Abstract
Background
Joint effusions identified by MRI may accompany osteomyelitis and determining whether the joint effusion is septic or reactive has important implications on patient care.
Objective
Determine the incidence of epiphyseal marrow edema, joint effusions, perisynovial edema and epiphyseal non-enhancement in the setting of pediatric metaphyseal osteomyelitis and whether this may be used to predict coexisting septic arthritis.
Materials and methods
Following IRB approval, we retrospectively evaluated children who underwent MRI and orthopedic surgical consultation for suspected musculoskeletal infection between January 2011 and September 2013. Criteria for inclusion in the study were microbiologically/pathologically proven infection, MRI prior to surgical intervention, long bone involvement and age 0–18 years. MRI exams were independently reviewed by two faculty pediatric radiologists to confirm the presence of appendicular metaphyseal osteomyelitis, to evaluate extent of edema, to determine subjective presence of a joint effusion and to assess perisynovial edema and epiphyseal non-enhancement. Any discrepant readings were reviewed in consensus. Charts and operative notes were reviewed to confirm the diagnosis of osteomyelitis and septic arthritis.
Results
One hundred and three joints with metaphyseal osteomyelitis were identified (mean age: 7.1 years; M:F 1.3:1), of whom 53% (55/103) had joint effusions, and of those, 75% (41/55) had surgically confirmed septic arthritis. The incidence of coexisting septic arthritis was 40% in the setting of epiphyseal edema, 74% in epiphyseal edema and effusion, 75% with perisynovial edema, 76% with epiphyseal non-enhancement and 77% when all four variables were present. Of these, the only statistically significant variable, however, was the presence of a joint effusion with a P-value of <0.0001 via Fisher exact test. Statistical significance for coexisting septic arthritis was also encountered when cases were subdivided into intra-articular vs. extra-articular metaphyses (P-value = 0.0499). No statistically significant difference was found between patients younger than 24 months and those older than 24 months.
Conclusion
Patients with joint effusions identified by MRI, in the setting of metaphyseal osteomyelitis, should be presumed to have septic arthritis until proven otherwise. Epiphyseal extension of edema, perisynovial edema and epiphyseal non-enhancement in the setting of metaphyseal osteomyelitis are not helpful predictors in differentiating reactive and pyogenic joint effusions. Osteomyelitis at a site with an intra-articular metaphyses, however, is more likely to have concurrent septic arthritis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
MRI characteristics of complications of osteomyelitis, including surgically significant bone, subperiosteal and soft-tissue abscesses are well described. MRI is an important early tool to determine location and extent of infection as well as assist with preoperative planning when surgically significant complications of osteomyelitis are identified [1–3]. One quandary regarding MRI is whether the presence of joint effusion, in the setting of nearby metaphyseal osteomyelitis, represents an innocuous, non-pyogenic reactive effusion or concurrent septic arthritis. This distinction is important because septic arthritis requires specific surgical irrigation and debridement of the joint compared with isolated osteomyelitis. In non-complicated osteomyelitis, management more commonly includes medical rather than surgical treatment. Therefore, knowing whether an effusion is reactive or pyogenic is important to optimize patient care [1, 4–7].
Several studies investigating MRI predictors of septic arthritis have shown considerable overlap of imaging features of pyogenic and non-pyogenic joint effusions [8, 9]. In pediatric and adult patients, MR findings of synovial thickening, perisynovial and bone marrow edema and enhancement and the lack of contralateral joint effusions favor septic arthritis rather than transient synovitis, but there is overlap of findings in pyogenic and non-pyogenic causes of a joint effusion [8, 9].
In our experience, when nearby osteomyelitis is present, not all cases of a coexisting joint effusion have represented pyogenic septic arthritis. To our knowledge, no study has addressed whether the extent of nearby osteomyelitis identified by MRI may help predict septic arthritis. Therefore, the purpose of this study was to determine the incidence of concomitant septic arthritis in the setting of appendicular metaphyseal osteomyelitis in children and whether epiphyseal extent of edema may help predict when a nearby joint effusion actually represents coexisting septic arthritis. Additionally, previously investigated characteristics of perisynovial edema and epiphyseal non-enhancement were evaluated.
Materials and methods
Patient selection
Following IRB approval, retrospective review was performed of all patients who underwent orthopedic consult for musculoskeletal infections and had MRI between January 2011 and September 2013 at an urban, tertiary care children’s hospital. The orthopedic surgeons at our institution maintain a comprehensive spreadsheet of all patients on which they are consulted. Each entry was reviewed for relevance to osteoarticular infections and cross-checked in the picture archiving system (PACS) (Phillips iSite, Foster City, CA, USA) for MRI. In addition, a separate PACS search was performed using Primordial© keyword search solution (Primordial Design Inc., San Mateo, CA, USA) to include all MRI studies performed for musculoskeletal infection in the same time period. Criteria for inclusion in the study were as follows: (a) MRI prior to surgical intervention, (b) infection involving the metaphysis of a long bone, (c) no medical comorbidities (sickle cell, immunosuppression, etc.) or history of direct innoculation osteomyelitis, (d) acute infection confirmed with microbiology and/or surgical pathology, and (e) patient age between 0 and 18 years. This resulted in a study population of 103 joints in 97 patients.
Imaging technique
All 103 joints were imaged using a 1.5-T or 3.0-T Achieva MR system (Philips Healthcare, Best, The Netherlands). On the basis of age and body size, a quadrature-body, XL Torso (Philips Healthcare) or cardiac coil was used for the region of interest. Imaging was then focused with a smaller field of view at the location of signal abnormality and the following sequences were obtained: T1-weighted coronal or axial, fluid sensitive (short tau inversion recovery [STIR], T2-weighted fat-saturated, or proton density fat-saturated) coronal and axial, and T1-weighted fat-saturated contrast-enhanced (0.1 mmol/kg IV gadopentetate dimeglumine [Magnevist, Bayer HealthCare, Whippany, NJ, USA]) sequences in two or three planes with fat suppression.
Imaging review and data collection
MRI exams were independently reviewed by two faculty pediatric radiologists with a combined 37 years of experience (G.S.B. 28 years, J.H.K. 9 years) to confirm the presence of appendicular metaphyseal osteomyelitis, evaluate extent of edema, determine subjective presence of a joint effusion and assess perisynovial edema and epiphyseal non-enhancement. Any discrepant readings were reviewed in consensus. Imaging evidence of osteomyelitis was defined as increased fluid-sensitive signal with corresponding decreased T1 signal and abnormal enhancement (Fig. 1) with additional supportive imaging findings including bone and soft-tissue abscesses and periosteal edema. When there was isolated marrow edema only, a study was labeled as osteomyelitis when there was clinical and surgical documentation of infection. All studies from the orthopedic surgical consult list were re-reviewed by imaging and chart review to confirm final diagnosis. Extent was recorded as involving the metaphysis with or without epiphyseal extension (Fig. 2). Subjective determination of a joint effusion was documented as present or not present. When applicable, the ipsilateral, non-involved joint or contralateral joint was used as a reference (Fig. 2). Positive perisynovial edema was defined as greater than 50% circumferential pericapsular edema-like signal. Positive epiphyseal non-enhancement was documented as present when there was either focal or diffuse non-enhancement of the epiphyseal cartilage and/or ossification center on postcontrast imaging (Fig. 3). The variable of epiphyseal extension of edema and epiphyseal non-enhancement could both be positive when fluid-sensitive sequences show epiphyseal edema but postcontrast images demonstrate non-enhancing foci. Measurements for synovial thickness were not evaluated because there are no known normative standards for measurements to determine pathology in children that account for different appendicular joint locations, relative size of effusion, and differing ages and size of children. Chart and operative notes were reviewed to confirm the diagnosis of osteomyelitis with or without septic arthritis, at which time additional parameters (i.e. age, gender, etc.) were recorded. Joint effusion was considered septic based on laboratory evaluation, including an organism present on culture or Gram stain of synovial aspirate, a synovial fluid cell count of >20,000 white blood cells per cubic millimeter, or the presence of grossly purulent material in the joint at the time of surgery. Diagnosis of osteomyelitis was based on clinical examination, laboratory studies and culture of bone aspiration with supportive MRI and/or surgical findings. When there was discrepancy between imaging and clinical diagnosis, imaging was re-reviewed, and surgical and microbiological diagnosis was taken as the reference diagnosis.
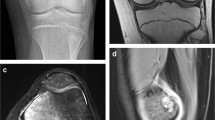
Isolated metaphyseal osteomyelitis without septic arthritis in a 4-year, 5-month-old girl presenting with limping, fever and knee swelling. Coronal T1 (a), sagittal proton density fat saturation (b), and postcontrast sagittal T1 proton density fat saturation (c) MRI sequences demonstrate distal femoral metaphyseal osteomyelitis (white asterisk), no epiphyseal extension of marrow edema and no effusion, but a large subperiosteal abscess (black asterisk)
Metaphyseal osteomyelitis with marrow edema extending to the epiphysis without coexisting septic arthritis in a 10-year, 5-month-old boy presenting with left knee pain for 5 days, no trauma, and elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Coronal T1 (a), coronal STIR (b) and axial STIR (c) MRI sequences demonstrate proximal tibial metaphyseal osteomyelitis (black arrowhead) with marrow edema that extends to the epiphysis (black asterisk) and a physiological amount of fluid in the joint, i.e. no joint effusion (white arrow)
Metaphyseal osteomyelitis of the proximal femur with hip effusion surgically proven as concurrent septic arthritis in a 4-year, 1-month-old girl presenting with poorly localized left lower extremity pain for roughly 1 week, fever and inability to bear weight. Coronal T1 (a), coronal STIR (b) and postcontrast coronal T1 fat saturation (c) MRI images demonstrate left proximal femoral metaphyseal heterogeneous low T1, increased STIR signal and heterogeneous enhancement (white asterisks) of the marrow consistent with early osteomyelitis with a joint effusion (white arrows). The decreased enhancement of the left femoral capital epiphysis is an example of what was recorded as positive for the variable of epiphyseal non-enhancement
Results
General
Ninety-seven children with metaphyseal long bone osteomyelitis were identified with a total of 103 joints. The average patient age was 7.1 years with standard deviation of 4.6 years. The median was 7 years with a minimum age of 1 month and maximum age of 15.4 years. The lower quartile was 2.8 years and the upper quartile was 10.7 years; therefore, 50% of the children were between the ages of approximately 3 and 11 years. Fifty-six percent of the patients were male, while 44 were female for a 1.3:1 male-to-female ratio. Distribution of metaphyseal osteomyelitis in the appendicular skeleton is presented in Table 1 with 34% occurring around the knee, 36% around the ankle, 16% at the hip, 6% at the shoulder, 4% at the wrist and 3% around the elbow. The overall incidence of coexisting septic arthritis in the setting of metaphyseal osteomyelitis irrespective of extent of edema, presence of joint effusion, perisynovial edema or epiphyseal non-enhancement or age was 40% (41/103).
Intra- vs. extra-articular metaphyses
At sites where the metaphysis is intra-articular (proximal femur, proximal radius, distal fibula and proximal humerus), the rate of coexisting septic arthritis was 55% (17/31) (Table 2). When joints were divided between those with intra-articular metaphyses and those with extra-articular metaphyses, a statistically significant positive relationship was identified between those with intra-articular metaphyses and coexisting septic arthritis (P-value = 0.0499).
Effusions (reactive vs. pyogenic)
Of the 103 sites of osteomyelitis, 55 (53%) had coexisting joint effusions. Of these, 25% (14/55) were reactive/non-pyogenic while 75% (41/55) represented coexisting septic arthritis (pyogenic effusion) (Tables 2 and 3). No cases of coexisting septic arthritis were identified that did not have MR identified effusion. Mean age at which the effusion was reactive was 5.5 years (standard deviation 5.3) versus 7.4 years (standard deviation 4.7) when the effusion was pyogenic. Using a Wilcoxon rank test, the difference in age was not statistically significant with a P-value of 0.2160. Additionally, presence of effusion and the relationship with coexisting septic arthritis were divided in the age groups younger than 24 months and older than 24 months because of differences in vascular anatomy (Table 3). In the category of patients younger than 24 months, 69% of effusions (9/13) represented co-existing septic arthritis rather than reactive effusions. The overall rate of coexisting septic arthritis in this age group was 43% (9/21). In the category of patients older than 24 months, 76% (32/42) of effusions represented concurrent septic arthritis. Using Fisher exact test, there was no statistically significant difference between the two age groups in predicting whether an effusion represented coexisting septic arthritis versus reactive effusion with a P-value of 0.7188.
The incidence of effusion and septic arthritis was also analyzed by joint location and summarized in Table 1.
Extent of marrow edema
When evaluating extent of bone marrow edema, 29% (30/103) of cases demonstrated edema-like signal in the metaphysis only, while the remainder, 71% (73/103), demonstrated extension of signal into the epiphysis (Table 2). When edema extended to the epiphysis, 40% (29/73) of cases represented coexisting septic arthritis. When edema was present in the metaphysis only, 40% (12/30) of cases represented coexisting septic arthritis. When epiphyseal edema was present, 53% (39/73) of cases also demonstrated joint effusion and of these 74% (29/39) represented coexisting septic arthritis. Forty-seven percent (34/73) of patients with epiphyseal edema did not have effusions. When marrow edema was present in the metaphysis only, 53% (16/30) demonstrated joint effusions of which 75% (12/16) represented coexisting septic arthritis. No effusion was present in 47% (14/30) of the cases with only metaphyseal marrow edema. In summary, there was no statistically significant difference in the presence of epiphyseal edema or the presence of effusion and epiphyseal edema in predicting coexisting septic arthritis via Fisher exact test with a P-value of 1 and 1, respectively (Tables 2 and 3). In the context of children younger than 24 months, 67% (14/21) demonstrated epiphyseal extension of edema of which 57% (8/14) were confirmed as coexisting septic arthritis. In children older than 24 months, 72% (59/82) demonstrated epiphyseal extension of edema of which 36% (21/59) represented coexisting septic arthritis. The proportions of coexisting septic arthritis in these two age categories when epiphyseal edema was present is not statistically significant by a Fisher exact test but does suggest a trend with a P-value of 0.2234.
Organism cultured
In regard to the rate of organism recovery in the setting of musculoskeletal infection, bacteria were cultured in 94% (97/103) of cases. Ninety percent were caused by Staphylococcus aureus. Methicillin-resistant Staphyloccocus aureus (MRSA) represented 36% (37/103) of the total infections and 41% (17/41) of the coexisting septic arthritis cases. Methicillin-sensitive Staphylococcus aureus (MSSA) represented 49% (50/103) of the total infections and 51% (21/41) of the coexisting septic arthritis cases. The type of bacteria cultured by joint location and source of sample (joint, bone, blood, etc.) is provided in Table 1.
A subset analysis of patients with documented joint effusions by MRI and additional variables in the setting of metaphyseal osteomyelitis (n = 55) are shown in Table 3.
Perisynovial edema was present in 87% of patients with MRI-documented joint effusion (48/55). Of these patients, 75% (36/48) represented cases of coexisting septic arthritis. For the 7 joints without perisynovial edema, coexisting septic arthritis was present in 71% (5/7). In regard to epiphyseal non-enhancement, two studies could not be evaluated secondary to lack of intravenous contrast. Thirty-two percent (17/53) of patients with MRI-documented joint effusion demonstrated focal or diffuse epiphyseal non-enhancement. Of these patients, 76% (13/17) represented coexisting septic arthritis When epiphyseal non-enhancement was not present, 75% (27/36) had coexisting septic arthritis. Epiphyseal extension of edema was present in 71% (39/55) of cases with effusion. When epiphyseal edema and joint effusion were present, septic arthritis was confirmed in 74% (29/39). When evaluating the above variables in the setting of positive joint effusion, none of them was statistically significant, all with a P-value of 1 using a Fisher exact test. For cases in which all variables were positive in the setting of metaphyseal osteomyelitis (combination of a joint effusion, epiphyseal edema, perisynovial edema and epiphyseal non-enhancement), the incidence of coexisting septic arthritis in the setting of metaphyseal osteomyelitis was 77% (10/13).
Discussion
Although there has been dramatic change in the prognosis and disease course in acute osteoarticular infections during the last century, acute osteoarticular infections are still a source of morbidity and even mortality, especially when concurrent septic arthritis and osteomyelitis are initially overlooked [6, 10, 11]. In our study, the presence of a coexisting joint effusion in the setting of metaphyseal osteomyelitis was found to represent concurrent septic arthritis in a majority of cases (75%) with a P-value of <0.0001. Additionally, the feature of osteomyelitis at an intra-articular metaphysis correlated positively with coexisting septic arthritis (P-value = 0.0499). The other categories of age younger than or older than 24 months, presence or lack of concurrent intra-articular (epiphyseal) edema, perisynovial edema or epiphyseal non-enhancement did not predict whether an effusion was reactive or pyogenic. Therefore, patients with metaphyseal osteomyelitis and effusion, regardless of the above factors, should be considered high risk for septic arthritis and appropriate work-up and management should be initiated [12].
The unique vascular anatomy in the neonate has long been cited as a cause for coexistence of osteomyelitis and septic arthritis. As in most children, the route of infection is hematogenous and bacteria seed the metaphysis due to slow flow in tiny hairpin vessels [10]. However, unique to the neonatal period, the cartilaginous epiphyses (before appearance of secondary ossification centers) receive blood directly from the metaphysis, explaining the reported higher incidence of concurrent infection in this age group [13]. Additionally, the neonatal periosteum is thinner and more likely to perforate, spilling infection into the joint and adjacent soft tissues [14]. McCarthy et al. [11] quote a rate of 76% and, more recently, Montgomery et al. [6] quote a rate of 78% for concurrent septic arthritis in the setting of neonatal osteomyelitis. In our study population, the percent of patients younger than 2 years with coexisting septic arthritis was 44%; however, children in this age group only represented 20% of the overall population. Since the epiphysis and metaphysis have a separate blood supply beyond the neonatal period, it is postulated that rates of concomitant osteomyelitis and septic arthritis should decrease in incidence. Including all pediatric age groups, the reported incidence of concurrent septic arthritis and osteomyelitis is 17–33% [11, 14–20], which is lower than our rate of 40%; however, we did not investigate cases of septic arthritis only. In our study, there was no statistical difference in cases of coexisting septic arthritis in the setting of metaphyseal osteomyelitis in children younger than versus older than 2 years of age (P-value = 0.8053) (Table 2).
On MR imaging, features of pyogenic and reactive effusions have significant overlap including size of effusions, synovial thickening and perisynovial edema, and this has been previously published [8, 9, 21, 22]. We did evaluate some of these additional parameters and found that the most consistent predictor of a coexisting pyogenic arthritis was solely the presence of a joint effusion, P-value <0.0001 (Tables 2 and 3). Perisynovial edema did not help with predicting the presence of coexisting septic arthritis (P-value = 1). Also in our study, the presence of epiphyseal nonenhancement was not anymore helpful in predicting coexisting septic arthritis (P-value = 1) in contradistinction to the findings recently published by Kim et al. [23] although our study populations differed since all our patients had coexisting metaphyseal osteomyelitis and conventional postcontrast imaging was performed rather than dynamic contrast imaging.
The potential relationship between extent of edema in the setting of osteomyelitis and presence of septic arthritis was investigated because we postulated that the proximity of marrow edema would correspond directly to the chance of having concurrent septic arthritis. Additionally, at some joints, the metaphysis is also intra-articular. These sites include the proximal femur, proximal radius, distal fibula and proximal humerus [24]. We hypothesized that the presence of intracapsular (epiphyseal) edema and location at a joint with an intra-articular metaphysis would increase the risk of septic arthritis. Location at a joint with an intra-articular metaphysis was statistically significant (P-value = 0.0499), while presence of epiphyseal edema was not (P-value = 1), (Table 2).
Because of the significant impact on morbidity and mortality, Montgomery et al. [6] investigated parameters to improve earlier detection of coexisting osteomyelitis in the setting of septic arthritis, in which they coined the term concurrent infections. In contrast to our study, Montgomery et al. [6] started with patients with a known diagnosis of septic arthritis and looked for concurrent osteomyelitis. Our study examined patients with osteomyelitis and looked for concurrent diagnoses of septic arthritis. Additionally, our study focused on MR imaging features with clinical and surgical correlation, whereas Montgomery et al. [6] evaluated age, location, duration of symptoms, imaging performed and lab values, without documentation of whether MR was utilized during the work-up. Montgomery et al. [6] found an overall incidence of concurrent septic arthritis and osteomyelitis of 21.5%. In their study, patients were divided into four age group categories and no average age was provided. In a separate study involving 50 adult cases of septic arthritis, 33 cases of biopsy-proven septic arthritis and osteomyelitis were identified for an incidence of 66% [8]. Our study design and patient population were intrinsically different and our rate of 40% may not be directly comparable to these two previous studies.
In our study, we found a 0% incidence of surgically confirmed septic arthritis in cases with no evidence of an effusion on MRI. Therefore, in the setting of metaphyseal osteomyelitis, no MR evidence of effusion has a 100% negative predictive value for coexisting septic arthritis. This is discordant with a prior study that noted that 30% of adults with septic arthritis had no effusions identifiable by MRI [8]. In that study, the percentage included a relatively high number of small joint infections, specifically metatarsophalangeal and interphalangeal joints of the feet. When only large joints were analyzed, the rate of septic arthritis without effusion dropped to 9%, which is more in line with our study findings.
There are several limitations of this retrospective study. In terms of overall incidence, coexisting septic arthritis and osteomyelitis could be considered a matter of “what came first, the chicken or the egg?” For example, Montgomery et al. [6] published their incidence of concurrent osteomyelitis in the setting of septic arthritis (21.5%), while our incidence refers to concurrent septic arthritis in the setting of osteomyelitis (40%). Therefore, the rates cannot be directly compared. Additionally, the goal of this study was to facilitate diagnosis and management in the era when nearly all patients with suspected osteomyelitis are referred for MRI at our institution and to help answer the practical question of whether to suggest septic arthritis when a joint effusion is present in the setting of osteomyelitis and to identify additional features such as intraosseous, subperiosteal or intra-muscular abscesses that would require surgical intervention. Another limitation is that the presence or absence of a joint effusion was made subjectively since there are no known normative values on MRI for what constitutes normal joint fluid volume versus joint effusion. Lastly, our study has a slight selection bias as four out of a total of 107 cases of osteomyelitis seen at our institution during this time period were not referred for an orthopedic consultation. These four cases were not included in the study cohort.
Conclusion
Adjacent joint effusions identified on MRI in children with appendicular osteomyelitis should be presumed to be pyogenic, and this should be conveyed to the treating surgeon to help facilitate surgical planning. However, our findings may not be applicable to the small joints of the hands and feet due to an insufficient number of cases. The presence or absence of transphyseal intra-articular epiphyseal extension of edema related to metaphyseal osteomyelitis cannot be used as a predictor of whether a joint effusion is reactive or pyogenic.
References
Dodwell ER (2013) Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr 25:58–63
Kan JH, Young RS, Yu C et al (2010) Clinical impact of gadolinium in the MRI diagnosis of musculoskeletal infection in children. Pediatr Radiol 40:1197–1205
Kan JH, Hilmes MA, Martus JE et al (2008) Value of MRI after recent diagnostic or surgical intervention in children with suspected osteomyelitis. AJR Am J Roentgenol 191:1595–1600
Pääkkönen M, Peltola H (2013) Treatment of acute septic arthritis. Pediatr Infect Dis J 32:684–685
Liu C, Bayer A, Cosgrove SE et al (2011) Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292
Montgomery CO, Siegel E, Blasier RD et al (2013) Concurrent septic arthritis and osteomyelitis in children. J Pediatr Orthop 33:464–467
Dartnell J, Ramachandran M, Katchburian M (2012) Haematogenous acute and subacute paediatric osteomyelitis. J Bone Joint Surg 94B:584–595
Karchevsky M, Schweitzer ME, Morrison WB et al (2004) MRI findings of septic arthritis and associated osteomyelitis in adults. AJR Am J Roentgenol 182:119–122
Yang WJ, Im SA, Lim GY et al (2006) MR imaging of transient synovitis: differentiation from septic arthritis. Pediatr Radiol 36:1154–1158
Trueta J, Agerholm M (1948) Acute haematogenous osteomyelitis; diagnosis and treatment. Overseas Postgrad Med J 2:311–322
McCarthy J, Dormans J, Kozin S (2005) Musculoskeletal infection in children: basic treatment principles and recent advancements. Instr Course Lect 54:515–528
Pääkkönen M, Peltola H (2013) Bone and joint infections. Pediatr Clin N Am 60:425–436
Offiah AC (2006) Acute osteomyelitis, septic arthritis and discitis: differences between neonates and older children. Eur J Radiol 60:221–232
Frank G, Mahoney HM, Eppes SC (2005) Musculoskeletal infections in children. Pediatr Clin N Am 52:1083–1106
Jackson MA, Burry VF, Olson LC (1992) Pyogenic arthritis associated with adjacent osteomyelitis: identification of the sequela-prone child. Pediatr Infect Dis J 11:9–13
Perlman MH, Patzakis MJ, Kumar PJ et al (2000) The incidence of joint involvement with adjacent osteomyelitis in pediatric patients. J Pediatr Orthop 20:40–43
Song KM, Sloboda JF (2001) Acute hematogenous osteomyelitis in children. J Am Acad Orthop Surg 9:166–175
Sucato DJ, Schwend R, Gillespie R (1997) Septic arthritis of the hip in children. JAAOS 5:249–260
Wang CL, Wang SM, Yang YJ et al (2003) Septic arthritis in children: relationship of causative pathogens, complications, and outcome. J Microbiol Immunol Infect 36:41–46
Welkon CJ, Long SS, Fisher MC et al (1986) Pyogenic arthritis in infants and children: a review of 95 cases. Pediatr Infect Dis 5:669–676
Strouse PJ, Londy F, DiPietro MA et al (1999) MRI evaluation of infectious and non-infectious synovitis: preliminary studies in a rabbit model. Pediatr Radiol 29:367–371
Graif M, Schweitzer ME, Deely D et al (1999) The septic versus nonseptic inflamed joint: MRI characteristics. Skelet Radiol 28:616–620
Kim EY, Kwack KS, Cho JH et al (2012) Usefulness of dynamic contrast-enhanced MRI in differentiating between septic arthritis and transient synovitis in the hip joint. AJR Am J Roentgenol 198:428–433
Stans AA (2014) Muskuloskeletal infection. In: Lovell W, Weinstein SL, Flynn JM (eds) Lovell and Winter’s pediatric orthopaedics, 7th edn. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia
Acknowledgments
The authors would like to sincerely extend their appreciation to Robbie Schallert for his contribution to data organization and statistical analysis.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
K. Schallert, E., Herman Kan, J., Monsalve, J. et al. Metaphyseal osteomyelitis in children: how often does MRI-documented joint effusion or epiphyseal extension of edema indicate coexisting septic arthritis?. Pediatr Radiol 45, 1174–1181 (2015). https://doi.org/10.1007/s00247-015-3293-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-015-3293-0