Abstract
Partial or total iris defects (aniridia), either congenital or acquired, may represent a therapeutic and surgical challenge. Not only present these patients with several visual disabilities and concomitant eye diseases (e.g., corneal opacity, cataract, glaucoma), they experience considerable aesthetic impairment. Recently, a new biocompatible foldable and custom-made silicon iris prosthesis was introduced (Customflex® Artificialiris; HumanOptics, Erlangen, Germany), which enabled development of new surgical techniques for the reconstruction of anterior segment in patients with iris defects in aphakic or pseudophakic eyes, whether with intact or compromised capsular support. Currently, several surgical techniques are reported for artificial iris implantation including implantation in the capsular bag or ciliary sulcus, four-floating suture technique, and scleral fixation techniques in combination with IOL implantation. This chapter provides a systematic overview of current surgical approaches for the artificial iris implantation with a thorough step-by-step technique description and case presentation including important surgical, pre- and postoperative considerations, and the reported cosmetic and functional results.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Artificial iris
- Anterior segment reconstruction
- Aniridia
- Ocular trauma
- Iris prosthesis
- Iris defects
- Globe rupture
- Penetrating injury
- Four-floating suture technique
- Modified Yamane technique
- Canabrava
- 6.0 polypropylene suture
- Artificial iris-IOL complex
-
Artificial iris (AI) can be implanted using four different surgical techniques.
-
8 years after anterior segment reconstruction, PKP, AI, and IOL implantation, the BCVA is 20/20.
-
No CME up to 8 years after AI implantation when using four-floating suture technique.
-
Modified Yamane AI and IOL implantation.
-
1.8 mm injector is used for AI implantation into the bag.
Introduction
Iris defects, either partial or total (aniridia), may be congenital or acquired. Congenital aniridia is most commonly caused by mutations in PAX6, FOXC1, PITX2, and CYP1B1 genes [1]. Acquired iris defects develop secondary to penetrating or blunt ocular trauma and iatrogenic trauma (e.g., due to iris tumor resections) or can be associated with iridocorneal endothelial (ICE) syndromes due to severe iris atrophy or other developmental ocular anomalies [2]. These patients present with several visual disabilities such as increased sensitivity to glare, reduced visual acuity, contrast sensitivity, lost depth of focus, and aberration errors. Moreover, aesthetic impairment can be considerable.
Although various conservative treatment approaches are available, such as tinted anti-glare spectacles, [3] colored contact lenses, [4] and lamellar intrastromal corneal tattooing, [5] they may yield insufficient functional and aesthetic results [3]. Surgical reconstruction of iris defects, on the other hand, can be challenging [6, 7]. In case of smaller circumscribed iris defects, pupil reconstruction can be achieved by direct iris suture placement [8]. However, if total aniridia or large and multiple complex iris defects exist, surgical reconstruction with an implantation of artificial iris prosthesis may be preferred. [6, 7] Currently, there are several implants available on the market such as Morcher (Stuttgart, Germany), which achieved good clinical outcomes, [6, 7] and Ophtec (Groningen, the Netherlands) artificial iris implants. Unfortunately, some of these systems usually require large corneal incisions of at least 10 mm [2]. Since 2002, a new foldable silicone-based custom-made iris prosthesis (Customflex® Artificialiris; HumanOptics, Erlangen, Germany) [9] is available, and several novel surgical iris reconstruction techniques for medical and aesthetic rehabilitation were introduced with promising results [3, 8]. In addition to the silicone-based iris prosthesis, a hydrophobic acrylic foldable iris diaphragm either with or without optic which can be implanted in capsular bag or ciliary sulcus is also available on the market (Reper-NN LTD.; Russia) [10, 11]. In the following chapter, we will describe in a step-by-step fashion the major surgical approaches for artificial iris implantation and their advantages and disadvantages. In addition, most common surgical and postoperative complications as well as follow-up results will be discussed.
Artificial Iris Design
A Short History of Artificial Iris Devices
The first prosthetic iris device was implanted already in the 1960s by Peter Choyce [12]. The devices were made of polymethyl methacrylate (PMMA) and were implanted directly into the angle [12]. They were abandoned as they were likely to cause glaucoma and corneal failure [12]. In 1991, the next generation of prosthetic iris devices came on the market that were designed by Sundmacher et al. along with Morcher GMBH [12]. They were still made of PMMA optics with a black outer PMMA diaphragm, thus requiring extremely large incisions [12]. Subsequently, Volker Rasch and Morcher developed an injectable multipiece iris prosthesis that was a capsular tension ring-type device which was implanted in the capsular bag and was first implanted by Kenneth Rosenthal and then later by Robert Osher in 1996 [12]. In the following years, Morcher and Ophtec each developed several different artificial iris devices designed with or without optics offering also more color options, which improved cosmesis. However, they still did not exactly match the fellow eye, and some still required large incisions (Morcher) [12].
Custom-Made Silicon Iris Prosthesis
In the early 2000s, HumanOptics along with Hans Reinhard Koch started to design a silicone-based custom iris prosthetic device [9, 12]. In 2011, the new foldable and biocompatible custom-made silicon iris prosthesis was approved in the European Economic Area by Conformite Europeenne marking [8] and in 2018 by US Food and Drug Administration in the United States [3]. The surface of the hydrophobic-pigmented silicon artificial iris (Customflex® Artificialiris; HumanOptics, Erlangen, Germany) is custom-made [9]. It consists of a black optically opaque posterior surface and a colored individually designed and hand-painted anterior surface based on patient’s high-resolution photographs of his iris remnants and/or the healthy iris of the fellow eye. In case of intraoperative suture placement, it can be manufactured with an embedded fiber meshwork to prevent suture migration. The total artificial iris diameter is 12.8 mm, which can be trephined to custom dimensions during surgery. It has a fixed pupil diameter of 3.35 mm and is thicker at the pupillary margin (0.40 mm). The thickness decreases to 0.25 mm at the periphery. It is designed for posterior chamber implantation in pseudophakic or aphakic patients, alone or combined with an IOL. [9] On the horizon are also several other devices including a self-regulating artificial iris capable of self-changing pupil size [13, 14].
Preoperative Patient Evaluation
Artificial iris (AI) implants are indicated in patients with partial or complete aniridia in pseudophakic or aphakic eyes, either with or without remaining lens capsular support. As AI may induce cataract formation, it should not be used in phakic eyes [8, 15]. Table 29.1 summarizes most common indications for AI implantation such as traumatic aniridia due to penetrating eye injury, iatrogenic iris defects, traumatic mydriasis after blunt ocular trauma, congenital aniridia, Urrets-Zavalia syndrome, Axenfeld-Rieger syndrome, and ICE syndrome [2]. The overall complexity of the anterior segment disease will determine which surgical approach the surgeon will use. Thus, to achieve the best postoperative results, careful preoperative planning and detailed patient history is required before iris reconstruction surgery is performed.
First, all patients should undergo precise clinical evaluation and several preoperative measurements, which include best corrected visual acuity, intraocular pressure measurement, endothelial cell density evaluation, axial length, keratometry, the white-to-white (WTW) diameter measured horizontally and vertically, and macular optical coherence tomography. In case of obscured optical clarity, an additional ultrasound examination should be obtained to exclude severe posterior segment disease. Second, to achieve the best postoperative aesthetic results, high-resolution true-color photographs of both eyes are mandatory. The surgeon needs to preoperatively decide if the AI will be fixated with sutures to order the appropriate design (with polymer fiber meshwork).
Surgical Techniques
In the past few years, several surgical techniques for AI implantation have been developed in regard to (a) size and extend of the iris defect, (b) the preoperative lens status (e.g., phakic, pseudophakic, aphakic), and (c) whether the lens capsular support is present or not. Based on these preoperative considerations, three major implantation techniques can be described: (1) partial AI implantation; (2) complete AI implantation into either capsular bag or into ciliary sulcus; and (3) complete AI implantation with scleral fixation.
Basic Surgical Considerations
In cases of sufficient capsular support, AI implantation should be performed through a small corneal incision (minimum 2.8 mm and maximum 7.0 mm) [8] using either forceps or an injector system. The foldable iris implant is then directly inserted into a capsular bag or ciliary sulcus (as discussed in more detail below), which can be additionally sutured to sclera or residual iris using Prolene sutures to achieve better implant position and stability. In aphakic eyes, this procedure can be combined with an IOL implantation as the total thickness of an artificial iris (0.25–0.4 mm), IOL (0.5–1.0 mm), and the residual iris tissue (about 0.5 mm) does not exceed the thickness of a natural human lens (3.5–5.0 mm) [8]. If necessary, the artificial iris can intraoperatively be trimmed with a trephine to the desirable diameter according to the patient’s WTW measurements (approximately WTW distance minus 0.5 mm) [2, 3, 8].
Partial Artificial Iris Implantation
This technique may be suitable in selected cases of smaller iris defects affecting 1–3 clock hours [8]. First, the AI implant with a fiber meshwork is cut with scissors to the appropriate size and then inserted with forceps into the anterior chamber. The AI segment is then sutured in place to the patients’ own iris remnant. This procedure is reported to be time consuming [8].
Complete Artificial Iris Implantation into Capsular Bag or Ciliary Sulcus
Sulcus-Fixation in Pseudophakic Eyes
This technique is used in preoperatively pseudophakic eyes with a stable IOL placed in the capsular bag with a large iris defects (> 2 clock hours) or with a persistent mydriasis. An injector system is used to implant the foldable artificial iris through a small corneal or scleral incision (2.8 mm) into the anterior chamber that is unfolded in the ciliary sulcus. This approach does not require suturing; thus it is quick and easy to perform. [8] However, as presented in Fig. 29.1, contact between the AI and intraocular structures might cause late and chronic intraocular low-grade inflammation recalcitrant to medical therapy, which resolved after AI was explanted. Similar to our surgical case presented in Fig. 29.1, we needed to explant AI implants from two other patients that underwent AI implantation into sulcus in another facility and presented to us with chronic low-grade inflammation. We hypothesized that in those cases, intraocular inflammation that occurred late after surgery might be a direct result of contact between AI and intraocular structures. Bahadur et al. also reported of required artificial iris exchange in five patients commonly due to corneal decompensation [16]. Original models included Ophtec, Morcher BrightOcular, and HumanOptics [16]. Three of the original artificial irises were passively placed in capsular bag or ciliary sulcus, and all five were exchanged with Morcher or HumanOptics, and all were implanted with suture fixation to sclera, although final visual acuity in most cases remained poor especially due to corneal decompensation and glaucoma [16]. However, recently in the US Food and Drug Administration (FDA) prospective, non-randomized, multicenter study, which determined the safety and effectiveness of the CustomFlexTM Artificial Iris for the treatment of eyes with congenital or acquired full or partial iris defects including 447 eyes, had zero cases that required device explant due to chronic inflammation [17, 18]. Although only 44 of 447 (9,84%) eyes underwent AI passive sulcus fixation without suturing with the last follow-up visit being scheduled 12 months postoperatively, [17, 18] it was speculated that inflammation incidence was lowered by modifying the device edge with circular trephination [17, 18].
Postoperative results of artificial iris implantation into sulcus. (a) Preoperative image of a patient that presented with posttraumatic mydriasis after blunt ocular trauma. First, we performed posttraumatic cataract extraction. At the time of surgery, capsular tension ring and intraocular lens were implanted into the capsular bag. The capsular tension ring was sutured to the sclera using 9.0 polypropylene suture. Due to posttraumatic mydriasis, we decided to implant an artificial iris. (b) Postoperative image of left eye 12 months after artificial iris implantation into the sulcus. (c) The aesthetic outcome was great, and the patient was very satisfied. Visual acuity was 20/20. However, 2 years later, her left eye became red and painful. Visual acuity dropped. On examination we found light anterior chamber inflammation. (d) Optical coherence tomography showed cystoid macular edema. (e) Ultrasound biomicroscopy revealed contact between the artificial iris and ciliary body and/or iris pigment epithelium, which might cause rubbing. She was started on topical corticosteroids and nonsteroidal anti-inflammatory drops, but the cystoid macular edema persisted; therefore intravitreal triamcinolone injections were started. Nevertheless, due to recalcitrant inflammation, we had to explant the artificial iris. We hypothesized that contact between the artificial iris and intraocular structures induced chronic inflammation resistant to medical treatment
Capsular Bag Implantation with a Combined Standard Cataract Surgery
This technique is safe and suitable if the patient has a preexisting cataract and a large iris defect (Video 29.1). In a large case series of 96 cases in the Figueiredo and Snyder study, 91% of artificial iris devices were implanted into the capsular bag [19]. Despite multiple ocular comorbidities, postoperative results showed no cases in which the iris device explant was required. [19] First, a standard cataract extraction is performed taking care to perform a minimal corneal incision of 2.2 mm and a planned capsulorhexis diameter of 6 mm. Phacoemulsification and implantation of an IOL is performed in a standard fashion (Figs. 29.2 and 29.3). It is mandatory to implant a capsular tension ring (CTR) to prevent future capsular shrinkage and decentration of the AI. Iridectomies are not necessary [8]. However, manipulation needs to be gentle to prevent rupture of continuous curvilinear capsulorhexis edge, as this would prevent from further artificial iris implantation into capsular bag.
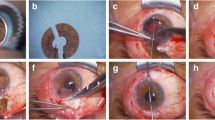
Artificial iris implantation into capsular bag. In our opinion, the safest way to implant an artificial iris is implantation into capsular bag. Herein, we present a case of artificial iris implantation and cataract removal in a 20-year-old patient after penetrating eye injury to the left eye. (a) First, intumescent cataract extraction with a capsular tension ring (CTR) and IOL implantation was performed. Of note, if artificial iris will be implanted into a capsular bag, it is mandatory to insert a CTR into the capsular bag. Next, the capsulorhexis was enlarged. (b) The artificial iris was trephined to a 9.0 mm diameter, (c) folded, and bimanually inserted into a 1.8 mm injector. (d) The folded artificial iris was injected into the anterior chamber through a 2.2 mm incision and glided into the capsular bag using Ogawa hook as the second instrument. The artificial iris was unfolded bimanually. The periphery of the unfolded artificial iris is glided into the capsular bag by elevating the continuous curvilinear capsulorhexis (CCC) rim with one instrument and using the second instrument to glide it below. The manipulation needs to be gentle to prevent rupture of CCC edge, as this would prevent from further artificial iris implantation into capsular bag. Thus, the CCC should be big enough to allow artificial iris fixation into the bag
Postoperative results of artificial iris implantation into capsular bag. Preoperative (a) and 10-month postoperative (b, c) images after artificial iris implantation into the capsular bag of the left eye of a 20-year-old patient with posttraumatic aniridia and cataract (the same patient as in Fig. 29.2) due to penetrating eye injury (perforation through cornea). (a) Patient presented with posttraumatic corneal leukoma, white cataract, and iris defect. (b, c) The postoperative cosmetic appearance was excellent with visual acuity of 20/20 10 months after surgery. No intraoperative or postoperative complications occurred
Complete Artificial Iris Implantation with Scleral Fixation
If capsular support is compromised and extensive iris defects are present, scleral fixation can be used in several surgical variants.
Four-Floating Suture Technique (Pfeifer Technique)
To avoid contact with the AI and the intraocular structures, we modified previous fixation techniques. The four-floating suture technique enables AI implantation with no contact to the intraocular structures as it floats on the four sutures that are fixated to the sclera (Fig. 29.4, Video 29.2).
Schematic representation of four-floating suture technique (Pfeifer technique). (a) The sutures are tied to the artificial iris using a lasso technique. (b, c) The artificial iris is centered with the four sutures that are fixated to the sclera. Care must be taken that the edge of the artificial iris is not in contact with pigment epithelium of the remaining iris or ciliary body (d). (b, c) Ideally, there should be a red reflex barely seen between the edge of the artificial iris and the limbus
Thus, to avoid contact between AI and the intraocular structures, in this technique AI without meshwork is trimmed to 10.5 mm diameter using a trephine and sutured using four 9.0 polypropylene sutures. The sutures are tied to the artificial iris using a lasso technique (Fig. 29.4a). These sutures are attached to long-curved needles. Surgeon is sitting at the 12 o’clock position, where the main incision is performed. Anatomical center of the cornea is marked, and four additional points 2 mm behind the limbus that are 90 degrees apart are marked too. A 30 gauge thin-wall-needle is introduced from outside and through the sclera 2 mm behind the limbus near the 7 o’clock position. The 9.0 polypropylene suture on the long-curved-needle is introduced through the main incision at 12 o’clock and docked into the 30 gauge needle. The needle is brought out of the eye and left there. The same is done at the mark near the 4 o’clock position. Sutures are pulled until the AI is brought adjacent to the main incision. AI is folded and pushed through the main incision into the anterior chamber and unfolded. Two remaining needles are brought into the anterior chamber through the main incision and docked into the 30 gauge needles at 10:30 and 1:30 and pulled out of the sclera. The AI is centered by pulling the sutures and tightened to the sclera using Szurman zigzag suturing technique. At the end a knot is done, and conjunctiva is sutured or glued around the limbus. The incisions performed with a 30 gauge needle are watertight. If the incisions are done using a MVR knife, they should be sutured or glued to be watertight. Care must be taken that the edge of the AI is not in contact with the pigment epithelium of the remaining iris or ciliary body (Fig. 29.4d). Ideally there should be a red reflex that is barely seen between the edge of the AI and the limbus (Fig. 29.4c, d). Using this technique, AI alone (Figs. 29.5 and 29.6) or attached to an IOL can be implanted (Video 29.3).
Artificial iris implantation using four-floating sutures technique into a pseudophakic eye. When implanting the artificial iris into a pseudophakic eye, slight technique modifications are used. (a) First, artificial iris implant is trephined to diameter of 10.5 mm, and 9.0 polypropylene suture is used to do the lasso suture around the artificial iris four times. (b) The two needles are docked into the 26 gauge cannula or 30 gauge needle, which were introduced through sclera into posterior chamber 2 mm behind the limbus. The needles are pooled out the eye, and (c) artificial iris is folded and implanted into the anterior chamber. Docking is repeated. Two trailing sutures are placed at the proximal sites. The artificial iris is centered, and sutures are fixed to the sclera using the zigzag technique
Postoperative results of artificial iris implantation using four-floating suture technique. Preoperative (a) and 6-year postoperative (b, c) images after artificial iris implantation in the right eye of a 46-year-old patient with posttraumatic mydriasis after blunt ocular trauma (the same patient as in Fig. 29.5) using four-floating suture technique with visual acuity of 20/20 on last follow-up
AI can also be implanted at the time of a penetrating keratoplasty (Figs. 29.7 and 29.8). Concomitantly, aphakia is treated by implanting an AI attached to an IOL (AI-IOL complex). Intraocular lens is sutured to the artificial iris through haptics using loop sutures, as presented in Fig. 29.7. This is done at four places. After artificial iris-IOL complex preparation, the cornea is trephined, and 30 gauge needles are introduced through the sclera 2 mm behind the limbus and docked with the needles of the sutures, which are brought out the eye on four places that were marked previously. Alternatively, the needles can penetrate the sclera 2 mm behind the limbus from inside out. The artificial iris-IOL complex is then pushed through the 8 mm opening in the cornea and centered by pulling the sutures. Finally, the sutures are secured into the sclera using the Szurman zigzag technique. The surgical technique is safe and reproducible, with good aesthetic and functional outcomes (Fig. 29.8).
Artificial iris-IOL complex implantation using four-floating suture technique combined with penetrating keratoplasty. (a) Intraocular lens is sutured to the artificial iris through haptics using a loop suture, which is done at four sites. After artificial iris-IOL complex preparation, the cornea is trephined, and 30 gauge needles are introduced through the sclera 2 mm behind the limbus and docked with the needles of the sutures, which are brought out the eye on four places that were marked previously. Alternatively, the needles can penetrate the sclera 2 mm behind the limbus from inside out. (b) The artificial iris-IOL complex is then pushed through the 8 mm opening in the cornea and (c) centered by pulling the sutures. (d) Finally, the sutures are secured into the sclera using the Szurman zigzag technique
Postoperative results of artificial iris-IOL complex implantation combined with penetrating keratoplasty. Preoperative (a) and 7-year postoperative (b, c) images after artificial iris-IOL complex implantation in the right eye of a 71-year-old patient with subtotal traumatic aniridia and aphakia after penetrating ocular trauma. The procedure was combined with penetrating keratoplasty (the same patient as in Fig. 29.7). Best spectacle corrected visual acuity was 20/32, and contact lens corrected visual acuity was 20/20, with no postoperative inflammation
Artificial Iris-IOL Complex Scleral Fixation Technique: The Modified Yamane Technique
In aphakic patients, AI can be implanted in combination with an intraocular lens using a modified Yamane technique (Video 29.4), as is step-by-step presented in Fig. 29.9. In short, AI of overall 12.8 mm in diameter is trimmed to 10.5 mm. Using a 30 gauge needle, tunnels on each side are made through the artificial iris 0.75 mm away from its edge. Next, each haptic of three-piece intraocular lens is introduced into the needle on each side, and the optics of an IOL are pushed to make contact with the center of an artificial iris. The center of the cornea and two points 2 mm behind limbus 180 degrees apart are marked (two marks on each side 2 mm apart). Anterior chamber maintainer is placed, set to 30 mmHg. With superior approach, the frown incision is done about 5.5 mm wide with diamond knife set to 250 microns, and next the stab incision is done. The safety suture is placed through the artificial iris. After that the complex of IOL/artificial iris is implanted into the anterior chamber. Scleral tunnel at markings 2 mm behind limbus in 10 degrees angle is made parallel to the limbus using 30 gauge needle, bent 45 degrees, and the haptic is docked into the lumen of a needle using 23 gauge forceps through side stab incision. The same is done on the other side, using 30 gauge needle bent 90 degrees and 23 gauge forceps, entering through the main corneal incision to dock IOL haptic into the needle’s lumen. Both needles are pulled out simultaneously to externalize both haptics outside the sclera, then first haptic is grabbed with forceps, needle is detached, and haptic is flanged using cautery. Then the same is performed on the second haptic. Both haptics are pushed and buried into the sclera. Anterior chamber maintainer and safety suture are removed. Finally the incisions are sutured and hydrated. Good aesthetic and functional outcomes are achieved (Fig. 29.10).
Artificial iris-IOL complex implantation using Yamane technique into an aphakic eye. Artificial iris can be implanted in combination with an intraocular lens using Yamane technique in aphakic patients. (a) First, artificial iris is trephined to 10.5 mm. (b) The artificial iris is penetrated 0.75 mm from its edge in a 10 degree angle, and the haptic of the IOL is docked into a 30 gauge thin-wall-needle and (c) pulled out on the front side of the artificial iris. The same is done on the opposite side. (d) Artificial iris-IOL complex is ready for implantation. (e) The diamond knife is set at 0.25 mm. (f) The 4.5–5.0 mm frown incision is performed. Using bevel knife, the tunnel is performed, and entrance into anterior chamber is initiated using stiletto knife. (g) The artificial iris-IOL complex is grasped with a forceps and introduced into the anterior chamber. Care must be taken not to damage the haptics. If needed, the incision can be enlarged. (h) Safety suture placed near the edge of the artificial iris prevents slippage of the complex into the vitreous cavity. (i) The leading haptic is docked into the 30 gauge thin-wall-needle. (j) The needle is released, and the trailing haptic is docked into the needle on the opposite side. (k) Both needles are pulled out of the eye in the direction of the tunnel. The artificial iris-IOL complex centers nicely. (l) As the blue haptic is visualized on the left side, the needle is released, and the opposite haptic is grabbed using the forceps. After pulling it out of the needle, a flange is performed using cautery. (m) Then the leading haptic is grasped, pulled out of the needle, and another flange is created on the opposite side. (n) The haptics are pushed under the conjunctiva and slightly into the sclera. The bluish color should be visualized through the conjunctiva at the end of surgery if secondary centering of the artificial iris-IOL complex is needed
Postoperative results of artificial iris-IOL complex implantation using Yamane technique into an aphakic eye. Preoperative (a, c) and 3-month postoperative (b, d) images after IOL explantation due to broken haptic and implantation of an artificial iris-IOL complex using Yamane technique into left eye of a patient (same patient as in Fig. 29.9) after globe rupture
Artificial Iris-IOL Complex Scleral Fixation Technique: Pfeifer-Canabrava Technique
The latest modification for AI implantation is the Pfeifer-Canabrava technique (Video 29.5, Fig. 29.11) . Here the 6.0 polypropylene suture is used. Firstly, 4.5 mm frown incision is performed. Four points 2 millimeter behind the sclera are marked 90 degrees apart. The 6.0 polypropylene suture is brought into the anterior chamber and docked into the 30-gauge thin-wall-needle opposite the main incision 2 mm behind the limbus. Next, the second suture is done in a similar way. The AI is trimmed to 10.5 mm, and a plate haptic IOL is centered onto the pupil of the AI. Then the 30 gauge needle is pushed through the leading haptic of the IOL and AI, and 6.0 polypropylene suture is docked into the lumen of the needle. The suture is brought out on the other side, and a flange is performed. Then the proximal sutures are placed 2 mm behind the limbus and guided out of anterior chamber through the IOL and AI using a 30 gauge needle. The next flange is created. The AI-IOL complex is implanted into the anterior chamber. By pulling on sutures the AI-IOL complex is centered. The sutures are cut and pulled, and four flanges are performed 2 mm behind the limbus. The AI is nicely centered, and the sutures are shortened using cautery. The conjunctiva is pulled so that the flanges jump under it. The main incision is sutured. Similarly, when performing this technique, the AI-IOL complex is postoperatively suspended in the anterior chamber. There is no contact between the AI and surrounding intraocular structures or tissues. Good aesthetic outcomes can be achieved (Fig. 29.12).
Artificial iris-IOL complex implantation using Pfeifer-Canabrava technique. (a) The 6.0 polypropylene suture is introduced through the plate haptic IOL and AI. (b) Next, at one end of the suture, the flange is created using cautery. (c) The other end of the 6.0 polypropylene suture is brought into the anterior chamber and docked into the 30-gauge thin-wall-needle opposite the main incision 2 mm behind the limbus. (d) In this image the suture is brought out and a flange is performed. The AI is nicely centered, and the sutures are shortened using cautery. (e) The conjunctiva is pulled so that the flanges jump under it
Postoperative results of artificial iris-IOL complex implantation using Pfeifer-Canabrava technique into an aphakic eye. Preoperative (a) and 12-month postoperative (b) images after implantation of an artificial iris-IOL complex into the right eye of a 61-year-old aphakic patient (same patient as in Fig. 29.11) after globe rupture and anterior segment reconstruction with nice aesthetic appearance
When using this surgical technique, care must be taken that there is always positive pressure in the anterior chamber. The pars plana infusion cannula is introduced into the anterior chamber perilimbal through the cornea. The intraocular pressure is set at 15 mm of mercury in all cases. When the 30 gauge needles are introduced through the sclera, IOP is raised to 30 mm of mercury.
Artificial Iris and Non-foldable IOL Implantation Sutured to Sclera
Mayer et al. [3, 8, 20] described a similar technique, where the AI and IOL were implanted and fixated to the sclera separately. The sutures of IOL and from the AI are placed before implantation. First, a non-foldable poly(methyl methacrylate) IOL (Morcher 81B) is implanted through 7.0 mm sclerocorneal incision and sutured to sclera with 10–0 polypropylene usually at 3 o’clock and 9 o’clock. In the same way the artificial iris is implanted and sutured to sclera at the 6 o’clock and 12 o’clock, thus reducing the axial tilt. The main incision is closed with 10-0 nylon sutures [8].
Artificial Iris and IOL Complex Implantation Sutured to the Sclera
Mayer et al. [8] also described alternative surgical techniques in which AI-IOL complex is implanted and sutured to sclera. First, the IOL is sewn on the back of an artificial iris implant through the haptics, near optic from posterior direction, and then turned around on the front side to go back through the iris. The complex is then implanted as a folded sandwich into the eye through a 5.5–6.0 mm sclerocorneal incision and sutured to sclera at 3 and 9 o’clock position. Prior to implantation, two concave iridectomies 180 degrees apart are cut with scissors, and distal haptics are cut off to reduce the size of the foldable complex, allowing smaller incisions and easier implantation. This technique includes only two scleral attachment points, which can be time sparing.
Another surgical approach so-called slip-and-slide technique was described, which allows implantation of artificial iris combined with an IOL through small incision (3.0 mm), thus reducing surgically induced astigmatism. The components of artificial iris and plate-haptic IOL are fixated with knotless sutures together at four points, but can be inserted separately and then assembled after implantation and finally sutured to sclera. It can be performed with a non-toric or toric IOL. [21]
Other Surgical Approaches
Open-Sky Implantation During a Perforating Keratoplasty
Artificial iris implantation can be combined with a penetrating keratoplasty, as also in detail described above. In this case, the cornea is trephined, and AI with iridectomies is implanted directly through the corneal aperture into the ciliary sulcus or as presented also above with the four-floating sutures fixated into the sclera. Finally, a donor corneal transplant is sutured. [8]
A Sectoral Artificial Iris Implantation in Phakic Eye
The implant is cut according to the size of the iris defect and implanted through 3.5 mm sclerocorneal incision. Scleral side is sutured to the sclera with 10-0 polypropylene suture and remaining part to the natural iris tissue with Siepser slip-knot technique. This technique has proved to be successful. [22] In our case series, we have not used this technique.
Clinical Outcomes, Complications and Their Management
Significant advances in AI prosthesis development enabled new surgical approaches for anterior segment reconstruction in acquired or congenital iris defects, which already showed promising functional and aesthetic results. It needs to be emphasized that majority of patients present with complex eye comorbidities and other structural deformities (e.g., after trauma); thus postoperative functional outcomes can be importantly influenced by the preoperative posterior pole and overall eye health. Nevertheless, many patients report improvement in visual acuity and quality of vision (e.g., reduced glare, photophobia) [23]. In addition, most patients can achieve good cosmetic outcomes.
After AI implantation, there are several reported complications that need to be addressed medically or surgically and are often directly related to other preoperative eye conditions (e.g., posttraumatic ocular hypertension or glaucoma) and/or to the surgical procedure itself rather than being caused by the intraocular device [24].
The most commonly reported complications in literature are (1) persistent intraocular inflammation and macular edema (21%), which was in majority successfully managed by continuous topical nonsteroidal therapy or parabulbar injection of betamethasone; (2) increased intraocular pressure (>20 mmHg) or glaucoma development (5.9–9%), which in most cases was sufficiently managed by topical antiglaucoma medications, some (3.9–15%) required further surgical management such as glaucoma valve implantation; (3) postoperative hypotony (9%) that was managed by at least one hyaluronic acid injection into the anterior chamber; (4) loss of endothelial cell count, which lead to corneal decompensation and required corneal transplantation (11–18%); and (5) artificial iris decentration or malposition (5.9–12%). Our surgical technique of AI repositioning is shown in Figs. 29.13 and 29.14 and Video 29.6.
Artificial iris repositioning. (a) AI is subluxated and slightly mobile. (b) The 9.0 polypropylene loop suture is docked into the 27 G cannula behind the AI and brought out of the sclera through 23 G incision 2 mm behind the limbus. (c, d) The loop of the distal part of the suture is pulled over the AI through the same incision. (e, f) The lasso is performed around the AI and tightened. The AI is centered by pooling the suture. Zigzag scleral fixation is performed. (g) Conjunctiva is glued using fibrin glue. (h) The AI is nicely centered
Postoperative results of artificial iris repositioning. Preoperative (a, d) and postoperative (b, c, e) images after subluxated artificial iris repositioning (same patient as in Fig. 29.13)
Other rare but vision-threatening complications included retinal detachment (2%) and phthisis bulbi, which was probably due to severe preoperative trauma and endophthalmitis [20, 25]. Recently, Rickmann et al. [23] reported additional artificial iris-related complications such as darkening of the iris remnants and glaucoma development that were clearly associated with implants with integrated fiber mesh, but not to those without [23].
Table 29.2 summarizes our clinical outcomes in 18 consecutive patients, who underwent complex anterior segment reconstruction with the implantation of AI from 2013 to 2020. The majority of our patients (16/18) had posttraumatic aniridia or posttraumatic mydriasis with concomitant anterior segment injury, two presented with congenital aniridia. Three patients had an AI-IOL complex implantation with scleral fixation (modified Yamane technique). 12 patients had the AI implanted using four-floating suture technique . Two patients had AI implanted into the capsular bag and one AI-IOL complex implantation with modified Canabrava technique. Great aesthetic results were achieved in the majority of cases. Likewise, 2/3 of cases reported improvement in visual acuity. Importantly, except for the one patient (Fig. 29.1) that the AI was implanted into the sulcus, in our case series with a long follow-up period (first operation in 2013), no postoperative inflammation or macular edema was observed when there was correct AI placement without touching any eye structure. We can only speculate that this could be a direct reflection of our surgical technique which ensures floating AI or AI-IOL complex on suspension sutures or haptics without touching or rubbing against any other eye structure. The most common postoperative complications that occurred were elevated intraocular pressure (12%), artificial iris decentration (6%), corneal decompensation (6%), and phthisis bulbi in one patient. However, in this case this might be a consequence of severe eye trauma and multiple prior surgical interventions, which included corneal transplantation, vitrectomy due to retinal detachment, and several glaucoma procedures.
To sum up, iris defect reconstruction with an artificial iris implantation using described surgical approaches is safe; however due to the complex nature and concomitant eye diseases, close and long-term patient follow-up is essential to achieve best cosmetic and functional outcomes.
Conclusions
Iris defects can be successfully reconstructed with an AI implantation using various surgical approaches as presented in this chapter. The procedures are safe with good functional and aesthetic outcomes in majority of patients. However, even though postoperative complications are usually rare and related to concomitant eye diseases and/or direct surgical trauma rather than due to the device itself, surgeons need to be aware and know how to implant the AI and handle the most common postoperative complications. Therefore, proper surgical technique and close and long-term follow-up are mandatory for successful surgical outcomes.
Take-Home Message
-
AI has to be implanted in the way it does not and will not come into the contact with intraocular tissue.
-
AI must be trephined to 10.5 mm or less when implanted using four-floating suture technique (sulcus) or 9.0 mm when implanted into the capsular bag.
-
If inflammation is present after AI implantation quick action should be taken to reposition the AI in the way it will not be in contact to intraocular tissue or explant it.
-
Intracapsular AI implantation is the 1st choice technique if capsular bag is present.
-
AI without meshwork is used in 100% of cases. When suturing the lasso is performed around the AI, or the IOL is being used as support. AI should float in anterior chamber fixed to the sclera using four sutures or IOL haptics if no capsular bag is present.
References
Samant M, Chauhan BK, Lathrop KL, Nischal KK. Congenital aniridia: etiology, manifestations and management. Expert Rev Ophthalmol. 2016;11:135–44.
Riedl JC, Schuster AK, Vossmerbaeumer U. Indications and surgical techniques for implantation of the Artificial Iris®. Ophthalmologe. 2020;117:786–90.
Yildirim TM, Khoramnia R, Masyk M, Son H-S, Auffarth GU, Mayer CS. Aesthetics of iris reconstruction with a custom-made artificial iris prosthesis. PLoS One. 2020;15:e0237616.
Jung JW, Han SH, Kim SA, Kim EK, Seo KY, Kim T-I. Evaluation of pigment location in tinted soft contact lenses. Cont Lens Anterior Eye. 2016;39:210–6.
Remky A, Redbrake C, Wenzel M. Intrastromal corneal tattooing for iris defects. J Cataract Refract Surg. 1998;24:1285–7.
Burk SE, Da Mata AP, Snyder ME, Cionni RJ, Cohen JS, Osher RH. Prosthetic iris implantation for congenital, traumatic, or functional iris deficiencies. J Cataract Refract Surg. 2001;27:1732–40.
Osher RH, Burk SE. Cataract surgery combined with implantation of an artificial iris. J Cataract Refract Surg. 1999;25:1540–7.
Mayer C, Tandogan T, Hoffmann AE, Khoramnia R. Artificial iris implantation in various iris defects and lens conditions. J Cataract Refract Surg. 2017;43:724–31.
Humanoptics. https://www.humanoptics.com/en/Humanoptics.
Pozdeyeva NA, Pashtayev NP, Lukin VP, Batkov YN. Artificial iris-lens diaphragm in reconstructive surgery for aniridia and aphakia. J Cataract Refract Surg. 2005;31:1750–9.
Walter T. Parker, David R. Hardten MES (2019) Iris prosthesis implantation. Video atlas of anterior segment repair and reconstruction. https://doi.org/10.1055/b-0039-172073.
Na J-H, Park SC, Sohn Y, Lee S-D. Realizing the concept of a scalable artificial iris with self-regulating capability by reversible photoreaction of spiropyran dyes. Biomaterials. 2013;34:3159–64.
Shareef FJ, Sun S, Kotecha M, Kassem I, Azar D, Cho M. Engineering a light-attenuating artificial iris. Investig Ophthalmol Vis Sci. 2016;57:2195–202.
Koch KR, Heindl LM, Cursiefen C, Koch HR. Artificial iris devices: benefits, limitations, and management of complications. J Cataract Refract Surg. 2014;40:376–82.
Bahadur GG, Miller KM. Artificial iris exchange. J Cataract Refract Surg. 2020;46:1630–6.
https://clinicaltrials.gov/ct2/show/nct01860612. Safety and effectiveness of the customflex artificial iris prosthesis for the treatment of iris defects.
https://www.Accessdata.Fda.Gov/Cdrh_Docs/Pdf17/P170039b.Pdf. Safety and effectiveness of the customflex artificial iris prosthesis for the treatment of iris defects.
Figueiredo GB, Snyder ME. Long-term follow-up of a custom-made prosthetic iris device in patients with congenital aniridia. J Cataract Refract Surg. 2020;46:879–87.
Mayer CS, Laubichler AE, Khoramnia R, Tandogan T, Prahs P, Zapp D, Reznicek L. Challenges and complication management in novel artificial iris implantation. J Ophthalmol. 2018;2018:3262068.
Wolf A, Shajari M. Slip-and-slide technique for combined small-incision artificial iris and IOL implantation. J Cataract Refract Surg. 2020;46:1433–5.
Magnus J, Trau R, Mathysen DGP, Tassignon M-J. Safety of an artificial iris in a phakic eye. J Cataract Refract Surg. 2012;38:1097–100.
Rickmann A, Szurman P, Januschowski K, Waizel M, Spitzer MS, Boden KT, Szurman GB. Long-term results after artificial iris implantation in patients with aniridia. Graefe’s Arch Clin Exp Ophthalmol. 2016;254:1419–24.
Bonnet C, Miller KM. Safety and efficacy of custom foldable silicone artificial iris implantation: prospective compassionate-use case series. J Cataract Refract Surg. 2020;46:893–901.
Spitzer MS, Nessmann A, Wagner J, Yoeruek E, Bartz-Schmidt KU, Szurman P, Szurman GB. Customized humanoptics silicone iris prosthesis in eyes with posttraumatic iris loss: outcomes and complications. Acta Ophthalmol. 2016;94:301–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Electronic Supplementary Materials
Capsular Bag Implantation with a Combined Standard Cataract Surgery (MP4 420567 kb)
Four floating Suture Technique (Pfeifer technique) (MP4 2320 kb)
Artificial iris implantation using 4-floating sutures technique (MP4 655462 kb)
AI and IOL implantation using Yamane technique (MP4 591890 kb)
AI Canabrava technique (MP4 497863 kb)
IA repositioning (MP4 36941 kb)
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pfeifer, V., Marzidovšek, M., Lužnik, Z. (2022). Artificial Iris Implantation: Overview of Surgical Techniques. In: Alió, J.L., Dick, H.B., Osher, R.H. (eds) Cataract Surgery . Essentials in Ophthalmology. Springer, Cham. https://doi.org/10.1007/978-3-030-94530-5_29
Download citation
DOI: https://doi.org/10.1007/978-3-030-94530-5_29
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94529-9
Online ISBN: 978-3-030-94530-5
eBook Packages: MedicineMedicine (R0)