Abstract
Systemic anti-cancer therapy (SACT) plays an integral role in the treatment of early and advanced gynaecological cancers. Therapeutics need to balance efficacy with toxicity and require intensive development from pre-clinical research to reach clinical practice. Conventional cytotoxic chemotherapy forms the mainstay of treatment for most gynaecological cancers but there is an increasing role for personalised medicine. Cancer management targeted to specific targets in cancer pathogenesis are becoming increasingly used and PARP inhibitors have led the way in ovarian cancer. Immunotherapy has dramatically altered cancer treatments in the past decade and has an increasing role in gynaecological cancers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Systemic anti-cancer treatment (SACT) including chemotherapy, immunotherapy, and targeted therapy form a key part of the multimodality management of patients with gynaecological cancers. Surgery and radiotherapy can be used for local control and debulking of gynaecological cancers and can be curative alone for early-stage cancers. SACT is required for the treatment of metastatic and micro-metastatic disease with a variety of mechanisms of action.
The hallmarks of cancer were first described by Hanahan and Weinberg in 2000 and updated in 2011 [1]. They describe the characteristics of cancer and can be therapeutic SACT targets. In this chapter we aim to summarise the underlying biology, pharmacology, and data for SACT in gynaecological cancers with a focus on ovarian and endometrial cancer. Newer developments including the role for PARP inhibitors and immunotherapy will be discussed (Fig. 11.1).
Hallmarks of cancer described and updated with emerging hallmarks in 2011. The figure features capabilities involved tumour pathogenesis, metastasis, and cancer cell survival. Created with BioRender.com (Adapted from Hanahan and Weinberg [1])
11.1.1 Systemic Anti-cancer Treatment Principles
SACT including chemotherapy aims to stop the unregulated growth of cancer cells and metastasis from their site of origin. Chemotherapy can be cytotoxic, that is killing cells including cancer cells, or cytostatic, stopping cancer cell growth and spread. However, chemotherapy is indiscriminate and will have an effect on all actively dividing cells. The aim is to have a therapeutic effect with the least possible toxicity on normal tissues. Traditionally cytotoxic chemotherapy dose is limited by bone marrow toxicity or toxicity to other rapidly dividing tissues, for example, mucous membranes in the GI tract, although newer targeted agents and immunotherapy have their own toxicity profiles. The pharmacological principle of therapeutic index is used in drug development to identify the maximal tolerated dose. The difficulty with anticancer agents is their narrow therapeutic index and it is the responsibility of a medical oncologist is to distinguish between activity and toxicity in drug development trials, and to balance clinical activity and toxicity in clinical practice. Following pre-clinical drug development active agents are included in phase 1 trials, establishing safety and a maximal tolerated dose to use in a phase 2 trial. Phase 2 trials aim to establish efficacy before evaluation against a current treatment or placebo in a phase 3 trials (summarised in Table 11.1).
SACT aims to kill cancer cells or stop their growth through their action on the cell cycle and interaction with DNA, RNA and cellular proteins. Different chemotherapeutic drugs may have activity at different stages of the cell cycle, or action on cellular signalling pathways. There are checkpoints between each phase of the cell cycle (Fig. 11.2) which must be met to proceed to the next phase, if not met, apoptosis, or programmed cell death is triggered. A key part of the cell cycle checkpoints is the need to ensure DNA integrity is maintained through homologous recombination repair, base excision repair and mismatch repair pathways. Cellular growth signalling pathways are driven by cell surface receptors linked to intracellular kinase, for example the mitogen-activated protein kinase (MAPK) pathway and nuclear receptors, for example the oestrogen receptor pathway. These pathways have been exploited in the newer therapeutics of targeted agents and immunotherapy which will be discussed later.
Chemotherapy agents can be classified according to their mechanism of action and those with activity in gynaecological cancers include platinum derivatives, taxanes and anthracyclines (Table 11.2). Unfortunately, drug resistance develops in cancer cells through upregulation of alternative pathways, for example through increasing cellular drug efflux pumps or a failure of apoptosis following DNA damage. This leads to growth of a genetically resistant clone of cells, leading to a loss of clinical effectiveness and requires a change in treatment where available.
Chemotherapy dosing and scheduling differs depending on the pharmacokinetics of each drug and method of administration established during clinical trials. The most common regime for intravenous cytotoxic agents is to be administered intravenously every 3 weeks to allow recovery of toxicity before the next administration. Dosing can be uniform or calculated either by body surface area [2], body weight or in the case of carboplatin using the Calvert formula using estimated or actual glomerular filtration rate by area under the curve (AUC) [3]. Prior to each SACT treatment cycle patients are evaluated for signs of toxicity. Alterations can then be made in supportive medications, for example, additional anti-emetics or in the dosing of chemotherapy. Toxicity assessment and identification of adverse events can use the grading criteria set out by the National Institutes of Health Common Terminology Criteria for Adverse Events (CTCAE) [4]. Further guidance on management and monitoring is available from licensing authorities and in the UK in the summary of product characteristics available from the electronic medicine compendium [5].
Response assessment is an integral part of non-surgical cancer management and can use clinical, biochemical and radiological methods. Clinical assessment of response will depend on patient’s symptoms and signs but may be clear, for example, the frequency of abdominal paracentesis required in patients with malignant ascites. Biochemical response assessment will depend on the primary site of gynaecological cancer. Serous ovarian cancers may secrete CA-125 which can be used to assess response to chemotherapy. The Gynaecologic Cancer Intergroup GCIG CA-125 response criteria were defined and used in clinical trials as a validated marker of biochemical progression during first line treatment and response in relapsed disease [6]. Other biochemical markers include AFP and HCG in germ cell tumours and include inhibin for granulosa cell tumours [7]. The role of circulating tumour cells and/or circulating tumour DNA (ctDNA) is under evaluation and may enter clinical practice in the future as both a diagnostic and response assessment tool [8]. Radiological response can be assessed through cross sectional imaging, most commonly CT and MRI, although PET-CT has an important role in cervical cancer and is increasingly being used in the management of other gynaecological malignancies [9]. Radiological response in clinical trials is assessed using the RECIST criteria [10] with a similar reporting format advocated in clinical practice outside of clinical trials.
Personalised medicine and the role for targeted cancer treatments is an increasing possibility in cancer medicine. Personalised medicine aims to use a management strategy for an individual patient taking into account the patient’s tumour biology and likelihood of response to particular therapy whilst reducing potential toxicity. In gynaecological cancer this has been through the introduction of PARP inhibitors, initially in those patients with a germline BRCA mutation and later for all patients. These targeted agents have a different side effect profile compared to traditional cytotoxic chemotherapy and specific mechanisms of action linked to cancer biology. For example, NTRK (neurotrophic tyrosine kinase) fusion positive solid tumours, for example in uterine sarcoma, can be targeted with drugs such as larotrectinib and entrectinib [11]. There are other targeted anti-cancer treatments used in gynaecological cancer and these are summarised in Table 11.3.
Anti-angiogenic agents are used in the treatment of ovarian and cervical cancer, in particular the monoclonal antibody bevacizumab in combination with chemotherapy and as a maintenance agent post induction chemotherapy. Hormonal agents also have a role in those cancers with an underlying hormonal driver such as endometroid endometrial cancer.
SACT can be used in gynaecological cancers in the adjuvant, neo-adjuvant and metastatic setting. Adjuvant treatment aims to increase survival by reducing the risk of relapse and is used after definitive local control to target a much smaller group of cancer cells once the bulk has been improved through surgery. Neo-adjuvant treatment aims to control symptomatic or bulky disease and may facilitate less morbid surgery. It can also give an indication of the underlying disease biology at post-surgery pathological assessment and likelihood of achieving long term cancer cure [12]. Neo(adjuvant) treatment also has oncological advantages in that it treats cancer when cancer cells are most susceptible to chemotherapy. SACT in the advanced, incurable cancer setting has two main aims, to alter the disease course and in doing so improve survival, and to improve symptoms (palliation).
11.1.2 Systemic Anti-cancer Therapy in Ovarian, Fallopian Tube and Primary Peritoneal Cancer
SACT is an integral part of the treatment of patients with ovarian, fallopian tube and primary peritoneal cancer at early and advanced stages.
11.1.2.1 Early Stage Disease (FIGO I–II)
Platinum based chemotherapy in early-stage disease (FIGO I-II) has been shown to reduce the risk of recurrence and improve overall survival. The ICON 1 (International Collaborative Ovarian Neoplasm) trial and ACTION trials demonstrated a significant improvement in relapse free survival and overall survival [13, 14]. This benefit was confirmed in a meta-analysis by the Cochrane group including an analysis of five prospective clinical trials showing that adjuvant chemotherapy has a survival advantage over observation following surgery. Chemotherapy options include single agent carboplatin, at a dose of AUC 5 or 6, or carboplatin and paclitaxel for 6 cycles scheduled every 3 weeks [15]. ESMO, NCCN and UK guidelines recommend adjuvant chemotherapy [16,17,18]. There is evidence of benefit across risk groups and the ESMO recommendations are summarised in Table 11.4. Response rates to non-serous epithelial ovarian cancer histology is poorer than serous and there is little data to guide recommendations in these subtypes.
11.1.2.2 Advanced Disease (FIGO III–IV)
Primary debulking surgery is the standard of care where complete cytoreduction is probable and the patient’s fitness and burden of disease makes surgery possible. However neoadjuvant chemotherapy followed by interval debulking surgery has been shown to be non-inferior to primary surgery followed by adjuvant chemotherapy [19]. For advanced disease the standard of current standard chemotherapy is intravenous carboplatin AUC 5/6 and paclitaxel 175mg/m2 every 3 weeks for 6 cycles. There is potentially a role for intraperitoneal chemotherapy and HIPEC (hyperthermic intraperitoneal chemotherapy) but is currently confined to centres where the technical ability is possible and clinical trials [18, 20]. There is no survival benefit from adding in a third chemotherapy drug [18, 21]. Anti-angiogenics have been trialled as a maintenance treatment post adjuvant chemotherapy in advanced disease and bevacizumab is funded in the UK currently for any patients with stage 4 or incompletely resected Stage 3C disease (>1 cm residual disease). Maintenance bevacizumab has a progression free survival advantage in this group when given for 18 cycles (12 months) following surgery or where surgery is not feasible [22, 23]. Bevacizumab is given intravenously every 3 weeks and has side effects including hypertension, proteinuria, venous and arterial thromboembolic events, and gastrointestinal toxicity including rarely perforation [22, 23]. There is a role for PARP inhibitors as a maintenance treatment post adjuvant chemotherapy which will be discussed later in this chapter.
11.1.2.3 Relapsed Disease: Focus on High Grade Serous Ovarian Cancer
Patients need a rigorous surveillance routine post first line treatment as relapse rates are high and further surgery and systemic treatment may be feasible. Secondary debulking surgery should be considered in appropriate patients and followed by further systemic therapy. The decision to offer further systemic therapy on relapse is based on symptoms, performance status and radiological findings. There is no survival benefit in starting systemic therapy based on rising CA125 alone [24]. In those patients suitable for further chemotherapy with a longer treatment free interval (TFI) combination treatment with platinum rechallenge is recommended. For those patients with short TFI (less than 6 months) or progressing on first line therapy an alternative single agent chemotherapy is equally effective and less toxic [16]. The choice of further chemotherapy depends on patient factors including patient choice, performance status, toxicity from previous treatment and any hypersensitivity reactions, and the treatment and platinum free interval. Response rates to platinum chemotherapy fall on a continuum from around 50–60% to less than 20% depending upon platinum free interval. Resistance to platinum chemotherapy can be intrinsic to the tumour and progression may occur early, or develop later after first or subsequent line of platinum chemotherapy. GCIG categories are summarised in Table 11.5 and definitions based on the probability of responding to further platinum chemotherapy [25].
Combination platinum chemotherapy options for recurrent disease include carboplatin and pegylated liposomal doxorubicin (PLD), gemcitabine or paclitaxel [26,27,28,29,30]. Different schedules are possible but in general patients are offered six cycles of platinum based chemotherapy with imaging response assessment halfway through. Each regime has shown a progressive free survival benefit of between 9 and 12 months. Bevacizumab in combination with platinum based therapy and continued as maintenance treatment has also shown a benefit in progression free survival and increased response rates in the recurrent ovarian cancer setting but availability in clinical practice will depend on local funding arrangements [30].
Patients with a short platinum free interval are conventionally treated with single agent chemotherapy and the most efficacious options include weekly paclitaxel, PLD and gemcitabine. Response rates to these agents have been shown to be increased with the addition of bevacizumab [31] However, response rates are generally low, in the region of 20% and further dose dense platinum chemotherapy can have a role for some patients. For example, cisplatin in a dose dense schedule has shown evidence of high response rates [32]. There is also a role for hormonal therapy in relapsed ovarian cancer especially in later lines of therapy. Hormonal therapy with tamoxifen or AI has shown modest overall response rates and evidence of disease control. Data is largely from retrospective case series with response rates of 15% [33], and in the Paragon phase 2 trial anastrozole showed evidence of clinical benefit in 35% of patients [34] although the ESGO-ESMO consensus guideline statement highlights the uncertain benefit [16]. Hormonal therapy does have a role in non-epithelial ovarian tumours such as granulosa cell tumours and is recommended by ESMO [35].
11.1.3 PARP Inhibitors in Ovarian Cancer
PARP inhibitors have changed the treatment landscape in advanced high-grade platinum sensitive ovarian cancer. There are multiple pathways involved in DNA repair that can be affected in cancer and exploited through SACT (Fig. 11.3a).
(a) DNA repair pathways including relevant proteins in gynaecological cancers. PARP Poly (ADP-ribose) polymerase, BRCA Breast cancer gene. (b) Effect of PARP inhibitors on ovarian cancer cells with BRCA mutations. (Created with BioRender.com)
Approximately 50% of patients with high grade serous ovarian cancer have defects in DNA repair via homologous recombination (HR) due to germline or somatic mutations, summarised in Fig. 11.4 [36]. Defective DNA repair is an important target both through platinum-based chemotherapy inducing crosslinking and DNA damage and can be exploited through PARP inhibitors. PARP inhibitors use the concept of synthetic lethality in ovarian cancer whereby a defect in one gene, for example BRCA1 has little effect but when combined with another deficit leads to cell death [37]. PARP is a DNA repair pathway enzyme required to repair single strand breaks in DNA through the base excision repair pathway. PARP inhibitors stop this process and lead to double stranded DNA breaks. In patients with germline or somatic deficiencies in this pathway, through BRCA 1 or 2 mutation or loss of other proteins involved in homologous DNA repair, DNA repair cannot continue leading to cell death (summarised in Fig. 11.3b).
Frequency of HR alterations in high grade serous ovarian cancer. Approximately 50% may have deficiencies in HRD. (Adapted from Konstantinopoulos et al. [40])
Patients can be tested for BRCA1 and BRCA2 germline and tumour mutations to guide both future cancer risk and individual cancer management. There are other mutations in DNA repair pathways that lead to homologous recombination deficiency (HRD). There are different methods of assessing for HRD. This can be by tumour next generation sequencing or proprietary assays. For example, the Myriad Genetics myChoice assay was used in the PAOLA-1 trial to guide maintenance therapy post first line chemotherapy [38] and Foundation Medicine next generation sequencing can assess for mutations in homologous recombination pathway genes and LOH (loss of heterozygosity) [39].
PARP inhibitors were first introduced as a maintenance treatment following platinum based chemotherapy for patients with recurrent ovarian cancer and have subsequently been shown to improve outcomes following first line treatment for patients with stage 3 or 4 high grade ovarian serous ovarian, fallopian tube or primary peritoneal cancer. Efficacy is more pronounced in patients with a BRCA mutation or homologous repair deficiency (HRD) but clinical benefit is also seen in patients without HRD. Olaparib was the first agent used in clinical trials and has a shown a very significant relapse free and overall survival benefit in patients with a germline or somatic BRCA mutation in the first line setting and following treatment for a platinum sensitive relapse [41,42,43]. Niraparib is licensed as a maintenance treatment post first or subsequent platinum sensitive relapse following platinum-based chemotherapy irrespective of BRCA or HRD status [44]. Rucaparib is licensed as a maintenance treatment post chemotherapy in a platinum sensitive relapse irrespective of BRCA or HRD status [45]. More recently the combination of Olaparib and bevacizumab as a maintenance treatment post platinum-based chemotherapy has shown evidence of efficacy compared to PARP inhibitor alone and has been approved in the UK for patients that are HRD positive [46].
PARP inhibitors do have adverse events associated with their use. Haematological toxicity including anaemia, neutropenia and thrombocytopenia can be common and may require dose alternations [42,43,44]. There are some specific toxicities associated with certain drugs. For example, niraparib is associated with hypertension and requires regular monitoring of blood pressure when starting and rucaparib can cause deranged liver function. PARPi can also be associated with systemic symptoms including fatigue, nausea, and anorexia although often this is short lived. Patients also need to be counselled regarding rare but serious toxicity of an increased risk of myelodysplasia and acute myeloid leukaemia (MDS/AML) with PARPi treatment. A systematic review published in 2020 of over 5000 patients treated with PARPi reported a significantly increased risk of MDS/AML, OR 2.63 (0.73% vs. 0.41% in normal controls) [47] and in long term data from the SOLO2 trial, use of PARPi in platinum pre-treated BRCA mutant patients increased the rate of MDS/AML from 4 to 8% [48].
11.1.4 Targeted Treatment in Low Grade Serous Ovarian Cancer
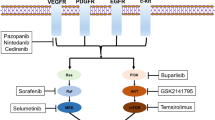
Low grade serous ovarian cancer (LGSOC) is a rare subtype of ovarian cancer which often presents at a younger age with a different molecular pathogenesis with alternations in the RAS and MAPK (mitogen-activated protein kinase) signalling pathways, described in Fig. 11.5. Treatment is centred on surgery and optimal cytoreduction as often there is a much lower response to platinum-based chemotherapy, less than 25% compared to 60–70% in high grade serous cancers [49]. Hormonal therapy can form part of the first line treatment with a survival benefit shown in a retrospective series following adjuvant chemotherapy [50]. In relapsed disease response rates for platinum based and other chemotherapy is poor.
RAS-MAPK signalling pathway (Created with BioRender.com)
MEK inhibitors have been developed and trialled in this setting. The first, ENGOTov11/MILO study using binimetinib compared to chemotherapy showed no significant benefit [51]. However more recently the LOGS trial using trametinib compared to physician’s choice of chemotherapy or hormonal therapy showed a statistically significant benefit for progression free and overall survival compared to standard of care. There was also a much-improved overall response rate, around 26% compared to only 6% in the control group [52]. In particular further chemotherapy showed a response rate of 9% for paclitaxel, 3% for PLD and 0% for topotecan. Hormonal therapy with letrozole had a response rate of 13.6%. However, there is toxicity with MEKi most commonly diarrhoea, nausea, skin rashes, and change in heart function. Further trials including in combination with other treatments are ongoing.
11.1.5 Systemic Anti-cancer in Endometrial Cancer
SACT and radiotherapy form a key part of the management of patients with early and advanced uterine cancer. Currently risk stratification is based on pathological findings including histology type, grade, and presence or absence of lymphovascular space invasion (LVSI). Although currently not routinely available in clinical practice in the future it may be feasible to refine this further using molecular pathology, to assess for POLE and p53 mutation status and presence of mismatch repair deficiency [53]. Risk groups have been defined in the 2020 ESGO/ESTRO/ESP guidelines and summarised in Table 11.6.
Patients in the high-intermediate risk groups with or without complete nodal staging may be considered for chemotherapy especially in high grade disease with significant LVSI. Patients in the advanced, metastatic, and high-risk groups should be counselled regarding the benefits of adjuvant chemotherapy.
The PORTEC-3 trial studied patients with high-risk features comparing radiotherapy to concurrent chemoradiotherapy and four cycles of adjuvant carboplatin and paclitaxel. The trial demonstrated an overall survival benefit for chemotherapy over radiotherapy alone which was most pronounced in patients with serous histology and stage 3 disease [54]. Studies are ongoing to define molecular subtypes with a risk of relapse and how adjuvant treatment can be tailored to risk. For example, the benefit of adjuvant chemotherapy in stage 1 and 2 clear cell cancers has not been consistently demonstrated across clinical trials [53]. In practice in the UK if chemotherapy is recommended to patients would be 4–6 cycles of carboplatin AUC5/6 and paclitaxel 175 mg/m2 every 3 weeks followed by external beam radiotherapy and vaginal brachytherapy where indicated.
For advanced endometrial cancer maximal cytoreduction followed by chemotherapy should be considered after specialist MDT assessment. Patients with oligometastatic disease should be considered for local control including surgery, radiotherapy including stereotactic radiotherapy or other ablative techniques. In patients with unresectable disease SACT can have a role in improving patient’s symptoms and improving overall survival [55]. Systemic treatment options including platinum-based chemotherapy with the combination of carboplatin and paclitaxel. This was shown to be non-inferior and less toxic than the previously used regime of cisplatin, doxorubicin plus paclitaxel [56]. Beyond first line treatment there is a paucity of high-quality data and patients should be considered for clinical trials. Active chemotherapy agents include paclitaxel, anthracyclines and for high grade serous endometrial cancers with a long platinum free interval re-challenge with platinum can be considered.
Alternatively hormonal based therapy can be considered. This can have high response rates especially for hormone receptor positive, lower grade cancers. This is discussed in more detail in the next chapter and is advocated in international guidelines [53, 57].
11.1.6 Immunotherapy in Endometrial Cancer
Immunotherapy has revolutionised the treatment of malignancy and clinical trials have been ongoing in the role for immunotherapy in gynaecological cancer. Immunotherapy has been reviewed in detail by Waldman [58]. In summary immunotherapy or checkpoint inhibition aims to use the patient’s immune system to target cancer. Summarised by Chen and Mellman in the cancer immunity cycle, cancer cells produce new antigens that are recognised by the immune system however tumours develop means of avoiding the immune system through upregulation of immune checkpoint pathways [59]. Programmed cell death protein (PD-1), programmed cell death protein ligand 1 (PDL-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) targeted agents were the first to be introduced to clinical practice. Anti-PD1 drugs include: pembrolizumab, nivolumab and dostarlimab. Anti-PD-L1 agents: atezolizumab, durvalumab, and avelumab. Immunotherapy currently has shown evidence of efficacy and clinical benefit in mismatch repair deficit advanced endometrial cancer and advanced cervical cancer (Fig. 11.6).
Immune system regulation and role of checkpoint inhibitors. (Created with BioRender.com)
Endometrial cancer can be associated with mismatch repair deficiency through germline deficiencies, in Lynch syndrome, or sporadic mutations in mismatch repair proteins. Immunotherapy has been studied in patients with recurrent or metastatic endometrial cancer following platinum-based chemotherapy. Dostarlimab, a PD-1 targeting agent, has shown evidence of high response rates and anti-tumour activity. Pembrolizumab and nivolumab have shown evidence in dMMR tumours and in the United States have been licensed by the Federal Drug Agency (FDA) as a pan-tumour indication for mismatch repair deficiency (dMMR) or microsatellite instability high (MSI-H) cancers or tumour with a high tumour mutational burden (TMB) [60].
Cancer treatment with immunotherapy is a rapidly evolving field and is under investigation in combination with targeted agents. In the LEAP-001 (NCT03884101) trial the combination of pembrolizumab and lenvatinib, a multi-kinase targeting TKI, is being investigated are used in combination for patients with mismatch repair proficient or deficient advanced endometrial cancer. In ovarian cancer immunotherapy is also being trialled in combination with PARP inhibitors in the ATHENA trial (NCT03522246) and dostarlimab and niraparib in combination (NCT04679064).
Immunotherapy has a very different toxicity profile to conventional chemotherapy due to its mechanism of action effecting the immune system. Checkpoint inhibitors targeting PD-1, PD-L1 and CTLA-4 can lead to a wide range of immune related adverse events effecting any organ. This can be mild requiring only symptomatic treatment through to life threatening organ dysfunction that can be fatal. Commonly this can lead to rashes, endocrinopathies, pneumonitis, colitis, and rarely fatal effects such as myocarditis [61]. Immune related side effects can happen at any time during or after treatment with immunotherapy and can have a significant impact on quality of life. Immune related side effects need specialist management from oncologists and medical specialists.
11.1.7 Cervical, Vulval and Rare Gynaecological Cancers
Systemic anti-cancer therapy also forms an integral role in the treatment of cervical, vulvar and other rarer gynaecological cancers such as non-epithelial ovarian cancer, germ cell tumours and gestational trophoblastic disease. Common regime used for germ cell tumor is the combination of Bleomycin, Etoposide and cisplatin for four cycles. For gestational trophoblastic neoplasia (GTN) Methotrexate is used for low risk disease while combination of Etoposide, Methotrexate and Dactinomycin- cyclophosphamide and vincristine (EMACO regime) is used for high risk GTN. Chemotherapy is also used a radiosensitiser in concurrent chemoradiation protocols used in the treatment of gynaecological cancers. Treatment is guided by national and international guidelines, for example ESMO and NCCN [62, 35].
11.2 Conclusions
Chemotherapy and other systemic anti-cancer treatment form a key part of the management of gynaecological cancers in combination with surgery and radiotherapy. Conventional chemotherapy using platinum based and taxanes have a significant role in the treatment of gynaecological cancers. There is an established role for targeted therapies including PARP inhibitors and other protein kinase inhibitors especially in ovarian cancer. Immunotherapy has revolutionised the treatment of cancers and has an evolving role in endometrial cancer with future work ongoing in combination with other agents. The trade-off is toxicity. This can be dose related but can also be unpredictable and life threatening. There is much to be done to refine SACT in patients’ management and how improve the lives of patients living with and beyond cancer.
Key Points
-
Chemotherapy in early and advanced stage disease should be considered where appropriate in a patient’s cancer journey.
-
Personalised cancer medicine is evolving and aims to maximise benefit whilst limited toxicity.
-
PARP inhibitors have changed the prospects for patients with ovarian cancer and recommended for patients with platinum sensitive disease.
-
Immunotherapy has an evolving role in gynaecological cancers and most applicable to patients with mismatch repair deficient endometrial cancer.
References
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. https://doi.org/10.1016/j.cell.2011.02.013.
du Bois D, du Bois EF. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Int Med. 1916;17((6_2):863–71. https://doi.org/10.1001/archinte.1916.00080130010002.
Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7(11):1748–56. https://doi.org/10.1200/JCO.1989.7.11.1748.
https://ctep.cancer.gov/protocolDevelopment/adverse_effects.htm
Rustin GJS, Vergote I, Eisenhauer E, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the gynecological cancer intergroup (GCIG). Int J Gynecol Cancer. 2011;21:419–23.
Lyubimova NV, Beyshembaev AM, Kushlinskiy DN, Zordania KI, Adamyan LV. Granulosa cell tumors of the ovary and inhibin B. Bull Exp Biol Med. 2011;150(5):635–8. https://doi.org/10.1007/s10517-011-1209-z.
Roncati L, Lusenti B. Liquid biopsy to monitor early relapse of gynaecological malignancies. Eur J Gynaecol Oncol. 2020;41(6):849–51. https://doi.org/10.31083/j.ejgo.2020.06.5430.
Narayanan P, Sahdev A. The role of 18F-FDG PET CT in common gynaecological malignancies. Br J Radiol. 2017;90(1079):20170283. https://doi.org/10.1259/bjr.20170283.
Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1—Update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7. https://doi.org/10.1016/j.ejca.2016.03.081.
Drilon A, Laetsch TW, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–9.
Kerr DJ, et al. Oxford textbook of oncology. 3rd ed. Oxford: Oxford University Press; 2016.
Colombo N, Guthrie D, Chiari S, Parmar M, Qian W, Swart AM, Torri V, Williams C, Lissoni A, Bonazzi C. International collaborative ovarian neoplasm trial 1: a randomized trial of adjuvant chemotherapy in women with early-stage ovarian cancer. J Natl Cancer Inst. 2003;95(2):125–32. https://doi.org/10.1093/jnci/95.2.125.
Trimbos JB, Parmar M, Vergote I, Guthrie D, Bolis G, Colombo N, Vermorken JB, Torri V, Mangioni C, Pecorelli S. International collaborative ovarian neoplasm trial 1 and adjuvant chemotherapy in ovarian neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst. 2003;95(2):105–12. https://doi.org/10.1093/jnci/95.2.105.
Lawrie TA, Winter-Roach BA, Heus P, Kitchener HC. Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer. Cochrane Database Syst Rev. 2015;2015(12):CD004706. https://doi.org/10.1002/14651858.CD004706.pub5.
Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019;30(5):672–705. https://doi.org/10.1093/annonc/mdz062.
Armstrong DK, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(2):191–226.
Fotopoulou C, Hall M, Cruickshank D, et al. British Gynaecological Cancer Society (BGCS) epithelial ovarian/fallopian tube/primary peritoneal cancer guidelines: recommendations for practice. Eur J Obstet Gynecol Reprod Biol. 2017;213:123–39. https://doi.org/10.1016/j.ejogrb.2017.04.016.
Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Eng J Med. 2010;363(10):943–53. https://doi.org/10.1056/nejmoa0908806.
Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2016;2016(1):CD005340. https://doi.org/10.1002/14651858.CD005340.pub4.
du Bois A, Weber B, Rochon J, et al. Addition of epirubicin as a third drug to carboplatin-paclitaxel in first-line treatment of advanced ovarian cancer: a prospectively randomized gynecologic cancer intergroup trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group and the Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens. J Clin Oncol. 2006;24:1127–35.
Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Eng J Med. 2011;365(26):2473–83. https://doi.org/10.1056/nejmoa1104390.
Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Eng J Med. 2011;365(26):2484–96. https://doi.org/10.1056/nejmoa1103799.
Rustin GJS, van der Burg MEL, Griffin CL, et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010;376(9747):1155–63. https://doi.org/10.1016/S0140-6736(10)61268-8.
Wilson M, et al. Fifth ovarian cancer consensus conference of the gynecologic cancer intergroup: recurrent disease. Ann Oncol. 2017;28:727–32.
Gladieff L, Ferrero A, de Rauglaudre G, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in partially platinum-sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial. Ann Oncol. 2012;23(5):1185–9. https://doi.org/10.1093/annonc/mdr441.
Wagner U, Marth C, Largillier R, et al. Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs paclitaxel and carboplatin in platinum-sensitive ovarian cancer patients. Br J Cancer. 2012;107(4):588–91. https://doi.org/10.1038/bjc.2012.307.
ICON and AGO Collaborators. Paclitaxel plus platinum based chemotherapy versus conventional platinum based chemotherapy in women with relapsed ovarian cancer: the ICON/ AGO-OVAR 2.2 trial. Lancet. 2003;361:2009–106.
Pfisterer J, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24(29):4699–707.
Aghajanian C, Blank SV, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–45.
Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–8.
Van der Burg ME, de Wit R, Van Putton WL, et al. Weekly cisplatin and daily oral etoposide is highly effective in platinum pre-treated ovarian cancer. B J Cancer. 2002;7:19–25.
George A, et al. The role of hormonal therapy in patients with relapsed high-grade ovarian carcinoma: a retrospective series of tamoxifen and letrozole. BMC Cancer. 2017;17:456. https://doi.org/10.1186/s12885-017-3440-0.
Kok PS, Beale P, O’Connell RL, et al. PARAGON (ANZGOG-0903): a phase 2 study of anastrozole in asymptomatic patients with estrogen and progesterone receptor-positive recurrent ovarian cancer and CA125 progression. J Gynecol Oncol. 2019;30(5):e86. https://doi.org/10.3802/jgo.2019.30.e86.
Ray-Coquard I, Morice P, Lorusso D, Prat J, Oaknin A, Pautier P, Colombo N. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv1–iv18. https://doi.org/10.1093/annonc/mdy001.
Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5(11):1137–54. https://doi.org/10.1158/2159-8290.CD-15-0714.
Ashworth A, Lord CJ. Synthetic lethal therapies for cancer: what’s next after PARP inhibitors? Nat Rev Clin Oncol. 2018;15(9):564–76. https://doi.org/10.1038/s41571-018-0055-6.
https://medicines.astrazeneca.co.uk/home/oncology/hrd-tbrca-testing/hrd-testing.html
Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 2015;5(11):1137–54. https://doi.org/10.1158/2159-8290.CD-15-0714. Epub 2015 Oct 13. PMID: 26463832; PMCID: PMC4631624.
Ledermann JA, Harter P, Gourley C, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17(11):1579–89. https://doi.org/10.1016/S1470-2045(16)30376-X.
Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Eng J Med. 2018;379(26):2495–505. https://doi.org/10.1056/nejmoa1810858.
Poveda A, Floquet A, Ledermann JA, et al. Final overall survival (OS) results from SOLO2/ENGOT-ov21: a phase III trial assessing maintenance olaparib in patients (pts) with platinum-sensitive, relapsed ovarian cancer and a BRCA mutation. J Clin Oncol. 2020;22(5):620–31. https://doi.org/10.1200/jco.2020.38.15_suppl.6002.
Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Eng J Med. 2016;37(32)):2968–73. https://doi.org/10.1056/nejmoa1611310.
Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949–61. https://doi.org/10.1016/S0140-6736(17)32440-6.
Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Eng J Med. 2019;381(25):2416–28. https://doi.org/10.1056/nejmoa1911361.
Morice PM, Leary A, et al. Myelodysplastic syndromeand acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol. 2021;8(2):e122–34.
Poveda A, Floquet A, et al. SOLO2/ENGOTOV21 investigators. Olaparib tablets as maintainance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation: a final analysis of a double blind, randomised, placebo controlled phase 3 trial. Lancet Oncol. 2021;22(5):620–31. https://doi.org/10.1016/S1470-2045(21)00073-5.
Grabowski JP, Harter P, Heitz F, et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol Oncol. 2016;140(3):457–62. https://doi.org/10.1016/j.ygyno.2016.01.022.
Gershenson DM, Bodurka DC, Coleman RL, Lu KH, Malpica A, Sun CC. Hormonal maintenance therapy for women with low-grade serous cancer of the ovary or peritoneum. J Clin Oncol. 2017;35(10):1103. https://doi.org/10.1200/JCO.2016.71.0632.
Monk BJ, Grisham RN, Banerjee S, et al. MILO/ENGOT-ov11: binimetinib versus physician’s choice chemotherapy in recurrent or persistent low-grade serous carcinomas of the ovary, fallopian tube, or primary peritoneum. J Clin Oncol. 2020;38(32):3753. https://doi.org/10.1200/JCO.20.01164.
Gershenson D. A randomised Phase II/III study to assess the efficacy of trametinib in patients with recurrent or progressive low grade serous or peritoneal cancer. Gynecol Oncol. 2020;159(Suppl 1):v897–8. https://doi.org/10.1016/j.ygyno.2020.06.045.
Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31(1):12–39. https://doi.org/10.1136/ijgc-2020-002230.
de Boer SM, Powell ME, Mileshkin L, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20(9):1273–85. https://doi.org/10.1016/S1470-2045(19)30395-X.
Vale CL, Tierney J, Bull SJ, Symonds PR. Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma. Cochrane Database Syst Rev. 2012;2012(8):CD003915. https://doi.org/10.1002/14651858.CD003915.pub4.
Miller D, Filiaci V, Fleming G, Mannel R, Cohn D, Matsumoto T, Tewari K, DiSilvestro P, Pearl M, Zaino R. Late-breaking abstract 1: randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125(3):771. https://doi.org/10.1016/j.ygyno.2012.03.034.
Sundar S, Balega J, Crosbie E, et al. BGCS uterine cancer guidelines: recommendations for practice. Eur J Obstet Gynecol Reprod Biol. 2017;213:71–97. https://doi.org/10.1016/j.ejogrb.2017.04.015.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68. https://doi.org/10.1038/s41577-020-0306-5.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. https://doi.org/10.1016/j.immuni.2013.07.012.
Yoshino T, et al. JSCO-ESMO-ASCO-JSMO-TOS: international expert consensus recommendationfor tumour agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann Oncol. 2020;31(7):861–72. https://doi.org/10.1016/j.annonc.2020.03.299.
Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv119–42. https://doi.org/10.1093/annonc/mdx225.
Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv72–83. https://doi.org/10.1093/annonc/mdx220.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tilby, M., Williams, S., Pascoe, J. (2022). Chemotherapy in Gynaecological Cancers and Newer Developments. In: Singh, K., Gupta, B. (eds) Gynecological Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-94110-9_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-94110-9_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94109-3
Online ISBN: 978-3-030-94110-9
eBook Packages: MedicineMedicine (R0)