Abstract
I. Introduction
A. Noninvasive monitoring of oxygenation has become a standard procedure in neonatology.
B. Pulse oximetry (SpO2) is based on using the pulsatile variations in optical density of tissues in the red and infrared wavelengths to compute arterial oxygen saturation without the need for calibration.
C. The method was invented in 1972 by Takuo Aoyagi, and its clinical application was first reported in 1975 by Susumu Nakajima, a surgeon, and his associates.
D. There is a small discrepancy between SaO2 and the SpO2. The SaO2 denotes measurement of arterial oxygen saturation by invasive methods (performing arterial blood gas with co-oximetry) and SpO2 measures pulse oximetry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
-
I.
Introduction
-
A.
Noninvasive monitoring of oxygenation has become a standard procedure in neonatology.
-
B.
Pulse oximetry (SpO2) is based on using the pulsatile variations in optical density of tissues in the red and infrared wavelengths to compute arterial oxygen saturation without the need for calibration.
-
C.
The method was invented in 1972 by Takuo Aoyagi, and its clinical application was first reported in 1975 by Susumu Nakajima, a surgeon, and his associates.
-
D.
There is a small discrepancy between SaO2 and the SpO2. The SaO2 denotes measurement of arterial oxygen saturation by invasive methods (performing arterial blood gas with co-oximetry) and SpO2 measures pulse oximetry.
-
A.
-
II.
Advantages
-
A.
Saturation is a basic physiologic determinant of tissue oxygen delivery.
-
B.
High sensitivity to detect hypoxemia.
-
C.
No warm-up or equilibration time.
-
D.
Immediate and continuous readout.
-
E.
Pulse-by-pulse detection of rapid or transient changes in saturation.
-
F.
Substantially lower maintenance.
-
G.
Skin burns from probe are very rare compared to transcutaneous monitoring.
-
H.
Minimal effect of motion, light, perfusion, and temperature with the advent of “Signal Extraction Technology” in pulse oximetry which removes the noise from the artifact signals.
-
A.
-
III.
Disadvantages
-
A.
Failure to detect hyperoxia at functional saturation of more than 94% and may impede weaning of oxygen as high PaO2 is not recognized.
-
B.
Not reliable in cases of severe hypotension or marked edema.
-
C.
May provoke unnecessary evaluation of transient clinically insignificant desaturation events with older pulse oximeters.
-
D.
Pulsatile veins may cause falsely low SpO2 readings because the oximeter cannot differentiate between venous and arterial pulsations (e.g., in newborns with hyperdynamic circulation).
-
A.
-
IV.
Terminology in Pulse Oximetry
-
A.
Functional and fractional saturation
-
1.
Functional saturation . Any forms of hemoglobin in the sample which do not bind oxygen in a reversible way are not included in calculating functional hemoglobin saturation. Pulse oximetry can measure functional saturation from only two forms of hemoglobin, oxyhemoglobin (HbO2) and deoxyhemoglobin (Hb), which is calculated by:
$$ Functional\ saturation=\frac{HbO_2}{HbO_2+ Hb}\times 100 $$-
2.
Fractional saturation . The fractional saturation is defined as the ratio of the amount of hemoglobin saturated with oxygen to all other forms of hemoglobin, including dyshemoglobin (CoHb and Met Hb). The co-oximeters used in blood gas laboratories measure fractional saturation, as they use many wavelengths of light and are thus able to measure all types of hemoglobin present.
$$ Fractional\ saturation=\frac{HbO_2}{HbO_2+ Hb+\mathrm{CoHb}+\mathrm{MetH}}\times 100 $$-
3.
Pulse oximeters can measure only functional saturation. Some instruments display fractional saturation measurements, which are derived by subtracting 2% from the functional saturation. It is important to be aware of what the instrument is reading.
-
1.
-
B.
Bias and Precision.
Normal level of dyshemoglobin is <2%. The mean of the difference (error) between oxygen saturation and oxyhemoglobin (SpO2 and HbO2) measured by a co-oximeter is called bias and the standard deviation of this is called precision.
-
C.
Averaging time . Pulse oximetry oxygen saturation values are obtained by averaging from (period ranging from 2–16 secs) preceding measurements. This is displayed as a single oxygen saturation value, a running average of preceding measurements. Shorter averaging time detect lower saturation and frequency of desaturation episodes.
-
A.
-
V.
Practical Considerations
-
A.
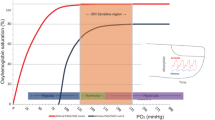
Oxyhemoglobin dissociation curve and pulse oximetry (Chap. 6)
-
B.
Presence of abnormal hemoglobins (dyshemoglobin)
-
1.
Carboxyhemoglobin. SpO2 is overestimated in presence of CoHb (e.g., neonatal jaundice, hemolysis).
-
2.
Methemoglobin. SpO2 decreases in proportion to the percentage of MetHb present.
-
1.
-
C.
Reduced perfusion states
-
1.
Hypothermia does not cause problem if the temperature is >30 °C.
-
2.
Hypovolemia. Loss of signal (presence of signal does not indicate adequate perfusion).
-
1.
-
D.
Anemia does not cause problem as long as Hb is >5 g /dL.
-
E.
Effect of pigments
-
1.
Bilirubin has no influence except if there is acute hemolysis (CoHb).
-
2.
Meconium staining of skin can cause falsely low SpO2 readings.
-
1.
-
F.
Venous pulsations (e.g., tricuspid regurgitation) may cause falsely low SpO2 readings.
-
G.
Abnormal absorption spectrum of hemoglobin (e.g., Hb Köln) may affect the reliability of pulse oximetry but is extremely rare.
-
A.
-
VI.
Technical considerations
-
A.
Calibration and accuracy
-
1.
Quality of signal. Before interpreting an SpO2 reading, the quality of signal received by the probe should be confirmed by a good plethysmographic waveform and/or heart rate similar to that on the ECG monitor.
-
2.
Differing software among brands. There are small differences between the measurements obtained with different brands of pulse oximeters.
-
3.
Inaccuracy increases when saturation is <75–80%: The bias and precision between SpO2 and HbO2 measured by co-oximetry:
-
(a)
0.5% and 2.5%, respectively, when SpO2 is >90%
-
(b)
1.9% and 2.7%, respectively, when SpO2 is 80–90%
-
(c)
5.8% and 4.8%, respectively, when SpO2 is <80%.
-
(a)
-
1.
-
B.
Delay of response
-
1.
Response time is faster if probe is centrally placed, 50–60% earlier detection by sensors placed centrally (ear, cheek, tongue) than by sensors placed peripherally (finger, toe).
-
2.
Depends on averaging time. The shortest averaging time should be selected, although this usually increases sensitivity to motion.
-
3.
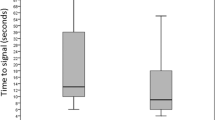
Attaching the sensor to the infant and then to the extension cable of the oximeter provides slightly earlier readings (mean difference of 10 seconds).
-
1.
-
C.
Motion artifact: The performance of pulse oximeters is affected by motion. To overcome this, several brands of pulse oximeters are now equipped with new algorithms like “adaptative probe off detection technology” that cancel noise signal that is common to both wavelengths.
-
D.
Interference from other light sources
-
1.
Fluctuating light sources : shielding the probe with cloth or opaque material can overcome the problem of light interference.
-
2.
Incorrectly placed probe (optical shunt or penumbra effect). Part of the light is transmitted without any tissue absorption. This is particularly so if too large a probe is used.
-
1.
-
E.
Electrical or magnetic interface
-
1.
When using pulse oximetry in an MRI suite, care should be taken to use specially designed equipment in order to avoid interference with SpO2 or even burns from ferrous metals.
-
2.
Electrocautery can also cause failure of pulse oximetry.
-
1.
-
A.
-
VII.
Advances in Pulse Oximetry
-
A.
Perfusion index (PI): The new-generation pulse oximeters provide PI, which is the ratio of pulsatile signal (arterial blood) indexed against non-pulsatile signal (venous blood, skin, and tissues). Trend of PI provides relative assessment of pulse strength and indicator of peripheral perfusion. The normal PI in term newborn infants has been reported at 0.7 (tenth percentile) and 0.5 (third percentile).
-
B.
Pleth variability index (PVI): This has been used to assess the fluid status in adults, but there are no data evaluating its use in newborn infants.
-
C.
Oxygen saturation histogram: provides graphical representation of distribution of oxygen saturation in different ranges over a time period. Trend of oxygen saturation histogram could help in weaning respiratory support and titration of oxygen.
-
D.
Other measurements: A few of the new-generation pulse oximeters have incorporated noninvasive measurement of co-oximetry to provide continuous hemoglobin measurements (SpHb). This has been shown to have moderate correlation with total hemoglobin measured from the laboratory. Some of the monitors have additional measurement of end tidal carbon dioxide. All of these parameters need to be validated in further studies.
-
A.
-
VIII.
Clinical Use of Pulse Oximetry
-
A.
Optimizing oxygen therapy: Meta-analysis of the oxygen saturation target studies concluded that, within the widely used SpO2 target range of 85–95%, targeting the “lower” range (85–89%) compared to the “higher” range (91–95%) for preterm infants <28 weeks’ gestation significantly increased the relative risks of mortality and necrotizing enterocolitis and significantly reduced the risk of severe ROP.
-
B.
Delivery room stabilization/resuscitation: Pulse oximetry during resuscitation provides real-time clinical information about heart rate and oxygen saturation and facilitates important decision-making related to interventions, such as positive pressure ventilation, cardiac compression, and oxygen titration.
-
C.
Newborn screening: Pulse oximetry has now been accepted as a standard screening tool for early detection of cyanotic congenital heart disease and other neonatal morbidities like sepsis. This has been adopted by pediatric societies around the world as part of the routine newborn evaluation.
-
A.
-
IX.
Rules for the optimal use of pulse oximetry
-
A.
Verify probe integrity before use.
-
B.
Avoid mixing probes and monitors of different brands.
-
C.
Check the quality of signal received by the probe (good waveform or true heart rate).
-
D.
Maintain probe positioning under direct visual control.
-
E.
Consider physiologic limitations of SpO2 and interpret accordingly.
-
F.
In case of doubt, check patient’s condition.
-
G.
Check arterial blood gases if saturation is persistently below 80%.
-
H.
Remember that high SaO2 may indicate significant hyperoxemia.
-
A.
Suggested Reading
Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, Donath SM, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010;125:e1340–7.
Hay WW Jr, Rodden DJ, Collins SM, et al. Reliability of conventional and new pulse oximetry in neonatal patients. J Perinatol. 2002;22:360–6.
Morgan C, Newell SJ, Ducker DA, et al. Continuous neonatal blood gas monitoring using a multiparameter intra-arterial sensor. Arch Dis Child. 1999;80:F93–8.
Moyle JTB, Hahn CEW. In: Adams AP, editor. Principles and practice series: pulse oximetry. London: BMJ Books; 1998.
Poets CF, Southhall DP. Noninvasive monitoring of oxygenation in infants and children: practical considerations and areas of concern. Pediatrics. 1994;3:737–46.
Richardson DK, Eichenwald EC. Blood gas monitoring and pulmonary function tests. In: Cloherty JP, Stark AR, editors. Manual of neonatal care. New York: Lippincott-Raven; 1998. p. 354–5.
Saugstad OD, Aune D. Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation studies. Neonatology. 2014;105(1):55–63.
Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet. 2012;379:2459e64.
Tin W, Lal M. Principles of pulse oximetry and its clinical application in neonatal medicine. Semin Fetal Neonatal Med. 2015;20:192–7.
Vagedes J, Poets CF, Dietz K. Averaging time, desaturation level, duration and extent. Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F265–6. https://doi.org/10.1136/archdischild-2012-302543.
Veyckemans F. Equipment, monitoring and environmental conditions. In Bissonnette B, Dalens BJ, editors. Pediatric anesthesia – principles and practice. New York: McGraw-Hill; 2002. p. 442–445. 2014; 105:55e63.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gupta, S., Loganathan, P., Tin, W. (2022). Clinical Controversies in Pulse Oximetry. In: Donn, S.M., Mammel, M.C., van Kaam, A.H. (eds) Manual of Neonatal Respiratory Care. Springer, Cham. https://doi.org/10.1007/978-3-030-93997-7_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-93997-7_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93996-0
Online ISBN: 978-3-030-93997-7
eBook Packages: MedicineMedicine (R0)