Abstract
Neurofilaments (NF) are the most abundant cytoskeletal component of large myelinated axons from adult central and peripheral nervous system. Here, we provide an overview of the complementary approaches, including biochemistry, cell biology and transgenic technology that were used to investigate the assembly, axonal transport and functions of NF in normal and pathological situations. Following their synthesis and assembly in the cell body, NFs are transported along the axon. This process is finely regulated via phosphorylation of the carboxy-terminal part of the two high-molecular-weight subunits of NF. The correct formation of an axonal network of NF is crucial for the establishment and maintenance of axonal calibre and consequently for the optimisation of conduction velocity. The frequent disorganisation of NF network observed in several neuropathologies support their contribution. However, despite the presence of NF mutations found in some patients, the exact relations between these mutations, the abnormal NF organisation and the pathological process remain a challenging field of investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The neuronal cytoskeleton is composed of three interconnected structures: the actin microfilaments (MFs), microtubules (MTs) and intermediate filaments (IFs). The diameter of IFs (10 nm) is “intermediate” between microfilaments (6 nm) and myosin filaments (15 nm) [1]. Neurofilaments (NF) are the major IFs present in adult neurons and their expression is restricted to neuronal cell types. Neurons express differentially several IF proteins depending on their developing stage or their localisation in the nervous system: nestin (200 kDa), three NF subunits (called NFL (light, 68 kDa), NFM (medium, 160 kDa) and NFH (heavy, 205 kDa)), α-internexin (66 kDa), peripherin (57 kDa) and synemin (41 kDa) [2–6].
The main role recognised for NF is to increase the axonal calibre of myelinated axons and consequently their conduction velocity. They also contribute to the dynamic properties of the axonal cytoskeleton during neuronal differentiation, axon outgrowth, regeneration and guidance [7]. Perturbations of their metabolism and organisation are frequently associated with various neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD) and Charcot-Marie-Tooth disease (CMT). An intense production and investigation of several transgenic models revealed new mechanisms underlying the normal and pathological biology of NF. However, different aspects concerning NF biology and function are still unsolved. In this review, we tempted to cover the current knowledge related to NF structure, assembly, expression, metabolism and functions. We will also discuss various transgenic models and NF involvement in neurodegenerative disorders.
Composition and Structure of Neurofilaments
Fibrous networks within neurons, initially named neurofibrils, were first described in the nineteenth century. With the development of electron microscopy, it was shown that neurofibrils were comprised of ~10-nm-diameter filaments, which were called NF and later classified in the IF family. IF proteins form a large family of proteins of various sizes (40–280 kDa) and primary structure, expressed differentially according to cell types (for recent reviews, see [8–10]). They are classified into six types on the basis of similarities in sequence and gene structure. IFs expressed by mammalian neurons consist of α-internexin, the NF triplet proteins designated as NFL, NFM and NFH for, respectively, low-, medium- and high-molecular-weight subunits (type IV), peripherin, synemin (type IV) and nestin (type VI). Originally, it was assumed that NF were composed only by NFL, NFM and NFH, but recent studies indicated that other proteins such as α-internexin and peripherin are also co-assembled with NF [11, 12]. This review is focused on NF triplet proteins hereafter referred to as NF subunits or NF proteins, but it is important to keep in mind that other IF proteins also participate to the formation of neuronal intermediate filaments.
NFs represent the main cytoskeletal elements in mature neurons. They account for 13% of total proteins and 54% of the Triton-insoluble proteins [13]. The molecular weights of NFL, NFM and NFH predicted from the DNA sequences are respectively 61.5, 102.5 and 112.5 kDa in human. However, due to their high content of negatively charged amino acids (glutamic acids) in their sequences and their extensive post-translational modifications (phosphorylation and glycosylation), they display higher apparent molecular weights on sodium dodecyl sulphate polyacrylamide gel electrophoresis (68, 160 and 205 kDa, respectively, for NFL, NFM and NFH). NFM and NFH alone or combinations of NFM and NFH fail to form filaments in the absence of NFL. NFL alone is able to form homopolymers in vitro and in cells transfected with NFL but not in rodents [14–20]. As opposed to mouse or rat NFL, human NFL has been reported to form homopolymers in vivo [19]. In rodent, NF are obligate heteropolymers composed of the NFL, NFM and NFH subunits [21] with a subunit stoichiometry of 4:2:1 [22]. This ratio varies during neuronal development. Moreover, others IF proteins such as peripherin in the peripheral nervous system (PNS) and α-internexin in the central nervous system (CNS) can co-assemble with NFL, NFM and NFH to form NF [11, 12]. Today, it must be admitted that these proteins are integral components of NF, as well as NFL, NFM and NFH. Yuan et al. [12] demonstrated that α-internexin can be considered as a fourth subunit of NF in adult CNS. They co-purified α-internexin with the NF triplet proteins from Triton-insoluble fraction in amounts comparable with those of the others subunits. Moreover, α-internexin with all three NF subunits co-assemble into single filamentous network in SW13vim(−) cells and is co-localised with NFM on the same NF in optic nerve in mice. They calculated a subunit stoichiometry of 4:2:2:1 (NFL–α-internexin–NFM–NFH) in optic nerve and spinal cord. Therefore, NF must be considered as the IFs of neurons, which can be comprised of up to four or more different proteins depending on neuronal type and developmental stage.
As all IF proteins, NF triplet proteins share a common tripartite structure, with non-helical amino and carboxy-terminal regions (called the head and tail domains) flanking a ~46-nm-long central α-helical rod domain composed of approximately 310 amino acids [23] (Fig. 1). This rod domain contains highly conserved motifs. Every seventh residue of its more central portion is hydrophobic, providing a hydrophobic seal on the helical surface necessary for the formation of coiled coil structures. Head and tail domains of the NF subunits are less conserved. The head domain is short and rich for Ser and Thr. The tail domain is the distinctive feature of NF proteins. For NFL, this region is short and contains many glutamic acid residues (E segment) while tail domains of NFM and NFH are longer and also contain E segments. Moreover, the carboxy-terminal domains of NFM and NFH contain numerous repeats of phosphorylation sites Lys–Ser–Pro (KSP, up to 51 repeats are present in mouse NFH; Fig. 1). The Ser residue of these repeats is highly phosphorylated in vivo [24, 25].
Schematic representation of mouse, rat and human NF subunits. The molecular weights predicted from the DNA sequences are shown. The three subunits share a highly conserved central α-helical domain of approximately 310 amino acids that is flanked by non-α-helical amino and carboxy-terminal end domains called, respectively, heads and tails. The tail domains are of variable size and contain many glutamic acid residues (green) and lysine for NFH (deep blue). The tail domains of NFM and NFH also contain multiple KSP repeats (orange) which are heavily phosphorylated (arrowheads). This graph is made according to the sequences and phosphorylation sites (determined experimentally or by similarity) provided by UniProtKB/Swiss-Prot database (mouse NFL: P08551, rat NFL: P19527, human NFL: P07196, mouse NFM: P08553, rat NFM: P12839, human NFM: P07197, mouse NFH: P19246, rat NFH: P16884, human NFH: P12036)
Assembly of Neurofilaments
As soon as the different NF subunits are expressed, they co-assemble to form an IF. This assembly does not require nucleotide binding or hydrolysis but is strongly dependent on ionic strength, pH and temperature [26]. The first step of NF formation is the dimerisation of NFL with either NFM or NFH via the association of their conserved rod domains to form parallel side-to-side coiled coil dimers. Two coiled coil dimers line up in a half-staggered manner, forming an anti-parallel tetramer [27]. Tetramers combine to form protofilaments, which finally assemble to constitute the final 10-nm filament.
It has long been considered that NFL subunits constitute the core of the NF whereas NFM and NFH are arranged around this core. However, immunoelectron microscopy using antibodies directed against head, rod or tail domains of individual NF subunits showed that all three NF proteins are incorporated integrally into filaments [28, 29]. Tail domains of NFM and NFH form lateral projections extending from the filament backbone [17, 30]. These sidearms participate to the stabilisation of the filament network by forming cross-bridges between NF and other cytoskeletal elements or organelles.
Head and rod domains of NFL and NFM, and especially their post-translational modifications, are essential for the NF assembly [31–35]. Phosphorylation of Ser-51 and Ser-55 on NFL [36] and phosphorylation of Ser-23 on NFM by protein kinase A (PKA) and protein kinase C (PKC) [37] were shown to regulate in vivo NF assembly. Indeed, the phosphorylation of NFL head domain prevents their assembly or cause disassembly when incorporated into filaments [38]. It is also interesting to note that the phosphate present on Ser-55 of NFL is turned over rapidly following NFL synthesis in neurons [36], suggesting a possible role in the blockade of NF assembly before their transport into neurites. The generation of a transgenic mouse with a mutant NFL transgene in which Ser-55 was mutated to Asp to mimic permanent phosphorylation resulted in pathological accumulation of NF in brain neuronal cell bodies [39]. Finally, polymerisation of NFL protein in vitro was inhibited by phosphorylation of NFL head domain by protein kinase N (PKN) [40]. Together, these results indicate that the transient phosphorylation of head domains in perikarya prevents the polymerisation of NF subunits [35]. NFM and NFH head domains are also modified by O-linked N-acetyl glucosamine (O-GlcNAc) on sites close to phosphorylation sites [41]. Similar sites are present in the NFH head and tail domains [42]. This proximity could suggest that these post-translational modifications may influence each other and play a yet unknown role in filament assembly.
Finally, it was shown that NUDEL, a mammalian homologue of the Aspergillus nidulans nuclear distribution molecule NudE, is involved in NF assembly [43]. This protein associates directly with soluble pool of NFL and indirectly with NFH subunit. By interacting with NFL, NUDEL promotes the incorporation of NF subunits in the network during NF assembly but does not assemble with NF proteins. Moreover, genetic knockdown of NUDEL disrupts NF stoichiometry which, in turn, results in impaired NF assembly and transport [43].
In vitro interactions occurring between NF allow the formation of a viscoelastic network resistant to important deformation, suggesting the importance of cross-bridges for NF mechanical properties [44, 45]. Kreplak et al. [46] used atomic force microscopy to test the mechanical properties of single NF. They showed that NF can be stretched more than threefold, with an average of 2.6-fold, suggesting that NF may indeed function as mechanical shock absorbers in vivo.
Expression of Neurofilaments
In human, genes coding for NFL and NFM (NEFL and NEFM genes) are very closely linked on chromosome 8 (8p21) [47, 48] while NFH gene (NEFH gene) is located on chromosome 22 (22q12.2) [49]. In mice, NEFL and NEFM genes are located on chromosome 14 (14 D3 for NEFL and 14 D1 for NEFM) while NEFH gene is located on chromosome 11 (11 A1–A5). The expression of the three NF subunits is finely regulated during nervous system development and coincides with neuron differentiation [7]. This expression is controlled at the transcriptional level but also through post-transcriptional regulation of mRNA localisation, stability and translational efficiency (for recent review, see [50]). When axons reached their targets, NFL is the first subunit to be expressed, together with α-internexin and peripherin, and then it is rapidly followed by NFM expression [51, 52]. At this early stage, the axoskeleton is composed mainly by MTs while NFL and NFM are expressed only at low levels. This unique composition of the cytoskeleton is also found during axonal regeneration in the PNS, and thus it has been suggested that it may improve growth [52–57]. The appearance of NFH occurs after synaptogenesis and is accompanied by robust up-regulation of NFL and NFM expression [52]. At this advanced stage, NF of mature composition may further enhance axon stability and calibre. Interestingly, the increased NF density is accompanied by a decreased MT density [58], suggesting a fine balance between these two networks. In cultured neuroblastoma cells, the level of mRNAs coding for NF is proportional to their protein levels, and NFL and NFM mRNAs appear several days before the expression of NFH mRNA. The sequential appearance of NF proteins follows that of their mRNAs, with an early expression of NFL and NFM and a later expression of NFH [59]. Apart from Giasson and Mushynski [60], several studies observed that the expression levels of NFL and NFM are mutually regulated and independent from NFH [61, 62]. This could be related to their genomic localisation, but the molecular mechanisms are still unknown.
Axotomy is followed by a strong down-regulation of NF mRNAs and proteins in PNS [63–65] and CNS [66–69], leading to reduced levels of axonally transported NF in injured neurons [65, 70, 71]. Then, during regeneration of injured axons in PNS, the expression of NF subunits is strongly up-regulated [71–74]. This reversion does not occur when regeneration is prevented [75, 76] as well as in mammalian CNS axons which normally do not regenerate [66, 67]. However, in transected axons of the spinal cord from lamprey, where NFM expression is initially suppressed, Jacobs et al. [77] observed an up-regulation of this subunit only in axons that successfully regenerate, while NFM levels remain low in those that do not regenerate. Similarly, increased amounts of NFM mRNA and protein are observed during successful optic nerve regeneration in Xenopus laevis [78].
Post-translational Modifications of Neurofilaments
Phosphorylation of Neurofilaments
The phosphorylation is the best-documented post-translational modification of NF proteins. Multiple aspects of NF’s biology, including their assembly and their axonal transport, are regulated by their phosphorylation status. Moreover, aberrant NF phosphorylation is a pathological hallmark of many human neurodegenerative disorders (for detailed reviews, see [79, 80]).
NF proteins are the most extensively phosphorylated proteins in neurons with up to 51 sites of phosphorylation located on the C-terminal domain of NFH [24, 81–85] (Fig. 1). This phosphorylation is topographically regulated, with a proximo-distal gradient consisting of an intense phosphorylation in axons and little or no phosphorylation in cell bodies and dendrites [7, 86–89]. Phosphorylation sites are located on the amino-terminal and carboxy-terminal domains of the three NF subunits. These sites are the targets of, respectively, second messenger-dependent kinases [90–92] and second messenger-independent kinases [25, 91, 92]. Phosphorylation of head domain arises mainly in cell body soon after the synthesis of NF proteins while the phosphorylation of tails domains coincides with their entry into the axon [93–95].
As mentioned above, phosphorylation of NFL and NFM head domains by PKA, PKC and PKN prevents the assembly of NF or leads to their disassembly [36–38, 40]. In vitro studies also revealed Rho-associated kinases and calcium–calmodulin-dependent protein kinase II phosphorylation sites in the NFL head domain [96, 97].
NFM and NFH tail domains are the most extensively phosphorylated regions. Most of these phosphorylation sites are KSP repeats, although other Ser–Thr-containing motifs are also phosphorylated. Many roles have been attributed to the phosphorylation of KSP motifs, including the formation of cross-bridges between NF or with MTs, the expansion of the axonal calibre, the slowing of the NF axonal transport and the integration of NF in a stationary pool [18, 47, 87, 98–103]. Phosphorylation of KSP sites depends on two families of Pro-directed kinases: the cyclin-kinase Cdk5 and the microtubule-associated protein (MAP) kinases. Signal transduction cascades leading to the activation of these kinases could be triggered by growth factors [104, 105], Ca2+ influx [106], integrins [107] and myelination [89]. The link between the myelination and the phosphorylation of NFM and NFH sidearms was first suggested by the decreased phosphorylation of NF in the dysmyelinated mouse mutant Trembler [108] and by their poor phosphorylation in non-myelinated axonal domains like the initial segment and nodes of Ranvier [108–111]. The binding of myelin-associated glycoprotein (MAG) to axonal receptors was proposed to activate a signalisation cascade leading to the phosphorylation of NF in myelinated regions [112, 113], but the molecular cascade of this process as well as its regulation are still unclear.
Cdk5 preferentially phosphorylates KSPXK motifs of NFH in vitro and in vivo [114–121], preventing the binding of dephosphorylated NFH to MTs [122]. But the majority of KSP repeats in rat–mouse NF tail domains are phosphorylated by MAP kinases, including extracellular signal-regulated kinases 1 and 2 which phosphorylates KSPXXK and KSPXXXK motifs of NFH [120], stress-activated protein kinase which is responsible for NFH tail domain phosphorylation on KSPXE motifs in cell body under stress-activated conditions [60, 123], glycogen synthetase kinase 3 (GSK3) which phosphorylates some of the KSP sites in bovine NFM [124] and few sites on NFH [117], p38 kinase [125, 126] and c-Jun N-terminus kinase 1 and 3 (JNK1/3) [123, 127, 128].
Ser–Thr residues in the glutamic acid region of the three subunits tail domains are phosphorylated by casein kinase I (CKI) [91, 129–133] while casein kinase II (CKII) also phosphorylates Ser-473 on the short-tail domain of NFL [134, 135].
Phosphorylation of head and tail domains are intimately related. Zheng et al. [136] showed that phosphorylation of NFM head domain by PKA reduces the phosphorylation of tail domain by MAP kinases in vitro and in vivo. Moreover, mutation of Ser-1, 23 and 46 residues to Ala in the head domain of NFM prevents PKA phosphorylation in transfected NIH3T3 cells and fails to inhibit tail domain phosphorylation by MAP kinases [136]. These results highlight a regulatory mechanism by which phosphorylation of NF head domains could prevent NF assembly and C-terminal phosphorylation in cell body, protecting the neuron from abnormal accumulation of phosphorylated NF in perikarya.
The phosphorylation state of NF proteins in the different neuronal compartments depends on a dynamic balance between the activities of kinases and phosphatases. Since NF head domain phosphorylation inhibits NF assembly [36–38], their dephosphorylation is necessary to allow the polymerisation of NF proteins prior to their transport into the axon. The extensive enzymatic dephosphorylation of NF induces a progressive loss of their capacity to interconnect in vitro into a reticulated network, measured by the formation of highly viscous gels in purified preparations of NF [137]. Finally, dephosphorylation of NF tail domains facilitates their degradation at the terminals [138] and regulates their interaction with other cytoskeletal proteins. Dephosphorylation of head and tail domains of NF subunits is mainly (60%) catalysed by phosphatase 2A [139–142]. phosphatase 1 also contributes to the dephosphorylation of NF but to a lower extent (10–20%) [142].
Glycosylation and Glycation of Neurofilaments
NFs are also post-translationally modified by attachment of O-GlcNAc to individual Ser and Thr residues. O-GlcNAc is a common modification of cytosolic and nuclear proteins that regulates protein stability, subcellular localisation and protein–protein interactions [143]. Like phosphorylation, O-glycosylation is dynamic and often reciprocal to phosphorylation at the same sites or adjacent to them (Fig. 1). Dong et al. [41, 42] identified several O-GlcNAc sites on NFL head domain (Thr-21, Ser-27, Ser-34 and Ser-48) and NFM head (Thr-19, Ser-34 and Thr-48) and tail domains (Thr-431). NFH is also extensively modified by O-GlcNAc in the head domain (Thr-53, Ser-54 and Ser-56) and at multiple sites within the KSP repeat motifs in the tail domain, although the exact sites remain to be identified. In purified NF proteins, the O-GlcNAc modifications occur at a stoichiometry of approximately 0.1, 0.15 and 0.3 mol of GlcNAc per mole of, respectively, NFL, NFM and NFH [41, 42]. The function of these modifications is still elusive, but several clues suggest a role in the NF assembly. For example, all O-glycosylation sites within head domains are located in regions essential for in vivo NF assembly, close to the phosphorylation sites involved in this process. O-glycosylation of NF head domains could reciprocally modulate its phosphorylation and consequently the assembly and dynamics of NF. Further investigations are necessary to elucidate the precise mechanism regulating NF O-glycosylation, the relation between NF phosphorylation and O-glycosylation and the distribution of O-glycosylated NF. Antibodies that specifically recognise O-glycosylated epitopes in NF subunits could be an important tool to elucidate these questions. To this end, Lüdemann et al. [144] generated a monoclonal antibody specifically directed against an O-glycosylated epitope in the tail domain of NFM. They showed that O-glycosylated NFM is enriched in the axons of human neurons in situ, together with hyperphosphorylated NF, indicating a synchronous phosphorylation and O-glycosylation of the tail domain of NFM within the axon. However, the O-glycosylation of NFM and the activity of MAP kinases are reversibly regulated, suggesting reciprocal regulation between phosphorylation of the KSP region and O-glycosylation.
The first evidence of NF glycation, also called non-enzymatic glycosylation, was reported in peripheral nerves in diabetes mellitus [145]. A possible role for this modification in familial and sporadic ALS was also suggested [146].
Nitration, Oxidation and Ubiquitination of Neurofilaments
In addition to phosphorylation and O-glycosylation, NF undergo nitration, oxidation and ubiquitination. Nitration of NFL subunit was reported in the normal rat brain using a proteomic analysis [147]. NF nitration was also detected in NF-rich inclusions in motoneurons of sporadic ALS cases [148]. This modification is catalysed by superoxide dismutase 1 (SOD1) in vitro on four Tyr residues of NFL, one in the head domain (Tyr-17) and three in the rod domain (Tyr-138, Tyr-177 and Tyr-265) [149]. It is interesting to note that Tyr-17 is essential for the polymerisation of NF while the other three Tyr residues are located within the coiled coil structure of the rod domain and are likely involved in intermolecular hydrophobic interactions. The nitration change normally hydrophobic residues into negatively charged hydrophilic residues, thereby disrupting the assembly and stability of NF. Consequently, it was proposed that SOD1-catalysed nitration of NF may contribute to motoneurons dysfunction in ALS [149, 150].
The oxidation of NF arises during ageing and Wallerian degeneration and was found in neurodegenerative disorders. Oxidised NFs are more susceptible to calpain proteolysis and form dense aggregates and bundles of laterally aggregated filaments [151]. The incubation of disassembled NFL with SOD1 and H2O2 causes the formation of dityrosine crosslink and the aggregation of NFL protein proportionally to the concentration of hydrogen peroxide [152]. Antioxidant molecules inhibit these effects. Finally, ubiquitination of NF proteins has been suggested by Gou and Leterrier [153] as a possible mechanism for NF degradation.
Degradation of Neurofilaments
After their assembly in the perikaryon, NFs are slowly transported by the slow axonal transport toward the nerve terminal [154] where they are degraded. A degradation of NF over the entire length of axons in mouse sciatic nerve has also been reported during Wallerian degeneration in transected fragments of nerve [155] and in a context of axonal NF deficiency [156]. Nixon and Logvinenko [103] suggested that such a degradation of NF proteins in axons could account for non-homogeneous distribution of NF in axons.
Calcium-activated proteases such as calpain are found in human tissues and degrade NF from squid, rat, bovine and worm [157–161]. NF proteins that are usually absent in synaptic terminals accumulate following leupeptin treatment, a protease inhibitor. This indicates that their normal absence in synapses is due to degradation by calcium-activated proteases [162]. The initial evidence that calcium plays a role in the degradation of NF came from observations showing disintegration of NF in rat peripheral nerve fibres after exposure to calcium [163]. Purification of calcium-activated neutral proteases in rat peripheral nerve or spinal cord resulted in the identification of calpain I (or μ-calpain) and calpain II (or m-calpain), as defined respectively by the micromolar or millimolar levels of calcium required for their activation [164, 165]. Calpain II is present in glial cells while calpain I is predominantly expressed in neurons [166]. At endogenous sub-micromolar calcium concentrations, Nixon et al. [167] demonstrated a limited proteolysis of NFM as a post-translational modification during the axonal transport.
Most calcium-activated neutral proteases show a high degree of substrate specificity with IFs [168]. Unlike many proteases, calpain specificity appears to be determined by conformational factors and primary amino acid sequences. It usually catalyses limited cleavage of its substrates. Participation of calpain and calcium-activated neutral proteases in NF turnover is plausible as reflected by the occurrence in normal brain of characteristic NF protease-resistant fragments throughout the neuraxis. These fragments could be retrogradely transported and could regulate the synthesis, assembly and delivery of NF in accordance with their turnover level at remote sites [158, 162]. Inhibition of calpain proteolytic activity in transected axon abolishes growth cone formation suggesting a central role in the reorganisation of the axonal cytoskeleton during its transition from a stable differentiated state into a dynamically extending structure [169].
NF are also degraded by non-specific proteases like lysosomal cathepsin D, trypsin and α-chymotrypsin. Such trypsin proteolytic strategies were used to analyse the spatial architecture of NF [170, 171]. Cathepsin D plays an important role in NF metabolism. The content of cathepsin D is probably more than 1,000-fold greater than that of a calcium-activated neutral proteases [172]. Purified brain cathepsin D was shown to degrade NF proteins from rat, mouse, bovine and human tissues, and some characteristic fragments produced by this hydrolysis were shown to be normally present in brain [173–175].
In addition to their participation in the turnover process, proteases produce NF-derived peptides that could be active. The hypothesis of a possible regulation of gene expression by NF proteins led Traub et al. [176] to show that subunit proteins of NF bind to RNA and single-stranded DNA. The DNA-binding sites are located in the amino-terminal domain [177] and are preserved during the digestion of NF by calcium-activated proteases. However, the capacity of NF to affect the DNA or RNA fragments to which they bind is unknown.
Post-translational modifications can regulate proteolysis of NF, as illustrated for phosphorylation which protect NF from proteolysis by calpains [138, 178]. Aluminium, a neurotoxin which causes NF protein phosphorylation and accumulation in neuronal perikarya [179, 180], inhibits calpain-mediated proteolysis of NF [181]. These results raise the possibility that kinase or phosphatase activity might determine the rate of turnover of NF proteins. Gou and Leterrier [153] also indicated that ubiquitination facilitates the proteolysis of NF.
Calcium-activated neutral proteases play important roles in tissue injury. Many pathological states induce an increase of free calcium within the axon leading to a massive proteolysis of NF [182, 183]. The degradation of NF in transected rat sciatic nerve is reduced if the influx of calcium into the axoplasm is prevented or if calcium-activated neutral proteases activities are inhibited [155]. Such enzymatic fragmentation in transected nerves generates protease-resistant NF fragments which may represent, after externalisation into the endoneurium, a mechanism responsible for the generation of auto-antibodies to NF proteins detected in Parkinson’s disease and in several neurological diseases [184–187].
Abnormal aggregations of NF are a hallmark of several human neuropathological situations. In ALS, they accumulate in cell bodies or in the proximal part of axons from motor neurons [188, 189]. They accumulate in Lewy bodies of PD [190], in neurofibrillary tangles (NFTs) of AD [191] and, following intoxication by aluminium, hexanedione, acrylamide or β, β‘-iminodipropionitrile (IDPN) [192–195]. In all these pathological situations, the cellular and molecular mechanisms used to eliminate the neurofilamentous aggregates are still unknown. It has been shown that trypsin-like proteases are expressed in neurons [196–198], and they accumulate within pathological neurofilamentous aggregates [199]. Tsuji et al. [200] showed increased levels of calpains in the cytosolic fraction of AD brains when compared to control brains. Fasani et al. [201] showed that NF isolated from NFH-LacZ transgenic mice (in which NF are sequestered in cell bodies [202]) are more sensitive to exogenous trypsin and α-chymotrypsin than normal NF. Moreover, an increased trypsin immuno-labelling is detected in perikarya from such mice compared to wild-type animals. These results suggest that when NFs are sequestered in the cell body, their amount is controlled by an increased susceptibility to trypsin-like proteolysis and an increased production of proteases [201].
As mentioned above, it has long been considered that NF degradation only occurs at the axon terminals. However, a degradation of NF over the entire length of axons in mouse sciatic nerve was recently highlighted by Millecamps et al. [156]. They generated transgenic mice with doxycycline-regulated expression of human NFL (hNFL) with or without endogenous mouse NFL proteins (respectively, tTA;hNFL;NFL+/− and tTA;hNFL;NFL−/− mice). The doxycycline administration in drinking water of tTA;hNFL;NFL−/− mice silences the expression of hNFL and the pre-existing protein subsequently disappears in their sciatic nerve, with an estimated half-life of 3 weeks. This loss is synchronised over the entire length of the sciatic nerve, suggesting a homogeneous and not a local degradation of hNFL. In contrast, no detectable loss of hNFL protein was observed in presence of stationary NF network in sciatic nerve from tTA;hNFL;NFL+/− mice, even after 4.5 months of doxycycline treatment. After 8 months of treatment, 35% of hNFL protein was still remaining [156]. These results show that a pre-existing NF network is a key determinant of half-lives of NF proteins by reducing their turnover rate and support the view that NF proteins can spend several months, if not years, in long NF-rich peripheral axons. This long life makes them potential targets for oxidation or other harmful modifications, which in turn may cause NF disorganisation.
Interaction of Neurofilaments with Proteins and Organelles
As parts of a complex and dynamic network, NF interact with several partners and these interactions are principally mediated through NF-associated proteins that can modulate function (enzyme) and structure (linker protein) of NF (Table 1). Linker proteins are responsible for the interaction between the different filaments or organelles, whereas enzymes (principally kinases and phosphatases) modulate NF architecture, assembly and spacing.
In neurons, NF and MTs are two major components of the cytoskeleton. Dynamic interactions between these elements are crucial for the axoskeleton and are regulated mostly by phosphorylation. This was documented by several biochemical studies showing that tubulin and/or MTs are able to interact directly with NF both in vitro and in vivo [203–209]. This direct interaction was also illustrated by quick freeze deep etch electron microscopy [30, 210]. Dephosphorylation of NF by alkaline phosphatase promotes the NF–MT interaction mediated by carboxy-terminal domain of NFH [18] suggesting spatio-temporal regulation of NF–MT interaction by kinases. Tau protein kinase II specific phosphorylation of NFH tail domain has been described to dissociate NF and MTs [122]. NF–MT interaction was also shown to be mediated by MAPs such as MAP2 [30].
Stable tubule only polypeptide (STOP) proteins (named also MTAP6 or MAP6) are a family of cytoskeleton associated proteins responsible for the MT cold stability. These proteins were initially isolated from rat brain cold-stable MTs [211, 212] and were shown to induce MT cold stability in vitro when added to labile MTs [213, 214] or when expressed in cells normally devoid of stable MTs [215]. They are abundant in neurons, which contain a large amount of stable MTs [216, 217], and associate preferentially to cold- and drug-stable polymers [218]. STOP proteins were shown to be associated with NF by both biochemical co-purification of NF and their co-precipitation with NF in axonal spheroids of ALS [219]. In NFH-LacZ transgenic mice, STOP proteins were also found to co-accumulate with NF in the perikaryon. While further investigations are necessary to understand the mechanism of STOP–NF interactions and their functional relevance, these data indicate that STOP proteins could be considered as a cytoskeletal integrator and a marker of ALS spheroids [219].
Several studies indicate a direct interaction between motor protein dynein and kinesin with NF [220–225], mediated principally by the phosphorylated carboxy-terminal domains of NFM and NFH [222, 223, 225]. These motor proteins could contribute to the transport of NF along axons and dendrites (for review, see [226]). For example, co-immunoprecipitation experiments with anti-dynein antibody induced selectively co-precipitation of phosphorylated NF, while anti-kinesin selectively co-precipitated hypophosphorylated NF [225]. Recently, atomic force microscopy allowed a direct evaluation of the interaction between NF and cytoplasmic dynein [223]. Yeast two-hybrid and affinity chromatography assays also identified a direct binding between dynein intermediate chain and NFM [223], possibly involved in the saltatory bi-directional axonal transport of NF in the neuron.
Associated proteins mediate interaction of NF with vesicles. Biochemical preparations such as synapsin I immunoprecipitation [227] or nearest neighbour analysis for brain synapsin I [228] have shown that this protein can link non-secretory vesicles directly to NFL subunit.
Bullous pemphigoid antigen 1 neural isoform (BPAG1n, 280 kDa) has originally been described as a major cytoskeletal integrator connecting actin filaments to NF [229]. In BPAG1−/− mice, electron microscope analysis along sensory axons revealed regional swellings filled with lysosomal vesicles and disorganised arrays of NF [229, 230]. Co-transfection of BPAG1n with NFL and NFH in SW13 cells show that BPAG1n is able to link NF and actin networks [229]. Natural mutations of BPAG1 [231] cause the well-known mouse model of neurological disorder dystonia muscularum (dt/dt) characterised by a disorganised cytoskeleton in the sensory nervous system. It was thus suggested that a loss of BPAG1 interactions with IFs is important to the pathogenesis in dt/dt mice. However, Eyer et al. [232] showed that pathogenesis of axonopathy in dt/dt mice was independent of axonal NF as demonstrated by matting dt/dt mice and NFH-LacZ transgenic mice in which NF aggregated in the cell body of neurons [202]. Similar results were obtained by matting dt/dt mice with knockout NFL mice [233], confirming that the presence or absence of NF in the axon does not affect the appearance of the dt/dt phenotype. Moreover, the ability of the neuronal splice isoform of BPAG1 to connect actin filaments to NF is now called into question by works of Leung et al. [234]. Indeed, they found that the only neuronal isoform of BPAG1 (named BPAG1a) lacks an IF binding domain. However, BPAG1a has an actin binding domain and a microtubule actin domain, suggesting that this protein could play a role in maintaining the structural organisation of the neuronal cytoskeleton [235].
Electron microscopy investigation in Dieters’ neurons from lateral vestibular nucleus of rabbit brain allowed to visualise MF-mediated interaction of NF with the plasma membrane and the nuclear pores [236]. These authors suggested that NF may play a linking role between plasma membrane and nucleus. As a matter of fact, during membrane depolarisation, early transduction signal mediator such as Ca2+ or cyclic adenosine monophosphate could mediate NF rearrangement and contribute to a modification of the DNA transcription at the site of the nuclear pore complex [236]. Moreover, it has been shown that NF were able to bind DNA, RNA and histone H1 nuclear protein, suggesting a possible role of NF in the regulation of transcription processes [176, 177, 237].
Finally, using electron microscopy on transected squid giant axons, it has been shown that NF formed dynamic complex with smooth endoplasmic reticulum. Modification and rearrangement of such complex could be involved in some neurodegenerative diseases [238]. Cross-bridges between mitochondria and NF were also emphasised by ultrastructural studies [210, 239]. Since mitochondria do not move along NF in neuronal processes deprived of MTs and MFs [240], it was postulated that NF serve as a docking site for these organelles and regulate their spatial distribution along axons. The overexpression of NFH in culture cells induces the selective perikaryal retention of mitochondria [241, 242], suggesting that regulated NF–mitochondria binding is required for normal translocation of mitochondria in axons. Wagner et al. [243] provided evidences that NFM and NFH sidearms mediate this interaction between NF and mitochondria and proposed that porin molecules or other cytoskeleton-binding proteins of the mitochondrial outer membrane mediate this interaction. They also revealed that the binding of NF to mitochondria depends on the potential of the mitochondrial membranes, suggesting that the conformation or the organisation of the partner of NF on the mitochondrial outer membrane is modified by the membrane potential of the organelle. Finally, it seems that phosphorylation of NF may regulate their binding affinity for mitochondria in a potential-dependent manner [243].
Neurofilaments Contribute to and Modulate the Axonal Calibre
NFs play an essential role in growth and maintenance of axonal calibre. This was first suggested when Friede and Samorajski [244] observed that increased NF numbers and densities are correlated with increased axonal calibres. Moreover, the axonal radial growth coincides with the entry of NF into axons during axonal development or regeneration [245, 246]. The recent use of various animal models clearly showed the importance of NF in the control of the axonal diameter. These models include mice knockout for NF genes (Table 2), mice expressing human (Table 3), mouse (Table 4) and modified NF subunits (Table 5). But the first evidence of the implication of NF in axonal radial growth in an animal model was obtained in Japanese quails. Indeed, an important axonal atrophy was observed in quiver quails characterised by the absence of NF in their axons caused by non-sense mutation in NEFL gene [247–249]. The first evidence in mice was provided by the production of transgenic mice overexpressing human NFH [250] (Table 3). These mice develop perikaryal accumulations of NF, resulting in a deficiency of axonal NF and in an axonal atrophy. This was further confirmed by the generation of NFH-LacZ transgenic mice in which expression of a NFH-β-galactosidase fusion protein provokes the perikaryal aggregation of NF, leading to a 50% reduction of axonal calibres [202, 251] (Fig. 2; Table 5). Finally, the targeted disruption of NEFL gene in mice caused the lack of axonal NF and strongly reduced diameter of myelinated axons [61] (Table 2). Multiple studies analysed the importance of the number and stoichiometric proportion of each NF subunit in the axonal calibre determination. Transgenic mice overexpressing murine NFL have a two to threefold increase in the number of NF but the diameter of their axons is only slightly modified [252, 253] (Table 4), suggesting that the number of NF by itself is not the main determinant of axonal diameter. Yet, triple heterozygous knockout mice (NFL+/−; NFM+/−; NFH+/−) in which integrity of NF network and normal subunit stoichiometry are preserved exhibit a 40% decrease of NF content and a 50% decrease of axonal diameter in L5 ventral root [254]. The individual increase in each of the three NF subunits inhibits radial axonal growth, and the simultaneous increase of NFM and NFH exacerbates this axonal atrophy [253]. In contrast, the co-overexpression of either NFL–NFM or NFL–NFH increases the axonal calibre [253, 255], suggesting that NFL in combination with either NFM or NFH is sufficient to promote radial growth. Altogether, these results indicate that both number of NF and a precise stoichiometry of their subunits are essential in the expansion of axonal diameter. To determine the specific contributions of NFM and NFH subunits in the axonal size, Elder et al. [62] made null mutant mice deficient for NFM. The axonal calibre was strongly reduced as well as NFL mRNA and protein levels. Modification of NFM expression was also reported in NFL−/− mice [61] suggesting that the levels of NFL and NFM are mutually regulated, reinforcing the view that the stoichiometry of each NF subunit is crucial for the establishment of a proper axonal calibre. Three different NFH-null mice were produced and revealed that this subunit contributes to a lesser extent than NFM to the determination of the axonal diameter [256–258]. However, subtle differences exist between these models. Indeed, Elder et al. [256] reported no modification in NFM protein level but a significant reduction of the axonal calibre, while Rao et al. [257] and Zhu et al. [258] observed an up-regulation of NFM (respectively, 100% and 20%) but only minor modification of the axonal diameter, with a slight decrease in the large-calibre axons. As suggested by Hirokawa and Takeda [259], this divergence could be explained in part by the chronological differences between the data ([257] and [258] 3 months; [256] 4 months), suggesting a later effect of NFH on the axonal radial growth. It is also important to note that Rao et al. [257] and Zhu et al. [258] reported a compensatory increase in MT density and NFM phosphorylation in their NFH-null mice, complicating the conclusions about the exact role of NFH in axonal radial growth.
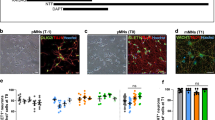
The expression of the NFH-β-galactosidase fusion protein in NFH-LacZ mice causes the aggregation of NF in neuronal cell bodies, inducing their depletion from the axonal compartment and the decrease of the axonal calibre. a, b Cross sections of Wt (a) and NFH-LacZ (b) spinal cord. Note the presence of massive aggregates (asterisks) in transgenic motoneurons while such accumulations are absent from control neurons. Bar, 20 μm. c, d Electron micrographs showing the axonal cytoskeleton in axons from Wt (c) and NFH-LacZ (d) sciatic nerve. NF (asterisks) are the most abundant cytoskeletal component in control axons while they are absent from transgenic axons. In contrast, the density of MTs (arrowheads) in axons deprived of NF is strongly increased. Bar, 200 nm. e, f Typical cross section views of spinal cord from Wt (e) and NFH-LacZ (f) mice. Transgenic axons have smaller diameters and proportionally thinner myelin sheaths than control axons. Bar, 5 μm. g, h Typical cross section views of sciatic nerve from Wt (g) and NFH-LacZ (h) mice. As in spinal cord, the axonal diameter is strongly reduced in transgenic sciatic nerve. However, axons from NFH-LacZ mice recruit inappropriately thick myelin relative to their absolute reduced calibres. Bar, 5 μm. i Serial sections of spinal cord showing an axon (green for Wt and red for NFH-LacZ mice) at the level of a node of Ranvier. Despite the absence of axonal NF in transgenic axons, the extent of axonal constriction at nodes is similar between control and NFH-LacZ fibres. Bar, 2 μm. j Double immunohistochemistry showing the molecular organisation of nodes of Ranvier in sciatic nerve and in spinal cord from 4-month-old Wt and NFH-LacZ mice. The localisation of sodium channels (pan-Na, Nav1.2, Nav1.6), βIV-spectrin, Caspr and Kv1.1 potassium channels is not modified by the absence of axonal NF. Nav1.6 is co-localised with βIV-spectrin at nodes while Nav1.2 is absent from matures nodes. Caspr and Kv1.1 concentrate in paranodes and juxtaparanodes, respectively. Bar, 10 μm
It has long been suspected that the phosphorylation status of NFM and NFH carboxy-terminal domains controls axon calibre by regulating NF transport [98, 99] and/or interfilament spacing [85, 89, 246, 260]. KSP repeated motifs on NFM and NFH sidearms are variably phosphorylated, principally after the entry of NF into the axon [86–88, 94]. It has been suggested that phosphorylation of KSP repeats could increase the total negative charges on sidearms and thus causes their lateral extension by repulsive interactions [47, 87], increasing NF spacing and axonal calibre. In agreement with this assumption, Brown and Hoh [261] used atomic force microscopy to show the presence of a weak repulsive force around the core of the filament. Moreover, this repulsive force is absent in homopolymers of NFL or trypsinised native filaments which lack the sidearms present in native filaments and attenuated when the filaments are enzymatically dephosphorylated [262]. As there are more KSP repeats in NFH than in NFM (51 vs. seven in mice), it was thought that this subunit should contribute more to the axonal radial growth. This idea was reinforced by the observation that phosphorylated NFH sidearms are less pliant and larger structures than dephosphorylated sidearms [263]. Moreover, a 50% reduction in the level of axonal NFH in mice overexpressing NFM decreases axonal calibre [264] (Table 4) and modest increases in NFH slightly enhance radial growth in transgenic mice [265]. However, production of NFH-null mice [256–258], as well as mice expressing NFH deprived of its carboxy-terminal domain (NFHtailΔ mice) [266] (Table 5), demonstrated no major modification in NF spacing and axonal radial growth, except a delay in the acquisition of a normal axonal calibre. However, as mentioned above, NFH-null mice are characterised by a compensatory increase for MT density and NFM phosphorylation. Similarly, the phosphorylation level of NFM is higher in NFHtailΔ mice [266], making difficult to determine the exact implication of NFH in axonal calibre. In contrast, disruption of the NFM gene [62], or deletion of its carboxy-terminal domain in NFMtailΔ mice [267, 268], reduced the interfilament spacing and axonal calibre, showing a preponderant role of NFM in determining axonal diameter. Finally, it is interesting to note that axonal calibre in double transgenic NFM/HtailΔ mice from 6 months of age is similar to that of NFMtailΔ mice, while NF spacing is reduced and NF network is disorganised [267]. This suggests a role for NFH tail domain in NF–NF interactions but not in the control of axonal calibre. Another possibility could be that a yet unknown molecule may be involved in the spatial spacing of NF and may bind differently to NFM and NFH.
The axonal radial growth is closely related to the myelination. In vitro myelination induces the increase of axonal calibre [269] but demyelination in vivo causes a local axonal atrophy [270, 271] and modifications in the axoskeletal organisation [245]. A severe axonal atrophy is also observed in hypomyelinated tracts from shiverer mutant mice [272]. A link between myelination and phosphorylation of NF has been proposed following the analysis of the dysmyelinated mouse mutant Trembler. This mutant revealed a decreased NF phosphorylation correlated with an increased NF density and reduced axonal calibres [108]. Moreover, NFM and NFH sidearms are highly phosphorylated in myelinated axonal segments and poorly phosphorylated in non-myelinated domains like the initial segment and the node of Ranvier where axonal calibre is reduced [108–111]. The axonal radial growth does not require the formation of a compact myelin but only the axon ensheathment by the myelin-forming cell [246], suggesting that a molecule localised in the adaxonal membrane of the glial cell regulates the axonal calibre. Because MAG is enriched in this region and starts to be expressed when the axonal calibre expands, it has been suggested that MAG plays a crucial role for the axonal size expansion [273, 274]. A defect in the radial growth of myelinated axons was observed in MAG-deficient mice, together with a reduction of NF phosphorylation in myelinated fibres [112]. It was proposed that the binding of MAG to axonal receptors activates a signalling cascade leading to the phosphorylation of NF and consequently induces the axonal radial growth in myelinated regions [112, 113, 267, 275]. However, we recently show that the relative extent of calibre reduction at nodes of Ranvier is similar between axons containing or not NF [251], suggesting that NF and consequently their phosphorylation are not responsible for the axonal calibre difference between internodes and nodes. Thus, the axonal size reduction at nodes of Ranvier could result from a constrictive pressure exerted by paranodal loops of the myelinating cells.
Neurofilaments Contribute to the Axonal Conduction Properties
Ultrastructure of myelinated fibres is optimised for maximal conduction velocity through the axonal calibre [276–278], internodal length [279–282], myelin thickness [283–285], as well as geometry and molecular organisation of the nodes of Ranvier [286, 287]. As internodal length and myelin thickness are proportional to the axonal size [288–290], NF are key players for modulating the axonal conduction.
Several animal models with abnormal NF expression or distribution highlighted implication of NF in the conduction properties. Quiver quails expressing a mutated NFL display a reduced conduction velocity proportional to the decrease of the axonal calibre [291]. Similarly, consistent with axonal atrophy, lower conduction velocities are observed in NFL−/− mice and NFM−/− mice [292], in mice expressing human NFH (hNFH mice) [293], in NFMtailΔ mice [267] and in NFH-LacZ transgenic mice [251, 294]. These modifications are not restricted to the conduction velocity but other electrophysiological parameters are also affected in these animals. These include altered auditory evoked potentials in quiver quails [295], prolongation of refractory period in NFM−/− mice [292], decreased resting membrane potential, prolonged duration of action potential and decreased inward and outward rectification in hNFH mice [293], reduced amplitude of the compound action potential and abnormalities of somaesthetic and auditive-evoked potentials in NFH-LacZ mice [251], suggesting multiple implications of NF in conduction. This is reinforced by the fact that conduction velocity, refractory period as well as correlation between the rate of rise and decay of action potential and conduction velocity are significantly modified in NFH−/− mice despite normal axonal diameter, g-ratio and internodal length [292] providing strong evidence that NF are involved in defining not only the structural but also the functional integrity of myelinated axons. Kriz et al. [292, 293] proposed that NFH may have a specific role in modulating ion channel function, but the exact molecular mechanism is still unclear even if it appeared that localisation of Na+ and K+ channels in, respectively, node and juxtaparanode is unaffected by the lack of axonal NF [251]. It should also be mentioned that, in contrast to NFH−/− mice, conduction velocity is not altered in NFHtailΔ mice [267], indicating no implication of NFH sidearm in this parameter. Finally, it cannot be excluded that absence of axonal NF also affects the axoplasmic resistance.
The conduction velocity also depends on myelin thickness and internodal length, which are proportional to axonal diameter. Optimum conduction velocities are achieved when internodal lengths are ∼100 times the axonal calibre and for g-ratio values comprised between 0.6 and 0.7 [281, 283, 285]. To determine whether changes in myelin sheath dimensions can contribute to electrophysiological defects observed in absence of axonal NF, we measured g-ratio and internodal length in NFH-LacZ transgenic mice [251]. It appeared that myelin thickness is differently regulated in CNS and PNS in response to reduced axon calibres. The calibre-reduced axons from NFH-LacZ mice are invested with proportionally thinner myelin in CNS without modifications of the g-ratio, while in PNS axons are overmyelinated compared to their reduced diameter (g-ratio of 0.52 vs. 0.63 in transgenic and control PNS). A similar disparity was reported in NFM−/− and NFH−/− mice [296]. Surprisingly, the axonal atrophy in both CNS and PNS from NFH-LacZ mice does not affect the internodal length. Consequently, internodal lengths are ∼200 times the diameter of NF-deficient axons. According to Rushton [283], such a ratio increases internal resistance and reduces the capacity to activate sodium channels. These results indicate that myelin dimensions are not optimal for conduction in absence of axonal NF.
The geometry and composition of nodes of Ranvier are also crucial for the propagation of the nerve impulse. Both in PNS and CNS, nodes are characterised by an important constriction of the axon reaching approximately 30% to 15% of the internodal size [297, 298]. This constriction promotes higher conduction velocities by reducing the nodal capacity through a smaller nodal area. It also reduces the contribution of the paranodal axolemmal membrane by restricting conductance along the periaxonal pathway [299]. Two models have been considered to explain the nodal constriction [300]. First, the contraction model suggests that myelinating cells exert a pressure via their paranodal loops sufficient to reduce the axonal diameter and/or to limit the axonal radial growth at nodes. The high content of contractile proteins (filamentous actin, myosin) and mitochondria in paranodal loops consolidates this assumption [297, 301]. Secondly, the NF model suggests that reduction of axon calibre at nodes is due to a densely packed hypophosphorylated NF network while larger internodes contain spaced hyperphosphorylated NF [108–111]. However, we recently showed that the extent of axonal constriction at nodes is similar with and without axonal NF [251], indicating that they are not required for the establishment of the nodal ultrastructure and thus arguing in favour of the contraction model.
In conclusion, NFs are crucial for the correct conduction of the nerve impulse and therefore their defects could contribute to neurodegenerative processes. They increase the conduction velocity by promoting the axonal radial growth and are essential to achieve optimal g-ratio and internodal length. On the other hand, they are not necessary for the formation and maintenance of ultrastructure and molecular organisation of nodes of Ranvier.
Axonal Transport of Neurofilaments
Neurons are highly polarised cells and their axonal length can reach more than 1 m in humans. Most proteins are synthesised in cell bodies and transported down the axon through a mechanism called axonal transport. Weiss and Hiscoe [302] demonstrated for the first time this process using ligation of sciatic nerves. This leads to an axonal swelling proximal to the ligation and to an axonal shrinkage in distal region. When ligation is removed, material accumulated in proximal region moved down the nerve at 1 to 2 mm/day. These observations emphasised the existence of a flow of material from the cell body to the nerve terminals. In the 1960s, an important step in characterising the axonal transport was achieved using radiolabelled newly synthesised proteins following injections of [3H] amino acids or [35S] methionine in sciatic nerve or retinal ganglion cells from living animals [303–305]. The distance travelled by labelled proteins showed that the axonal transport is divided in two major categories depending on their speed of transport [306]: the fast axonal transport (~250 to 400 mm/day in mammals) conveys mitochondria, neurotransmitters, channel proteins, lysosomes and endosomes [307–309] and the slow axonal transport (~0.1 to 4 mm/day) conveys axonal cytoskeleton and cytosolic proteins. The slow axonal transport can also be divided into two rate components: the slow component a (0.1–1 mm/day), containing NF and MTs and the slow component b (2–4 mm/day), containing actin, spectrin and other cytoplasmic proteins [154, 310].
It was first speculated that the various rates of transport were due to the association of cargoes with different molecular motors. Members of the kinesin family and cytoplasmic dynein were identified as the major motors responsible for the fast axonal transport in, respectively, the anterograde (toward the terminal end) and retrograde (toward the cell body) directions [311, 312]. However, the identity of motors responsible for the slow axonal transport of NF remained unknown. This was partially elucidated by two studies analysing the transport of green-fluorescent-protein (GFP)-tagged NF subunits transfected into cultured sympathetic neurons [313, 314]. Surprisingly, the authors observed that both GFP-NFM and GFP-NFH move at rates of up to 1 μm/s, corresponding to the rates of molecular motors ensuring the fast axonal transport. This suggests that motors used for slow and fast axonal transport are identical. However, contrary to fast axonal transport of cargoes that moved continuously, NFs are transported intermittently in axons because their fast movements are interrupted by prolonged pauses. Only a small fraction of NF is moving at any given time since it was evaluated that NF spent 97% of their time pausing [315]. Thus, the overall speed of axonal transport would not depend on the motors involved but on the duration of association between cargoes and motors. Components moving with the fast axonal transport could be attached to the motor for a long period while interaction between components moving with the slow axonal transport and their motors would be short. Another interesting discovery emerging from these studies is the description of a bi-directional transport of the NF but with a predominant anterograde prevalence. Similar results were obtained with extruded squid axoplasm [316] and along MTs in vitro [222]. Altogether, these data indicate that the overall slow axonal transport of NF is the result of a combination between fast bi-directional movements and long-lasting pauses.
The implication of kinesin and dynein in axonal transport of the NF was later confirmed by various observations. First, several evidences suggest a direct interaction between NF and kinesin or dynein [220–223, 317]. The inhibition or depletion of dynein [225, 318] affect the axonal transport of NF and lead to their proximal accumulation, while microinjection of anti-kinesin and anti-dynein antibody affect anterograde and retrograde NF transport in cultured dorsal root ganglia neurons [224]. Finally, targeted disruption of neuronal kinesin heavy chain KIF5A induces the accumulation of NF in cell bodies of peripheral sensory neurons [319], suggesting that KIF5A may be a NF motor.
Millecamps et al. [156] have provided new insights into the mechanisms of axonal transport of NF. As described above, they generated transgenic mice with doxycycline-controlled expression of hNFL, with or without endogenous mouse NFL proteins (respectively, tTA;hNFL;NFL+/− and tTA;hNFL;NFL−/− mice). They showed that the presence of the axonal NF array strongly slows down the axonal transport of NF. Indeed, when doxycycline treatment of tTA;hNFL;NFL−/− mice is stopped; the reappearance of hNFL occurred in synchrony along the sciatic nerve within 1 week, indicating a fast axonal transport of NF in axons deprived of stationary NF network. They estimated a rate of ~10 mm/day in axons with low NF content compared to ~1 mm/day in axons with a dense NF array. The authors suggested that these differences could be due to a decreased number of pauses during the travel of NF and/or to decreased interactions with a stationary pool.
The forms in which NF are transported (subunits or polymers) and the contribution of their phosphorylation were sources of intense investigations for many years. One model suggested that NF polymerise in cell bodies immediately after synthesis, and they are subsequently transported as filaments. On the opposite, the subunit model proposed that polymers are essentially stationary and that NF could be transported as free subunits or small oligomers that integrate stationary cytoskeletal polymers. Both hypotheses were based on various observations (for review, see [320, 321]). For example, the metabolic labelling of cytoskeletal proteins with [35S] methionine shows a synchronised movement of the three NF subunits, in favour of the polymer transport model [154, 322]. Moreover, the majority of NF in axons is polymers and it is difficult to detect free monomers. Conversely, in fluorescence recovery after photobleaching experiments, no movement of the bleached area was observed and the recovery of fluorescence is gradual [323], supporting the subunit transport hypothesis. In the same way, the existence of a subunit–small oligomer transport is suggested by the finding that NFM is transported along axons after injection of a recombinant adenovirus encoding tagged NFM protein in transgenic mice deprived of axonal NF [324], even if a diffusion of this protein cannot be completely excluded [325]. An important advance was made by analysing the transport of GFP-NFM and GFP-NFH in transfected neurons [313, 314]. In these studies, intact filaments (1–15 μm of length) represent 95% of the moving structures observed, strongly supporting the polymer model. The transport of NF polymers was recently confirmed by Yan and Brown [326] who showed that NF move in the form of assembled polymers in axons of cultured neonatal mouse sympathetic neurons and, more interestingly, that moving and stationary NF are complex heteropolymers also containing peripherin and α-internexin along >85% of their length [327]. Yuan et al. [328] also demonstrated that α-internexin is a key determinant for the axonal transport of NF in CNS. They observed that NFM can be transported into axons of the optic nerve in absence of NFL and NFH, while the additional deletion of α-internexin abolishes this transport, indicating that NFM monomers alone are not efficiently transported. The finding that a small number of IFs are present in optic axons from NFL−/−;NFH−/− mice but not from α-internexin−/−;NFL−/− mice suggests that NFM is able to associate with α-internexin to form IFs. The co-localisation of α-internexin and NFM on the same filament [12] reinforced this assumption. Thus, it seems that hetero-oligomer composed of at least NFM and α-internexin is the minimal form in which NF proteins can be transported in CNS axons. It is also interesting to note that the deletion of NFH or α-internexin does not affect the NF transport [266, 328] while the loss of both proteins causes the selective acceleration of the axonal transport of NFL and NFM subunits, suggesting redundant roles of α-internexin and NFH in axonal transport of NF.
Another controversial subject concerns the role played by phosphorylation in the axonal transport of NF. Phosphorylation of NF, in particular NFH, has long been considered to decrease their transport rate. The entry of NFH into axons during development coincides with reduced axonal transport of NF [51, 329]. Moreover, the phosphorylation level of NF correlates with a slow transport of radiolabelled NF in projections of the rat L5 dorsal root ganglion [100], while hypophosphorylated NF in mouse optic axons move faster than hyperphosphorylated ones [101]. Similarly, lack of NFH accelerates NF transport [258], while increased NFH expression selectively slows NF transport [265]. Finally, NF containing NFH mutated to generate constitutively non-phosphorylated NFH move faster than those containing wild-type NFH, while NF containing NFH mutated to generate constitutively phosphorylated NFH move slower [102]. All these studies support the view that the rate of NF transport is inversely correlated to their phosphorylation state. However, the analysis of NF axonal transport in optic nerve from NFMtailΔ mice and NFHtailΔ mice shows no modification in the rate of NF transport [266, 268, 330] suggesting that phosphorylation of NFM and NFH sidearm is not directly involved in this process. Alternatively, a yet unknown molecule could bind differently to these mutated NF and may modulate their transport. It should also be noted that phosphorylation of both NFM and NFH subunits are tightly regulated. Indeed, phosphorylation of NFM is significantly increased in NFH−/− mice and in NFHtailΔ mice [258, 266] while an increased phosphorylation of NFH is observed in NFM−/− mice and in NFMtailΔ mice [62, 268]. The measure of NF transport in NFMtailΔ mice and NFHtailΔ mice could thus be biased by this compensatory phosphorylation. It would be interesting to study the NF transport in double transgenic NFMtailΔ/NFHtailΔ mice in order to answer this question.
Several possibilities could explain how phosphorylation regulates the axonal transport of NF. One main observation is that phosphorylation of NF controls their association with molecular motors. In particular, phosphorylation of NF promotes their release from kinesin [221, 317] and increase their affinity for dynein [225]. Consequently, the phosphorylation of NF would cause their slowing by reducing their anterograde transport by the kinesin and by supporting their retrograde transport by the dynein. Thus, the long pauses during their travel would correspond to the moments when NFs are dissociated from their motors. This model is consistent with the intermittent movement of NF into axons [313, 314] and with mathematical modelling of NF axonal transport [331]. Future investigations should focus on determining the precise nature of the motors that drive the movements of NF and the exact function of phosphorylation as well as signals and pathways regulating it. It is particularly important to determine which carboxy-terminal phosphorylation sites are involved in this process and whether the phosphorylation sites located in NF head domains, which are involved in NF assembly, also play a role in their transport.
Neurofilaments and Pathologies
The discovery of mutations in NF proteins associated with several neurodegenerative diseases argues for the participation of NF in these pathologies. Moreover, abnormal accumulations of NF are a pathological hallmark of many human neurodegenerative disorders, including ALS, AD, PD, CMT, giant axonal neuropathy (GAN), dementia with Lewy bodies, spinal muscular atrophy, progressive supranuclear palsy and diabetic neuropathy. Multiple factors can potentially induce the accumulation of NF, including dysregulation of NF gene expression, NF mutations, defective axonal transport, abnormal post-translational modifications and proteolysis. Here, we will review some neurodegenerative disorders that involve NF abnormalities and toxic agents responsible for NF accumulations.
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis, also called Lou Gehrig’s disease, is a late-onset progressive motor neuron disease characterised by intraneuronal aggregates of NF, termed spheroids, in affected neurons [332–337]. About 90% of ALS cases are sporadic while approximately 10% are inherited in a dominant manner. Although the mechanisms leading to the accumulation of NF in ALS remain unclear, the overexpression of NFL [338], NFM [264] or hNFH [250] provokes NF aggregations and morphological alterations similar to those found in ALS. Remarkably, the motor neuron disease caused by excess hNFH proteins can be rescued by overexpression of human NFL in a dosage-dependent fashion [255], suggesting the importance of subunit stoichiometry in ALS pathogenesis. The 70% reduction of NFL mRNA in degenerating neurons of ALS [339, 340] reinforces this assumption. Evidence that aberrant NF accumulation can contribute to the neuronal death came from the observation that the expression of a mutated NFL subunit causes the aggregation of NF leading to a selective degeneration of spinal motor neurons and to a severe atrophy of skeletal muscles [341]. Codon deletions or insertions in the KSP repeat motifs of NFH have been identified in unrelated patients with sporadic ALS [342–344]. However, two others studies failed to identify variants in the NF genes linked to sporadic and familial ALS [345, 346]. Together, these data suggest that mutations in the NF genes are not a systematic common cause of ALS but could be a risk factor for sporadic ALS.
Mutations in SOD1, the most abundant cytosolic enzyme, account for 20% of all the familial cases. As for ALS patients, mice expressing mutant SOD1 display NF accumulation [347, 348] and exhibit a phenotype similar to that of mice overexpressing NFL or hNFH. Moreover, axonal transport of NF is perturbed in SOD1G37R [348, 349], SOD1G85R [349] and SOD1G93A [350] mice. To determine whether NFs are involved in SOD1-mediated disease, mice expressing mutant SOD1 were mated with transgenic mice with altered NF protein content. The withdrawal of NF from the axonal compartment and their perikaryal accumulation induced by the expression of NFH-β-galactosidase fusion protein conferred no beneficial effect in SOD1G37R [232], indicating that axonal NF are not necessary for SOD1-mediated disease. This was also confirmed in SOD1G85R deprived of NFL but the absence of axonal NF in these mice prolongs their life span by approximately 15% [351]. Surprisingly, overexpression of mouse NFL or mouse NFH in SOD1G93A mice [352] and overexpression of hNFH in SOD1G37R mice [353] also increase their life span by, respectively, 15% and 65%. This suggests a protective effect of perikaryal accumulation of NF proteins in motor neuron disease caused by mutant SOD1. However, the mechanism of protection is still unclear, even if it seems that perikaryal accumulation of NF proteins rather than axonal NF deficiency is responsible for slowing disease in these models. Indeed, the formation of large perikaryal aggregates and the massive depletion of axonal NF due to the expression of the human NFH43 allele cause more positive effects than human NFH44 allele which induces smaller aggregates and more axonal NF [353]. Moreover, the disruption of one allele for each NF gene induces a 40% decrease of axonal NF proteins content and an important axonal atrophy without perikaryal accumulation of NF in SOD1G37R mice but does not extend their life span nor does it alleviate the loss of motor axons [254].
Several hypotheses were proposed to explain this protective effect of perikaryal aggregates in SOD1-mediated disease. Thanks to their multiple calcium-binding sites, NF proteins may act as calcium chelators. According to this, a significant neuroprotection was obtained by overexpressing the calcium-binding protein calbindin-D28k in cultured motor neurons [354]. Ehlers et al. [355] have shown that NFs are involved in localisation of N-methyl d-aspartate (NMDA) receptors in the neuronal plasma membrane by interacting with the NMDA NR1 subunit. It cannot thus be excluded that accumulation of NF can interfere with glutamate receptor function and prevent glutamate excitotoxicity. Finally, Nguyen et al. [356] proposed that perikaryal accumulations of NF in motor neurons may alleviate ALS pathogenesis by acting as a phosphorylation sink for cyclin-dependent kinase 5 dysregulation induced by mutant SOD1, thereby reducing the detrimental hyperphosphorylation of tau and other neuronal substrates. This was supported by the fact that NF accumulations contain hyperphosphorylated NFM and NFH subunits in ALS patients [357] and in SOD1 mutant mice [347, 358] while they are normally not phosphorylated in cell bodies. However, removal of NFM and NFH sidearms led to a delay of disease in SOD1 mutant mice rather than the acceleration predicted by a kinase dysregulation model [359], indicating that perikaryal phosphorylation of NF is not an essential mechanistic contributor to reduced toxicity of SOD1 mutants. Alternatively, NF removal from the axonal compartment contributes to a more flexible axoplasm capable of enhancing axonal transport which is impaired in SOD1 mice [349, 360].
Peripherin, a type III IF protein, is also a component of spheroids in ALS [189, 347, 361]. The overexpression of wild-type peripherin in mice led to the formation of cytoplasmic protein aggregates and induces the selective loss of motor neurons during ageing [362, 363]. Because NFL mRNA levels are reduced in cases of ALS [339, 340], Beaulieu et al. [362] generated double transgenic mice overexpressing peripherin and deficient for NFL (Per;NFL−/− mice). They observed that the onset of peripherin-mediated disease is accelerated by the deficiency of NFL. In absence of NFL, peripherin interacts with NFM and NFH to form disorganised IF structures [11]. This could explain why the number of IF inclusion bodies is increased in Per;NFL−/− mice, leading to an earlier neuronal death. In contrast, peripherin toxicity can be attenuated by co-expression of NFL [364], illustrating the importance of IF protein stoichiometry. Another example of protection came from the generation of double transgenic mice overexpressing both peripherin and NFH in which peripherin-mediated disease is completely abolished [365]. Excess of NFH shifted the intracellular localisation of inclusion bodies from the axonal to the perikaryal compartment of motor neurons, suggesting that the toxicity of peripherin inclusions may be related to their axonal localisation, possibly by blocking the axonal transport. All these results underline the crucial role of IF protein stoichiometry in the formation, localisation and toxicity of neuronal inclusion bodies in ALS.
Alzheimer Disease
AD is the most common type of dementia characterised by progressive cognitive deterioration and excessive loss of memory, together with declining activities and behavioural changes. One major neuropathological change occurring in affected neurons of AD patients is the perikaryal formation of NFTs composed of tau, NF and other cytoskeleton proteins [366, 367]. It is still controversial whether NFTs represent a primary causative factor in AD. Moreover, the mechanism responsible for NFT formation is not completely understood, even if it seems that dysregulation of tau metabolism is more involved than alterations of NF proteins [368]. NF in NFT appear as paired helical filaments and differ from normal NF in their resistance to solubilisation by detergents [369], suggesting that they form highly compacted structures. Another characteristic of NF in NFT is their extensive phosphorylation [370–372], probably caused by a down-regulation of protein phosphatase 2A [373–376]. Deng et al. [377] suggested that the hyperphosphorylation and accumulation of NF in AD brain might be caused by impaired brain glucose uptake–metabolism. Indeed, they observed in AD brain and in a rat model mimicking the decreased glucose uptake–metabolism observed in brains of patients with AD a decrease in O-GlcNAcylation and an increased phosphorylation level of KSP repeats from NFM. They also showed that O-GlcNAcylation and phosphorylation of NFM are reciprocally regulated in cultured neuroblastoma cells and in metabolically active rat brain slices, providing a new mechanism of regulation of NF phosphorylation and a possible explanation on the hyperphosphorylation and accumulation of NF in AD brain.
Parkinson Disease
PD is a progressive disorder of the CNS affecting dopaminergic neurons of the substantia nigra. A neuropathological hallmark of PD is the formation of ubiquitinated protein inclusions named Lewy bodies, composed of α-synuclein, NF proteins, ubiquitin and proteasome subunits [378–382]. NF in Lewy bodies undergo inappropriate phosphorylation and proteolysis [383, 384] and NFL and NFH mRNAs are decreased in PD [190].
Mutations in the parkin gene is the major cause of familial PD [385–387] but a point mutation in the NEFM gene was also reported in a French–Canadian patient who developed the disease at the age of 16 [388]. This mutation consists in a substitution of Ser for Gly at residue 336, a highly conserved region in the rod domain 2B of NFM. However, three other family members also carried the G336S mutation but are unaffected, arguing against the implication of NFM mutation in pathogenesis of PD. Moreover, the G336S mutation does not affect the assembly and the distribution of NF in vitro [389]. The screening of 102 French–Canadian PD patients failed to identify this mutation, indicating that G336S mutation is not common even in a PD population of similar ethnic background and does not play a major role for the development of PD [390]. Similarly, G336S mutation was never found in 322 sporadic and familial PD patients of German origin [391]. Nevertheless, this study identified in NEFM gene from two patients a Pro-to-Gln substitution at residue 725 and a deletion of Val in position 829, two highly conserved sites. Finally, no mutation of the NEFL gene has been identified in 328 sporadic and familial PD patients [392]. These results suggest that mutations in NF genes are not a primary cause of PD even if the rare variants of the NEFM gene identified may act as susceptibility factors for PD.
Charcot-Marie-Tooth
CMT is the most common form of hereditary peripheral neuropathy affecting both sensory and motor nerves [393]. Patients with CMT progressively develop a weakness of muscles and become unable to walk. CMT neuropathies are classified into several categories, including CMT1, CMT2, CMT3, CMT4 and CMTX. Types 1, 3, 4 and X are demyelinating whereas type 2 is an axonal neuropathy. Accumulation of NF in CMT2 was reported for the first time by Vogel et al. [394]. Several mutations of NEFL gene, located throughout the three functional domains of the NFL protein (head, rod and tail), are associated with CMT type 2E or CMT type 1F. The first mutation was identified by Mersinayova et al. [395] in a large Russian family with CMT2. This missense mutation in the rod domain 2B consists by the substitution of a highly conserved Gln for Pro at position 333 (Q333P). The second reported mutation was another substitution in the non-helical head domain of NFL (Pro to Arg at residue 8) found in members of a Belgian family with a severe CMT phenotype [396]. The expression of NFLP8R or NFLQ333P in cultured cells disrupts both NF assembly and axonal transport of NF and induces the accumulation of mitochondria in cell bodies and proximal axons [397–399]. These mutations also affect anterograde and retrograde fast axonal transport and cause fragmentation of the Golgi apparatus and degeneration of neuritic processes in culture neurons [399], providing possible mechanisms by which these mutants could be involved in axonal degeneration and CMT pathogenesis.
The Pro at codon 22 is also the target of several mutations. A Pro-to-Ser substitution (P22S) was observed in nine members of a large Slovenian CMT2 family, with a complete co-segregation between this mutation and the dominantly inherited CMT2 phenotype [400]. P22S mutation was also detected in an Italian family. Examination of nerve biopsies in these patients revealed a primary axonopathy characterised by giant axons with swellings composed almost entirely of aggregated NF [401]. A Pro-to-Thr substitution (P22T) was detected in unrelated Japanese patients with CMT disease [402]. P22 NFL mutant proteins are unable to assemble into filaments and form aggregates in vitro [126, 399]. It was shown that P22S and P22T mutations abolish the phosphorylation of the adjacent Thr21 which normally inhibits filament assembly [126] and thus could explain the formation of NF aggregates by these mutants. It is interesting to note that phosphorylation of NFL head domain by PKA alleviates aggregates in cortical neurons, providing a potential therapeutic approach to dissociate NF aggregates in CMT [126].
Thereafter, other mutations of NEFL gene were reported in cases of CMT. Jordanova et al. [403] screened 323 patients with CMT or related peripheral neuropathies and identified six disease-associated missense mutations and one 3-bp in-frame deletion in the NEFL gene. In the same way, Fabrizi et al. [404] screened 177 patients and identified four mutations in the head and rod domains of NFL, including a novel Leu268Pro substitution and a novel del322Cys_326 Asn deletion. The majority of these mutants form aggregates, except E7K and D469N. The harmful effect of NFL mutations could produce defect in axonal transport after the formation of large accumulation of NF in cell bodies and axons. The first duplication–insertion mutation of NFL in a patient with CMT was reported by Leung et al. [405]. Unlike other NFL mutations inducing the formation of NF aggregates and a blockade of their axonal transport, this mutation appeared to provoke neuronal degeneration probably through both aggregation and de-stabilisation of the neuronal IF network.
Goryunov et al. [406] showed that mutations of myotubularin-related protein 2, which lead to the CMT4B form, can cause NFL aggregation in culture cells, indicating that mutation of NFL is not necessary to induce NF aggregations in CMT. Finally, an implication of NF in demyelinating CMT cannot be excluded. Nerves from patients expressing NFLL268P or NFLE89K show evidence of Schwann cell abnormalities [403, 404] and abnormalities of NF phosphorylation occur in demyelinated axonal segments [407].
Neuronal Intermediate Filament Inclusion Disease
Neuronal intermediate filament inclusion disease (NIFID), also called neurofilament inclusion body disease or neurofilament inclusion disease, is a recently described neurological disorder of early onset with a heterogeneous clinical phenotype, including fronto-temporal dementia and pyramidal and extrapyramidal signs [408–410]. The symptoms comprise behavioural and personality changes and, less often, memory loss, cognitive impairment, language deficits and motor weakness [411]. The pathological phenotype consists in neuronal loss, gliosis, swollen neurons and presence of large IF inclusions in the cell body of many neurons. These inclusions contain neither tau nor α-synuclein [408–410] but are rich in NF triplet proteins (phosphorylated and unphosphorylated epitopes) and especially α-internexin [412, 413]. The number of IF aggregates is higher in areas with little neuronal loss and lower in sites of intense neuronal degeneration. Cairns et al. [412] proposed that the formation of these inclusions is an early event in the pathogenesis of NIFID and that these aggregates are released and degraded into the extracellular space following degeneration of the neuron. The precise mechanism leading to the formation of these aggregates is unknown, but defect in axonal transport and/or gene expression may be involved. A mutation analysis of patients with NIFID revealed no pathogenic variants for all type IV neuronal IF, SOD1 and NUDEL [414]. The role of these IF inclusions in the pathogenesis of NIFID also remains to be elucidated.
Diabetic Neuropathy
Diabetes is a disease in which low levels of insulin or abnormal resistance to insulin’s effects induce perturbed glucose metabolism and inappropriately high blood sugar. Diabetic neuropathy is a peripheral nerve disorder caused by diabetes affecting principally sensory nerves and dorsal root ganglia and characterised by slowing conduction velocity, impairment of axonal transport, axonal atrophy and a reduced capacity for nerve regeneration. All these features of nerve function depend on the integrity of the axonal cytoskeleton and in particular on the NF network. Consistent with this, multiple abnormalities of NF biology have been identified in models of diabetes. Medori et al. [415, 416] observed in rats with streptozotocin-induced diabetes and in BioBreeding rats (a model of spontaneous type I diabetes) an impairment of the axonal transport of NF, actin and tubulin concomitant with a proximal increase and a distal decrease of axonal cross-sectional area. The distal axonal atrophy is accompanied by an important loss of NF in this region [417]. Accumulations of highly phosphorylated NF epitopes are present in proximal axonal segments of dorsal root ganglia sensory neurons from diabetic patients [418]. An increase of NF phosphorylation, correlated with activation of JNK, was also detected in lumbar dorsal root ganglia from rat models [419]. Finally, significantly decreased mRNA levels for the three NF subunits as well as reduced NF numbers and densities within large myelinated sensory axons were reported in long-term diabetic models [420]. All these results suggest that NF abnormalities may contribute to the development of diabetic neuropathy or may be affected by this disease.
However, slowing of conduction velocity in diabetic models occurs much earlier than loss of NF investment or axonal atrophy [420]. To further elucidate the contribution of NF in diabetic neuropathy pathogenesis, Zochodne et al. [294] analysed the effect of streptozotocin-induced diabetes in transgenic NFH-LacZ mice characterised by NF-deficient axons. An accelerated diabetic neuropathy was observed in these mice. Indeed, superimposing diabetes on axons without NF was associated with an earlier reduction of both conduction velocity and nerve action potential amplitudes and increased axonal atrophy. This indicates that changes in NF expression, transport or post-translational modifications cannot account alone for conduction slowing and atrophy in diabetic neuropathy, but their presence may help axons to resist diabetic damage.
Giant Axonal Neuropathy
GAN is a rare progressive neurodegenerative disorder affecting both PNS and CNS which generally appears in infancy or early childhood [421, 422]. First signs of GAN usually begin in the PNS but as the disorder progresses the CNS becomes involved, causing a progressive decline in mental function, loss of control of body movement and seizures. GAN is caused by mutations in the GAN gene, which codes for the gigaxonin [423]. The major cytopathological hallmark of GAN is the presence of masses of NF producing focal enlargements in the distal regions of axons associated with a reduced number of MTs [424]. In contrast, axonal segments proximal to the swellings exhibit a reduction in number of NF [425]. Disorganisation and accumulation of other types of IFs are also found in skin fibroblasts, Schwann cells and muscle fibres [426–430]. The minimal distance separating NF in sural nerve axons of a patient with GAN is decreased compared to controls and, more surprisingly, the mean diameter of NF is increased (12.4 nm in GAN compared with 10.1 nm in controls) [431]. The mechanism of distal axonal accumulation of NF is still unclear. An acceleration of their axonal transport was observed in optic nerve from experimentally induced GAN rat model, concomitant with a proximal decreased content of NF and their distal accumulation [432]. The authors proposed that acceleration of NF transport in the presence of a normal rate of NF protein synthesis and insertion into transport system would lead to the formation of distal axonal swellings with packed NF.
To determine how disruption of gigaxonin’s function leads to GAN, Ding et al. [433] generated gigaxonin-deficient mice. Despite the development of a progressive deterioration for motor function, fertility and life span of these mice are normal and giant axons are never seen. However, these animals display enlarged axons with densely packed NF, leading to the segregation of axonal organelles, a feature characteristic of human GAN pathology. This is accompanied by an axonal loss at the age of 9–12 months. Gigaxonin can interact with the light chain of MAP1B [434], tubulin folding cofactor B (TBCB, [435]) and with MAP8 [433] and can control their ubiquitin-mediated degradation. GAN-associated mutations of gigaxonin perturb its association with MAP1B-LC, TBCB and MAP8 while gigaxonin ablation results in the accumulation of these partners [433, 435, 436]. These data suggest a crucial role of gigaxonin in the maintenance of a normal cytoskeleton network. However, the exact implication of cytoskeleton abnormalities on neurodegeneration in GAN remains to be established.
Toxic Agents that Disorganise the Neurofilament Network
IDPN is a toxin that segregates MTs from NF and thereby causes their abnormal accumulation. Its administration results in severe reduction or blockade of NF transport [437], with NF accumulation in the proximal region of axons [192] and segregation of NF and MTs distal to this region [194]. The aggregation of NF occurs through abnormal cross-linking of hyperphosphorylated NF [438]. The susceptibility of various neurons to these effects depends on their NF content; NF-rich large-calibre axons being the most affected [439]. Unlike normal mice, NFH-null mutant mice do not develop swellings of motor axons when treated with IDPN [258], demonstrating that NFH protein is a key mediator of IDPN-induced axonopathy.
The injection of aluminium chloride in spinal cord of rabbits causes the formation of NF tangles in neuronal perikarya and proximal parts of dendrites [193]. NF transport is maintained in the distal region of the axon, resulting in lack of NF in axonal segments immediately distal to the block [179]. NFs sequestered in cell bodies are highly phosphorylated [440], which may account for their abnormal axonal transport. Moreover, aluminium inhibits NF degradation and dephosphorylation [181, 441, 442] and reduces the assembly of newly synthesised NF subunits into NF [443].
The effects of acrylamide are very similar to those of aluminium. During the development of experimental acrylamide neuropathy, NF transport is inhibited and NFs accumulate in proximal axons with formation of axonal swellings [195]. The administration of acrylamide also increases the expression of mRNA for NF proteins and the phosphorylation of NF in rats [444–446] but decreases their degradation [447]. However, acrylamide-induced neurotoxicity is not initiated exclusively through its action on axonal NF because NF-deficient mutant quiver quails, crayfish (a species lacking NF) and NFH-LacZ mice are sensitive to neurotoxic effects of acrylamide [448–450].
Arsenic also disrupts the organisation of NF network. Protein analysis of sciatic nerves from rats treated with arsenite (the inorganic form of arsenic) showed disappearance of NF [451]. DeFuria and Shea [452] demonstrate that arsenite decreases NF transport and induces the perikaryal accumulation of phosphorylated NF in NB2/d1 cells and in cultured dorsal root ganglion neurons. These effects were prevented by inhibiting JNK and GSK-3β.
Finally, lead exposure during the development of a chicken model of auditory temporal processing results in decreased amount and phosphorylation of NFM within the axons connecting auditory nuclei in the avian brainstem [453]. During the mouse development, lead exposure increases phosphorylation of both NFM and NFH within auditory brainstem nuclei. Moreover, neuritic beadings immuno-labelled for NF are observed following lead exposure both in vivo and in vitro, suggesting an impairment of the axonal transport [454].
Conclusion
This review attempted to summarise current knowledge about NF biology. These last years, our understanding of the regulation, structure and functions of NF in normal and pathological conditions has been considerably improved by using transgenic mice models. However, several aspects of the NF biology remain elusive and further investigations are required to solve these issues. While it is clearly established that NF play a central role in the growth and maintenance of the axonal calibre, the exact mechanism employed is still unclear. In particular, it remains to elucidate why sidearms from NFM contribute more than those of NFH in the axonal radial growth, how the axonal calibre is modulated along its length, and how myelinating cells regulate the axonal architecture.
The recent development of techniques such as time-lapse imaging coupled with the use of NF proteins tagged with fluorescent probes allowed important advances in characterising the axonal transport of NF. It is now well accepted that kinesin and dynein convey NF, but the mechanisms regulating the interaction of NF with these motor proteins or with the stationary pool of NF should help to clarify how the axonal transport of NF is regulated. It also appears that α-internexin is an integral component of NF and participates in their axonal transport in CNS. Whether peripherin plays an analogous role in PNS remains to be determined. Finally, it will be crucial to understand how the normal or disorganised NF networks interact and possibly affect the fast axonal transport in order to evaluate to which extent abnormal accumulations of NF contribute to the neurodegenerative processing observed in human neuropathologies.
Discoveries of NF mutations associated with several neurodegenerative diseases as well as the production of various mouse models provide promising avenues to understand the possible implication of NF in these pathologies. However, it appeared that NF aggregation can play both detrimental and beneficial functions. While perikaryal accumulations are generally well tolerated, axonal aggregates are often toxic. It will be important to elucidate the reasons of this discrepancy and the pathways involved in the toxic effects of axonal NF aggregates. A possibility would be that axonal neurofilamentous aggregations affect more profoundly the fast axonal transport than perikaryal aggregations by altering the microtubular assembly together with their associated proteins as observed with STOP proteins aggregated with NF in axonal spheroids in ALS patients. Finally, recent findings highlighted that perturbations in post-transcriptional regulation of NF expression could also play a role in neurodegeneration by affecting the NF homeostasis in neurons. The identification and characterisation of new NF RNA binding proteins and their functions and regulation represent challenging issues. Alternatively, the presence of NF fragments resulting from abnormal degradation of NF following their aggregation could also affect the axoskeleton dynamics. These possibilities represent promising research areas to explore the implication of NF accumulations routinely observed in neurodegenerative diseases.
Abbreviations
- AD:
-
Alzheimer’s disease
- ALS:
-
amyotrophic lateral sclerosis
- BPAG1n:
-
bullous pemphigoid antigen 1 neural isoform
- CaMKII:
-
calcium–calmodulin-dependent protein kinase II
- CKI/II:
-
casein kinase I and II
- CMT:
-
Charcot-Marie-Tooth disease
- CNS:
-
central nervous system
- GAN:
-
giant axonal neuropathy
- GSK3:
-
glycogen synthetase kinase 3
- IDPN:
-
β, β‘-iminodipropionitrile
- IF:
-
intermediate filaments
- JNK1/3:
-
c-Jun N-terminus kinase 1 and 3
- KSP:
-
lysine–serine–proline
- MAG:
-
myelin-associated glycoprotein
- MAP:
-
microtubule-associated protein
- MF:
-
microfilaments
- MT:
-
microtubules
- NF:
-
neurofilaments
- NFH:
-
heavy neurofilament subunit
- NFL:
-
light neurofilament subunit
- NFM:
-
medium-sized neurofilament subunit
- NFT:
-
neurofibrillary tangles
- NIFID:
-
neuronal intermediate filament inclusion disease
- O-GlcNAc:
-
O-linked N-acetyl glucosamine
- PD:
-
Parkinson’s disease
- PKA:
-
protein kinase A
- PKC:
-
protein kinase C
- PKN:
-
protein kinase N
- PNS:
-
peripheral nervous system
- PP2A:
-
phosphatase 2A
- SAPK:
-
stress-activated protein kinase
- SC:
-
slow component
- SOD1:
-
superoxide dismutase 1
- STOP:
-
stable tubule only polypeptide
- TBCB:
-
tubulin folding cofactor B
References
Ishikawa H, Bischoff R, Holtzer H (1968) Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol 38:538–555
Portier MM, de Nechaud B, Gros F (1983) Peripherin, a new member of the intermediate filament protein family. Dev Neurosci 6:335–344
Kaplan MP, Chin SS, Fliegner KH, Liem RK (1990) Alpha-internexin, a novel neuronal intermediate filament protein, precedes the low molecular weight neurofilament protein (NF-L) in the developing rat brain. J Neurosci 10:2735–2748
Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60:585–595
Julien JP, Mushynski WE (1998) Neurofilaments in health and disease. Prog Nucleic Acid Res Mol Biol 61:1–23
Izmiryan A, Cheraud Y, Khanamiryan L, Leterrier JF, Federici T, Peltekian E, Moura-Neto V, Paulin D, Li Z, Xue ZG (2006) Different expression of synemin isoforms in glia and neurons during nervous system development. Glia 54:204–213
Nixon RA, Shea TB (1992) Dynamics of neuronal intermediate filaments: a developmental perspective. Cell Motil Cytoskeleton 22:81–91
Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U (2007) Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol 8:562–573
Kim S, Coulombe PA (2007) Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev 21:1581–1597
Oshima RG (2007) Intermediate filaments: a historical perspective. Exp Cell Res 313:1981–1994
Beaulieu JM, Robertson J, Julien JP (1999) Interactions between peripherin and neurofilaments in cultured cells: disruption of peripherin assembly by the NF-M and NFH subunits. Biochem Cell Biol 77:41–45
Yuan A, Rao MV, Sasaki T, Chen Y, Kumar A, Veeranna, Liem RK, Eyer J, Peterson AC, Julien JP, Nixon RA (2006) Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J Neurosci 26:10006–10019
Morris JR, Lasek RJ (1982) Stable polymers of the axonal cytoskeleton: the axoplasmic ghost. J Cell Biol 92:192–198
Geisler N, Weber K (1981) Self-assembly in Vitro of the 68,000 molecular weight component of the mammalian neurofilament triplet proteins into intermediate-sized filaments. J Mol Biol 151:565–571
Liem RK, Hutchison SB (1982) Purification of individual components of the neurofilament triplet: filament assembly from the 70 000-Dalton subunit. Biochemistry 21:3221–3226
Gardner EE, Dahl D, Bignami A (1984) Formation of 10-nanometer filaments from the 150 K-Dalton neurofilament protein in vitro. J Neurosci Res 11:145–155
Hisanaga S, Hirokawa N (1988) Structure of the peripheral domains of neurofilaments revealed by low angle rotary shadowing. J Mol Biol 202:297–305
Hisanaga S, Hirokawa N (1990) Molecular architecture of the neurofilament. II. Reassembly process of neurofilament L protein in vitro. J Mol Biol 211:871–882
Carter J, Gragerov A, Konvicka K, Elder G, Weinstein H, Lazzarini RA (1998) Neurofilament (NF) assembly; divergent characteristics of human and rodent NF-L subunits. J Biol Chem 273:5101–5108
Jacomy H, Zhu Q, Couillard-Despres S, Beaulieu JM, Julien JP (1999) Disruption of type IV intermediate filament network in mice lacking the neurofilament medium and heavy subunits. J Neurochem 73:972–984
Lee MK, Xu Z, Wong PC, Cleveland DW (1993) Neurofilaments are obligate heteropolymers in vivo. J Cell Biol 122:1337–1350
Scott D, Smith KE, O’Brien BJ, Angelides KJ (1985) Characterization of mammalian neurofilament triplet proteins. Subunit stoichiometry and morphology of native and reconstituted filaments. J Biol Chem 260:10736–10747
Geisler N, Kaufmann E, Fischer S, Plessmann U, Weber K (1983) Neurofilament architecture combines structural principles of intermediate filaments with carboxy terminal extensions increasing in size between triplet proteins. Embo J 2:1295–1302
Julien JP, Mushynski WE (1982) Multiple phosphorylation sites in mammalian neurofilament polypeptides. J Biol Chem 257:10467–10470
Julien JP, Mushynski WE (1983) The distribution of phosphorylation sites among identified proteolytic fragments of mammalian neurofilaments. J Biol Chem 258:4019–4025
Angelides KJ, Smith KE, Takeda M (1989) Assembly and exchange of intermediate filament proteins of neurons: neurofilaments are dynamic structures. J Cell Biol 108:1495–1506
Heins S, Wong PC, Muller S, Goldie K, Cleveland DW, Aebi U (1993) The rod domain of NF-L determines neurofilament architecture, whereas the end domains specify filament assembly and network formation. J Cell Biol 123:1517–1533
Balin BJ, Clark EA, Trojanowski JQ, Lee VM (1991) Neurofilament reassembly in vitro: biochemical, morphological and immuno-electron microscopic studies employing monoclonal antibodies to defined epitopes. Brain Res 556:181–195
Balin BJ, Lee VM (1991) Individual neurofilament subunits reassembled in vitro exhibit unique biochemical, morphological and immunological properties. Brain Res 556:196–208
Hirokawa N, Glicksman MA, Willard MB (1984) Organization of mammalian neurofilament polypeptides within the neuronal cytoskeleton. J Cell Biol 98:1523–1536
Gill SR, Wong PC, Monteiro MJ, Cleveland DW (1990) Assembly properties of dominant and recessive mutations in the small mouse neurofilament (NF-L) subunit. J Cell Biol 111:2005–2019
Wong PC, Cleveland DW (1990) Characterization of dominant and recessive assembly-defective mutations in mouse neurofilament NF-M. J Cell Biol 111:1987–2003
Chin SS, Macioce P, Liem RK (1991) Effects of truncated neurofilament proteins on the endogenous intermediate filaments in transfected fibroblasts. J Cell Sci 99(Pt 2):335–350
Ching GY, Liem RK (1993) Assembly of type IV neuronal intermediate filaments in nonneuronal cells in the absence of preexisting cytoplasmic intermediate filaments. J Cell Biol 122:1323–1335
Ching GY, Liem RK (1999) Analysis of the roles of the head domains of type IV rat neuronal intermediate filament proteins in filament assembly using domain-swapped chimeric proteins. J Cell Sci 112(Pt 13):2233–2240
Sihag RK, Nixon RA (1991) Identification of Ser-55 as a major protein kinase A phosphorylation site on the 70-kDa subunit of neurofilaments. Early turnover during axonal transport. J Biol Chem 266:18861–18867
Sihag RK, Jaffe H, Nixon RA, Rong X (1999) Serine-23 is a major protein kinase A phosphorylation site on the amino-terminal head domain of the middle molecular mass subunit of neurofilament proteins. J Neurochem 72:491–499
Hisanaga S, Gonda Y, Inagaki M, Ikai A, Hirokawa N (1990) Effects of phosphorylation of the neurofilament L protein on filamentous structures. Cell Regul 1:237–248
Gibb BJ, Brion JP, Brownlees J, Anderton BH, Miller CC (1998) Neuropathological abnormalities in transgenic mice harbouring a phosphorylation mutant neurofilament transgene. J Neurochem 70:492–500
Mukai H, Toshimori M, Shibata H, Kitagawa M, Shimakawa M, Miyahara M, Sunakawa H, Ono Y (1996) PKN associates and phosphorylates the head-rod domain of neurofilament protein. J Biol Chem 271:9816–9822
Dong DL, Xu ZS, Chevrier MR, Cotter RJ, Cleveland DW, Hart GW (1993) Glycosylation of mammalian neurofilaments. Localization of multiple O-linked N acetyl glucosamine moieties on neurofilament polypeptides L and M. J Biol Chem 268:16679–16687
Dong DL, Xu ZS, Hart GW, Cleveland DW (1996) Cytoplasmic O-GlcNAc modification of the head domain and the KSP repeat motif of the neurofilament protein neurofilament-H. J Biol Chem 271:20845–20852
Nguyen MD, Shu T, Sanada K, Lariviere RC, Tseng HC, Park SK, Julien JP, Tsai LH (2004) A NUDEL-dependent mechanism of neurofilament assembly regulates the integrity of CNS neurons. Nat Cell Biol 6:595–608
Leterrier JF, Kas J, Hartwig J, Vegners R, Janmey PA (1996) Mechanical effects of neurofilament cross-bridges. Modulation by phosphorylation, lipids, and interactions with F-actin. J Biol Chem 271:15687–15694
Rammensee S, Janmey PA, Bausch AR (2007) Mechanical and structural properties of in vitro neurofilament hydrogels. Eur Biophys J 36:661–668
Kreplak L, Bar H, Leterrier JF, Herrmann H, Aebi U (2005) Exploring the mechanical behavior of single intermediate filaments. J Mol Biol 354:569–577
Myers MW, Lazzarini RA, Lee VM, Schlaepfer WW, Nelson DL (1987) The human mid-size neurofilament subunit: a repeated protein sequence and the relationship of its gene to the intermediate filament gene family. Embo J 6:1617–1626
Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA (2002) Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A 99:16899–16903
Lees JF, Shneidman PS, Skuntz SF, Carden MJ, Lazzarini RA (1988) The structure and organization of the human heavy neurofilament subunit (NF-H) and the gene encoding it. Embo J 7:1947–1955
Thyagarajan A, Strong MJ, Szaro BG (2007) Post-transcriptional control of neurofilaments in development and disease. Exp Cell Res 313:2088–2097
Willard M, Simon C (1983) Modulations of neurofilament axonal transport during the development of rabbit retinal ganglion cells. Cell 35:551–559
Carden MJ, Trojanowski JQ, Schlaepfer WW, Lee VM (1987) Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci 7:3489–3504
Shaw G, Osborn M, Weber K (1981) An immunofluorescence microscopical study of the neurofilament triplet proteins, vimentin and glial fibrillary acidic protein within the adult rat brain. Eur J Cell Biol 26:68–82
Shaw G, Weber K (1982) Differential expression of neurofilament triplet proteins in brain development. Nature 298:277–279
Pachter JS, Liem RK (1984) The differential appearance of neurofilament triplet polypeptides in the developing rat optic nerve. Dev Biol 103:200–210
Zhao Y, Szaro BG (1995) The optic tract and tectal ablation influence the composition of neurofilaments in regenerating optic axons of Xenopus laevis. J Neurosci 15:4629–4640
Walker KL, Yoo HK, Undamatla J, Szaro BG (2001) Loss of neurofilaments alters axonal growth dynamics. J Neurosci 21:9655–9666
Cuenca N, Fernandez E, de Juan J, Carreres J, Iniguez C (1987) Postnatal development of microtubules and neurofilaments in the rat optic nerve: a quantitative study. J Comp Neurol 263:613–617
Breen KC, Anderton BH (1991) Temporal expression of neurofilament polypeptides in differentiating neuroblastoma cells. Neuroreport 2:21–24
Giasson BI, Mushynski WE (1997) Study of proline-directed protein kinases involved in phosphorylation of the heavy neurofilament subunit. J Neurosci 17:9466–9472
Zhu Q, Couillard-Despres S, Julien JP (1997) Delayed maturation of regenerating myelinated axons in mice lacking neurofilaments. Exp Neurol 148:299–316
Elder GA, Friedrich VL Jr, Bosco P, Kang C, Gourov A, Tu PH, Lee VM, Lazzarini RA (1998) Absence of the mid-sized neurofilament subunit decreases axonal calibers, levels of light neurofilament (NF-L), and neurofilament content. J Cell Biol 141:727–739
Wong J, Oblinger MM (1987) Changes in neurofilament gene expression occur after axotomy of dorsal root ganglion neurons: an in situ hybridization study. Metab Brain Dis 2:291–303
Goldstein ME, Weiss SR, Lazzarini RA, Shneidman PS, Lees JF, Schlaepfer WW (1988) mRNA levels of all three neurofilament proteins decline following nerve transection. Brain Res 427:287–291
Oblinger MM, Lasek RJ (1988) Axotomy-induced alterations in the synthesis and transport of neurofilaments and microtubules in dorsal root ganglion cells. J Neurosci 8:1747–1758
Mikucki SA, Oblinger MM (1991) Corticospinal neurons exhibit a novel pattern of cytoskeletal gene expression after injury. J Neurosci Res 30:213–225
Tetzlaff W, Alexander SW, Miller FD, Bisby MA (1991) Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J Neurosci 11:2528–2544
Hoffman PN, Pollock SC, Striph GG (1993) Altered gene expression after optic nerve transection: reduced neurofilament expression as a general response to axonal injury. Exp Neurol 119:32–36
McKerracher L, Essagian C, Aguayo AJ (1993) Temporal changes in beta-tubulin and neurofilament mRNA levels after transection of adult rat retinal ganglion cell axons in the optic nerve. J Neurosci 13:2617–2626
Hoffman PN, Lasek RJ (1980) Axonal transport of the cytoskeleton in regenerating motor neurons: constancy and change. Brain Res 202:317–333
Hoffman PN, Thompson GW, Griffin JW, Price DL (1985) Changes in neurofilament transport coincide temporally with alterations in the caliber of axons in regenerating motor fibers. J Cell Biol 101:1332–1340
Hoffman PN, Cleveland DW (1988) Neurofilament and tubulin expression recapitulates the developmental program during axonal regeneration: induction of a specific beta-tubulin isotype. Proc Natl Acad Sci U S A 85:4530–4533
Muma NA, Hoffman PN, Slunt HH, Applegate MD, Lieberburg I, Price DL (1990) Alterations in levels of mRNAs coding for neurofilament protein subunits during regeneration. Exp Neurol 107:230–235
Wong J, Oblinger MM (1990) A comparison of peripheral and central axotomy effects on neurofilament and tubulin gene expression in rat dorsal root ganglion neurons. J Neurosci 10:2215–2222
Tetzlaff W, Bisby MA, Kreutzberg GW (1988) Changes in cytoskeletal proteins in the rat facial nucleus following axotomy. J Neurosci 8:3181–3189
Jiang YQ, Pickett J, Oblinger MM (1994) Comparison of changes in beta-tubulin and NF gene expression in rat DRG neurons under regeneration-permissive and regeneration prohibitive conditions. Brain Res 637:233–241
Jacobs AJ, Swain GP, Snedeker JA, Pijak DS, Gladstone LJ, Selzer ME (1997) Recovery of neurofilament expression selectively in regenerating reticulospinal neurons. J Neurosci 17:5206–5220
Gervasi C, Thyagarajan A, Szaro BG (2003) Increased expression of multiple neurofilament mRNAs during regeneration of vertebrate central nervous system axons. J Comp Neurol 461:262–275
Grant P, Pant HC (2000) Neurofilament protein synthesis and phosphorylation. J Neurocytol 29:843–872
Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC (2007) Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res 313:2098–2109
Jones SM, Williams RC Jr. (1982) Phosphate content of mammalian neurofilaments. J Biol Chem 257:9902–9905
Geisler N, Vandekerckhove J, Weber K (1987) Location and sequence characterization of the major phosphorylation sites of the high molecular mass neurofilament proteins M and H. FEBS Lett 221:403–407
Goldstein ME, Sternberger LA, Sternberger NH (1987) Varying degrees of phosphorylation determine microheterogeneity of the heavy neurofilament polypeptide (Nf-H). J Neuroimmunol 14:135–148
Lee VM, Otvos L Jr., Carden MJ, Hollosi M, Dietzschold B, Lazzarini RA (1988) Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc Natl Acad Sci U S A 85:1998–2002
Pant HC, Veeranna (1995) Neurofilament phosphorylation. Biochem Cell Biol 73:575–592
Sternberger LA, Sternberger NH (1983) Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A 80:6126–6130
Glicksman MA, Soppet D, Willard MB (1987) Posttranslational modification of neurofilament polypeptides in rabbit retina. J Neurobiol 18:167–196
Oblinger MM, Brady ST, McQuarrie IG, Lasek RJ (1987) Cytotypic differences in the protein composition of the axonally transported cytoskeleton in mammalian neurons. J Neurosci 7:453–462
Nixon RA, Paskevich PA, Sihag RK, Thayer CY (1994) Phosphorylation on carboxyl terminus domains of neurofilament proteins in retinal ganglion cell neurons in vivo: influences on regional neurofilament accumulation, interneurofilament spacing, and axon caliber. J Cell Biol 126:1031–1046
Sihag RK, Jeng AY, Nixon RA (1988) Phosphorylation of neurofilament proteins by protein kinase C. FEBS Lett 233:181–185
Sihag RK, Nixon RA (1989) In vivo phosphorylation of distinct domains of the 70-kiloDalton neurofilament subunit involves different protein kinases. J Biol Chem 264:457–464
Sihag RK, Nixon RA (1990) Phosphorylation of the amino-terminal head domain of the middle molecular mass 145-kDa subunit of neurofilaments. Evidence for regulation by second messenger-dependent protein kinases. J Biol Chem 265:4166–4171
Nixon RA, Lewis SE (1986) Differential turnover of phosphate groups on neurofilament subunits in mammalian neurons in vivo. J Biol Chem 261:16298–16301
Nixon RA, Lewis SE, Marotta CA (1987) Posttranslational modification of neurofilament proteins by phosphate during axoplasmic transport in retinal ganglion cell neurons. J Neurosci 7:1145–1158
Nixon RA, Lewis SE, Dahl D, Marotta CA, Drager UC (1989) Early posttranslational modifications of the three neurofilament subunits in mouse retinal ganglion cells: neuronal sites and time course in relation to subunit polymerization and axonal transport. Brain Res Mol Brain Res 5:93–108
Hashimoto R, Nakamura Y, Goto H, Wada Y, Sakoda S, Kaibuchi K, Inagaki M, Takeda M (1998) Domain- and site-specific phosphorylation of bovine NF-L by Rho associated kinase. Biochem Biophys Res Commun 245:407–411
Hashimoto R, Nakamura Y, Komai S, Kashiwagi Y, Tamura K, Goto T, Aimoto S, Kaibuchi K, Shiosaka S, Takeda M (2000) Site-specific phosphorylation of neurofilament-L is mediated by calcium/calmodulin-dependent protein kinase II in the apical dendrites during long-term potentiation. J Neurochem 75:373–382
Nixon RA, Brown BA, Marotta CA (1982) Posttranslational modification of a neurofilament protein during axoplasmic transport: implications for regional specialization of CNS axons. J Cell Biol 94:150–158
Lewis SE, Nixon RA (1988) Multiple phosphorylated variants of the high molecular mass subunit of neurofilaments in axons of retinal cell neurons: characterization and evidence for their differential association with stationary and moving neurofilaments. J Cell Biol 107:2689–2701
Archer DR, Watson DF, Griffin JW (1994) Phosphorylation-dependent immunoreactivity of neurofilaments and the rate of slow axonal transport in the central and peripheral axons of the rat dorsal root ganglion. J Neurochem 62:1119–1125
Jung C, Yabe JT, Lee S, Shea TB (2000) Hypophosphorylated neurofilament subunits undergo axonal transport more rapidly than more extensively phosphorylated subunits in situ. Cell Motil Cytoskeleton 47:120–129
Ackerley S, Thornhill P, Grierson AJ, Brownlees J, Anderton BH, Leigh PN, Shaw CE, Miller CC (2003) Neurofilament heavy chain side arm phosphorylation regulates axonal transport of neurofilaments. J Cell Biol 161:489–495
Nixon RA, Logvinenko KB (1986) Multiple fates of newly synthesized neurofilament proteins: evidence for a stationary neurofilament network distributed nonuniformly along axons of retinal ganglion cell neurons. J Cell Biol 102:647–659
Li BS, Veeranna, Gu J, Grant P, Pant HC (1999) Activation of mitogen-activated protein kinases (Erk1 and Erk2) cascade results in phosphorylation of NF-M tail domains in transfected NIH 3T3 cells. Eur J Biochem 262:211–217
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183
Li BS, Veeranna, Grant P, Pant HC (1999) Calcium influx and membrane depolarization induce phosphorylation of neurofilament (NF-M) KSP repeats in PC12 cells. Brain Res Mol Brain Res 70:84–91
Li BS, Zhang L, Gu J, Amin ND, Pant HC (2000) Integrin alpha(1) beta(1)-mediated activation of cyclin-dependent kinase 5 activity is involved in neurite outgrowth and human neurofilament protein H Lys–Ser–Pro tail domain phosphorylation. J Neurosci 20:6055–6062
de Waegh SM, Lee VM, Brady ST (1992) Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell 68:451–463
Reles A, Friede RL (1991) Axonal cytoskeleton at the nodes of Ranvier. J Neurocytol 20:450–458
Mata M, Kupina N, Fink DJ (1992) Phosphorylation-dependent neurofilament epitopes are reduced at the node of Ranvier. J Neurocytol 21:199–210
Hsieh ST, Crawford TO, Griffin JW (1994) Neurofilament distribution and organization in the myelinated axons of the peripheral nervous system. Brain Res 642:316–326
Yin X, Crawford TO, Griffin JW, Tu P, Lee VM, Li C, Roder J, Trapp BD (1998) Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J Neurosci 18:1953–1962
Dashiell SM, Tanner SL, Pant HC, Quarles RH (2002) Myelin-associated glycoprotein modulates expression and phosphorylation of neuronal cytoskeletal elements and their associated kinases. J Neurochem 81:1263–1272
Lew J, Winkfein RJ, Paudel HK, Wang JH (1992) Brain proline-directed protein kinase is a neurofilament kinase which displays high sequence homology to p34cdc2. J Biol Chem 267:25922–25926
Shetty KT, Link WT, Pant HC (1993) cdc2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: isolation and characterization. Proc Natl Acad Sci U S A 90:6844–6848
Hisanaga S, Uchiyama M, Hosoi T, Yamada K, Honma N, Ishiguro K, Uchida T, Dahl D, Ohsumi K, Kishimoto T (1995) Porcine brain neurofilament-H tail domain kinase: its identification as cdk5/p26 complex and comparison with cdc2/cyclin B kinase. Cell Motil Cytoskeleton 31:283–297
Guidato S, Tsai LH, Woodgett J, Miller CC (1996) Differential cellular phosphorylation of neurofilament heavy side-arms by glycogen synthase kinase-3 and cyclin-dependent kinase-5. J Neurochem 66:1698–1706
Sun D, Leung CL, Liem RK (1996) Phosphorylation of the high molecular weight neurofilament protein (NF-H) by Cdk5 and p35. J Biol Chem 271:14245–14251
Bajaj NP, Miller CC (1997) Phosphorylation of neurofilament heavy-chain side-arm fragments by cyclin-dependent kinase-5 and glycogen synthase kinase-3alpha in transfected cells. J Neurochem 69:737–743
Veeranna, Amin ND, Ahn NG, Jaffe H, Winters CA, Grant P, Pant HC (1998) Mitogen-activated protein kinases (Erk1,2) phosphorylate Lys–Ser–Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J Neurosci 18:4008–4021
Sharma M, Sharma P, Pant HC (1999) CDK-5-mediated neurofilament phosphorylation in SHSY5Y human neuroblastoma cells. J Neurochem 73:79–86
Miyasaka H, Okabe S, Ishiguro K, Uchida T, Hirokawa N (1993) Interaction of the tail domain of high molecular weight subunits of neurofilaments with the COOH-terminal region of tubulin and its regulation by tau protein kinase II. J Biol Chem 268:22695–22702
Brownlees J, Yates A, Bajaj NP, Davis D, Anderton BH, Leigh PN, Shaw CE, Miller CC (2000) Phosphorylation of neurofilament heavy chain side-arms by stress activated protein kinase-1b/Jun N-terminal kinase-3. J Cell Sci 113(Pt 3):401–407
Guan RJ, Khatra BS, Cohlberg JA (1991) Phosphorylation of bovine neurofilament proteins by protein kinase FA (glycogen synthase kinase 3). J Biol Chem 266:8262–8267
Ackerley S, Grierson AJ, Banner S, Perkinton MS, Brownlees J, Byers HL, Ward M, Thornhill P, Hussain K, Waby JS, Anderton BH, Cooper JD, Dingwall C, Leigh PN, Shaw CE, Miller CC (2004) p38alpha stress-activated protein kinase phosphorylates neurofilaments and is associated with neurofilament pathology in amyotrophic lateral sclerosis. Mol Cell Neurosci 26:354–364
Sasaki T, Gotow T, Shiozaki M, Sakaue F, Saito T, Julien JP, Uchiyama Y, Hisanaga S (2006) Aggregate formation and phosphorylation of neurofilament-L Pro22 Charcot-Marie-Tooth disease mutants. Hum Mol Genet 15:943–952
Giasson BI, Mushynski WE (1996) Aberrant stress-induced phosphorylation of perikaryal neurofilaments. J Biol Chem 271:30404–30409
O’Ferrall EK, Robertson J, Mushynski WE (2000) Inhibition of aberrant and constitutive phosphorylation of the high-molecular-mass neurofilament subunit by CEP-1347 (KT7515), an inhibitor of the stress-activated protein kinase signaling pathway. J Neurochem 75:2358–2367
Dosemeci A, Floyd CC, Pant HC (1990) Characterization of neurofilament-associated protein kinase activities from bovine spinal cord. Cell Mol Neurobiol 10:369–382
Floyd CC, Grant P, Gallant PE, Pant HC (1991) Principal neurofilament-associated protein kinase in squid axoplasm is related to casein kinase I. J Biol Chem 266:4987–4994
Link WT, Dosemeci A, Floyd CC, Pant HC (1993) Bovine neurofilament-enriched preparations contain kinase activity similar to casein kinase I—neurofilament phosphorylation by casein kinase I (CKI). Neurosci Lett 151:89–93
Hollander BA, Bennett GS, Shaw G (1996) Localization of sites in the tail domain of the middle molecular mass neurofilament subunit phosphorylated by a neurofilament-associated kinase and by casein kinase I. J Neurochem 66:412–420
Bennett GS, Quintana R (1997) Identification of Ser–Pro and Thr–Pro phosphorylation sites in chicken neurofilament-M tail domain. J Neurochem 68:534–543
Xu ZS, Liu WS, Willard M (1990) Identification of serine 473 as a major phosphorylation site in the neurofilament polypeptide NF-L. J Neurosci 10:1838–1846
Nakamura Y, Hashimoto R, Kashiwagi Y, Wada Y, Sakoda S, Miyamae Y, Kudo T, Takeda M (1999) Casein kinase II is responsible for phosphorylation of NF-L at Ser-473. FEBS Lett 455:83–86
Zheng YL, Li BS, Veeranna, Pant HC (2003) Phosphorylation of the head domain of neurofilament protein (NF-M): a factor regulating topographic phosphorylation of NF-M tail domain KSP sites in neurons. J Biol Chem 278:24026–24032
Leterrier JF, Eyer J (1987) Properties of highly viscous gels formed by neurofilaments in vitro. A possible consequence of a specific inter-filament cross-bridging. Biochem J 245:93–101
Pant HC (1988) Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem J 256:665–668
Sacher MG, Athlan ES, Mushynski WE (1994) Increased phosphorylation of the amino-terminal domain of the low molecular weight neurofilament subunit in okadaic acid-treated neurons. J Biol Chem 269:18480–18484
Saito T, Shima H, Osawa Y, Nagao M, Hemmings BA, Kishimoto T, Hisanaga S (1995) Neurofilament-associated protein phosphatase 2A: its possible role in preserving neurofilaments in filamentous states. Biochemistry 34:7376–7384
Veeranna, Shetty KT, Link WT, Jaffe H, Wang J, Pant HC (1995) Neuronal cyclin-dependent kinase-5 phosphorylation sites in neurofilament protein (NF-H) are dephosphorylated by protein phosphatase 2A. J Neurochem 64:2681–2690
Strack S, Westphal RS, Colbran RJ, Ebner FF, Wadzinski BE (1997) Protein serine/threonine phosphatase 1 and 2A associate with and dephosphorylate neurofilaments. Brain Res Mol Brain Res 49:15–28
Slawson C, Hart GW (2003) Dynamic interplay between O-GlcNAc and O-phosphate: the sweet side of protein regulation. Curr Opin Struct Biol 13:631–636
Ludemann N, Clement A, Hans VH, Leschik J, Behl C, Brandt R (2005) O-glycosylation of the tail domain of neurofilament protein M in human neurons and in spinal cord tissue of a rat model of amyotrophic lateral sclerosis (ALS). J Biol Chem 280:31648–31658
Ryle C, Leow CK, Donaghy M (1997) Nonenzymatic glycation of peripheral and central nervous system proteins in experimental diabetes mellitus. Muscle Nerve 20:577–584
Chou SM, Wang HS, Taniguchi A, Bucala R (1998) Advanced glycation end products in neurofilament conglomeration of motoneurons in familial and sporadic amyotrophic lateral sclerosis. Mol Med 4:324–332
Suzuki Y, Tanaka M, Sohmiya M, Ichinose S, Omori A, Okamoto K (2005) Identification of nitrated proteins in the normal rat brain using a proteomics approach. Neurol Res 27:630–633
Chou SM, Wang HS, Taniguchi A (1996) Role of SOD-1 and nitric oxide/cyclic GMP cascade on neurofilament aggregation in ALS/MND. J Neurol Sci 139(Suppl):16–26
Crow JP, Ye YZ, Strong M, Kirk M, Barnes S, Beckman JS (1997) Superoxide dismutase catalyzes nitration of tyrosines by peroxynitrite in the rod and head domains of neurofilament-L. J Neurochem 69:1945–1953
Reynolds MR, Berry RW, Binder LI (2007) Nitration in neurodegeneration: deciphering the “Hows” “nYs”. Biochemistry 46:7325–7336
Troncoso JC, Costello AC, Kim JH, Johnson GV (1995) Metal-catalyzed oxidation of bovine neurofilaments in vitro. Free Radic Biol Med 18:891–899
Kim NH, Jeong MS, Choi SY, Hoon Kang J (2004) Oxidative modification of neurofilament-L by the Cu,Zn-superoxide dismutase and hydrogen peroxide system. Biochimie 86:553–559
Gou JP, Leterrier JF (1995) Possible involvement of ubiquitination in neurofilament degradation. Biochem Biophys Res Commun 217:529–538
Hoffman PN, Lasek RJ (1975) The slow component of axonal transport. Identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol 66:351–366
Schlaepfer WW (1974) Calcium-induced degeneration of axoplasm in isolated segments of rat peripheral nerve. Brain Res 69:203–215
Millecamps S, Gowing G, Corti O, Mallet J, Julien JP (2007) Conditional NF-L transgene expression in mice for in vivo analysis of turnover and transport rate of neurofilaments. J Neurosci 27:4947–4956
Pant HC, Gainer H (1980) Properties of a calcium-activated protease in squid axoplasm which selectively degrades neurofilament proteins. J Neurobiol 11:1–12
Schlaepfer WW, Lee C, Lee VM, Zimmerman UJ (1985) An immunoblot study of neurofilament degradation in situ and during calcium-activated proteolysis. J Neurochem 44:502–509
Gallant PE, Pant HC, Pruss RM, Gainer H (1986) Calcium-activated proteolysis of neurofilament proteins in the squid giant neuron. J Neurochem 46:1573–1581
Perlmutter LS, Gall C, Baudry M, Lynch G (1990) Distribution of calcium-activated protease calpain in the rat brain. J Comp Neurol 296:269–276
Eagles PA, Gilbert DS, Maggs A (1981) The location of phosphorylation sites and Ca2+-dependent proteolytic cleavage sites on the major neurofilament polypeptides from Myxicola infundibulum. Biochem J 199:101–111
Roots BI (1983) Neurofilament accumulation induced in synapses by leupeptin. Science 221:971–972
Schlaepfer WW (1971) Experimental alterations of neurofilaments and neurotubules by calcium and other ions. Exp Cell Res 67:73–80
Murachi T, Tanaka K, Hatanaka M, Murakami T (1980) Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Adv Enzyme Regul 19:407–424
Murachi T (1990) Calpain and calpastatin. Rinsho Byori 38:337–346
Hamakubo T, Kannagi R, Murachi T, Matus A (1986) Distribution of calpains I and II in rat brain. J Neurosci 6:3103–3111
Nixon RA, Brown BA, Marotta CA (1983) Limited proteolytic modification of a neurofilament protein involves a proteinase activated by endogenous levels of calcium. Brain Res 275:384–388
Nelson WJ, Traub P (1982) Intermediate (10 nm) filament proteins and the Ca2+-activated proteinase specific for vimentin and desmin in the cells from fish to man: an example of evolutionary conservation. J Cell Sci 57:25–49
Gitler D, Spira ME (1998) Real time imaging of calcium-induced localized proteolytic activity after axotomy and its relation to growth cone formation. Neuron 20:1123–1135
Chin TK, Eagles PA, Maggs A (1983) The proteolytic digestion of ox neurofilaments with trypsin and alpha-chymotrypsin. Biochem J 215:239–252
Chin TK, Harding SE, Eagles PA (1989) Characterization of two proteolytically derived soluble polypeptides from the neurofilament triplet components NFM and NFH. Biochem J 264:53–60
Malik MN, Fenko MD, Iqbal K, Wisniewski HM (1983) Purification and characterization of two forms of Ca2+-activated neutral protease from calf brain. J Biol Chem 258:8955–8962
Nixon RA, Marotta CA (1984) Degradation of neurofilament proteins by purified human brain cathepsin D. J Neurochem 43:507–516
Banay-Schwartz M, Dahl D, Hui KS, Lajtha A (1987) The breakdown of the individual neurofilament proteins by cathepsin D. Neurochem Res 12:361–367
Suzuki H, Takeda M, Nakamura Y, Kato Y, Tada K, Hariguchi S, Nishimura T (1988) Neurofilament degradation by bovine brain cathepsin D. Neurosci Lett 89:240–245
Traub P, Vorgias CE, Nelson WJ (1985) Interaction in vitro of the neurofilament triplet proteins from porcine spinal cord with natural RNA and DNA. Mol Biol Rep 10:129–136
Wang Q, Tolstonog GV, Shoeman R, Traub P (2001) Sites of nucleic acid binding in type I-IV intermediate filament subunit proteins. Biochemistry 40:10342–10349
Goldstein ME, Sternberger NH, Sternberger LA (1987) Phosphorylation protects neurofilaments against proteolysis. J Neuroimmunol 14:149–160
Bizzi A, Crane RC, Autilio-Gambetti L, Gambetti P (1984) Aluminum effect on slow axonal transport: a novel impairment of neurofilament transport. J Neurosci 4:722–731
Troncoso JC, Sternberger NH, Sternberger LA, Hoffman PN, Price DL (1986) Immunocytochemical studies of neurofilament antigens in the neurofibrillary pathology induced by aluminum. Brain Res 364:295–300
Nixon RA, Clarke JF, Logvinenko KB, Tan MK, Hoult M, Grynspan F (1990) Aluminum inhibits calpain-mediated proteolysis and induces human neurofilament proteins to form protease-resistant high molecular weight complexes. J Neurochem 55:1950–1959
Posmantur R, Hayes RL, Dixon CE, Taft WC (1994) Neurofilament 68 and neurofilament 200 protein levels decrease after traumatic brain injury. J Neurotrauma 11:533–545
Banik NL, Matzelle DC, Gantt-Wilford G, Osborne A, Hogan EL (1997) Increased calpain content and progressive degradation of neurofilament protein in spinal cord injury. Brain Res 752:301–306
Bahmanyar S, Moreau-Dubois MC, Brown P, Cathala F, Gajdusek DC (1983) Serum antibodies to neurofilament antigens in patients with neurological and other diseases and in healthy controls. J Neuroimmunol 5:191–196
Elizan TS, Casals J, Yahr MD (1983) Antineurofilament antibodies in postencephalitic and idiopathic Parkinson’s disease. J Neurol Sci 59:341–347
Stefansson K, Marton LS, Dieperink ME, Molnar GK, Schlaepfer WW, Helgason CM (1985) Circulating autoantibodies to the 200,000-Dalton protein of neurofilaments in the serum of healthy individuals. Science 228:1117–1119
Toh BH, Gibbs CJ Jr., Gajdusek DC, Tuthill DD, Dahl D (1985) The 200- and 150-kDa neurofilament proteins react with IgG autoantibodies from chimpanzees with kuru or Creutzfeldt-Jakob disease; a 62-kDa neurofilament-associated protein reacts with sera from sheep with natural scrapie. Proc Natl Acad Sci U S A 82:3894–3896
Hirano A (1991) Cytopathology of amyotrophic lateral sclerosis. Adv Neurol 56:91–101
Corbo M, Hays AP (1992) Peripherin and neurofilament protein coexist in spinal spheroids of motor neuron disease. J Neuropathol Exp Neurol 51:531–537
Hill WD, Arai M, Cohen JA, Trojanowski JQ (1993) Neurofilament mRNA is reduced in Parkinson’s disease substantia nigra pars compacta neurons. J Comp Neurol 329:328–336
Leigh PN, Dodson A, Swash M, Brion JP, Anderton BH (1989) Cytoskeletal abnormalities in motor neuron disease. An immunocytochemical study. Brain 112(Pt 2):521–535
Chou SM, Hartmann HA (1965) Electron microscopy of focal neuroaxonal lesions produced by beta-beta-iminodipropionitrile (IDPN) in rats. I. The advanced lesions. Acta Neuropathol 4:590–603
Kadota T, Kadota K (1978) Neurofilament hypertrophy induced in the rabbit spinal cord after intracisternal injection of aluminum chloride (author’s transl). J Toxicol Sci 3:57–67
Papasozomenos SC, Autilio-Gambetti L, Gambetti P (1981) Reorganization of axoplasmic organelles following beta, beta’-iminodipropionitrile administration. J Cell Biol 91:866–871
Gold BG, Griffin JW, Price DL (1985) Slow axonal transport in acrylamide neuropathy: different abnormalities produced by single-dose and continuous administration. J Neurosci 5:1755–1768
Gschwend TP, Krueger SR, Kozlov SV, Wolfer DP, Sonderegger P (1997) Neurotrypsin, a novel multidomain serine protease expressed in the nervous system. Mol Cell Neurosci 9:207–219
Yamashiro K, Tsuruoka N, Kodama S, Tsujimoto M, Yamamura Y, Tanaka T, Nakazato H, Yamaguchi N (1997) Molecular cloning of a novel trypsin-like serine protease (neurosin) preferentially expressed in brain. Biochim Biophys Acta 1350:11–14
Scarisbrick IA, Isackson PJ, Ciric B, Windebank AJ, Rodriguez M (2001) MSP, a trypsin-like serine protease, is abundantly expressed in the human nervous system. J Comp Neurol 431:347–361
Chou SM, Taniguchi A, Wang HS, Festoff BW (1998) Serpin = serine protease-like complexes within neurofilament conglomerates of motoneurons in amyotrophic lateral sclerosis. J Neurol Sci 160(Suppl 1):S73–79
Tsuji T, Shimohama S, Kimura J, Shimizu K (1998) m-Calpain (calcium-activated neutral proteinase) in Alzheimer’s disease brains. Neurosci Lett 248:109–112
Fasani F, Bocquet A, Robert P, Peterson A, Eyer J (2004) The amount of neurofilaments aggregated in the cell body is controlled by their increased sensitivity to trypsin-like proteases. J Cell Sci 117:861–869
Eyer J, Peterson A (1994) Neurofilament-deficient axons and perikaryal aggregates in viable transgenic mice expressing a neurofilament-beta-galactosidase fusion protein. Neuron 12:389–405
Runge MS, Laue TM, Yphantis DA, Lifsics MR, Saito A, Altin M, Reinke K, Williams RC Jr. (1981) ATP-induced formation of an associated complex between microtubules and neurofilaments. Proc Natl Acad Sci U S A 78:1431–1435
Leterrier JF, Wong J, Liem RK, Shelanski ML (1984) Promotion of microtubule assembly by neurofilament-associated microtubule-associated proteins. J Neurochem 43:1385–1391
Aamodt EJ, Williams RC Jr. (1984) Association of microtubules and neurofilaments in vitro is not mediated by ATP. Biochemistry 23:6031–6035
Heimann R, Shelanski ML, Liem RK (1985) Microtubule-associated proteins bind specifically to the 70-kDa neurofilament protein. J Biol Chem 260:12160–12166
Flynn G, Purich DL (1987) GTP regeneration influences interactions of microtubules, neurofilaments, and microtubule-associated proteins in vitro. J Biol Chem 262:15443–15447
Minami Y, Sakai H (1983) Network formation by neurofilament-induced polymerization of tubulin: 200 K subunit of neurofilament triplet promotes nucleation of tubulin polymerization and enhances microtubule assembly. J Biochem 94:2023–2033
Minami Y, Endo S, Sakai H (1984) Participation of 200 K or 150 K subunit of neurofilament in construction of the filament core with 70 K subunit and promotion of tubulin polymerization by incorporated 200 K subunit. J Biochem 96:1481–1490
Hirokawa N (1982) Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J Cell Biol 94:129–142
Job D, Rauch CT, Fischer EH, Margolis RL (1982) Recycling of cold-stable microtubules: evidence that cold stability is due to substoichiometric polymer blocks. Biochemistry 21:509–515
Denarier E, Fourest-Lieuvin A, Bosc C, Pirollet F, Chapel A, Margolis RL, Job D (1998) Nonneuronal isoforms of STOP protein are responsible for microtubule cold stability in mammalian fibroblasts. Proc Natl Acad Sci U S A 95:6055–6060
Job D, Fischer EH, Margolis RL (1981) Rapid disassembly of cold-stable microtubules by calmodulin. Proc Natl Acad Sci U S A 78:4679–4682
Margolis RL, Rauch CT, Job D (1986) Purification and assay of a 145-kDa protein (STOP145) with microtubule-stabilizing and motility behavior. Proc Natl Acad Sci U S A 83:639–643
Bosc C, Cronk JD, Pirollet F, Watterson DM, Haiech J, Job D, Margolis RL (1996) Cloning, expression, and properties of the microtubule-stabilizing protein STOP. Proc Natl Acad Sci U S A 93:2125–2130
Pirollet F, Rauch CT, Job D, Margolis RL (1989) Monoclonal antibody to microtubule-associated STOP protein: affinity purification of neuronal STOP activity and comparison of antigen with activity in neuronal and nonneuronal cell extracts. Biochemistry 28:835–842
Pirollet F, Derancourt J, Haiech J, Job D, Margolis RL (1992) Ca(2+)-calmodulin regulated effectors of microtubule stability in bovine brain. Biochemistry 31:8849–8855
Guillaud L, Bosc C, Fourest-Lieuvin A, Denarier E, Pirollet F, Lafanechere L, Job D (1998) STOP proteins are responsible for the high degree of microtubule stabilization observed in neuronal cells. J Cell Biol 142:167–179
Letournel F, Bocquet A, Dubas F, Barthelaix A, Eyer J (2003) Stable tubule only polypeptides (STOP) proteins co-aggregate with spheroid neurofilaments in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 62:1211–1219
Yabe JT, Pimenta A, Shea TB (1999) Kinesin-mediated transport of neurofilament protein oligomers in growing axons. J Cell Sci 112(Pt 21):3799–3814
Yabe JT, Jung C, Chan WK, Shea TB (2000) Phospho-dependent association of neurofilament proteins with kinesin in situ. Cell Motil Cytoskeleton 45:249–262
Shah JV, Flanagan LA, Janmey PA, Leterrier JF (2000) Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin. Mol Biol Cell 11:3495–3508
Wagner OI, Ascano J, Tokito M, Leterrier JF, Janmey PA, Holzbaur EL (2004) The interaction of neurofilaments with the microtubule motor cytoplasmic dynein. Mol Biol Cell 15:5092–5100
Theiss C, Napirei M, Meller K (2005) Impairment of anterograde and retrograde neurofilament transport after anti-kinesin and anti-dynein antibody microinjection in chicken dorsal root ganglia. Eur J Cell Biol 84:29–43
Motil J, Chan WK, Dubey M, Chaudhury P, Pimenta A, Chylinski TM, Ortiz DT, Shea TB (2006) Dynein mediates retrograde neurofilament transport within axons and anterograde delivery of NF from perikarya into axons: regulation by multiple phosphorylation events. Cell Motil Cytoskeleton 63:266–286
Hirokawa N (1998) Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279:519–526
Goldenring JR, Lasher RS, Vallano ML, Ueda T, Naito S, Sternberger NH, Sternberger LA, DeLorenzo RJ (1986) Association of synapsin I with neuronal cytoskeleton. Identification in cytoskeletal preparations in vitro and immunocytochemical localization in brain of synapsin I. J Biol Chem 261:8495–8504
Steiner JP, Ling E, Bennett V (1987) Nearest neighbor analysis for brain synapsin I. Evidence from in vitro reassociation assays for association with membrane protein(s) and the Mr = 68,000 neurofilament subunit. J Biol Chem 262:905–914
Yang Y, Dowling J, Yu QC, Kouklis P, Cleveland DW, Fuchs E (1996) An essential cytoskeletal linker protein connecting actin microfilaments to intermediate filaments. Cell 86:655–665
Guo L, Degenstein L, Dowling J, Yu QC, Wollmann R, Perman B, Fuchs E (1995) Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell 81:233–243
Brown A, Bernier G, Mathieu M, Rossant J, Kothary R (1995) The mouse dystonia musculorum gene is a neural isoform of bullous pemphigoid antigen 1. Nat Genet 10:301–306
Eyer J, Cleveland DW, Wong PC, Peterson AC (1998) Pathogenesis of two axonopathies does not require axonal neurofilaments. Nature 391:584–587
Yang Y, Bauer C, Strasser G, Wollman R, Julien JP, Fuchs E (1999) Integrators of the cytoskeleton that stabilize microtubules. Cell 98:229–238
Leung CL, Zheng M, Prater SM, Liem RK (2001) The BPAG1 locus: alternative splicing produces multiple isoforms with distinct cytoskeletal linker domains, including predominant isoforms in neurons and muscles. J Cell Biol 154:691–697
Young KG, Kothary R (2007) Dystonin/Bpag1—a link to what? Cell Motil Cytoskeleton 64:897–905
Metuzals J, Mushynski WE (1974) Electron microscope and experimental investigations of the neurofilamentous network in Deiters’ neurons. Relationship with the cell surface and nuclear pores. J Cell Biol 61:701–722
Traub P, Perides G, Kuhn S, Scherbarth A (1987) Interaction in vitro of non-epithelial intermediate filament proteins with histones. Z Naturforsch [C] 42:47–63
Metuzals J, Fishman HM, Robb IA (1995) The neurofilamentous network-smooth endoplasmic reticulum complex in transected squid giant axon. Biol Bull 189:216–218
Leterrier JF, Rusakov DA, Nelson BD, Linden M (1994) Interactions between brain mitochondria and cytoskeleton: evidence for specialized outer membrane domains involved in the association of cytoskeleton-associated proteins to mitochondria in situ and in vitro. Microsc Res Tech 27:233–261
Morris RL, Hollenbeck PJ (1995) Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J Cell Biol 131:1315–1326
Straube-West K, Loomis PA, Opal P, Goldman RD (1996) Alterations in neural intermediate filament organization: functional implications and the induction of pathological changes related to motor neuron disease. J Cell Sci 109(Pt 9):2319–2329
Szebenyi G, Smith GM, Li P, Brady ST (2002) Overexpression of neurofilament H disrupts normal cell structure and function. J Neurosci Res 68:185–198
Wagner OI, Lifshitz J, Janmey PA, Linden M, McIntosh TK, Leterrier JF (2003) Mechanisms of mitochondria-neurofilament interactions. J Neurosci 23:9046–9058
Friede RL, Samorajski T (1970) Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat Rec 167:379–387
Hoffman PN, Griffin JW, Price DL (1984) Control of axonal caliber by neurofilament transport. J Cell Biol 99:705–714
Sanchez I, Hassinger L, Paskevich PA, Shine HD, Nixon RA (1996) Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. J Neurosci 16:5095–5105
Yamasaki H, Itakura C, Mizutani M (1991) Hereditary hypotrophic axonopathy with neurofilament deficiency in a mutant strain of the Japanese quail. Acta Neuropathol 82:427–434
Yamasaki H, Bennett GS, Itakura C, Mizutani M (1992) Defective expression of neurofilament protein subunits in hereditary hypotrophic axonopathy of quail. Lab Invest 66:734–743
Ohara O, Gahara Y, Miyake T, Teraoka H, Kitamura T (1993) Neurofilament deficiency in quail caused by nonsense mutation in neurofilament-L gene. J Cell Biol 121:387–395
Cote F, Collard JF, Julien JP (1993) Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Cell 73:35–46
Perrot R, Lonchampt P, Peterson AC, Eyer J (2007) Axonal neurofilaments control multiple fiber properties but do not influence structure or spacing of nodes of Ranvier. J Neurosci 27:9573–9584
Monteiro MJ, Hoffman PN, Gearhart JD, Cleveland DW (1990) Expression of NF-L in both neuronal and nonneuronal cells of transgenic mice: increased neurofilament density in axons without affecting caliber. J Cell Biol 111:1543–1557
Xu Z, Marszalek JR, Lee MK, Wong PC, Folmer J, Crawford TO, Hsieh ST, Griffin JW, Cleveland DW (1996) Subunit composition of neurofilaments specifies axonal diameter. J Cell Biol 133:1061–1069
Nguyen MD, Lariviere RC, Julien JP (2000) Reduction of axonal caliber does not alleviate motor neuron disease caused by mutant superoxide dismutase 1. Proc Natl Acad Sci U S A 97:12306–12311
Meier J, Couillard-Despres S, Jacomy H, Gravel C, Julien JP (1999) Extra neurofilament NF-L subunits rescue motor neuron disease caused by overexpression of the human NF-H gene in mice. J Neuropathol Exp Neurol 58:1099–1110
Elder GA, Friedrich VL Jr., Kang C, Bosco P, Gourov A, Tu PH, Zhang B, Lee VM, Lazzarini RA (1998) Requirement of heavy neurofilament subunit in the development of axons with large calibers. J Cell Biol 143:195–205
Rao MV, Houseweart MK, Williamson TL, Crawford TO, Folmer J, Cleveland DW (1998) Neurofilament-dependent radial growth of motor axons and axonal organization of neurofilaments does not require the neurofilament heavy subunit (NF-H) or its phosphorylation. J Cell Biol 143:171–181
Zhu Q, Lindenbaum M, Levavasseur F, Jacomy H, Julien JP (1998) Disruption of the NF-H gene increases axonal microtubule content and velocity of neurofilament transport: relief of axonopathy resulting from the toxin beta,beta’-iminodipropionitrile. J Cell Biol 143:183–193
Hirokawa N, Takeda S (1998) Gene targeting studies begin to reveal the function of neurofilament proteins. J Cell Biol 143:1–4
Gotow T, Takeda M, Tanaka T, Hashimoto PH (1992) Macromolecular structure of reassembled neurofilaments as revealed by the quick-freeze deep-etch mica method: difference between NF-M and NF-H subunits in their ability to form cross-bridges. Eur J Cell Biol 58:331–345
Brown HG, Hoh JH (1997) Entropic exclusion by neurofilament sidearms: a mechanism for maintaining interfilament spacing. Biochemistry 36:15035–15040
Kumar S, Hoh JH (2004) Modulation of repulsive forces between neurofilaments by sidearm phosphorylation. Biochem Biophys Res Commun 324:489–496
Aranda-Espinoza H, Carl P, Leterrier JF, Janmey P, Discher DE (2002) Domain unfolding in neurofilament sidearms: effects of phosphorylation and ATP. FEBS Lett 531:397–401
Wong PC, Marszalek J, Crawford TO, Xu Z, Hsieh ST, Griffin JW, Cleveland DW (1995) Increasing neurofilament subunit NF-M expression reduces axonal NF-H, inhibits radial growth, and results in neurofilamentous accumulation in motor neurons. J Cell Biol 130:1413–1422
Marszalek JR, Williamson TL, Lee MK, Xu Z, Hoffman PN, Becher MW, Crawford TO, Cleveland DW (1996) Neurofilament subunit NF-H modulates axonal diameter by selectively slowing neurofilament transport. J Cell Biol 135:711–724
Rao MV, Garcia ML, Miyazaki Y, Gotow T, Yuan A, Mattina S, Ward CM, Calcutt NA, Uchiyama Y, Nixon RA, Cleveland DW (2002) Gene replacement in mice reveals that the heavily phosphorylated tail of neurofilament heavy subunit does not affect axonal caliber or the transit of cargoes in slow axonal transport. J Cell Biol 158:681–693
Garcia ML, Lobsiger CS, Shah SB, Deerinck TJ, Crum J, Young D, Ward CM, Crawford TO, Gotow T, Uchiyama Y, Ellisman MH, Calcutt NA, Cleveland DW (2003) NF-M is an essential target for the myelin-directed “outside-in” signaling cascade that mediates radial axonal growth. J Cell Biol 163:1011–1020
Rao MV, Campbell J, Yuan A, Kumar A, Gotow T, Uchiyama Y, Nixon RA (2003) The neurofilament middle molecular mass subunit carboxyl-terminal tail domains is essential for the radial growth and cytoskeletal architecture of axons but not for regulating neurofilament transport rate. J Cell Biol 163:1021–1031
Windebank AJ, Wood P, Bunge RP, Dyck PJ (1985) Myelination determines the caliber of dorsal root ganglion neurons in culture. J Neurosci 5:1563–1569
Aguayo AJ, Attiwell M, Trecarten J, Perkins S, Bray GM (1977) Abnormal myelination in transplanted Trembler mouse Schwann cells. Nature 265:73–75
Pollard JD, McLeod JG (1980) Nerve grafts in the Trembler mouse. An electrophysiological and histological study. J Neurol Sci 46:373–383
Kirkpatrick LL, Witt AS, Payne HR, Shine HD, Brady ST (2001) Changes in microtubule stability and density in myelin-deficient shiverer mouse CNS axons. J Neurosci 21:2288–2297
Sternberger NH, Quarles RH, Itoyama Y, Webster HD (1979) Myelin-associated glycoprotein demonstrated immunocytochemically in myelin and myelin-forming cells of developing rat. Proc Natl Acad Sci U S A 76:1510–1514
Trapp BD, Andrews SB, Cootauco C, Quarles R (1989) The myelin-associated glycoprotein is enriched in multivesicular bodies and periaxonal membranes of actively myelinating oligodendrocytes. J Cell Biol 109:2417–2426
Lunn MP, Crawford TO, Hughes RA, Griffin JW, Sheikh KA (2002) Anti-myelin-associated glycoprotein antibodies alter neurofilament spacing. Brain 125:904–911
Gasser HS, Grundfest H (1939) Axon diameters in relation to the spike dimensions and the conduction velocity in mammalian A fibers. Am J Physiol 127:393–414
Hursh JB (1939) Conduction velocity and diameter of nerve fibers. Am J Physiol 127:131–139
Hutchinson NA, Koles ZJ, Smith RS (1970) Conduction velocity in myelinated nerve fibres of Xenopus laevis. J Physiol 208:279–289
Huxley AF, Stampfli R (1949) Evidence for saltatory conduction in peripheral myelinated nerve fibres. J Physiol 108:315–339
Goldman L, Albus JS (1968) Computation of impulse conduction in myelinated fibers; theoretical basis of the velocity-diameter relation. Biophys J 8:596–607
Brill MH, Waxman SG, Moore JW, Joyner RW (1977) Conduction velocity and spike configuration in myelinated fibres: computed dependence on internode distance. J Neurol Neurosurg Psychiatry 40:769–774
Court FA, Sherman DL, Pratt T, Garry EM, Ribchester RR, Cottrell DF, Fleetwood-Walker SM, Brophy PJ (2004) Restricted growth of Schwann cells lacking Cajal bands slows conduction in myelinated nerves. Nature 431:191–195
Rushton WA (1951) A theory of the effects of fibre size in medullated nerve. J Physiol 115:101–122
Hodgkin AL (1964) The ionic basis of nervous conduction. Science 145:1148–1154
Smith RS, Koles ZJ (1970) Myelinated nerve fibers: computed effect of myelin thickness on conduction velocity. Am J Physiol 219:1256–1258
Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ (2001) Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron 30:369–383
Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B (2001) Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron 30:385–397
Murray JA, Blakemore WF (1980) The relationship between internodal length and fibre diameter in the spinal cord of the cat. J Neurol Sci 45:29–41
Friede RL, Meier T, Diem M (1981) How is the exact length of an internode determined. J Neurol Sci 50:217–228
Fried K, Hildebrand C, Erdelyi G (1982) Myelin sheath thickness and internodal length of nerve fibres in the developing feline inferior alveolar nerve. J Neurol Sci 54:47–57
Sakaguchi T, Okada M, Kitamura T, Kawasaki K (1993) Reduced diameter and conduction velocity of myelinated fibers in the sciatic nerve of a neurofilament-deficient mutant quail. Neurosci Lett 153:65–68
Kriz J, Zhu Q, Julien JP, Padjen AL (2000) Electrophysiological properties of axons in mice lacking neurofilament subunit genes: disparity between conduction velocity and axon diameter in absence of NF-H. Brain Res 885:32–44
Kriz J, Meier J, Julien JP, Padjen AL (2000) Altered ionic conductances in axons of transgenic mouse expressing the human neurofilament heavy gene: A mouse model of amyotrophic lateral sclerosis. Exp Neurol 163:414–421
Zochodne DW, Sun HS, Cheng C, Eyer J (2004) Accelerated diabetic neuropathy in axons without neurofilaments. Brain 127:2193–2200
Sheykholeslami K, Kaga K, Mizutani M (2001) Auditory nerve fiber differences in the normal and neurofilament deficient Japanese quail. Hear Res 159:117–124
Elder GA, Friedrich VL Jr., Lazzarini RA (2001) Schwann cells and oligodendrocytes read distinct signals in establishing myelin sheath thickness. J Neurosci Res 65:493–499
Berthold CH, Rydmark M (1983) Electron microscopic serial section analysis of nodes of Ranvier in lumbosacral spinal roots of the cat: ultrastructural organization of nodal compartments in fibres of different sizes. J Neurocytol 12:475–505
Hildebrand C, Remahl S, Persson H, Bjartmar C (1993) Myelinated nerve fibres in the CNS. Prog Neurobiol 40:319–384
Halter JA, Clark JW Jr. (1993) The influence of nodal constriction on conduction velocity in myelinated nerve fibers. Neuroreport 4:89–92
Zimmermann H (1996) Accumulation of synaptic vesicle proteins and cytoskeletal specializations at the peripheral node of Ranvier. Microsc Res Tech 34:462–473
Zimmermann H, Vogt M (1989) Membrane proteins of synaptic vesicles and cytoskeletal specializations at the node of Ranvier in electric ray and rat. Cell Tissue Res 258:617–629
Weiss P, Hiscoe H (1948) Experiments in the mechanism of nerve growth. J Exp Zool 107:315–395
Droz B, Leblond CP (1962) Migration of proteins along the axons of the sciatic nerve. Science 137:1047–1048
Lasek RJ (1967) Bidirectional transport of radioactively labelled axoplasmic components. Nature 216:1212–1214
Lasek RJ (1968) Axoplasmic transport of labeled proteins in rat ventral motoneurons. Exp Neurol 21:41–51
Grafstein B, Forman DS (1980) Intracellular transport in neurons. Physiol Rev 60:1167–1283
Dahlstrom A, Haggendal J, Heiwall PO, Larsson PA, Saunders NR (1974) Intra-axonal transport of neurotransmitters in mammalian neurons. Symp Soc Exp Biol 229–247
Lombet A, Laduron P, Mourre C, Jacomet Y, Lazdunski M (1985) Axonal transport of the voltage-dependent Na+ channel protein identified by its tetrodotoxin binding site in rat sciatic nerves. Brain Res 345:153–158
Hollenbeck PJ (1996) The pattern and mechanism of mitochondrial transport in axons. Front Biosci 1:d91–d102
Black MM, Lasek RJ (1980) Slow components of axonal transport: two cytoskeletal networks. J Cell Biol 86:616–623
Brady ST (1985) A novel brain ATPase with properties expected for the fast axonal transport motor. Nature 317:73–75
Vale RD, Reese TS, Sheetz MP (1985) Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 42:39–50
Roy S, Coffee P, Smith G, Liem RK, Brady ST, Black MM (2000) Neurofilaments are transported rapidly but intermittently in axons: implications for slow axonal transport. J Neurosci 20:6849–6861
Wang L, Ho CL, Sun D, Liem RK, Brown A (2000) Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nat Cell Biol 2:137–141
Trivedi N, Jung P, Brown A (2007) Neurofilaments switch between distinct mobile and stationary states during their transport along axons. J Neurosci 27:507–516
Prahlad V, Helfand BT, Langford GM, Vale RD, Goldman RD (2000) Fast transport of neurofilament protein along microtubules in squid axoplasm. J Cell Sci 113(Pt 22):3939–3946
Jung C, Lee S, Ortiz D, Zhu Q, Julien JP, Shea TB (2005) The high and middle molecular weight neurofilament subunits regulate the association of neurofilaments with kinesin: inhibition by phosphorylation of the high molecular weight subunit. Brain Res Mol Brain Res 141:151–155
LaMonte BH, Wallace KE, Holloway BA, Shelly SS, Ascano J, Tokito M, Van Winkle T, Howland DS, Holzbaur EL (2002) Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron 34:715–727
Xia CH, Roberts EA, Her LS, Liu X, Williams DS, Cleveland DW, Goldstein LS (2003) Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J Cell Biol 161:55–66
Baas PW, Brown A (1997) Slow axonal transport: the polymer transport model. Trends Cell Biol 7:380–384
Hirokawa N, Funakoshi ST, Takeda S (1997) Slow axonal transport: the subunit transport model. Trends Cell Biol 7:384–388
Lasek RJ, Garner JA, Brady ST (1984) Axonal transport of the cytoplasmic matrix. J Cell Biol 99:212s–221s
Okabe S, Miyasaka H, Hirokawa N (1993) Dynamics of the neuronal intermediate filaments. J Cell Biol 121:375–386
Terada S, Nakata T, Peterson AC, Hirokawa N (1996) Visualization of slow axonal transport in vivo. Science 273:784–788
Popov S, Poo MM (1992) Diffusional transport of macromolecules in developing nerve processes. J Neurosci 12:77–85
Yan Y, Brown A (2005) Neurofilament polymer transport in axons. J Neurosci 25:7014–7021
Yan Y, Jensen K, Brown A (2007) The polypeptide composition of moving and stationary neurofilaments in cultured sympathetic neurons. Cell Motil Cytoskeleton 64:299–309
Yuan A, Rao MV, Kumar A, Julien JP, Nixon RA (2003) Neurofilament transport in vivo minimally requires hetero-oligomer formation. J Neurosci 23:9452–9458
Hoffman PN, Lasek RJ, Griffin JW, Price DL (1983) Slowing of the axonal transport of neurofilament proteins during development. J Neurosci 3:1694–1700
Yuan A, Nixon RA, Rao MV (2006) Deleting the phosphorylated tail domain of the neurofilament heavy subunit does not alter neurofilament transport rate in vivo. Neurosci Lett 393:264–268
Brown A, Wang L, Jung P (2005) Stochastic simulation of neurofilament transport in axons: the “stop-and-go” hypothesis. Mol Biol Cell 16:4243–4255
Carpenter S (1968) Proximal axonal enlargement in motor neuron disease. Neurology 18:841–851
Averback P (1981) Unusual particles in motor neuron disease. Arch Pathol Lab Med 105:490–493
Delisle MB, Carpenter S (1984) Neurofibrillary axonal swellings and amyotrophic lateral sclerosis. J Neurol Sci 63:241–250
Hirano A, Donnenfeld H, Sasaki S, Nakano I (1984) Fine structural observations of neurofilamentous changes in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 43:461–470
Munoz DG, Greene C, Perl DP, Selkoe DJ (1988) Accumulation of phosphorylated neurofilaments in anterior horn motoneurons of amyotrophic lateral sclerosis patients. J Neuropathol Exp Neurol 47:9–18
Murayama S, Bouldin TW, Suzuki K (1992) Immunocytochemical and ultrastructural studies of upper motor neurons in amyotrophic lateral sclerosis. Acta Neuropathol 83:518–524
Xu Z, Cork LC, Griffin JW, Cleveland DW (1993) Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell 73:23–33
Bergeron C, Beric-Maskarel K, Muntasser S, Weyer L, Somerville MJ, Percy ME (1994) Neurofilament light and polyadenylated mRNA levels are decreased in amyotrophic lateral sclerosis motor neurons. J Neuropathol Exp Neurol 53:221–230
Wong NK, He BP, Strong MJ (2000) Characterization of neuronal intermediate filament protein expression in cervical spinal motor neurons in sporadic amyotrophic lateral sclerosis (ALS). J Neuropathol Exp Neurol 59:972–982
Lee MK, Marszalek JR, Cleveland DW (1994) A mutant neurofilament subunit causes massive, selective motor neuron death: implications for the pathogenesis of human motor neuron disease. Neuron 13:975–988
Figlewicz DA, Krizus A, Martinoli MG, Meininger V, Dib M, Rouleau GA, Julien JP (1994) Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum Mol Genet 3:1757–1761
Tomkins J, Usher P, Slade JY, Ince PG, Curtis A, Bushby K, Shaw PJ (1998) Novel insertion in the KSP region of the neurofilament heavy gene in amyotrophic lateral sclerosis (ALS). Neuroreport 9:3967–3970
Al-Chalabi A, Andersen PM, Nilsson P, Chioza B, Andersson JL, Russ C, Shaw CE, Powell JF, Leigh PN (1999) Deletions of the heavy neurofilament subunit tail in amyotrophic lateral sclerosis. Hum Mol Genet 8:157–164
Rooke K, Figlewicz DA, Han FY, Rouleau GA (1996) Analysis of the KSP repeat of the neurofilament heavy subunit in familiar amyotrophic lateral sclerosis. Neurology 46:789–790
Vechio JD, Bruijn LI, Xu Z, Brown RH Jr., Cleveland DW (1996) Sequence variants in human neurofilament proteins: absence of linkage to familial amyotrophic lateral sclerosis. Ann Neurol 40:603–610
Tu PH, Raju P, Robinson KA, Gurney ME, Trojanowski JQ, Lee VM (1996) Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc Natl Acad Sci U S A 93:3155–3160
Borchelt DR, Wong PC, Becher MW, Pardo CA, Lee MK, Xu ZS, Thinakaran G, Jenkins NA, Copeland NG, Sisodia SS, Cleveland DW, Price DL, Hoffman PN (1998) Axonal transport of mutant superoxide dismutase 1 and focal axonal abnormalities in the proximal axons of transgenic mice. Neurobiol Dis 5:27–35
Williamson TL, Cleveland DW (1999) Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci 2:50–56
Zhang B, Tu P, Abtahian F, Trojanowski JQ, Lee VM (1997) Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J Cell Biol 139:1307–1315
Williamson TL, Bruijn LI, Zhu Q, Anderson KL, Anderson SD, Julien JP, Cleveland DW (1998) Absence of neurofilaments reduces the selective vulnerability of motor neurons and slows disease caused by a familial amyotrophic lateral sclerosis-linked superoxide dismutase 1 mutant. Proc Natl Acad Sci U S A 95:9631–9636
Kong J, Xu Z (2000) Overexpression of neurofilament subunit NF-L and NF-H extends survival of a mouse model for amyotrophic lateral sclerosis. Neurosci Lett 281:72–74
Couillard-Despres S, Zhu Q, Wong PC, Price DL, Cleveland DW, Julien JP (1998) Protective effect of neurofilament heavy gene overexpression in motor neuron disease induced by mutant superoxide dismutase. Proc Natl Acad Sci U S A 95:9626–9630
Roy J, Minotti S, Dong L, Figlewicz DA, Durham HD (1998) Glutamate potentiates the toxicity of mutant Cu/Zn-superoxide dismutase in motor neurons by postsynaptic calcium-dependent mechanisms. J Neurosci 18:9673–9684
Ehlers MD, Fung ET, O’Brien RJ, Huganir RL (1998) Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci 18:720–730
Nguyen MD, Lariviere RC, Julien JP (2001) Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron 30:135–147
Manetto V, Sternberger NH, Perry G, Sternberger LA, Gambetti P (1988) Phosphorylation of neurofilaments is altered in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 47:642–653
Morrison BM, Gordon JW, Ripps ME, Morrison JH (1996) Quantitative immunocytochemical analysis of the spinal cord in G86R superoxide dismutase transgenic mice: neurochemical correlates of selective vulnerability. J Comp Neurol 373:619–631
Lobsiger CS, Garcia ML, Ward CM, Cleveland DW (2005) Altered axonal architecture by removal of the heavily phosphorylated neurofilament tail domains strongly slows superoxide dismutase 1 mutant-mediated ALS. Proc Natl Acad Sci U S A 102:10351–10356
Vande Velde C, Garcia ML, Yin X, Trapp BD, Cleveland DW (2004) The neuroprotective factor Wlds does not attenuate mutant SOD1-mediated motor neuron disease. Neuromolecular Med 5:193–203
Migheli A, Pezzulo T, Attanasio A, Schiffer D (1993) Peripherin immunoreactive structures in amyotrophic lateral sclerosis. Lab Invest 68:185–191
Beaulieu JM, Nguyen MD, Julien JP (1999) Late onset of motor neurons in mice overexpressing wild-type peripherin. J Cell Biol 147:531–544
Beaulieu JM, Jacomy H, Julien JP (2000) Formation of intermediate filament protein aggregates with disparate effects in two transgenic mouse models lacking the neurofilament light subunit. J Neurosci 20:5321–5328
Robertson J, Beaulieu JM, Doroudchi MM, Durham HD, Julien JP, Mushynski WE (2001) Apoptotic death of neurons exhibiting peripherin aggregates is mediated by the proinflammatory cytokine tumor necrosis factor-alpha. J Cell Biol 155:217–226
Beaulieu JM, Julien JP (2003) Peripherin-mediated death of motor neurons rescued by overexpression of neurofilament NF-H proteins. J Neurochem 85:248–256
Ishii T, Haga S, Tokutake S (1979) Presence of neurofilament protein in Alzheimer’s neurofibrillary tangles (ANT). An immunofluorescent study. Acta Neuropathol 48:105–112
Nukina N, Kosik KS, Selkoe DJ (1987) Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to cross-reaction with tau protein. Proc Natl Acad Sci U S A 84:3415–3419
Schmidt ML, Lee VM, Trojanowski JQ (1990) Relative abundance of tau and neurofilament epitopes in hippocampal neurofibrillary tangles. Am J Pathol 136:1069–1075
Selkoe DJ, Ihara Y, Salazar FJ (1982) Alzheimer’s disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science 215:1243–1245
Sternberger NH, Sternberger LA, Ulrich J (1985) Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci U S A 82:4274–4276
Lee VM, Otvos L Jr., Schmidt ML, Trojanowski JQ (1988) Alzheimer disease tangles share immunological similarities with multiphosphorylation repeats in the two large neurofilament proteins. Proc Natl Acad Sci U S A 85:7384–7388
Wang J, Tung YC, Wang Y, Li XT, Iqbal K, Grundke-Iqbal I (2001) Hyperphosphorylation and accumulation of neurofilament proteins in Alzheimer disease brain and in okadaic acid-treated SY5Y cells. FEBS Lett 507:81–87
Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K (1995) Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem 65:732–738
Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K (1993) Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem 61:921–927
Gong CX, Wang JZ, Iqbal K, Grundke-Iqbal I (2003) Inhibition of protein phosphatase 2A induces phosphorylation and accumulation of neurofilaments in metabolically active rat brain slices. Neurosci Lett 340:107–110
Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2005) Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci 22:1942–1950
Deng Y, Li B, Liu F, Iqbal K, Grundke-Iqbal I, Brandt R, Gong CX (2008) Regulation between O-GlcNAcylation and phosphorylation of neurofilament-M and their dysregulation in Alzheimer disease. Faseb J 22:138–145
Goldman JE, Yen SH, Chiu FC, Peress NS (1983) Lewy bodies of Parkinson’s disease contain neurofilament antigens. Science 221:1082–1084
Galloway PG, Mulvihill P, Perry G (1992) Filaments of Lewy bodies contain insoluble cytoskeletal elements. Am J Pathol 140:809–822
Iwatsubo T, Yamaguchi H, Fujimuro M, Yokosawa H, Ihara Y, Trojanowski JQ, Lee VM (1996) Purification and characterization of Lewy bodies from the brains of patients with diffuse Lewy body disease. Am J Pathol 148:1517–1529
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A 95:6469–6473
Trimmer PA, Borland MK, Keeney PM, Bennett JP Jr., Parker WD Jr. (2004) Parkinson’s disease transgenic mitochondrial cybrids generate Lewy inclusion bodies. J Neurochem 88:800–812
Forno LS, Sternberger LA, Sternberger NH, Strefling AM, Swanson K, Eng LF (1986) Reaction of Lewy bodies with antibodies to phosphorylated and non-phosphorylated neurofilaments. Neurosci Lett 64:253–258
Pappolla MA (1986) Lewy bodies of Parkinson’s disease. Immune electron microscopic demonstration of neurofilament antigens in constituent filaments. Arch Pathol Lab Med 110:1160–1163
Leroy E, Anastasopoulos D, Konitsiotis S, Lavedan C, Polymeropoulos MH (1998) Deletions in the Parkin gene and genetic heterogeneity in a Greek family with early onset Parkinson’s disease. Hum Genet 103:424–427
Abbas N, Lucking CB, Ricard S, Durr A, Bonifati V, De Michele G, Bouley S, Vaughan JR, Gasser T, Marconi R, Broussolle E, Brefel-Courbon C, Harhangi BS, Oostra BA, Fabrizio E, Bohme GA, Pradier L, Wood NW, Filla A, Meco G, Denefle P, Agid Y, Brice A (1999) A wide variety of mutations in the Parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson’s Disease Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson’s Disease. Hum Mol Genet 8:567–574
Lucking CB, Durr A, Bonifati V, Vaughan J, De Michele G, Gasser T, Harhangi BS, Meco G, Denefle P, Wood NW, Agid Y, Brice A (2000) Association between early-onset Parkinson’s disease and mutations in the Parkin gene. N Engl J Med 342:1560–1567
Lavedan C, Buchholtz S, Nussbaum RL, Albin RL, Polymeropoulos MH (2002) A mutation in the human neurofilament M gene in Parkinson’s disease that suggests a role for the cytoskeleton in neuronal degeneration. Neurosci Lett 322:57–61
Perez-Olle R, Lopez-Toledano MA, Liem RK (2004) The G336S variant in the human neurofilament-M gene does not affect its assembly or distribution: importance of the functional analysis of neurofilament variants. J Neuropathol Exp Neurol 63:759–774
Han F, Bulman DE, Panisset M, Grimes DA (2005) Neurofilament M gene in a French-Canadian population with Parkinson’s disease. Can J Neurol Sci 32:68–70
Kruger R, Fischer C, Schulte T, Strauss KM, Muller T, Woitalla D, Berg D, Hungs M, Gobbele R, Berger K, Epplen JT, Riess O, Schols L (2003) Mutation analysis of the neurofilament M gene in Parkinson’s disease. Neurosci Lett 351:125–129
Rahner N, Holzmann C, Kruger R, Schols L, Berger K, Riess O (2002) Neurofilament L gene is not a genetic factor of sporadic and familial Parkinson’s disease. Brain Res 951:82–86
Skre H (1974) Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet 6:98–118
Vogel P, Gabriel M, Goebel HH, Dyck PJ (1985) Hereditary motor sensory neuropathy type II with neurofilament accumulation: new finding or new disorder? Ann Neurol 17:455–461
Mersiyanova IV, Perepelov AV, Polyakov AV, Sitnikov VF, Dadali EL, Oparin RB, Petrin AN, Evgrafov OV (2000) A new variant of Charcot-Marie-Tooth disease type 2 is probably the result of a mutation in the neurofilament-light gene. Am J Hum Genet 67:37–46
De Jonghe P, Mersivanova I, Nelis E, Del Favero J, Martin JJ, Van Broeckhoven C, Evgrafov O, Timmerman V (2001) Further evidence that neurofilament light chain gene mutations can cause Charcot-Marie-Tooth disease type 2E. Ann Neurol 49:245–249
Brownlees J, Ackerley S, Grierson AJ, Jacobsen NJ, Shea K, Anderton BH, Leigh PN, Shaw CE, Miller CC (2002) Charcot-Marie-Tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum Mol Genet 11:2837–2844
Perez-Olle R, Leung CL, Liem RK (2002) Effects of Charcot-Marie-Tooth-linked mutations of the neurofilament light subunit on intermediate filament formation. J Cell Sci 115:4937–4946
Perez-Olle R, Lopez-Toledano MA, Goryunov D, Cabrera-Poch N, Stefanis L, Brown K, Liem RK (2005) Mutations in the neurofilament light gene linked to Charcot-Marie-Tooth disease cause defects in transport. J Neurochem 93:861–874
Georgiou DM, Zidar J, Korosec M, Middleton LT, Kyriakides T, Christodoulou K (2002) A novel NF-L mutation Pro22Ser is associated with CMT2 in a large Slovenian family. Neurogenetics 4:93–96
Fabrizi GM, Cavallaro T, Angiari C, Bertolasi L, Cabrini I, Ferrarini M, Rizzuto N (2004) Giant axon and neurofilament accumulation in Charcot-Marie-Tooth disease type 2E. Neurology 62:1429–1431
Yoshihara T, Yamamoto M, Hattori N, Misu K, Mori K, Koike H, Sobue G (2002) Identification of novel sequence variants in the neurofilament-light gene in a Japanese population: analysis of Charcot-Marie-Tooth disease patients and normal individuals. J Peripher Nerv Syst 7:221–224
Jordanova A, De Jonghe P, Boerkoel CF, Takashima H, De Vriendt E, Ceuterick C, Martin JJ, Butler IJ, Mancias P, Papasozomenos S, Terespolsky D, Potocki L, Brown CW, Shy M, Rita DA, Tournev I, Kremensky I, Lupski JR, Timmerman V (2003) Mutations in the neurofilament light chain gene (NEFL) cause early onset severe Charcot-Marie-Tooth disease. Brain 126:590–597
Fabrizi GM, Cavallaro T, Angiari C, Cabrini I, Taioli F, Malerba G, Bertolasi L, Rizzuto N (2007) Charcot-Marie-Tooth disease type 2E, a disorder of the cytoskeleton. Brain 130:394–403
Leung CL, Nagan N, Graham TH, Liem RK (2006) A novel duplication/insertion mutation of NEFL in a patient with Charcot-Marie-Tooth disease. Am J Med Genet A 140:1021–1025
Goryunov D, Nightingale A, Bornfleth L, Leung C, Liem RK (2008) Multiple disease-linked myotubularin mutations cause NFL assembly defects in cultured cells and disrupt myotubularin dimerization. J Neurochem 104:1536–1552
Sahenk Z (1999) Abnormal Schwann cell-axon interactions in CMT neuropathies. The effects of mutant Schwann cells on the axonal cytoskeleton and regeneration-associated myelination. Ann N Y Acad Sci 883:415–426
Bigio EH, Lipton AM, White CL 3rd, Dickson DW, Hirano A (2003) Frontotemporal and motor neurone degeneration with neurofilament inclusion bodies: additional evidence for overlap between FTD and ALS. Neuropathol Appl Neurobiol 29:239–253
Cairns NJ, Perry RH, Jaros E, Burn D, McKeith IG, Lowe JS, Holton J, Rossor MN, Skullerud K, Duyckaerts C, Cruz-Sanchez FF, Lantos PL (2003) Patients with a novel neurofilamentopathy: dementia with neurofilament inclusions. Neurosci Lett 341:177–180
Josephs KA, Holton JL, Rossor MN, Braendgaard H, Ozawa T, Fox NC, Petersen RC, Pearl GS, Ganguly M, Rosa P, Laursen H, Parisi JE, Waldemar G, Quinn NP, Dickson DW, Revesz T (2003) Neurofilament inclusion body disease: a new proteinopathy? Brain 126:2291–2303
Cairns NJ, Grossman M, Arnold SE, Burn DJ, Jaros E, Perry RH, Duyckaerts C, Stankoff B, Pillon B, Skullerud K, Cruz-Sanchez FF, Bigio EH, Mackenzie IR, Gearing M, Juncos JL, Glass JD, Yokoo H, Nakazato Y, Mosaheb S, Thorpe JR, Uryu K, Lee VM, Trojanowski JQ (2004) Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology 63:1376–1384
Cairns NJ, Zhukareva V, Uryu K, Zhang B, Bigio E, Mackenzie IR, Gearing M, Duyckaerts C, Yokoo H, Nakazato Y, Jaros E, Perry RH, Lee VM, Trojanowski JQ (2004) alpha-internexin is present in the pathological inclusions of neuronal intermediate filament inclusion disease. Am J Pathol 164:2153–2161
Uchikado H, Shaw G, Wang DS, Dickson DW (2005) Screening for neurofilament inclusion disease using alpha-internexin immunohistochemistry. Neurology 64:1658–1659
Momeni P, Cairns NJ, Perry RH, Bigio EH, Gearing M, Singleton AB, Hardy J (2006) Mutation analysis of patients with neuronal intermediate filament inclusion disease (NIFID). Neurobiol Aging 27:778 e771–778 e776
Medori R, Autilio-Gambetti L, Monaco S, Gambetti P (1985) Experimental diabetic neuropathy: impairment of slow transport with changes in axon cross-sectional area. Proc Natl Acad Sci U S A 82:7716–7720
Medori R, Jenich H, Autilio-Gambetti L, Gambetti P (1988) Experimental diabetic neuropathy: similar changes of slow axonal transport and axonal size in different animal models. J Neurosci 8:1814–1821
Yagihashi S, Kamijo M, Watanabe K (1990) Reduced myelinated fiber size correlates with loss of axonal neurofilaments in peripheral nerve of chronically streptozotocin diabetic rats. Am J Pathol 136:1365–1373
Schmidt RE, Beaudet LN, Plurad SB, Dorsey DA (1997) Axonal cytoskeletal pathology in aged and diabetic human sympathetic autonomic ganglia. Brain Res 769:375–383
Fernyhough P, Gallagher A, Averill SA, Priestley JV, Hounsom L, Patel J, Tomlinson DR (1999) Aberrant neurofilament phosphorylation in sensory neurons of rats with diabetic neuropathy. Diabetes 48:881–889
Scott JN, Clark AW, Zochodne DW (1999) Neurofilament and tubulin gene expression in progressive experimental diabetes: failure of synthesis and export by sensory neurons. Brain 122(Pt 11):2109–2118
Berg BO, Rosenberg SH, Asbury AK (1972) Giant axonal neuropathy. Pediatrics 49:894–899
Igisu H, Ohta M, Tabira T, Hosokawa S, Goto I (1975) Giant axonal neuropathy. A clinical entity affecting the central as well as the peripheral nervous system. Neurology 25:717–721
Bomont P, Cavalier L, Blondeau F, Ben Hamida C, Belal S, Tazir M, Demir E, Topaloglu H, Korinthenberg R, Tuysuz B, Landrieu P, Hentati F, Koenig M (2000) The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat Genet 26:370–374
Pfeiffer J, Schlote W, Bishoff A, Boltshauser E, Müller GS (1977) Generalized giant axonal neuropathy. A filament-forming disease of neuronal, endothelial, glial and Schwann cells in a patient without kinky hair. Acta Neuropathol (Berl.) 40:213–218
Asbury AK, Gale MK, Cox SC, Baringer JR, Berg BO (1972) Giant axonal neuropathy—a unique case with segmental neurofilamentous masses. Acta Neuropathol 20:237–247
Fois A, Balestri P, Farnetani MA, Berardi R, Mattei R, Laurenzi E, Alessandrini C, Gerli R, Ribuffo A, Calvieri S (1985) Giant axonal neuropathy. Endocrinological and histological studies. Eur J Pediatr 144:274–280
Mohri I, Taniike M, Yoshikawa H, Higashiyama M, Itami S, Okada S (1998) A case of giant axonal neuropathy showing focal aggregation and hypophosphorylation of intermediate filaments. Brain Dev 20:594–597
Takebe Y, Koide N, Takahashi G (1981) Giant axonal neuropathy: report of two siblings with endocrinological and histological studies. Neuropediatrics 12:392–404
Treiber-Held S, Budjarjo-Welim H, Reimann D, Richter J, Kretzschmar HA, Hanefeld F (1994) Giant axonal neuropathy: a generalized disorder of intermediate filaments with longitudinal grooves in the hair. Neuropediatrics 25:89–93
Yang Y, Allen E, Ding J, Wang W (2007) Giant axonal neuropathy. Cell Mol Life Sci 64:601–609
Donaghy M, King RH, Thomas PK, Workman JM (1988) Abnormalities of the axonal cytoskeleton in giant axonal neuropathy. J Neurocytol 17:197–208
Monaco S, Autilio-Gambetti L, Zabel D, Gambetti P (1985) Giant axonal neuropathy: acceleration of neurofilament transport in optic axons. Proc Natl Acad Sci U S A 82:920–924
Ding J, Allen E, Wang W, Valle A, Wu C, Nardine T, Cui B, Yi J, Taylor A, Jeon NL, Chu S, So Y, Vogel H, Tolwani R, Mobley W, Yang Y (2006) Gene targeting of GAN in mouse causes a toxic accumulation of microtubule-associated protein 8 and impaired retrograde axonal transport. Hum Mol Genet 15:1451–1463
Ding J, Liu JJ, Kowal AS, Nardine T, Bhattacharya P, Lee A, Yang Y (2002) Microtubule-associated protein 1B: a neuronal binding partner for gigaxonin. J Cell Biol 158:427–433
Wang W, Ding J, Allen E, Zhu P, Zhang L, Vogel H, Yang Y (2005) Gigaxonin interacts with tubulin folding cofactor B and controls its degradation through the ubiquitin-proteasome pathway. Curr Biol 15:2050–2055
Allen E, Ding J, Wang W, Pramanik S, Chou J, Yau V, Yang Y (2005) Gigaxonin-controlled degradation of MAP1B light chain is critical to neuronal survival. Nature 438:224–228
Griffin JW, Hoffman PN, Clark AW, Carroll PT, Price DL (1978) Slow axonal transport of neurofilament proteins: impairment of beta,beta’-iminodipropionitrile administration. Science 202:633–635
Eyer J, McLean WG, Leterrier JF (1989) Effect of a single dose of beta,beta’-iminodipropionitrile in vivo on the properties of neurofilaments in vitro: comparison with the effect of iminodipropionitrile added directly to neurofilaments in vitro. J Neurochem 52:1759–1765
Griffin JW, Parhad I, Gold B, Price DL, Hoffman PN, Fahnestock K (1985) Axonal transport of neurofilament proteins in IDPN neurotoxicity. Neurotoxicology 6:43–53
Bizzi A, Gambetti P (1986) Phosphorylation of neurofilaments is altered in aluminium intoxication. Acta Neuropathol 71:154–158
Shea TB, Balikian P, Beermann ML (1992) Aluminum inhibits neurofilament protein degradation by multiple cytoskeleton-associated proteases. FEBS Lett 307:195–198
Shea TB, Beermann ML (1994) Multiple interactions of aluminum with neurofilament subunits: regulation by phosphate-dependent interactions between C-terminal extensions of the high and middle molecular weight subunits. J Neurosci Res 38:160–166
Shea TB, Wheeler E, Jung C (1997) Aluminum inhibits neurofilament assembly, cytoskeletal incorporation, and axonal transport. Dynamic nature of aluminum-induced perikaryal neurofilament accumulations as revealed by subunit turnover. Mol Chem Neuropathol 32:17–39
Howland RD, Alli P (1986) Altered phosphorylation of rat neuronal cytoskeletal proteins in acrylamide induced neuropathy. Brain Res 363:333–339
Gold BG, Price DL, Griffin JW, Rosenfeld J, Hoffman PN, Sternberger NH, Sternberger LA (1988) Neurofilament antigens in acrylamide neuropathy. J Neuropathol Exp Neurol 47:145–157
Endo H, Kittur S, Sabri MI (1994) Acrylamide alters neurofilament protein gene expression in rat brain. Neurochem Res 19:815–820
Tanii H, Hayashi M, Hashimoto K (1988) Neurofilament degradation in the nervous system of rats intoxicated with acrylamide, related compounds or 2,5-hexanedione. Arch Toxicol 62:70–75
Sickles DW, Pearson JK, Beall A, Testino A (1994) Toxic axonal degeneration occurs independent of neurofilament accumulation. J Neurosci Res 39:347–354
Takahashi A, Mizutani M, Agr B, Itakura C (1994) Acrylamide-induced neurotoxicity in the central nervous system of Japanese quails. Comparative studies of normal and neurofilament-deficient quails. J Neuropathol Exp Neurol 53:276–283
Stone JD, Peterson AP, Eyer J, Oblak TG, Sickles DW (2001) Neurofilaments are nonessential to the pathogenesis of toxicant-induced axonal degeneration. J Neurosci 21:2278–2287
Vahidnia A, Romijn F, Tiller M, van der Voet GB, de Wolff FA (2006) Arsenic-induced toxicity: effect on protein composition in sciatic nerve. Hum Exp Toxicol 25:667–674
DeFuria J, Shea TB (2007) Arsenic inhibits neurofilament transport and induces perikaryal accumulation of phosphorylated neurofilaments: roles of JNK and GSK-3beta. Brain Res 1181:74–82
Lurie DI, Brooks DM, Gray LC (2006) The effect of lead on the avian auditory brainstem. Neurotoxicology 27:108–117
Jones LG, Prins J, Park S, Walton JP, Luebke AE, Lurie DI (2008) Lead exposure during development results in increased neurofilament phosphorylation, neuritic beading, and temporal processing deficits within the murine auditory brainstem. J Comp Neurol 506:1003–1017
Frappier T, Regnouf F, Pradel LA (1987) Binding of brain spectrin to the 70-kDa neurofilament subunit protein. Eur J Biochem 169:651–657
Frappier T, Stetzkowski-Marden F, Pradel LA (1991) Interaction domains of neurofilament light chain and brain spectrin. Biochem J 275(Pt 2):521–527
Hao R, MacDonald RG, Ebadi M, Schmit JC, Pfeiffer RF (1997) Stable interaction between G-actin and neurofilament light subunit in dopaminergic neurons. Neurochem Int 31:825–834
Haddad LA, Smith N, Bowser M, Niida Y, Murthy V, Gonzalez-Agosti C, Ramesh V (2002) The TSC1 tumor suppressor hamartin interacts with neurofilament-L and possibly functions as a novel integrator of the neuronal cytoskeleton. J Biol Chem 277:44180–44186
Rao MV, Engle LJ, Mohan PS, Yuan A, Qiu D, Cataldo A, Hassinger L, Jacobsen S, Lee VM, Andreadis A, Julien JP, Bridgman PC, Nixon RA (2002) Myosin Va binding to neurofilaments is essential for correct myosin Va distribution and transport and neurofilament density. J Cell Biol 159:279–290
Kim OJ, Ariano MA, Lazzarini RA, Levine MS, Sibley DR (2002) Neurofilament-M interacts with the D1 dopamine receptor to regulate cell surface expression and desensitization. J Neurosci 22:5920–5930
Dubois M, Strazielle C, Julien JP, Lalonde R (2005) Mice with the deleted neurofilament of low molecular weight (Nefl) gene: 2. Effects on motor functions and spatial orientation. J Neurosci Res 80:751–758
Elder GA, Friedrich VL Jr., Margita A, Lazzarini RA (1999) Age-related atrophy of motor axons in mice deficient in the mid-sized neurofilament subunit. J Cell Biol 146:181–192
Elder GA, Friedrich VL Jr., Pereira D, Tu PH, Zhang B, Lee VM, Lazzarini RA (1999) Mice with disrupted midsized and heavy neurofilament genes lack axonal neurofilaments but have unaltered numbers of axonal microtubules. J Neurosci Res 57:23–32
Julien JP, Tretjakoff I, Beaudet L, Peterson A (1987) Expression and assembly of a human neurofilament protein in transgenic mice provide a novel neuronal marking system. Genes Dev 1:1085–1095
Beaudet L, Cote F, Houle D, Julien JP (1993) Different posttranscriptional controls for the human neurofilament light and heavy genes in transgenic mice. Brain Res Mol Brain Res 18:23–31
Ma D, Descarries L, Julien JP, Doucet G (1995) Abnormal perikaryal accumulation of neurofilament light protein in the brain of mice transgenic for the human protein: sequence of postnatal development. Neuroscience 68:135–149
Ma D, Descarries L, Micheva KD, Lepage Y, Julien JP, Doucet G (1999) Severe neuronal losses with age in the parietal cortex and ventrobasal thalamus of mice transgenic for the human NF-L neurofilament protein. J Comp Neurol 406:433–448
Mathieu JF, Ma D, Descarries L, Vallee A, Parent A, Julien JP, Doucet G (1995) CNS distribution and overexpression of neurofilament light proteins (NF-L) in mice transgenic for the human NF-L: aberrant accumulation in thalamic perikarya. Exp Neurol 132:134–146
Lee VM, Elder GA, Chen LC, Liang Z, Snyder SE, Friedrich VL Jr., Lazzarini RA (1992) Expression of human mid-sized neurofilament subunit in transgenic mice. Brain Res Mol Brain Res 15:76–84
Vickers JC, Morrison JH, Friedrich VL Jr, Elder GA, Perl DP, Katz RN, Lazzarini RA (1994) Age-associated and cell-type-specific neurofibrillary pathology in transgenic mice expressing the human midsized neurofilament subunit. J Neurosci 14:5603–5612
Elder GA, Friedrich VL Jr., Liang Z, Li X, Lazzarini RA (1994) Enhancer trapping by a human mid-sized neurofilament transgene reveals unexpected patterns of neuronal enhancer activity. Brain Res Mol Brain Res 26:177–188
Tu PH, Elder G, Lazzarini RA, Nelson D, Trojanowski JQ, Lee VM (1995) Overexpression of the human NFM subunit in transgenic mice modifies the level of endogenous NFL and the phosphorylation state of NFH subunits. J Cell Biol 129:1629–1640
Gama Sosa MA, Friedrich VL Jr, DeGasperi R, Kelley K, Wen PH, Senturk E, Lazzarini RA, Elder GA (2003) Human midsized neurofilament subunit induces motor neuron disease in transgenic mice. Exp Neurol 184:408–419
Collard JF, Cote F, Julien JP (1995) Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature 375:61–64
Xu Z, Tung VW (2000) Overexpression of neurofilament subunit M accelerates axonal transport of neurofilaments. Brain Res 866:326–332
Tu PH, Robinson KA, de Snoo F, Eyer J, Peterson A, Lee VM, Trojanowski JQ (1997) Selective degeneration of Purkinje cells with Lewy body-like inclusions in aged NFHLACZ transgenic mice. J Neurosci 17:1064–1074
Letournel F, Bocquet A, Perrot R, Dechaume A, Guinut F, Eyer J, Barthelaix A (2006) Neurofilament high molecular weight-green fluorescent protein fusion is normally expressed in neurons and transported in axons: a neuronal marker to investigate the biology of neurofilaments. Neuroscience 137:103–111
Acknowledgements
We thank Drs. J.P. Julien and A.C. Peterson for critical reading of this manuscript and helpful scientific suggestions. This work was supported by grants from the Association Française contre les Myopathies, Institut National sur le Cancer and Association pour la Recherche sur la Sclérose en Plaques to J. Eyer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perrot, R., Berges, R., Bocquet, A. et al. Review of the Multiple Aspects of Neurofilament Functions, and their Possible Contribution to Neurodegeneration. Mol Neurobiol 38, 27–65 (2008). https://doi.org/10.1007/s12035-008-8033-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-008-8033-0