Abstract
Regenerative rehabilitation is an emerging field combining regenerative medicine strategies with evidence-based rehabilitation practices. The overall aim of regenerative rehabilitation is to repair and regenerate organ systems and recover function following injury or illness. This review presents recent advances in regenerative rehabilitation with attention to historical context. The state of the field of development and implementation of therapeutic strategies across anatomic and physiologic systems is discussed. Providing an overview of relevant strategies and examples of early successes, the importance of physiologic functional outcomes is emphasized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
In his statements on the history of rehabilitation from the Vietnam War, Paul Brown noted, “Leaders and innovators in the field were asking the question—‘Where does treatment cease and rehabilitation start?’ The true significance of this basically rhetorical question lay in the implication that treatment and rehabilitation were indistinguishable…” (Burkhalter 1994). Using a more thoughtful approach to the importance of the indistinguishable aspects of treatment and rehabilitation, regenerative rehabilitation has recently expanded as a field to provide patients with multi-pronged approaches to restore function after injury, disease, or illness. While the origins of rehabilitation and regenerative medicine treatments can be traced back thousands of years, the establishment of regenerative rehabilitation as a discipline has occurred relatively recently.
Strategies for rehabilitation can be traced back to the ancient Chinese, who used movement to relieve pain thousands of years ago through the practice of Qigong. Greek physician Herodicus used exercise to prevent disease in the fifth century B.C.E.; Roman physician Galen used rehabilitation for military injuries in the second century C.E., and during the Middle Ages philosopher-physician Maimonides used exercise for health (Kleisiaris et al. 2014). Similarly, the use of regenerative medicine techniques for various treatments can be found in the early use of biomaterials by the Egyptians during the Neolithic age, the Europeans during the Middle Ages, and as early as 600 A.D. by the Mayan civilization (Marin et al. 2020). The historic review of the field of regenerative rehabilitation herein seeks to draw attention to notable recent successes and describe progress in preclinical research and clinical translation. Through this review, we focus on clinically meaningful and system-specific functional outcomes as the field collectively works to develop regenerative rehabilitation practices that repair, regenerate, and rejuvenate the body following injury or disease.
1.2 Rehabilitation
The objective of rehabilitation, or physical therapy, is to act upon the systems of the body to facilitate physiologically beneficial adaptations. The medical specialty of Physical Medicine and Rehabilitation aims to restore functional limitations resulting from various pathological conditions or injuries by incorporating the expertise of practitioners involved in all stages of the rehabilitative process (Atanelov et al. 2015). Examples include skeletal muscle hypertrophy and/or bone mineral deposition following injury or surgery, or to attenuate declines in patients suffering chronic diseases, such as sarcopenia or heart failure. Toward the beginning of the twentieth century, patients were often restricted to bedrest and prolonged immobilization following acute or chronic injury, subsequently followed by a slow progression back into activity. Unfortunately, bedrest reduces the physiological load on the musculoskeletal system needed to induce physiological adaptations. This results in atrophy, net bone resorption, and poor functional outcomes (Koukourikos et al. 2014). In contrast, early weight-bearing and ambulation following surgical procedures to repair injuries, such as ankle fracture and anterior cruciate ligament (ACL) injury, have been associated with reduced in-patient hospitalization time, earlier return to full ambulation, and earlier return to work or sport (Simanski et al. 2006; Dehghan et al. 2016).
1.2.1 Early History of Rehabilitation
The practice of rehabilitation dates back to written accounts of physical healing techniques around 400 B.C. However, it was not until World War I that rehabilitation methodologies and education became more prominent in the United States as physicians began practicing formal physiotherapy, or physical medicine and rehabilitation, to rehabilitate injured and disabled military personnel. Although physicians prescribed various physical treatments, no standard physical therapy practices had yet been established. Consequently, the American Medical Association (AMA) Council on Physical Therapy was established in 1926 to broadly identify effective physical rehabilitative treatments. At the forefront of this movement was Dr. John Coulter, a military physician who served during World War I and as faculty at Northwestern University Medical School. Coulter was a leader in the educational development of physical therapy practices. Notably, his collaboration with basic scientists and other medical and surgical practitioners helped to establish the legitimacy of rehabilitative practices. In particular, his collaboration with Dr. Frank Krusen, founder of one of the first academic departments of physical medicine in the United States at Temple University Medical School, later resulted in establishing the American Academy of Physical Medicine and Rehabilitation in 1947. The practice of rehabilitation became more prevalent and accelerated during and immediately following World War II. During this time period, more injuries were survivable. The focus of physical medicine shifted from the recovery of ambulation to improving patients’ physical, mental, emotional, vocational, and social capacities.
1.2.2 Progressing to Evidence-Based Practices in Rehabilitation
In recent years, the rehabilitation field has moved to incorporate sound methodological research and evidence-based practice (EBP) into the clinic. One of the first descriptions of EBP in rehabilitation was in 1992 at McMaster University in Ontario, Canada. Since then, EBP has been defined as “the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients/clients” (Sackett et al. 1996). Evidence-based practice encompasses three main principles. First, it integrates therapists’ individual expertise, proficiency, and judgment based on clinical experience, with the best available clinical evidence from systematic and sound methodological research. Second, individual patient preferences and values must be considered in selecting the best procedures and practices for the patients’ condition and the severity of that condition. Third, healthcare economics must be considered, with specific attention paid to the availability, quality and cost of treatments, facilities, and health insurance. Taken together, multiple factors must be considered when implementing an EBP rehabilitation program for patients across a wide spectrum of pathological conditions and injuries.
It is well known that physical activity and resistance exercise confer substantial health benefits, with consistent, progressive training shown to improve a variety of health and physical outcomes. These outcomes include muscular strength, endurance, power, and cardiorespiratory fitness. The latter includes increased maximal oxygen uptake and decreased heart rate and blood pressure for a given absolute submaximal intensity, among other physiological variables. Increased levels of physical activity and exercise are inversely associated with premature mortality, cardiovascular disease, stroke, osteoporosis, type II diabetes, and metabolic syndrome, among other progressive deleterious conditions. Specific types of exercises are recommended to improve long-term health and functional outcomes across a wide spectrum of pathological conditions and injuries.
The purpose of prescribing rehabilitative exercise is to impart various mechanical stimuli, loads, and/or forces on body systems to effect beneficial responses. These stimuli are needed to transmit biochemical signals and subsequently activate intracellular signaling cascades; a process referred to as mechanotransduction. These include tensile (pulling), compressive (pushing), shear (parallel, opposite), torsional (twisting), and vibrational forces. Mechanotransduction is critical for eliciting a molecular response involving the activation of transcription factors necessary for net protein production, extracellular matrix (ECM) synthesis, skeletal or cardiac muscle hypertrophy, and bone mineral deposition, among others. For example, protein synthesis rates within skeletal muscle fibers are primarily controlled by the mechanistic target of rapamycin (mTOR) enzyme, which is activated by the mechanical stretch of muscle and several anabolic hormones, including insulin-like growth factor and growth hormone (Schoenfeld 2010; Mavalli et al. 2010). In fact, mTOR has been reported (Dreyer et al. 2006) to increase during and immediately following an acute bout of resistance exercise, subsequently leading to phosphorylation and activation of downstream target proteins to increase net protein production. Ultimately, the application of whole-body or regional mechanical stimuli must be specifically tailored to obtain the desired beneficial adaptations.
To impart specific adaptations on physiologic systems, different rehabilitation regimens are used in patients depending on age, specific pathological conditions, and injuries. The foundation of most common types of rehabilitation includes body weight, resistance, flexibility exercises, and aerobic activity. Body weight and resistance exercises impart tensile and compressive forces that activate intracellular signaling cascades. These induce skeletal muscle hypertrophy, increase net bone mineral deposition, and improve cardiovascular function. For example, loading specific skeletal muscle groups and bones has been shown to prevent, attenuate, or reverse bone mass loss in patients with osteoporosis. It has also been shown to reduce pain and disability in patients with osteoarthritis (American College of Sports Medicine et al. 2009; Garber et al. 2011; Messier 2008). Common indications for body weight and resistance exercises include vascular (e.g., myocardial infarction, heart failure), neurological (e.g., multiple sclerosis, stroke, amyotrophic sclerosis), and various musculoskeletal conditions (e.g., fractures, joint arthroplasty). Dynamic and static flexibility exercises are prescribed to improve range of motion (ROM) and enhance patients’ ability to perform weight-bearing activities, activities of daily living, and, in the case of athletes, improve sports performance through increased speed and rate of force production. In addition to commonly implemented rehabilitation methods, other therapies have been developed to improve functional outcomes, including electrical, magnetic, or mechanical stimulation, whole-body or targeted vibration, and blood flow restriction. Although these approaches have demonstrated some promise in improving functional outcomes across clinical populations, there is a lack of evidence to suggest specific timing and dosing guidelines to optimize therapeutic benefit. A multimodal rehabilitation program may act synergistically to confer optimal physiological adaptations on the body, specific to the stimuli enacted, to enhance functional outcomes.
1.2.3 Overarching Goals of Rehabilitation
Rehabilitation programs are designed to include distinct phases to progressively improve outcomes related to pain, joint ROM, muscular strength, and overall function by the end of each stage. Prior to the advancement from one phase to the next, a series of functional tests are typically administered to ensure patients are ready to progress. Tests may include loading percentage, number of repetitions for resistance exercises, or performance intensity of a particular task, such as walking or jogging.
Generally, the goal of the first phase of rehabilitation following injury or a surgical procedure is to protect the injured area. In this phase, immobilization may be prescribed, such as unloading of lower extremity injuries with a cast or a sling for upper extremity injuries. The goal of the second phase is to increase tissue tolerance to loading by slowly progressing to weight-bearing exercises such as walking. During this phase, activities may include performing active and passive ROM exercises, submaximal isometric exercises for affected muscle groups, and progressive loading. Examples of the latter include unloaded cycling or abdominal stability exercises for lower extremity injuries. Goals of the third phase include achieving full ROM of the affected joint, increasing tissue tolerance to loading, and improving strength and endurance with exercises such as jogging or biking. The final rehabilitation phase is aimed to return patients to performing activities at pre-injury or pre-surgery levels.
Each progressive phase of rehabilitation varies in length of time and types of exercises performed based upon the heterogeneity, complexity and severity of the patients’ injury, and pain tolerance level (Myer et al. 2006). For example, mild hamstring strains generally require up to 1 month to rehabilitate. More complex injuries such as ACL injury with subsequent surgical reconstruction may require 9 months or longer to rehabilitate before patients return to full activity. As hamstring strain injuries primarily impact the muscle, do not involve bone or require surgery, an unloading phase is generally not prescribed. Instead, the first rehabilitative phase (acute management) includes early loading of the injured muscle (Heiderscheit et al. 2010). The functional recovery phase follows with the goal of recovering muscle strength and ROM, in addition to graded running to maintain cardiorespiratory fitness. Then activities specific to an individual’s sport or vocation are implemented. Finally, testing to return to full activity is performed. Individuals are encouraged to continue specific functional exercises to prevent re-injury following clearance for full activity. Rehabilitation phases are general guidelines. Even across studies examining optimal strategies to rehabilitate homogeneous mild hamstring injuries, varying numbers of phases and types of loading have been reported (Heiderscheit et al. 2010; Lightsey et al. 2018; Wangensteen et al. 2017). Further investigations are required to determine the optimal timing and loading strategies for each stage of rehabilitation across various injuries and pathological conditions to promote efficient repair.
1.3 Regenerative Medicine
Regenerative medicine is based on the principles of cell and tissue biology to facilitate the restoration of tissue and function. Regenerative medicine has been described as the “process of creating living, functional tissues to repair or replace tissue or organ function lost due to age, disease, damage, or congenital defects” by the United States National Institutes of Health (NIH). Regenerative medicine includes an array of emerging technologies, such as biomaterials, stem cell therapies, engineered organs (e.g., trachea, urinary bladder), and/or tissues (e.g., skin, muscle, cartilage) to promote regeneration in affected body regions. While rehabilitation can improve tissue restoration and functional outcomes, there are countless conditions for which rehabilitation alone is not sufficient.
1.3.1 History of Biomaterials
The use of natural materials in medical procedures had a long history of success before modern biomaterial-based regenerative medicine emerged as a field in the mid-twentieth century. The use of sutures may be the oldest form of a regenerative biomaterial, with some reports indicating ancient Egyptians used linen sutures during the Neolithic age. Europeans used catgut sutures during the Middle Ages, and nacre dental implants were successfully used as early as 600 A.D. by the Mayan civilization (Biomaterials Science 2020). Although the first contact lenses were not developed until the nineteenth century, Leonardo Da Vinci is credited for developing the concept in 1508. Many synthetic polymers were developed for military use during World War II and subsequently used by surgeons as experimental treatments when necessary. For example, polymethylmethacrylate (PMMA) was found to be biologically inert, or producing minimal foreign body reaction, after fighter pilots were injured with PMMA windshield shrapnel (Williams and Isaacson 2014). This prompted the use of PMMA as intraocular lens replacements while various other materials began to be tested for biocompatibility.
Historically, biomaterials were designed to be inert and simply replace damaged tissue rather than assisting in regeneration. To be successful, biomaterials need to have the necessary mechanical properties for a specific application in addition to being biocompatible. If a material is cytotoxic, the surrounding tissue will die and thus it is not biocompatible. Even when not cytotoxic, materials may prove incompatible by activating the immune system’s foreign body response. A foreign body response may create a fibrous capsule around the implant, altering its physical properties and damaging the surrounding tissue. In many applications today, surface modifications are made to the biomaterial to reduce the foreign body response and/or provoke beneficial effects in the surrounding tissue.
While attempting to solve the problem of inert metallic bone implants from being rejected, Hench et al. developed a bioactive glass material that integrates with the existing bone (Hench et al. 1971). The interface between the glass and bone was bound with an active hydroxyapatite layer instead of a fibrous capsule. The bound interface was not only stronger but also reduced the risk of rejection compared to inert metallic implants. This study provided a new framework for thinking about biomaterials by demonstrating inertness is not always advantageous. Instead of trying to avoid biological interaction, materials can be designed to positively influence the surrounding tissue. Currently, the most widely accepted definition of a biomaterial is “any substance or combination of substances, other than drugs, synthetic or natural in origin, which can be used for any period of time, which augments or replaces partially or totally any tissue, organ or function of the body, in order to maintain or improve the quality of life of the individual” (Marin et al. 2020).
1.3.2 Modern Biomaterials
Modern biomaterials can be broken down broadly into three classes: ceramics/glasses, metals, and polymers. Ceramics have the highest tensile strength but lowest ductility of the three biomaterial categories, making them suitable for bone and dental implants. They are often used in articulations of metal joint replacements because of their friction resistance. Porous ceramics are not suitable for load-bearing but may provide an ideal bone implant by allowing existing bone to grow within the pores and create a strong interface. Glasses and glass-ceramics have become popular choices for bone implants due to the mechanical properties and ability to integrate bioactive components. As of 2016, it was reported Hench’s 45S5 Bioglass® has been used in over 1.5 million patients (Hench et al. 1971).
Metallic biomaterials are used commonly in joint replacements and bone fixation, dental implants, and vascular stents. Common types include stainless steel, cobalt-based alloys, and titanium-based alloys. These are generally preferred over other materials because of their mechanical strength and toughness, corrosion resistance, and light weight. In addition, they can be processed below their recrystallization temperature, known as cold working. This creates imperfections in the crystalline structure that inhibit the movement of atoms, further increasing strength and hardness (Biomaterials Science 2020). Stainless steels are commonly used as expandable vascular stents because of their ductility and subsequent strain hardening. Furthermore, metals allow for endothelial growth over their surface which reduces the risk of blood clotting. By the early 2000s, drug-eluting metallic stents proved superior to bare metal stents for reducing clots and are regularly used today (Silvain et al. 2014). Metallic implants are thought to promote the regeneration of healthy vascular tissue. However, due to their strength and general inability to degrade, metallic biomaterials used for applications such as fracture fixation do not promote regeneration. Instead, metallic fixation has a stress shielding effect, limiting the load placed on the bone, and consequently reduces bone remodeling due to Wolff’s law. Therefore, degradable materials, such as polymers, have begun to be tested for viability in bone fixation to encourage bone remodeling and ultimately function (Kulkarni et al. 1971; Cai et al. 2019).
Polymers comprise a wide range of possible bulk material and surface properties and may be more useful for tissue regeneration than metals or ceramics due to potential biodegradability. Polymers consist of multiple repeating molecular subunits, or “mers,” which form chains that cross-link together to form the bulk material. They can be synthetically made (e.g., polyethylene, polypropylene, etc.) or occur naturally (e.g., collagen, silk, wool, etc.). Polymers can consist of a single type of repeat unit, called a homopolymer, or can be made up of two or more types of repeat units called a copolymer. The amount of cross-linking within a polymer material can dictate the elastic modulus and toughness of that material. Polymers with lower cross-linking density have a low modulus and are known as elastomers, as they can stretch and return to the prior shape. Plastic polymers can withstand higher stress than elastomers with greater ductility and toughness. Finally, brittle polymers are heavily cross-linked, resulting in lower ductility but increased strength, similar to ceramics. Because of this wide range, polymers have been used in tissue engineering, drug delivery, vascular and skin grafts, and joint replacements, among others. Moreover, highly cross-linked and hydrophilic polymers can form hydrogels, which are advantageous in a variety of applications. The highly cross-linked structure of hydrogels allows for withstanding tensile stress, while hydrophilicity allows for stretch and elasticity.
1.3.3 Tissue Engineering
The field of tissue engineering began to emerge in the 1970s. Bell et al. first reported that collagen hydrogels cultured with fibroblasts undergo contraction and form skin-like structures (Bell et al. 1979). Around the same time, Yannas and Burke examined the ability of a porous, cross-linked collagen-glycosaminoglycan scaffold to regenerate skin in vivo. The scaffolds were implanted into the wound beds of excised skin of guinea pigs and found to regenerate skin remarkably well (Yannas et al. 1982; Yannas and Burke 1980). These findings led to the first clinical uses of artificial skin and provided a basis for engineering other types of tissue.
Providing a conceptual framework, the tissue engineering triad guides the successful development of new constructs (Almouemen et al. 2019). The first pillar of the triad is a biomaterial scaffold, or matrix, which provides structure and an organized template upon which stem cells and regenerated tissue can align. The scaffold’s mechanical properties should closely resemble the native ECM of the desired tissue. Thus, natural polymers such as collagen, silk, cellulose, and various proteins are often used as tissue engineering scaffolds due to the resemblance of native ECM. Importantly, because natural polymers are comprised of proteins, they have the advantage of innate bioactivity. This encompasses the second pillar of the tissue engineering triad: appropriate signaling and biophysical cues. Natural materials contain binding sites for various growth factors and cell attachment, which can dictate cellular differentiation and proliferation. They can also be enzymatically degraded, allowing for replacement by an endogenously produced matrix. Additionally, modifying polymer alignment and pore size within the scaffold can enhance organization of regenerated tissue and allow nutrients and metabolites to enter and exit the region. The third pillar of the triad is the need for cells; specifically, progenitor cells that will regenerate the desired tissue. This can be accomplished by the scaffold attracting endogenous cells when implanted, or by seeding the scaffold before implantation.
Today, tissue engineering using decellularized scaffolds has been attempted for just about every organ in the body with varying degrees of success (Yu et al. 2016; Corona and Greising 2016). Although these naturally derived scaffolds offer many advantages as previously discussed, they typically lack the necessary mechanical properties for some applications and are difficult to produce with consistent properties. Synthetic polymer scaffolds can be produced with more precision, consistency, and stronger mechanical properties, yet do not degrade as readily and are not innately bioactive materials. Consequently, composite scaffolds and hydrogels which combine natural and synthetic polymeric aspects are becoming more common in tissue engineering.
1.3.4 Cellular Therapy
Perhaps a more simplified approach to regeneration than transplantation of engineered tissues is the use of stem cell therapy. Instead of introducing a foreign biomaterial and managing the potential host responses, stem cells can be injected or delivered to the desired tissue and directly contribute to regeneration. Hematopoietic (HSCs) and mesenchymal stem cells (MSCs) are the most common types used for tissue regeneration today, though embryonic (ESCs) and induced pluripotent stem cells (iPSCs) theoretically have greater potential. The first use of the term “stem cell” in the literature dates back to the late nineteenth century by Ernst Haeckel (Ramalho-Santos and Willenbring 2007). However, stem cell research did not truly gain momentum until the late twentieth century. Today MSCs as they are referred to, are “adult” stem cells primarily harvested from bone marrow. It is now known that other types of tissue can be sources of MSCs, including adipose, umbilical cord, amniotic fluid, and dental pulp. While the term MSC was first coined by Arnold Caplan in 1991 (Caplan 1991), previous studies identified the osteogenic capacity of bone marrow, likely due to the presence of such cells (Tavassoli and Crosby 1968). MSCs are multipotent, meaning they can differentiate to form multiple types of tissue, including muscle, bone, cartilage, fat, and connective tissue. While MSCs have been useful for the aforementioned applications, pluripotent stem cells’ ability to differentiate into any type of tissue presents a likely regenerative advantage. Thomson et al. were the first to isolate human ESCs (Thomson et al. 1998), which are pluripotent and can self-renew indefinitely. However, because the embryo (blastocyst) from which these cells are taken needs to be destroyed, there is controversy regarding the ethics of harvesting ESCs. In 2006, Takahashi and Yamanaka developed a method for converting mature fibroblast cells to induced pluripotent stem cells (iPSCs) for which Yamanaka received the Nobel Prize in 2012 (Takahashi and Yamanaka 2006). This was a breakthrough in the field of stem cell research, as iPSCs have the virtually unlimited proliferative ability, and avoid ethical issues surrounding the use of ESCs, significantly advancing the field of stem cell biology and regeneration.
In theory, multipotent and pluripotent stem cells have the ability to cure a host of diseases, yet the only current US FDA-approved stem cell products are HSCs derived from cord blood to treat blood disorders, mainly for end-stage cancer patients. Common adverse reactions to HSC treatment include acute and chronic bacterial infections and graft-versus-host disease, with a range of severity (Omrani and Almaghrabi 2017; Zhao et al. 2019). Thus, the primary limitations of obtaining FDA approval are safety and efficacy, as numerous severe complications exist with treatment and little to no improvement has been demonstrated with treatment for some conditions. Nonetheless, hundreds of clinics providing autologous stem cell therapies have opened throughout the U.S. and globally. While often justified by the use of autologous tissue, the legality under which many operate is questionable. Though long-term complications are not well understood at this point, numerous patients have reported tumor formation years after an unapproved treatment (Bauer et al. 2018). Likewise, MSCs have been shown to promote metastatic growth in vitro and in vivo (Wang et al. 2015).
While there is a promising future for stem cell therapies, refinements need to be made to reduce adverse complications and improve efficacy. Appropriate methods to effectively deliver cells remain a significant barrier to use. MSCs have been tested in animal models for the treatment of various conditions including skeletal muscle injury, stroke, peripheral nerve injury, cartilage damage, and osteoarthritis among others (Goldman et al. 2017; Wilke et al. 2007; Guercio et al. 2012; Horita et al. 2006). The most common methods of cell delivery are injection either directly into the tissue of interest or the systemic circulation. Yet only a small proportion of cells appear to engraft at the treated site in most cases with, approximately 90% of cells lost within the first few hours following transplantation (Mooney and Vandenburgh 2008). Moreover, cells display a weak ability to migrate from the injection site. Inconsistent results between studies with similar treatments are perhaps linked to inconsistencies in cell engraftment due to these issues.
1.3.5 Combined Approaches in Regenerative Medicine
Combinatorial approaches that use stem cells in conjunction with biomaterials may help address the problem of stem cell loss by promoting cell adhesion and encouraging cell engraftment within the body region of interest. Current means of the combination include seeding a 3D biomaterial scaffold with stem cells ex vivo and subsequent implantation of the engineered tissue construct. Challenges of creating a useful tissue construct include the efficiency of cell seeding and obtaining a uniform distribution of cells within the 3D scaffold (Martin et al. 2004). Early attempts at cell seeding often used static loading methods, resulting in an uneven distribution of cells throughout the scaffold and poor seeding efficiency. This process is better accomplished by using a bioreactor, which typically provides mechanical stimulation to the cells in addition to maintaining an appropriate physiological environment. Mechanical stress or stimulation has been shown to increase in vitro bioactivity of cells in 3D matrices (Butler et al. 2000), and thus allows improved tissue regeneration outcomes.
Bioreactors designed to grow tissues “in situ” have also been widely employed in regenerative medicine research. Considerations for bioreactor designs include scaffold type, environmental control, mass transport of nutrients and regulatory molecules, physical signals, and scale (Biomaterials Science 2020). It is important to note that ideal bioreactor conditions will vary based on the specific type of tissue desired. As previously discussed, scaffold types can include naturally- or synthetically-derived 3D polymer matrices or a composite of the two. Environmental control of parameters including temperature, pH, and gas diffusion is an advantageous feature of the bioreactor approach compared to standard laboratory tissue culture systems. Gas exchange units can precisely regulate oxygen and carbon dioxide at physiological levels, in turn controlling pH of the culture medium and metabolic activity of seeded cells. Moreover, mass transport of molecules, gas, and nutrients into the porous scaffold and subsequent waste removal has been a major obstacle. Under static conditions, little substance successfully migrates into scaffold pores, including cells, resulting in a shell on the exterior of the scaffold. By manipulating fluid flow rates, proper transport and uniform distribution of cells and molecules can occur. One recent approach has been to surgically implant bioreactors into patients themselves during the incubation period in an effort to overcome some of these limitations (Watson et al. 2020), with bioreactor harvest prior to tissue implantation.
1.3.6 Composite Tissue Regeneration
A long-term unrealized goal of regenerative medicine will be the complete regeneration of composite tissues in the manner of amphibians and other self-regenerating animals. Strategies to unlock the body’s natural healing responses have been pursued using various approaches. While pioneering work has been done, clinically significant results have remained elusive. Early work in electrical stimulation replicated the processes of limb regeneration from salamanders through the formation of a blastemal of germ cells, akin to ESCs which then recapitulated severed limbs. Reversal of the naturally occurring biological currents could likewise abort amphibian limb regeneration (Becker and Spadaro 1972). This work progressed to mammals with some indication of a partial healing response akin to blastemal formation (Becker and Spadaro 1972), and this work has been replicated recently (Leppik et al. 2015). Recent investigations have attempted to refine this approach through the use of tailored small molecule modulation of the underlying bioelectrical circuits that have been shown to direct spatial patterning and direct anatomic regrowth (Mathews and Levin 2018).
Building on this work, a more in-depth study of the underlying mechanisms of these early experiments has been pursued. A host of growth factor, cellular, and small molecule approaches have been pursued. Cultured human placental cells are believed to exert a favorable paracrine effect from a host of endogenous growth factors. Given their intrinsic lack of immunogenicity, they have been found safe and effective for use in lower extremity critical limb ischemia and hip fracture when applied locally (Modarai and Patel 2019; Winkler et al. 2018). Signaling molecules such as small interfering RNA (siRNA) have been tested preclinically for the healing of a variety of insults by stimulating endogenous repair processes (Hu et al. 2019). Small molecule interventions have also found application in this regard (Billin et al. 2016). Further advances in composite tissue regeneration are expected to result in simultaneous regrowth of multiple tissues, with the potential to reduce or eliminate the need for wound micromanagement following injury and pathology.
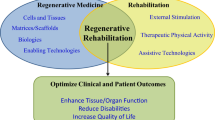
1.4 Regenerative Rehabilitation
Regenerative medicine and rehabilitation science were established and have evolved primarily as separate disciplines. Yet, when implemented alone, each may fail to fully recover tissue function. Recent calls have been made to combine these two approaches into a single therapy, termed regenerative rehabilitation. The concept of regenerative rehabilitation broadly covers all tissues and organ systems of the body. It is defined as therapy that “integrates regenerative technologies with rehabilitation clinical practices to restitute function and quality of life in patients with disabilities due to otherwise irreparable tissues or organs damaged by disease or trauma” (Perez-Terzic and Childers 2014). Regenerative rehabilitation is an emerging and rapidly expanding field, as evidenced by the rise in publications per year since 2000. The overarching goal of this combined approach is to synergistically improve clinical outcomes by restoring damaged or lost tissue and recovering tissue functionality to a pre-pathological or pre-injured state.
The concept of regenerative rehabilitation was initially formalized and integrated within the past decade (Perez-Terzic and Childers 2014). The first department of Rehabilitation and Regenerative Medicine was established at Columbia University in New York and has been led by Dr. Joel Stein since 2008 (Perez-Terzic and Childers 2014). Subsequently, Columbia’s Stem Cell Initiative research program became integrated with the University’s rehabilitation medicine and education entities. Since this establishment and the integration of these entities, several institutions throughout the United States have integrated regenerative medicine techniques into academic rehabilitation departments, including for example: the Rehabilitation Medicine Research Center within the Physical Medicine and Rehabilitation Department at Mayo Clinic; the McGowan Institute for Regenerative Medicine at the University of Pittsburgh; the Stanford Institute for Stem Cell Biology and Regenerative Medicine; and the Department of Physical Therapy and Rehabilitation Science at the University of California, San Francisco. These institutions, along with others, are part of a larger International Consortium for Regenerative Rehabilitation. The Consortium’s mission is to bring together leading scientists and clinicians to form new interdisciplinary collaborations and exchange ideas for the development and translation of technologies that restore function and enhance patients’ quality of life (Willett et al. 2020). Beginning in 2015, the Alliance of Regenerative Rehabilitation Research and Training (AR3T) program at the University of Pittsburgh received NIH funding to support the expansion of research and scientific knowledge, expertise, and methodologies across regenerative medicine and rehabilitation science disciplines. Broadly advancing the field of regenerative rehabilitation, a growing number of researchers have recently begun investigating the efficacy of integrating regenerative and rehabilitation strategies for various traumatic injuries and chronic pathologies (Ambrosio and Rando 2018). Prominent examples from various fields are subsequently discussed.
1.4.1 Early Successes of Regenerative Rehabilitation
Despite its relative infancy, progress has been made in the study and implementation of regenerative rehabilitation approaches. The purpose of these combined approaches is to enhance the local microenvironment, tissue plasticity, and functional capacity following injury or in a pathological condition (Fig. 1.1). To date, most of this research has been conducted in the preclinical setting. There are currently only limited clinical research reports, largely case studies and series. Combined approaches implemented across fields of study have included rehabilitation in the form of physical activity, electrical, magnetic, or mechanical stimulation, and ultrasonography. These rehabilitation practices have been performed prior to and/or following a regenerative strategy, such as stem cell or growth factor delivery and/or scaffold implantation, with varying success reported. Examples of injuries and pathologies for which initial regenerative rehabilitation therapies have made substantial progress include ischemic conditions, such as stroke and peripheral arterial disease (PAD).
The goal of regenerative rehabilitation is to elicit a synergistic effect when combining regenerative medicine approaches with rehabilitation practices to enhance tissue repair and regeneration and recover muscle function. Regenerative rehabilitation practices have been investigated across fields, with various techniques employed across injuries and pathologies. BMSC bone marrow-derived stem cells, PRP platelet-rich plasma, ROM range of motion, DASH disabilities of the arm, shoulder, and hand, MFA musculoskeletal function assessment, VO2 oxygen consumption
In the central nervous system (CNS), the primary effectors of neural plasticity and remodeling are electrical and neurotrophic signaling. It is therefore the goal to elicit plasticity through these mechanisms following CNS trauma, such as ischemic stroke. Stroke is a leading cause of disability worldwide (Krause et al. 2019). In the United States, approximately 800,000 people suffer from a stroke yearly, and at least two-thirds of stroke survivors require rehabilitation (Virani et al. 2020). During an ischemic stroke, vessel occlusion and cessation of cerebral blood flow leads to a lack of oxygen and glucose to the area fed by the occluded vessel. This results in neuronal cell death by necrosis in the initial phase following injury and long-term apoptosis due to oxidative stress, chronic inflammation, and glial scar formation at and surrounding the infarct region (Portis and Sanberg 2017; Tam et al. 2014). Following stroke, patients may experience various disabilities, including paralysis, hemiplegia, or hemiparesis, all leading to decreased ambulation; lack of coordination or balance; sensory disturbances such as pain; difficulty using or understanding language; and problems with thinking and memory, among others. Currently, the only FDA-approved treatment for ischemic stroke is tissue plasminogen activator (tPA), a naturally occurring protein that must be administered within approximately three hours of a stroke event to dissolve the clot and restore blood flow (Powers et al. 2018). Patients are generally then prescribed rehabilitation that includes aerobic exercise, such as treadmill walking or ergometer cycling. Patients may also be prescribed non-invasive repetitive transcranial magnetic stimulation (rTMS) and/or transcranial direct current stimulation (tDCS) to enhance neuromuscular activation and cortical reorganization. Stimulation-based rehabilitation has been reported to improve, but not fully recover ambulation, muscle strength, balance, and postural control following stroke (Moritz and Ambrosio 2017; Teskey et al. 2003). The clinical study of regenerative strategies has been limited to small trials or case studies, primarily using stem cell therapies over the past two decades. Significantly more research has been conducted in the preclinical setting using rodent models of stroke.
Early animal models of ischemic stroke, or cerebral ischemia (e.g., middle cerebral arterial occlusion; MCAO), were developed in the late 1970s. These stroke models have brought insight into tissue damage mechanisms following cerebral vessel occlusion. The models have also been used to examine the efficacy of regenerative therapies, both alone and in combination with rehabilitation strategies, to improve structural and functional outcomes following stroke (Fluri et al. 2015). Preclinical regenerative strategies implemented following MCAO have included: stem cells, such as MSCs, neural stem cells (NSC), and neural progenitor cells (NPC); decellularized or hydrogel-based scaffolds seeded with or without stem cells; and pharmacological treatments (Gopalakrishnan et al. 2019). Preclinical rehabilitation regimens have included: treadmill running; enriched environments that provide various sensory, social, motor, and visual stimuli; and electrical stimulation and rTMS. In a rat model of MCAO and mouse model of hypoxic-ischemic brain injury, the efficacy of various combinations of regenerative rehabilitation strategies has been examined. The majority of these studies reported combining MSCs derived from various sources, such as adipose tissue and bone marrow with treadmill exercise or with an enriched environment (Cho et al. 2016; Zhang et al. 2015; Sasaki et al. 2016).
Across studies, results have demonstrated improved angiogenesis, neurogenesis, and synaptogenesis while modulating inflammation and decreasing apoptosis, glial scar formation, and infarct volume. Additionally, in most studies, a beneficial synergistic effect was reported on behavioral, sensory, and motor function outcomes. Tests to examine these outcomes included: the limb placement test; cylinder and ladder walking tests to evaluate forelimb symmetry; Roger’s test to assess simple motor function, such as reflexes; grip strength; and the rotarod test to examine coordination and balance. More recently, the use of MSCs combined with ipsilesional cathodal current stimulation (Morimoto et al. 2018) as well as NSCs combined with rTMS (Peng et al. 2019) supports similar improved structural and functional outcomes to studies combining stem cell therapies and rehabilitation, such as treadmill running. It is evident that regenerative rehabilitation approaches can synergistically facilitate neuroprotection and enhance structural and functional outcomes following stroke. This is an important observation, as stem cells, particularly MSCs, are being considered for stroke treatment in the clinical setting. Phase I/II trials have shown initial safety and efficacy of bone-marrow-derived MSCs in combination with rehabilitation (e.g., physical, occupational, and speech therapies) to improve clinical outcomes following stroke, including scores on the NIH Stroke Scale (NIHSS) and Fugle-Meyer assessment (Steinberg et al. 2016). More investigations are needed to identify the optimal combination of regenerative rehabilitation strategies for patients following stroke and the appropriate timing to initiate these therapies in the clinic.
The field of cardiology has also seen success in implementing regenerative rehabilitation approaches. One such area is limb PAD, a major vascular complication that affects over 200 million people worldwide and up to 20% of individuals over the age of 65 (Shu and Santulli 2018). PAD is characterized by progressive blockage of at least one peripheral artery in the lower extremity by plaque, resulting in stenosis or occlusion and leading to decreased blood flow to leg muscles. This disease can lead to chronic limb ischemia and tissue loss, requiring amputation in severe cases. Approximately 40% of PAD patients have intermittent claudication, or muscle pain, cramping, or aching in the calf or thigh. Currently, rehabilitation prescribed for PAD patients includes walking, with intensity guided by pain. Regenerative strategies have included delivery of endothelial progenitor cells (EPCs) in animal models (Yu et al. 2009; Hu et al. 2008) and in clinical trials (Lara-Hernandez et al. 2010). Endothelial progenitor cells, which are isolated from mononuclear cells, are well known for their ability to self-renew and their potential to differentiate into functional endothelial cells (Napoli et al. 2011), supporting the effectiveness of EPCs to improve ischemia-related organ dysfunction through enhanced angiogenesis. In clinical trials, EPCs have been shown to be safe and effective in improving tissue perfusion and ankle-brachial index measurements, the gold standard method by which PAD is diagnosed, while decreasing leg pain at rest and/or improving pain-free walking time (Van Tongeren et al. 2008; Higashi et al. 2004; Bartsch et al. 2007). Others, however, have reported null results for these measures (Franz et al. 2011). It is noteworthy that study endpoints have ranged between 1- and 13-months post-EPC treatment, and different processing techniques and EPC concentrations have been used, which may explain conflicting results across clinical studies. Preclinically, in a rat model of critical limb ischemia, investigators (Yeh et al. 2012) implemented a combined approach delivering bone marrow-derived EPCs and extracorporeal shock wave therapy, providing mechanical stimulation through high-energy acoustic waves. This combined approach has been observed to improve hindlimb angiogenesis and restore blood flow to levels observed in uninjured animals. Importantly, these improvements were greater than with either approach alone. However, no measures of function were included in these analyses. These findings are important as EPCs and shock wave therapy are clinically available (Sun et al. 2011; Napoli et al. 2011); further clinical investigation is warranted to determine the efficacy of this combined therapy along with other approaches.
1.5 Historic Systems Biology Approach to Regenerative Rehabilitation
Across injuries and pathological conditions, it is evident that substantial progress has been made in the fields of regenerative medicine and rehabilitation. Initial findings suggest that regenerative rehabilitation approaches can act synergistically to improve tissue architecture and function. Currently, approaches are at various stages of success and translation across the body. Herein, using a systems biology approach, we provide highlights of recent work and emphasize functional evaluation of regenerative rehabilitation.
1.5.1 Central and Peripheral Nervous System
A host of debilitating conditions can affect the CNS and PNS. Regenerative rehabilitation for previously intractable nervous system injuries has come a long way in the past decade, yet still faces substantial challenges. Despite the development of stem cell therapies, for the most part, significant injury to the brain and spinal cord remains largely irreversible from a functional standpoint. Historically, focus has been on the management of long-term disability, reduction of the rate of loss of residual function, and preservation of quality of life. Physical and occupational therapy plays a critical role, along with appropriate medical management and social support. However, substantial progress in regenerative rehabilitation for injuries to the brain, spinal cord, and nerves promises to substantially improve the outlook in the coming years.
1.5.1.1 Central Nervous System
Vascular insults and cellular degenerative processes can lead to a vast number of CNS lesions affecting nearly every possible function. Until recently, the mainstays of therapy have been medical management to prevent ongoing declines, and physical and occupational rehabilitation to preserve activities of daily living. Due to the complexity of injuries, there is a need to study the relative contributions of rehabilitation and regenerative therapies independently. Improvements in functional recovery have been demonstrated clinically following stem cell transplantation. Combination with growth factors may further reduce inflammation, stimulate neurogenesis and improve stem cell survival (Asgharzade et al. 2020). Repetitive transcranial stimulation to alter brain excitability is also now being used clinically and found to have use in subcortical stroke, particularly when applied to the unaffected hemisphere (Ito et al. 2020). Pharmacologic interventions may serve as adjuvants to recovery and some including citicoline, fluoxetine, niacin, and levodopa are in clinical trials or current use (Szelenberger et al. 2020). Exercise, including increasing use of robotic assistance has been demonstrated to induce neural plasticity and improve motor function by regenerating neurons and intra-hemispheric connections, along with functional reorganization in unaffected areas (Xing and Bai 2020). The window for exercise rehabilitation intervention is now thought to be greater than 6 months (Szelenberger et al. 2020). Stem cell therapies have also been used to treat neurodegenerative diseases including various forms of dementia, with some initial promise demonstrated (Sivandzade and Cucullo 2021).
1.5.1.1.1 Spinal Cord
More than 300,000 people are estimated to live with spinal cord injury in the United States, with new cases approaching 20,000 per year due primarily to falls, gunshot, and motor vehicle accidents (Kiyotake et al. 2020). The current standard of care includes: early decompression surgery to repair vertebrae, medical management attempting to increase spinal perfusion, hypothermia, and rehabilitation. The outlook for recovery generally remains poor for moderate to severe injuries (Khorasanizadeh et al. 2019). While there are a great deal of potential solutions currently being studied, neither regenerative medicine or rehabilitation approaches have yet led to full recovery from moderate to severe injuries (Chhabra and Sarda 2017). Regenerative approaches have included a wide range of cellular products alone and in combination with biomaterials, molecular therapies and/or drugs (Ashammakhi et al. 2019), including some in clinical stage development. The wide array of rehabilitation approaches currently in development includes direct electrical (Courtine and Sofroniew 2019), transcranial (de Araujo et al. 2020), and pharmacologic stimulation (Hayashi et al. 2010), as well as conventional locomotor rehabilitation techniques. Numerous electrically conductive biomaterials are also in preclinical development in an effort to merge regenerative and rehabilitative approaches (Kiyotake et al. 2020).
1.5.1.1.2 Traumatic Brain Injury
Traumatic brain injury (TBI) has been a signature insult of recent wars and is common in civilians as well, with more than 280,000 hospitalizations and 56,000 deaths in 2014 (Capizzi et al. 2020). Roughly 20% of injuries are moderate to severe. Traumatic injuries to the CNS, including the brain (i.e., TBI) and spinal cord, are less common and less well studied than medical causes of CNS injury. As a result, progress in regenerative rehabilitation has not been as great as with medical causes. As a more diffuse process, TBI is more poorly understood, associated more commonly with cognitive deficits, and is more difficult to treat. Despite this, there is limited evidence emerging for modest improvements in even severe TBI symptoms, cognitive function, and favorable neuroplasticity with both cognitive rehabilitation and non-invasive brain stimulation techniques (Galetto and Sacco 2017). Immersive virtual reality rehabilitation has also played an increasing role (Maggio et al. 2019). Vagal nerve stimulation has demonstrated limited benefit in recovery from both stroke and TBI (Wu et al. 2020; Pruitt et al. 2016) and may have a favorable impact on a number of inflammatory conditions as well (Johnson and Wilson 2018).
1.5.1.2 Peripheral Nerve Injury
While injured peripheral nerves tend to regenerate reliably, the slow speed of Wallerian degeneration limits the speed of distal axonal regrowth past the point of injury to 1 mm/day. Even with modern diagnostics, including electromyograms and nerve conduction testing, it remains impossible to determine with confidence whether or not injured nerves will recover function (Saunders and Rose 2021). As a result, watchful waiting generally remains the norm for injury repair due to ongoing diagnostic limitations. Treatment with autologous nerve grafts may commit patients to longer periods of denervation with greater risk for atrophy of target muscles, ultimately rendering such repairs useless. Decellularized cadaveric human nerve grafts have proven to be effective tissue sparing solutions and an early win for repair of traumatic injuries. Emerging regenerative technologies aim to overturn this paradigm. Interventions to improve diagnostic accuracy, preserve partial nerve conduction, and support denervated target end organs during regrowth are currently in development (Gurjar et al. 2021). Methods to preserve nerve continuity and short-circuit the process of Wallerian degeneration, including fusion of the nerve ends with chemical sealants, could potentially shift the current watchful waiting paradigm to one of surgical emergency akin to that of traumatic vascular repair (Riley et al. 2017).
1.5.2 Cardiovascular System
Meaningful regenerative and rehabilitative outcomes are dependent on well-perfused tissues. In serious injuries and vascular occlusive events early, definitive, and sustained restoration of blood flow is at the heart of limb and vital organ salvage. Failure to do so invariably leads to loss of target organ function and in some cases amputation with a permanent disability. In serious traumatic injuries, arterial damage, laceration, and thrombosis often require urgent vascular reconstruction to save tissues from ischemia, necrosis, and further amputation. Once the large vessels have been restored, attention must be turned to microvascular damage to prevent tissue ischemia. On the macro level, while regenerative vascular replacements have advanced to clinical-stage investigational therapies including trauma indications (Amiel et al. 2006), little has changed in surgical practice in the last 30 years. There are two current methods for vascular reconstruction: harvesting of autologous vein or using synthetic graft materials. While each has advantages, there are important limitations for both. Autologous vessels can be time-consuming to harvest, in some cases inadequate for the intended use, and difficult or impossible to use, particularly in complex multi-limb trauma (Fox et al. 2005). Donor-site morbidity is also an issue including infection, scarring, leg edema, and loss of potential graft material in young patients who later develop atherosclerotic disease (Terada et al. 1999). Contamination of open traumatic injuries likewise creates challenges for use of synthetic grafts, commonly of teflon (ePTFE) or dacron due to high risk of bacterial infection (Owens et al. 2007). In addition to conventional open repair procedures, endovascular techniques have gained increasing use in trauma in recent years (Johnson 2010; DuBose et al. 2015).
Advances in biomaterials are currently being investigated in large animal models for the potential to improve clinical outcomes when coated onto synthetic vascular grafts and reduce infection rates (Liu et al. 2018). These have had greater application in recent years for developing interventions for cardiovascular disease (Stapleton et al. 2020). Recent advances in decellularized scaffolds, vascular cell seeding, and the design of bioactive polymers for in situ arterial regeneration have yielded promising results, but are not yet approved for clinical use (Ong et al. 2017). The ideal engineered vascular graft material would behave like a native vessel, but spare the harvest of autologous tissue. Originally developed as hemodialysis grafts for patients with end-stage renal disease (Kirkton et al. 2019), investigational regenerative medicine technologies advancing through the clinic (i.e., the human acellular vessel, or HAV) have shown significant preliminary promise for battlefield trauma applications (Morrison et al. 2019).
While truly novel regenerative options for vascular trauma remain largely experimental, options for those requiring rehabilitation from cardiovascular disease are more numerous. The primary financial interest for developing treatment modalities for injured vessels is the proliferation of cardiovascular disease, including coronary heart, cerebrovascular, and PAD. The emphasis on cardiovascular disease has resulted in a technology gap concerning vascular trauma. However, conventional grafts, and even those that are tissue-engineered suffer from patency issues as smaller calibers below 6 mm vessel diameter (Pashneh-Tala et al. 2016). Many current vascular repair treatments focus on treating occluded or stenosed vessels via stent-graft insertion, angioplasty, or vascular grafts (Pashneh-Tala et al. 2016). Importantly, many vascular repair solutions have been studied in the context of diseased rather than injured vessels. As a result, many products are indicated to repair, bypass, or improve vessel patency. Although many of these solutions can be used to replace vessels in cases of traumatic vascular wounds, they may not have been expressly designed to do so. The primary disadvantage in this regard is the need to grow vessels ex vivo, often based on tissue culture from patient biopsies and/or bioreactors. An immediately implantable off-the-shelf solution is needed in severe trauma, though only one example of a tissue-engineered product has made it to clinical stage development to date (Morrison et al. 2019).
Traumatic injury rehabilitation tends to be focused more on specific rehabilitation of the effector organs, primarily muscle, nerve, and soft tissue. While there are no rehabilitation interventions specifically aimed at blood vessels currently, recent findings suggest exercise under partial ischemic conditions may improve limb rehabilitation outcomes (Day 2018). Rehabilitation programs for PAD affecting the limbs are better defined, with supervised treadmill rehabilitation found to be of benefit across a meta-analysis of randomized trials (McDermott 2018). Cardiovascular injury rehabilitation paradigms vary between traumatic injury and occlusive disease. Cardiac rehabilitation programs are the norm with graded exercise programs, increasingly performed at home following revascularization procedures, including surgical coronary artery bypass grafting, endovascular interventions, and medically managed vascular events (Ambrosetti et al. 2020). These programs in combination with lipid management, dietary interventions, and tobacco cessation have been shown to improve outcomes as well as physiologic indicators, such as increased peak VO2 (Izawa 2020). Vascular rehabilitation related to stroke specifically is described above.
1.5.3 Skeletal Muscle System
Skeletal muscle makes up approximately 40% of body mass and is essential for movement and locomotion, postural control, respiratory activity, and heat production. Although skeletal muscle has a robust capacity for regeneration and repair following acute injury, endogenous healing mechanisms are impaired in more complex or traumatic skeletal muscle injuries or chronic pathologies, such as volumetric muscle loss (VML), muscular dystrophies (e.g., Duchenne’s and limb girdle), and sarcopenia. These conditions contribute to muscle atrophy and associated deficits in muscle function, leading to poor quality of life. Patients may also suffer from a host of related chronic comorbidities, such as fibrosis and inflammation. The heterogenous nature of skeletal muscle pathologies and related comorbidities make the establishment of standardized care difficult. Therefore, it is essential to investigate various regenerative rehabilitation approaches to treat complex skeletal muscle pathologies.
Duchenne and limb girdle muscular dystrophies are debilitating conditions characterized by skeletal muscle atrophy and weakness. Duchenne muscular dystrophy (DMD) is the most common genetic disease among pediatric onset dystrophies. Patients have a mutation in the dystrophin gene, resulting in a lack of the structural protein, dystrophin, contributing to fragile muscle tissue, atrophy, and weakness (Emery 2002; Hoffman et al. 1987). Although rehabilitation is currently not prescribed for patients with DMD due to lack of standard guidelines and perhaps fear of exacerbating outcomes (Markert et al. 2012), clinical trials have recently been conducted to examine the safety and efficacy of aerobic (NCT03319030) and isometric resistance (NCT02421523) exercise to improve muscle strength in ambulatory and non-ambulatory boys with DMD. In the preclinical setting, rehabilitation, including treadmill and wheel running and isometric resistance exercise, has been shown to improve ambulation, maximal isometric force and contractility rates, as well as decrease the number of regenerating muscle fibers as determined by histology (Lindsay et al. 2019; Call et al. 2010).
In addition to rehabilitation, regenerative strategies, including cellular therapies, have also been examined. The first attempt to transplant cells in a dystrophin-deficient mdx mouse model occurred in 1989 with the transplantation of healthy mouse satellite cells which improved numbers of dystrophin positive muscle cells (Partridge et al. 1989). More recently, iPS cells have been investigated. Within the past decade, human artificial chromosome (HAC) and CRISPR technologies have been used to successfully repair the dystrophin gene of DMD patient-derived iPS cells (Li et al. 2015; Kazuki et al. 2010). The corrected iPS cells were then differentiated into skeletal muscle cells, with subsequent analysis showing the full-length dystrophin mRNA to be present (Li et al. 2015). These results provide an important framework for iPS cell-based gene therapy for genetic disorders such as DMD. In the mdx mouse model, transplantation of autologous iPS cells has restored dystrophin to the diseased muscle, while improving, but not fully recovering, muscle function compared to wild-type mice (Darabi et al. 2012). Collectively, this work indicates that strategies are needed to improve dystrophin levels and that rehabilitation is needed to enhance muscle function to improve long-term outcomes for patients with DMD.
Severe skeletal muscle injury, such as VML, is another example of a muscle pathology that would benefit from a combined regenerative rehabilitation strategy (Saunders and Rose 2021). Volumetric muscle loss was initially defined in 2011 as “the traumatic or surgical loss of skeletal muscle with resultant functional impairment” (Grogan et al. 2011). Current treatment strategies include wound closure to mitigate infection and scar tissue debridement. Following initial wound repair the most common approach to treat the muscle remaining after VML is rehabilitation, but limited functional recovery has been reported preclinically and clinically with rehabilitation alone (Garg et al. 2015). Regenerative strategies have also been investigated in treating skeletal muscle injury. A recent systematic review and meta-analysis (Greising et al. 2019) reviewed the effectiveness of various regenerative approaches in animal models of VML injury. Decellularized scaffolds combined with stem cells and/or progenitor cells were found to have the greatest probability to improve muscle function compared to untreated animals. Although a treatment approach that combines the preceding regenerative strategy with rehabilitation may work to synergistically improve functional outcomes following VML, few studies have examined this combinatorial approach. Combined approaches have reported functional improvements resulting from, for example, implantation of biomaterials within the defect area, seeded with or without stem cells, followed by rehabilitation, although functional deficits persist (Greising et al. 2019). In the clinic, a paucity of case studies and series have been conducted to investigate the efficacy of regenerative rehabilitation strategies to improve functional outcomes post-VML. A case study of a combat injured soldier who underwent rehabilitation prior to and following surgical implantation of a decellularized scaffold into the VML defect area indicated an ~20% improvement in isometric force 27 weeks post-operatively, but functional deficits were still evident (Gentile et al. 2014). More recently, a clinical trial (Dziki et al. 2016) conducted in 13 VML-afflicted patients, 7–120 months removed from the injury date, received a decellularized scaffold followed by early rehabilitation. Despite ~37% and 27% improvements in force production and ROM, respectively, significant functional deficits remained. While it is evident that this type of heterogenous injury necessitates a more involved regenerative rehabilitation approach, it is necessary to investigate the combination of therapies that optimally enhance tissue regeneration and functionality following injury.
1.5.4 Skeletal System
Fractures of the bone are one of the most common traumatic injuries in humans and result from high-energy trauma such as motor vehicle accidents, military combat, or hard hits in contact sports. Like skeletal muscle, bone displays incredible plasticity following injury. According to the mechanostat theory, bone adapts its strength and geometry when subjected to mechanical loading to meet the functional demands (Frost 2003). Clinically, rehabilitation, including early weight-bearing exercise, low magnitude electrical stimulation, and low-intensity pulsed ultrasonography (LIPUS), is generally prescribed across the healing process to elicit various stimuli for adaptation (Kristiansen et al. 1997; Heckman et al. 1994; Kubiak et al. 2013). However, complex or compromised fractures, including open fractures that involve not only bone but also the surrounding muscle, nerves, and vasculature, fail to heal, resulting in a non-union fracture. In cases of open fracture, infection mitigation and internal or external fixation is the first treatment priority. Patients also commonly receive a bone graft, currently considered a gold standard treatment, using either an autograft or allograft. Yet, bone grafts have limitations, including donor site morbidity, failed bone tissue integration from the host, and vascularization issues, often leading to a delay in healing and further delayed rehabilitation (Ho-Shui-Ling et al. 2018).
Complex fractures are an ideal instance where regenerative rehabilitation can improve return to function sooner. Potential regenerative medicine strategies to treat complex bone fracture have been largely investigated in the preclinical setting, including use of bone tissue engineering, gene, and growth factor (e.g., bone morphogenetic protein-2 and 7) therapies, MSC delivery (e.g., adipose-, bone marrow-derived), and bioengineered decellularized scaffold implantation (Trohatou and Roubelakis 2017). Some of these strategies have been evaluated in ongoing or recently completed clinical case studies or trials. Clinically, the first report of an autologous bone marrow-derived MSC-seeded hydroxyapatite biomaterial implanted in large (4–8 cm) bone segmental defects of three patients was published in 2001 (Quarto et al. 2001). By 12 months following implantation, all patients had experienced complete bone union and full recovery of limb function. Although similar strategies have been used in the clinic since this report, various issues have been raised, including the high complexity and high-cost burdens associated with implementing cell-based engineering therapies.
Strategies implementing regenerative and rehabilitation approaches in combination have been investigated in animal models of fracture. These strategies have included electrical stimulation initiated following implantation of scaffolds incorporated with MSCs (Leppik et al. 2018) and a LIPUS regimen initiated following MSC injection (Cheung et al. 2013). A combined strategy of electrical stimulation applied after implantation of a scaffold seeded with adipose tissue-derived MSCs was first investigated in 2018 in a rat model of femoral fracture (Leppik et al. 2018). Initial in vitro analysis showed electrical stimulation following cell seeding on the scaffold increased osteogenic differentiation, similar to findings from previous studies (Hardy et al. 2015). A subsequent in vivo analysis showed the combined treatment strategy to improve bone healing up to 8 weeks following fracture compared to uninjured animals. Evidence of healing included increased bone formation and vessel density assessed histologically; bone strength evaluated by 3-point bending tests; and osteogenic gene expression. Finally, others (Cheung et al. 2013) have shown MSC injection followed by 4 weeks of LIPUS in a rat model of femoral fracture improves fracture healing. Healing was evidenced by increased callus width and area, evaluated by radiology, and greater bone volume, measured via microcomputed tomography, compared to uninjured animals and injured animals administered MSCs without LIPUS. Although it is evident that these combined strategies may facilitate improved structural and functional bone outcomes following fracture, further study is needed to elucidate the mechanisms underlying these combined approaches and to determine an optimal therapy combination for translation into the clinical setting.
1.5.5 Connective Tissue
Connective tissue comes in a variety of types and can be found in all systems of the body; however, this section will focus on the dense connective tissues of the musculoskeletal system, specifically tendons and ligaments. While both tissues are comprised of collagen, elastin, proteoglycans, fibroblasts, and water, the percentage of each component differs, leading to different mechanical properties. Tendons typically have a higher collagen composition coupled with lower elastin, proteoglycan, and water content (Rumian et al. 2007). This leads to high stiffness and tensile strength, advantageous for force transmission from muscle to bone that generally occurs in a uniaxial direction. Ligaments serve as support structures for joints, connecting bone to bone, and thus not only need high strength but also enough elasticity to withstand forces from various directions. Therefore, ligaments typically have lower collagen and higher elastin content than tendons. When injured, both tendon and ligaments display a range of outcomes based on anatomical location and severity of injury, but in most cases, the injured tissue never regains full strength. Rehabilitation alone in some combination of targeted exercise strengthening, stretching, ultrasound, cryotherapy, or massage is typically recommended for partial tears and mild tendinopathy (Papadopoulos and Mani 2020; Edwards et al. 2016; Maffulli et al. 2004). Full-thickness tears and more progressive tendinopathy often require surgical treatment followed by rehabilitation modalities. While the most common surgical approach for ligament rupture is reconstruction using autologous or allogenic tendon grafts, donor site morbidity and infection risk remain significant limitations (Hardy et al. 2017; Weitzel et al. 2002). Furthermore, of athletes that undergo ACL reconstruction, only about a third achieve pre-injury level of play after 2 years and the long-term risk of osteoarthritis increases dramatically (Sepulveda et al. 2017), particularly for athletes that experience more than one ACL rupture. Tendon ruptures are commonly repaired with similar methods and limitations, or by anchoring the tendon to the bone. In all cases, the prevalence of re-rupture is high.
Novel strategies for tendon and ligament regeneration have included synthetic biomaterial grafts, cell therapy (Agung et al. 2006), growth factors and/or gene therapy, or a combination of these in tissue engineering approaches. Though synthetic ACL grafts have been developed and experimentally used for over a century (Corner 1914), they have been remarkably unsuccessful to date (Legnani et al. 2010; Ventura et al. 2010). Likewise, optimism regarding a standalone cell therapy for tendon and ligament regeneration has generally faded (Hirzinger et al. 2014; Pas et al. 2017), yet there remains a promise for its use in tissue engineering. Furthermore, while platelet-rich plasma (PRP) emerged in the 1980s to combat blood loss during cardiac surgery (Ferrari et al. 1987), its use as a therapy for musculoskeletal injuries has grown in popularity over the past decade (Kia et al. 2018). Platelet-rich plasma is proposed to stimulate healing by the release and subsequent regulation of various growth factors from platelets (Boswell et al. 2012). In a rat model of Achilles tendon injury, PRP treatment improved outcomes of stiffness and force at failure after 3 and 5 days of muscle unloading using Botox injections; yet, mechanical loading was necessary for these effects to continue after 14 days (Virchenko and Aspenberg 2006). Similarly, animal models of tendon tissue engineering have shown improved outcomes when stem cells are used in conjunction with mechanical stimulation prior to implantation (Juncosa-Melvin et al. 2006; Juncosa-Melvin et al. 2007) however, when mechanical tension is removed, these beneficial changes are lost and inflammation increases (Bayer et al. 2014). Together these findings highlight the potential importance of a structured rehabilitation program in combination with regenerative strategies to maintain mechanical loading and thus the tissue architecture.
1.6 Importance of Functional Outcomes
As an emerging field leveraging the disciplines of regenerative medicine and rehabilitation, goals to optimize functional repair, recovery, and/or regeneration of various tissues across the body must be centered on the functional physiologic outcomes to advance EBP approaches. As more and more regenerative rehabilitation interventions reach the clinic, there is an ongoing need for investigations to determine how best to evaluate recovery and restoration of functional capacity. Ultimately, standardized physiologic outcomes are required and should dove-tail into clinical outcome assessment and patient-centered outcomes as outlined by the United States FDA. Requiring evaluations both prior to and following the implementation of regenerative rehabilitation strategies is essential. Often patient-focused outcomes can be impactful in these instances, and qualitative self or caregiver assessments of: daily physical activity, extremity function, physical function, and walking speed can be used to understand ongoing efficacy.
Again, using a systems biology approach (Fig. 1.1), functional measures commonly assessed in the clinical setting to determine improvements during the rehabilitative process are discussed. Across the cardiovascular system, well-established outcomes evaluate changes in exercise capacity and endurance by the use of the submaximal and maximal VO2 tests, resting and submaximal heart rate, blood pressure, and ejection fraction. Additionally, these are often combined with respiratory function tests, such as vital capacity, maximal inspiratory pressure, and maximal expiratory pressures. Within the central and peripheral nervous systems, a variety of functional tests are used, including the Fugl-Meyers Assessment, Wolf Motor Arm Function Test, Action Research Arm Test, 6-min walk test, and Berg Balance Scale. All in efforts to assess the ongoing efficacy of interventions. Moving into the musculoskeletal system, broadly function of the extremities is often predicated on skeletal muscle function. Muscle function, or strength, is often evaluated using isokinetic or isometric testing or task-specific movements, such as single-leg jumps. The evaluation of muscle function in these ways is useful for skeletal muscle, bone, and connective tissue.
1.7 Future Directions in Regenerative Rehabilitation
The field of regenerative rehabilitation has come a long way in the last two decades with the advent of cellular and regenerative therapies. Automation, adjunctive therapies, and a greater understanding of effective rehabilitation paradigms through study have advanced recovery of functional deficits. The long-term goal is to translate these combined strategies to the clinical setting to optimize patients’ function and quality of life. Given the wide array of potential debilitating insults and illnesses, a highly ambitious and broad research agenda lies ahead before the true promise of converging regenerative and rehabilitative modalities can be realized.
The rehabilitation field continues to suffer from insufficient outcome evidence from randomized clinical trials. The ideal ‘prescription’ of therapy regimens likely to be beneficial in many cases remains an educated guess, often tied to traditional approaches based on an intuitive understanding of the pathology. Studying and standardizing function outcomes in controlled experiments will help to better quantify the timing, intensity, and specific physiologic approaches to physical medicine regimens. Randomized clinical trials of regenerative rehabilitation regimens will be essential in this regard, comparing novel physical medicine prescriptions to commonly accepted standards of care. The variability encountered will also help to better define the need for patient-centric approaches based on specific responses encountered during locomotor interventions. Advances in automated, individualized self-guided rehabilitation programs are likely to be of great benefit in this regard, by providing data-driven feedback to both the patient and the treatment team to guide therapy. This will in turn improve the capacity for self-care and adherence to prescribed regimens. Unfortunately, funding for large, controlled trials remains anemic, in part due to the relative lack of potential commercial indications. In contrast, emerging rehabilitative adjuncts including device-based electrical and mechanical stimulation, immersive virtual reality, and robotic assistance are likely to attract significant commercial backing and help to lead the field forward.
In contrast, the closely aligned field of regenerative medicine has rapidly expanded into a multibillion-dollar industry and promises to grow exponentially over the coming decade. As more potential approaches to unlocking the body’s natural healing powers are discovered, the pace of development is only expected to increase. Simple autologous therapies such as PRP injection for soft tissue injury, or MSCs for cosmetic and medical applications have gained substantial clinical acceptance in recent years. Autologous ex vivo constructs including personalized meniscal cartilage replacements grown from biopsy specimens are likely to be approved soon. Decellularized scaffolds are also on the verge of approval for several applications. Products combining scaffolds, growth factors, and small molecules are also on the horizon, along with adjunctive therapies to support immunomodulation and growth. These early interventions are ultimately likely to be supplanted by composite tissue-agnostic approaches. Proof of principle has already been found for a plethora of technologies to include siRNAs, modulation of bioelectrical circuits, electrical stimulation, antiaging factors, and pluripotent stem cell therapies. These are likely to dominate the field in coming decades, promising to unlock the potential for full regeneration of lost and damaged tissues.
References
Agung M, Ochi M, Yanada S, Adachi N, Izuta Y, Yamasaki T, Toda K (2006) Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc 14(12):1307–1314. https://doi.org/10.1007/s00167-006-0124-8
Almouemen N, Kelly HM, O’Leary C (2019) Tissue engineering: understanding the role of biomaterials and biophysical forces on cell functionality through computational and structural biotechnology analytical methods. Comput Struct Biotechnol J 17:591–598. https://doi.org/10.1016/j.csbj.2019.04.008
Ambrosetti M, Abreu A, Corra U, Davos CH, Hansen D, Frederix I, Iliou MC, Pedretti RF, Schmid JP, Vigorito C, Voller H, Wilhelm M, Piepoli MF, Bjarnason-Wehrens B, Berger T, Cohen-Solal A, Cornelissen V, Dendale P, Doehner W, Gaita D, Gevaert AB, Kemps H, Kraenkel N, Laukkanen J, Mendes M, Niebauer J, Simonenko M, Zwisler AO (2020) Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol. https://doi.org/10.1177/2047487320913379
Ambrosio F, Rando TA (2018) The regenerative rehabilitation collection: a forum for an emerging field. NPJ Regen Med 3:20. https://doi.org/10.1038/s41536-018-0058-z
American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS (2009) American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 41(7):1510–1530. https://doi.org/10.1249/MSS.0b013e3181a0c95c
Amiel GE, Komura M, Shapira O, Yoo JJ, Yazdani S, Berry J, Kaushal S, Bischoff J, Atala A, Soker S (2006) Engineering of blood vessels from acellular collagen matrices coated with human endothelial cells. Tissue Eng 12(8):2355–2365. https://doi.org/10.1089/ten.2006.12.2355
Asgharzade S, Talaei A, Farkhondeh T, Forouzanfar F (2020) Combining growth factor and stem cell therapy for stroke rehabilitation, a review. Curr Drug Targets 21(8):781–791. https://doi.org/10.2174/1389450121666200107100747
Ashammakhi N, Kim HJ, Ehsanipour A, Bierman RD, Kaarela O, Xue C, Khademhosseini A, Seidlits SK (2019) Regenerative therapies for spinal cord injury. Tissue Eng Part B Rev 25(6):471–491. https://doi.org/10.1089/ten.TEB.2019.0182
Atanelov L, Stiens SA, Young MA (2015) History of physical medicine and rehabilitation and its ethical dimensions. AMA J Ethics 17(6):568–574. https://doi.org/10.1001/journalofethics.2015.17.6.mhst1-1506
Bartsch T, Brehm M, Zeus T, Kogler G, Wernet P, Strauer BE (2007) Transplantation of autologous mononuclear bone marrow stem cells in patients with peripheral arterial disease (the TAM-PAD study). Clin Res Cardiol 96(12):891–899. https://doi.org/10.1007/s00392-007-0569-x
Bauer G, Elsallab M, Abou-El-Enein M (2018) Concise review: a comprehensive analysis of reported adverse events in patients receiving unproven stem cell-based interventions. Stem Cells Transl Med 7(9):676–685. https://doi.org/10.1002/sctm.17-0282
Bayer ML, Schjerling P, Herchenhan A, Zeltz C, Heinemeier KM, Christensen L, Krogsgaard M, Gullberg D, Kjaer M (2014) Release of tensile strain on engineered human tendon tissue disturbs cell adhesions, changes matrix architecture, and induces an inflammatory phenotype. PLoS One 9(1):e86078. https://doi.org/10.1371/journal.pone.0086078
Becker RO, Spadaro JA (1972) Electrical stimulation of partial limb regeneration in mammals. Bull N Y Acad Med 48(4):627–641
Bell E, Ivarsson B, Merrill C (1979) Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A 76(3):1274–1278. https://doi.org/10.1073/pnas.76.3.1274
Billin AN, Bantscheff M, Drewes G, Ghidelli-Disse S, Holt JA, Kramer HF, McDougal AJ, Smalley TL, Wells CI, Zuercher WJ, Henke BR (2016) Discovery of novel small molecules that activate satellite cell proliferation and enhance repair of damaged muscle. ACS Chem Biol 11(2):518–529. https://doi.org/10.1021/acschembio.5b00772
Biomaterials Science (2020) A history of biomaterials, 4th edn. Academic. https://doi.org/10.1016/B978-0-12-816137-1.00002-7
Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA (2012) Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy 28(3):429–439. https://doi.org/10.1016/j.arthro.2011.10.018
Burkhalter W (1994) Surgery in Vietnam: orthopaedic surgery. OTSG Department of the Army, Washington, DC
Butler DL, Goldstein SA, Guilak F (2000) Functional tissue engineering: the role of biomechanics. J Biomech Eng 122(6):570–575. https://doi.org/10.1115/1.1318906
Cai M, Liu H, Jiang Y, Wang J, Zhang S (2019) A high-strength biodegradable thermoset polymer for internal fixation bone screws: preparation, in vitro and in vivo evaluation. Colloids Surf B Biointerfaces 183:110445. https://doi.org/10.1016/j.colsurfb.2019.110445
Call JA, McKeehen JN, Novotny SA, Lowe DA (2010) Progressive resistance voluntary wheel running in the mdx mouse. Muscle Nerve 42(6):871–880
Capizzi A, Woo J, Verduzco-Gutierrez M (2020) Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am 104(2):213–238. https://doi.org/10.1016/j.mcna.2019.11.001
Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9(5):641–650. https://doi.org/10.1002/jor.1100090504
Cheung WH, Chin WC, Wei FY, Li G, Leung KS (2013) Applications of exogenous mesenchymal stem cells and low intensity pulsed ultrasound enhance fracture healing in rat model. Ultrasound Med Biol 39(1):117–125. https://doi.org/10.1016/j.ultrasmedbio.2012.08.015
Chhabra HS, Sarda K (2017) Clinical translation of stem cell based interventions for spinal cord injury—are we there yet? Adv Drug Deliv Rev 120:41–49. https://doi.org/10.1016/j.addr.2017.09.021
Cho SR, Suh H, Yu JH, Kim HH, Seo JH, Seo CH (2016) Astroglial activation by an enriched environment after transplantation of mesenchymal stem cells enhances angiogenesis after hypoxic-ischemic brain injury. Int J Mol Sci 17(9). https://doi.org/10.3390/ijms17091550
Corner EM (1914) Notes of a case illustrative of an artificial anterior crucial ligament, demonstrating the action of that ligament. Proc R Soc Med 7 (Clin Sect):120–121
Corona BT, Greising SM (2016) Challenges to acellular biological scaffold mediated skeletal muscle tissue regeneration. Biomaterials 104:238–246. https://doi.org/10.1016/j.biomaterials.2016.07.020
Courtine G, Sofroniew MV (2019) Spinal cord repair: advances in biology and technology. Nat Med 25(6):898–908. https://doi.org/10.1038/s41591-019-0475-6
Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, Perlingeiro RC (2012) Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10(5):610–619. https://doi.org/10.1016/j.stem.2012.02.015
Day B (2018) Personalized blood flow restriction therapy: how, when and where can it accelerate rehabilitation after surgery? Arthroscopy 34(8):2511–2513. https://doi.org/10.1016/j.arthro.2018.06.022
de Araujo AVL, Ribeiro FPG, Massetti T, Potter-Baker KA, Cortes M, Plow EB, da Silva TD, Tonks J, Anghinah R, Magalhaes FH, Fregni F, de Mello Monteiro CB (2020) Effectiveness of anodal transcranial direct current stimulation to improve muscle strength and motor functionality after incomplete spinal cord injury: a systematic review and meta-analysis. Spinal Cord 58(6):635–646. https://doi.org/10.1038/s41393-020-0438-2
Dehghan N, McKee MD, Jenkinson RJ, Schemitsch EH, Stas V, Nauth A, Hall JA, Stephen DJ, Kreder HJ (2016) Early weightbearing and range of motion versus non-weightbearing and immobilization after open reduction and internal fixation of unstable ankle fractures: a randomized controlled trial. J Orthop Trauma 30(7):345–352. https://doi.org/10.1097/BOT.0000000000000572
Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB (2006) Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576(Pt 2):613–624. https://doi.org/10.1113/jphysiol.2006.113175
DuBose JJ, Savage SA, Fabian TC, Menaker J, Scalea T, Holcomb JB, Skarupa D, Poulin N, Chourliaras K, Inaba K, Rasmussen TE, Group APS (2015) The American Association for the Surgery of Trauma PROspective Observational Vascular Injury Treatment (PROOVIT) registry: multicenter data on modern vascular injury diagnosis, management, and outcomes. J Trauma Acute Care Surg 78(2):215–222.; Discussion 222–213. https://doi.org/10.1097/TA.0000000000000520
Dziki J, Badylak S, Yabroudi M, Sicari B, Ambrosio F, Stearns K, Turner N, Wyse A, Boninger ML, Brown EHP, Rubin JP (2016) An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. NPJ Regen Med 1:16008. https://doi.org/10.1038/npjregenmed.2016.8
Edwards P, Ebert J, Joss B, Bhabra G, Ackland T, Wang A (2016) Exercise rehabilitation in the non-operative management of rotator cuff tears: a review of the literature. Int J Sports Phys Ther 11(2):279–301
Emery AE (2002) The muscular dystrophies. Lancet 359(9307):687–695
Ferrari M, Zia S, Valbonesi M, Henriquet F, Venere G, Spagnolo S, Grasso MA, Panzani I (1987) A new technique for hemodilution, preparation of autologous platelet-rich plasma and intraoperative blood salvage in cardiac surgery. Int J Artif Organs 10(1):47–50
Fluri F, Schuhmann MK, Kleinschnitz C (2015) Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther 9:3445–3454. https://doi.org/10.2147/DDDT.S56071
Fox CJ, Gillespie DL, O’Donnell SD, Rasmussen TE, Goff JM, Johnson CA, Galgon RE, Sarac TP, Rich NM (2005) Contemporary management of wartime vascular trauma. J Vasc Surg 41(4):638–644. https://doi.org/10.1016/j.jvs.2005.01.010
Franz RW, Shah KJ, Johnson JD, Pin RH, Parks AM, Hankins T, Hartman JF, Wright ML (2011) Short- to mid-term results using autologous bone-marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. Vasc Endovascular Surg 45(5):398–406. https://doi.org/10.1177/1538574411405545
Frost HM (2003) Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol 275(2):1081–1101. https://doi.org/10.1002/ar.a.10119
Galetto V, Sacco K (2017) Neuroplastic changes induced by cognitive rehabilitation in traumatic brain injury: a review. Neurorehabil Neural Repair 31(9):800–813. https://doi.org/10.1177/1545968317723748
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports Medicine (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43(7):1334–1359. https://doi.org/10.1249/MSS.0b013e318213fefb
Garg K, Ward CL, Hurtgen BJ, Wilken JM, Stinner DJ, Wenke JC, Owens JG, Corona BT (2015) Volumetric muscle loss: persistent functional deficits beyond frank loss of tissue. J Orthop Res 33(1):40–46. https://doi.org/10.1002/jor.22730
Gentile NE, Stearns KM, Brown EH, Rubin JP, Boninger ML, Dearth CL, Ambrosio F, Badylak SF (2014) Targeted rehabilitation after extracellular matrix scaffold transplantation for the treatment of volumetric muscle loss. Am J Phys Med Rehabil 93(11 Suppl 3):S79–S87. https://doi.org/10.1097/PHM.0000000000000145
Goldman SM, Henderson BEP, Corona BT (2017) Evaluation of bone marrow mononuclear cells as an adjunct therapy to minced muscle graft for the treatment of volumetric muscle loss injuries. Stem Cell Res Ther 8(1):142. https://doi.org/10.1186/s13287-017-0589-z
Gopalakrishnan A, Shankarappa SA, Rajanikant GK (2019) Hydrogel scaffolds: towards restitution of ischemic stroke-injured brain. Transl Stroke Res 10(1):1–18. https://doi.org/10.1007/s12975-018-0655-6
Greising SM, Corona BT, McGann C, Frankum JK, Warren GL (2019) Therapeutic approaches for volumetric muscle loss injury: a systematic review and meta-analysis. Tissue Eng Part B Rev. https://doi.org/10.1089/ten.TEB.2019.0207
Grogan BF, Hsu JR, Skeletal Trauma Research Consortium (2011) Volumetric muscle loss. J Am Acad Orthop Surg 19(Suppl 1):S35–S37
Guercio A, Di Marco P, Casella S, Cannella V, Russotto L, Purpari G, Di Bella S, Piccione G (2012) Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int 36(2):189–194. https://doi.org/10.1042/CBI20110304
Gurjar AA, Manto KM, Estrada JA, Kaufman M, Sun D, Talukder MAH, Elfar JC (2021) 4-aminopyridine: a single-dose diagnostic agent to differentiate axonal continuity in nerve injuries. Mil Med 186(Suppl 1):479–485. https://doi.org/10.1093/milmed/usaa310
Hardy JG, Villancio-Wolter MK, Sukhavasi RC, Mouser DJ, Aguilar D Jr, Geissler SA, Kaplan DL, Schmidt CE (2015) Electrical stimulation of human mesenchymal stem cells on conductive nanofibers enhances their differentiation toward osteogenic outcomes. Macromol Rapid Commun 36(21):1884–1890. https://doi.org/10.1002/marc.201500233
Hardy A, Casabianca L, Andrieu K, Baverel L, Noailles T, Junior French Arthroscopy S (2017) Complications following harvesting of patellar tendon or hamstring tendon grafts for anterior cruciate ligament reconstruction: systematic review of literature. Orthop Traumatol Surg Res 103(8S):S245–S248. https://doi.org/10.1016/j.otsr.2017.09.002
Hayashi Y, Jacob-Vadakot S, Dugan EA, McBride S, Olexa R, Simansky K, Murray M, Shumsky JS (2010) 5-HT precursor loading, but not 5-HT receptor agonists, increases motor function after spinal cord contusion in adult rats. Exp Neurol 221(1):68–78. https://doi.org/10.1016/j.expneurol.2009.10.003
Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF (1994) Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am 76(1):26–34. https://doi.org/10.2106/00004623-199401000-00004
Heiderscheit BC, Sherry MA, Silder A, Chumanov ES, Thelen DG (2010) Hamstring strain injuries: recommendations for diagnosis, rehabilitation, and injury prevention. J Orthop Sports Phys Ther 40(2):67–81. https://doi.org/10.2519/jospt.2010.3047
Hench LL, Splinter RJ, Allen WC, Greenlee TK (1971) Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res 5(6):117–141
Higashi Y, Kimura M, Hara K, Noma K, Jitsuiki D, Nakagawa K, Oshima T, Chayama K, Sueda T, Goto C, Matsubara H, Murohara T, Yoshizumi M (2004) Autologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemia. Circulation 109(10):1215–1218. https://doi.org/10.1161/01.CIR.0000121427.53291.78
Hirzinger C, Tauber M, Korntner S, Quirchmayr M, Bauer HC, Traweger A, Tempfer H (2014) ACL injuries and stem cell therapy. Arch Orthop Trauma Surg 134(11):1573–1578. https://doi.org/10.1007/s00402-014-2060-2
Hoffman EP, Brown RH Jr, Kunkel LM (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51(6):919–928
Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD (2006) Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res 84(7):1495–1504. https://doi.org/10.1002/jnr.21056
Ho-Shui-Ling A, Bolander J, Rustom LE, Johnson AW, Luyten FP, Picart C (2018) Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 180:143–162. https://doi.org/10.1016/j.biomaterials.2018.07.017
Hu Z, Zhang F, Yang Z, Yang N, Zhang D, Zhang J, Cao K (2008) Combination of simvastatin administration and EPC transplantation enhances angiogenesis and protects against apoptosis for hindlimb ischemia. J Biomed Sci 15(4):509–517. https://doi.org/10.1007/s11373-008-9243-1
Hu B, Weng Y, Xia XH, Liang XJ, Huang Y (2019) Clinical advances of siRNA therapeutics. J Gene Med 21(7):e3097. https://doi.org/10.1002/jgm.3097
Ito A, Kubo N, Liang N, Aoyama T, Kuroki H (2020) Regenerative rehabilitation for stroke recovery by inducing synergistic effects of cell therapy and neurorehabilitation on motor function: a narrative review of pre-clinical studies. Int J Mol Sci 21(9). https://doi.org/10.3390/ijms21093135
Izawa H (2020) Cardiac rehabilitation as therapeutic strategy after coronary artery bypass grafting. Circ J 84(3):378–379. https://doi.org/10.1253/circj.CJ-20-0046
Johnson CA (2010) Endovascular management of peripheral vascular trauma. Semin Intervent Radiol 27(1):38–43. https://doi.org/10.1055/s-0030-1247887
Johnson RL, Wilson CG (2018) A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res 11:203–213. https://doi.org/10.2147/JIR.S163248
Juncosa-Melvin N, Shearn JT, Boivin GP, Gooch C, Galloway MT, West JR, Nirmalanandhan VS, Bradica G, Butler DL (2006) Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng 12(8):2291–2300. https://doi.org/10.1089/ten.2006.12.2291
Juncosa-Melvin N, Matlin KS, Holdcraft RW, Nirmalanandhan VS, Butler DL (2007) Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng 13(6):1219–1226. https://doi.org/10.1089/ten.2006.0339
Kazuki Y, Hiratsuka M, Takiguchi M, Osaki M, Kajitani N, Hoshiya H, Hiramatsu K, Yoshino T, Kazuki K, Ishihara C, Takehara S, Higaki K, Nakagawa M, Takahashi K, Yamanaka S, Oshimura M (2010) Complete genetic correction of ips cells from Duchenne muscular dystrophy. Mol Ther 18(2):386–393. https://doi.org/10.1038/mt.2009.274
Khorasanizadeh M, Yousefifard M, Eskian M, Lu Y, Chalangari M, Harrop JS, Jazayeri SB, Seyedpour S, Khodaei B, Hosseini M, Rahimi-Movaghar V (2019) Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine 1–17. https://doi.org/10.3171/2018.10.SPINE18802
Kia C, Baldino J, Bell R, Ramji A, Uyeki C, Mazzocca A (2018) Platelet-rich plasma: review of current literature on its use for tendon and ligament pathology. Curr Rev Musculoskelet Med 11(4):566–572. https://doi.org/10.1007/s12178-018-9515-y
Kirkton RD, Santiago-Maysonet M, Lawson JH, Tente WE, Dahl SLM, Niklason LE, Prichard HL (2019) Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med 11(485). https://doi.org/10.1126/scitranslmed.aau6934
Kiyotake EA, Martin MD, Detamore MS (2020) Regenerative rehabilitation with conductive biomaterials for spinal cord injury. Acta Biomater. https://doi.org/10.1016/j.actbio.2020.12.021
Kleisiaris CF, Sfakianakis C, Papathanasiou IV (2014) Health care practices in ancient Greece: the hippocratic ideal. J Med Ethics Hist Med 7:6
Koukourikos K, Tsaloglidou A, Kourkouta L (2014) Muscle atrophy in intensive care unit patients. Acta Inform Med 22(6):406–410. https://doi.org/10.5455/aim.2014.22.406-410
Krause M, Phan TG, Ma H, Sobey CG, Lim R (2019) Cell-based therapies for stroke: are we there yet? Front Neurol 10:656. https://doi.org/10.3389/fneur.2019.00656
Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR (1997) Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am 79(7):961–973. https://doi.org/10.2106/00004623-199707000-00002
Kubiak EN, Beebe MJ, North K, Hitchcock R, Potter MQ (2013) Early weight bearing after lower extremity fractures in adults. J Am Acad Orthop Surg 21(12):727–738. https://doi.org/10.5435/JAAOS-21-12-727
Kulkarni RK, Moore EG, Hegyeli AF, Leonard F (1971) Biodegradable poly(lactic acid) polymers. J Biomed Mater Res 5(3):169–181. https://doi.org/10.1002/jbm.820050305
Lara-Hernandez R, Lozano-Vilardell P, Blanes P, Torreguitart-Mirada N, Galmes A, Besalduch J (2010) Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Ann Vasc Surg 24(2):287–294. https://doi.org/10.1016/j.avsg.2009.10.012
Legnani C, Ventura A, Terzaghi C, Borgo E, Albisetti W (2010) Anterior cruciate ligament reconstruction with synthetic grafts. A review of literature. Int Orthop 34(4):465–471. https://doi.org/10.1007/s00264-010-0963-2
Leppik LP, Froemel D, Slavici A, Ovadia ZN, Hudak L, Henrich D, Marzi I, Barker JH (2015) Effects of electrical stimulation on rat limb regeneration, a new look at an old model. Sci Rep 5:18353. https://doi.org/10.1038/srep18353
Leppik L, Zhihua H, Mobini S, Thottakkattumana Parameswaran V, Eischen-Loges M, Slavici A, Helbing J, Pindur L, Oliveira KMC, Bhavsar MB, Hudak L, Henrich D, Barker JH (2018) Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci Rep 8(1):6307. https://doi.org/10.1038/s41598-018-24892-0
Li HL, Fujimoto N, Sasakawa N, Shirai S, Ohkame T, Sakuma T, Tanaka M, Amano N, Watanabe A, Sakurai H, Yamamoto T, Yamanaka S, Hotta A (2015) Precise correction of the dystrophin gene in Duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Rep 4(1):143–154. https://doi.org/10.1016/j.stemcr.2014.10.013
Lightsey HM, Kantrowitz DE, Swindell HW, Trofa DP, Ahmad CS, Lynch TS (2018) Variability of United States online rehabilitation protocols for proximal hamstring tendon repair. Orthop J Sports Med 6(2):2325967118755116. https://doi.org/10.1177/2325967118755116
Lindsay A, Larson AA, Verma M, Ervasti JM, Lowe DA (2019) Isometric resistance training increases strength and alters histopathology of dystrophin-deficient mouse skeletal muscle. J Appl Physiol (1985) 126(2):363–375. https://doi.org/10.1152/japplphysiol.00948.2018
Liu RH, Ong CS, Fukunishi T, Ong K, Hibino N (2018) Review of vascular graft studies in large animal models. Tissue Eng Part B Rev 24(2):133–143. https://doi.org/10.1089/ten.TEB.2017.0350
Maffulli N, Sharma P, Luscombe KL (2004) Achilles tendinopathy: aetiology and management. J R Soc Med 97(10):472–476. https://doi.org/10.1258/jrsm.97.10.472
Maggio MG, De Luca R, Molonia F, Porcari B, Destro M, Casella C, Salvati R, Bramanti P, Calabro RS (2019) Cognitive rehabilitation in patients with traumatic brain injury: a narrative review on the emerging use of virtual reality. J Clin Neurosci 61:1–4. https://doi.org/10.1016/j.jocn.2018.12.020
Marin E, Boschetto F, Pezzotti G (2020) Biomaterials and biocompatibility: an historical overview. J Biomed Mater Res A 108(8):1617–1633. https://doi.org/10.1002/jbm.a.36930
Markert CD, Case LE, Carter GT, Furlong PA, Grange RW (2012) Exercise and Duchenne muscular dystrophy: where we have been and where we need to go. Muscle Nerve 45(5):746–751. https://doi.org/10.1002/mus.23244
Martin I, Wendt D, Heberer M (2004) The role of bioreactors in tissue engineering. Trends Biotechnol 22(2):80–86. https://doi.org/10.1016/j.tibtech.2003.12.001
Mathews J, Levin M (2018) The body electric 2.0: recent advances in developmental bioelectricity for regenerative and synthetic bioengineering. Curr Opin Biotechnol 52:134–144. https://doi.org/10.1016/j.copbio.2018.03.008
Mavalli MD, DiGirolamo DJ, Fan Y, Riddle RC, Campbell KS, van Groen T, Frank SJ, Sperling MA, Esser KA, Bamman MM, Clemens TL (2010) Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J Clin Invest 120(11):4007–4020. https://doi.org/10.1172/JCI42447
McDermott MM (2018) Exercise rehabilitation for peripheral artery disease: a review. J Cardiopulm Rehabil Prev 38(2):63–69. https://doi.org/10.1097/HCR.0000000000000343
Messier SP (2008) Obesity and osteoarthritis: disease genesis and nonpharmacologic weight management. Rheum Dis Clin North Am 34(3):713–729. https://doi.org/10.1016/j.rdc.2008.04.007
Modarai B, Patel AS (2019) PLX-PAD treatment of critical limb ischaemia: a clinically effective cell therapy at long last? Eur J Vasc Endovasc Surg 57(4):546. https://doi.org/10.1016/j.ejvs.2019.02.004
Mooney DJ, Vandenburgh H (2008) Cell delivery mechanisms for tissue repair. Cell Stem Cell 2(3):205–213. https://doi.org/10.1016/j.stem.2008.02.005
Morimoto J, Yasuhara T, Kameda M, Umakoshi M, Kin I, Kuwahara K, Kin K, Okazaki M, Takeuchi H, Sasaki T, Toyoshima A, Tajiri N, Agari T, Borlongan CV, Date I (2018) Electrical stimulation enhances migratory ability of transplanted bone marrow stromal cells in a rodent ischemic stroke model. Cell Physiol Biochem 46(1):57–68. https://doi.org/10.1159/000488409
Moritz CT, Ambrosio F (2017) Regenerative rehabilitation: combining stem cell therapies and activity-dependent stimulation. Pediatr Phys Ther 29(Suppl 3):S10–S15. https://doi.org/10.1097/PEP.0000000000000378
Morrison JJ, McMahon J, DuBose JJ, Scalea TM, Lawson JH, Rasmussen TE (2019) Clinical implementation of the Humacyte human acellular vessel: implications for military and civilian trauma care. J Trauma Acute Care Surg 87(1S Suppl 1):S44–S47. https://doi.org/10.1097/TA.0000000000002350
Myer GD, Paterno MV, Ford KR, Quatman CE, Hewett TE (2006) Rehabilitation after anterior cruciate ligament reconstruction: criteria-based progression through the return-to-sport phase. J Orthop Sports Phys Ther 36(6):385–402. https://doi.org/10.2519/jospt.2006.2222
Napoli C, Hayashi T, Cacciatore F, Casamassimi A, Casini C, Al-Omran M, Ignarro LJ (2011) Endothelial progenitor cells as therapeutic agents in the microcirculation: an update. Atherosclerosis 215(1):9–22. https://doi.org/10.1016/j.atherosclerosis.2010.10.039
Omrani AS, Almaghrabi RS (2017) Complications of hematopoietic stem cell transplantation: bacterial infections. Hematol Oncol Stem Cell Ther 10(4):228–232. https://doi.org/10.1016/j.hemonc.2017.05.018
Ong CS, Zhou X, Huang CY, Fukunishi T, Zhang H, Hibino N (2017) Tissue engineered vascular grafts: current state of the field. Expert Rev Med Devices 14(5):383–392. https://doi.org/10.1080/17434440.2017.1324293
Owens BD, Kragh JF Jr, Macaitis J, Svoboda SJ, Wenke JC (2007) Characterization of extremity wounds in operation Iraqi freedom and operation enduring freedom. J Orthop Trauma 21(4):254–257. https://doi.org/10.1097/BOT.0b013e31802f78fb
Papadopoulos ES, Mani R (2020) The role of ultrasound therapy in the management of musculoskeletal soft tissue pain. Int J Low Extrem Wounds 19(4):350–358. https://doi.org/10.1177/1534734620948343
Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM (1989) Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature 337(6203):176–179. https://doi.org/10.1038/337176a0
Pas H, Moen MH, Haisma HJ, Winters M (2017) No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med 51(13):996–1002. https://doi.org/10.1136/bjsports-2016-096794
Pashneh-Tala S, MacNeil S, Claeyssens F (2016) The tissue-engineered vascular graft-past, present, and future. Tissue Eng Part B Rev 22(1):68–100. https://doi.org/10.1089/ten.teb.2015.0100
Peng JJ, Sha R, Li MX, Chen LT, Han XH, Guo F, Chen H, Huang XL (2019) Repetitive transcranial magnetic stimulation promotes functional recovery and differentiation of human neural stem cells in rats after ischemic stroke. Exp Neurol 313:1–9. https://doi.org/10.1016/j.expneurol.2018.12.002
Perez-Terzic C, Childers MK (2014) Regenerative rehabilitation: a new future? Am J Phys Med Rehabil 93(11 Suppl 3):S73–S78. https://doi.org/10.1097/PHM.0000000000000211
Portis SM, Sanberg PR (2017) Regenerative rehabilitation: an innovative and multifactorial approach to recovery from stroke and brain injury. Cell Med 9(3):67–71. https://doi.org/10.3727/215517917X693393
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL, American Heart Association Stroke Council (2018) 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49(3):e46–e110. https://doi.org/10.1161/STR.0000000000000158
Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, Hays SA, Kilgard MP, Rennaker RL (2016) Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury. J Neurotrauma 33(9):871–879. https://doi.org/10.1089/neu.2015.3972
Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M (2001) Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 344(5):385–386. https://doi.org/10.1056/NEJM200102013440516
Ramalho-Santos M, Willenbring H (2007) On the origin of the term “stem cell”. Cell Stem Cell 1(1):35–38. https://doi.org/10.1016/j.stem.2007.05.013
Riley DC, Boyer RB, Deister CA, Pollins AC, Cardwell NL, Kelm ND, Does MD, Dortch RD, Bamba R, Shack RB, Thayer WP (2017) Immediate enhancement of nerve function using a novel axonal fusion device after neurotmesis. Ann Plast Surg 79(6):590–599. https://doi.org/10.1097/SAP.0000000000001242
Rumian AP, Wallace AL, Birch HL (2007) Tendons and ligaments are anatomically distinct but overlap in molecular and morphological features—a comparative study in an ovine model. J Orthop Res 25(4):458–464. https://doi.org/10.1002/jor.20218
Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS (1996) Evidence based medicine: what it is and what it isn’t. BMJ 312(7023):71–72. https://doi.org/10.1136/bmj.312.7023.71
Sasaki Y, Sasaki M, Kataoka-Sasaki Y, Nakazaki M, Nagahama H, Suzuki J, Tateyama D, Oka S, Namioka T, Namioka A, Onodera R, Mikami T, Wanibuchi M, Kakizawa M, Ishiai S, Kocsis JD, Honmou O (2016) Synergic effects of rehabilitation and intravenous infusion of mesenchymal stem cells after stroke in rats. Phys Ther 96(11):1791–1798. https://doi.org/10.2522/ptj.20150504
Saunders D, Rose L (2021) Regenerative rehabilitation of catastrophic extremity injury in military conflicts and a review of recent developmental efforts. Connect Tissue Res 62(1):83–98. https://doi.org/10.1080/03008207.2020.1776707
Schoenfeld BJ (2010) The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res 24(10):2857–2872. https://doi.org/10.1519/JSC.0b013e3181e840f3
Sepulveda F, Sanchez L, Amy E, Micheo W (2017) Anterior cruciate ligament injury: return to play, function and long-term considerations. Curr Sports Med Rep 16(3):172–178. https://doi.org/10.1249/JSR.0000000000000356
Shu J, Santulli G (2018) Update on peripheral artery disease: epidemiology and evidence-based facts. Atherosclerosis 275:379–381. https://doi.org/10.1016/j.atherosclerosis.2018.05.033
Silvain J, Cayla G, Collet JP, Fargeot C, Montalescot G (2014) [Coronary stents: 30 years of medical progress]. Med Sci (Paris) 30(3):303–310. https://doi.org/10.1051/medsci/20143003019
Simanski CJ, Maegele MG, Lefering R, Lehnen DM, Kawel N, Riess P, Yucel N, Tiling T, Bouillon B (2006) Functional treatment and early weightbearing after an ankle fracture: a prospective study. J Orthop Trauma 20(2):108–114. https://doi.org/10.1097/01.bot.0000197701.96954.8c
Sivandzade F, Cucullo L (2021) Regenerative stem cell therapy for neurodegenerative diseases: an overview. Int J Mol Sci 22(4). https://doi.org/10.3390/ijms22042153
Stapleton L, Zhu Y, Woo YJ, Appel E (2020) Engineered biomaterials for heart disease. Curr Opin Biotechnol 66:246–254. https://doi.org/10.1016/j.copbio.2020.08.008
Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, Case C, McGrogan M, Yankee EW, Schwartz NE (2016) Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke 47(7):1817–1824. https://doi.org/10.1161/STROKEAHA.116.012995
Sun CK, Shao PL, Wang CJ, Yip HK (2011) Study of vascular injuries using endothelial denudation model and the therapeutic application of shock wave: a review. Am J Transl Res 3(3):259–268
Szelenberger R, Kostka J, Saluk-Bijak J, Miller E (2020) Pharmacological Interventions and Rehabilitation Approach for Enhancing Brain Self-repair and Stroke Recovery. Curr Neuropharmacol 18(1):51–64. https://doi.org/10.2174/1570159X17666190726104139
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. https://doi.org/10.1016/j.cell.2006.07.024
Tam RY, Fuehrmann T, Mitrousis N, Shoichet MS (2014) Regenerative therapies for central nervous system diseases: a biomaterials approach. Neuropsychopharmacology 39(1):169–188. https://doi.org/10.1038/npp.2013.237
Tavassoli M, Crosby WH (1968) Transplantation of marrow to extramedullary sites. Science 161(3836):54–56. https://doi.org/10.1126/science.161.3836.54
Terada Y, Fukuda S, Tohda E, Kigawa I, Wanibuchi Y, Mitsui T (1999) Venous function and delayed leg swelling following saphenectomy in coronary artery bypass grafting. Jpn J Thorac Cardiovasc Surg 47(11):559–562. https://doi.org/10.1007/BF03218062
Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA (2003) Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res 25(8):794–800. https://doi.org/10.1179/016164103771953871
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147. https://doi.org/10.1126/science.282.5391.1145
Trohatou O, Roubelakis MG (2017) Mesenchymal stem/stromal cells in regenerative medicine: past, present, and future. Cell Reprogram 19(4):217–224. https://doi.org/10.1089/cell.2016.0062
Van Tongeren RB, Hamming JF, Fibbe WE, Van Weel V, Frerichs SJ, Stiggelbout AM, Van Bockel JH, Lindeman JH (2008) Intramuscular or combined intramuscular/intra-arterial administration of bone marrow mononuclear cells: a clinical trial in patients with advanced limb ischemia. J Cardiovasc Surg (Torino) 49(1):51–58
Ventura A, Terzaghi C, Legnani C, Borgo E, Albisetti W (2010) Synthetic grafts for anterior cruciate ligament rupture: 19-year outcome study. Knee 17(2):108–113. https://doi.org/10.1016/j.knee.2009.07.013
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, MSV E, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee (2020) Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141(9):e139–e596. https://doi.org/10.1161/CIR.0000000000000757
Virchenko O, Aspenberg P (2006) How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop 77(5):806–812. https://doi.org/10.1080/17453670610013033
Wang W, Zhong W, Yuan J, Yan C, Hu S, Tong Y, Mao Y, Hu T, Zhang B, Song G (2015) Involvement of Wnt/beta-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget 6(39):42276–42289. https://doi.org/10.18632/oncotarget.5514
Wangensteen A, Bahr R, Van Linschoten R, Almusa E, Whiteley R, Witvrouw E, Tol JL (2017) MRI appearance does not change in the first 7 days after acute hamstring injury-a prospective study. Br J Sports Med 51(14):1087–1092. https://doi.org/10.1136/bjsports-2016-096881
Watson E, Tatara AM, van den Beucken J, Jansen JA, Wong ME, Mikos AG (2020) An ovine model of in vivo bioreactor-based bone generation. Tissue Eng Part C Methods 26(7):384–396. https://doi.org/10.1089/ten.TEC.2020.0125
Weitzel PP, Richmond JC, Altman GH, Calabro T, Kaplan DL (2002) Future direction of the treatment of ACL ruptures. Orthop Clin North Am 33(4):653–661. https://doi.org/10.1016/s0030-5898(02)00017-2
Wilke MM, Nydam DV, Nixon AJ (2007) Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res 25(7):913–925. https://doi.org/10.1002/jor.20382
Willett NJ, Boninger ML, Miller LJ, Alvarez L, Aoyama T, Bedoni M, Brix KA, Chisari C, Christ G, Dearth CL, Dyson-Hudson TA, Evans CH, Goldman SM, Gregory K, Gualerzi A, Hart J, Ito A, Kuroki H, Loghmani MT, Mack DL, Malanga GA, Noble-Haeusslein L, Pasquina P, Roche JA, Rose L, Stoddart MJ, Tajino J, Terzic C, Topp KS, Wagner WR, Warden SJ, Wolf SL, Xie H, Rando TA, Ambrosio F (2020) Taking the next steps in regenerative rehabilitation: establishment of a new interdisciplinary field. Arch Phys Med Rehabil 101(5):917–923. https://doi.org/10.1016/j.apmr.2020.01.007
Williams D, Isaacson B (2014) The 5 hallmarks of biomaterials success: an emphasis on orthopaedics. Adv Biosci Biotechnol 5:283–293
Winkler T, Perka C, von Roth P, Agres AN, Plage H, Preininger B, Pumberger M, Geissler S, Hagai EL, Ofir R, Pinzur L, Eyal E, Stoltenburg-Didinger G, Meisel C, Consentius C, Streitz M, Reinke P, Duda GN, Volk HD (2018) Immunomodulatory placental-expanded, mesenchymal stromal cells improve muscle function following hip arthroplasty. J Cachexia Sarcopenia Muscle 9(5):880–897. https://doi.org/10.1002/jcsm.12316
Wu D, Ma J, Zhang L, Wang S, Tan B, Jia G (2020) Effect and safety of transcutaneous auricular vagus nerve stimulation on recovery of upper limb motor function in subacute ischemic stroke patients: a randomized pilot study. Neural Plast 2020:8841752. https://doi.org/10.1155/2020/8841752
Xing Y, Bai Y (2020) A review of exercise-induced neuroplasticity in ischemic stroke: pathology and mechanisms. Mol Neurobiol 57(10):4218–4231. https://doi.org/10.1007/s12035-020-02021-1
Yannas IV, Burke JF (1980) Design of an artificial skin. I. Basic design principles. J Biomed Mater Res 14(1):65–81. https://doi.org/10.1002/jbm.820140108
Yannas IV, Burke JF, Orgill DP, Skrabut EM (1982) Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 215(4529):174–176. https://doi.org/10.1126/science.7031899
Yeh KH, Sheu JJ, Lin YC, Sun CK, Chang LT, Kao YH, Yen CH, Shao PL, Tsai TH, Chen YL, Chua S, Leu S, Yip HK (2012) Benefit of combined extracorporeal shock wave and bone marrow-derived endothelial progenitor cells in protection against critical limb ischemia in rats. Crit Care Med 40(1):169–177. https://doi.org/10.1097/CCM.0b013e31822d74d0
Yu JX, Huang XF, Lv WM, Ye CS, Peng XZ, Zhang H, Xiao LB, Wang SM (2009) Combination of stromal-derived factor-1alpha and vascular endothelial growth factor gene-modified endothelial progenitor cells is more effective for ischemic neovascularization. J Vasc Surg 50(3):608–616. https://doi.org/10.1016/j.jvs.2009.05.049
Yu Y, Alkhawaji A, Ding Y, Mei J (2016) Decellularized scaffolds in regenerative medicine. Oncotarget 7(36):58671–58683. https://doi.org/10.18632/oncotarget.10945
Zhang YX, Yuan MZ, Cheng L, Lin LZ, Du HW, Chen RH, Liu N (2015) Treadmill exercise enhances therapeutic potency of transplanted bone mesenchymal stem cells in cerebral ischemic rats via anti-apoptotic effects. BMC Neurosci 16:56. https://doi.org/10.1186/s12868-015-0196-9
Zhao L, Chen S, Yang P, Cao H, Li L (2019) The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther 10(1):182. https://doi.org/10.1186/s13287-019-1287-9
Acknowledgments
The authors gratefully acknowledge helpful comments and review from Dr. Jarrod Call. Additionally, we acknowledge the work of many colleagues who contributed to the improvement of the understanding of the current state of the field for regenerative rehabilitation, across our collective goals for the advancement of new solutions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
This work was supported by funding from the Department of Defense, Clinical & Rehabilitative Medicine Research Program: W81XWH-18-1-0710 and W81XWH-20-1-0885 (SMG) and National Institutes of Health T32-AR050938 (CJRP). Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense or National Institutes of Health.
Declaration of Interests
The authors declare that they have no potential or actual conflict of interest.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Raymond-Pope, C.J., Hoffman, D.B., Saunders, D.L., Greising, S.M. (2022). Historical Perspectives of Regenerative Rehabilitation: Recovering and Restoring Functional Capacity. In: Greising, S.M., Call, J.A. (eds) Regenerative Rehabilitation. Physiology in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-95884-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-95884-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-95883-1
Online ISBN: 978-3-030-95884-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)