Abstract
The purpose of present study was to evaluate active mobilization effect of mesenchymal stem cells (MSCs) into injured tissues after intraarticular injection of MSCs, and to evaluate their contribution to tissue regeneration. MSCs, which were obtained from green fluorescent protein (GFP) transgenic Sprague–Dawley (SD) rat and cultivated, were injected into normal SD rats in which multiple tissues had been injured including anterior cruciate ligament (ACL), medial meniscus, and articular cartilage of the femoral condyles. At 4 weeks after injection of MSCs, fluorescent microscopic observation, immunohistochemical or histological examinations were performed to evaluate mobilization of MSCs into injured tissue and their contribution to tissue regeneration. In the group of 1 × 106 MSCs injection, GFP positive cells could mobilize into the injured ACL alone in all 8 knees. In the group of 1 × 107 MSCs injection, GFP positive cells were observed in the injured site of ACL in all 8 knees and in the injured site of medial meniscus and cartilage of femoral condyles in 6 of 8 knees. More interestingly, extracellular matrix stained by toluidine blue was present around GFP positive cells in the injured femoral condyles cartilage and medial meniscus, indicating tissue regeneration. Intraarticularly injected MSCs could mobilize into the injured tissues, and probably contributed to tissue regeneration. This study demonstrated the possibility of intraarticular injection of MSCs for the treatment of intraarticular tissue injuries including ACL, meniscus, or cartilage. If this treatment option is established, it can be minimally invasive compared to conventional surgeries for these tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute trauma-induced injury of cruciate ligaments and meniscus in the knee joint is common for the orthopedic surgeon. If the injuries remain untreated, articular cartilage damage often occurs. Therefore, injury of the cruciate ligaments and meniscus is sometimes subject to operation. In particular, it is well known that articular cartilage has very limited natural healing potential and cartilage injury of a loaded area evolves to the osteoarthritis of the knee joint [11]. Recently, for anterior cruciate ligament (ACL) or meniscal injuries, arthroscopically assisted surgeries are performed. For cartilage injuries, there are many treatments such as osteochondral autograft [1, 3, 12] and bone marrow stimulation [2, 20, 21] or autologous chondrocyte implantation using a monolayer culture system to treat cartilage defect [4, 13, 18]. We have developed transplantation of tissue-engineered cartilage, which have been made ex vivo in a three-dimensional culture system using atelocollagen gel as a scaffold, and demonstrated short-time successful results [10, 15, 16]. However, each procedure for ACL, meniscus, or cartilage injuries has its own disadvantages with invasive surgery or anesthesia. If these intraarticular tissue injuries can be treated by the injection of so-called stem cells that can differentiate into various tissues, it can be less invasive treatment requiring less or no surgical procedures.
Currently, mesenchymal stem cells (MSCs) from bone marrow are well known to have a capacity to differentiate into various types of lineage such as cartilage, bone, and adipose tissue [19]. There is significant interest in MSCs as cell sources of tissue engineering [5]. More interestingly, when the organ is injured, transplanted bone marrow cells are mobilized into the injured organ to repair the organ [17]. Based on this fact, we hypothesized that intraarticularly injected MSCs can mobilize into the injured tissue and contribute to injured tissue regeneration.
The purpose of the present study was to evaluate the active mobilization effect of MSCs into injured tissues after intraarticular injection of MSCs, and to evaluate their contribution to tissue regeneration.
Materials and methods
The research protocol of this experiment was reviewed and approved by the ethical committee of our university.
Preparation of MSCs from Green rat
Eight-week-old male enhanced green fluorescent protein (GFP) definite expressed transgenic Sprague–Dawley (SD) rats (Green rat, Japan SLC Co., Ltd, Hamamatsu, Japan) were used for this study (Fig. 1Aa). Most cells in the Green rat contain GFP and luminescence of these cells can be observed by a fluorescent microscope as long as the cells survive. If luminescence of cells is weak, we can also perform immunostaining for GFP. Therefore, it is quite easy to detect the GFP positive cells under a fluorescent microscope or with immunostaining when the GFP positive cells are grafted into a GFP negative rat.
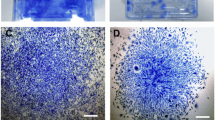
Fluorescent microscopic view, cell proliferation, and chondrogenesis of MSCs isolated from Green rat. A a Green rat. b Fluorescent microscopic view of MSCs from Green rat. Magnification 100-fold. The scale indicates 50 μm. B Cell proliferation of GFP positive MSCs (GFP-MSCs). MSCs were isolated from bone marrow aspirates of Green rats, and were cultured with 10% FBS–DMEM. Population doublings level (PDL) is regarded as zero for culture starting immediately after the primary culture of cells, and calculated to increase according to the equation—log2{(the number of collected cells)/(the number of seeded cells)}. C Evaluation of chondrogenesis of Green rat MSCs using RT-PCR analysis. In D(+) group (the pellet cultured with chondrogenic medium containing TGF-β3 and dexamethasone), PCR products of aggrecan, type II collagen, and GAPDH appeared at 322, 448, and 449 bp, respectively, whereas in D(−) group (cultured with the medium not contain TGF-β3 and dexamethasone), those of products did not appear. The data shown are typical of three independent experiments (n = 3)
Under intraperitoneal anesthesia with sodium pentobarbital (35 mg/kg), the proximal medial surface of each tibia was exposed through a small incision. Two milliliters of bone marrow was aspirated with a 23-gauge needle that was fastened to a 10-ml syringe containing 0.2 ml of heparin (1,000 units/ml). The aspirates were washed twice with Dulbecco’s phosphate-buffered saline without calcium and magnesium [PBS(−), Gibco, Grand Island, NY, USA] and centrifuged at 1,500 rpm for 5 min. The supernatant was discarded. The cells were suspended in culture media consisting of Dulbecco’s modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS, Sigma-Aldrich Corp., St Louis, MO, USA) and penicillin–streptomycin–fungizone (Bio-Whittaker, Walkersville, MD, USA), and were seeded on 100-mm culture dishes and incubated in a humidified 5% CO2–95% air atmosphere at 37°C. The medium was first changed 5 days after culture and every 3 days thereafter. Ten to 14 days after incubation, the cells usually proliferated up to confluence. Then the adherent cells were released from the dishes by exposure to 0.25% trypsin and 0.02% EDTA, and rinsed twice with culture medium. The cells were counted in a hemocytometer and seeded at 2 × 105 cells on each dish. Before the cells were approaching confluence again, the adherent cells were harvested with the same methods, then were detected the adherent MSCs on the culture dish, a fluorescent microscope (508 nm of wavelength, with activation at 489 nm of wavelength, Leica Microsystem AG, Wetzlar, Germany) was used. Finally, to use MSCs injection, the cells were suspended in saline at 1 × 107 or 1 × 108 cells/ml.
Chondrogenic differentiation of MSCs of Green rat
Before intraarticular injection of MSCs, the chondrogenic differentiation potential of MSCs of Green rat was investigated. A modified version of Johnstone’s pellet culture system [9] was used to induce chondrogenesis. MSCs (2 × 105 cells) were placed in a 15-ml polypropylene tube (Greiner BioOne, Frickenhausen, Germany) and centrifuged to form a pellet. The pellet was cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2 in 1 ml of chondrogenic medium containing high-glucose DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10 ng/ml transforming growth factor (TGF)-β3 (Sigma, St Louis, MO, USA), 10−7 M dexamethasone (Sigma, St Louis, MO, USA), 50 μg/ml ascorbic acid-2-phosphate sesquimagnesium salt (Sigma, St Louis, MO, USA), 40 μg/ml l-proline (Nacalai tesque, Kyoto, Japan), ITS-A supplement (Invitrogen, Carlsbad, CA, USA; 10 μg/ml insulin, 6.7 ng/ml sodium selenite, 5.5 μg/ml transferrin, 110 μg/ml sodium pyruvate), and 1.25 mg/ml bovine serum albumin (Sigma, St Louis, MO, USA). As a negative control, MSCs were cultured under the similar conditions, but TGF-β3 and dexamethasone were not added to the medium. The medium was changed every 3–4 days. To evaluate chondrogenesis of MSCs, after 21 days of culture, reverse transcription-polymerase chain reaction (RT-PCR) analysis using type II collagen and aggrecan primers was performed.
RNA preparation and RT-PCR analysis
Total RNA was prepared from the pellets of Green rat MSCs (passage 4) using the RNeasy micro kit (Qiagen, Tokyo, Japan). Prepared RNA was converted to cDNA using the Superscript™ First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. PCR was performed in a Minicycler (PTC-150, Bio-Rad, Hercules, CA). PCR amplification conditions for aggrecan, type II collagen, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were as follows: 94°C for 2 min followed by 35 cycles of 94°C for 15 s, 56°C (or for GAPDH: 58°C) for 30 s, and 68°C for 1 min. The reaction products were resolved by electrophoresis on a 2% agarose gel and visualized with ethidium bromide. The GAPDH primers were designed ourselves, the aggrecan and type II collagen primers were as described [6]. The primers were as follows:
aggrecan (forward): 5-TAGAGAAGAAGAGGGGTTAGG-3,
aggrecan (reverse): 5-AGCAGTAGGAGCCAGGGTTAT-3,
type II collagen (forward): 5-GAAGCACATCTGGTTTGGAG-3,
type II collagen (reverse): 5-TTGGGGTTGAGGGTTTTACA-3,
GAPDH (forward): 5-GCCAAAAGGGTCATCATCTC-3,
GAPDH (reverse): 5-GCCTGCTTCACCACCTTCTT-3.
Surgical protocol and intraarticular injection
Thirty-two normal SD rats (12-week-old, male, Japan SLC; they do not express GFP) were anesthetized with sodium pentobarbital (35 mg/kg, i.p.). After both knee joints were exposed through medial parapatellar incision, ACL, medial meniscus, and femoral condyles cartilage were injured with a surgical knife. In the ACL, a transverse incision, which is perpendicular to ligament direction, was created in the middle of ACL. Meniscal injury was created in the central avascular area of the meniscus. Cartilage injury was created in the weight bearing area of femoral condyle. At 3 days after the surgery, in MSCs injection group, 100 μl cell suspension containing 1 × 106 or 1 × 107 cells of Green rat MSCs was injected into the injured joint via a medial approach with a 26-gauge needle under general anesthetization using sodium pentobarbital. We injected MSCs into 8 knees for each cell-transplanted group. The contralateral knees were served as a control in which only 100 μl saline was injected. On the other hand, in the sham-operation group, the knee joints were exposed with the same incision, and were closed without operation for tissue injury. Three days after surgery, 100 μl of the cell suspension containing 1 × 106 or 1 × 107 cells of GFP labeled MSCs was injected with same method above under anesthesia. We injected MSCs in 8 knees for each sham-operation group. All rats moved freely in the cage after surgery.
Fluorescent microscopic observation and immunohistochemical or histological examination
To evaluate whether injected MSCs could mobilize into the injured tissue at 4 weeks after MSCs injection, SD rats were sacrificed with an intraperitoneal injection of sodium pentobarbital at 4 weeks after MSCs injection. After both knee joints were exposed, ACL, medial menisci, and femoral condyles were obtained in each group. The tissues were fixed in 4% paraformaldehyde and decalcified in EDTA. They were embedded in plastic legin (Technovit 8100®, Okenshoji Co., Ltd, Tokyo, Japan) and cut into 5-μm sections. GFP positive cells on unstained slices were observed under a fluorescent microscope. To detect the injected GFP positive cells, immunohistochemical examination using anti-GFP monoclonal antibody (JL-8, BD Biosciences, Clontech, Tokyo, Japan) was also performed. Initially, the GFP luminescence was planned to be observed with a fluorescence microscope. However, because the wavelength of the marrow cells from SD rats was often similar to that of GFP, the implanted MSCs were observed using anti-GFP monoclonal antibody immunostaining in this study, when detection of GFP positive cell was difficult or obscure. For histological examination, the sections were stained with hematoxylin–eosin (HE) or toluidine blue.
Results
Morphology, expansion, and chondrogenic differentiation of MSCs
Mesenchymal stem cells of Green rats on culture dishes had green fluorescence (Fig. 1Ab), and they were proliferating at least up to 35 days, maintaining a spindle shape (Fig. 1B). To obtain large number of cells for MSCs injection, we decided to use MSCs up to passage 4. RT-PCR analysis of the pellet cultures of Green rat MSCs demonstrated that type II collagen and aggrecan mRNA expression was able to be detected in the pellets cultured with chondrogenic differentiation medium (Fig. 1C, D + ), while in the pellets cultured without TGF-β3 and dexamethasone, mRNA expression of those markers could not be detected (Fig. 1C, D −). Thus, these results obtained by RT-PCR analysis demonstrated that Green rat MSCs have chondrogenic differentiation potential.
Fluorescent microscopic observation and immunohistochemical or histological examination
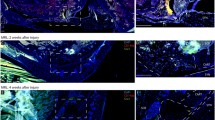
In the group in which 1 × 106 MSCs were injected into the tissue injured knee joints, examination with the fluorescent microscope and HE staining showed that injected MSCs were able to mobilize in the injured portion of the ACLs in all 8 knees (Fig. 2c). In contrast, injected MSCs were not able to mobilize in cartilage of the femoral condyles and the medial menisci at 8 knees (Fig. 2a, b, e, f). There was no remarkable tissue regeneration in this group.
Typical of fluorescent microscopic views (a–d) and HE staining (e–g) in the group in which 1 × 106 MSCs were injected into the tissue injured knee joints. GFP positive cells could not be observed in the injured portion of medial femoral condyles (a) and medial menisci (b) in all 8 knees. GFP positive cells could mobilize in the injured portion of the ACL in all 8 knees (c, square of dotted line). d An enlarged image of square of dotted line. a, c, e, g Magnification 200-fold. The scale indicates 50 μm. b, f Magnification 100-fold. The scale indicates 100 μm
In the group in which 1 × 106 MSCs were injected in the sham-operation knee, examination with the fluorescent microscope showed that GFP positive cells were not observed on the medial femoral condyles cartilage, medial menisci, and ACL with the fluorescence microscope (data not shown).
In the group of 1 × 107 MSCs were injected into the tissue injured knee joints, GFP positive cells were observed in the injured portion of the femoral condyles cartilage (Fig. 3a), medial menisci (Fig. 3c, arrows) in 6 of 8 knees and the ACL (data not shown) in all 8 knees, respectively. Moreover, extracellular matrix stained by toluidine blue was present around GFP positive cells in the injured portion of the femoral condyles cartilage and medial menisci (Fig. 3b, d).
Typical of fluorescent microscopic views (a), immunohistochemical staining using anti-GFP antibody (c) and toluidine blue staining (b, d) in the group in which 1 × 107 MSCs were injected into the tissue injured knee joints. GFP positive cells were observed in the injured portion of the medial femoral condyles cartilage (a), medial menisci (c, the arrows show GFP positive cells). Extracellular matrix stained by toluidine blue was present around GFP positive cells in the injured portion of the medial femoral condyles cartilage (b) and medial menisci (d). a, b, c, d Magnification 400-fold. a, c The scale indicates 25 μm. b, d The scale indicates 50 μm
In the group of 1 × 107 MSCs were injected in the sham-operation knee, GFP positive cells were sparsely adhered to normal ACL and medial meniscus in 5 of 8 knees (data not shown).
In the groups in which multiple tissue injuries were created, but any MSCs were not injected, no GFP positive cells were detected in the injured ACL, meniscus, or cartilage of femoral condyle, showing no apparent tissue regeneration at the injured site.
Unexpectedly, free bodies of scar tissue with fibroblastic-like cells were observed in the knee joints in 5 of 8 knees (data not shown) in the tissue injured knee and sham-operated knee in which 1 × 107 MSCs were injected, although scar tissues were not observed in the group of 1 × 106 MSCs injection.
Discussion
In this study, after multiple tissue injuries including ACL, medial meniscus, and cartilage of femoral condyles had been created, ex vivo expanded MSCs were injected into the joints. Results demonstrated the mobilization of MSCs into the injured site of those tissues. More interestingly, those mobilized MSCs contributed to the tissue regeneration, showing extracellular matrix synthesis around the cells. Especially, mobilization of MSCs and tissue regeneration was clearly observed when 1 × 107 MSCs were injected into the joints of SD rats.
Orthopedic surgeons often have a chance to treat patients with ACL injuries, meniscal injuries, or cartilage injuries in the knee joints. Because those tissues have limited healing potential due to their own anatomical characteristics, surgical treatments are often indicated after adequate periods of conservative treatments. There have been many surgical treatments for ACL, meniscus, or cartilage injuries, and favorable clinical results have been reported. However, even though we can choose any arthroscopically assisted surgical treatments, some incisions and lumbar or general anesthesia is necessary.
One simple potential strategy for tissue repair is the transplantation of pluripotent stem cells such as MSCs to the injured site by intraarticular injection of the cells to the knee joint for the repair of ACL or meniscal injuries or cartilage defects.
Among several pluripotent stem cells, MSCs from bone marrow have been in the spotlight as cell sources for cartilage repair. MSCs are the cell population of undifferentiated cells isolated from adult tissue, which have the capacity to differentiate into mesodermal lineages, such as bone, cartilage, fat, muscle, or other tissues. MSCs from bone marrow can be expanded in culture and differentiated into the desired lineage with the application of specific growth factors or bioactive molecules. MSCs have several potential advantages for tissue regeneration. We believe that these MSCs from bone marrow are the most clinically promising stem cells [5, 19].
In this study, we confirmed chondrogenic-differentiating potential of MSCs from Green rats as shown in RT-PCR evaluation for type II collagen and aggrecan. As for the differentiation of MSCs to different lineages and their contribution for tissue regeneration, we have reported several studies. Ito et al. transplanted MSCs hybridized with hydroxyapatite to the tibias of SD rats without co-culture. They demonstrated that excellent bone formation and differentiation of transplanted MSCs of Green rats into osteoblast-like cells, showing their osteogenic potential [7]. Izuta et al. reported that using an organ culture model, transplanted MSCs from Green rats into meniscal defect could survive and proliferate with synthesis of abundant extracellular matrix around the cells [8]. Yamasaki et al. seeded MSCs from Green rats into freeze-thawed normal meniscus as a scaffold, and demonstrated that transplanted MSCs survived and contributed to synthesis of extracellular matrix [24]. Thus, transplantation of MSCs has been proven to be an effective method for tissue regeneration.
There is an interesting phenomenon about bone marrow cell transplantation for tissue repair. Petersen et al. transplanted conventional bone marrow of male rat to syngeneic female rat with an injured liver. They reported that a proportion of the regenerated hepatic cells were shown to be donor bone marrow-derived [17]. Possible explanation for this fact is that some kind of signal, such as cytokines from the injured site regulated the transplanted cells and affect on a differentiation of the cell into the site-specific cells.
We also confirmed cell mobilization to injured ACL, or meniscus, or cartilage when MSCs were transplanted in the joints. When 1 × 107 MSCs were injected into the joints, transplanted cells mobilized more effectively to the injured tissue compared to injection of 1 × 106 MSCs.
Regarding the contribution of MSCs for tissue regeneration, there have been several studies. Toma et al. demonstrated that directly injected MSCs into the heart could differentiate into myocardial cell [22]. Wakitani et al. reported that MSCs embedded in collagen gel was transplanted to 3 mm × 6 mm of full-thickness cartilage defect of rabbits, and excellent repair of the defected cartilage was observed successfully [23]. These results suggest that MSCs could differentiate into several lineages depended on transplanted site, namely, have in situ differentiation potential in the transplanted site. In this study, MSCs were injected into knee joint in which ACL, meniscus, and cartilage injuries had been created. We demonstrated that transplanted MSCs could mobilize in the injured tissues and generate extracellular matrix stained by toluidine blue in the cartilage and the meniscus, showing in situ differentiation of MSCs. To elucidate the mobilization mechanism of MSCs, detection of cytokines released from the injured site or expression analysis of receptors on MSCs received signals of cytokines will be definitely necessary in future studies.
Recently, Murphy et al. injected 1 × 107 cells of MSCs into the caprine knee joint of osteoarthritis induced by complete excision of the medial meniscus and resection of the ACL [14]. They observed that at 6 weeks after the injection, degeneration of the articular cartilage, osteophytic remodeling, and subchondral sclerosis were reduced compared with control group. Our results is consistent with their results regarding MSCs’ contribution for injured tissue regeneration, although we have focused acute trauma, such as ACL, meniscus, or cartilage injuries rather than osteoarthritis. Moreover and difference from their study, we made a group in which MSCs were transplanted into sham-operation group to evaluate the mobilization effect of MSCs to injured tissue.
The most problematic phenomenon was the fact that injected 1 × 107 MSCs generated free bodies of scar tissue in the joint (data not shown). Although an apparent explanation for generation of those scar tissues is not clearly demonstrated, it may indicate adverse effect of transplantation of large number of MSCs. Since generation of scar tissue leads to arthrofibrosis that may cause considerable dysfunction of the joints, further investigations will be definitely indispensable for actual clinical application. To resolve this adverse effect, optimal cell number of injected MSCs will need to be determined in future studies. Other more appropriate cell delivery systems, in which MSCs can be mobilized to an injured side will be necessary.
Conclusions
A large number of MSCs injected into the multiple tissue injured knee joint could mobilize to the injured area, contributing to tissue regeneration. This finding shows that the MSCs injection can be a useful option for repair of intraarticular injuries of the joints.
References
Berlet GC, Marscia A, Miniaci A (1999) Treatment of unstable osteochondritis dissecans lesions of the knee using autogenous osteochondral grafts (mosaicplasty). Arthroscopy 15:312–316
Blevins FT, Steadman JR, Rodrigo JJ, Silliman J (1998) Treatment of articular defects in athletes. An analysis of functional outcome and lesion appearance. Orthopedics 21:761–768
Bobic V (1996) Arthroscopic osteochondral autograft transplantation in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 3:262–264
Brittberg M, Lindahl A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Caplan AI, Bruder SP (2001) Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med 7:259–264
Hanada K, Solchaga LA, Caplan AI, Hering TM, Goldberg VM, Yoo JU, Johnstone B (2001) BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J Cell Biochem 81:284–294
Ito Y, Tanaka N, Fujimoto Y, Yasunaga Y, Ishida O, Agung M, Ochi M (2004) Bone formation using novel interconnected porous calcium hydroxyapatite ceramic hybridized with cultured marrow stromal stem cells derived from Green rat. J Biomed Mater Res A 69:454–461
Izuta Y, Ochi M, Adachi N, Deie M, Yamasaki T, Shinomiya R (2005) Meniscal repair using bone marrow-derived mesenchymal stem cells: experimental study using green fluorescent protein transgenic rats. Knee 12:217–223
Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU (1998) In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238:265–272
Katsube K, Ochi M, Uchio Y, Maniwa S, Matsusaki M, Tobita M, Iwasa J (2000) Repair of articular cartilage defects with cultured chondrocytes in atelocollagen gel. Comparison with cultured chondrocytes in suspension. Arch Orthop Trauma Surg 120:121–127
Mankin HJ (1982) The response of articular cartilage to mechanical injury. J Bone Joint Surg Am 64:460–466
Matsusue Y, Yamamoto T, Hama H (1993) Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy 9:318–321
Minas T (2001) Autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop Relat Res 391(Suppl):349S–361S
Murphy JM, Fink DJ, Hunziker EB, Barry FP (2003) Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 48:3464–3474
Ochi M, Uchio Y, Tobita M, Kuriwaka M (2001) Current concepts in tissue engineering technique repair of cartilage defect. Artif Organs 25:172–179
Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J (2002) Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defect of the knee. J Bone Joint Surg Br 84:571–578
Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP (1999) Bone marrow as a potential source of hepatic oval cells. Science 284:1168–1170
Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A (2000) Two- to 9-year outcome after autologous chondrocytes transplantation of the knee. Clin Orthop Relat Res 374:212–234
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Steadman RJ, Rodkey WG, Rodrigo JJ (2001) Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res 391(Suppl.):362S–369S
Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG (2003) Outcomes of microfracture for traumatic defects of the knee. Average 11-year follow-up. Arthroscopy 19:477–484
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD (2002) Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105:93–98
Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM (1994) Mesenchymal cells-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am 76:579–592
Yamasaki T, Deie M, Shinomiya R, Izuta Y, Yasunaga Y, Yanada S, Sharman P, Ochi M (2005) Meniscal regeneration using tissue engineering with a scaffold derived from a rat meniscus and mesenchymal stromal cells derived from rat bone marrow. J Biomed Mater Res A 75:23–30
Acknowledgments
We thank Dr Tomohiro Nakano of Hiroshima University, for their technical assistance with histological analysis and encouragement. The present study was supported in part by Grants-in-Aid for Scientific Research (No. 16209045) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The reported experiments comply with the current laws of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agung, M., Ochi, M., Yanada, S. et al. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc 14, 1307–1314 (2006). https://doi.org/10.1007/s00167-006-0124-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-006-0124-8