be important in the three-dimensional dynamics of muscle contraction. Extracellular water in muscle regulates ion concentrations and pH, which affect muscle contraction and force development during exercise [49]. Activity moves fluid from blood plasma into muscle due to increased hydrostatic [50, 51] and osmotic forces [52, 53]. At the onset of muscle activity, the hydrostatic pressure gradient from the vascular toward the interstitial space of muscle moves fluid into the muscle [54, 55]. Muscular activity increases the osmotic pressure in the interstitial space due to the accumulation of exercise-related metabolites [52, 56, 57], which is proportional to the intensity of muscle action [58]. This leads to a further reduction in plasma volume, which is only partially made up for by reflex decrease of capillary pressure and increased hydrostatic pressure toward the vascular space, increased plasma osmolality, and some lymphatic return [59, 60]. With prolonged submaximal exercise, lactate is removed from active muscle to the vascular space at a higher rate leading to further changes in osmotic pressure gradients [61, 62]. Overall, a net increase in activity-induced intramuscular fluid volume has been observed after different kinds of activities such as short-term high-intensity exercise [63,64,65,66], and prolonged isometric contraction [67]. Furthermore, the accumulation of metabolites within muscle cells is related to the fiber type distribution in the muscle, where the increase is greater in fast-twitch type 2 fibers [68].
The intramuscular fluid pressure developed during active muscle contraction is estimated to be proportional to the developed muscle force, and influences muscle shape as it shortens longitudinally and swells radially [69,70,71]. Studies on isolated muscles have demonstrated that increased muscle fluid volume leads to an increase in passive muscle tension [35]. The passive tension at a given length changes in proportion to the volume change, and a measurable change in force can be observed with volume changes as small as 5% in isolated muscles [72]. The changes in muscle volume may also influence active contractile force by limiting radial transmission of muscle force [73]. Passive forces contribute substantially to normal movement, such as walking, and biarticular muscles play a role in passively transferring energy across joints [74]. The passive force contributions occur at the end ranges of joint motions (i.e., peak hip extension, peak knee extension, and peak ankle dorsiflexion) and hence increased passive resistance can compromise the overall active and passive range of motion [74]. Thus, intramuscular fluid can increase compression of contractile muscle tissue, reduce radial transmission of muscle force, increase passive tension in muscle, and reduce overall range of motion.
Intramuscular Fluid Affects the Viscosity of the Ground Substance of the ECM
The ECM is highly dynamic, and its composition is balanced by continuous production, degradation, and remodeling of its components to maintain homeostasis. The ECM regulates the biomechanical properties of tissues, maintains the structural integrity of muscles, and regulates cell growth and tissue function in health and disease [75,76,77]. GAGs are key components of the ECM, as they are responsible for most of its physical properties, and they modulate cellular behavior (see below). Hyaluronan (hyaluronic acid, HA), traditionally regarded as a space-filling ground substance is the most abundant GAG in the ECM, found in the endomysium, perimysium, and epimysium of muscle [78, 79], as well as in the loose connective tissue or fascia surrounding muscle [80, 81]. HA is the only nonsulfated GAG, composed of a repeating disaccharide of glucuronic acid and N-acetyl glucosamine, that forms long chains or polymers assuming molecular weights of the order of 105 to 107 Da and an extended length of 0.25–25 μm [82]. It is synthesized by HA synthases (HAS 1-3) located on the plasma membrane, and HA molecules of different molecular weights are extruded from the cytoplasm to the ECM through the HAS pores that link the intracellular with the extracellular space (Fig. 6.3) [83]. The chemical structure of HA, in particular the presence of -OH groups, makes it highly hydrophilic enabling the molecule to retain water and swell which makes it an ideal space-filling molecule. Under physiological conditions, molecules of high molecular weight HA start to entangle at concentrations of less than 1 g/l and assume an expanded random coil structure surrounded by water molecules, which occupies a very large volume. Most of the volume of high molecular weight HA is water, which is not bound by the polymer. The polymer shape is constantly changing, but the water still contributes to the effective size of each molecule because of its frictional interaction with closely spaced polymer segments. The time-average shape of HA can be described as a sphere, with greatest density of chain segments near the center. Furthermore, the effective sphere-like volume of a wormlike HA chain grows exponentially as the molecular weight of HA increases, accounting for its unique hydrodynamic properties which affect tissue hydration, viscosity, and physical stiffness [84].
Structure and synthesis of hyaluronan (HA). (a) Molecular structure of a HA disaccharide unit. HA is a negatively charged polysaccharide composed of repeating disaccharide units of glucuronic acid (GlcA; blue) and N-acetylglucosamine (GlcNAc). (b) Secondary structure of a HA tetrasaccharide with water. Hydrogen bonds are represented by red dashed lines. (c) Predicted structure of mammalian HAS. HAS enzymes contain multiple membrane-spanning regions at both the amino and carboxyl terminus and catalytic sites at the central part of the molecule. (d) Schematic illustration of HA synthesis and secretion. HAS enzymes catalyze the alternative addition of UDP-GlcA and UDP-GlcNAc to the nascent HA chain and extrude it through the plasma membrane. (From Kobayashi T (2020), with permission)
Muscle Hydration, Lubrication, Viscoelasticity, and ECM Stiffness
The production and degradation of HA, its hydrodynamic properties, and distribution throughout the ECM significantly affect intramuscular fluid dynamics, ECM viscoelasticity, and the passive resistance of the ECM. As noted above, as the concentration and molecular weight of HA increases, it entrains more water within its hydrodynamic volume, and the viscosity of the solution increases exponentially (a 10 mg/ml solution of 1.5 × 106 Da HA has a viscosity 5000× that of water) due to macromolecular crowding of the polymer (Fig. 6.4) [85, 86]. Viscosity is the resistance to flow of a liquid. Under shear stress, the viscosity of HA drops rapidly while maintaining elasticity, making it an ideal biological lubricant [87]. However, the molecular weight and viscosity of HA solutions affects the lubrication of tissues. For example, at the cartilage-cartilage interface, the relative effectiveness of friction reduction (especially static friction, the resistance to start up motion) is dependent on the molecular weight of HA: the higher the molecular weight, the lower the friction [88]. This is thought to be due to a “viscous boundary layer” of HA at the surface of cartilage that facilitates low-velocity high-load movements [89]. In contrast, for high-velocity low-load movements, such as in the endomysium and perimysium of muscle, the thickness of the HA-containing boundary is large compared with the diameter of the molecules [79]. Here friction would be predicted to increase with increased HA concentration and viscosity, negatively affecting lubrication, i.e., the higher the molecular weight, the greater the friction [90]. Viscoelasticity is the time-dependent resistance to loading or deformation. Hyaluronan solutions also exhibit viscoelastic behavior that is highly dependent on HA concentration, temperature, pH, and the ionic strength of the solution [91]. HA shows nonlinear viscoelastic behavior with higher concentration, temperatures, and with increased or decreased pH [92,93,94,95,96], implying that a muscle with higher HA concentrations, and hence higher viscoelasticity will show greater passive resistance to stretch or contraction. Thus, the concentration and rheological properties of HA in the ECM of muscle can contribute significantly to increased passive resistance during movement [97, 98].
Hydrodynamic properties and viscosity of hyaluronan (HA). (a) HA chains of increasing molecular weight (from left to right) 0.1, 0.5, 1, 3 and 6 million have hydrodynamic diameters of approximately 50, 140, 210, 400 and 600 nm, respectively in physiological saline solution. (b) The effective hydrodynamic domain of each chain is modeled as a sphere, the volume of which is dependent on the molecular weight to the 1.8 power. (c) Experimental data for HA in physiological saline shows a marked increase in viscosity with increasing concentration and intrinsic viscosity, as expected for nonideal solutions. (Adapted from Cowman MK (2015), with permission)
Effect of Exercise, Immobility, and Overuse on Hyaluronan Concentration
The HA in muscle is primarily of high molecular weight (>4 million Da), whereas the HA contained in lymph is primarily of low molecular weight. Volume loading produces a preferential increase in the flux of low molecular weight HA, although the maximum daily removal of HA by lymph is <1% of the tissue content in a homeostatic state [99]. In human muscle, HA is especially abundant in the perimysium (Fig. 6.5). HA muscle concentrations show large interindividual variation at rest, with no correlation between muscle and serum HA levels in the healthy state. Exercise does not immediately change muscle HA concentration, but serum HA increases significantly and decreases rapidly to lower than resting levels by 30-min postexercise [79]. However, inducing muscle hypertrophy, for example by synergist elimination after Achilles tenectomy in mice increased the concentration of HA in the ECM of the compensating plantaris muscle and increased the expression of HA synthases within a variety of cell types [100]. The hypertrophic stimulus significantly increased muscle HA concentration 2.8-fold after two days, which remained significantly increased at seven days, and then decreased gradually toward control levels by 14 days (Fig. 6.6). Endogenous hyaluronidase genes, HYAL1 and HYAL2, were also highly expressed in skeletal muscle but did not change after the tenectomy. These results indicate that HA levels change dynamically in response to a hypertrophic stimulus and various cell types participate in its synthesis. Similar prior studies in rats also showed hypertrophy of the compensating muscle after synergist elimination, accompanied by large increases in the ECM, particularly thickening of the endomysium [101] and increase in the muscle’s wet weight after 21 days mediated by interleukin-6 [102]; however HA was not quantified. Taken together, these studies demonstrate that muscle HA synthesis increases in response to exercise overload, and can contribute to thickening of the ECM. Interestingly, unloading of the rat soleus muscle by immobilization of the ankle joint resulted in muscle shortening and increase in muscle HA content four weeks postimmobilization compared to controls. However, thickening and disorganization of endomysial collagen fibrils only became apparent by 12 weeks postimmobilization (Fig. 6.7) [103]. Thus, disruption of HA homeostasis by both muscle overloading and unloading leads to changes in muscle HA content, and precedes changes in collagen fiber organization and content in the muscle ECM.
Location of hyaluronan (HA) in human muscle. (a) Light micrograph of histological section of quadriceps femoris muscle obtained at surgery stained with brown staining biotinylated HA binding protein (HABP). The endomysium (arrows) and perimysium (asterisks) are rich in HA. (b) Control slide treated with Streptomyces hyaluronidase showed no staining, indicating that the HABP specifically reacted with HA and not with any of the other GAGs. (From Piehl-Aulin K (1991), with permission)
Upregulation of hyaluronan (HA) and tenascin-C (TN-C) during compensatory hypertrophy in the mouse plantaris muscle. (a and a’) In control muscles, HA (labeled with hyaluronic acid binding protein, red) was expressed at low levels and TN-C (green) was restricted to the aponeurosis (a). (b and b’) Two days after Achilles tenectomy, the epimysium became enriched with HA (arrowheads), and both HA and TN-C appeared to infiltrate the basal lamina surrounding individual myofibers (white laminin). (c and c’) HA and TN-C were ubiquitous within the interstitial space after seven days. (d and d’) By 14 days, HA and TN-C expression had decreased within the muscle body, whereas the epimysium was still strongly labeled for HA compared with the control (a). Bar 200 μm; x 10. (Modified from Calve S (2012), with permission)
Upregulation of hyaluronan (HA) and thickening of soleus muscle endomysia after immobilization of a rat ankle joint. (a) Control muscles, HA (labeled with hyaluronic acid binding protein, brown) was expressed at low levels. (b) Four weeks after immobilization the endomysium and perimysium are strongly labeled for HA compared with the control; bar 50 μm. (c) Scanning electron micrographs (SCM) of soleus muscle endomysia four weeks after immobilization. (d) SCM of soleus muscle endomysia 12 weeks after immobilization; bar 1 μm. (Modified from Okita M (2004), with permission)
The Hyaluronan Hypothesis of Muscle Stiffness: its Role in Spasticity, Fibrosis, and Contracture
The Hyaluronan Hypothesis of Muscle Stiffness
The hyaluronan hypothesis of muscle stiffness postulated that the excessive deposition of HA in the ECM of muscle contributes to the development of muscle stiffness by dramatically altering its viscosity [104, 105]. As alluded to earlier in this chapter, HA is a high molecular weight GAG in the ECM, where it serves as a lubricant, allowing contracting muscle fibers to glide past each other and facilitate force transmission [79, 106]. The hyaluronan hypothesis is based on several findings. First, paresis and immobility after stroke lead to rapid muscle atrophy [107]. Second, immobility results in a relative increase in the proportion of the ECM which is initially composed of space-filling HA and subsequently results in the deposition of collagen as shown in animal models [103]. Third, at high concentrations, hyaluronan and protein-crosslinked assemblies of HA aggregate [108], and interact with water molecules to dramatically increase the viscoelasticity of the ECM [90]. These large, aggregated HA molecules cannot be cleared from the muscle particularly when mobility is reduced. Thus, hyperviscous HA in the ECM can increase the passive stiffness of the ECM, and reduce lubrication and gliding during force transmission causing myofibers, muscle fascicles, and whole muscles to be stuck together. Finally, as discussed below, hyperreflexia and “muscle overactivity” in patients with spasticity, as well as compensatory muscle overuse can trigger excessive production of HA by mechanically overloading specific muscles.
Evidence for the hyaluronan hypothesis is obtained from clinical data which showed that treatment with the enzyme hyaluronidase, which hydrolyzes high molecular weight HA into smaller fragments, led to a dramatic reduction in resistance to passive motion measured using the Modified Ashworth Scale , and increased passive and active range of motion [104] (see Chap. 13). In addition, T1 rho (T1ρ) muscle MRI which images intramuscular GAG content showed increased T1ρ relaxation times in patients with poststroke muscle stiffness, and a reduction to more normal levels after treatment with the enzyme hyaluronidase (Fig. 6.8) [109]. A more recent study with biexponential T1ρ muscle MRI quantified the structure of HA in relation to its association with water molecules, and showed that the accumulated HA in stiff muscles traps intramuscular free water, which is released after treatment with hyaluronidase [110]. The affinity of HA for water in stiff muscles can also be observed as a hypoechoic signal on gray scale ultrasound imaging [111]. Additionally, the demonstration of increased muscle viscosity in spastic muscles of stroke survivors using ultrasound shear wave velocity measurements and muscle modeling [112,113,114] also supports the hyaluronan hypothesis.
Imaging hyaluronan (HA) in post stroke muscle stiffness using T1ρ muscle MRI. (a) T1ρ maps of representative slices overlaid over anatomy in a control subject. (b) T1ρ maps of three representative slices overlaid over anatomy in a patient with poststroke muscle stiffness prior to hyaluronidase injection treatment. (c) T1ρ maps of the same patient in (b) at approximately similar slice locations following hyaluronidase injection treatment. Note the difference in shape of the muscle before and after the injections. (Modified from Menon R (2019), with permission)
Spasticity Versus Muscle Stiffness
The hyperreflexia associated with spasticity became synonymous with “muscle overactivity” with the availability of botulinum toxin injections for focal treatment of muscle overactivity [115, 116], as explained in Chap. 1. Muscle overactivity then became undifferentiated from hypertonia and muscle stiffness, although it has been recognized that paralysis, muscle shortening, and muscle overactivity are three separate disabling factors in patients with spasticity that may need to be treated differently [117] (see also Chaps. 2 and 3). It has been suggested that paresis leads to muscle shortening via nonreflex (i.e., non-neural) increases in passive resistance to movement or stretch, which may increase the sensitivity to stretch and hence produce muscle overactivity via reflex mechanisms [118, 119]. However, the pathophysiologic basis of non-neural muscle shortening and its relationship to neural reflex mechanisms and muscle overactivity has been difficult to elucidate. The finding above that muscle unloading from paresis and immobility leads to an increase in HA in muscle ECM [103], which increases its viscosity and intrinsic stiffness, suggests that HA may be the missing link that connects paresis, muscle shortening, and spasticity [105].
Muscle stretch is sensed by muscle spindle receptors which reside within the HA-rich perimysium of the muscle. The muscle spindles are fusiform structures consisting of a bundle of intrafusal fibers, classified as nuclear bag and chain fibers, enclosed in a connective tissue capsule. The muscle spindle capsule consists of two distinct portions, the inner and the outer capsule [120]. The inner capsule encloses the intrafusal fibers within an innermost axial space, whereas the outer capsule is multilayered and encloses a fluid-filled space in the equatorial region of the spindle called the periaxial space. HA is abundant in the axial and periaxial spaces of the muscle spindles, in all layers of the spindle capsule, as well as in the endoneurium and in the space in between individual axons in the perimysium (Fig. 6.9) [121]. The presence of HA in the periaxial fluid has been shown to be responsible for the transcapsular potential which increases the sensitivity of the sensory endings to mechanical stimuli [122]. Alteration in the viscosity of the HA solution in the muscle spindle can thus increase the sensitivity of the muscle spindle to stretch based on models of mechanosensation [123]. Furthermore, a muscle that is in a shortened position shows increased sensitivity to stretch [124]. Taken together, paresis and immobility can lead to HA accumulation in the ECM of muscle which may increase the stretch-sensitivity of the muscle spindle particularly in shortened paretic muscles. This along with the excitatory-inhibitory imbalance in the spinal cord interneuronal network after upper motor neuron lesions (see Chap. 1), may further potentiate stretch reflex hyperexcitability that is characteristic of spastic muscles. The finding that muscle overloading (due to compensatory overuse of specific muscles and potentially reflex overactivity) increases HA synthesis [100], suggests that the excessive HA can further increase the viscosity and intrinsic stiffness of the ECM and contribute to stiffness of the whole muscle. Thus, HA accumulation may be central to both the non-neural and neural mechanisms that produce increased resistance to passive joint motion in patients with spasticity demonstrated in Chap. 4.
Muscle spindle structure, location and hyaluronan (HA) content. (a) Schematic drawing of the structure of the muscle spindle in the equatorial region. The intrafusal fibers are shown in the axial spaces within the inner capsule. (b) Serial cross-sections of human lumbrical muscle stained with brown staining biotinylated HA binding protein (HABP) (a) and toluidine blue (b) showing a neurovascular bundle (A artery, V vein, N nerve) and a muscle spindle (MS). Notice the strong HABP staining in (a) that fills the capsular space of the muscle spindle and surrounds the nerve fibres in the perimysium; bar 50 μm. (Modified from Pedroso-Domellof F (1998), with permission)
Role of Hyaluronan in the Progression to Fibrosis and Contracture
As discussed in Chap. 5, the thickness and collagen content of the ECM in the endomysium, perimysium, and epimysium are increased in chronically spastic muscles that are contractured [125]. Therefore, increased collagen content has been associated with muscle stiffness in patients with spasticity. If this is the case, muscles with higher collagen content and disorganization would be expected to be stiffer. Interestingly, surgical samples of spastic muscle bundles showed significantly lower passive mechanical stiffness when compared with nonspastic muscle bundles [126]. Thus, recent studies do not support a role for increased content and disorganization of collagen in the development of passive muscle stiffness [127, 128]. In contrast, the viscoelastic properties of spastic muscles of stroke survivors under passive conditions were shown to be significantly altered using ultrasound shear wave velocity measurements, which were used in pure elastic and viscoelastic (Voigt) muscle models [112,113,114]. The effect of stroke was mainly evident in the viscous parameter, and the differences between stroke-affected and nonaffected muscles were more evident at large joint angles, suggesting that the increase in passive muscle stiffness poststroke was largely due to an increase in muscle viscosity.
The viscoelasticity or stiffness of the ECM has important implications for cell–ECM adhesion, cell spreading, migration, differentiation, and even organoid formation [129,130,131]. The molecular weight of HA is associated with different biological functions: low molecular weight HA is usually associated with proinflammatory response, proangiogenic activity, and migration and proliferation of cells, whereas high molecular weight HA is linked to cellular differentiation and anti-inflammatory effects. HA binding receptors include specific cell-surface receptors such as CD44 and RHAMM (receptor for hyaluronic-acid-mediated motility), as well as hyaluronan receptor for endocytosis (HARE), hyaluronan-binding protein 1 (HABP1), and lymphatic vessel endothelial receptor for hyaluronan 1 (LYVE1). HA interacts with cell surfaces in at least two ways — it can bind to cell-surface receptors to induce the transduction of a range of intracellular signals, either directly or by activating other receptors, and/or it can be retained at the cell surface by sustained transmembrane interactions with its synthases. Either means of retention can generate a voluminous pericellular matrix, or “coat” that incorporates several other HA-binding molecules. This “provisional matrix” provides a hydrated environment in which HA functions as a microenvironmental cue that regulates cell behavior [132]. HA in the pericellular matrix controls the deposition of fibronectin and collagen and modulates the induction of myofibroblasts [133], which are a specialized form of fibroblast responsible for the excessive production of collagen leading to fibrosis and tissue destruction in multiple diseases [134,135,136].
The differentiation of fibroblasts to myofibroblasts is primarily driven by mechanical tension, and cytokines such as TGF-β, and fibronectin [137, 138]. Several studies have shown that HA is intimately connected with the maintenance of the myofibroblast phenotype [139,140,141,142,143,144,145]. Removal of cell-surface HA is known to destabilize focal adhesions involved in cell attachment [146], suggesting that HA in the cross-linked pericellular matrix may cooperate with focal adhesions to provide the mechanical tension needed to maintain the myofibroblast phenotype. Association of HA with the cell-surface receptor CD44 influences the positioning of TGF-β receptors which can have an impact on TGF-β signaling [147], and blocking the synthesis of HA in fibroblasts inhibits the fibroblast-to-myofibroblast conversion [144]. HA also interacts with the fibrillar collagens in the ECM to modulate the mechanical properties of collagen and alter the contractile forces that can be generated by the cells [148]. In fact, the addition of HA to a collagen preparation with a slow rate of fibril formation led to an acceleration in fibril formation, and injection of the collagen solution into tissue promoted the migration of fibroblast-like cells into the region occupied by the injected collagen [149]. Overexpression of HA synthases has also been shown to regulate the invasiveness of fibroblasts, promote fibrosis, and control fibroblast senescence in a lung model of fibrosis [150, 151]. These studies suggest that targeting HA accumulation or synthesis could be an effective therapeutic approach to prevent fibrosis (Fig. 6.10). However, one study suggested that removal of HA may increase the expression and accumulation of collagen I and fibronectin [133], which may point to the balance of HA necessary for tissue homeostasis – both too little and too much may signal a tipping point toward fibrosis. Despite the gaps in a complete mechanistic understanding of fibrosis, increased levels of HA have been shown to precede fibrosis in several organs [152,153,154,155,156]. Fibrosis is the end stage of many different diseases and often results from a chronic inflammatory insult, although remodeling of the ECM toward a fibrotic phenotype does not have to involve an inflammatory component [157,158,159]. Processes that result in muscle fibrosis include chronic inflammation [160], denervation [161], neurotoxin injection [162, 163], and direct trauma [161, 164, 165]. Muscle contractures that occur secondary to cerebral palsy, stroke and other chronic neurological conditions, and muscular diseases are due to fibrosis [126, 166, 167], and surgical approaches are used to release these contractures. However, there is no current therapy for recovery from fibrosis.
Schematic model for evolution of muscle stiffness to fibrosis and contracture. (a) Cross-section of a normal skeletal muscle. (b) Development of muscle stiffness due to the accumulation of hyaluronan (HA) in the extracellular space, which increases the viscosity of the ECM, and causes the muscle fibers to stick together. (c) Continued HA accumulation initiates fibrosis leading to the replacement of HA by collagen, thickened endomysium and perimysium, and further muscle fiber atrophy. Note that whereas fibrosis is irreversible, muscle stiffness may be reversible, representing a potential therapeutic target. (Modified from Menon R (2019), with permission)
Conclusion
Spastic paresis due to neurologic injury from an upper motor neuron lesion results in both disruption of active force generation and excessive increase in passive muscle forces that generate resistance to movement. The three-dimensional geometry of muscle, the structure and function of the extracellular matrix (ECM), and changes in intramuscular fluid volume, hyaluronan (HA) content, and ECM viscosity influence the development of passive mechanical stiffness and form the basis for understanding the hyaluronan hypothesis of muscle stiffness. Both muscle unloading, due to paresis and immobility, and muscle overloading due to spastic muscle overactivity and compensatory overuse of specific muscles in individuals with spasticity can disrupt ECM homeostasis leading to the accumulation, biophysical alteration, and aggregation of HA in muscle, which in turn increases the viscoelasticity of the ECM, and leads to muscle stiffness. The HA-rich matrix in turn controls the deposition of fibronectin and collagen and modulates the induction of myofibroblasts that are responsible for the excessive production of collagen leading to fibrosis and eventually to contracture. A better understanding of the role of HA in the progression to fibrosis in muscle could lead to the prevention of contractures and the consequent disability.
References
Shishmarev D. Excitation-contraction coupling in skeletal muscle: recent progress and unanswered questions. Biophys Rev. 2020;12(1):143–53.
Hill AV. The mechanics of active muscle. Proc R Soc Lond B Biol Sci. 1953;141(902):104–17.
Lieber RL, Murray WM, Clark DL, Hentz VR, Friden J. Biomechanical properties of the brachioradialis muscle: implications for surgical tendon transfer. J Hand Surg Am. 2005;30(2):273–82.
Lieber RL, Binder-Markey BI. Biochemical and structural basis of the passive mechanical properties of whole skeletal muscle. J Physiol. 2021;599:3809.
Wang K, McCarter R, Wright J, Beverly J, Ramirez-Mitchell R. Regulation of skeletal muscle stiffness and elasticity by titin isoforms: a test of the segmental extension model of resting tension. Proc Natl Acad Sci U S A. 1991;88(16):7101–5.
Lieber RL, Ponten E, Burkholder TJ, Friden J. Sarcomere length changes after flexor carpi ulnaris to extensor digitorum communis tendon transfer. J Hand Surg Am. 1996;21(4):612–8.
Lieber RL, Friden J. Intraoperative measurement and biomechanical modeling of the flexor carpi ulnaris-to-extensor carpi radialis longus tendon transfer. J Biomech Eng. 1997;119(4):386–91.
Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol. 2011;589(Pt 10):2625–39.
Ward SR, Winters TM, O'Connor SM, Lieber RL. Non-linear scaling of passive mechanical properties in fibers, bundles, fascicles and whole rabbit muscles. Front Physiol. 2020;11:211.
Smith LR, Fowler-Gerace LH, Lieber RL. Muscle extracellular matrix applies a transverse stress on fibers with axial strain. J Biomech. 2011;44(8):1618–20.
Ward SR, Eng CM, Smallwood LH, Lieber RL. Are current measurements of lower extremity muscle architecture accurate? Clin Orthop Relat Res. 2009;467(4):1074–82.
Azizi E, Brainerd EL, Roberts TJ. Variable gearing in pennate muscles. Proc Natl Acad Sci U S A. 2008;105(5):1745–50.
Holt NC, Danos N, Roberts TJ, Azizi E. Stuck in gear: age-related loss of variable gearing in skeletal muscle. J Exp Biol. 2016;219(Pt 7):998–1003.
Eng CM, Azizi E, Roberts TJ. Structural determinants of muscle gearing during dynamic contractions. Integr Comp Biol. 2018;58(2):207–18.
Gans C. Fiber architecture and muscle function. Exerc Sport Sci Rev. 1982;10:160–207.
Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, et al. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001;534(Pt. 2):613–23.
Purslow PP, Delage JP. General anatomy of the muscle fasciae. In: Fascia: the tensional network of the human body. London: Churchill Livingstone; 2012. p. 5–10.
Purslow PP. The structure and role of intramuscular connective tissue in muscle function. Front Physiol. 2020;11:495.
Purslow PP, Trotter JA. The morphology and mechanical properties of endomysium in series-fibred muscles: variations with muscle length. J Muscle Res Cell Motil. 1994;15(3):299–308.
Trotter JA. Functional morphology of force transmission in skeletal muscle. A brief review. Acta Anat (Basel). 1993;146(4):205–22.
Trotter JA, Purslow PP. Functional morphology of the endomysium in series fibered muscles. J Morphol. 1992;212(2):109–22.
Pardo JV, Siliciano JD, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements ("costameres") mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci U S A. 1983;80(4):1008–12.
Ervasti JM. Costameres: the Achilles' heel of Herculean muscle. J Biol Chem. 2003;278(16):13591–4.
Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, et al. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol. 2011;589(Pt 5):1195–208.
Passerieux E, Rossignol R, Letellier T, Delage JP. Physical continuity of the perimysium from myofibers to tendons: involvement in lateral force transmission in skeletal muscle. J Struct Biol. 2007;159(1):19–28.
Purslow PP. Muscle fascia and force transmission. J Bodyw Mov Ther. 2010;14(4):411–7.
Millman BM. The filament lattice of striated muscle. Physiol Rev. 1998;78(2):359–91.
Williams CD, Salcedo MK, Irving TC, Regnier M, Daniel TL. The length-tension curve in muscle depends on lattice spacing. Proc Biol Sci. 2013;280(1766):20130697.
Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184(1):170–92.
Williams CD, Regnier M, Daniel TL. Elastic energy storage and radial forces in the myofilament lattice depend on sarcomere length. PLoS Comput Biol. 2012;8(11):e1002770.
Roberts TJ, Eng CM, Sleboda DA, Holt NC, Brainerd EL, Stover KK, et al. The multi-scale, three-dimensional nature of skeletal muscle contraction. Physiology (Bethesda). 2019;34(6):402–8.
Borg TK, Caulfield JB. Morphology of connective tissue in skeletal muscle. Tissue Cell. 1980;12(1):197–207.
Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44(3):318–31.
Gindre J, Takaza M, Moerman KM, Simms CK. A structural model of passive skeletal muscle shows two reinforcement processes in resisting deformation. J Mech Behav Biomed Mater. 2013;22:84–94.
Sleboda DA, Roberts TJ. Incompressible fluid plays a mechanical role in the development of passive muscle tension. Biol Lett. 2017;13(1):20160630.
Blemker SS, Pinsky PM, Delp SL. A 3D model of muscle reveals the causes of nonuniform strains in the biceps brachii. J Biomech. 2005;38(4):657–65.
Sinha U, Sinha S, Hodgson JA, Edgerton RV. Human soleus muscle architecture at different ankle joint angles from magnetic resonance diffusion tensor imaging. J Appl Physiol (1985). 2011;110(3):807–19.
Street SF. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol. 1983;114(3):346–64.
Huijing PA, Baan GC, Rebel GT. Non-myotendinous force transmission in rat extensor digitorum longus muscle. J Exp Biol. 1998;201(Pt 5):683–91.
Huijing PA. Epimuscular myofascial force transmission: a historical review and implications for new research. International Society of Biomechanics Muybridge Award Lecture, Taipei, 2007. J Biomech. 2009;42(1):9–21.
Yucesoy CA, Baan G, Huijing PA. Epimuscular myofascial force transmission occurs in the rat between the deep flexor muscles and their antagonistic muscles. J Electromyogr Kinesiol. 2010;20(1):118–26.
Turrina A, Martinez-Gonzalez MA, Stecco C. The muscular force transmission system: role of the intramuscular connective tissue. J Bodyw Mov Ther. 2013;17(1):95–102.
Wood LK, Kayupov E, Gumucio JP, Mendias CL, Claflin DR, Brooks SV. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J Appl Physiol (1985). 2014;117(4):363–9.
Meyer G, Lieber RL. Muscle fibers bear a larger fraction of passive muscle tension in frogs compared with mice. J Exp Biol. 2018;221(Pt 22):jeb182089.
Azizi E, Deslauriers AR, Holt NC, Eaton CE. Resistance to radial expansion limits muscle strain and work. Biomech Model Mechanobiol. 2017;16(5):1633–43.
Siebert T, Till O, Blickhan R. Work partitioning of transversally loaded muscle: experimentation and simulation. Comput Methods Biomech Biomed Engin. 2014;17(3):217–29.
Siebert T, Till O, Stutzig N, Gunther M, Blickhan R. Muscle force depends on the amount of transversal muscle loading. J Biomech. 2014;47(8):1822–8.
Ruttiman RJ, Sleboda DA, Roberts TJ. Release of fascial compartment boundaries reduces muscle force output. J Appl Physiol (1985). 2019;126(3):593–8.
Sjogaard G, Saltin B. Extra- and intracellular water spaces in muscles of man at rest and with dynamic exercise. Am J Phys. 1982;243(3):R271–80.
Jacobsson S, Kjellmer I. Accumulation of fluid in exercising skeletal muscle. Acta Physiol Scand. 1964;60:286–92.
Kjellmer I. The effect of exercise on the vascular bed of skeletal muscle. Acta Physiol Scand. 1964;62:18–30.
Lundvall J. Tissue hyperosmolality as a mediator of vasodilatation and transcapillary fluid flux in exercising skeletal muscle. Acta Physiol Scand Suppl. 1972;379:1–142.
Lundvall J, Mellander S, Westling H, White T. Fluid transfer between blood and tissues during exercise. Acta Physiol Scand. 1972;85(2):258–69.
Folkow B, Haglund U, Jodal M, Lundgren O. Blood flow in the calf muscle of man during heavy rhythmic exercise. Acta Physiol Scand. 1971;81(2):157–63.
Nicolaides AN, Zukowski AJ. The value of dynamic venous pressure measurements. World J Surg. 1986;10(6):919–24.
Meyer RA, Prior BM. Functional magnetic resonance imaging of muscle. Exerc Sport Sci Rev. 2000;28(2):89–92.
Bjornberg J. Forces involved in transcapillary fluid movement in exercising cat skeletal muscle. Acta Physiol Scand. 1990;140(2):221–36.
Jenner G, Foley JM, Cooper TG, Potchen EJ, Meyer RA. Changes in magnetic resonance images of muscle depend on exercise intensity and duration, not work. J Appl Physiol (1985). 1994;76(5):2119–24.
Senay LC Jr, Pivarnik JM. Fluid shifts during exercise. Exerc Sport Sci Rev. 1985;13:335–87.
Greenleaf JE, Convertino VA, Stremel RW, Bernauer EM, Adams WC, Vignau SR, et al. Plasma [Na+], [Ca2+], and volume shifts and thermoregulation during exercise in man. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(6):1026–32.
Gladden LB. Muscle as a consumer of lactate. Med Sci Sports Exerc. 2000;32(4):764–71.
Stallknecht B, Vissing J, Galbo H. Lactate production and clearance in exercise. Effects of training. A mini-review. Scand J Med Sci Sports. 1998;8(3):127–31.
Willwacher S, Sleboda DA, Mahlich D, Bruggemann GP, Roberts TJ, Bratke G. The time course of calf muscle fluid volume during prolonged running. Physiol Rep. 2020;8(9):e14414.
Ploutz-Snyder LL, Convertino VA, Dudley GA. Resistance exercise-induced fluid shifts: change in active muscle size and plasma volume. Am J Phys. 1995;269(3 Pt 2):R536–43.
Raja MK, Raymer GH, Moran GR, Marsh G, Thompson RT. Changes in tissue water content measured with multiple-frequency bioimpedance and metabolism measured with 31P-MRS during progressive forearm exercise. J Appl Physiol (1985). 2006;101(4):1070–5.
Shi J, Zheng YP, Chen X, Huang QH. Assessment of muscle fatigue using sonomyography: muscle thickness change detected from ultrasound images. Med Eng Phys. 2007;29(4):472–9.
Jensen BR, Jorgensen K, Sjogaard G. The effect of prolonged isometric contractions on muscle fluid balance. Eur J Appl Physiol Occup Physiol. 1994;69(5):439–44.
Prior BM, Ploutz-Snyder LL, Cooper TG, Meyer RA. Fiber type and metabolic dependence of T2 increases in stimulated rat muscles. J Appl Physiol (1985). 2001;90(2):615–23.
Heukelom B, van der Stelt A, Diegenbach PC. A simple anatomical model of muscle, and the effects of internal pressure. Bull Math Biol. 1979;41(6):791–802.
Daggfeldt K. Muscle bulging reduces muscle force and limits the maximal effective muscle size. J Mech Med Biol. 2006;6:229–39.
Winters TM, Sepulveda GS, Cottler PS, Kaufman KR, Lieber RL, Ward SR. Correlation between isometric force and intramuscular pressure in rabbit tibialis anterior muscle with an intact anterior compartment. Muscle Nerve. 2009;40(1):79–85.
Sleboda DA, Wold ES, Roberts TJ. Passive muscle tension increases in proportion to intramuscular fluid volume. J Exp Biol. 2019;222(Pt 21):jeb209668.
Sleboda DA, Roberts TJ. Internal fluid pressure influences muscle contractile force. Proc Natl Acad Sci U S A. 2020;117(3):1772–8.
Whittington B, Silder A, Heiderscheit B, Thelen DG. The contribution of passive-elastic mechanisms to lower extremity joint kinetics during human walking. Gait Posture. 2008;27(4):628–34.
Csapo R, Gumpenberger M, Wessner B. Skeletal muscle extracellular matrix - what do we know about its composition, regulation, and physiological roles? A narrative review. Front Physiol. 2020;11:253.
Amorim S, Reis CA, Reis RL, Pires RA. Extracellular matrix mimics using Hyaluronan-based biomaterials. Trends Biotechnol. 2021;39(1):90–104.
Manou D, Caon I, Bouris P, Triantaphyllidou IE, Giaroni C, Passi A, et al. The complex interplay between extracellular matrix and cells in tissues. Methods Mol Biol. 2019;1952:1–20.
Laurent C, Johnson-Wells G, Hellstrom S, Engstrom-Laurent A, Wells AF. Localization of hyaluronan in various muscular tissues. A morphological study in the rat. Cell Tissue Res. 1991;263(2):201–5.
Piehl-Aulin K, Laurent C, Engstrom-Laurent A, Hellstrom S, Henriksson J. Hyaluronan in human skeletal muscle of lower extremity: concentration, distribution, and effect of exercise. J Appl Physiol (1985). 1991;71(6):2493–8.
Stecco C, Stern R, Porzionato A, Macchi V, Masiero S, Stecco A, et al. Hyaluronan within fascia in the etiology of myofascial pain. Surg Radiol Anat. 2011;33(10):891–6.
Fede C, Angelini A, Stern R, Macchi V, Porzionato A, Ruggieri P, et al. Quantification of hyaluronan in human fasciae: variations with function and anatomical site. J Anat. 2018;233(4):552–6.
Laurent TC. Biochemistry of hyaluronan. Acta Otolaryngol Suppl. 1987;442:7–24.
Kobayashi T, Chanmee T, Itano N. Hyaluronan: metabolism and function. Biomol Ther. 2020;10(11):1525.
Cowman MK, Lee HG, Schwertfeger KL, McCarthy JB, Turley EA. The content and size of Hyaluronan in biological fluids and tissues. Front Immunol. 2015;6:261.
Cowman MK, Hernandez M, Kim JR. Macromolecular crowding in the biomatrix. In: Balazs EA, editor. Structure and function of biomatrix control of cell behavior and gene expression. Edgewater: Matrix Biology Institute; 2012. p. 45–66.
Hascall V, Esko JD. Hyaluronan. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al., editors. Essentials of Glycobiology. 3rd ed. New York: Cold Spring Harbor; 2015.
Cowman MK, Chen CC, Pandya M, Yuan H, Ramkishun D, LoBello J, et al. Improved agarose gel electrophoresis method and molecular mass calculation for high molecular mass hyaluronan. Anal Biochem. 2011;417(1):50–6.
Kwiecinski JJ, Dorosz SG, Ludwig TE, Abubacker S, Cowman MK, Schmidt TA. The effect of molecular weight on hyaluronan's cartilage boundary lubricating ability--alone and in combination with proteoglycan 4. Osteoarthr Cartil. 2011;19(11):1356–62.
Yakubov GE, McColl J, Bongaerts JH, Ramsden JJ. Viscous boundary lubrication of hydrophobic surfaces by mucin. Langmuir. 2009;25(4):2313–21.
Cowman MK, Schmidt TA, Raghavan P, Stecco A. Viscoelastic properties of Hyaluronan in physiological conditions. F1000Res. 2015;4:622.
Gibbs DA, Merrill EW, Smith KA, Balazs EA. Rheology of hyaluronic acid. Biopolymers. 1968;6(6):777–91.
Caspersen MB, Roubroeks JP, Qun L, Shan H, Fogh J, Ruidong Z, et al. Thermal degradation and stability of sodium hyaluronate in solid state. Carbohydr Polym. 2014;107:25–30.
Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340(5):791–809.
Matteini P, Dei L, Carretti E, Volpi N, Goti A, Pini R. Structural behavior of highly concentrated hyaluronan. Biomacromolecules. 2009;10(6):1516–22.
Snetkov P, Zakharova K, Morozkina S, Olekhnovich R, Uspenskaya M. Hyaluronic acid: the influence of molecular weight on structural, physical, physico-chemical, and degradable properties of biopolymer. Polymers (Basel). 2020;12(8):1800.
Shimada E, Matsumura G. Viscosity and molecular weight of hyaluronic acids. J Biochem. 1975;78(3):513–7.
Meyer GA, McCulloch AD, Lieber RL. A nonlinear model of passive muscle viscosity. J Biomech Eng. 2011;133(9):091007.
Elliott DM, Robinson PS, Gimbel JA, Sarver JJ, Abboud JA, Iozzo RV, et al. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann Biomed Eng. 2003;31(5):599–605.
Armstrong SE, Bell DR. Relationship between lymph and tissue hyaluronan in skin and skeletal muscle. Am J Physiol Heart Circ Physiol. 2002;283(6):H2485–94.
Calve S, Isaac J, Gumucio JP, Mendias CL. Hyaluronic acid, HAS1, and HAS2 are significantly upregulated during muscle hypertrophy. Am J Physiol Cell Physiol. 2012;303(5):C577–88.
Williams PE, Goldspink G. Connective tissue changes in surgically overloaded muscle. Cell Tissue Res. 1981;221(2):465–70.
White JP, Reecy JM, Washington TA, Sato S, Le ME, Davis JM, et al. Overload-induced skeletal muscle extracellular matrix remodelling and myofibre growth in mice lacking IL-6. Acta Physiol (Oxf). 2009;197(4):321–32.
Okita M, Yoshimura T, Nakano J, Motomura M, Eguchi K. Effects of reduced joint mobility on sarcomere length, collagen fibril arrangement in the endomysium, and hyaluronan in rat soleus muscle. J Muscle Res Cell Motil. 2004;25(2):159–66.
Raghavan P, Lu Y, Mirchandani M, Stecco A. Human recombinant hyaluronidase injections for upper limb muscle stiffness in individuals with cerebral injury: a case series. EBioMedicine. 2016;9:306–13.
Stecco A, Stecco C, Raghavan P. Peripheral mechanisms of spasticity and treatment implications. Curr Phys Med Rehabil Rep. 2014;2(2):121–7.
Huijing PA, Jaspers RT. Adaptation of muscle size and myofascial force transmission: a review and some new experimental results. Scand J Med Sci Sports. 2005;15(6):349–80.
Scherbakov N, Sandek A, Doehner W. Stroke-related sarcopenia: specific characteristics. J Am Med Dir Assoc. 2015;16(4):272–6.
Zhao M, Yoneda M, Ohashi Y, Kurono S, Iwata H, Ohnuki Y, et al. Evidence for the covalent binding of SHAP, heavy chains of inter-alpha-trypsin inhibitor, to hyaluronan. J Biol Chem. 1995;270(44):26657–63.
Menon RG, Raghavan P, Regatte RR. Quantifying muscle glycosaminoglycan levels in patients with post-stroke muscle stiffness using T1rho MRI. Sci Rep. 2019;9(1):14513.
Menon RG, Raghavan P, Regatte RR. Pilot study quantifying muscle glycosaminoglycan using bi-exponential T1rho mapping in patients with muscle stiffness after stroke. Sci Rep. 2021;11(1):13951.
Stecco A, Pirri C, Caro R, Raghavan P. Stiffness and echogenicity: development of a stiffness-echogenicity matrix for clinical problem solving. Eur J Transl Myol. 2019;29(3):8476.
Rasool G, Wang AB, Rymer WZ, Lee SSM. Shear waves reveal viscoelastic changes in skeletal muscles after hemispheric stroke. IEEE Trans Neural Syst Rehabil Eng. 2018;26(10):2006–14.
Wang AB, Perreault EJ, Royston TJ, Lee SSM. Changes in shear wave propagation within skeletal muscle during active and passive force generation. J Biomech. 2019;94:115–22.
Rasool G, Wang AB, Rymer WZ, Lee SS. Altered viscoelastic properties of stroke-affected muscles estimated using ultrasound shear waves - preliminary data. Annu Int Conf IEEE Eng Med Biol Soc. 2016;2016:2869–72.
Brin MF. Dosing, administration, and a treatment algorithm for use of botulinum toxin A for adult-onset spasticity. Spasticity Study Group. Muscle Nerve Suppl. 1997;6:S208–20.
Gracies JM, Elovic E, McGuire J, Simpson DM. Traditional pharmacological treatments for spasticity. Part I: local treatments. Muscle Nerve Suppl. 1997;6:S61–91.
Gracies JM. Pathophysiology of impairment in patients with spasticity and use of stretch as a treatment of spastic hypertonia. Phys Med Rehabil Clin N Am. 2001;12(4):747–68, vi.
Gracies JM. Pathophysiology of spastic paresis. II: emergence of muscle overactivity. Muscle Nerve. 2005;31(5):552–71.
Singer B, Dunne J, Allison G. Reflex and non-reflex elements of hypertonia in triceps surae muscles following acquired brain injury: implications for rehabilitation. Disabil Rehabil. 2001;23(17):749–57.
Barker D, Banks RW. The muscle spindle. In: Engel AG, Franzini-Armstrong C, editors. Myology 1. 2nd ed. New York: McGraw-Hill; 1994. p. 333–60.
Pedrosa-Domellof F, Hellstrom S, Thornell LE. Hyaluronan in human and rat muscle spindles. Histochem Cell Biol. 1998;110(2):179–82.
Fukami Y. Studies of capsule and capsular space of cat muscle spindles. J Physiol. 1986;376:281–97.
Song Z, Banks RW, Bewick GS. Modelling the mechanoreceptor's dynamic behaviour. J Anat. 2015;227(2):243–54.
Edin BB, Vallbo AB. Stretch sensitization of human muscle spindles. J Physiol. 1988;400:101–11.
de Bruin M, Smeulders MJ, Kreulen M, Huijing PA, Jaspers RT. Intramuscular connective tissue differences in spastic and control muscle: a mechanical and histological study. PLoS One. 2014;9(6):e101038.
Lieber RL, Runesson E, Einarsson F, Friden J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve. 2003;28(4):464–71.
Chapman MA, Pichika R, Lieber RL. Collagen crosslinking does not dictate stiffness in a transgenic mouse model of skeletal muscle fibrosis. J Biomech. 2015;48(2):375–8.
Smith LR, Barton ER. Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am J Physiol Cell Physiol. 2014;306(10):C889–98.
Pelham RJ Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94(25):13661–5.
Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature. 2020;584(7822):535–46.
Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4(7):528–39.
Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277(7):4589–92.
Evanko SP, Potter-Perigo S, Petty LJ, Workman GA, Wight TN. Hyaluronan controls the deposition of fibronectin and collagen and modulates TGF-beta1 induction of lung Myofibroblasts. Matrix Biol. 2015;42:74–92.
Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13(1):7–12.
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170(6):1807–16.
Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180(4):1340–55.
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–63.
Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–3.
Albeiroti S, Soroosh A, de la Motte CA. Hyaluronan's role in fibrosis: a pathogenic factor or a passive player? Biomed Res Int. 2015;2015:790203.
Jenkins RH, Thomas GJ, Williams JD, Steadman R. Myofibroblastic differentiation leads to hyaluronan accumulation through reduced hyaluronan turnover. J Biol Chem. 2004;279(40):41453–60.
Meran S, Thomas D, Stephens P, Martin J, Bowen T, Phillips A, et al. Involvement of hyaluronan in regulation of fibroblast phenotype. J Biol Chem. 2007;282(35):25687–97.
Meran S, Thomas DW, Stephens P, Enoch S, Martin J, Steadman R, et al. Hyaluronan facilitates transforming growth factor-beta1-mediated fibroblast proliferation. J Biol Chem. 2008;283(10):6530–45.
Simpson RM, Meran S, Thomas D, Stephens P, Bowen T, Steadman R, et al. Age-related changes in pericellular hyaluronan organization leads to impaired dermal fibroblast to myofibroblast differentiation. Am J Pathol. 2009;175(5):1915–28.
Webber J, Jenkins RH, Meran S, Phillips A, Steadman R. Modulation of TGFbeta1-dependent myofibroblast differentiation by hyaluronan. Am J Pathol. 2009;175(1):148–60.
Webber J, Meran S, Steadman R, Phillips A. Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J Biol Chem. 2009;284(14):9083–92.
Twarock S, Tammi MI, Savani RC, Fischer JW. Hyaluronan stabilizes focal adhesions, filopodia, and the proliferative phenotype in esophageal squamous carcinoma cells. J Biol Chem. 2010;285(30):23276–84.
Ito T, Williams JD, Fraser DJ, Phillips AO. Hyaluronan regulates transforming growth factor-beta1 receptor compartmentalization. J Biol Chem. 2004;279(24):25326–32.
Allison DD, Wight TN, Ripp NJ, Braun KR, Grande-Allen KJ. Endogenous overexpression of hyaluronan synthases within dynamically cultured collagen gels: implications for vascular and valvular disease. Biomaterials. 2008;29(20):2969–76.
Tsunenaga M, Nishiyama T, Horii I, Nakayama Y, Arai K, Hayashi T. Effect of hyaluronate on physicochemical and biological properties of collagen solution which could be used as collagen filler. Connect Tissue Res. 1992;28(1–2):113–23.
Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, et al. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208(7):1459–71.
Li Y, Liang J, Yang T, Monterrosa Mena J, Huan C, Xie T, et al. Hyaluronan synthase 2 regulates fibroblast senescence in pulmonary fibrosis. Matrix Biol. 2016;55:35–48.
Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1819–28.
Suresh K, Scheid E, Klotz L, Venkateswaran V, Gauldie J, Foley R. Induction of specific human cytotoxic T cells using dendritic cells transduced with an adenovector encoding rat epidermal growth factor receptor 2. Int J Oncol. 2011;39(4):907–13.
Chandra SR, Shenoy RK, Karthikeyan SK, Chithra P, Annapoorni CS. Two cases of a rare treatable limb girdle muscle disease. J Assoc Physicians India. 2011;59:321–5.
Vijaya Laxmi S, Thirupathi Reddy Y, Suresh Kuarm B, Narsimha Reddy P, Crooks PA, Rajitha B. Synthesis and evaluation of chromenyl barbiturates and thiobarbiturates as potential antitubercular agents. Bioorg Med Chem Lett. 2011;21(14):4329–31.
Kumar V, Murthy AK, Suresh KP. Glutathione S-transferase M1 and T1 status and the risk of laryngeal cancer: a meta-analysis. Asian Pac J Cancer Prev. 2011;12(9):2221–6.
Eckes B, Nischt R, Krieg T. Cell-matrix interactions in dermal repair and scarring. Fibrogenesis Tissue Repair. 2010;3:4.
Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175(1):3–16.
Sivakumar P, Das AM. Fibrosis, chronic inflammation and new pathways for drug discovery. Inflamm Res. 2008;57(9):410–8.
Meyer GA, Lieber RL. Skeletal muscle fibrosis develops in response to desmin deletion. Am J Physiol Cell Physiol. 2012;302(11):C1609–20.
Lewis DM, Pardoe MJ, Webb SN. The effect of denervation on the mechanical and electrical properties of cat skeletal muscle [proceedings]. J Physiol. 1978;277:48P–9P.
Thacker BE, Tomiya A, Hulst JB, Suzuki KP, Bremner SN, Gastwirt RF, et al. Passive mechanical properties and related proteins change with botulinum neurotoxin A injection of normal skeletal muscle. J Orthop Res. 2012;30(3):497–502.
Binder-Markey BI, Dewald JPA, Murray WM. The biomechanical basis of the claw finger deformity: a computational simulation study. J Hand Surg Am. 2019;44(9):751–61.
Carraro U, Dalla Libera L, Catani C, Danieli-Betto D. Chronic denervation of rat diaphragm: selective maintenance of adult fast myosin heavy chains. Muscle Nerve. 1982;5(7):515–24.
Omer GE Jr. Evaluation and reconstruction of the forearm and hand after acute traumatic peripheral nerve injuries. J Bone Joint Surg Am. 1968;50(7):1454–78.
Nuckolls GH, Kinnett K, Dayanidhi S, Domenighetti AA, Duong T, Hathout Y, et al. Conference report on contractures in musculoskeletal and neurological conditions. Muscle Nerve. 2020;61(6):740–4.
Smith LR, Irianto J, Xia Y, Pfeifer CR, Discher DE. Constricted migration modulates stem cell differentiation. Mol Biol Cell. 2019;30(16):1985–99.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Raghavan, P., Stecco, A., Menon, R., Cowman, M.K., Regatte, R. (2022). Mechanisms of Development of Passive Mechanical Muscle Stiffness. In: Raghavan, P. (eds) Spasticity and Muscle Stiffness. Springer, Cham. https://doi.org/10.1007/978-3-030-96900-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-96900-4_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-96899-1
Online ISBN: 978-3-030-96900-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)