Abstract
Concussions account for the majority of traumatic brain injuries sustained annually. While the term concussion or mild traumatic brain injury denotes a less severe form of injury, epidemiological studies indicate that the high prevalence and persisting symptoms in a small percentage of those afflicted contribute to a substantial ongoing burden to healthcare resources. Understanding the neuropathophysiology responsible for the manifestation of clinical symptoms is paramount to the development of diagnostic tools and therapeutics. A review of the many physiological responses associated with concussion including the role of inflammation, hemodynamics, and metabolic regulation cannot be covered in sufficient detail in a single chapter. White matter injury is known to be an important contributor to outcome after traumatic brain injury. As such, this section focuses on our current understanding of the neurophysiology of concussion in the context of white matter injury and the development of tools to address concussion in this context. Specifically, we describe the current state of white matter imaging, cytoskeletal alterations and proteolysis in axons, mechanisms of ionic dysregulation, military trauma, and biomarker development in concussion. The pathophysiology of concussion is complex as the biomechanical transduction of force on the brain’s tissues is translated to the level of physical interaction between various cell types and subsequently affects signaling pathways at a subcellular level. Collectively, these contribute to dysfunction and disruption of homeostasis in white matter tissue. The type of initial force, such as primary blast or blunt trauma, further contributes to this complex biological puzzle as the brain’s response to mTBI is heterogeneous and varied.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
The clinical diagnosis of concussion largely relies on external indicators of neurological injury for diagnosis. The ever-evolving definition of concussion has been described as “a clinical syndrome of biomechanically induced alteration of brain function typically affecting memory and orientation, which may involve loss of consciousness” [1, 2]. Despite the vague clinical description, advances in imaging technologies, the development of clinical biomarkers, and preclinical animal studies have been used to identify subtle but significant microstructural abnormalities and identified molecular cascades occurring in the absence of macroscopic injury to the brain, which collectively contribute to changes in cognitive status. These pathophysiological events highlight the contribution of concussions as an important contributor to the spectrum of traumatic brain injury (TBI) despite being classified as a mild traumatic event. As technologies evolve, the ability to reliably and accurately detect subtle cellular changes in cerebral structure and function gives hope for the more accurate diagnosis of injuries, with potential prognostic and therapeutic applications. Understanding the link between biophysical transduction of force from tissue to cellular and even ultrastructural scales for an injury type that is notoriously heterogeneous is challenging from a preclinical modeling perspective. In turn, this challenge is amplified when it comes to developing therapeutic strategies [3].
This chapter focuses on our current understanding of the pathophysiology associated with these processes and their contribution to the evolving pathophysiology of concussion. Although many of the mechanisms described below are also applicable to moderate and severe TBIs, it can be assumed that these cellular processes are also shared with mild TBI albeit at a lesser degree. There are, however, notable differences between injury severities which further suggest that not all mechanisms of secondary injury are applicable or relevant across the injury spectrum of TBI. Preclinical models have helped to bridge the gaps in knowledge from the limitations of noninvasive methods in the clinic and provide a better understanding of the discrete molecular and cellular events unfolding within the brain. Although developing clinically relevant, accurate, and reliable models of clinical concussion remains a challenge, animal models, computer simulations, and in vitro systems remain an important component of understanding the pathophysiology of concussion [4, 5].
Normal and Abnormal Physiological States
It is interesting to consider that even under normal conditions there exists considerable pliability and tolerance within an otherwise static central nervous system (CNS). For example, the very act of bending the spine or rapid rotation of the head involves some substantial biomechanical force transduction to axons within the CNS. Body movement lends itself to some degree of nerve fiber stretching and bending or inertial motion of otherwise static tissues but ultimately returning to a normal state [6]. In vitro studies have demonstrated that axons have a high tolerance to stretch and exhibit a delayed elasticity phenomenon, whereby axons subject to up to 65% tensile strain generally recover their original shape afterward [7].
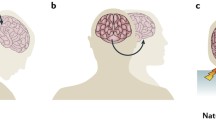
Similarly, the brain is largely protected from inertial acceleration by the fluidity of the brain encased in a rigid skull surrounded by cerebral spinal fluid (CSF). Dramatic and instantaneous acceleration or deceleration (<50 ms) beyond the cushioning capacity of the CSF generates substantial linear and rotational shear forces that result in axonal injury [8]. In particular, the anisotropic properties of white matter tissue in the brain lend itself to higher levels of strain and shear depending on the direction and magnitude of the force being applied [9,10,11]. Microcompartment in vitro studies further support the argument that axons or neurites are more susceptible to injury than the cell soma [12]. In the same vein, computational models of axons that incorporate cytoskeletal dynamics between actin and spectrin, as well as the anchoring protein, ankyrin, point to greater intrinsic stiffness in axons relative to the cell bodies which further imply increased susceptibility to injury relative to other brain structures [13]. While imaging studies of mTBI patients support the vulnerability of white matter in concussion, the underlying pathophysiology remains a complex issue to resolve (Fig. 2.1).
(a) A schematic representation of the coronal section of a human brain. At the macroscopic level, rotational forces resulting from head trauma can translate into inertial movement of the brain resulting in deformation of tissues and brain structures. These forces include shear strain on white matter tracts that are particularly vulnerable to excessive inertial loading. (b) At a microscopic scale, the biomechanical transduction of forces on white matter tracts can result in rapid stretching of axons regardless of the orientation of fibers. Studies suggest that shear strain rarely results in primary axotomy. The majority of axons return to their normal state. However, a subset of axons develops morphological characteristics associated with diffuse axonal injury. These include abnormal morphology, such as axonal swelling and varicosities associated with activation of molecular pathways and disruption of cytoskeletal networks
Imaging White Matter Injury in Concussion
Diffuse axonal injury (DAI) is a common component across all TBI severities [9, 14,15,16]. However, the dispersed and subtle structural alterations associated with concussion are inherently difficult to verify clinically, despite being the most likely contributor to morbidity after injury. Computed tomography (CT) and magnetic resonance imaging (MRI) are largely limited to the detection of macroscopic abnormalities and lack the sensitivity to detect the subtle white matter changes that may occur with concussions [17, 18]. Biomarker studies, improved imaging technologies, and animal models of TBI have demonstrated that the absence of overt changes in brain following mTBI and concussion does not exclude the occurrence of pathophysiological changes which contribute to morbidity.
Diffuse tensor imaging (DTI) is an advanced MRI sequence that provides a more sensitive technique for identifying injuries to white matter tracts [18]. The underlying principle is that DTI estimates rates of water diffusion in different spatial directions, with white matter tracts exhibiting highly directional, anisotropic diffusion parallel to fiber bundles [18, 19]. Its application in TBI involves the detection of changes in diffusion properties within axonal fiber bundles, which presumably indicate some form of physical anomaly in the white matter tracts [20]. While there is no doubt that DTI provides some information on structural disturbances to the brain after TBI , to date, the most effective application of this relatively new imaging modality has been in moderate and severe TBIs. Its potential application in concussion is still in proof-of-concept stage [21, 22]. Although noninvasive imaging of mTBI pathology has not been fully translated into clinical utility [23], there is well-documented postmortem evidence of axonal pathology associated with TBI that continues to be bolstered by complementary preclinical animal studies [5, 14]. Bridging the uncertainty of pathophysiological mechanisms in human cases of concussion with what is known from more severe forms of TBI and animal models will help further validate the use of imaging modalities in characterizing concussion pathophysiology.
Axonal Injury at the Microscopic Level
The collective contribution of subcellular structural alterations, metabolic changes, and ionic shifts in the brain results in impairment of neurotransmission that contributes to the morbidity associated with concussion [24]. Computational modeling conducted at the tissue, cellular [25, 26], and ultrastructural levels [27] has demonstrated the physical transfer of strain, shear, and stresses from macroscopic to microscopic scales in greater detail [8]. These studies are useful in conjunction with histological studies which highlight the complex physical relationship between individual axons within white matter bundles [28] and other constituents of the brain including glia, myelin sheathing, and vascular cells [6]. Differing viscoelastic properties among varying cell types and structures create a complex environment when transduction of biomechanical forces is taken into consideration.
The axonal membrane demonstrates a spectrum of injury responses dependent on the force of injury. For example, in fluid percussion injured cats, mTBI was shown not to result in disrupted axonal membranes but was still associated with neurofilament disruption and compaction. However, with increasing injury severity, axonal membrane permeability becomes a pathological feature of injury [29,30,31]. Similarly, in vitro studies have demonstrated that mammalian axonal membranes display a remarkable tolerance to stretch injury, with uptake of low molecular weight fluorescent dyes only in instances of higher strain rates resulting in primary axotomy [7]. A direct translation of critical injury thresholds between in vitro and in vivo injury models is difficult to implement, due to the simplicity of in vitro systems compared to the heterogeneity of cells and tissue composition of in vivo systems. However, both injury models agree that primary axotomy resulting from initial strain or impact is in fact a rare occurrence. Experimental evidence suggests that axonal injury or disconnection is an evolving and progressive condition [10, 28]. Human postmortem studies have confirmed that the development of neurofilament misalignment and the formation of retraction bulbs is a delayed process even in severe cases of TBI which suggests that primary axotomy is a rare occurrence at the time of impact [32]. Detailed in vitro investigation has demonstrated that stretch injury conditions that mimic in vivo injury forces result in a heterogeneous response among cytoskeletal elements. For example, early periodic breaks in the microtubule structure were reported [33], while fast transported beta-amyloid precursor protein (β-APP) and slow transported heavy neurofilament (NF200) demonstrated different morphological deposition patterns along injured axons [34]. The described process of events involves the disruption of microtubule networks which are generally regarded as rigid structure within axons and pathways for axonal transport [10, 14]. The misalignment and disruption of the microtubule network set into motion a molecular sequence of failure in axonal transport, resulting in the accumulation of proteins within axons and the formation of axonal varicosities. Collectively, these studies suggest that concussions are likely not a cause of primary axotomy, but rather a sequence of molecular events that unfolds after trauma leads to axonal dysfunction or disconnection. Understanding these processes and their course of evolution is paramount to the development of therapeutic strategies.
Despite a relative resilience of the axonal membrane to shear forces, at the subcellular level the complex organization of the cytoskeleton involves dynamic reorganization in response to structural disruption [6]. The response to cytoskeletal disruption can manifest in the form of axonal swellings. Similar to a motor accident on a highway creating congestion as incoming traffic continues to backlog, transported proteins accumulate within disrupted networks of microtubules and neurofilaments that no longer support continuous paths to traverse. This characteristic histopathology finding is a hallmark for diffuse axonal injury resulting in axonal swellings and eventually disconnection bulbs [35]. β-APP has been used as a general histopathology marker of axonal injury in this regard [36]. Mild TBI has been shown to result in deposition of β-APP in human postmortem studies [37]. These findings have also been replicated in a swine model of rotational acceleration injury [38]. Furthermore, these animal studies provide evidence that axonal injury can occur in the absence of loss of consciousness [38], leading to a greater appreciation of the sensitivity of the brain to mild traumatic forces.
Mechanisms of Ionic Dysregulation and Calcium Pathways
Based on the disruption of cytoskeletal networks and the relative impermeability of cell membranes to primary axotomy, the question arises as to how cytoskeletal disruption is initiated. Although primary physical disruption likely plays some role, it is the delayed evolution of axonal pathology that suggests active cellular mechanisms are involved in the development of axonal pathology. One major event in concussion at the cellular level is shifted in ionic equilibrium within neurons and glia which lead to dysregulation of calcium signaling pathways with detrimental consequences. Large early increases in extracellular glutamate and potassium posttrauma have been demonstrated in rat models of mTBI [39, 40]. The altered neurotransmitter levels and ion flux coincide with spreading waves of depolarization observed experimentally [41] as well as clinically [42, 43]. The increased concentration of extracellular glutamate is presumably responsible for large influxes of extracellular calcium mediated by ionotropic N-methyl D-aspartate (NMDA) receptors [44], which further contribute to cellular depolarization through voltage-gated sodium channels. Interestingly, sublethal stretch injury in vitro in primary neuronal cultures has been shown to increase susceptibility to subsequent NMDA challenge [45]. Although performed in vitro, these findings suggest a state of increased vulnerability of the brain to repeat concussion.
Alterations in ion concentrations within intact axons have also been shown in vitro to be due to the activation of mechano-sensitive voltage-gated sodium channels [46]. The proposed sequence of events involves influx of sodium resulting in subsequent activation of voltage-gated calcium channels as well as reversal of sodium–calcium (Na+-Ca2+) exchangers [47]. The net effect is an increase in intra-axonal calcium, which results in the activation of calcium-sensitive proteases, such as calpain, or further release of intracellular calcium stores from the mitochondria or endoplasmic reticulum leading to a feedforward process of calcium overload [48]. While these mechanisms hold true for more severe forms of axonal injury, other data indicate that intracellular calcium stores are likely involved in the initial calcium spike observed after mild trauma [49]. Moreover, there are biphasic responses to calcium dynamics in the cell, suggesting that the initial phase is a result of intracellular calcium, while subsequent calcium waves are propagated through primarily extracellular sources [49, 50]. These data highlight the complexity and ever-changing physiology of cell receptors and signaling in response to injury and further highlight the difficulties in treating these conditions.
Calcium plays a critical role in numerous cell functions and acts as a linchpin in the activation of pathophysiological processes linked to axonal and neuronal degeneration [51]. The numerous processes initiated by calcium include excessive protease activation, phosphatase activation, initiation of apoptosis, mitochondrial failure, and the reversal of sodium–calcium exchangers leading to membrane depolarization [48, 52]. Importantly, the route of calcium entry, also referred to as the “source specificity hypothesis,” [44] determines subsequent activation of discrete downstream signaling pathways adding another layer of complexity to the calcium signaling process [48, 53].
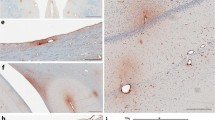
One important consequence of calcium influx is the activation of calpains, a family of cysteine proteases with numerous cell functions including cytoskeletal remodeling, cell signaling, differentiation, and vesicular trafficking under physiological conditions [53, 54]. The two calpains relevant to diseases in the CNS are activated at micro- and millimolar concentrations of calcium [55], denoted as either μ-calpain, m-calpain, or calpain-1 and calpain-2, respectively [56]. While studies suggest that TBI results in influxes of calcium in the millimolar range [51], it has been difficult to parse out the individual contributions of each isoform due to overlapping cleavage targets and a lack of isoform-specific inhibitors [56]. However, several studies point toward μ-calpain as the mediator of neurodegeneration after insult [57]. Regardless, calpain activation is associated with the cleavage of alpha II spectrin, an important structural component of the axonal cytoskeleton [58]. The breakdown of intact 280 kDa alpha II spectrin to a lower molecular weight product of 145/150 kDa is a hallmark indicator of calpain-mediated axonal injury [59]. The specificity of calpain’s cleavage site makes it an attractive target as a clinical biomarker of axonal injury as it has been shown to be detectable in CSF after injury [59]. Moreover, alpha II spectrin is further cleaved into a 120 kDa product associated with cleaved caspase-3 activity [60]. Although concussion typically does not result in significant neuronal loss, there is evidence that apoptosis can occur in some instances [61]. Thus, alpha II spectrin may be useful to delineate the underlying injury pathways of axonal injury or delayed cell death occurring after mTBI (Fig. 2.2).
Cartoon depicts some of the molecular mechanisms described in in vitro studies believed to contribute to secondary axonal injury after trauma. Mechano-sensitive voltage-gated sodium channels are activated by physical insult resulting in sodium ion influx into axons. The increased sodium ion concentration in axons causes reversal of sodium–calcium exchangers. Increased calcium ion influx can mobilize calpains resulting in proteolysis of cytoskeletal proteins such as alpha II spectrin. Shedding of spectrin into the interstitial spaces finds its way into peripheral circulation and can be detected in some instances as a serum biomarker. Physical damage to microtubule networks results in the disruption of axonal transport. Beta-amyloid precursor protein accumulates within axons due to transport failure resulting in axonal swelling. This has been shown to result in axonal disconnection
Military Concussion
Military operations over the last two decades have highlighted a shift in warfare injuries, with a larger percentage attributable to survivable blast exposure and head trauma [62]. Blast exposures and mTBI contribute to a large segment of the brain-injured cohort and have become increasingly recognized as a potentially unique form of mTBI [63]. Evidence suggests that non-fatal blast exposure in the absence of contusion also results in axonal injury but with patterns distinct from conventional head trauma [64]. Data from animal models of subclinical blast trauma point toward significant and delayed alterations in heavy neurofilament expression, which is in turn associated with impaired electrophysiological function in white matter and behavioral impairments [65, 66]. In these studies of mild non-blunt force trauma, calpain-mediated breakdown of alpha II spectrin has been observed in the absence of neuronal cell loss [66, 67]. Mild blast studies have also demonstrated neurovascular changes including disruption of the blood–brain barrier (BBB) involving degeneration of astrocytic endfeet following blast exposure [68, 69]. There is also evidence to suggest that soldiers exposed to blast injuries develop deposits of phosphorylated tau, a hallmark indicator of chronic traumatic encephalopathy [70]. Although tau deposition is generally attributed to repetitive head trauma, there is well-documented postmortem and in vivo imaging studies describing tau deposition years following a single TBI event highlighting the complexity of the response to various modalities of TBI [71, 72]. It should also be noted that blast modeling in animals, particularly the defining criteria for mild or low-level blast and its correlation to human low-level blast exposure, is not well-established or standardized [73]. Moreover, blast injuries in human cases are often confounded with comorbidities including post-traumatic stress disorder (PTSD) and limited to imaging studies which provide little insight into the underlying molecular mechanisms of injury.
The Biology of Biomarkers for Concussion
Biomarkers have the potential to noninvasively diagnose the presence of concussion. However, their clinical application has been met with limited success. The shortcomings of biomarker utility in concussion are in part due to the broad clinical definition based on external symptoms, while the underlying physiological criteria have not been specifically defined [74]. This disconnect is also complicated by evidence pointing to the pathophysiology of concussion extending beyond the window of clinical symptoms [75,76,77]. These imply a disconnect between the current gold standard of clinical diagnosis for concussion and the molecular tools meant to supplant this standard. Despite these challenges, the principles of biomarker development are based on our understanding of cellular and subcellular changes occurring in neural tissues in response to trauma which are presumably important contributors to clinical outcome.
Cleavage substrates of calpain are presumably released from injured neurons or axotomized axons and find their way into the CSF and blood. These biomarkers provide insight into proteolytic cleavage targets and an opportunity for the development of a noninvasive diagnostic tool. Neurofilaments have also been evaluated clinically as biomarkers of axonal injury and provide some insight into the pathophysiology of axonal injury after concussion. Neurofilaments subtypes consisting of light, medium, and heavy chains are the largest contributors to the intermediate filament family that make up the neuronal cytoskeleton along with actin microfilaments and the larger caliber microtubules [78]. In animal models, there is a demonstrated correlation between injury severity and the amount of detectable serum and CSF phosphorylated heavy neurofilament [79]. Serum presence of neurofilament light (NFL) chain after repetitive head injury has been shown to correlate with injury severity in repetitively concussed athletes [80]. Serum presence of NFL chain also correlates with CSF levels. Interestingly, elevated NFL was detected up to 3 months post-injury in boxers [81], suggesting a prolonged shedding or injury to axons, which further supports that notion of concussion as an ongoing neurodegenerative process.
A common theme in studies of white matter injury is that heterogeneity exists among axons, not only in composition (e.g., myelinated vs unmyelinated), but also in their response to injury. In vitro studies have demonstrated that the neurofilament response to stretch varies depending on the degree of mechanical insult applied [82]. The varied response to different degrees of mechanical injury in vitro is consistent with in vivo observations which indicate differing immunoreactivity subtypes following TBI [83]. Similarly, neurofilament compaction, believed to be a result of phosphatase activity on the sidearm structures [84], does not occur in the same axons as those exhibiting microtubule destabilization and impaired axonal transport [85,86,87]. The varying axonal response to injury across these studies were reported under conditions of moderate to severe modeled TBI and whether these mechanisms are involved in concussion remains to be further elucidated. However, these studies are valuable for demonstrating the heterogenic axonal response to TBI.
Mild stretch injury induces increased neurofilament immunoreactivity in axons [82] which also correlates with increases in heavy neurofilament expression observed in fluid percussion models and mild blast in vivo models [66, 88]. Similarly, increases in phosphorylated heavy neurofilament have been detected in serum samples from boxers [89]. Phosphorylated heavy neurofilaments are predominantly localized in long axons [90], which make them a potential marker for axonal injury. Numerous clinical and animal studies have examined the prognostic and diagnostic value of serum and CSF biomarkers. However, their meaningful application in human concussion and mTBI remains to be further clarified due to technical hurdles in terms of thresholds of detection and whether the select measures are truly indicative of underlying pathophysiology [91, 92] (Fig. 2.3).
In vitro studies suggest that axon membrane permeability is not a common occurrence in mTBI. Thus, calcium entry either occurs from reversal of sodium–calcium exchangers or secondary calcium release from the axonal endoplasmic reticulum. Other observed mechanisms of calcium-induced injury to axons include activation of phosphatases resulting in loss of sidearms projections in neurofilaments. Sidearm projections are believed to be responsible for maintaining axon caliber and their loss results in neurofilament compaction contributing to changes to axon diameter. Phosphorylated neurofilaments have been detected in serum samples from concussed patients. While it is assumed that membrane disruption is responsible for the shedding of proteins such as neurofilaments and GFAP, there remains the issue as to how these proteins end up in peripheral circulation given that concussed patients and mild TBI animal models demonstrated little evidence of axotomy
Biomarker studies have been useful in identifying some generalized cellular changes in addition to neurofilament shedding and disruption. One such example is the detection of glial fibrillary acidic protein (GFAP). GFAP is an astrocytic scaffolding protein whose presence in serum has recently been shown to correlate with MRI abnormalities [93] and has been detected in serum samples of mild and moderate TBI patients [94]. As with all biomarkers of TBI, the reliability of GFAP as an indicator of mTBI is not firmly established. For example, no reported changes in GFAP were found in serum samples from Olympic boxers, while phosphorylated tau, a suspected indicator of chronic traumatic encephalopathy, was detected [95]. There are numerous reasons for the discrepancies between these findings including timing of sampling, type of injuries sustained, and methods used for detection. Similar to the issues surrounding the clinical usefulness of DTI in the detection of concussion, further studies are required to better understand the physiology and temporal sequence of protein shedding into the blood from the CNS for the application of biomarkers to be both reliable and accurate [91, 96].
Repetitive Concussion and Implications for Neurodegenerative Diseases
Animal studies examining GFAP immunoreactivity after mTBI have demonstrated an increase in GFAP expression after injury and increased the presence of microglial activation [97]. Not surprisingly, repetitive head injury in rodents also demonstrates increased the presence of GFAP expression and immune reactivity in brain tissue [98]. In addition to increase in GFAP expression in reactive astrocytes, increased presence of phosphorylated tau (p-tau) protein in rodent models [98] is consistent with the neuropathology of chronic traumatic encephalopathy (CTE). CTE is the term used to describe the neurodegenerative condition characterized by perivascular neurofibrillary tangles of p-tau which frequently occurs in tandem with tau expressing astrocytic tangles seen in a significant number of repetitively head-injured cases [99, 100]. Although there is no clear consensus on whether CTE constitutes a distinct clinical condition [101] or is part of a larger spectrum of tauopathies, the diagnosis for CTE can only be confirmed through postmortem histological analysis and is graded in four stages based on the distribution and density of p-tau [100, 102]. Given that stages of CTE pathology can be categorized, the molecular course of events suggests an evolution of injury but also the potential for intervention at limiting the progression of CTE.
In addition to the association with neurodegenerative tauopathies, concussion and mTBI are also associated with an increased risk of developing dementias [103]. The link between concussion and dementia demonstrates an overlap in pathology and molecular pathways with some evidence pointing toward concussion as an accelerator for those at risk or increasing risk of development of neurodegenerative disorders such as Alzheimer’s disease [104]. At a basic research level, understanding the progression of tauopathies has been difficult despite the recent development of numerous models of mild and repetitive brain trauma [105,106,107]. Although histological examination in these models demonstrates tau deposition [105], the time course of detection is relatively acute compared to the life-long development in human cases of concussion. The overlapping pathology of other neurodegenerative disorders poses a challenge in trying to parse the effects of tau deposition in the sole context of mTBI, considering the natural progression of dementias and AD. This necessitates longitudinal studies when attempting to establish risk of disease development. This further highlights the challenges in modeling human concussion in animals [4, 108] and extrapolating information between species and reconciling what are shared mechanisms of injury progression, regardless of temporal discrepancies.
Emerging Research
An area of important clinical significance in the acute management of brain trauma is the integrity of the BBB and its effects on cerebrovascular dynamics. While the majority of the work to date has focused on the effects on concussion on white matter injury, there is evidence from animal models that BBB and neurovascular disruption are also components of mTBI [106, 109, 110]. A recent mTBI case study indicates potential detection of neurovascular compromise suggesting that the neurovascular unit may also be at risk in addition to neurons and glia [111]. A critical limitation in the clinical evaluation of BBB is the lack of direct assessment on its integrity. Serum biomarkers are a surrogate measure of a presumed leakiness of the BBB, allowing passage of otherwise impermeable proteins into peripheral circulation and vice versa. However, as indicated, the reliability and the interpretation of serum biomarkers, particularly in light of the discovery of the perivascular glymphatic system [112] and a sinus-associated lymphatic system [113] in the brain poses new questions about the mechanism of protein leakage into circulation following TBI [112, 114].
These recent anatomical findings bring to light the complexity of the brain and our limited understanding, even in its native state. There is an obvious need for further study in understanding the interactions between anatomical structures at the cellular and molecular levels in the context of concussion. Advances in imaging technologies in parallel with advanced molecular techniques are on the verge of providing an integrated understanding of preclinical and clinical pathophysiological mechanisms underlying concussion.
References
Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250–7.
Mullally WJ. Concussion. Am J Med. 2017;130:885–92.
Meaney DF, Morrison B, Dale BC. The mechanics of traumatic brain injury: a review of what we know and what we need to know for reducing its societal burden. J Biomech Eng. 2014;136:021008.
Park E, Baker AJ. Translational mild traumatic brain injury research: bridging the gap between models and clinical uncertainty. In: Wang KKW, editor. Neurotrauma. Oxford University Press; Madison Ave., New York, NY, USA. 2018.
Petraglia AL, Dashnaw ML, Turner RC, Bailes JE. Models of mild traumatic brain injury: translation of physiological and anatomic injury. Neurosurgery. 2014;75(Suppl 4):S34–49.
Kirkcaldie MT, Collins JM. The axon as a physical structure in health and acute trauma. J Chem Neuroanat. 2016;76:9–18.
Smith DH, Wolf JA, Lusardi TA, Lee VM, Meaney DF. High tolerance and delayed elastic response of cultured axons to dynamic stretch injury. J Neurosci. 1999;19:4263–9.
Meaney DF, Smith DH. Biomechanics of concussion. Clin Sports Med. 2011;30:19–31, vii.
Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. J Head Trauma Rehabil. 2003;18:307–16.
Smith DH. Neuromechanics and pathophysiology of diffuse axonal injury in concussion. Bridge (Wash D C). 2016;46:79–84.
Giordano C, Cloots RJ, van Dommelen JA, Kleiven S. The influence of anisotropy on brain injury prediction. J Biomech. 2014;47:1052–9.
Grevesse T, Dabiri BE, Parker KK, Gabriele S. Opposite rheological properties of neuronal microcompartments predict axonal vulnerability in brain injury. Sci Rep. 2015;5:9475.
Zhang Y, Abiraman K, Li H, Pierce DM, Tzingounis AV, Lykotrafitis G. Modeling of the axon membrane skeleton structure and implications for its mechanical properties. PLoS Comput Biol. 2017;13:e1005407.
Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35–43.
Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59.
Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1–12.
Bazarian JJ, Blyth B, Cimpello L. Bench to bedside: evidence for brain injury after concussion--looking beyond the computed tomography scan. Acad Emerg Med. 2006;13:199–214.
Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–92.
Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–67.
Le TH, Mukherjee P, Henry RG, Berman JI, Ware M, Manley GT. Diffusion tensor imaging with three-dimensional fiber tractography of traumatic axonal shearing injury: an imaging correlate for the posterior callosal “disconnection” syndrome: case report. Neurosurgery. 2005;56:189.
Yuh EL, Hawryluk GW, Manley GT. Imaging concussion: a review. Neurosurgery. 2014;75(Suppl 4):S50–63.
Asken BM, DeKosky ST, Clugston JR, Jaffee MS, Bauer RM. Diffusion tensor imaging (DTI) findings in adult civilian, military, and sport-related mild traumatic brain injury (mTBI): a systematic critical review. Brain Imaging Behav. 2018;12:585–612.
Churchill NW, Hutchison MG, Richards D, Leung G, Graham SJ, Schweizer TA. The first week after concussion: blood flow, brain function and white matter microstructure. Neuroimage Clin. 2017;14:480–9.
Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(Suppl 4):S24–33.
Karami G, Grundman N, Abolfathi N, Naik A, Ziejewski M. A micromechanical hyperelastic modeling of brain white matter under large deformation. J Mech Behav Biomed Mater. 2009;2:243–54.
Abolfathi N, Naik A, Sotudeh Chafi M, Karami G, Ziejewski M. A micromechanical procedure for modelling the anisotropic mechanical properties of brain white matter. Comput Methods Biomech Biomed Engin. 2009;12:249–62.
Cloots RJ, van Dommelen JA, Nyberg T, Kleiven S, Geers MG. Micromechanics of diffuse axonal injury: influence of axonal orientation and anisotropy. Biomech Model Mechanobiol. 2011;10:413–22.
Povlishock JT. Pathobiology of traumatically induced axonal injury in animals and man. Ann Emerg Med. 1993;22:980–6.
Pettus EH, Christman CW, Giebel ML, Povlishock JT. Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. J Neurotrauma. 1994;11:507–22.
Kilinc D, Gallo G, Barbee KA. Mechanically-induced membrane poration causes axonal beading and localized cytoskeletal damage. Exp Neurol. 2008;212:422–30.
Kilinc D, Gallo G, Barbee KA. Mechanical membrane injury induces axonal beading through localized activation of calpain. Exp Neurol. 2009;219:553–61.
Christman CW, Grady MS, Walker SA, Holloway KL, Povlishock JT. Ultrastructural studies of diffuse axonal injury in humans. J Neurotrauma. 1994;11:173–86.
Tang-Schomer MD, Patel AR, Baas PW, Smith DH. Mechanical breaking of microtubules in axons during dynamic stretch injury underlies delayed elasticity, microtubule disassembly, and axon degeneration. FASEB J. 2010;24:1401–10.
Tang-Schomer MD, Johnson VE, Baas PW, Stewart W, Smith DH. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp Neurol. 2012;233:364–72.
Povlishock JT, Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma. 1995;12:555–64.
Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett. 1993;160:139–44.
Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–6.
Browne KD, Chen XH, Meaney DF, Smith DH. Mild traumatic brain injury and diffuse axonal injury in swine. J Neurotrauma. 2011;28:1747–55.
Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900.
Takahashi H, Manaka S, Sano K. Changes in extracellular potassium concentration in cortex and brain stem during the acute phase of experimental closed head injury. J Neurosurg. 1981;55:708–17.
Bouley J, Chung DY, Ayata C, Brown RH Jr, Henninger N. Cortical spreading depression denotes concussion injury. J Neurotrauma. 2019;36:1008–17.
Oka H, Kako M, Matsushima M, Ando K. Traumatic spreading depression syndrome. Review of a particular type of head injury in 37 patients. Brain. 1977;100:287–98.
Shaw NA. The neurophysiology of concussion. Prog Neurobiol. 2002;67:281–344.
Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–37.
Arundine M, Chopra GK, Wrong A, et al. Enhanced vulnerability to NMDA toxicity in sublethal traumatic neuronal injury in vitro. J Neurotrauma. 2003;20:1377–95.
Iwata A, Stys PK, Wolf JA, et al. Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J Neurosci. 2004;24:4605–13.
Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci. 2001;21:1923–30.
Stirling DP, Stys PK. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol Med. 2010;16:160–70.
Staal JA, Dickson TC, Gasperini R, Liu Y, Foa L, Vickers JC. Initial calcium release from intracellular stores followed by calcium dysregulation is linked to secondary axotomy following transient axonal stretch injury. J Neurochem. 2010;112:1147–55.
Weber JT, Rzigalinski BA, Willoughby KA, Moore SF, Ellis EF. Alterations in calcium-mediated signal transduction after traumatic injury of cortical neurons. Cell Calcium. 1999;26:289–99.
Weber JT. Altered calcium signaling following traumatic brain injury. Front Pharmacol. 2012;3:60.
Buki A, Povlishock JT. All roads lead to disconnection?--Traumatic axonal injury revisited. Acta Neurochir. 2006;148:181–93; discussion 93–4.
Ma M. Role of calpains in the injury-induced dysfunction and degeneration of the mammalian axon. Neurobiol Dis. 2013;60:61–79.
Zatz M, Starling A. Calpains and disease. N Engl J Med. 2005;352:2413–23.
Dayton WR. Comparison of low- and high-calcium-requiring forms of the calcium-activated protease with their autocatalytic breakdown products. Biochim Biophys Acta. 1982;709:166–72.
Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801.
Geddes JW, Saatman KE. Targeting individual calpain isoforms for neuroprotection. Exp Neurol. 2010;226:6–7.
Goodman SR, Zimmer WE, Clark MB, Zagon IS, Barker JE, Bloom ML. Brain spectrin: of mice and men. Brain Res Bull. 1995;36:593–606.
Pineda JA, Wang KK, Hayes RL. Biomarkers of proteolytic damage following traumatic brain injury. Brain Pathol. 2004;14:202–9.
Wang KK, Posmantur R, Nath R, et al. Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J Biol Chem. 1998;273:22490–7.
Riggs JE. Delayed diffuse neurodegeneration after cerebral concussion. Mil Med. 2001;166:1029–30.
Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398–402.
Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–63.
Ryu J, Horkayne-Szakaly I, Xu L, et al. The problem of axonal injury in the brains of veterans with histories of blast exposure. Acta Neuropathol Commun. 2014;2:153.
Park E, Eisen R, Kinio A, Baker AJ. Electrophysiological white matter dysfunction and association with neurobehavioral deficits following low-level primary blast trauma. Neurobiol Dis. 2013;52:150–9.
Park E, Gottlieb JJ, Cheung B, Shek PN, Baker AJ. A model of low-level primary blast brain trauma results in cytoskeletal proteolysis and chronic functional impairment in the absence of lung barotrauma. J Neurotrauma. 2011;28:343–57.
Hernandez A, Tan C, Plattner F, et al. Exposure to mild blast forces induces neuropathological effects, neurophysiological deficits and biochemical changes. Mol Brain. 2018;11:64.
Shetty AK, Mishra V, Kodali M, Hattiangady B. Blood brain barrier dysfunction and delayed neurological deficits in mild traumatic brain injury induced by blast shock waves. Front Cell Neurosci. 2014;8:232.
Gama Sosa MA, De Gasperi R, Perez Garcia GS, et al. Low-level blast exposure disrupts gliovascular and neurovascular connections and induces a chronic vascular pathology in rat brain. Acta Neuropathol Commun. 2019;7:6.
McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10:S242–53.
Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22:142–9.
Gorgoraptis N, Li LM, Whittington A, et al. In vivo detection of cerebral tau pathology in long-term survivors of traumatic brain injury. Sci Transl Med. 2019;11:eaaw1993.
Elder GA, Stone JR, Ahlers ST. Effects of low-level blast exposure on the nervous system: is there really a controversy? Front Neurol. 2014;5:269.
Asken BM. Concussion biomarkers: deviating from the garden path. JAMA Neurol. 2019;76:515–6.
Meier TB, Bellgowan PS, Singh R, Kuplicki R, Polanski DW, Mayer AR. Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol. 2015;72:530–8.
Prichep LS, McCrea M, Barr W, Powell M, Chabot RJ. Time course of clinical and electrophysiological recovery after sport-related concussion. J Head Trauma Rehabil. 2013;28:266–73.
Vagnozzi R, Signoretti S, Floris R, et al. Decrease in N-acetylaspartate following concussion may be coupled to decrease in creatine. J Head Trauma Rehabil. 2013;28:284–92.
Perrot R, Berges R, Bocquet A, Eyer J. Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol. 2008;38:27–65.
Anderson KJ, Scheff SW, Miller KM, et al. The phosphorylated axonal form of the neurofilament subunit NF-H (pNF-H) as a blood biomarker of traumatic brain injury. J Neurotrauma. 2008;25:1079–85.
Shahim P, Tegner Y, Marklund N, et al. Astroglial activation and altered amyloid metabolism in human repetitive concussion. Neurology. 2017;88:1400–7.
Zetterberg H, Hietala MA, Jonsson M, et al. Neurochemical aftermath of amateur boxing. Arch Neurol. 2006;63:1277–80.
Chung RS, Staal JA, McCormack GH, et al. Mild axonal stretch injury in vitro induces a progressive series of neurofilament alterations ultimately leading to delayed axotomy. J Neurotrauma. 2005;22:1081–91.
Johnson VE, Stewart W, Weber MT, Cullen DK, Siman R, Smith DH. SNTF immunostaining reveals previously undetected axonal pathology in traumatic brain injury. Acta Neuropathol. 2016;131:115–35.
Okonkwo DO, Pettus EH, Moroi J, Povlishock JT. Alteration of the neurofilament sidearm and its relation to neurofilament compaction occurring with traumatic axonal injury. Brain Res. 1998;784:1–6.
DiLeonardi AM, Huh JW, Raghupathi R. Impaired axonal transport and neurofilament compaction occur in separate populations of injured axons following diffuse brain injury in the immature rat. Brain Res. 2009;1263:174–82.
Stone JR, Singleton RH, Povlishock JT. Intra-axonal neurofilament compaction does not evoke local axonal swelling in all traumatically injured axons. Exp Neurol. 2001;172:320–31.
Marmarou CR, Povlishock JT. Administration of the immunophilin ligand FK506 differentially attenuates neurofilament compaction and impaired axonal transport in injured axons following diffuse traumatic brain injury. Exp Neurol. 2006;197:353–62.
Park E, Liu E, Shek M, Park A, Baker AJ. Heavy neurofilament accumulation and alpha-spectrin degradation accompany cerebellar white matter functional deficits following forebrain fluid percussion injury. Exp Neurol. 2007;204:49–57.
Neselius S, Zetterberg H, Blennow K, Marcusson J, Brisby H. Increased CSF levels of phosphorylated neurofilament heavy protein following bout in amateur boxers. PLoS One. 2013;8:e81249.
Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983;80:6126–30.
Agoston DV, Shutes-David A, Peskind ER. Biofluid biomarkers of traumatic brain injury. Brain Inj. 2017;31:1195–203.
Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol. 2013;9:201–10.
Gill J, Latour L, Diaz-Arrastia R, et al. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology. 2018;91:e1385–e9.
Papa L, Lewis LM, Falk JL, et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann Emerg Med. 2012;59:471–83.
Neselius S, Zetterberg H, Blennow K, et al. Olympic boxing is associated with elevated levels of the neuronal protein tau in plasma. Brain Inj. 2013;27:425–33.
Gan ZS, Stein SC, Swanson R, et al. Blood biomarkers for traumatic brain injury: a quantitative assessment of diagnostic and prognostic accuracy. Front Neurol. 2019;10:446.
Hylin MJ, Orsi SA, Zhao J, et al. Behavioral and histopathological alterations resulting from mild fluid percussion injury. J Neurotrauma. 2013;30:702–15.
Petraglia AL, Plog BA, Dayawansa S, et al. The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surg Neurol Int. 2014;5:184.
McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–35.
McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25:350–64.
LoBue C, Schaffert J, Cullum CM. Chronic traumatic encephalopathy: understanding the facts and debate. Curr Opin Psychiatry. 2020;33:130–5.
McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64.
Pattinson CL, Gill JM. Risk of dementia after TBI – a cause of growing concern. Nat Rev Neurol. 2018;14:511–2.
Fakhran S, Yaeger K, Alhilali L. Symptomatic white matter changes in mild traumatic brain injury resemble pathologic features of early Alzheimer dementia. Radiology. 2013;269:249–57.
Kane MJ, Angoa-Perez M, Briggs DI, Viano DC, Kreipke CW, Kuhn DM. A mouse model of human repetitive mild traumatic brain injury. J Neurosci Methods. 2012;203:41–9.
Tagge CA, Fisher AM, Minaeva OV, et al. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain. 2018;141:422–58.
Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra60.
Dewitt DS, Perez-Polo R, Hulsebosch CE, Dash PK, Robertson CS. Challenges in the development of rodent models of mild traumatic brain injury. J Neurotrauma. 2013;30:688–701.
Johnson VE, Weber MT, Xiao R, et al. Mechanical disruption of the blood-brain barrier following experimental concussion. Acta Neuropathol. 2018;135:711–26.
Abdul-Muneer PM, Schuetz H, Wang F, et al. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med. 2013;60:282–91.
Chan ST, Evans KC, Rosen BR, Song TY, Kwong KK. A case study of magnetic resonance imaging of cerebrovascular reactivity: a powerful imaging marker for mild traumatic brain injury. Brain Inj. 2015;29:403–7.
Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra11.
Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–41.
Plog BA, Dashnaw ML, Hitomi E, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35:518–26.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Park, E., Baker, A.J. (2022). The Pathophysiology of Concussion. In: Schweizer, T.A., Baker, A.J. (eds) Tackling the Concussion Epidemic. Springer, Cham. https://doi.org/10.1007/978-3-030-93813-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-93813-0_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93812-3
Online ISBN: 978-3-030-93813-0
eBook Packages: MedicineMedicine (R0)