Abstract
The distinct movements of macropinosome formation and maturation have corresponding biochemical activities which occur in a defined sequence of stages and transitions between those stages. Each stage in the process is regulated by variously phosphorylated derivatives of phosphatidylinositol (PtdIns) which reside in the cytoplasmic face of the membrane lipid bilayer. PtdIns derivatives phosphorylated at the 3’ position of the inositol moiety, called 3’ phosphoinositides (3’PIs), regulate different stages of the sequence. 3’PIs are synthesized by numerous phosphoinositide 3’-kinases (PI3K) and other lipid kinases and phosphatases, which are themselves regulated by small GTPases of the Ras superfamily. The combined actions of these enzymes localize four principal species of 3’PI to distinct domains of the plasma membrane or to discrete organelles, with distinct biochemical activities confined to those domains. Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3) and phosphatidylinositol (3,4)-bisphosphate (PtdIns(3,4)P2) regulate the early stages of macropinosome formation, which include cell surface ruffling and constrictions of circular ruffles which close into macropinosomes. Phosphatidylinositol 3-phosphate (PtdIns3P) regulates macropinosome fusion with other macropinosomes and early endocytic organelles. Phosphatidylinositol (3,5)-bisphosphate (PtdIns(3,5)P2) mediates macropinosome maturation and shrinkage, through loss of ions and water, and subsequent traffic to lysosomes. The different characteristic rates of macropinocytosis in different cell types indicate levels of regulation which may be governed by the cell’s capacity to generate 3’PIs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Increased appreciation for the importance of macropinocytosis in health and disease has highlighted limits to our understanding of the mechanisms of its regulation. The formation of macropinosomes requires several distinct movements of cytoplasm which must be coordinated spatially and temporally. The movements include transient extension of curved protrusions from the plasma membrane followed by retraction and contraction of those protrusions that close them into membrane-bounded macropinosomes derived from plasma membrane. Subsequent intracellular movements of macropinosomes are accompanied by their fusion with endosomes and lysosomes (collectively referred to as the endolysosomal network (Huotari and Helenius 2011)), or by reversal of the process through fusion with plasma membrane. These activities are mediated by the assembly, contraction, and disassembly of the actin filament network that underlies plasma membrane protrusions, by localized fusion and fission between the macropinosome and endolysosomes, and by regulated flux of water, ions, and solutes across the bounding membrane of the macropinosome. The movements are analogous to the movements that phagocytic cells use to ingest particles. However, unlike phagocytosis, the movements of macropinocytosis occur without a particle surface to guide the process. The organization of cytoplasm and signaling molecules during macropinosome morphogenesis varies between cell types and different kinds of stimulation. In all cases, macropinosomes form through a self-organized series of distinct chemical activities which require mechanisms to coordinate the timing of their activation and inhibition.
3’ phosphoinositides (3’PIs) are essential to many of these activities and their coordination. Although 3’PIs provide no mechanical or structural support for macropinosome morphogenesis, they do serve to organize the component activities in space and time. This chapter summarizes the known roles for 3’PIs in the component activities and overall organization of macropinocytosis.
Phosphoinositides and the Enzymes that Affect their Abundance
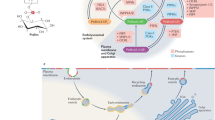
Phosphatidylinositol (PtdIns) is a minor species of phospholipid in cellular membranes, localizing primarily in the leaflet of the membrane lipid bilayer that faces the cytosolic space (inner leaflet). The hydroxyl groups of the inositol sugar moiety of PtdIns (Fig. 7.1a) are readily modified by phosphorylation. Thus, PtdIns is substrate for enzymes that generate phosphatidylinositol 3-phosphate (PtdIns3P), PtdIns4P, and PtdIns5P, which are substrates for lipid kinases that generate phosphatidylinositol (4,5)-bisphosphate (PtdIns(4,5)P2), PtdIns(3,4)P2, and PtdIns(3,5)P2. Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3) is generated by phosphorylation of PtdIns(4,5)P2 (Fig. 7.1b). These phosphoinositides may be dephosphorylated by lipid phosphatases; for example, the phosphatase PTEN (phosphatase and tensin-homolog) synthesizes PtdIns(4,5)P2 from PtdIns(3,4,5)P3 (Di Paolo and De Camilli 2006).
(a) The chemical structure of phosphatidylinositol (PtdIns). The blue box highlights the hydrophilic inositol moiety, the phosphodiester linkage to glycerol, and the positions of the hydroxyls which may be variously phosphorylated. The pink box highlights the hydrophobic diacylglycerol moiety which resides in the membrane lipid bilayer. (b) Essential pathways of 3’PI metabolism. 3’PIs most relevant to macropinocytosis are indicated in red, the principal pathways of their synthesis and degradation for macropinocytosis are indicated with blue arrows, and the essential enzymes are indicated in purple font. Overlays indicate the biochemical activities associated with ruffling (green), macropinosome formation (blue), and macropinosome maturation (orange). PI3K I: class I PI3K ; PI3K II: class II PI3K ; PI3K III: Vps34 or class III PI3K . Other labels are indicated in the text
The 3’PIs in metazoan cells are PtdIns3P, PtdIns(3,4)P2, PtdIns(3,5)P2, and PtdIns(3,4,5)P3, which are synthesized and degraded by localized activities of phosphoinositide kinases and phosphatases. For example, PtdIns3P may be synthesized by the phosphorylation of PdtIns by the class III phosphoinositide 3-kinase (PI3K ) VPS34, by the dephosphorylation of PtdIns(3,4)P2 by INPP4, or by dephosphorylation of PtdIns(3,5)P2 by Fig. 4 (Di Paolo and De Camilli 2006). The different 3’PIs distribute into different and characteristic membrane compartments. The reversibility of the phosphorylation reactions allows for the abundance and location of different phosphoinositide species to be regulated rapidly. Also, phosphoinositides diffuse laterally in the plane of the membrane leaflet, conferring on them the ability to integrate laterally the membrane-associated chemical activities within an organelle or membrane domain. Thus, the cytosolic surface of a macropinosome or phagosome can be rapidly enriched in, or rapidly depleted of, a single species of 3’PI (Henry et al. 2004).
Phosphoinositides can also be hydrolyzed by reactions which are less readily reversed. Phospholipase C (PLC) hydrolyzes PtdIns(4,5)P2 on the glycerol side of the phosphodiester bond, yielding diacylglycerol (DAG) and inositol (1,4,5)-trisphosphate (InsP3). Other important phospholipases, D, A1 and A2 (PLCD, PLCA1, PLCA2), primarily hydrolyze phospholipid substrates other than phosphoinositides. The reaction products of phospholipases have potent biological activities. Although 3’PIs are not hydrolyzed by phospholipases, the activities of PLCγ1 and PLCγ2 are regulated in part by PtdIns(3,4,5)P3 and PtdIns(3,4)P2 (Falasca et al. 1998), which may organize PLC-mediated signaling spatially inside cells through their localization and concentrations.
Enzymes that Synthesize or Degrade 3’PIs
Metazoan cells have three classes of PI3K (Jean and Kiger 2014; Vanhaesebroeck et al. 2012). Class I PI3Ks, which synthesize PtdIns(3,4,5)P3 from PtdIns(4,5)P2 (Toker and Cantley 1997; Vanhaesebroeck et al. 2012), are comprised of two subsets. PI3K class IA includes the catalytic proteins p110α (protein symbol: PIK3CA), p110β (PIK3CB), and p110δ (PIK3CD), and the regulatory proteins p85α, p55α and p50α (PIK3R1), p85β (PIK3R2), p55γ (PIK3R3), and p150 (PIK3R4). They can be activated by tyrosine kinase receptor signaling, and dimers containing p110β can also be activated by heterotrimeric G proteins. Class IB PI3K is comprised of the catalytic protein p110γ (PIK3CG), and the regulatory protein p101 (PIK3R5), which are activated by heterotrimeric G proteins. Class I PI3K catalytic proteins have domains that bind to the small GTPase Ras (p110α, p110δ, and p110γ) or to the GTPases Rac and Cdc42 (p110β), which increase PI3K catalytic activity (Fruman et al. 2017). Class II PI3Ks (PIK3C2A, PIK3C2B, PIK3C2G) synthesize PtdIns3P from PtdIns, and PtdIns(3,4)P2 from PtdIns4P. Class III PI3K (PIK3C3), also called VPS34, synthesizes PtdIns3P from PtdIns.
Some 3’PIs are synthesized by phosphatases or lipid kinases other than PI3K . SHIP1 (INPP5D) and SHIP2 (INPPL1) are 5’PI phosphatases that hydrolyze PtdIns(3,4,5)P3 to produce PtdIns(3,4)P2. Inositol polyphosphate-4-phosphatase (INPP4) synthesizes PtdIns3P from PtdIns(3,4)P2 (Maekawa et al. 2014). PIKfyve (PIP5K3) is a 5’PI kinase that synthesizes PtdIns(3,5)P2 from PtdIns3P (Shisheva 2012). 3’PI phosphatases relevant to macropinocytosis include myotubularin-related protein 6 (MTMR6: PtdIns3P to PtdIns (Maekawa et al. 2014)), INPP4 (PtdIns(3,4)P2 to PtdIns3P; (Maekawa et al. 2014)), Fig. 4 (PtdIns(3,5)P2 to PtdIns3P; (McCartney et al. 2014)), and PTEN (PtdIns(3,4,5)P3 to PtdIns(4,5)P2 and PtdIns(3,4)P3 to PtdIns-4P) (Goulden et al. 2019; Jiao et al. 2020; S. M. Kim et al. 2018). PTEN is an important inhibitor of class I PI3K functions (Maehama et al. 2001). PTEN deletion occurs in many cancers, highlighting the significance of PtdIns(3,4,5)P3 in supporting cell growth (S. M. Kim et al. 2018).
The strong association of PI3K metabolism with cancer and other diseases has led to the development of many inhibitors of PI3K and related enzymes. Broad specificity PI3K inhibitors include wortmannin and LY294002, both of which inhibit macropinocytosis (Araki et al. 1996). Numerous inhibitors of class I PI3Ks are in clinical use or various stages of evaluation. Class III (VPS34) PI3K inhibitors, including 3-methyladenine, VPS34-IN1, and SAR405 (Araki et al. 2006; Bago et al. 2014; Miller et al. 2010; Ronan et al. 2014), have potential for therapeutic treatments in cancer. Class II PI3K inhibitors have not been described. The PIKfyve inhibitor apilimod has been used to treat autoimmune disease and cancer (de Campos et al. 2020; Ikonomov et al. 2019).
How Phosphoinositides Organize Cytoplasm
A membrane lipid bilayer containing phosphoinositides presents a surface decorated with variously phosphorylated inositide sugars anchored to the membrane by their diacylglycerol moieties. Cytoplasmic proteins with phosphoinositide-binding domains concentrate at these membrane surfaces by diffusion and binding, where they are activated allosterically or by their increased proximity to membrane-localized binding proteins. Many kinases, phosphatases, and hydrolases bind 3’PIs, as do proteins that regulate small GTPases of the Ras superfamily which can modulate other effectors allosterically. Thus, an organelle membrane enriched in a particular 3’PI recruits and activates a distinct combination of enzyme activities that stabilize the identity of that domain, execute a defined set of effector activities, and guide its transition to a specific different identity. The identities of some membranous compartments in cells, especially those that comprise the endolysosomal system, are transient and vectorial, meaning that the molecular profile of the organelle membranes remains stable for a limited period before changing to another specific profile as the organelle ages. During the two to five minutes it takes to form a macropinosome, the biochemical profile of the membrane changes transiently and sequentially from that of the plasma membrane to that of early endosomes. A newly formed macropinosome is enriched in the GTPase Rab5a. Active Rab5a activates the class III PI3K VPS34, which synthesizes PtdIns3P, thereby increasing concentrations of PtdIns3P in the macropinosome membrane. The GTPase-activating protein (GAP) that inactivates Rab5a is itself activated by PtdIns3P (Law et al. 2017). Thus, increasing concentrations of PtdIns3P on the macropinosome membrane activate feedback inhibition that leads to the loss of Rab5a and the arrival of Rab7 (Langemeyer et al. 2020). Consequently, the Rab5a-positive, PtdIns3P-rich membrane of the nascent macropinosome transitions after several minutes to another profile depleted of those molecules and enriched in Rab7 and PtdIns(3,5)P2.
These chemistries underlie the progression of different stages during macropinosome maturation. The characteristic profiles organize the local effector activities: actin polymerization, actomyosin contractility, membrane fusion, and the transitions to different stages of the maturation sequence. These transitions sometimes define decision branch-points between two maturation routes. In phagocytosis, 3’PI concentrations must reach thresholds for commitment to particle ingestion (Zhang et al. 2010). In this way, 3’PIs can integrate and direct the activities of cytoplasm.
Localization and Mapping of 3’PIs and Associated Chemistries
Much of what is known about the organization of 3’PIs in macropinocytosis has been discovered through fluorescence microscopy (Maekawa and Fairn 2014). When expressed in cells, fluorescent protein (FP) chimeras of PI-binding domains can concentrate near membranes enriched for the target PI. The net synthesis and degradation of the target PIs can be monitored by confocal microscopy or by ratiometric widefield fluorescence microscopy of the FP chimeras (Araki et al. 2007; Hoppe and Swanson 2004; Vieira et al. 2001). For example, a yellow fluorescent protein (YFP) chimera with the PtdIns(3,4,5)P3-binding PH domain of the enzyme Bruton’s tyrosine kinase (YFP-BtkPH), when expressed inside a cell, distributes uniformly through the cytoplasm of an unstimulated cell. When stimulation increases class I PI3K activity, the YFP-BtkPH concentrates on membranes enriched in PtdIns(3,4,5)P3. This has allowed study of the distributions and dynamics of PtdIns(3,4,5)P3 in living cells during macropinosome formation (Araki et al. 2007; Yoshida et al. 2009). A drawback of this method is that high levels of expression of 3’PI-binding FP chimeras can interfere with the 3’PI-dependent reactions they are meant to reveal (Wills et al. 2018; Wills et al. 2021). Control experiments are needed to ensure such artifacts do not alter the essential 3’PI dynamics significantly.
The Cellular Activities Essential for Macropinocytosis
Some cancer cells exhibit macropinocytosis constitutively. In many non-transformed cells, however, macropinocytosis occurs in response to stimulation of cell surface receptors, most notably growth factor receptors, which initiate cell movements that lead to macropinosome formation. The morphologies of these movements vary widely among cell types and even within a single cell. The protrusions which close into macropinosomes are called ruffles, which are curved folds of plasma membrane with underlying meshworks of actin filaments. The actin filaments inside ruffles are polymerized into roughly planar arrays of parallel filaments or cross-linked networks of filaments. Actin polymerization occurs either at the distal margins of ruffles, which are enriched in the growing ends of actin filaments, or at branch-points along the sides of actin filaments. Actin cross-linking proteins may reinforce the meshwork structure (Sasaki et al. 2001). The organization of ruffles is regulated by cytoplasmic microtubules (Rosania and Swanson 1996; Waterman-Storer et al. 1999). In actively macropinocytic cells observed on coverslips, ruffles extend as protrusions from the dorsal surface (i.e., the surface facing away from the coverslip; Fig. 7.2). Most ruffles form as curved sheet-like extensions, which either continue growing into fully circular, crater-shaped extensions of the cell surface or close back against the cell as a cresting wave, trapping extracellular fluid into plasma membrane-derived vesicles. In a cell which is spread out on a coverslip, single ruffles that cover large areas of the surface, called circular dorsal ruffles, often appear after acute stimulation with growth factors. They mature by constricting centripetally and forming macropinosomes near the ruffling regions. More commonly, the ruffles on a cell surface are smaller and short-lived, lasting only one to five minutes. They resolve by receding back into the cell or by closing into macropinosomes. Closure was thought to occur at the distal margins of ruffles (J. A. Swanson 2008). This occurs sometimes but the more common processes involve either an asymmetric wavelike closing against the cell (Quinn et al. 2021) or a circumferential constriction of the macropinocytic cup near the base of the circular ruffles, creating macropinosomes which are small relative to the ruffles that precede them (Fig. 7.3). The basal constriction that closes into a macropinosome may involve a twisting movement of tent-pole like actin bundles within the ruffle (Condon et al. 2018), or other kinds of constrictions away from the distal margin of the ruffle (Quinn et al. 2021).
Summary of the stages of macropinocytosis and the corresponding 3’PIs. The stages of macropinosome formation and maturation are indicated as side view sections progressing from left to right. The predominate phosphoinositides at each stage are indicated. Overlays indicate the membrane movements associated with ruffling (green), macropinosome formation (blue), and macropinosome maturation (orange). Small ruffles on a quiescent surface are activated by Arf6 and PtdIns-4P to generate early ruffles, enriched in PtdIns(4,5)P2 and active Cdc42. As ruffles enlarge, concentrations of PtdIns(3,4,5)P3 increase, as well as the activities of Ras, Rac, and Rab35. Ras and Rab35 promote the feedback amplification of class I PI3K and Rac activities. Closing macropinosomes are enriched in PtdIns(3,4,5)P3 and PtdIns(3,4)P2. During or just after closure, Rac is deactivated and the activities of Rab5 and Vps34 increase. Nascent macropinosomes are stabilized by elevated concentrations of PtdIns3P and by increased activities of Rab5, Rab35, Rab20, Rab21, and Arf6. Maturing macropinosomes fuse with other macropinosomes and with endolysosomes. Increasing concentrations of PtdIns(3,5)P2 on macropinosomes increase the activities of TPC1, TPC2, and Septins
Once a macropinosome has separated from plasma membrane as an intracellular organelle, which we refer to here as macropinosome closure, it takes either of two routes. It may reverse course and return to the plasma membrane (Feliciano et al. 2011) or begin a series of changes that ultimately lead the macropinosome to merge with endolysosomes. The regurgitation may simply be a redistribution of plasma membrane following incomplete closure of the macropinosome. Macropinosomes that do not recycle immediately to the plasma membrane swell initially, transforming from irregular shapes into more rounded shapes, then shrink by the export of the ingested sodium and chloride and the osmotically obliged water (Freeman et al. 2020). The ions introduced into cytoplasm by export from macropinosomes are expelled from the cell by plasma membrane ion transporters such as the Na/K ATPase, thereby allowing equilibration of cell volume (Freeman et al. 2020). Macropinosome shrinkage allows the formation of small vesicles or narrow membranous tubules which break away from the macropinosome and return membrane and plasma membrane proteins to the cell surface (Freeman et al. 2020; Kerr et al. 2006). Meanwhile, newly formed macropinosomes migrate from the cell periphery to perinuclear positions, first fusing with others of their kind or with early endosomes, then merging with endolysosomes (Racoosin and Swanson 1993). This later stage of maturation sometimes occurs by transient and reversible connections between the macropinosome and the endolysosomes, which has been called pyranhalysis or kiss-and-run (Willingham and Yamada 1978; Yoshida et al. 2015b). Eventually the macropinosome merges completely into the endolysosomal network, where the internalized macromolecular solutes are degraded by acid hydrolases. Thus, in a cell which is continuously forming macropinosomes, internalized water and ions move across macropinosome membranes into cytoplasm and out of the cell, internalized membrane is recycled to plasma membrane via recycling tubules, and extracellular macromolecules are scavenged efficiently for hydrolytic degradation to smaller molecules that support cell metabolism.
3’PI-Dependent Activities of Macropinocytosis
Macropinocytosis requires the localized synthesis of 3’PIs at different stages of the process (Fig. 7.3). PtdIns(3,4,5)P3 and PtdIns(3,4)P2 are concentrated in circular ruffles and closing macropinosomes (Yoshida et al. 2009). PtdIns3P is concentrated in newly formed macropinosomes (Araki et al. 2006). Quantitative fluorescence microscopy of 3’PI-binding FP chimeras expressed in macrophages showed that macropinocytic cups formed in response to stimulation with Colony-stimulating Factor-1 (CSF-1) exhibited a sequence of associated phosphoinositides during their formation and closure; PtdIns(4,5)P2 increased first in circular ruffles, followed by transient, sequential peaks of PtdIns(3,4,5)P3, PtdIns(3,4)P2, and PtdIns3P as macropinosomes closed into the cell (Welliver and Swanson 2012). Imaging of other cell types indicated similar patterns of PtdIns(4,5)P2 and 3’PIs during macropinocytosis (Araki et al. 2007; Porat-Shliom et al. 2008). Genetic analysis of macropinocytosis in Caenorhabditis elegans embryos demonstrated an essential sequence of 3’PI-modifying enzymes necessary for macropinosome formation and maturation that was consistent with the fluorescence microscopic studies (Maekawa et al. 2014). The implied sequence of 3’PIs in the C. elegans study was PtdIns(3,4,5)P3, PtdIns(3,4)P2, PtdIns3P, PtdIns. PtdIns(3,5)P2 is not readily visualized in living cells, but experimental manipulation of PIKfyve activity indicates the importance of PtdIns(3,5)P2 for late stages of macropinosome maturation (Krishna et al. 2016). With this sequence of phosphoinositides in the various stages of macropinocytosis, we next review the roles for the principal 3’PI species in the underlying biochemical activities.
Class I PI3K Is Necessary for Some But not all Ruffling
The four class I PI3K catalytic proteins have been implicated in macropinocytosis to varying degrees depending on their levels of expression and the receptor pathways that initiate the process. They can be activated by growth factor receptors, Toll-like receptors (TLR), chemokine receptors, and G-protein-coupled receptors (GPCR). p110β is regulated by inputs from both GPCR and tyrosine kinase receptors, and functions as a coincidence detector or integrator of signaling inputs (Bresnick and Backer 2019). Receptors bind to PI3K directly or to adapter proteins that recruit and activate p85 regulatory proteins (Fruman et al. 2017). PtdIns(3,4,5)P3 synthesis increases by allosteric activation of PI3K catalytic proteins and by the increased proximity of the enzymes to their substrates. The actin cytoskeleton, organized by cytoplasmic microtubules (Rosania and Swanson 1996) or some other structural feature of the cups themselves, facilitates amplification of PI3K activities in plasma membrane domains circumscribed by ruffles (Erami et al. 2017; Pacitto et al. 2017; Yoshida et al. 2018). FP chimeras of PH domains show increased concentrations of PtdIns(3,4,5)P3 and PtdIns(3,4)P2 in the membranes associated with ruffles (Araki et al. 2007; Yoshida et al. 2018). The phosphatase PTEN lowers levels of PtdIns(3,4,5)P3 in cells and provides a brake on stimulation of PI3K leading to macropinocytosis. PTEN deletion or inhibition often stimulates macropinocytosis (S. M. Kim et al. 2018). For some cells, class I PI3K is required for ruffling, a necessary prerequisite for macropinosome formation. In murine embryonic fibroblasts (MEF) and PTEN-deficient cancer cells, p110β is required for ruffling and macropinocytosis in response to PDGF (Salloum et al. 2019). In contrast, PI3K inhibition in macrophages inhibits macropinosome closure but not ruffling in response to CSF-1 (Araki et al. 1996). In summary, class I PI3Ks are required for both ruffling and closure in some cells, but only for closure in others.
How do class I PI3Ks organize ruffling? The localized and oriented polymerization of actin filaments beneath ruffles requires the small GTPase Rac, an essential regulator of ruffling and macropinosome formation (Buckley et al. 2020; Fujii et al. 2013; Grimmer et al. 2002). The Rac effectors p21-activated kinase-1 (Pak1) and WAVE are important for macropinocytosis (Dharmawardhane et al. 2000; Veltman et al. 2016). Phosphorylated Pak1 binds to and activates LIM kinase, which phosphorylates proteins that regulate actin filament dynamics, including filament-uncapping proteins and the filament-severing protein cofilin (Delorme et al. 2007). Pak1 also activates Ctb1/BARS, a protein essential to macropinosome closure which works through activation of phospholipase D (Haga et al. 2009). Rac also activates WAVE, which stimulates ruffle extension by activating Arp2/3, which mediates the formation of actin filament branches on other actin filaments (Eden et al. 2002). PtdIns(3,4,5)P3-dependent guanine nucleotide exchange factors (GEFs) which activate Rac include Tiam-1, Vav, and TRIO (Bai et al. 2015). For cells that require PI3K for ruffling, these Rac GEFs may initiate the ruffles leading to macropinocytosis. However, as inhibition of class I PI3K does not inhibit ruffling in all circumstances, it is not certain that the PtdIns(3,4,5)P3-dependent Rac GEFs are necessary for macropinocytosis.
The mechanism by which ruffles become circular is still unclear. The signaling pathway and machinery of large circular dorsal ruffle formation are distinct from those of relatively small circular ruffles or macropinocytic cups formed by the curling of peripheral ruffles (Itoh and Hasegawa 2013). The mechanism of large circular dorsal ruffle formation is well characterized in PDGF-stimulated fibroblasts. The formation of circular ruffles from peripheral ruffles is not perturbed by PI3K inhibitors in macrophages or EGF-stimulated A431 cells (Araki et al. 2007; Araki et al. 1996). However, the formation of large circular dorsal ruffles (> 20 μm in diameter) observed in some types of culture cells, such as PDGF-stimulated fibroblasts, is dependent on class I PI3K activity (Salloum et al. 2019; Wymann and Arcaro 1994). In cells that form circular dorsal ruffles, increased PtdIns(3,4,5)P3 concentrations persist for several minutes within the domain of plasma membrane circumscribed by the actin-rich ruffles (Yoshida et al. 2018). These domains may facilitate PI3K amplification (Pacitto et al. 2017). Macropinosomes form at the base of the contracting circular dorsal ruffles. Lanzetti et al. showed that Rab5 organizes circular dorsal ruffle formation through coordinated activities of PI3K , Ras, and Rac (Lanzetti et al. 2004). SH3YL1 (SH3 domain containing Ysc84-like 1), which binds to PtdIns(3,4,5)P3, is an important regulator of dorsal ruffle formation (Hasegawa et al. 2011). PtdIns(3,4)P2 synthesis from PtdIns(3,4,5)P3 by the 5’PI phosphatase SHIP2, which also binds to SH3YL1, is correlated with formation of the circular ruffles. ARAP1 (Arf GAP with Rho GAP domain, ankyrin repeat, and PH domain 1), which is an Arf GAP with multiple PH domains that bind to PtdIns(3,4,5)P3, localizes to the membrane inside the circular ruffles after PDGF-stimulation. ARAP1 and its substrate Arf1/5 are involved in the ring size control of circular ruffles (Hasegawa et al. 2012). The actin cytoskeleton machineries N-WASP, WAVE, and Arp2/3, which are effectors of Rac1, are also localized to circular ruffles (Krueger et al. 2003; Legg et al. 2007; Suetsugu et al. 2003). Also, the F-actin-bundling protein actinin-4 localizes in circular ruffles of macrophages (Araki et al. 2000) and PDGF-stimulated fibroblasts (Lanzetti et al. 2004). PtdIns(4,5)P2 and PdtIns(3,4,5)P3 differentially regulate actinin flexibility and actin-bundling function through their binding to the calponin homology domain 2 of α-actinin (Corgan et al. 2004; Fraley et al. 2003). The Rab5 GAP RN-tre interacts with both F-actin and actinin-4 and is also necessary for circular ruffle formation (Lanzetti et al. 2004).
As mentioned above, Rac1 is indispensable for membrane ruffling. However, strong overexpression of constitutively active Rac1 produces long straight linear ruffles in RAW264 cells, indicating that a Rac1 effector promotes ruffle formation but not circularization of the ruffles (Ikeda et al. 2017). Lanzetti et al. (2004) showed the same result in PDGF-stimulated MEFs. Local and temporal modulation of Rac1 activity within a small cell surface area may be required for circular ruffle formation.
Oncogenic Ras stimulates macropinocytosis in many cells (Bar-Sagi and Feramisco 1986). Stimulation may occur through the binding of GTP-Ras to the Ras-binding domains of PI3K catalytic subunits p110α, p110δ, or p110γ, which leads to local generation of PtdIns(3,4,5)P3. H-Ras-dependent macropinocytosis in HeLa cells leads to formation of PtdIns(3,4,5)P3-rich macropinosomes (Porat-Shliom et al. 2008). Alternatively, stimulation of macropinocytosis may be due to the effects of oncogenic Ras on the redistribution of cholesterol to plasma membrane which consequently increases Rac localization to plasma membrane (Ramirez et al. 2019). The requirement for wild-type Ras in macropinocytosis is uncertain, however, as deletion of H-, K-, and N-Ras in MEFs did not inhibit macropinocytosis (Palm et al. 2017).
Ruffling and macropinocytosis also require other Class I PI3K-dependent GTPases, including Arf6, Cdc42, RhoG, and Abi1. Arf6 is required for macropinocytosis in H-Ras-transformed HeLa and HT1080 cells (Porat-Shliom et al. 2008; Williamson and Donaldson 2019). The Arf6 GEF cytohesin 2 is activated by PI3K (Davies et al. 2014). Arf6 effectors are PI4P5K, WAVE, and JIP3/4, which together increase actin polymerization and recycling of internalized membrane to the plasma membrane. The RhoG GEF SGEF was shown to stimulate macropinocytosis (Ellerbroek et al. 2004). P-Rex1 is a PtdIns(3,4,5)P3-dependent GEF for RhoG (Damoulakis et al. 2014). RhoG activates Rac by binding to ELMO in complex with the Rac GEF DOCK180. Thus, 3’PIs may promote ruffling through RhoG, upstream of Rac. Abi1, in complex with Abl and PI3K p85, promotes macropinocytosis (Dubielecka et al. 2010; N. Kim et al. 2019; Kotula 2012). Rab-family GTPases can also activate class I PI3Ks for macropinocytosis. Rab35 immunoprecipitates with PI3K p85α and stimulates actin dynamics (Marat et al. 2012) and the formation of phagosomes (Egami et al. 2011), circular dorsal ruffles, and macropinosomes (Corallino et al. 2018). Rab35 inhibits Arf6 (Egami et al. 2015). The Rab35 effector ACAP2 is an Arf6 GAP which may be relevant to macropinocytosis (Kobayashi and Fukuda 2012). Rab8a is activated by TLRs and activates p110γ (Wall et al. 2019; Wall et al. 2017). Rab10 is recruited to PtdIns(3,4,5)P3-positive macropinosomes in macrophages and regulates the formation of recycling vesicles (Liu et al. 2020). However, Rab10 recruitment is PtdIns(3,4,5)P3-independent (Kawai et al. 2021).
Class I PI3K and Macropinosome Closure
Although class I PI3Ks are not always necessary for cell ruffling, they are nearly always required for macropinosome closure. The constriction that closes ruffles and cups into macropinosomes requires a PI3K-dependent contractile activity mediated by nonmuscle myosins (Araki et al. 1996; J.A. Swanson et al. 1999). High local concentrations of PtdIns(3,4,5)P3 and PtdIns(3,4)P2 at the base of the ruffles may activate PLCγ1 or PLCγ2, generating DAG from PtdIns(4,5)P2. The only known exception to this requirement for class I PI3K in macropinosome closure is in phorbol myristate acetate (PMA)-stimulated macropinocytosis, which does not require class I PI3K for ruffling or closure (Yoshida et al., 2015a). Moreover, PMA-elicited macropinosomes do not generate significant levels of PtdIns(3,4,5)P3 or PtdIns(3,4)P2 in murine bone marrow-derived macrophages (Yoshida et al. 2015b). This indicates that PMA bypasses class I PI3K-dependent activities necessary for closure; likely through mimicry of DAG, the product of PLCγ1. However, studies of other cells indicate roles for type I PI3K in PMA-stimulated macropinocytosis, so further studies will be needed to explain the different results.
Protein kinase C isoforms (PKCs) essential to macropinosome formation are activated by DAG and possibly also by calcium released by InsP3-binding channels in endoplasmic reticulum. PKC (Yoshida et al. 2015a) or another DAG-dependent activity (Ard et al. 2015) then stimulates the contractile activities of myosin that constrict the cup. Myosin 1B, myosin 1E, and myosin IF are also implicated in macropinosome formation in Dictyostelium discoideum (Brzeska et al. 2016; Chen et al. 2012). Because Myosin 1E and myosin 1F, which have a PtdIns(3,4,5)P3-binding tail homology region 1 (TH1) domain, are recruited to the membrane through interaction with PtdIns(3,4,5)P3 during chemotaxis and phagocytosis in neutrophils (Chen and Iijima 2012) and RAW macrophages (Ikeda et al. 2017), these isoforms of myosin may contribute to macropinosome closure in mammalian cells as well as Dictyostelium cells. Macropinocytosis is inhibited by the myosin II inhibitor blebbistatin (Jiang et al. 2010; Lou et al. 2014; Williamson and Donaldson 2019; Yoshida et al. 2015b) and by the myosin light chain kinase (MLCK) inhibitor ML-7 (Araki et al. 2003). Unlike the myosin I isoforms, the recruitment and contractile activity of myosin II is independent of PtdIns(3,4,5)P3 (Araki et al. 2003).
Macropinosome closure requires the inactivation of Rac. Fluorescence microscopy of YFP-BtkPH in macrophages showed a transient spike (ca. 90 sec) of PtdIns(3,4,5)P3 in cup membranes associated with closure of macropinosomes. Fluorescence resonance energy transfer (FRET)-based imaging showed a coincident spike of Rac activity, which suggested that Rac activation and deactivation are both necessary for macropinosome closure. Consistent with this idea, Fujii et al. (Fujii et al. 2013) showed that although Rac activity is necessary for the ruffling that creates macropinosomes, Rac must be inactivated to allow closure of the macropinosome. Experimentally forcing Rac to remain in its active, GTP-bound conformation inhibited macropinosomes from fully closing into the cell. This suggests that inactivation of Rac by a GTPase-activating protein (GAP) is necessary for closure. PtdIns(3,4,5)P3-binding GAPs for Rac and Cdc42 were shown to be necessary for phagocytosis of large particles (Schlam et al. 2015). Similar PtdIns(3,4,5)P3-dependent Rac GAP activities may be required for macropinosome closure.
PtdIns(3,4)P2 may have distinct functions in macropinocytosis. Most of the class I PI3K activities that increase PtdIns(3,4,5)P3 also increase PtdIns(3,4)P2, and both species can activate many class I PI3K-dependent activities. Specific roles for the dephosphorylation of PtdIns(3,4,5)P3 to PtdIns(3,4)P2 by SHIP-1 or SHIP-2 in ruffles have not been demonstrated; however, there are suggestions that PtdIns(3,4)P2 facilitates scission of macropinosomes from plasma membrane into the cytoplasm (Hawkins and Stephens 2016).
PtdIns3P Facilitates Macropinosome Formation and Macropinosome Maturation
All macropinocytosis requires synthesis of PtdIns3P. In metazoan cells, PtdIns3P accumulates on membranes of cups and nascent macropinosomes. In A431 cells, the class III PI3K inhibitor 3-methyladenine did not inhibit macropinosome formation but prevented accumulation of PtdIns-3P and EEA1 on macropinosomes, as well as homotypic fusion of macropinosomes and subsequent macropinosome maturation (Araki et al. 2006). PtdIns3P synthesis on macropinosomes may occur by a sequential cascade in cup membranes, in which PtdIns4P is phosphorylated to PtdIns(4,5)P2 and PtdIns(3,4,5)P3, then dephosphorylated to PtdIns(3,4)P2 then PtdIns3P (Welliver and Swanson 2012). Alternatively, macropinosomal PtdIns3P may be synthesized simply by VPS34-mediated phosphorylation of PtdIns. Which of these pathways to PtdIns3P predominates, and how these pathways are selected, remains unknown.
Rab5a is also required for macropinosome closure. Experimentally limiting Rab5a activation leads to the formation of unstable macropinosomes which either fail to close into the cell or fuse back with the plasma membrane without maturing (Feliciano et al. 2011). Rab5a may stabilize macropinosomes by recruiting and activating Vps34, thus promoting the synthesis of PtdIns3P (Christoforidis et al. 1999). PtdIns3P stabilizes macropinosomes for fusion with other endosomes and for continued maturation. The PtdIns3P-binding sorting nexins (e.g., SNX5) and other PtdIns3P-binding proteins associate with the tubular extensions of macropinosomes that mediate membrane recycling following shrinkage. Rab5a markedly accumulates on nascent macropinosomes after the PtdIns(3,4,5)P3 spike, coincident with the rise in PtdIns3P levels (Welliver and Swanson 2012). PtdIns3P-rich membrane may prevent regurgitation by promoting activation of CORVET-HOPS complexes, which regulate PtdIns3P-dependent homotypic fusion of endosomes (CORVET) and the Rab5 to Rab7 transition (HOPS) (Solinger and Spang 2013). Nascent macropinosomes lacking PtdIns3P often fail to mature, and in some cells macropinosomes recycle without fusing to endolysosomes (Hewlett et al. 1994). In EGF-stimulated macropinocytosis by A431 cells, PtdIns3P and EEA1 persisted on membrane of macropinosomes as long as the macropinosomes were present in the cells. Macropinosomes decreased in size and number with time but did not mature into late endosome/lysosomes (Araki et al. 2006; Hamasaki et al. 2004). Their content was not delivered to endolysosomes but instead recycled to extracellular space. It remains unclear why this cell behaves differently than most cells.
Synthesis of PtdIns(3,5)P2 by PIKfyve Mediates Macropinosome Shrinkage
PIKfyve activity is required for the shrinkage of macropinosomes that accompanies maturation (Krishna et al. 2016). Macropinosome shrinkage is mediated by the lysosomal cation channel TRPML1/MCOLN1 and by the two-pore channels TPC1 and TPC2, which mediate the PtdIns(3,5)P2-dependent export of sodium and calcium from macropinosomes (Freeman et al. 2020; Krishna et al. 2016). Depletion of PtdIns(3,5)P2 by PIKfyve inhibition prevents ingested sodium and chloride of internalized fluids from being transported out of macropinosomes via TPC1; consequently, water accumulates and distends the vacuolar compartments and thereby inhibits the return of membrane to the cell surface via recycling tubules (Freeman et al. 2020). PtdIns(3,5)P2 also regulates macropinosome fusion through recruitment of septin GTPases (Dolat and Spiliotis 2016).
Roles for PI3K in Macropinocytosis by Dictyostelium discoideum
The free-living amoeba Dictyostelium discoideum feeds by phagocytosis of smaller microbes and, to a limited extent, by macropinocytosis of extracellular fluids. Laboratory strains of Dictyostelium selected for axenic growth in liquid medium exhibit increased macropinocytosis, which allows sufficient ingestion of soluble nutrients to support their metabolism (Hacker et al. 1997). Macropinosomes of axenic strains form similarly to those of metazoan cells and wild-type Dictyostelium: protrusions of plasma membrane organize into cup-shaped cell extensions which constrict and close into macropinosomes. The macropinosomes of axenic strains are larger than those of wild-type strains because of spontaneous mutations in the gene for the Ras GAP NF-1. The deficiency of NF-1 increases the activity of Dictyostelium Ras proteins and associated PI3K activity in the domains of plasma membrane that form macropinocytic cups, which allows the formation of larger cups and macropinosomes. The versatility of Dictyostelium for genetics and fluorescence microscopy has allowed insightful and revealing analyses of the roles for 3’PIs in macropinosome formation.
PI3K is essential for macropinocytosis in Dictyostelium, but it does not synthesize 3’ phosphoinositides. Rather, the variously phosphorylated inositol headgroups are anchored to the membrane by an ether linkage between a fatty acid chain and the glycerol backbone, rather than an ester linkage, and the substrates for Dictyostelium PI3K isoforms and PTEN are plasmanylinositols (Clark et al. 2014). Thus, macropinosome formation in Dictyostelium is regulated by a molecule with the same inositol headgroup as PtdIns(3,4,5)P3, which we refer to here as PIP3 , but with a different lipid backbone. PIP3 is concentrated within the borders of the circular cup or patch of membrane, with a distinct boundary between the PIP3-rich interior and the PIP3-poor membrane outside the cup. In contrast to metazoan cells, PI3P does not accumulate in forming cups or nascent macropinosomes. PTEN localizes to plasma membrane outside of the cup, which suggests that its exclusion helps define the PIP3 patch. Dictyostelium PI3K1 and PI3K2 support ruffling and cup formation; PI3K4 supports closure (Hoeller et al. 2013). They are activated allosterically by GTP-RasG or GTP-RasS and their Ras-binding domains are required for macropinocytosis (Hoeller et al. 2013). PIP3 and active Ras coincide in the cup membranes, which suggests a positive feedback amplification mechanism involving Ras and PI3K (Veltman et al. 2016). Such feedback interactions have been identified in other motility systems (Thevathasan et al. 2013).
Macropinosome formation in Dictyostelium also requires the GTPase Rac and its effector SCAR/WAVE, which activates Arp2/3-based actin polymerization at the outer rim of the cup. Coronin and formins are also essential for macropinocytosis (Junemann et al. 2016; Kelsey et al. 2012). Thus, despite the different mechanisms between metazoans and Dictyostelium of inositol anchorage to membranes, the conserved requirement for Ras-regulated PI3K in macropinocytosis indicates the importance of anchored inositol phosphates for organizing actin into cups and macropinosomes.
Feedback Regulation of Macropinocytosis by 3’PIs
The cell’s ability to generate PtdIns(3,4,5)P3 and macropinosomes may be limited by metabolism or cellular dimensions. Macropinocytosis is a source of nutrients for some cancer cells, which suggests that ingestion may be regulated by nutrient supply or other metabolic needs. AMP kinase, whose activity increases when ATP levels are low, is required for macropinocytosis in starved PTEN-deficient cancer cells (S. M. Kim et al. 2018). Activation of Akt by PtdIns(3,4,5)P3 increases activity of the metabolic regulatory complex mTORC1 (Laplante and Sabatini 2012). mTORC1 can limit protein scavenging by macropinocytosis (Palm et al. 2017). mTORC1 is negatively regulated by TSC1/2, which is itself negatively regulated by Akt1. However, TSC2-deficiency, which increases mTORC1 activity, upregulates VPS34-dependent macropinocytosis (Filippakis et al. 2018), and this discrepancy is not simply explained.
The ability to synthesize 3’PIs necessary for macropinocytosis may be regulated by larger-scale feedback related to the actin and microtubule cytoskeleton, or to the dimensions of the vacuolar compartment or of the cell itself. PI3K is required for phagocytosis of large but not small particles (Cox et al. 1999), which suggests a role for 3’PIs in regulating the size of permissible gulps. Phagocytosis requires concentrations of PtdIns(3,4,5)P3 to exceed a threshold concentration for particle ingestion (Zhang et al. 2010). The cell’s ability to attain such concentrations of PtdIns(3,4,5)P3 in phagosomal or macropinocytic cups may be regulated by the cell’s capacity for enlargement. That is, 3’PIs may serve as permissive gates for invagination or for progression through the stages of macropinosome maturation.
Remaining Questions
Most of the essential components of macropinocytosis have been identified, yet we remain largely ignorant about how their activities are regulated overall. What factors regulate the characteristic rates or capacity of macropinocytosis in different cell types? If macropinocytosis occurs by self-organized chemistries, then what controls the magnitude of those reactions? Do 3’PIs regulate cup size, frequency of macropinosome formation, or macropinosome stability? If so, how? Why is only some ruffling PI3K-dependent? How does PtdIns(3,4,5)P3 or PtdIns(3,4)P2 organize cup closure? What regulates the curvature of ruffles? How does Ras regulate macropinocytosis ? Answers to these questions will likely reveal how macropinocytosis contributes to health and disease.
References
Araki N, Johnson MT, Swanson JA (1996) A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis in macrophages. J. Cell Biol. 135:1249–1260
Araki N, Hatae T, Yamada T, Hirohashi S (2000) Actinin-4 is preferentially involved in circular ruffling and macropinocytosis in mouse macrophages: analysis by fluorescence ratio imaging. J. Cell Sci. 113:3329–3340
Araki N, Hatae T, Furukawa A, Swanson JA (2003) Phosphoinositide-3-kinase-independent contractile activities associated with Fcgamma-receptor-mediated phagocytosis and macropinocytosis in macrophages. J Cell Sci 116(Pt 2):247–257. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12482911
Araki N, Hamasaki M, Egami Y, Hatae T (2006) Effect of 3-methyladenine on the fusion process of macropinosomes in EGF-stimulated A431 cells. Cell Struct Funct 31(2):145–157. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17146146
Araki N, Egami Y, Watanabe Y, Hatae T (2007) Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp Cell Res 313(7):1496–1507. https://doi.org/10.1016/j.yexcr.2007.02.012
Ard R, Mulatz K, Pomoransky JL, Parks RJ, Trinkle-Mulcahy L, Bell JC, Gee SH (2015) Regulation of Macropinocytosis by Diacylglycerol Kinase zeta. PLoS One 10(12):e0144942. https://doi.org/10.1371/journal.pone.0144942
Bago R, Malik N, Munson MJ, Prescott AR, Davies P, Sommer E et al (2014) Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. Biochem J 463(3):413–427. https://doi.org/10.1042/BJ20140889
Bai Y, Xiang X, Liang C, Shi L (2015) Regulating Rac in the nervous system: molecular function and disease implication of Rac GEFs and GAPs. Biomed Res Int 2015:632450. https://doi.org/10.1155/2015/632450
Bar-Sagi D, Feramisco JR (1986) Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 233:1061–1066
Bresnick AR, Backer JM (2019) PI3Kbeta-A versatile transducer for GPCR, RTK, and small GTPase signaling. Endocrinology 160(3):536–555. https://doi.org/10.1210/en.2018-00843
Brzeska H, Koech H, Pridham KJ, Korn ED, Titus MA (2016) Selective localization of myosin-I proteins in macropinosomes and actin waves. Cytoskeleton (Hoboken) 73(2):68–82. https://doi.org/10.1002/cm.21275
Buckley CM, Pots H, Gueho A, Vines JH, Munn CJ, Phillips BA et al (2020) Coordinated Ras and Rac activity shapes macropinocytic cups and enables phagocytosis of geometrically diverse bacteria. Curr Biol 30(15):2912–2926 e2915. https://doi.org/10.1016/j.cub.2020.05.049
Chen CL, Iijima M (2012) Myosin I: A new pip(3) effector in chemotaxis and phagocytosis. Commun Integr Biol 5(3):294–296. https://doi.org/10.4161/cib.19892
Chen CL, Wang Y, Sesaki H, Iijima M (2012) Myosin I links PIP3 signaling to remodeling of the actin cytoskeleton in chemotaxis. Sci Signal 5(209):ra10. https://doi.org/10.1126/scisignal.2002446
Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip S-C et al (1999) Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nature Cell Biol. 1:249–252
Clark J, Kay RR, Kielkowska A, Niewczas I, Fets L, Oxley D et al (2014) Dictyostelium uses ether-linked inositol phospholipids for intracellular signalling. Embo J 33(19):2188–2200. https://doi.org/10.15252/embj.201488677
Condon ND, Heddleston JM, Chew TL, Luo L, McPherson PS, Ioannou MS et al (2018) Macropinosome formation by tent pole ruffling in macrophages. J Cell Biol 217(11):3873–3885. https://doi.org/10.1083/jcb.201804137
Corallino S, Malinverno C, Neumann B, Tischer C, Palamidessi A, Frittoli E et al (2018) A RAB35-p85/PI3K axis controls oscillatory apical protrusions required for efficient chemotactic migration. Nat Commun 9(1):1475. https://doi.org/10.1038/s41467-018-03571-8
Corgan AM, Singleton C, Santoso CB, Greenwood JA (2004) Phosphoinositides differentially regulate alpha-actinin flexibility and function. Biochem J 378(Pt 3):1067–1072. https://doi.org/10.1042/BJ20031124
Cox D, Tseng CC, Bjekic G, Greenberg S (1999) A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem 274(3):1240–1247. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9880492
Damoulakis G, Gambardella L, Rossman KL, Lawson CD, Anderson KE, Fukui Y et al (2014) P-Rex1 directly activates RhoG to regulate GPCR-driven Rac signalling and actin polarity in neutrophils. J Cell Sci 127(Pt 11):2589–2600. https://doi.org/10.1242/jcs.153049
Davies JC, Tamaddon-Jahromi S, Jannoo R, Kanamarlapudi V (2014) Cytohesin 2/ARF6 regulates preadipocyte migration through the activation of ERK1/2. Biochem Pharmacol 92(4):651–660. https://doi.org/10.1016/j.bcp.2014.09.023
de Campos CB, Zhu YX, Sepetov N, Romanov S, Bruins LA, Shi CX et al (2020) Identification of PIKfyve kinase as a target in multiple myeloma. Haematologica 105(6):1641–1649. https://doi.org/10.3324/haematol.2019.222729
Delorme V, Machacek M, DerMardirossian C, Anderson KL, Wittmann T, Hanein D et al (2007) Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev Cell 13(5):646–662. https://doi.org/10.1016/j.devcel.2007.08.011
Dharmawardhane S, Schurmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM (2000) Regulation of macropinocytosis by p21-activated kinase-1. Mol. Biol. Cell 11:3341–3352
Di Paolo G, De Camilli P (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443(7112):651–657. https://doi.org/10.1038/nature05185
Dolat L, Spiliotis ET (2016) Septins promote macropinosome maturation and traffic to the lysosome by facilitating membrane fusion. J Cell Biol 214(5):517–527. https://doi.org/10.1083/jcb.201603030
Dubielecka PM, Machida K, Xiong X, Hossain S, Ogiue-Ikeda M, Carrera AC et al (2010) Abi1/Hssh3bp1 pY213 links Abl kinase signaling to p85 regulatory subunit of PI-3 kinase in regulation of macropinocytosis in LNCaP cells. FEBS Lett 584(15):3279–3286. https://doi.org/10.1016/j.febslet.2010.06.029
Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW (2002) Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 418(6899):790–793. https://doi.org/10.1038/nature00859
Egami Y, Fukuda M, Araki N (2011) Rab35 regulates phagosome formation through recruitment of ACAP2 in macrophages during FcgammaR-mediated phagocytosis. J Cell Sci 124(Pt 21):3557–3567. https://doi.org/10.1242/jcs.083881
Egami Y, Fujii M, Kawai K, Ishikawa Y, Fukuda M, Araki N (2015) Activation-inactivation cycling of Rab35 and ARF6 is required for phagocytosis of zymosan in RAW264 macrophages. J Immunol Res 2015:429439. https://doi.org/10.1155/2015/429439
Ellerbroek SM, Wennerberg K, Arthur WT, Dunty JM, Bowman DR, DeMali KA et al (2004) SGEF, a RhoG guanine nucleotide exchange factor that stimulates macropinocytosis. Mol Biol Cell 15(7):3309–3319. https://doi.org/10.1091/mbc.E04-02-0146
Erami Z, Khalil BD, Salloum G, Yao Y, LoPiccolo J, Shymanets A et al (2017) Rac1-Stimulated Macropinocytosis Enhances GbetaUpsilon Activation of PI3Kbeta. Biochem J 474:3903–3914. https://doi.org/10.1042/BCJ20170279
Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J (1998) Activation of phospholipase Cγ by PI 3-kinase-induced PH domain-mediated membrane targeting. Embo J 17(2):414–422. https://doi.org/10.1093/emboj/17.2.414
Feliciano WD, Yoshida S, Straight SW, Swanson JA (2011) Coordination of the Rab5 cycle on macropinosomes. Traffic 12(12):1911–1922. https://doi.org/10.1111/j.1600-0854.2011.01280.x
Filippakis H, Belaid A, Siroky B, Wu C, Alesi N, Hougard T et al (2018) Vps34-mediated macropinocytosis in Tuberous Sclerosis Complex 2-deficient cells supports tumorigenesis. Sci Rep 8(1):14161. https://doi.org/10.1038/s41598-018-32256-x
Fraley TS, Tran TC, Corgan AM, Nash CA, Hao J, Critchley DR, Greenwood JA (2003) Phosphoinositide binding inhibits alpha-actinin bundling activity. J Biol Chem 278(26):24039–24045. https://doi.org/10.1074/jbc.M213288200
Freeman SA, Uderhardt S, Saric A, Collins RF, Buckley CM, Mylvaganam S et al (2020) Lipid-gated monovalent ion fluxes regulate endocytic traffic and support immune surveillance. Science 367(6475):301–305. https://doi.org/10.1126/science.aaw9544
Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT (2017) The PI3K Pathway in Human Disease. Cell 170(4):605–635. https://doi.org/10.1016/j.cell.2017.07.029
Fujii M, Kawai K, Egami Y, Araki N (2013) Dissecting the roles of Rac1 activation and deactivation in macropinocytosis using microscopic photo-manipulation. Sci Rep 3:2385. https://doi.org/10.1038/srep02385
Goulden BD, Pacheco J, Dull A, Zewe JP, Deiters A, Hammond GRV (2019) A high-avidity biosensor reveals plasma membrane PI(3,4)P2 is predominantly a class I PI3K signaling product. J Cell Biol 218(3):1066–1079. https://doi.org/10.1083/jcb.201809026
Grimmer S, van Deurs B, Sandvig K (2002) Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci 115(Pt 14):2953–2962. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12082155
Hacker U, Albrecht R, Maniak M (1997) Fluid-phase uptake by macropinocytosis in Dictyostelium. J. Cell Sci. 110:105–112
Haga Y, Miwa N, Jahangeer S, Okada T, Nakamura S (2009) CtBP1/BARS is an activator of phospholipase D1 necessary for agonist-induced macropinocytosis. Embo J 28(9):1197–1207. https://doi.org/10.1038/emboj.2009.78
Hamasaki M, Araki N, Hatae T (2004) Association of early endosomal autoantigen 1 with macropinocytosis in EGF-stimulated A431 cells. Anat Rec A Discov Mol Cell Evol Biol 277(2):298–306. https://doi.org/10.1002/ar.a.20027
Hasegawa J, Tokuda E, Tenno T, Tsujita K, Sawai H, Hiroaki H et al (2011) SH3YL1 regulates dorsal ruffle formation by a novel phosphoinositide-binding domain. J Cell Biol 193(5):901–916. https://doi.org/10.1083/jcb.201012161
Hasegawa J, Tsujita K, Takenawa T, Itoh T (2012) ARAP1 regulates the ring size of circular dorsal ruffles through Arf1 and Arf5. Mol Biol Cell 23(13):2481–2489. https://doi.org/10.1091/mbc.E12-01-0017
Hawkins PT, Stephens LR (2016) Emerging evidence of signalling roles for PI(3,4)P2 in Class I and II PI3K-regulated pathways. Biochem Soc Trans 44(1):307–314. https://doi.org/10.1042/BST20150248
Henry RM, Hoppe AD, Joshi N, Swanson JA (2004) The uniformity of phagosome maturation in macrophages. J. Cell Biol. 164:185–194
Hewlett LJ, Prescott AR, Watts C (1994) The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol 124(5):689–703. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8120092
Hoeller O, Bolourani P, Clark J, Stephens LR, Hawkins PT, Weiner OD et al (2013) Two distinct functions for PI3-kinases in macropinocytosis. J Cell Sci 126(Pt 18):4296–4307. https://doi.org/10.1242/jcs.134015
Hoppe AD, Swanson JA (2004) Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell 15(8):3509–3519. https://doi.org/10.1091/mbc.E03-11-0847
Huotari J, Helenius A (2011) Endosome maturation. Embo J 30(17):3481–3500. https://doi.org/10.1038/emboj.2011.286
Ikeda Y, Kawai K, Ikawa A, Kawamoto K, Egami Y, Araki N (2017) Rac1 switching at the right time and location is essential for Fcgamma receptor-mediated phagosome formation. J Cell Sci 130(15):2530–2540. https://doi.org/10.1242/jcs.201749
Ikonomov OC, Sbrissa D, Shisheva A (2019) Small molecule PIKfyve inhibitors as cancer therapeutics: Translational promises and limitations. Toxicol Appl Pharmacol 383:114771. https://doi.org/10.1016/j.taap.2019.114771
Itoh T, Hasegawa J (2013) Mechanistic insights into the regulation of circular dorsal ruffle formation. J Biochem 153(1):21–29. https://doi.org/10.1093/jb/mvs138
Jean S, Kiger AA (2014) Classes of phosphoinositide 3-kinases at a glance. J Cell Sci 127(Pt 5):923–928. https://doi.org/10.1242/jcs.093773
Jiang J, Kolpak AL, Bao ZZ (2010) Myosin IIB isoform plays an essential role in the formation of two distinct types of macropinosomes. Cytoskeleton (Hoboken) 67(1):32–42. https://doi.org/10.1002/cm.20419
Jiao Z, Cai H, Long Y, Sirka OK, Padmanaban V, Ewald AJ, Devreotes PN (2020) Statin-induced GGPP depletion blocks macropinocytosis and starves cells with oncogenic defects. Proc Natl Acad Sci U S A 117(8):4158–4168. https://doi.org/10.1073/pnas.1917938117
Junemann A, Filic V, Winterhoff M, Nordholz B, Litschko C, Schwellenbach H et al (2016) A Diaphanous-related formin links Ras signaling directly to actin assembly in macropinocytosis and phagocytosis. Proc Natl Acad Sci U S A 113(47):E7464–E7473. https://doi.org/10.1073/pnas.1611024113
Kawai K, Nishigaki SM, Egami Y, Araki N (2021) Rab10-positive tubular structures represent a novel endocytic pathway that diverges from canonical macropinocytosis in RAW264 macrophages. BioRxiv. https://doi.org/10.1101/2021.01.03.425161
Kelsey JS, Fastman NM, Noratel EF, Blumberg DD (2012) Ndm, a coiled-coil domain protein that suppresses macropinocytosis and has effects on cell migration. Mol Biol Cell 23(17):3407–3419. https://doi.org/10.1091/mbc.E12-05-0392
Kerr MC, Lindsay MR, Luetterforst R, Hamilton N, Simpson F, Parton RG et al (2006) Visualisation of macropinosome maturation by the recruitment of sorting nexins. J Cell Sci 119(Pt 19):3967–3980. https://doi.org/10.1242/jcs.03167
Kim SM, Nguyen TT, Ravi A, Kubiniok P, Finicle BT, Jayashankar V et al (2018) PTEN deficiency and AMPK activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Discov 8(7):866–883. https://doi.org/10.1158/2159-8290.CD-17-1215
Kim N, Kim S, Nahm M, Kopke D, Kim J, Cho E et al (2019) BMP-dependent synaptic development requires Abi-Abl-Rac signaling of BMP receptor macropinocytosis. Nat Commun 10(1):684. https://doi.org/10.1038/s41467-019-08533-2
Kobayashi H, Fukuda M (2012) Rab35 regulates Arf6 activity through centaurin-beta2 (ACAP2) during neurite outgrowth. J Cell Sci 125(Pt 9):2235–2243. https://doi.org/10.1242/jcs.098657
Kotula L (2012) Abi1, a critical molecule coordinating actin cytoskeleton reorganization with PI-3 kinase and growth signaling. FEBS Lett 586(17):2790–2794. https://doi.org/10.1016/j.febslet.2012.05.015
Krishna S, Palm W, Lee Y, Yang W, Bandyopadhyay U, Xu H et al (2016) PIKfyve regulates vacuole maturation and nutrient recovery following engulfment. Dev Cell 38(5):536–547. https://doi.org/10.1016/j.devcel.2016.08.001
Krueger EW, Orth JD, Cao H, McNiven MA (2003) A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol Biol Cell 14(3):1085–1096. https://doi.org/10.1091/mbc.E02-08-0466
Langemeyer L, Borchers AC, Herrmann E, Fullbrunn N, Han Y, Perz A et al (2020) A conserved and regulated mechanism drives endosomal Rab transition. Elife 9:e56090. https://doi.org/10.7554/eLife.56090
Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP (2004) Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature 429(6989):309–314. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15152255
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149(2):274–293. https://doi.org/10.1016/j.cell.2012.03.017
Law F, Seo JH, Wang Z, DeLeon JL, Bolis Y, Brown A et al (2017) The VPS34 PI3K negatively regulates RAB-5 during endosome maturation. J Cell Sci 130(12):2007–2017. https://doi.org/10.1242/jcs.194746
Legg JA, Bompard G, Dawson J, Morris HL, Andrew N, Cooper L et al (2007) N-WASP involvement in dorsal ruffle formation in mouse embryonic fibroblasts. Mol Biol Cell 18(2):678–687. https://doi.org/10.1091/mbc.e06-06-0569
Liu Z, Xu E, Zhao HT, Cole T, West AB (2020) LRRK2 and Rab10 coordinate macropinocytosis to mediate immunological responses in phagocytes. Embo J e104862:doi:10.15252/embj.2020104862
Lou J, Low-Nam ST, Kerkvliet JG, Hoppe AD (2014) Delivery of CSF-1R to the lumen of macropinosomes promotes its destruction in macrophages. J Cell Sci 127(Pt 24):5228–5239. https://doi.org/10.1242/jcs.154393
Maehama T, Taylor GS, Dixon JE (2001) PTEN and myotubularin: Novel phosphoinositide phosphatases. Annu. Rev. Biochem. 70:247–279
Maekawa M, Fairn GD (2014) Molecular probes to visualize the location, organization and dynamics of lipids. J Cell Sci 127(22):4801–4812. https://doi.org/10.1242/jcs.150524
Maekawa M, Terasaka S, Mochizuki Y, Kawai K, Ikeda Y, Araki N et al (2014) Sequential breakdown of 3-phosphorylated phosphoinositides is essential for the completion of macropinocytosis. Proc Natl Acad Sci U S A 111(11):E978–E987. https://doi.org/10.1073/pnas.1311029111
Marat AL, Ioannou MS, McPherson PS (2012) Connecdenn 3/DENND1C binds actin linking Rab35 activation to the actin cytoskeleton. Mol Biol Cell 23(1):163–175. https://doi.org/10.1091/mbc.E11-05-0474
McCartney AJ, Zhang Y, Weisman LS (2014) Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. Bioessays 36(1):52–64. https://doi.org/10.1002/bies.201300012
Miller S, Tavshanjian B, Oleksy A, Perisic O, Houseman BT, Shokat KM, Williams RL (2010) Shaping development of autophagy inhibitors with the structure of the lipid kinase Vps34. Science 327(5973):1638–1642. https://doi.org/10.1126/science.1184429
Pacitto R, Gaeta I, Swanson JA, Yoshida S (2017) CXCL12-induced macropinocytosis modulates two distinct pathways to activate mTORC1 in macrophages. J Leukoc Biol 101(3):683–692. https://doi.org/10.1189/jlb.2A0316-141RR
Palm W, Araki J, King B, DeMatteo RG, Thompson CB (2017) Critical role for PI3-kinase in regulating the use of proteins as an amino acid source. Proc Natl Acad Sci U S A 114(41):E8628–E8636. https://doi.org/10.1073/pnas.1712726114
Porat-Shliom N, Kloog Y, Donaldson JG (2008) A unique platform for H-Ras signaling involving clathrin-independent endocytosis. Mol Biol Cell 19(3):765–775. https://doi.org/10.1091/mbc.E07-08-0841
Quinn SE, Huang L, Kerkvliet JG, Swanson JA, Smith S, Hoppe AD et al (2021) The structural dynamics of macropinosome formation and PI3-kinase-mediated sealing revealed by lattice light sheet microscopy. Nat Commun 12:4838. https://doi.org/10.1038/s41467-021-25187-1
Racoosin EL, Swanson JA (1993) Macropinosome maturation and fusion with tubular lysosomes in macrophages. J. Cell Biol. 121:1011–1020
Ramirez C, Hauser AD, Vucic EA, Bar-Sagi D (2019) Plasma membrane V-ATPase controls oncogenic RAS-induced macropinocytosis. Nature 576(7787):477–481. https://doi.org/10.1038/s41586-019-1831-x
Ronan B, Flamand O, Vescovi L, Dureuil C, Durand L, Fassy F et al (2014) A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat Chem Biol 10(12):1013–1019. https://doi.org/10.1038/nchembio.1681
Rosania GR, Swanson JA (1996) Microtubules can modulate pseudopod activity from a distance inside macrophages. Cell Motil. Cytoskel. 34:230–245
Salloum G, Jakubik CT, Erami Z, Heitz SD, Bresnick AR, Backer JM (2019) PI3Kbeta is selectively required for growth factor-stimulated macropinocytosis. J Cell Sci 132(16):jcs231639. https://doi.org/10.1242/jcs.231639
Sasaki Y, Ohsawa K, Kanazawa H, Kohsaka S, Imai Y (2001) Iba1 is an actin-cross-linking protein in macrophages/microglia. Biochem Biophys Res Commun 286(2):292–297. https://doi.org/10.1006/bbrc.2001.5388
Schlam D, Bagshaw RD, Freeman SA, Collins RF, Pawson T, Fairn GD, Grinstein S (2015) Phosphoinositide 3-kinase enables phagocytosis of large particles by terminating actin assembly through Rac/Cdc42 GTPase-activating proteins. Nat Commun 6:8623. https://doi.org/10.1038/ncomms9623
Shisheva A (2012) PIKfyve and its Lipid products in health and in sickness. Curr Top Microbiol Immunol 362:127–162. https://doi.org/10.1007/978-94-007-5025-8_7
Solinger JA, Spang A (2013) Tethering complexes in the endocytic pathway: CORVET and HOPS. FEBS J 280(12):2743–2757. https://doi.org/10.1111/febs.12151
Suetsugu S, Yamazaki D, Kurisu S, Takenawa T (2003) Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev Cell 5(4):595–609. https://doi.org/10.1016/s1534-5807(03)00297-1
Swanson JA (2008) Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol 9(8):639–649. https://doi.org/10.1038/nrm2447
Swanson JA, Johnson MT, Beningo K, Post P, Mooseker M, Araki N (1999) A contractile activity that closes phagosomes in macrophages. J. Cell Sci. 112:307–316
Thevathasan JV, Tan E, Zheng H, Lin YC, Li Y, Inoue T, Fivaz M (2013) The small GTPase HRas shapes local PI3K signals through positive feedback and regulates persistent membrane extension in migrating fibroblasts. Mol Biol Cell 24(14):2228–2237. https://doi.org/10.1091/mbc.E12-12-0905
Toker A, Cantley LC (1997) Signalling through the lipid products of phosphoinositide 3-OH kinase. Nature 387:673–676
Vanhaesebroeck B, Stephens L, Hawkins P (2012) PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol 13(3):195–203. https://doi.org/10.1038/nrm3290
Veltman DM, Williams TD, Bloomfield G, Chen BC, Betzig E, Insall RH, Kay RR (2016) A plasma membrane template for macropinocytic cups. Elife 5:e20085. https://doi.org/10.7554/eLife.20085
Vieira OV, Botelho RJ, Rameh L, Brachmann SM, Matsuo T, Davidson HW et al (2001) Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 155:19–25
Wall AA, Luo L, Hung Y, Tong SJ, Condon ND, Blumenthal A et al (2017) Small GTPase Rab8a-recruited Phosphatidylinositol 3-Kinase gamma regulates signaling and cytokine outputs from endosomal toll-like receptors. J Biol Chem 292(11):4411–4422. https://doi.org/10.1074/jbc.M116.766337
Wall AA, Condon ND, Luo L, Stow JL (2019) Rab8a localisation and activation by Toll-like receptors on macrophage macropinosomes. Philos Trans R Soc Lond B Biol Sci 374(1765):20180151. https://doi.org/10.1098/rstb.2018.0151
Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED (1999) Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol 1(1):45–50. https://doi.org/10.1038/9018
Welliver TP, Swanson JA (2012) A growth factor signaling cascade confined to circular ruffles in macrophages. Biol Open 1(8):754–760. https://doi.org/10.1242/bio.20121784
Williamson CD, Donaldson JG (2019) Arf6, JIP3, and dynein shape and mediate macropinocytosis. Mol Biol Cell 30(12):1477–1489. https://doi.org/10.1091/mbc.E19-01-0022
Willingham MC, Yamada SS (1978) A mechanism for the destruction of pinosomes in cultured fibroblasts: Piranhalysis. J. Cell Biol. 78:480–487
Wills RC, Goulden BD, Hammond GRV (2018) Genetically encoded lipid biosensors. Mol Biol Cell 29(13):1526–1532. https://doi.org/10.1091/mbc.E17-12-0738
Wills RC, Pacheco J, Hammond GRV (2021) Quantification of Genetically Encoded Lipid Biosensors. Methods Mol Biol 2251:55–72. https://doi.org/10.1007/978-1-0716-1142-5_4
Wymann M, Arcaro A (1994) Platelet-derived growth factor-induced phosphatidylinositol 3-kinase activation mediates actin rearrangements in fibroblasts. Biochem J 298(Pt 3):517–520. https://doi.org/10.1042/bj2980517
Yoshida S, Hoppe AD, Araki N, Swanson JA (2009) Sequential signaling in plasma-membrane domains during macropinosome formation in macrophages. J Cell Sci 122(Pt 18):3250–3261. https://doi.org/10.1242/jcs.053207
Yoshida S, Gaeta I, Pacitto R, Krienke L, Alge O, Gregorka B, Swanson JA (2015a) Differential signaling during macropinocytosis in response to M-CSF and PMA in macrophages. Front Physiol 6:8. https://doi.org/10.3389/fphys.2015.00008
Yoshida S, Pacitto R, Yao Y, Inoki K, Swanson JA (2015b) Growth factor signaling to mTORC1 by amino acid-laden macropinosomes. J Cell Biol 211(1):159–172. https://doi.org/10.1083/jcb.201504097
Yoshida S, Pacitto R, Sesi C, Kotula L, Swanson JA (2018) Dorsal ruffles enhance activation of Akt by growth factors. J Cell Sci 131(22):jcs220517. https://doi.org/10.1242/jcs.220517
Zhang Y, Hoppe AD, Swanson JA (2010) Coordination of Fc receptor signaling regulates cellular commitment to phagocytosis. Proc Natl Acad Sci U S A 107(45):19332–19337. https://doi.org/10.1073/pnas.1008248107
Acknowledgments
The authors thank Jonathan Backer, David Friedman, and Rob Kay for helpful suggestions. J.A.S. is supported by the NIH.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Swanson, J.A., Araki, N. (2022). Roles for 3’ Phosphoinositides in Macropinocytosis. In: Commisso, C. (eds) Macropinocytosis. Subcellular Biochemistry, vol 98. Springer, Cham. https://doi.org/10.1007/978-3-030-94004-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-94004-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94003-4
Online ISBN: 978-3-030-94004-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)