Abstract
Breast density, or the amount of fibroglandular tissue in the breast, is a recognized and independent marker for breast cancer risk. In addition, breast density reduces the sensitivity of mammography due to a masking effect. Public awareness of the importance of breast density has resulted in legislation for reporting breast density for risk stratification purposes. To date, breast density assessment is performed with mammography and to some extent with magnetic resonance imaging. Data indicate that computerized, quantitative techniques in comparison with subjective, visual estimations are characterized by higher reproducibility and robustness. Standardized breast density assessment using automated volumetric quantitative methods has the potential to be useful for risk prediction, stratification, and determining the best screening plan for each woman. This chapter provides a comprehensive overview of the currently available imaging modalities for qualitative and quantitative breast density assessment and the current evidence on breast density and breast cancer risk assessment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Breast density is defined as the amount of fibroglandular breast components relative to fatty components within the breast. Fibroglandular breast components are composed of a mixture of connective, stromal, and parenchymal tissue (Boyd et al. 1992; Ghosh et al. 2008) and appear radiopaque on mammography; on the other hand, fatty components appear radiolucent. Large variations of breast tissue composition exist between women; breast composition also changes over the course of time and during the menstrual cycle, as influenced by endogenous and exogenous factors (Table 1) (Boyd et al. 2006; Byrne et al. 2017; Sterns and Zee 2000; van Duijnhoven et al. 2007). According to the American College of Radiology (ACR), 50% of women in the USA have high breast density, with 40% being categorized as having heterogeneously dense breasts (ACR category c) and 10% as having extremely dense breasts (ACR category d) (D’Orsi et al. 2013).
Based on a large twin study, Nguyen et al. reported that breast density is significantly influenced by the number of childbirths and by body mass index (BMI). Increased childbirths were found to be associated with a decrease of mammographic breast density as well as a corresponding breast cancer risk reduction of up to 4% per live birth (Nguyen et al. 2013). In studies on postmenopausal women, women with the greatest increase in weight and BMI experienced the greatest reduction in breast density (Wanders et al. 2015); however, higher BMI is also associated with higher breast cancer risk in this population (Keum et al. 2015; Huo et al. 2014). Using data from a longitudinal cohort, Hopper and colleagues reported a negative association between adolescent BMI at the age of 7–15 years and breast density at the age of 47–50 years, concluding that adolescent BMI is negatively associated with breast cancer risk (Hopper et al. 2016), in line with other publications in the literature (Harris et al. 2011; Andersen et al. 2014). Several studies have reported that lower BMI or a moderate reduction of body weight during adulthood, before or after menopause, has resulted in the reduction of postmenopausal breast cancer risk of up to 50% (Eliassen et al. 2006; Harvie et al. 2005). A recent study reported that breast density is associated with parity and BMI regardless of age (Krishnan et al. 2017).
2 Breast Density and the Risk for Breast Cancer
2.1 Masking Effect
Breast composition impacts the risk for breast cancer in different ways. Mammographic sensitivity for detecting breast cancer decreases as breast density increases (Kerlikowske et al. 2007; Boyd et al. 2007; McCormack and dos Santos 2006). Breast density is known for producing tissue overlap that leads to a masking effect. Two-dimensional imaging modalities including mammography are particularly susceptible to the masking effect. While the masking effect is a source of false-negative readings and correspondingly a low efficiency of screening examinations (Bailey et al. 2010), an increased density also leads to increased false positives and recall rates (Ballard-Barbash et al. 1997; Carney et al. 2003). High breast density leads to overlapping normal breast tissue, resulting in coalescent areas of breast parenchyma and obliteration of tissues with underlying tumors on imaging (D’Orsi et al. 2013; Rhodes et al. 2015). As a result, women with higher breast density are more often diagnosed with larger breast tumors and advanced stages with lymphatic involvement at initial diagnosis (Ghosh et al. 2008; Aiello et al. 2005; Roubidoux et al. 2004). Interval cancers also increase 6- to 17-fold in women with higher density breasts (Boyd et al. 2007; McCormack and dos Santos 2006).
Most of the evidence on the reduced sensitivity of mammography in dense breasts is from studies employing screen-film mammography (SFM) (D’Orsi et al. 2013; Price et al. 2013). With the introduction of full-field digital mammography (FFDM), the masking effect of dense breasts on cancer detection has been greatly reduced (Carney et al. 2003; Pisano et al. 2005). Kerlikowske et al. (2011) also showed that FFDM improves the detection of hormone receptor-negative breast cancers compared with SFM (FFDM 78.5% vs. SFM 65.8%, sensitivity p = 0.016, in women aged 40–79 years; 95.2% vs. 54.9%, sensitivity, p = 0.007, in women aged 40–49 years). As hormone receptor-negative breast cancers usually present with a higher grade, carry a poorer prognosis, and often manifest as interval cancers, they presumably constitute some proportion of the cancers masked at SFM screening in women with higher density categories. Recently, digital breast tomosynthesis (DBT), a three-dimensional imaging modality, has also been introduced. Several large-scale studies worldwide have investigated DBT in the screening setting, demonstrating an increase in cancer detection as well as a significant reduction in recall rates compared with FFDM, which is most likely attributable to a decreased masking effect (Destounis et al. 2015; Friedewald et al. 2014; McDonald et al. 2016). However, the value of DBT for breast cancer detection as related to breast density has not been fully elucidated. Ciatto et al. evaluated DBT in combination with FFDM in the STORM-1 trial, showing an improved cancer detection rate from 5.3 cancers to 8.1 cancers per 1000 screening examinations and a reduction of recalls by 17.2% (Ciatto et al. 2013). Bernardi et al. demonstrated similar results in the STORM-2 trial, showing cancer detection rates of up to 8.5 cancers per 1000 screening examinations when FFDM is combined with DBT, and up to 8.8 cancers per 1000 screening examinations when a synthesized two-dimensional mammographic image is reconstructed and combined with DBT. However, false-positive readings also increased when using DBT: 3.97% FFDM plus DBT and 4.45% synthetic FFDM plus DBT, respectively, compared with 3.42% for FFDM only (Bernardi et al. 2016).
2.2 Independent Risk Factor
Although the masking effect as related to breast density is an important issue to be considered, it must be noted that the association between breast density and risk for breast cancer is not merely a masking bias and cannot be explained by the reduced sensitivity of mammography alone. Conclusive data have shown that increased breast density is a strong and independent imaging biomarker for increased risk of breast cancer (McCormack and dos Santos 2006; Checka et al. 2012; Vachon et al. 2007; Boyd et al. 2010). Epithelial and glandular structures in the breast are the site of origin for most breast cancers; consequently, higher dense breast parenchyma is associated with an increased chance of future breast cancer development (Freer 2015). In a meta-analysis by McCormack et al. that investigated breast density as an independent risk factor for breast cancer, the relative risk associated with dense breasts was 2.92 for breasts that were 50–74% dense and 4.64 for breasts that were 75% or more dense (McCormack and dos Santos 2006). Boyd et al. summarized studies evaluating breast cancer risk with respect to quantitatively measured tissue density, and the odds ratio of the risk for breast cancer was found to range from 3.6 to 6.0 (Boyd et al. 2011).
Studies investigating breast density under screening conditions arrived at a similar conclusion regarding breast density as a strong predictor of breast cancer risk. Data from the TOMMY trial indicates that absolute measurements of fibroglandular tissue volume were significantly associated with increased breast cancer risk in higher density groups (Gilbert et al. 2015). After adjusting for age, a 2–3% increase of the odds of breast cancer was found per increase of 10 cm3 dense tissue depending on the automated breast density measurement system. The relative risk for breast cancer can differ based on whether a quantitative or qualitative approach is used to determine breast density. However, in either approach, higher breast density is associated with an increased relative risk. In their review, Destounis et al. reported that the relative risk for breast cancer was higher when using semiquantitative percentage calculation methods (up to 4.64) than when using subjective qualitative assessments (up to 3.98) to determine mammographic breast density (Destounis et al. 2017). This is concordant with other studies comparing qualitative and quantitative methods of density measurement that demonstrated an increased risk when using quantitative approaches (Jeffers et al. 2017; Keller et al. 2015). It must be pointed out that most studies that have investigated the association between breast density and breast cancer risk did not use ACR Breast Imaging Reporting and Data System (BI-RADS) categories but instead used quantitative measures or a different classification such as the Wolfe classification. The use of the BI-RADS categories results in a similar but milder association of risk with breast density (Freer 2015).
Many studies focusing on the association between mammographic breast density and relative risk of breast cancer have also compared women with almost entirely fatty breasts and women with extremely dense breasts, finding that the relative risk for breast cancer is 4–6 in women with extremely dense breasts compared with women with almost entirely fatty breasts (Sickles 2010). However, as only approximately 10% of women have almost entirely fatty breasts and another 10% have extremely dense breasts, the results are potentially misleading (D’Orsi et al. 2013). Compared with the average women, the relative risk for breast cancer is approximately 1.2 in women with heterogeneously dense breasts and 2.1 in women with extremely dense breasts.
Although the relative risk of breast density as a risk factor is much smaller than age, family history, reproductive history, and genetic mutations, it is not negligible as mammographically dense breasts are relatively common (approximately 50% of the screening population). Therefore, breast density contributes significantly more to cancer risk in the population than other much stronger but less common risk factors, such as a BRCA 1 or 2 mutation carrier or high-risk status (McCormack and dos Santos 2006; Freer 2015; Boyd et al. 2011). The consistent association between increased breast density and cancer risk emphasizes its potential for risk prediction and risk stratification; thus, it might become a valuable tool in determining the best individualized screening plan for each woman.
In the past decade, breast density notification laws have been passed with the intent of informing women about their own breast density and possible benefits from supplemental screening methods such as breast ultrasound (Hooley 2017). Currently, there are 38 states of the USA with a legal obligation to provide a patient and her primary care physician with her breast density status and the risk posed by breast density. In addition, breast density notification legislation laws are in progress in other states and will be issued shortly. Breast density legislation provides a unique opportunity to strengthen patient-provider relationships by encouraging physicians to engage women in a conversation about the limitations, risks, and benefits of screening, as well as to provide women with greater autonomy; however, ineffective transfer of information may cause anxiety and patient confusion, which emphasizes the need for innovative information tools creating a better understanding for risk and health-care management (Miles et al. 2019; Slanetz et al. 2015; Are You Dense? 2018).
3 Assessment Methods
3.1 Mammography
3.1.1 Subjective Qualitative Assessment
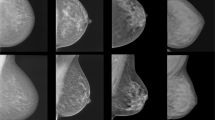
The assessment of breast density is usually performed based on the appearance of the amount of fibroglandular tissue relative to fatty tissue on mammography. To date, there are no recommendations or criteria for standardized assessment of breast density (Winkler et al. 2015; Colin et al. 2014). Methods range from the initial classification systems of Wolfe (1976) and Tabár (He et al. 2015) to the recent BI-RADS classification of the ACR, which is currently the most commonly used classification system. The differences of these classification systems are summarized in Table 2 (D’Orsi et al. 2013). The BI-RADS lexicon classification of breast density is mainly performed based on the subjective visual estimation. According to the current revised fifth edition of the BI-RADS atlas, published in 2013, breast density can be classified into ACR-MG-a, wherein the breasts are almost entirely fatty; ACR-MG-b, in which there are scattered areas of fibroglandular density; ACR-MG-c, wherein the breasts are heterogeneously dense, which may obscure small masses; and ACR-MG-d, in which the breasts are extremely dense, which lowers the sensitivity of mammography (D’Orsi et al. 2013). Women classified as either ACR-MG-a or -b are considered as having non-dense breasts, whereas women classified as either ACR-MG-c or -d are considered as having dense breasts. The revised fifth edition replaced a percentage categorization of total breast density with descriptive categories and identification of coalescent areas on the mammogram, acknowledging the possible masking of underlying breast masses, Fig. 1, and the potential benefit of supplemental screening (van der Waal et al. 2017).
Example images of the four breast density/composition categories defined by the fifth edition of the BI-RADS mammography atlas with descriptive categories indicating coalescent breast tissue with possible masking of underlying masses. ACR MG-a, the breasts are almost entirely fatty; ACR MG-b, there are scattered areas of fibroglandular density; ACR MG-c, the breasts are heterogeneously dense, which may obscure small masses; and ACR MG-d, the breasts are extremely dense, which lowers the sensitivity of mammography
Several studies have shown that subjective visual estimation of mammographic breast density is prone to error, with great inter- and intra-observer variability (Ciatto et al. 2012; Lee et al. 2015; Morrish et al. 2015; Wengert et al. 2016a). While training and experience can improve reader variability (Wengert et al. 2016a; Gao et al. 2008; Raza et al. 2016), subjective qualitative breast density assessment is not equipped to provide a reliable and reproducible objective assessment of breast density as a risk factor.
3.1.2 Objective Automated Quantitative Assessment
To overcome the limitations of subjective visual assessments, attempts have been made to develop automated quantitative technologies for breast density measurement. There are computer-aided semiautomated and fully automated measurement approaches available that allow either a two- or three-dimensional assessment of breast tissue structures. Cumulus™, the so-called gold standard of breast density assessment on mammography that has been validated by epidemiological studies, allows the estimation of the percentage area of dense breast tissue from mammographic images (Byng et al. 1994), yielding a higher reproducibility compared with BI-RADS visual assessment (Boyd et al. 2011). The limitation of Cumulus™ is that breast density measurements are derived from two-dimensional images and thus requires some user interaction, which renders it prone to bias. Recently, other three-dimensional mammography-based breast density measurement techniques have become available. Highnam (Highnam et al. 2007) and van Engeland (van Engeland et al. 2006) introduced fully automated approaches, Quantra (Morrish et al. 2015; Brandt et al. 2015; Wang et al. 2013) and Volpara (Lee et al. 2015; Morrish et al. 2015; Brandt et al. 2015; Wang et al. 2013), which allow mammography-based, volumetric, quantitative breast density measurements. Recently, yet another fully automated volumetric breast density measurement system “insight breast density,” which is integrated into the new MAMMOMAT Revelation (Siemens Healthineers, Erlangen, Germany) unit for three-dimensional mammography, has become available (Fig. 2).
Examples of increasing mammographic breast densities from left to right. Left craniocaudal (L-CC) and left mediolateral-oblique (L-MLO) were acquired with a Siemens MAMMOMAT Revelation (Siemens Healthcare GmbH, Erlangen, Germany). Density was assessed using the integrated insight breast density application, which calculates the total breast volume (Vol total, cm3) and the breast density volume (Vol BD, cm3 and %). Fully automated volumetric breast density measurements are displayed quantitatively and as the corresponding ACR BI-RADS category, A to D
Although the above approaches are fully automated, breast density calculation based on mammography may vary due to differences in tissue compression and breast positioning (Kopans 2008). All these approaches have in common a positive association between breast density and breast cancer risk. However, a paper from Gastounioti et al. (2016) discussed how the differences in quantitative breast density measurements are influenced by processed or raw mammographic images, as well as specific features of image acquisition, physical properties, and vendors.
3.2 Ultrasound
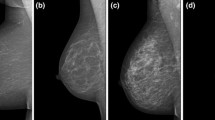
Ultrasound (US) of the breast is a ubiquitous, cost-effective, and reliable imaging modality, which is easily performed without the need for intravenous contrast application or ionizing radiation. To date, breast US cannot be reliably used for either a qualitative or quantitative breast density assessment. However, the latest version of the US BI-RADS atlas recommends an assessment of breast tissue composition with US using three descriptive categories: ACR-US-a, homogeneous background echotexture—fat; ACR-US-b, homogeneous background echotexture—fibroglandular; and ACR-US-c, heterogeneous background echotexture, Fig. 3 (D’Orsi et al. 2013). To overcome the drawback of handheld US, automated 3D whole-breast US (ABUS) has been introduced (Chae et al. 2013) and attempts have also been made to assess breast density with 3D ABUS using semiautomated techniques (Chen et al. 2016; Moon et al. 2011). Initial results suggest that ABUS might provide 3D volumetric imaging and accurate breast density measurement (Chen et al. 2016; Moon et al. 2011). US of the breast may be a valuable supplemental imaging modality to mammography in asymptomatic women with dense breast tissue to enable the detection of additional breast cancers invisible on mammography (Houssami and Ciatto 2011).
Example images of the three breast density/tissue composition categories defined by the fifth edition of the BI-RADS ultrasound atlas for screening-only purposes. ACR US-a, homogeneous background echotexture—fat; ACR US-b, homogeneous background echotexture—fibroglandular; and ACR US-c, heterogenous background echotexture
3.3 Magnetic Resonance Imaging
To address the problems of 2D mammography-based breast density assessment, promising approaches of volumetric, quantitative assessment of the amount of fibroglandular tissue on magnetic resonance imaging (MRI) have been developed and investigated.
In contrast to mammography, MRI allows radiation- and compression-free 3D imaging, which allows a standardized assessment of breast areas near the chest wall and axilla. MRI provides images related to the fat and water composition of the breast. Since the water composition is highly correlated with the prevalence of fibroglandular tissue, these images can be used for slice-by-slice segmentation of fibroglandular and fatty components and thereby allow quantitative breast density assessment (O’Flynn et al. 2015).
Many of the currently available approaches rely on the use of T1-weighted sequences, which provide grayscale images and therefore not enough tissue contrast to allow an objective assessment of breast parenchyma. In addition, most of these approaches require user interaction for breast area segmentation or threshold adjustments (van Engeland et al. 2006; Klifa et al. 2004, 2010; Lee et al. 1997; Thompson et al. 2009; Nie et al. 2008, 2010). Allowing accurate segmentation is one of the most important steps to precisely define breast and tissue borders. The boundaries for the segmentation are usually the anterior border of the major pectoral muscle and the anterior chest wall. The inferior border of the manubrium sterni and the submammary fold is the cranial and caudal boundaries. In addition, preferentially the variable subcutaneous fatty tissue of the cleavage should also be excluded from the segmentation. To overcome these, atlas- (Gubern-Merida et al. 2015; Wu et al. 2013) or template-aided (Wengert et al. 2015) semiautomated approaches with predefined breast models and automated adaption in real time have been investigated for an individual breast segmentation with high accuracy and robustness.
Meanwhile, there are already fully automated, volumetric measurement approaches for MRI-based measurements of the amount of fibroglandular breast tissue. Gubern-Mérida et al. (2014) used an expectation-maximization algorithm based on fuzzy C-means clustering, and Wu et al. (2013) developed a fully automated segmentation approach based on two-dimensional C-means clustering. Wengert et al. introduced an iterative segmentation for the separation of the bivariate signal intensity values on Dixon sequences, Fig. 4 (Wengert et al. 2015). The use of Dixon sequences for MRI-based measurements of the amount of fibroglandular tissue has been suggested previously (Graham et al. 1995) and tested with promising results (Wengert et al. 2015; Tagliafico et al. 2013, 2014), Fig. 5. Dixon sequences allow for improved reproducibility and accuracy of breast density measurements compared with conventional sequences (Wengert et al. 2016b, 2017). The integration of Dixon sequences into standard clinical dynamic contrast-enhanced MRI protocols, as well as for fibroglandular tissue quantification, is easily executed (Wengert et al. 2016b; Kuhl et al. 2014). Therefore, objective fibroglandular tissue segmentation derived from high-resolution Dixon sequences as the MRI-based reference standard for the assessment of the amount of FGT is a practical recommendation (Wengert et al. 2016b; Kuhl et al. 2014; Clauser et al. 2014; Mann et al. 2014).
Diagram of the process of fibroglandular tissue segmentation. For each individual breast and water/fat-based sequence, the program automatically segments an individual breast model, representing the identical 3D breast volume, with exclusion of the skin and the pectoralis muscle. (A) The signal intensity (SI) values of fat- and water-weighted pixel intensities were recorded and collected into a 2D histogram (top image). On the bottom, there is the 3D illustration of the histogram. (B) Thresholds for the corresponding fat and water SI values were automatically calculated by dividing the histogram into two regions half the distance between the two cluster peaks of the bimodal distribution of measured SI values. (C) Graphical illustration of the assignment for each voxel to be either fat tissue (red) or dense tissue (blue) into the 3D breast model. (Reprinted with permission from: Wengert GJ, Helbich TH, Vogl WD, et al. Introduction of an automated user independent quantitative volumetric magnetic resonance imaging breast density measurement system using the Dixon sequence: comparison with mammographic breast density assessment. Investigative Radiology. 2015;50(2):73–80. https://doi.org/10.1097/RLI.0000000000000102)
Examples of MRI-based breast density calculation; (a) fully automated measurements of fibroglandular (%gt) breast tissue based on the fat and water high-resolution Dixon images of a moderate (top row) and extremely dense breast (bottom row); with the corresponding threshold segmentation (template) and scatterplots, breast compartments are represented by the total segmented volume (cm3 volume), percentage of fibroglandular tissue (%gt), and percentage of fat tissue (%fat) (published in Wengert et al. 2015); (b) extract of the graphical computer interface illustrating the selection and thresholding process of the semiautomated assessment of fibroglandular breast tissue, with the output of the percentage of breast density (black circle). (Reprinted under a Creative Commons Attribution 4.0 International (CC BY 4.0) from Tagliafico A, Bignotti B, Tagliafigo G, et al. “Breast density assessment using a 3T MRI system: comparison among different sequences.” PLoS One. 2014;9(6):e99027. https://doi.org/10.1371/journal.pone.0099027)
A drawback of fully automated, volumetric MRI-based measurements is that the output of percentage values of breast density is not included in the current fifth edition of the ACR BI-RADS lexicon. The MRI BI-RADS lexicon currently contains the recommendation to assess the amount of fibroglandular tissue with MRI similar to mammography on a four-grade scale, Fig. 6: ACR-MRI-a, almost entirely fat; ACR-MRI-b, scattered fibroglandular tissue; ACR-MRI-c, heterogeneous fibroglandular tissue; and ACR-MRI-d, extreme fibroglandular tissue (D’Orsi et al. 2013). Recent studies have shown that subjective visual estimation of breast density on mammography and the amount of FGT on MRI are both prone to error with great inter- and intra-observer variability (Wengert et al. 2016a; Gao et al. 2008; Raza et al. 2016). While subjective visual estimation can be improved by reader training, similar to mammography, this seems a suboptimal solution compared with the objective quantitative MRI-based assessment of breast density as a risk factor (Lee et al. 2015; Morrish et al. 2015; Wengert et al. 2016a; Wang et al. 2013; Ciatto et al. 2005).
Example of T1-weighted high-resolution Dixon images of the four breast density/composition categories defined by the fifth edition of the BI-RADS MRI atlas with four categories similar to mammography. ACR MRI-a, almost entirely fat; ACR MRI-b, scattered fibroglandular tissue; ACR MRI-c, heterogeneous fibroglandular tissue, which may obscure small masses; and ACR MRI-d, extreme fibroglandular tissue. (Reprinted under a Creative Commons Attribution 4.0 International License from: Wengert GJ, Helbich, TH, Leithner D, et al. Multimodality Imaging of Breast Parenchymal Density and Correlation with Risk Assessment. Curr Breast Cancer Rep. 2019;11:23–33. https://doi.org/10.1007/s12609-019-0302-6)
4 New Avenues for Risk-Adapted Screening
While population-based screening programs using mammography with the aim of detecting breast cancer at an early stage have reduced cancer mortality by up to 49% (Broeders et al. 2012; Nickson et al. 2012), to date, there are no recommendations for risk-adapted screening.
Breast cancer risk estimation tools like the Gail and Tyrer-Cuzick models have been introduced with the purpose of identifying women who are at risk of developing breast cancer (Gail et al. 1989; Smith et al. 2014; Tyrer et al. 2004). The Gail model from the National Cancer Institute based on the general population is an eight-question tool using age, hormonal factors, benign disease, and number of fist-degree relatives who have already been diagnosed with breast cancer to estimate the relative risk of developing invasive breast cancer (Costantino et al. 1999). The Tyrer-Cuzick model uses similar risk factors from the Gail approach in conjunction with personal and genetic factors including the BRCA 1/2 genes for risk assessment of invasive breast cancer (Tyrer et al. 2004). However, it has been demonstrated that mammographic density is a stronger risk factor than any of the risk factors used in the Gail and Tyrer-Cuzick models; the combination of breast density with either the Gail or the Tyrer-Cuzick model resulted in a better breast cancer risk assessment (Brentnall et al. 2015). The Gail model, which is based on demographic and clinical data for breast cancer risk stratification, can be assessed online: https://www.mdcalc.com/gail-model-breast-cancer-risk. The Tyrer-Cuzick model providing a personal risk and risk of mutation carrier assessment can be found at http://ibis.ikonopedia.com/.
Moreover, the process of screening for breast cancer remains controversial with different recommendations between national breast cancer screening programs concerning the start points and the intervals for screening. A potential model for risk-adapted screening could include an initial risk stratification incorporating family and personal history, breast density assessed with mammography, and, potentially, lifestyle risk factors such as obesity (Mahoney et al. 2008) and alcohol (Zhang et al. 2007). Based on this model, women could be classified into different risk categories, e.g., low, intermediate, and high, and would undergo screening tailored to their individual risk.
Other avenues that can be explored for a more refined breast cancer risk stratification include the use of radiomics analyses and machine-learning techniques, such as deep learning.
Based on such refined risk stratification, women could then be offered risk-adapted screening with different imaging modalities. Low-risk women could continue to be screened with FFDM or, when available, DBT with synthesized mammography annually, biannually, or triennially based on national recommendations. Intermediate-risk women could undergo additional supplemental screening with US or MRI. High-risk women, who constitute a minority, could be offered MRI and mammography only in whom benefit has been demonstrated (e.g., BRCA 2 mutation carrier) (Phi et al. 2016). In this context, the Dutch DENSE trial investigates the effectiveness and cost-effectiveness of screening with mammography and MRI compared with those of screening with mammography alone in women with extremely dense breasts (Emaus et al. 2015). Recently published results showed that supplemental MRI screening of women with extremely dense breasts resulted in significantly fewer interval cancers compared to mammography as the sole screening methodology (2.5/1000 vs. 5.0/1000). The authors reported furthermore a cancer detection rate of 16.5/1000 women screened with MRI for breast cancer with a false-positive rate of 8.0% (Bakker et al. 2019). In addition, about 60% of the total screening population accepted the invitation of supplemental MRI screening. The most frequently stated reasons for not participating in this trial were MRI-related inconveniences, self-reported contraindications, and anxiety regarding the screening outcome (de Lange et al. 2018). Further results to better understand the role of MRI in this patient population are expected in the coming years after two rounds of screening are completed.
5 Summary
Breast density has recently become one of the hottest topics in breast imaging: firstly as it is an independent risk factor for breast cancer and secondly because high breast density reduces mammographic sensitivity due to a masking effect. Although the exact extent to which breast density is an independent risk factor remains controversial, there is consensus that the increased breast cancer risk is not solely attributable to the masking effect. This emphasizes the potential of breast density for cancer risk prediction and stratification, potentially becoming a valuable tool in determining the best screening plan for each woman and guiding supplemental screening methods. However, to be used in this context, breast density assessment must be reliable, reproducible, and accurate. Breast density has been predominantly assessed with mammography using qualitatively subjective visual inspection and the ACR BI-RADS classification. Due to substantial intra/inter-reader variability, semi/automated volumetric breast density measurement approaches with both mammography and MRI have been developed with excellent results. Initial attempts for automated volumetric breast density measurements with ABUS are promising. It is expected that these advances in breast density assessment will further define its role in breast cancer risk assessment and help tailoring breast cancer screening strategies to an individual woman’s risk, values, and preferences while also accounting for cost, potential harms, and important patient outcomes.
References
Aiello EJ, Buist DS, White E, Porter PL (2005) Association between mammographic breast density and breast cancer tumor characteristics. Cancer Epidemiol Biomarkers Prev 14(3):662–668
Andersen ZJ, Baker JL, Bihrmann K, Vejborg I, Sorensen TI, Lynge E (2014) Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast Cancer Res 16(1):R4
Are You Dense? 2008 (2018) https://www.areyoudenseadvocacy.org/dense
Bailey SL, Sigal BM, Plevritis SK (2010) A simulation model investigating the impact of tumor volume doubling time and mammographic tumor detectability on screening outcomes in women aged 40–49 years. J Natl Cancer Inst 102(16):1263–1271
Bakker MF, de Lange SV, Pijnappel RM, Mann RM, Peeters PHM, Monninkhof EM et al (2019) Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med 381(22):2091–2102
Ballard-Barbash R, Taplin SH, Yankaskas BC, Ernster VL, Rosenberg RD, Carney PA et al (1997) Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR Am J Roentgenol 169(4):1001–1008
Bernardi D, Macaskill P, Pellegrini M, Valentini M, Fanto C, Ostillio L et al (2016) Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. Lancet Oncol 17(8):1105–1113
Boyd NF, Jensen HM, Cooke G, Han HL (1992) Relationship between mammographic and histological risk factors for breast cancer. J Natl Cancer Inst 84(15):1170–1179
Boyd NF, Martin LJ, Yaffe MJ, Minkin S (2006) Mammographic density: a hormonally responsive risk factor for breast cancer. J Br Menopause Soc 12(4):186–193
Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E et al (2007) Mammographic density and the risk and detection of breast cancer. N Engl J Med 356(3):227–236
Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S (2010) Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst 102(16):1224–1237
Boyd NF, Martin LJ, Yaffe MJ, Minkin S (2011) Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res 13(6):223
Brandt KR, Scott CG, Ma L, Mahmoudzadeh AP, Jensen MR, Whaley DH et al (2015) Comparison of clinical and automated breast density measurements: implications for risk prediction and supplemental screening. Radiology 2015:151261
Brentnall AR, Harkness EF, Astley SM, Donnelly LS, Stavrinos P, Sampson S et al (2015) Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 17(1):147
Broeders M, Moss S, Nystrom L, Njor S, Jonsson H, Paap E et al (2012) The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen 19(Suppl 1):14–25
Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ (1994) The quantitative analysis of mammographic densities. Phys Med Biol 39(10):1629–1638
Byrne C, Ursin G, Martin CF, Peck JD, Cole EB, Zeng D et al (2017) Mammographic density change with estrogen and progestin therapy and breast cancer risk. J Natl Cancer Inst 109(9):djx001
Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM et al (2003) Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med 138(3):168–175
Chae EY, Shin HJ, Kim HJ, Yoo H, Baek S, Cha JH et al (2013) Diagnostic performance of automated breast ultrasound as a replacement for a hand-held second-look ultrasound for breast lesions detected initially on magnetic resonance imaging. Ultrasound Med Biol 39(12):2246–2254
Checka CM, Chun JE, Schnabel FR, Lee J, Toth H (2012) The relationship of mammographic density and age: implications for breast cancer screening. AJR Am J Roentgenol 198(3):W292–W295
Chen JH, Lee YW, Chan SW, Yeh DC, Chang RF (2016) Breast density analysis with automated whole-breast ultrasound: comparison with 3-D magnetic resonance imaging. Ultrasound Med Biol 42(5):1211–1220
Ciatto S, Houssami N, Apruzzese A, Bassetti E, Brancato B, Carozzi F et al (2005) Categorizing breast mammographic density: intra- and interobserver reproducibility of BI-RADS density categories. Breast 14(4):269–275
Ciatto S, Bernardi D, Calabrese M, Durando M, Gentilini MA, Mariscotti G et al (2012) A first evaluation of breast radiological density assessment by QUANTRA software as compared to visual classification. Breast 21(4):503–506
Ciatto S, Houssami N, Bernardi D, Caumo F, Pellegrini M, Brunelli S et al (2013) Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 14(7):583–589
Clauser P, Pinker K, Helbich TH, Kapetas P, Bernathova M, Baltzer PA (2014) Fat saturation in dynamic breast MRI at 3 Tesla: is the Dixon technique superior to spectral fat saturation? A visual grading characteristics study. Eur Radiol 24(9):2213–2219
Colin C, Schott AM, Valette PJ (2014) Mammographic density is not a worthwhile examination to distinguish high cancer risk women in screening. Eur Radiol 24(10):2412–2416
Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J et al (1999) Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst 91(18):1541–1548
D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA (2013) ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. American College of Radiology, Reston, VA
de Lange SV, Bakker MF, Monninkhof EM, Peeters PHM, de Koekkoek-Doll PK, Mann RM et al (2018) Reasons for (non)participation in supplemental population-based MRI breast screening for women with extremely dense breasts. Clin Radiol 73(8):759.e1–759.e9
Destounis SV, Morgan R, Arieno A (2015) Screening for dense breasts: digital breast tomosynthesis. AJR Am J Roentgenol 204(2):261–264
Destounis S, Arieno A, Morgan R, Roberts C, Chan A (2017) Qualitative versus quantitative mammographic breast density assessment: applications for the US and abroad. Diagnostics (Basel) 7(2):30
Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE (2006) Adult weight change and risk of postmenopausal breast cancer. JAMA 296(2):193–201
Emaus MJ, Bakker MF, Peeters PH, Loo CE, Mann RM, de Jong MD et al (2015) MR imaging as an additional screening modality for the detection of breast cancer in women aged 50–75 years with extremely dense breasts: The DENSE Trial Study Design. Radiology 277(2):527–537
Freer PE (2015) Mammographic breast density: impact on breast cancer risk and implications for screening. Radiographics 35(2):302–315
Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS et al (2014) Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 311(24):2499–2507
Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C et al (1989) Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 81(24):1879–1886
Gao J, Warren R, Warren-Forward H, Forbes JF (2008) Reproducibility of visual assessment on mammographic density. Breast Cancer Res Treat 108(1):121–127
Gastounioti A, Oustimov A, Keller BM, Pantalone L, Hsieh MK, Conant EF et al (2016) Breast parenchymal patterns in processed versus raw digital mammograms: a large population study toward assessing differences in quantitative measures across image representations. Med Phys 43(11):5862
Ghosh K, Brandt KR, Sellers TA, Reynolds C, Scott CG, Maloney SD et al (2008) Association of mammographic density with the pathology of subsequent breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev 17(4):872–879
Gilbert FJ, Tucker L, Gillan MG, Willsher P, Cooke J, Duncan KA et al (2015) The TOMMY trial: a comparison of TOMosynthesis with digital MammographY in the UK NHS Breast Screening Programme—a multicentre retrospective reading study comparing the diagnostic performance of digital breast tomosynthesis and digital mammography with digital mammography alone. Health Technol Assess 19(4):i–xxv, 1–136
Graham SJ, Stanchev PL, Lloyd-Smith JO, Bronskill MJ, Plewes DB (1995) Changes in fibroglandular volume and water content of breast tissue during the menstrual cycle observed by MR imaging at 1.5 T. J Magn Reson Imaging 5(6):695–701
Gubern-Merida A, Kallenberg M, Platel B, Mann RM, Marti R, Karssemeijer N (2014) Volumetric breast density estimation from full-field digital mammograms: a validation study. PLoS One 9(1):e85952
Gubern-Merida A, Kallenberg M, Mann RM, Marti R, Karssemeijer N (2015) Breast segmentation and density estimation in breast MRI: a fully automatic framework. IEEE J Biomed Health Inform 19(1):349–357
Harris HR, Tamimi RM, Willett WC, Hankinson SE, Michels KB (2011) Body size across the life course, mammographic density, and risk of breast cancer. Am J Epidemiol 174(8):909–918
Harvie M, Howell A, Vierkant RA, Kumar N, Cerhan JR, Kelemen LE et al (2005) Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev 14(3):656–661
He W, Hogg P, Juette A, Denton ER, Zwiggelaar R (2015) Breast image pre-processing for mammographic tissue segmentation. Comput Biol Med 67:61–73
Highnam R, Jeffreys M, McCormack V, Warren R, Davey Smith G, Brady M (2007) Comparing measurements of breast density. Phys Med Biol 52(19):5881–5895
Hooley RJ (2017) Breast density legislation and clinical evidence. Radiol Clin North Am 55(3):513–526
Hopper JL, Nguyen TL, Stone J, Aujard K, Matheson MC, Abramson MJ et al (2016) Childhood body mass index and adult mammographic density measures that predict breast cancer risk. Breast Cancer Res Treat 156(1):163–170
Houssami N, Ciatto S (2011) The evolving role of new imaging methods in breast screening. Prev Med 53(3):123–126
Huo CW, Chew GL, Britt KL, Ingman WV, Henderson MA, Hopper JL et al (2014) Mammographic density-a review on the current understanding of its association with breast cancer. Breast Cancer Res Treat 144(3):479–502
Jeffers AM, Sieh W, Lipson JA, Rothstein JH, McGuire V, Whittemore AS et al (2017) Breast cancer risk and mammographic density assessed with semiautomated and fully automated methods and BI-RADS. Radiology 282(2):348–355
Keller BM, Chen J, Daye D, Conant EF, Kontos D (2015) Preliminary evaluation of the publicly available Laboratory for Breast Radiodensity Assessment (LIBRA) software tool: comparison of fully automated area and volumetric density measures in a case-control study with digital mammography. Breast Cancer Res 17:117
Kerlikowske K, Ichikawa L, Miglioretti DL, Buist DS, Vacek PM, Smith-Bindman R et al (2007) Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst 99(5):386–395
Kerlikowske K, Hubbard RA, Miglioretti DL, Geller BM, Yankaskas BC, Lehman CD et al (2011) Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern Med 155(8):493–502
Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W et al (2015) Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst 107(3):djv088
Klifa C, Carballido-Gamio J, Wilmes L, Laprie A, Lobo C, Demicco E et al (2004) Quantification of breast tissue index from MR data using fuzzy clustering. Conf Proc IEEE Eng Med Biol Soc 3:1667–1670
Klifa C, Carballido-Gamio J, Wilmes L, Laprie A, Shepherd J, Gibbs J et al (2010) Magnetic resonance imaging for secondary assessment of breast density in a high-risk cohort. Magn Reson Imaging 28(1):8–15
Kopans DB (2008) Basic physics and doubts about relationship between mammographically determined tissue density and breast cancer risk. Radiology 246(2):348–353
Krishnan K, Baglietto L, Stone J, Simpson JA, Severi G, Evans CF et al (2017) Longitudinal study of mammographic density measures that predict breast cancer risk. Cancer Epidemiol Biomarkers Prev 26(4):651–660
Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB (2014) Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol 32(22):2304–2310
Lee NA, Rusinek H, Weinreb J, Chandra R, Toth H, Singer C et al (1997) Fatty and fibroglandular tissue volumes in the breasts of women 20–83 years old: comparison of X-ray mammography and computer-assisted MR imaging. AJR Am J Roentgenol 168(2):501–506
Lee HN, Sohn YM, Han KH (2015) Comparison of mammographic density estimation by Volpara software with radiologists’ visual assessment: analysis of clinical-radiologic factors affecting discrepancy between them. Acta Radiol 56(9):1061–1068
Mahoney MC, Bevers T, Linos E, Willett WC (2008) Opportunities and strategies for breast cancer prevention through risk reduction. CA Cancer J Clin 58(6):347–371
Mann RM, Mus RD, van Zelst J, Geppert C, Karssemeijer N, Platel B (2014) A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Invest Radiol 49(9):579–585
McCormack VA, dos Santos SI (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15(6):1159–1169
McDonald ES, Oustimov A, Weinstein SP, Synnestvedt MB, Schnall M, Conant EF (2016) Effectiveness of digital breast tomosynthesis compared with digital mammography: outcomes analysis from 3 years of breast cancer screening. JAMA Oncol 2(6):737–743
Miles RC, Lehman C, Warner E, Tuttle A, Saksena M (2019) Patient-reported breast density awareness and knowledge after breast density legislation passage. Acad Radiol 26(6):726–731
Moon WK, Shen YW, Huang CS, Luo SC, Kuzucan A, Chen JH et al (2011) Comparative study of density analysis using automated whole breast ultrasound and MRI. Med Phys 38(1):382–389
Morrish OW, Tucker L, Black R, Willsher P, Duffy SW, Gilbert FJ (2015) Mammographic breast density: comparison of methods for quantitative evaluation. Radiology 2015:141508
Nguyen TL, Schmidt DF, Makalic E, Dite GS, Stone J, Apicella C et al (2013) Explaining variance in the cumulus mammographic measures that predict breast cancer risk: a twins and sisters study. Cancer Epidemiol Biomarkers Prev 22(12):2395–2403
Nickson C, Mason KE, English DR, Kavanagh AM (2012) Mammographic screening and breast cancer mortality: a case-control study and meta-analysis. Cancer Epidemiol Biomarkers Prev 21(9):1479–1488
Nie K, Chen JH, Chan S, Chau MK, Yu HJ, Bahri S et al (2008) Development of a quantitative method for analysis of breast density based on three-dimensional breast MRI. Med Phys 35(12):5253–5262
Nie K, Chang D, Chen JH, Hsu CC, Nalcioglu O, Su MY (2010) Quantitative analysis of breast parenchymal patterns using 3D fibroglandular tissues segmented based on MRI. Med Phys 37(1):217–226
O’Flynn EA, Ledger AE, deSouza NM (2015) Alternative screening for dense breasts: MRI. AJR Am J Roentgenol 204(2):W141–W149
Phi XA, Saadatmand S, De Bock GH, Warner E, Sardanelli F, Leach MO et al (2016) Contribution of mammography to MRI screening in BRCA mutation carriers by BRCA status and age: individual patient data meta-analysis. Br J Cancer 114(6):631–637
Pisano ED, Gatsonis CA, Yaffe MJ, Hendrick RE, Tosteson AN, Fryback DG et al (2005) American College of Radiology Imaging Network digital mammographic imaging screening trial: objectives and methodology. Radiology 236(2):404–412
Price ER, Hargreaves J, Lipson JA, Sickles EA, Brenner RJ, Lindfors KK et al (2013) The California breast density information group: a collaborative response to the issues of breast density, breast cancer risk, and breast density notification legislation. Radiology 269(3):887–892
Raza S, Mackesy MM, Winkler NS, Hurwitz S, Birdwell RL (2016) Effect of training on qualitative mammographic density assessment. J Am Coll Radiol 13(3):310–315
Rhodes DJ, Radecki Breitkopf C, Ziegenfuss JY, Jenkins SM, Vachon CM (2015) Awareness of breast density and its impact on breast cancer detection and risk. J Clin Oncol 33(10):1143–1150
Roubidoux MA, Bailey JE, Wray LA, Helvie MA (2004) Invasive cancers detected after breast cancer screening yielded a negative result: relationship of mammographic density to tumor prognostic factors. Radiology 230(1):42–48
Sickles EA (2010) The use of breast imaging to screen women at high risk for cancer. Radiol Clin North Am 48(5):859–878
Slanetz PJ, Freer PE, Birdwell RL (2015) Breast-density legislation—practical considerations. N Engl J Med 372(7):593–595
Smith RA, Manassaram-Baptiste D, Brooks D, Cokkinides V, Doroshenk M, Saslow D et al (2014) Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 64(1):30–51
Sterns EE, Zee B (2000) Mammographic density changes in perimenopausal and postmenopausal women: is effect of hormone replacement therapy predictable? Breast Cancer Res Treat 59(2):125–132
Tagliafico A, Tagliafico G, Astengo D, Airaldi S, Calabrese M, Houssami N (2013) Comparative estimation of percentage breast tissue density for digital mammography, digital breast tomosynthesis, and magnetic resonance imaging. Breast Cancer Res Treat 138(1):311–317
Tagliafico A, Bignotti B, Tagliafico G, Astengo D, Martino L, Airaldi S et al (2014) Breast density assessment using a 3T MRI system: comparison among different sequences. PLoS One 9(6):e99027
Thompson DJ, Leach MO, Kwan-Lim G, Gayther SA, Ramus SJ, Warsi I et al (2009) Assessing the usefulness of a novel MRI-based breast density estimation algorithm in a cohort of women at high genetic risk of breast cancer: the UK MARIBS study. Breast Cancer Res 11(6):R80
Tyrer J, Duffy SW, Cuzick J (2004) A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23(7):1111–1130
Vachon CM, Pankratz VS, Scott CG, Maloney SD, Ghosh K, Brandt KR et al (2007) Longitudinal trends in mammographic percent density and breast cancer risk. Cancer Epidemiol Biomarkers Prev 16(5):921–928
van der Waal D, Ripping TM, Verbeek AL, Broeders MJ (2017) Breast cancer screening effect across breast density strata: a case-control study. Int J Cancer 140(1):41–49
van Duijnhoven FJ, Peeters PH, Warren RM, Bingham SA, van Noord PA, Monninkhof EM et al (2007) Postmenopausal hormone therapy and changes in mammographic density. J Clin Oncol 25(11):1323–1328
van Engeland S, Snoeren PR, Huisman H, Boetes C, Karssemeijer N (2006) Volumetric breast density estimation from full-field digital mammograms. IEEE Trans Med Imaging 25(3):273–282
Wanders JO, Bakker MF, Veldhuis WB, Peeters PH, van Gils CH (2015) The effect of weight change on changes in breast density measures over menopause in a breast cancer screening cohort. Breast Cancer Res 17:74
Wang J, Azziz A, Fan B, Malkov S, Klifa C, Newitt D et al (2013) Agreement of mammographic measures of volumetric breast density to MRI. PLoS One 8(12):e81653
Wengert GJ, Helbich TH, Vogl WD, Baltzer P, Langs G, Weber M et al (2015) Introduction of an automated user-independent quantitative volumetric magnetic resonance imaging breast density measurement system using the Dixon sequence: comparison with mammographic breast density assessment. Invest Radiol 50(2):73–80
Wengert GJ, Helbich TH, Woitek R, Kapetas P, Clauser P, Baltzer PA et al (2016a) Inter- and intra-observer agreement of BI-RADS-based subjective visual estimation of amount of fibroglandular breast tissue with magnetic resonance imaging: comparison to automated quantitative assessment. Eur Radiol 26(11):3917–3922
Wengert GJ, Pinker-Domenig K, Helbich TH, Vogl WD, Clauser P, Bickel H et al (2016b) Influence of fat-water separation and spatial resolution on automated volumetric MRI measurements of fibroglandular breast tissue. NMR Biomed 29(6):702–708
Wengert GJ, Pinker K, Helbich TH, Vogl WD, Spijker SM, Bickel H et al (2017) Accuracy of fully automated, quantitative, volumetric measurement of the amount of fibroglandular breast tissue using MRI: correlation with anthropomorphic breast phantoms. NMR Biomed. https://doi.org/10.1002/nbm.3705
Winkler NS, Raza S, Mackesy M, Birdwell RL (2015) Breast density: clinical implications and assessment methods. Radiographics 35(2):316–324
Wolfe JN (1976) Breast patterns as an index of risk for developing breast cancer. AJR Am J Roentgenol 126(6):1130–1137
Wu S, Weinstein SP, Conant EF, Kontos D (2013) Automated fibroglandular tissue segmentation and volumetric density estimation in breast MRI using an atlas-aided fuzzy C-means method. Med Phys 40(12):122302
Zhang SM, Lee IM, Manson JE, Cook NR, Willett WC, Buring JE (2007) Alcohol consumption and breast cancer risk in the Women’s Health Study. Am J Epidemiol 165(6):667–676
Acknowledgements
Funding was provided in part by the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement Nr. 688188.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wengert, G.J., Pinker, K., Helbich, T. (2022). Impact and Assessment of Breast Density. In: Fuchsjäger, M., Morris, E., Helbich, T. (eds) Breast Imaging . Medical Radiology(). Springer, Cham. https://doi.org/10.1007/978-3-030-94918-1_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-94918-1_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94917-4
Online ISBN: 978-3-030-94918-1
eBook Packages: MedicineMedicine (R0)