Abstract
Pediatric septic arthritis is a surgical emergency that requires multidisciplinary diagnosis and management. A high clinical suspicion with confirmatory laboratory and diagnostic imaging followed by prompt surgical decompression of the joint with irrigation and debridement alongside antibiotic administration is necessary to prevent long-term morbidity. The use of validated diagnostic algorithms, contemporary microbiologic testing capabilities, and advanced imaging can narrow a broad differential diagnosis and optimize time to treatment. Utilizing evidence-based treatment guidelines can minimize unnecessary surgical intervention and optimize outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords

Introduction and Epidemiology
Osteoarticular infections are a source of significant morbidity within the pediatric population. Expeditious diagnosis and multidisciplinary management are of paramount importance in an effort to mitigate complications and disability. Pediatric osteoarticular infections manifest as osteomyelitis and septic arthritis. Septic arthritis is defined as an acute infection of the synovial joints leading to inflammation and destruction of the affected joint structures, cartilage, and potential for extension to the metaphysis leading to secondary osteomyelitis in some joints. Therefore, mitigation of these complications is predicated upon judicious diagnosis, expeditious operative intervention, and antibiotic administration.
The literature provides a variable incidence of pediatric septic arthritis, which ranges from 4 to 12 per 100,000 [1,2,3,4,5,6,7,8]. The vast majority of these studies are retrospective case-control series with relatively small sample sizes [1,2,3,4,5,6,7,8] which may explain the wide variation in reported incidences. In contrast, Safdieh et al. reported epidemiologic data from a nationwide inpatient database analysis by querying hospital billing and diagnosis coding [6]. The authors demonstrated an incidence of 5.2 per 100,000 for septic arthritis [6]. This was similar to another inpatient database analysis that demonstrated a septic arthritis incidence of 4.2 per 100,000 [5]. The majority of studies suggest a static trend in the incidence of pediatric septic arthritis over time [1,2,3,4,5,6,7,8]. In resource-poor regions, the incidence of septic arthritis has been reported to be higher up to 20 per 100,000 [9].
Several risk factors and demographic associations have been noted in the literature. Okubo et al. demonstrated that pediatric septic arthritis was more prevalent among children who are male, white or black, and between ages 0 and 4 years [5]. In addition, low socioeconomic status seems to be associated with an increased prevalence of septic arthritis [5]. Furthermore, older children (10–14 years of age) were associated with increased rate of complications and sequelae such as osteomyelitis and bacteremia/septicemia [5]. The vast majority of septic arthritis cases involve the hip, knee, and ankles, although septic arthritis can be manifested in any synovial joint. Other risk factors for septic arthritis include immunodeficiency syndromes, sickle cell disease, prematurity, respiratory distress syndrome, and low birth weight [10].
Lyme disease is also a common entity that can cause infectious arthritis in pediatric patients. Lyme disease is classically a multisystem tick-borne disease caused by the spirochete, Borrelia burgdorferi, and is particularly common in the northeastern region of the United States. Lyme disease is noted to be the most common tick-borne disease in the United States [11]. It is associated with a bimodal age distribution among pediatric patients from ages 5–9 years and adults from ages 55–59 years [11]. According to the Centers for Disease Control and Prevention (CDC), the geographic distribution of high incidence areas with Lyme disease appears to be expanding and, therefore, should be a more prominent differential diagnosis alongside septic arthritis [11].
Pathology and Microbiology
Primary septic arthritis is most commonly the result of hematogenous spread. Local inoculation can also occur from joint penetration and trauma. An array of inflammatory biochemical processes is elicited following bacterial joint infection. Intra-articular bacteria release toxins that trigger a cytokine cascade from the synovial tissue . The resulting inflammatory acute phase response attracts leukocytes which produce a viscous cycle of cytokine-induced damaged via the release of collagenases and peptidases that can lead to cartilage damage within 8 hours of infection [12,13,14,15,16]. This aggressive response represents an orthopedic surgical emergency and can be quelled with expeditious source control via surgical intervention and antibiotic administration.
Secondarily, septic arthritis can occur from bacterial translocation from an adjacent source. The synovial joint capsule of the shoulders, elbows, hips, and ankles also contain the metaphysis. In these joints, extra-cortical extension of a metaphyseal osteomyelitis can seed the capsular joint space. Conversely, primary septic arthritis can cause a metaphyseal osteomyelitis via spread through the transphyseal network of vessels. This latter mode of metaphyseal spread is more common during the first 18 months of age [17]. Merlini et al. reviewed contrast-enhanced MRI studies and demonstrated that the transphyseal network of vessels can potentiate translocation of bacteria and cause concomitant hip osteomyelitis and septic arthritis in patients less than 18 months of age, which was a previous hypothesis proposed by Alderson et al.’s animal model study [17, 18].
Across all age groups, Staphylococcus aureus is the most commonly found bacterial cause of septic arthritis, with increasing rates of community-acquired methicillin-resistant Staphylococcus aureus (MRSA) [17] (Figs. 12.1 and 12.2). MRSA genotypes with two genes, Panton-Valentine leukocidin (pvl) and fibronectin-binding factor (fnbB), produce an aggressive form of septic arthritis [19]. MRSA genotypes that stem from an USA300 clone often carry both of these genes. Carrillo-Marquez et al. demonstrated that MRSA-USA300 is the predominant MRSA genotype that causes septic arthritis in children – 81% of MRSA infections [20]. This genotype is associated with longer duration of fever, higher incidence of bacteremia, osteomyelitis, thrombophlebitis, pulmonary embolism, and longer hospital stay [19, 20]. The severity of infection associated with MRSA-USA300 is linked to the gene products of pvl and fnbB. Pvl cytotoxin fenestrates the leukocyte cell membrane and instigates cell lysis, which releases more cytokines and fuels the inflammatory viscous cycle. This is aided by the function of fnbB, which encodes proteins that facilitate enhanced bacterial invasion, adhesion, and biofilm formation [19, 20].
Bacterial causes of osteoarticular infection [17]
This illustration depicts a three-dimensional (3D) computer-generated image of a group of methicillin-resistant Staphylococcus aureus (MRSA) bacteria, which were arranged in a cluster. ID # 19059, Center for Disease Control USA 2013/Antibiotic Resistance Coordination and Strategy Unit/Courtesy Jennifer Oosthuizen - Medical Illustrator. (With permission)
Certain pediatric populations are associated with other types of bacterial septic arthritis (Table 12.1). Namely, in infants, Group B Streptococcus is prevalent and is a common cause of many osteoarticular infections [21, 22]. Neisseria gonorrhoeae must be considered in sexual assault victims or in sexually active adolescents presenting with signs of septic arthritis. In addition, Kingella kingae is increasingly found in culture and polymerase chain reaction (PCR) analysis in septic arthritis cases in children younger than 4 years of age [23, 24]. Furthermore, Salmonella associated septic arthritis is more frequent in countries such as Zambia, Kenya, and Malawi where there is a higher prevalence of sickle cell disease and malaria [9].
The pathophysiology of Lyme arthritis has been extensively studied. Arthritis is the most prominent feature of Lyme disease [11]. Following a deer tick bite, B. burgdorferi modifies surface lipoproteins that promote infection to the synovial tissue and facilitate inflammation of the joint [25] (Fig. 12.3a, b). Acutely, arthritis results from synovial inflammation , preponderance of neutrophils, immune complex accumulation, and cytokine release [25]. Several host genetic factors have been studied that may predispose and facilitate progressive and refractory arthritis [26]. Following antibiotic treatment for acute Lyme arthritis , some patients can have recurrent polyarticular disease. Steere et al. have elucidated three main theories for this phenomenon: T-cell epitope mimicry, persistent or recurrent infection, and bystander activation (persistent immune reactivity despite eradication of B. burgdorferi) [25]. Even after antibiotic treatment, B. burgdorferi can remain intracellular and be detected with culture and PCR testing [27, 28].
(a) Photograph showing the blacklegged tick, Ixodes pacificus, known vector for the zoonotic spirochetal bacteria, Borrelia burgdorferi , which is the pathogen responsible for causing Lyme disease. ID # 7663/Center for Disease Control USA 2005/Courtesy James Gathany; William L. Nicholson, Ph.D. (With permission). (b) This digitally colorized scanning electron microscopic (SEM) image depicts a grouping of gram-negative, anaerobic, Borrelia burgdorferi bacteria, which had been derived from a pure culture. ID # 13176/Center for Disease Control USA 2011/Claudia Molins/Courtesy: Janice Haney Carr. (With permission)
Clinical Presentation and Examination
Septic arthritis is most commonly a monoarticular disease characterized by acute onset of pain, swelling, and joint immobility (pseudoparalysis). In general, fever is also reported. Initially, each patient must be assessed for associated life-threatening systemic conditions. Patients with fever, tachycardia, tachypnea, and hypotension should be investigated for sepsis. In addition, patients with nuchal rigidity should be concomitantly evaluated for meningitis. Similarly, auscultation of the heart and lungs may prompt evaluation for pneumonia, if there are abnormal breath sounds, or infectious endocarditis, if a cardiac murmur is present.
Initial diagnosis of septic arthritis in the emergency department is difficult. Vardiabasis et al. demonstrated that the initial emergency department diagnosis of septic arthritis was consistent only 42% of the time with the final definitive diagnosis [29]. This is particularly true in younger children who are non-ambulatory and/or non-verbal. Infants can have general malaise, distress, and pain upon moving the affected joint. Infants may not have fever or other systemic signs of infection. In the young child, diagnosis is also difficult as they can have distress with diaper changes, refusal to eat, and persistent crying. Patients of ambulatory age may refuse to bear weight and favor the contralateral extremity.
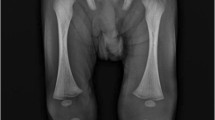
On examination, the affected joint may have swelling, effusion, warmth, and erythema (Fig. 12.4). Any attempt with short arc range of motion will produce pain and distress. In cases with septic hip arthritis, the child will present with pain and limp. In addition, capsular accumulation of fluid or abscess will prompt the child to place the hip joint in flexion, abduction, and external rotation in an effort to maximize intracapsular volume and limit stretching of the joint capsule, which triggers pain. In some instances, hip pain can be referred to the knee – which may be the chief presenting complaint. This is due to nerve innervation of the hip joint by articular branches of the obturator nerve that refer pain to the dermatomal distribution along the anteromedial thigh via cutaneous nerve branches. If increased intracapsular pressure causes a capsular tear, fluid collections can develop in the surrounding musculature, and hip joint instability should be considered.
In addition to septic arthritis, Lyme disease is an important consideration in a child who presents with joint pain and limp, particularly in endemic regions. It must be noted that Lyme can present many months after the initial tick bite and pain can be recurrent [30]. The Centers for Disease Control has provided criteria for clinical case definition along with laboratory diagnostic criteria (Table 12.2) [11]. Aiyer et al. reported on the distribution of joint involvement in patients with Lyme disease and demonstrated that the knee is most commonly involved in vast majority of cases, followed by the ankle (Fig. 12.5) [31]. Steere et al. demonstrated that 80% of patients diagnosed with Lyme disease had arthritis of some degree [32]. Symptoms were migratory and were noted, on average, 2 weeks after the onset of erythema migrans [32] (Fig. 12.6). This was associated with recurrent fatigue which lasted as long as 6 years [32]. The relapsing and remitting nature of these symptoms can distinguish Lyme from that of septic arthritis.
Adapted from case series from Aiyer et al. [31] Distribution of joints involved with Lyme arthritis in 155 patients
This clinical photograph depicts the pathognomonic erythematous rash in the pattern of a bull’s-eye, referred to as erythema migrans. The rash manifested at the site of a tick bite, signifies a case of Lyme disease, caused by the bacterium Borrelia burgdorferi, and transmitted to humans, by the bite of infected blacklegged ticks. ID # 9875 / Center for Disease Control 2007/ James Gathany / Courtesy: James Gathany. (With permission)
Investigations and Differential Diagnosis
Septic arthritis is a clinical diagnosis with laboratory and imaging studies utilized in an adjunctive fashion in an effort to corroborate a high pre-test probability.
Laboratory Studies
In addition to a white blood cell (WBC) count and polymorphonuclear cell-type predominance (left-shift) on the peripheral complete blood count (CBC) study, serum inflammatory markers are elevated in the setting of septic arthritis. Most centers test for acute phase reactants such as C-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR). The standard values for the ESR are dependent upon the testing laboratory and generally peak at 3–5 days within the onset of infection and normalize within 7–10 days after treatment (up to 4–6 weeks). An elevated CRP signifies an acute inflammatory response and peaks within 48 hours of onset of the infection and starts declining within 6 hours of treatment initiation. Pääkkönen et al. demonstrated that if both studies remain normal after a 3-day hospital stay, then septic arthritis is a low probability [33]. However, CRP elevation may not be apparent in some circumstances, particularly in K. kingae infections. Basmaci et al. demonstrated that in 10% of septic arthritis cases with S. aureus or K. kingae, the CRP was less than 10 mg/L [34]. Similarly, Ceroni et al. developed a diagnostic laboratory criterion to differentiate K. kingae septic arthritis from that of S. aureus [35]. The authors demonstrated that the CRP was consistently decreased in the setting of K. kingae infections [35]. CRP is not acutely elevated in cases of osteomyelitis or other chronic infections.
Many diagnostic algorithms have been developed to aid in the diagnosis of septic arthritis and distinguish this entity from transient synovitis [36,37,38,39,40,41,42]. The Kocher criteria was originally described to differentiate hip septic arthritis from transient synovitis of the hip (Table 12.3) [42]. The positive predictive probability was <0.2% for zero variables, 3% for one variable, 40% for two variables, 93.1% for 3 variables, and 99.6% for four variables. When applied to other patient populations, the Kocher criteria demonstrated diminished but good diagnostic positive predictive probabilities [41]. This criterion has been broadly applied to diagnose infections of other joints [42]. Other investigators have studied the utility of CRP and its role within diagnostic algorithms for septic arthritis [36,37,38,39,40]. Caird et al. performed a prospective randomized controlled trial to distinguish pediatric septic arthritis from transient synovitis of the hip and demonstrated that CRP >20 mg/L was a strong independent risk factor and the second-best predictor of septic arthritis [38]. This prospective trial reaffirmed the role of CRP in the diagnostic armamentarium for septic arthritis, as published by previous retrospective literature [36, 37]. CRP can also be utilized to trend the patient’s clinical response to antibiotic treatment and need for additional surgical intervention.
In addition to inflammatory markers, blood cultures should also be drawn prior to the administration of antibiotics to rule out bacteremia.
Imaging
Plain radiographs should be obtained as an initial test for patients who present with joint pain, inability to bear weight, and weakness. Plain radiographs serve to rule out trauma and neoplastic bone or soft tissue disorders. In the setting of septic arthritis, plain radiographs can demonstrate signs of joint space expansion with fluid, i.e., effusion, fat pad signs, or medial joint space widening in the setting of hip or shoulder septic arthritis [40]. The role of plain radiography in the setting of septic arthritis is to rule out other co-existing pathology such as fracture or subacute-chronic osteomyelitis.
Ultrasound has become widely adopted and utilized to diagnose joint effusions in a child suspected of septic arthritis. Most centers have become reliant upon ultrasound to diagnose deep joint effusions such as in the hip or shoulder as a prerequisite to joint aspiration [43]. Ultrasound is expeditious, readily available, and without radiation. In addition, in infants, ultrasound is a reliable imaging modality to assess the status of deep joint structures, such as the cartilaginous ossific nucleus, which cannot be visualized on radiographs in infants. If both hips are imaged, a difference in joint fluid volume >5 mm compared with the contralateral side is considered an indication for aspiration [44] (Fig. 12.7). Ultrasound is associated with a 5% false-negative rate and is suggested to be less reliable in patients with symptoms less than 24-hours or those with polyarticular involvement [45]. A negative ultrasound considerably lowers the probability of septic arthritis [46]. However, adjacent infections such as osteomyelitis and intramuscular infections must be ruled out with magnetic resonance imaging (MRI) if clinical suspicion for an osteoarticular infection is high [46]. In the setting of positive ultrasound findings, along with concerning clinical and laboratory studies , arthrocentesis is warranted. It should be noted that an ultrasound cannot differentiate fluid density between joint purulence and sterile effusions, as seen in the setting of inflammatory arthropathies.
To characterize the full extent of an osteoarticular infection, an MRI is an extremely valuable and indispensable tool (Fig. 12.8). MRI can also be utilized if there is a diagnostic quandary with equivocal ultrasound or laboratory results with high clinical suspicion and pre-test probability. Furthermore, an MRI is effective at identifying concomitant unsuspected adjacent osteoarticular infections [47, 48]. Monsalve et al. reviewed MRI studies of patients with septic arthritis of which 68% had concomitant unsuspected osteomyelitis [47]. As such, some authors have recommended routine MRI studies in patients suspected of septic arthritis in an effort to detect these related adjacent infections [47, 49]. However, other authors have expressed caution and developed algorithms to ensure appropriate use of cross-sectional imaging [50]. Rosenfeld et al. demonstrated that age above 3.6 years, CRP >13.8 mg/L, duration of symptoms >3 days, platelets <314 × 10 cells/μL, and ANC >8.6 × 10 cells/μL were risk factors for adjacent infection in the setting of pediatric septic arthritis [50]. Patients with three or more of these risk factors were associated with an 80% positive predictive value for adjacent osteoarticular infection [50].
In the setting of Lyme disease, Ecklund et al. identified three characteristic MRI findings – myositis, adenopathy , and lack of subcutaneous edema. These findings along with a consistent clinical picture of Lyme arthritis may prevent unnecessary surgical intervention [51].
Arthrocentesis
If the clinical picture and the serum laboratory and radiology studies are concerning for septic arthritis, the next best step in diagnosis is a joint aspiration. The sample should be obtained prior to the initiation of antibiotics. In most cases, aspiration of a superficial joint, such as the knee or elbow, can be performed at bedside with relative ease and accuracy with proper pain control for the child. For deep joints, such as the hip, an ultrasound-assisted arthrocentesis is often helpful and necessary. The joint aspirate sample should be sent to the laboratory expeditiously to evaluate, at a minimum, for gram stain/culture and synovial fluid cell count with differential. If an ultrasound-guided arthrocentesis fails to yield synovial fluid for laboratory analysis, a surgical arthrocentesis is recommended in the operating room under anesthesia. This sample should be sent for immediate analysis to aid in surgical decision-making.
The role of gram stain has been questioned. In a systematic review of the English literature, Kang et al. demonstrated that gram stain alone is only positive for approximately 30% of cases, which increased when the tissue was also cultured [10, 52]. Furthermore, Bram et al. demonstrated that gram stain is a poor screening tool for the detection of septic arthritis and the sensitivity further drops for the detection of gram-negative organisms [52]. As such , a negative gram stain must be taken into clinical context and further work-up must be performed if suspicion for septic arthritis remains high.
PCR studies of synovial fluid or tissue can expedite the identification of the causal organism and is an area of further research [53]. PCR studies are of particular utility if antibiotics were initiated prior to joint fluid aspiration, in which case, gram staining and culture become less worthwhile. In addition, PCR studies for the detection of K. kingae have garnered increased attention and practice [54]. In endemic regions, Lyme titer and PCR should also be obtained.
Synovial fluid total nucleated cell count ≥50,000/mm3 with increased percentage of polymorphonuclear cells (>90%) is classically associated with septic arthritis [55]. Margaretten et al. demonstrated the likelihood ratios for septic arthritis as a function of synovial fluid total nucleated cell count (Table 12.4) [55]. For counts ≥50,000/mm3, the likelihood ratio for septic arthritis is 2.9 (confidence interval (CI), 2.5–3.4) [55]. Despite these findings, the evidence for diagnostic cell count thresholds remains debated and the clinical picture must be given greater weight [56]. For example, Heyworth et al. demonstrated that hip septic arthritis was associated with synovial fluid cell counts of 25,000 to 75,000/mm3 [56]. In 17% of their cases, the synovial fluid WBC values were <50,000/mm3 [56].
Lyme Arthritis
Lyme arthritis and inflammatory arthritis may be polyarticular and migratory and accompany dermatologic findings. Titers for Lyme must be obtained if the patient resides in endemic areas and if the clinical suspicion is high. The CDC provides a diagnostic laboratory algorithm for Lyme disease (Fig. 12.9) [11]. A modified two-stage serologic test is utilized [57]. The initial ELISA or immunofluorescence assay is confirmed with a Western blot analysis. Negative IgG titers rule out Lyme disease [57]. PCR testing of Lyme from synovial fluid has a sensitivity of 80–85%; however, active infection is difficult to determine as the PCR can detect DNA from past infection with persistent B. burgdorferi genetic material [57]. In addition, there is no agreed upon threshold for synovial fluid total nucleated cell counts that define Lyme arthritis. Some authors have noted an average of 25,000 cells/mm3 but can be up to 100,000 cells/ [3, 58].
Differential Diagnosis
Trauma, neoplastic processes, and other osteoarticular infections must be considered in patients who present with joint pain consistent with septic arthritis. For hip pain specifically, Legg-Calve-Perthes disease, slipped capital femoral epiphysis, transient synovitis, osteomyelitis, and local intramuscular abscess can mimic signs of septic arthritis. Furthermore, conditions such as cellulitis, inflammatory arthritis, and hemarthroses must be assessed. To rule out adjacent osteomyelitis in the hip, some authors have recommended ilium and femoral neck bone marrow aspiration during surgical intervention, particularly if the MRI is equivocal [59]. Transient synovitis typically follows a history of an upper respiratory tract infection and is less likely to present with high fever and chills.
Clinical diagnostic rules, such as the Kocher criteria, have been developed and validated to help distinguish septic arthritis from other similar processes such as transient synovitis [42]. In addition, the range of the synovial fluid total nucleated cell count can be indicative of the disease process. In a systematic review of the literature with meta-analysis, Cruz et al. stratified synovial fluid cell counts with 95% confidence intervals (CI) as 5644–15,388 cells/mm3 for transient synovitis, 47,533–64,242 cells/mm3 for Lyme arthritis, and 105,432–260,214 cells/mm3 for septic arthritis [60].
Bockenstedt et al. provide an excellent comparison of various types of arthritis seen pediatric patients (Table 12.5).
Treatment
After establishing a diagnosis of septic arthritis, treatment is twofold and is optimized with multidisciplinary management and coordination with general pediatrics, orthopedic surgery, and infectious disease specialists. Adequate source control of the infection with joint irrigation and debridement is paramount. In addition, determination of the causal organism and subsequent antibiotic sensitivity is necessary for definitive antibiotic treatment. Prior to this, empiric antibiotics should be prescribed based upon evidence-based recommendations with regard to the most causal organism, patient’s age, microbiome of the region, and the patient’s clinical state. Typically, antibiotics should be held until a synovial fluid or tissue culture is obtained in an effort to tailor antibiotics to the causal bacteria – except in situations of sepsis and hemodynamic instability, where empiric broad spectrum antibiotics may be life-saving and should not be delayed.
Medical Intervention
Optimal empiric antibiotic selection for pediatric septic arthritis is predicated mainly upon the age of the patient, the patient’s immunization status (H. influenza), and the prevalence rate of MRSA in the local population. Regions with MRSA prevalence ≥10%, ICU patients, or immunocompromised hosts should be treated empirically for MRSA [61]. It should be noted that if vancomycin and gentamycin are prescribed, a target trough level must be monitored regularly and the antibiotic dosing be adjusted accordingly. In addition, the patient’s blood cell counts and hepatorenal function must be monitored in the setting of antibiotic therapy to assess for toxicity.
The empiric antibiotic treatment plan for children less than 3 months of age is typically vancomycin and gentamicin combination therapy [62, 63]. This regimen will cover MRSA, Group B Streptococcus, and gram-negative pathogens. The optimal dosing, frequency, and monitoring can vary, and a multidisciplinary dosing protocol should be discussed with infectious disease specialists and pharmacy. For older children, a first-generation cephalosporin is generally prescribed – or vancomycin if there is high rate of community-acquired MRSA. It is important to note that cephalosporins also covers K. kingae, whereas vancomycin does not. As such, if both MRSA and K. kingae are prevalent, a cephalosporin should be added along with vancomycin [24]. This is similar for N. gonorrhoeae as well along with the alternative, doxycycline. Following synovial fluid or tissue culture and antibiotic sensitivity, the antibiotics should be tailored appropriately.
The duration of antibiotic treatment is dependent upon how the patient performs clinically. In most cases, a short course of intravenous antibiotic treatment followed by 3 weeks of oral therapy is sufficient for septic arthritis [64]. Complicated septic arthritis may warrant longer intravenous antibiotic treatment. Several studies have assessed the optimal time to switch from intravenous to oral therapy [65,66,67,68,69]. Chou et al. demonstrated good clinical outcomes when oral antibiotics were initiated when a 50% reduction in CRP is observed with intravenous antibiotics [67]. Arnold et al. recommended that a CRP less than 2 mg/DL is an indicator of stopping antibiotic treatment [65]. Ultimately, the patient’s clinical picture will dictate optimal transition time and duration of treatment. The expediated switch to oral therapy is a departure from historic practices of keeping patients on intravenous antibiotics for several weeks, as similar outcomes were noted with both regimens alongside lesser complication profile with oral therapy [68, 69].
In addition to antibiotics, the role of corticosteroids has been assessed as an adjunctive method to reduce morbidity [70, 71]. Farrow et al. performed a systematic review and meta-analysis of both human and animal model trials in the setting of septic arthritis and demonstrated an overall consensus with regard to a reduction in duration of symptoms and inflammatory markers with corticosteroid therapy [70]. In addition, in the animal model data, corticosteroid plus antibiotic therapy was associated with a protective effect on cartilage. These findings were similar to another meta-analysis reported by Qin et al. [71]
For Lyme disease, adequate antibiotic therapy at the time of diagnosis is associated with good outcomes and can mitigate the risk for recurrent migratory arthritis [72]. Lyme arthritis is treated with antibiotics, and surgical intervention is typically not needed. It should be noted, as above, that persistent joint inflammation may be seen for several weeks or months after treatment. Clinical guidelines from the Infectious Diseases Society of America recommend 28 days of one of several oral antibiotics, typically doxycycline or a cephalosporin such as cefuroxime [73]. Dosing, need for intravenous antibiotics, and additional duration of therapy should be evaluated by infectious disease specialists [73].
Surgical Intervention
Septic arthritis represents a true orthopedic surgical urgency for which an irrigation and debridement of the infected joint is the cornerstone of treatment. After diagnosis and imaging to characterize the extent of infection, surgical intervention should be performed expeditiously. In the setting of hemodynamic instability, emergent surgical intervention is necessary for source control.
The type of surgical intervention, either an open arthrotomy or arthroscopic decompression, should be based upon surgeon comfort and the patient’s clinical status. Arthroscopic procedures can be performed in nearly all large joints, including the hip in young patients [74, 75]. Arthroscopic hip decompression with irrigation and debridement is typically performed utilizing lateral portals although medial portal arthroscopy has been theoretically proposed [76]. Open hip arthrotomy is more commonly performed. This is achieved utilizing a direct anterior or modified anterolateral approach. Care must be taken to avoid neurovascular structures, maintain perfusion to the hip, and avoid damage to chondral surfaces.
Arthroscopic knee irrigation and debridement is popular and performed readily by most trained orthopedic surgeons. Compared to open arthrotomy, arthroscopic knee decompression is associated with less need for repeat procedures, earlier knee range of motion, and weight-bearing [77]. In addition, arthroscopic knee irrigation and debridement can be performed if a repeat procedure is indicated. In the setting of a septic knee, the risk for revision decompression is associated with bacteremia, older children, and those with late presentation [77,78,79]. Revision surgery is indicated if the inflammatory markers do not improve after postoperative day two, continued wound drainage, or if the patient is clinically deteriorating.
Following surgical treatment , antibiotics should be prescribed with consultation from infectious disease specialists as detailed above. Surgical follow-up should be maintained for at least a year.
Complications
Timely diagnosis, surgical decompression, and antibiotics are typically associated with favorable outcomes for septic arthritis. However, late presentation, misdiagnosis, or delay in treatment can lead to significant morbidity and even mortality. Modern data from developed regions suggest that the 1-year rate of clinical dysfunction is 10%, compared with greater than 50% in resource-poor settings and historical data [80,81,82]. Advancements in diagnostic capabilities, testing, imaging, and care delivery were touted as reasons for this difference [81]. Misdiagnosis is associated with delay to arthrotomy as many culture-negative cases were attributed to juvenile inflammatory arthritis or other pathology that did not necessitate antibiotics or surgical intervention [80, 82]. In the study by Howard-Jones et al., limb-length discrepancy, hip instability, reduced range of motion, and recurrence of septic arthritis were the complications noted at 1 year [81].
Complications associated with septic arthritis of the lower extremity include limp, decreased range of motion, osteonecrosis of the femoral head in the hip or femoral condyle of the knee, limb-length discrepancy, hip instability, osteomyelitis, destruction of the epiphyseal plate, progressive ankylosis, chondral injury leading to early arthritis, and ankylosis leading to long term gait abnormalities [83,84,85]. Complications in the upper extremity include limb-length discrepancy, angular deformity, pain, and loss of motion. Prematurity and greater severity of illness is correlated with increased rate of long-term sequelae [80, 84]. For the hip specifically, classification systems have been reported in an effort to guide treatment [86,87,88,89]. Choi et al. proposed a modified radiographic classification based upon findings of Hunka et al. and proposed treatment regimens for each category [87,88,89].
Reconstructive options for deformity associated with sequelae of septic arthritis varied and complex. Goals should be aimed to optimize joint range of motion, pain control, and clinical function. Spiegel et al. proposed several treatment options for various sequelae of septic arthritis [90]. Leg length discrepancies can be addressed with epiphysiodesis, guided growth, osteotomies, or soft tissue releases. Hip instability can benefit from open reduction, pelvic support osteotomy, or arthroplasty depending upon the degree of arthritis. Contracture and loss of motion will often necessitate soft tissue releases. In the hip, abductor insufficiency from greater trochanter growth arrest may benefit from greater trochanteric transfer, pelvic support osteotomy, or hip arthrodesis [90]. Each treatment must be tailored to the patient’s functional demands, cultural expectations, and resources available to the surgeon and caretakers.
In an effort to minimize variation in care, clinical practice guidelines have been established at various institutions to prevent delay and optimize outcomes for pediatric patients with suspected septic arthritis [91,92,93]. Standard order sheets that reflect multidisciplinary consensus opinions have been established [91,92,93]. Kocher et al. demonstrated that the utilization of a clinical guideline at their institution was associated with lower follow -up inflammatory markers, radiation from imaging, presumptive drainage rate along with faster time to discharge, transition to oral antibiotic therapy, and greater compliance of recommended antibiotic therapy [93]. As such, adoption of institution-specific multidisciplinary clinical care guidelines can provide efficiency and optimize outcomes in the setting of pediatric septic arthritis.
Conclusion
Septic arthritis demands a multidisciplinary approach for diagnosis and management (Box 12.1). It is a diagnostic challenge and remains a true orthopedic surgical emergency. Timely diagnosis, surgical decompression, and antibiotic administration are crucial to optimize outcomes. Rates of complication and long-term sequelae from septic arthritis have diminished due to advancements in diagnostic capabilities, broad recognition, and heighted awareness among various providers. However, long-term morbidity, need for additional surgeries, and disability pose a significant challenge and is a realm of significant research and improvement.
Box 12.1 Key Learning Points
-
1.
Septic arthritis is a true orthopedic emergency.
-
2.
A multidisciplinary approach is key for diagnosis and management.
-
3.
Early diagnosis, surgical decompression, and antibiotics are essential for good outcome.
-
4.
Delayed diagnosis and inappropriate and inadequate treatment are associated with long-term sequela.
References
Christiansen P, Frederiksen B, Glazowski J, Scavenius M, Knudsen FU. Epidemiologic, bacteriologic, and long-term follow-up data of children with acute hematogenous osteomyelitis and septic arthritis: a ten-year review. J Pediatr Orthop B. 1999;8(4):302–5. Epub 1999/10/08.
Welkon CJ, Long SS, Fisher MC, Alburger PD. Pyogenic arthritis in infants and children: a review of 95 cases. Pediatr Infect Dis. 1986;5(6):669–76. Epub 1986/11/01.
Jackson MA, Nelson JD. Etiology and medical management of acute suppurative bone and joint infections in pediatric patients. J Pediatr Orthop. 1982;2(3):313–23. Epub 1982/08/01.
Gafur OA, Copley LA, Hollmig ST, Browne RH, Thornton LA, Crawford SE. The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines. J Pediatr Orthop. 2008;28(7):777–85. Epub 2008/09/25.
Okubo Y, Nochioka K, Marcia T. Nationwide survey of pediatric septic arthritis in the United States. J Orthop. 2017;14(3):342–6, Epub 2017/07/15.
Safdieh G, Silberman J, Nguyen J, Doyle SM, Blanco JS, Scher DM, et al. Pediatric septic arthritis and osteomyelitis in the USA: a national KID database analysis. HSS J. 2019;15(2):159–66. Epub 2019/07/23.
Malcius D, Trumpulyte G, Barauskas V, Kilda A. Two decades of acute hematogenous osteomyelitis in children: are there any changes? Pediatr Surg Int. 2005;21(5):356–9. Epub 2005/04/19.
Gillespie WJ. Epidemiology in bone and joint infection. Infect Dis Clin N Am. 1990;4(3):361–76. Epub 1990/09/01.
Lavy CB, Peek AC, Manjolo G. The incidence of septic arthritis in Malawian children. Int Orthop. 2005;29(3):195–6. Epub 2005/04/05.
Kang SN, Sanghera T, Mangwani J, Paterson JM, Ramachandran M. The management of septic arthritis in children: systematic review of the English language literature. J Bone Joint Surg. 2009;91(9):1127–33. Epub 2009/09/02.
Prevention CfDCa. Lyme Disease. Recent Surveillance Data; 2019 [cited 2020]; Available from: https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html#:~:text=In%202018%2C%20a%20total%20of,Disease%20Surveillance%20System%20(NNDSS).
Benvenuti M, An T, Amaro E, Lovejoy S, Mencio G, Martus J, et al. Double-edged sword: musculoskeletal infection provoked acute phase response in children. Orthop Clin North Am. 2017;48(2):181–97. Epub 2017/03/25.
Papathanasiou I, Malizos KN, Poultsides L, Karachalios T, Oikonomou P, Tsezou A. The catabolic role of toll-like receptor 2 (TLR-2) mediated by the NF-kappaB pathway in septic arthritis. J Orthop Res. 2011;29(2):247–51. Epub 2010/08/27.
Oronsky A, Ignarro L, Perper R. Release of cartilage mucopolysaccharide-degrading neutral protease from human leukocytes. J Exp Med. 1973;138(2):461–72. Epub 1973/08/01.
Harris ED Jr, Parker HG, Radin EL, Krane SM. Effects of proteolytic enzymes on structural and mechanical properties of cartilage. Arthritis Rheum. 1972;15(5):497–503. Epub 1972/09/01.
Smith RL, Schurman DJ, Kajiyama G, Mell M, Gilkerson E. The effect of antibiotics on the destruction of cartilage in experimental infectious arthritis. J Bone Joint Surg Am. 1987;69(7):1063–8. Epub 1987/09/01.
Merlini L, Anooshiravani M, Ceroni D. Concomitant septic arthritis and osteomyelitis of the hip in young children; a new pathophysiological hypothesis suggested by MRI enhancement pattern. BMC Med Imaging. 2015;15:17. Epub 2015/05/20.
Alderson M, Speers D, Emslie K, Nade S. Acute haematogenous osteomyelitis and septic arthritis--a single disease. An hypothesis based upon the presence of transphyseal blood vessels. J Bone Joint Surg. 1986;68(2):268–74. Epub 1986/03/01.
Dohin B, Gillet Y, Kohler R, Lina G, Vandenesch F, Vanhems P, et al. Pediatric bone and joint infections caused by Panton-valentine leukocidin-positive Staphylococcus aureus. Pediatr Infect Dis J. 2007;26(11):1042–8. Epub 2007/11/07.
Carrillo-Marquez MA, Hulten KG, Hammerman W, Mason EO, Kaplan SL. USA300 is the predominant genotype causing Staphylococcus aureus septic arthritis in children. Pediatr Infect Dis J. 2009;28(12):1076–80. Epub 2009/10/13.
Sankaran G, Zacharia B, Roy A, Purayil SP. Current clinical and bacteriological profile of septic arthritis in young infants: a prospective study from a tertiary referral Centre. Eur J Orthop Surg Traumatol. 2018;28(4):573–8. Epub 2018/02/11.
Branson J, Vallejo JG, Flores AR, Hulten KG, Mason EO, Kaplan SL, et al. The contemporary microbiology and rates of concomitant osteomyelitis in acute septic arthritis. Pediatr Infect Dis J. 2017;36(3):267–73. Epub 2016/11/22.
Williams N, Cooper C, Cundy P. Kingella kingae septic arthritis in children: recognising an elusive pathogen. J Child Orthop. 2014;8(1):91–5. Epub 2014/02/04.
Arnold JC, Bradley JS. Osteoarticular infections in children. Infect Dis Clin N Am. 2015;29(3):557–74. Epub 2015/08/28.
Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4(2):143–52. Epub 2004/03/26.
Guerau-de-Arellano M, Huber BT. Chemokines and toll-like receptors in Lyme disease pathogenesis. Trends Mol Med. 2005;11(3):114–20. Epub 2005/03/12.
Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330(4):229–34. Epub 1994/01/27.
Girschick HJ, Huppertz HI, Russmann H, Krenn V, Karch H. Intracellular persistence of Borrelia burgdorferi in human synovial cells. Rheumatol Int. 1996;16(3):125–32. Epub 1996/01/01.
Vardiabasis NV, Schlechter JA. Definitive diagnosis of children presenting to a pediatric emergency department with fever and extremity pain. J Emerg Med. 2017;53(3):306–12. Epub 2017/10/11.
Smith BG, Cruz AI Jr, Milewski MD, Shapiro ED. Lyme disease and the orthopaedic implications of Lyme arthritis. J Am Acad Orthop Surg. 2011;19(2):91–100. Epub 2011/02/05.
Aiyer A, Hennrikus W, Walrath J, Groh B, Ostrov B. Lyme arthritis of the pediatric lower extremity in the setting of polyarticular disease. J Child Orthop. 2014;8(4):359–65. Epub 2014/07/24.
Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107(5):725–31. Epub 1987/11/01.
Paakkonen M, Kallio MJ, Kallio PE, Peltola H. Sensitivity of erythrocyte sedimentation rate and C-reactive protein in childhood bone and joint infections. Clin Orthop Relat Res. 2010;468(3):861–6. Epub 2009/06/18.
Basmaci R, Ilharreborde B, Bonacorsi S, Kahil M, Mallet C, Aupiais C, et al. Septic arthritis in children with normal initial C-reactive protein: clinical and biological features. Arch Pediatr. 2014;21(11):1195–9. Epub 2014/10/06.
Ceroni D, Cherkaoui A, Combescure C, Francois P, Kaelin A, Schrenzel J. Differentiating osteoarticular infections caused by Kingella kingae from those due to typical pathogens in young children. Pediatr Infect Dis J. 2011;30(10):906–9. Epub 2011/04/16.
Singhal R, Perry DC, Khan FN, Cohen D, Stevenson HL, James LA, et al. The use of CRP within a clinical prediction algorithm for the differentiation of septic arthritis and transient synovitis in children. J Bone Joint Surg. 2011;93(11):1556–61. Epub 2011/11/08.
Sultan J, Hughes PJ. Septic arthritis or transient synovitis of the hip in children: the value of clinical prediction algorithms. J Bone Joint Surg. 2010;92(9):1289–93. Epub 2010/08/28.
Caird MS, Flynn JM, Leung YL, Millman JE, D'Italia JG, Dormans JP. Factors distinguishing septic arthritis from transient synovitis of the hip in children. A prospective study. J Bone Joint Surg Am. 2006;88(6):1251–7. Epub 2006/06/08.
Luhmann SJ, Jones A, Schootman M, Gordon JE, Schoenecker PL, Luhmann JD. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86(5):956–62. Epub 2004/05/01.
Jung ST, Rowe SM, Moon ES, Song EK, Yoon TR, Seo HY. Significance of laboratory and radiologic findings for differentiating between septic arthritis and transient synovitis of the hip. J Pediatr Orthop. 2003;23(3):368–72. Epub 2003/05/02.
Kocher MS, Mandiga R, Zurakowski D, Barnewolt C, Kasser JR. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86(8):1629–35. Epub 2004/08/05.
Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81(12):1662–70. Epub 1999/12/23.
Plumb J, Mallin M, Bolte RG. The role of ultrasound in the emergency department evaluation of the acutely painful pediatric hip. Pediatr Emerg Care. 2015;31(1):54–8. quiz 9-61. Epub 2015/01/07.
Moss SG, Schweitzer ME, Jacobson JA, Brossmann J, Lombardi JV, Dellose SM, et al. Hip joint fluid: detection and distribution at MR imaging and US with cadaveric correlation. Radiology. 1998;208(1):43–8. Epub 1998/07/01.
Gordon JE, Huang M, Dobbs M, Luhmann SJ, Szymanski DA, Schoenecker PL. Causes of false-negative ultrasound scans in the diagnosis of septic arthritis of the hip in children. J Pediatr Orthop. 2002;22(3):312–6. Epub 2002/04/19.
Montgomery NI, Epps HR. Pediatric septic arthritis. Orthop Clin North Am. 2017;48(2):209–16. Epub 2017/03/25.
Monsalve J, Kan JH, Schallert EK, Bisset GS, Zhang W, Rosenfeld SB. Septic arthritis in children: frequency of coexisting unsuspected osteomyelitis and implications on imaging work-up and management. AJR Am J Roentgenol. 2015;204(6):1289–95. Epub 2015/05/23.
Schallert EK, Kan JH, Monsalve J, Zhang W, Bisset GS 3rd, Rosenfeld S. Metaphyseal osteomyelitis in children: how often does MRI-documented joint effusion or epiphyseal extension of edema indicate coexisting septic arthritis? Pediatr Radiol. 2015;45(8):1174–81. Epub 2015/02/24.
Mignemi ME, Menge TJ, Cole HA, Mencio GA, Martus JE, Lovejoy S, et al. Epidemiology, diagnosis, and treatment of pericapsular pyomyositis of the hip in children. J Pediatr Orthop. 2014;34(3):316–25. Epub 2013/11/01.
Rosenfeld S, Bernstein DT, Daram S, Dawson J, Zhang W. Predicting the presence of adjacent infections in septic arthritis in children. J Pediatr Orthop. 2016;36(1):70–4. Epub 2015/01/13.
Ecklund K, Vargas S, Zurakowski D, Sundel RP. MRI features of Lyme arthritis in children. AJR Am J Roentgenol. 2005;184(6):1904–9. Epub 2005/05/24.
Bram JT, Baldwin KD, Blumberg TJ. Gram stain is not clinically relevant in treatment of pediatric septic arthritis. J Pediatr Orthop. 2018;38(9):e536–e40. Epub 2018/07/24.
El Houmami N, Bzdrenga J, Durand GA, Minodier P, Seligmann H, Prudent E, et al. Molecular tests that target the RTX locus do not distinguish between Kingella kingae and the recently described Kingella negevensis species. J Clin Microbiol. 2017;55(10):3113–22. Epub 2017/08/11.
Carter K, Doern C, Jo CH, Copley LA. The clinical usefulness of polymerase chain reaction as a supplemental diagnostic tool in the evaluation and the treatment of children with septic arthritis. J Pediatr Orthop. 2016;36(2):167–72. Epub 2015/04/19.
Margaretten ME, Kohlwes J, Moore D, Bent S. Does this adult patient have septic arthritis? JAMA. 2007;297(13):1478–88. Epub 2007/04/05.
Heyworth BE, Shore BJ, Donohue KS, Miller PE, Kocher MS, Glotzbecker MP. Management of pediatric patients with synovial fluid white blood-cell counts of 25,000 to 75,000 cells/mm(3) after aspiration of the hip. J Bone Joint Surg Am. 2015;97(5):389–95. Epub 2015/03/06.
Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme borreliosis. Clin Microbiol Rev. 2005;18(3):484–509. Epub 2005/07/16.
Bockenstedt LK, Wormser GP. Review: unraveling Lyme disease. Arthritis Rheumatol. 2014;66(9):2313–23. Epub 2014/06/27.
Schlung JE, Bastrom TP, Roocroft JH, Newton PO, Mubarak SJ, Upasani VV. Femoral neck aspiration aids in the diagnosis of osteomyelitis in children with septic hip. J Pediatr Orthopedics. 2018;38(10):532–6. Epub 2016/09/08.
Cruz AI Jr, Anari JB, Ramirez JM, Sankar WN, Baldwin KD. Distinguishing pediatric Lyme arthritis of the hip from transient synovitis and acute bacterial septic arthritis: a systematic review and meta-analysis. Cureus. 2018;10(1):e2112. Epub 2018/03/28.
Bachra BN. Calcification of connective tissue. Int Rev Connect Tissue Res. 1970;5:165–208. Epub 1970/01/01.
Kabak S, Halici M, Akcakus M, Cetin N, Narin N. Septic arthritis in patients followed-up in neonatal intensive care unit. Pediatr Int. 2002;44(6):652–7. Epub 2002/11/08.
Samora JB, Klingele K. Septic arthritis of the neonatal hip: acute management and late reconstruction. J Am Acad Orthop Surg. 2013;21(10):632–41. Epub 2013/10/03.
Castellazzi L, Mantero M, Esposito S. Update on the Management of Pediatric Acute Osteomyelitis and Septic Arthritis. Int J Mol Sci. 2016;1:17(6). Epub 2016/06/04.
Arnold JC, Cannavino CR, Ross MK, Westley B, Miller TC, Riffenburgh RH, et al. Acute bacterial osteoarticular infections: eight-year analysis of C-reactive protein for oral step-down therapy. Pediatrics. 2012;130(4):e821–8. Epub 2012/09/12.
Paakkonen M, Kallio MJ, Peltola H, Kallio PE. Antibiotic treatment and surgery for acute Hematogenous calcaneal osteomyelitis of childhood. J Foot Ankle Surg. 2015;54(5):840–3. Epub 2015/04/29.
Chou AC, Mahadev A. The use of C-reactive protein as a guide for transitioning to Oral antibiotics in pediatric Osteoarticular infections. J Pediatr Orthop. 2016;36(2):173–7. Epub 2015/05/02.
Keren R, Shah SS, Srivastava R, Rangel S, Bendel-Stenzel M, Harik N, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120–8. Epub 2014/12/17.
Peltola H, Paakkonen M. Acute osteomyelitis in children. N Engl J Med. 2014;370(4):352–60. Epub 2014/01/24.
Farrow L. A systematic review and meta-analysis regarding the use of corticosteroids in septic arthritis. BMC Musculoskelet Disord. 2015;16:241. Epub 2015/09/08.
Qin YF, Li ZJ, Li H. Corticosteroids as adjunctive therapy with antibiotics in the treatment of children with septic arthritis: a meta-analysis. Drug Des Devel Ther. 2018;12:2277–84. Epub 2018/08/09.
Dattwyler RJ, Luft BJ, Kunkel MJ, Finkel MF, Wormser GP, Rush TJ, et al. Ceftriaxone compared with doxycycline for the treatment of acute disseminated Lyme disease. N Engl J Med. 1997;337(5):289–94. Epub 1997/07/31.
Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089–134. Epub 2006/10/10.
Sanpera I, Raluy-Collado D, Sanpera-Iglesias J. Arthroscopy for hip septic arthritis in children. Orthop Traumatol Surg Res. 2016;102(1):87–9. Epub 2016/01/05.
Thompson RM, Gourineni P. Arthroscopic treatment of septic arthritis in very young children. J Pediatr Orthop. 2017;37(1):e53–e7. Epub 2015/09/24.
Edmonds EW, Lin C, Farnsworth CL, Bomar JD, Upasani VV. A medial portal for hip arthroscopy in children with septic arthritis: a safety study. J Pediatr Orthop. 2018;38(10):527–31. Epub 2016/09/08.
Johns B, Loewenthal M, Ho E, Dewar D. Arthroscopic versus open treatment for acute septic arthritis of the knee in children. Pediatr Infect Dis J. 2018;37(5):413–8. Epub 2017/09/26.
Telleria JJ, Cotter RA, Bompadre V, Steinman SE. Laboratory predictors for risk of revision surgery in pediatric septic arthritis. J Child Orthop. 2016;10(3):247–54. Epub 2016/05/14.
Agout C, Lakhal W, Fournier J, de Bodman C, Bonnard C. Arthroscopic treatment of septic arthritis of the knee in children. Orthop Traumatol Surg Res. 2015;101(8 Suppl):S333–6. Epub 2015/10/01.
Nunn TR, Cheung WY, Rollinson PD. A prospective study of pyogenic sepsis of the hip in childhood. J Bone Joint Surg Br. 2007;89(1):100–6. Epub 2007/01/30.
Howard-Jones AR, Isaacs D, Gibbons PJ. Twelve-month outcome following septic arthritis in children. J Pediatr Orthop B. 2013;22(5):486–90. Epub 2013/03/28.
Newman JH. Review of septic arthritis throughout the antibiotic era. Ann Rheum Dis. 1976;35(3):198–205. Epub 1976/06/01.
Fabry G, Meire E. Septic arthritis of the hip in children: poor results after late and inadequate treatment. J Pediatr Orthop. 1983;3(4):461–6. Epub 1983/09/01.
Wang CL, Wang SM, Yang YJ, Tsai CH, Liu CC. Septic arthritis in children: relationship of causative pathogens, complications, and outcome. J Microbiol Immunol Infect. 2003;36(1):41–6. Epub 2003/05/14.
Lunseth PA, Heiple KG. Prognosis in septic arthritis of the hip in children. Clin Orthop Relat Res. 1979;139:81–5. Epub 1979/03/01.
Forlin E, Milani C. Sequelae of septic arthritis of the hip in children: a new classification and a review of 41 hips. J Pediatr Orthop. 2008;28(5):524–8. Epub 2008/06/27.
Choi IH, Yoo WJ, Cho TJ, Chung CY. Operative reconstruction for septic arthritis of the hip. Orthop Clin North Am. 2006;37(2):173–83, vi. Epub 2006/04/28.
Choi IH, Shin YW, Chung CY, Cho TJ, Yoo WJ, Lee DY. Surgical treatment of the severe sequelae of infantile septic arthritis of the hip. Clin Orthop Relat Res. 2005;434:102–9. Epub 2005/05/03.
Hunka L, Said SE, MacKenzie DA, Rogala EJ, Cruess RL. Classification and surgical management of the severe sequelae of septic hips in children. Clin Orthop Relat Res. 1982;171:30–6. Epub 1982/11/01.
Spiegel DPJ, Banskota A, Shrestha O. Sequelae of septic arthritis of the hip. HELP Organization; 2007. p. 22.
Spruiell MD, Searns JB, Heare TC, Roberts JL, Wylie E, Pyle L, et al. Clinical care guideline for improving pediatric acute musculoskeletal infection outcomes. J Pediatric Infect Dis Soc. 2017;6(3):e86–93. Epub 2017/04/19.
Quick RD, Williams J, Fernandez M, Gottschalk H, Cosgrove P, Kahlden K, et al. Improved diagnosis and treatment of bone and joint infections using an evidence-based treatment guideline. J Pediatr Orthop. 2018;38(6):e354–e9. Epub 2018/05/05.
Kocher MS, Mandiga R, Murphy JM, Goldmann D, Harper M, Sundel R, et al. A clinical practice guideline for treatment of septic arthritis in children: efficacy in improving process of care and effect on outcome of septic arthritis of the hip. J Bone Joint Surg Am. 2003;85(6):994–9. Epub 2003/06/05.
Disclosure
No funds were received in support of this work. No benefits in any form have been or will be received from any commercial party related directly or indirectly to the subject of this manuscript
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nandyala, S.V., Herman, M.J., Kocher, M.S. (2022). Septic Arthritis. In: Belthur, M.V., Ranade, A.S., Herman, M.J., Fernandes, J.A. (eds) Pediatric Musculoskeletal Infections. Springer, Cham. https://doi.org/10.1007/978-3-030-95794-0_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-95794-0_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-95793-3
Online ISBN: 978-3-030-95794-0
eBook Packages: MedicineMedicine (R0)