Abstract
Introduction: The lung is an important and complicated organ that has profound impacts on the entire body when impacted by disease or illness. Due to its complexity, the human lung is difficult to study; lung models that can mimic this organ are key to better understanding and treating lung diseases. Various lung models have been developed over the years, but one important and recent model is the lung organoid model. Here we review human lung organoid models, including the main characteristics and potentials and their current and future applications for modelling lung development and diseases. Method: For the selection of literature cited, we used MEDLINE/Pubmed database. The keywords used in the MEDLINE research were: human lung development, lung organoids, lung stem cells, lung disease and repair, bioengineering lung. Results: Lung organoids, in layman's terms referred to as “mini lungs in a dish”, are 3D tissues that recapitulate the endogenous functions of the lungs. Lung organoids currently represent the closest model to the human pulmonary system. Human-derived three-dimensional (3D) models have been generated, allowing for a deeper understanding of cell-to-cell communication. They have also allowed researchers to better understand how diseases affect the lungs and determine potential treatment methods. Conclusions: Although the area of research using lung organoids is still relatively new, much has been learned from this model, and much more will continue to be learned. There is an urgent need to develop more complex organoid models containing mesenchymal tissues and vasculature to better understand lung diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Lung Development, Homeostasis and Regeneration
Different Cell Populations in Human Lungs and their Role in Lung Development
Introduction
The lung contains a wide variety of cell types, many of which are still poorly understood or yet to be discovered. Various lung researchers distinguish these cells differently, and there is no universal method of cell distinction, but this chapter will focus on the current research confirming 58 cell types in the human lung. These cell types are placed into four different categories: epithelial, endothelial, stromal, and immune cells [1, 2].
Epithelial Cells
Epithelial cells make up most of the body tissues, which line the internal and external surfaces of the body [3]. There are 15 known epithelial cells in the lung. Twelve of these cells reside in the airway, and three reside in the alveoli. The major epithelial airway cell types are ciliated, undifferentiated columnar, basal, and secretory cells [2, 4, 5]. The airway epithelium plays a pivotal role in the first line of defence against unwanted particulates and pathogens. It also plays a role in the maintenance of funnelling air into the airways to facilitate gas exchange [4, 6].
Within the 12 airway epithelial cell types, there are morphologically distinct types with different functions [6]. Columnar ciliated epithelial cells possess cilia, which aids in transporting mucus away from the lung into the throat [7]. Mucus cells, also known as goblet cells, secrete mucin and create a mucus layer necessary for trapping harmful substances in the airway lumen for secretion out of the lungs [8]. Serous cells, also known as secretory cells, perform various functions with the secretions they produce. These secretions keep the mucosa lining moist, humidify inhaled air, and clean the inhaled air from unwanted particulates and organisms [9]. Basal cells are stem cells capable of differentiating into the mucus and ciliated epithelial cells [6]. They help the attachment of columnar epithelium to the basal lamina by providing an area for cell-to-cell attachment [10]. Club cells are similar to basal cells in that they also stem cells and can give rise to ciliated and secretory cells [6]. Club cells produce surfactant proteins A, B, and D as well as metabolize xenobiotic compounds [11]. Pulmonary neuroendocrine cells (PNECs) are a rare type of epithelial cell that secrete biogenic amines and peptides, which play a major role in lung development and airway function [6]. Another group of rare cells in the human lung are known as ionocytes; these cells have a high expression of FoxI1 and the cystic fibrosis transmembrane regulator (CFTR), which may play a role in respiratory diseases such as cystic fibrosis (CF) [12].
The alveolus is where gas exchange occurs and contains epithelial cells that aid in maintaining lung homeostasis [13]. These cells are alveolar epithelial type 1 (AT1), alveolar epithelial type 2 (AT2), and AT2 signalling (AT2-s) cells [2]. AT1 cells occupy 96% of the surface of the alveoli and, although take up a huge portion of the surface, are extremely thin in order to allow passive gas diffusion [14−16]. AT2 cells synthesize surfactants to prevent the lungs from collapsing and differentiate into AT1 cells to facilitate lung repair and maintain homeostasis [16, 17].
Endothelial Cells
Endothelial cells are key regulators of vascular homeostasis via the inhibition of coagulation of the blood and accommodate blood flow levels within the lung [18]. These cells also enable efficient gas exchange in the lung by ventilation-perfusion matching [19]. The human lung contains nine endothelial cells and is placed into five different categories: artery, vein, capillary, bronchial vessel, and lymphatic cell [2].
Pulmonary artery endothelial cells (PAECs) and pulmonary vein endothelial cells (PVECs) have similar functions but are two distinct cells [20]. Both cells have calcium entry pathways and respond to inflammatory stimuli but at different rates [20]. There are three types of capillary cells within the human lung: aerocyte (aCap), intermediate, and general capillary (gCap) cells [2, 21]. The main function of all capillary cells of the lung is to perform leukocyte trafficking but each type of capillary cell expresses different genes that regulate this process [21]. Capillary cells have roles in hemostasis and lipid metabolism due to their production of pro-/anti-coagulants as well as fatty acids, respectively [21]. Bronchial artery endothelial cells (BAECs) and bronchial microvascular endothelial cells (BMVECs) play key roles in protein transudation [22]. Lymphatic cells contribute to alveolar clearance for greater efficiency in respiration [23]. These cells achieve this due to their close proximity to major airways and blood vessels involved in the process of respiration.
Stromal Cells
The lung contains nine different types of stromal cells [2]. Stromal cells are non-specific stem cells with the ability to become different cell types such as chondrocytes, osteoblasts, and adipocytes in order to replace old cells and aid in repair [24, 25]. Stromal cells also play a role in inflammation control and have been found in premature infants with lung issues such as acute respiratory distress syndrome (ARDS) [26]. Their prevalence suggests that these cells take part in lung regeneration, repair, and development.
Immune Cells
In the lung, there are 25 immune cell populations with various functions [2]. All of these cells are important for healthy lungs but some of the most important are neutrophils, macrophages, and lymphocytes which protect the lung by eliciting an immune response geared to remove and destroy unwanted pathogens [27].
Neutrophils, also known as polymorphonuclear leukocytes, are producers of highly reactive oxygen radicals that have been found to be involved in lung diseases such as ARDS and acute lung injury (ALI) [28, 29]. They are some of the first leukocytes to be activated by lung infection [29, 30]. Activated neutrophils phagocytose pathogens, resolve inflammation, clear damaged neutrophils, and modulate immune responses via cytokine release [28−30]. Macrophages work alongside neutrophils by clearing the dead or dying neutrophils as well as pathogens [30, 31]. They also release a wave of cytokines and chemokines to induce a rapid immune response by recruiting other cells [30, 31].
There are various subpopulations of lymphocytes, each with their own specialized functions, but their shared characteristics are their recruitment into the lung during infection, their involvement in the immune response via inhibition or recruitment of other cell types, and their production of anti-inflammatory signals [32, 33].
Cell Population Roles in Lung Development
Lung development is divided into five stages: embryonic, pseudoglandular, canalicular, saccular, and alveolarization [34]. A general overview of lung development is illustrated in Fig. 2.1. Within each stage, cells within the lung have specific functions that help progress lung development. Most research on lung development has been through studying mice; therefore, the following sections will briefly discuss mouse lung development and how it contrasts human lung development. The developmental periods beginning with embryonic (E) or postnatal day (P) will refer to mouse lung development stages and the developmental periods ending in post-conception weeks (pcw) will refer to human lung development.
Embryonic: E9-E12; 4–7 pcw
The development of the lung begins in the anterior foregut endoderm, which generates the respiratory endoderm [35]. The respiratory endoderm is what generates progenitor cells within the lung and begins on embryonic day 9.0 [34]. These progenitor cells, which are detected by the expression of Nkx2.1, then form the basic structure of the trachea and two lung buds that will form the left and right lobes of the lung [36]. The beginning of elongation of these lung buds into the mesenchyme is known as branching morphogenesis [5, 34]. The proximal progenitors generate PNECs, secretory cells, and goblet cells, whereas the distal progenitors produce AT1 and AT2 cells [34].
Pseudoglandular: E12-E15; 5–17 pcw
This stage begins when the bronchial tree is in the shape of a tubular gland and the epithelial tubules continue branching morphogenesis [5]. This is where maximal branching occurs [37]. The first 20 generations of the airway form by the end of this stage with primitive alveolar ducts [5, 38]. Columnar epithelial cells are found in the proximal airway and cuboidal epithelial cells are found in the distal airway [39]. The cells in the proximal airway differentiate into ciliated, non-ciliated, goblet, and basal cells. By the end of this stage, club cells are found in the trachea. Cells in the distal airway remain undifferentiated until branching morphogenesis is complete, occurring as late as the saccular stage in the human lung [39]. Undifferentiated Sox2/Sox9 double-positive cells are found in the distal epithelial tips in the human lung. The surrounding smooth muscle cells (SMCs) in the distal lung appear to play a role in branching morphogenesis due to recent studies showing a decrease in Sox2+/Sox9+ cells coinciding with decreased branching in fetal lung explants treated with a toxin [37]. This does not occur in mouse lung development since the proximal and distal lung cells are already expressing different Sox transcription factors in the pseudoglandular stage [37].
Canalicular: E16-E26; 16–26 pcw
The canalicular stage is the beginning of the development of alveolar sacs [5, 38]. In this stage, the Sox2+/Sox9+ cells are no longer present in human lungs due to the surrounding SMCs suppressing the Sox9+ cell population in the proximal region [37]. At this stage, human lung development mirrors mouse pseudo glandular lung development in that the proximal progenitors express Sox2+ cells and distal progenitors express Sox9+ cells [37]. The cuboidal epithelial cells differentiate into AT1 and AT2 cells [5]. The air-blood barrier forms with the help of the endothelium of the capillaries coming into contact with AT1 cells [5]. AT2 cells begin to produce surfactant as well as differentiate into AT1 cells [5, 36].
Saccular: E17-birth; 27–36 pcw
Branching morphogenesis stops in this stage and is the transitional stage into alveolarization [5]. This intermediate stage is when the distal branches narrow and form small saccules (primary septae), which become alveoli (secondary septae) in the alveolarization stage [5, 34]. The alveoli begin to grow, widen, and form [5]. The primary septa is covered by predominantly AT1 cells with some AT2 cells filling in the space. Smooth muscle cells begin to form a network of elastic fibres and collagen fibrils. This network allows for the development of the alveoli by providing a scaffold for continued lung maturation [5]. Mice are born during the saccular stage and their lungs continue into the alveolarization stage after birth [37]. This differs from humans: humans are born in the alveolarization stage. Both mouse and human lung development continue after birth with the maturation of alveoli and the alveolarization stage for both species are similar [37]. The following explanation of alveolarization focuses on human lung development, but the main processes that occur during these stages are nearly identical in mouse and human lung development.
Alveolarization: P4-P36; human birth- ~3–15 years
Alveolarization is the process by which primary septae become secondary septae [5, 40]. Alveolarization is separated into two stages: classical and continued alveolarization [5]. Human classical alveolarization ranges from birth to about three years and human continued alveolarization ranges from around two years of age to young adulthood, estimated to be between 15 and 21 years [5]. Previous research believed that human lung development concluded at around 8 years of age [41] but new studies have shown that the number of alveoli continues to develop around 15 years of age, with some subjects showing alveoli development into young adulthood (21 years) [42].
Classical Alveolarization: P4-P21; human birth-3 years.
The primary septae contain a double capillary network which is immature and inefficient for gas exchange. This stage is where the secondary septa are formed by the upfolding of one of the double capillary networks, resulting in secondary septae with a single capillary layer [5, 34]. This single layer formation, a process known as microvascular maturation, allows for the formation of alveoli [43, 44].
Continued Alveolarization: P14-P36; 3–21 years.
In continued alveolarization, microvascular maturation as well as classical alveolarization persist within the secondary septae, a process known as angiogenesis [5, 34, 43, 45]. This process moves distally over time and the alveoli mature as the child continues to grow. As the child gets older, alveolarization slows down.
The Challenge of Lung Modelling
The Complexity of the Human Lung
The lung is an extremely complex organ. Even with decades of research, researchers are still uncovering new knowledge about how the human lung functions.
Lack of Lung Tissue
There is limited access to lung tissue, especially fetal tissue, which is crucial to better understanding lung development. Most countries do not allow the use of human fetal tissue beyond 20 pcws, limiting the research on later lung development [46]. Later stages are typically studied using animal models, but various differences between animal and human lungs limits what researchers can learn about the human lung.
Differences Between Animal Models and the Human Lung
The types of animals used as a substitute for human tissues are rats, rabbits, mice, and rhesus monkeys [47−50]. There are various and significant differences between these animal models mentioned and human lungs. This is not meant to discredit the research performed on these animal models, but it is important to recognize that these studies have their limitations. Scientists have looked for other options for better understanding the human lung. The creation and use of lung organoids have become a practical option for researchers to study the human lung. Lung organoids remove two key issues in this field of research: the difficulty in obtaining human fetal lung tissue as well as the contrasting lung organization and cellular composition with the use of animal models.
Origin of Lung Organoid Cultures
Several groups have attempted to generate human lung organoids that can recapitulate essential features of human lungs in vitro. Generation of lung organoids usually includes endoderm induction, anterior-posterior patterning, lung specification, lung budding, branching morphogenesis, and maturation [51]. Currently, most lung organoids are developed either from human pluripotent stem cells (hPSCs) or stem cells isolated from primary tissues (Fig. 2.2). The resulting 3D human lung organoids are able to recapitulate various cell types, structures and some functions of mammalian lungs.
Overview of the generation of primary stem cell- and hPSC-derived lung organoids. Primary stem cells used for the generation of lung organoids are obtained from normal or diseased lung biopsies. Tissues are processed into a single cell suspension and then cultured in Matrigel to expand and form 3D organoid structures. hPSC-derived organoids are differentiated and developed from either ESCs or iPSCs. After differentiating into definitive endoderm and forming anterior foregut spheroids by modulating various signalling pathways, the cells can be embedded into Matrigel to further branch and form 3D lung organoids.
Lung Organoids Generated from Primary Stem Cells
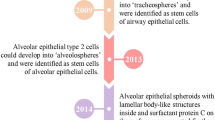
Several groups have made great efforts to generate human lung organoids from adult stem cells or fetal stem cells. These primary stem cells have the capability of self‐renewal and differentiation into multiple cell lineages, which have great potential in forming 3D lung organoids. 3D spheroid/organoid structures can be formed from lung progenitors including basal cells [52] and AT2 cells [17].
Hild and Jaffe described a method to generate 3D airway organoids from primary human airway basal cells [53]. Commercially available human bronchial epithelial cells are used as basal cells and made into a suspension in 5% Matrigel. The cell density is made to be 30,000 cells/mL and 20 µl are seeded onto each well of a Matrigel-coated 384-well plate to have 600 cells/well. Matrigel is added to each well on day 2 (48 h after incubation) and again on day 8. Lumen are observed after a week and the spheres begin to show differentiation after two weeks. These organoids give rise to basal cells, goblet cells, and multiciliated cells. Cultured bronchospheres can be used as a great model to study human airway epithelium growth, repair, and differentiation. Their ability to differentiate in as little as 14 days could be useful for quickly making these organoids for experiments. The capability of culturing 3D airway organoids in 384-well plates can also be applied to a high-throughput system for drug screening.
Sachs et al. reported an alternative approach for long-term culturing of human airway organoids from bronchoalveolar resections or lavage fluids [53]. Isolated epithelial cells collected either from solid lung tissues or broncho‐alveolar lavage fluid are embedded in basement membrane extract (BME). A 3D airway organoid with a polarized, pseudostratified airway epithelium containing basal, secretory, and multi‐ciliated cells are formed within several days. This relatively simple protocol of generating airway organoids from a small amount of routinely obtained patient samples (lavages, resections) provides a great model for drug screening and personalized treatment of lung diseases [53]. These organoids were stable for up to several months and most retained the diseases, mutations, and tumours that the patients had. This model showed that personalized medicine for lung disease could be made a possibility with lung organoids.
Another adult stem cell-derived human lung organoid model is presented by Tindle et al. in which the generated lung organoids contain both proximal and distal airway epithelial structures [54]. Deep lung biopsy samples from patients are used to generate a single cell suspension followed by 3D lung organoid formation in Matrigel. An advantage of this human lung organoid model is that it recapitulates the proximal and distal airways, including all 6 major lung epithelial cells: AT1, AT2, basal cells, goblet cells, ciliated cells, and club cells. Besides culturing and maintaining in 3D culture, these lung organoids can also be dissociated and cultured as a 2D monolayer for viral infection studies. The 2D monolayer favoured differentiation from AT2 to AT1 cells, making this a great model to studying this process or for studying AT1 cells.
Recent advancements made by Salahudeen et al. have described a method for the development of long-term culture of human distal lung airway and alveolar organoids [55]. They developed a feeder-free, chemically defined strategy to culture two types of human lung organoids derived clonally from single adult human AT2 cells or KRT5+ basal cells. The generated alveolar organoids are composed of homogenous AT2 cells capable of differentiating into AT1 cells while airway organoids contain two molecularly distinct distal airway basal cell subpopulations. Basal 1 cell population was characterized with proliferation and developmental programming. Basal 2 cell population was found to be enriched for structural, cytoskeletal, and calcium-binding protein genes. Both types of organoids were found to be stable for at least six months. The distal lung organoids were used to model COVID-19-associated pneumonia and since they were found to be useful in recapitulating disease, could be helpful for other lung diseases.
Youk et al. also described a long-term, feeder-free human 3D alveolar type 2 cell culture (h3AC) model derived from primary human lung tissue, which has a great potential in investigating the pathogenesis of SARS-CoV-2 and modelling other respiratory diseases [56]. After dissociating human AT2 (hAT2) cells obtained from distal parenchymal regions of healthy donor lungs and isolating through FACS using AT2 cell surface markers, sorted hAT2 single cells were embedded into Matrigel supplemented with growth factors that are essential in lung development to self-organize into an alveolar-like 3D structure. It was found that the differentiation from AT2 to AT1 cells was favoured in 2D culture. After six months, this 3D culture maintained normal karyotypes, but eight-month cultures lost some of their ability to form colonies as well as important markers such as the expression of pro-surfactant protein C (pro-SFTPC). Over several passages, h3ACs could still maintain functional mature hAT2 cells and were capable of AT1 cell differentiation when placed into 2D culture. Established h3ACs show a substantial SARS-CoV-2 infection with remarkable cellular and transcriptional changes post-infection compared to human 3D bronchial cultures generated previously by Sachs et al. and other 2D cell lines models [53, 57]. This 3D hAT2 cell culture serves as a great platform for viral infection studies to help better understand virus-host interaction and subsequent immune response in alveolar stem cells.
Lung Organoids Generated from Human Pluripotent Stem Cells (hPSCs)
Human lung organoids can also be generated from human pluripotent stem cells (hPSCs), including embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs). Generation of lung organoids from hPSCs usually requires a series of differentiation following developmental steps. In general, hPSCs are first specified to definitive endoderm (DE), followed by patterning to anterior foregut endoderm (AFE) and finally induced to lung lineage specification and maturation. These hPSC-derived lung organoids have great potential in providing models for studying human lung development, drug screening, and personalized medicine of various pulmonary diseases.
The first successful generation of AFE from hPSCs was achieved by Green et al. [58]. DE is induced from hPSCs with a high concentration of Activin A for four days and is confirmed with expression of DE markers CXCR4, c-KIT, and EPCAM [59]. To obtain an enriched culture of AFE cells after DE induction, Green et al. blocked bone morphogenetic protein (BMP) and transforming growth factor beta (TGFβ) signalling using NOGGIN and SB-431542 (SB), respectively. This group found that the removal of Activin A allowed for an increase in SOX2 and CDX2 expression, markers for anterior and posterior endoderm, respectively. They attempted to further differentiate the AFE cells by replacing NOGGIN/SB-431542 with WNT family member 3a (WNT3a), keratinocyte growth factor (KGF), fibroblast growth factor 10 (FGF10), BMP4, and epidermal growth factor (EGF). This led to the increase of markers P63, NKX2.1, NKX2.5, PAX1, and a decrease in SOX2. The addition of retinoic acid (RA) increased lung fate markers GATA6, FOXJ1, NKX2.1, and FOXP2. This study was important for future researchers to continue the process of developing lung organoids, as a majority of groups furthering the development of lung organoids used these growth factors and markers to advance the maturation of lung organoids. Huang et al. followed shortly by differentiating AFE cells into lung and airway progenitors and was able to achieve a higher progenitor yield. The differentiated lung and airway cells could further differentiate into basal, goblet, club, ciliated, AT1, and AT2 cells in vivo and in vitro [60, 61]. They used the protocols discussed by Green et al. to generate DE but used dorsomorphin (DSM) instead of NOGGIN and added IWP2, a WNT inhibitor. Huang et al. optimized the protocol inducing lung progenitors from AFE by exposing the cells to the same growth factors and the addition of CHIR99021 (CHIR), a glycogen synthase kinase (GSK) inhibitor. This increased the amount of NKX2.1+ FOXA2+ cells by almost 20% [60]. The cells were plated on fibronectin-coated plates and cultured with the previously mentioned growth factors to induce the lung progenitors to mature. The concentration of RA was changed along with the culturing time which increased FOXA2+ NKX2.1+ cells from less than 40% to over 80%. This continuation of Green et al.'s research significantly improved the maturation of cells into AFE, allowing for the differentiation into lung and airway cells.
Dye et al. reported a protocol to generate 3D lung organoids with a proximal airway-like structure along with distal alveolar-like epithelial structure composed of the basal, ciliated, club and alveolar cells [62]. Cells were treated with Activin A, followed by NOGGIN/SB, and then the addition of CHIR, FGF4, SB, and NOGGIN to generate AFE. These AFE cells were then stimulated with a hedgehog (HH) while inhibiting FGF using Smoothened agonist (SAG) and SU5402 (SU), respectively [62]. This resulted in spheroids that were NKX2.1+ FOXA2+ which were placed in Matrigel. These spheres were added to decellularized human lung matrices, allowing for multiciliated structures. They called these human lung organoids (HLOs) and HLOs to contain a small population of AT1 and AT2 cells and alveolar progenitor cells. Human lung organoids resemble human fetal lungs based on their global transcriptional profiles, making them a great model system for human lung development [62].
Chen et al. described a different strategy to generate lung bud organoids (LBOs) that can form airway and early alveolar structures to recapitulate human fetal lung development [63]. hPSCs were differentiated into DE and AFE in the same manner as shown by their previous work [60]. When adherent AFE cells were induced to ventral AFE, cell clumps spontaneously formed LBOs and expanded further when treated with FGF10, FGF7, BMP4, RA, and CHIR. These LBOs were cultured in suspension until day 20–25 followed by embedded in Matrigel for further generation of branching morphogenesis and maturation to lung and airway epithelial cells. LBOs can be cultured for an extended period of time, with maintenance capabilities in culture of more than 6 months. Both structural and transcriptomic data indicate that day 40 LBOs had reached the late second trimester of human gestation [64, 65]. LBOs were also transplanted under the kidney capsule of immunodeficient mice to determine if they could recapitulate lung development in vivo. This led to exhibited significant growth of airway structures undergoing branching morphogenesis showing proximodistal specification with evidence of early alveolar structures demonstrated by AT1 and AT2 cell markers [63].
McCauley et al. established a protocol to generate functional and expandable airway epithelial organoids [66]. Similar to other lung organoid generation approaches, hPSCs are first differentiated to DE, AFE, and then induced specifically to NKX2.1+ lung epithelial progenitors. These lung progenitors are then purified through FACS using cell surface markers CD47 and CD26 to sort out a high expression of CD47 and low or no expression of CD26. This is because cells that are CD47highCD26low have a high-level NKX2.1+ cells. The isolated progenitors can then be re-plated in Matrigel with FGF2, FGF10, corticosteroids, and cyclic-AMP to form 3D airway epithelial organoids. These organoids express secretory lineage markers and airway basal cell markers. In addition, Kotton's group also developed a strategy to generate isolated hPSC-derived alveolar epithelial type 2 cells (iAT2s), which can form 3D alveolospheres [67]. Using surfactant protein C (SFTPC) as a specific AT2 cell marker and NKX2.1 as a lung progenitor marker to establish SFTPC/NKX2.1 multifluorescent reporter hPSC lines, Jacob et al. purified SFTPC+ iAT2s differentiated from NKX2.1+ progenitors. This confirmed that SFTPC+ cells derive from NKX2.1+ cells. iAT2s were shown able to self-renew and proliferate to form 3D alveolospheres with mature AT2 functions, including the formation of lamellar bodies and the secretion of surfactants. This was in contrast to primary AT2 cells, which required mesenchymal feeders in order to form spheres. This was an important discovery because primary AT2 cells are difficult to maintain undifferentiated [68], allowing for alternative methods for studying AT2 cells.
Miller et al. designed a protocol to generate lung organoids from feeder-free hPSCs, specifically hESC cell lines H1 and H9 along with hiPSC cell lines UM63-1 and UM77-1 [51]. This protocol is capable of forming bud tip organoids as early as day 22 and HLOs after 50 days. Briefly, to generate both organoids, hPSCs are directed to the endoderm followed by foregut spheroids. These spheroids float into the media, are placed into Matrigel, and cultured for two more weeks to generate bud tip progenitor organoids. If the bud tip progenitor organoids are not passaged and allowed to continue growing in Matrigel, they become budded structures. Those that are passaged form bud tip progenitors. The budded structures can become bud tip progenitors if passaged as well. The bud tip progenitor organoids are useful for research involving undifferentiated cells, as they are similar to the human fetal lung progenitors found on the branching buds [51]. Human lung organoids, found to be similar to the human fetal lung, contained matured alveolar cell types such as AT1 and AT2 cells as well as mesenchymal cells. After being in culture for over 65 days, they will also be positive for basal stem cell marker P63+. Bud tip progenitor organoids are positive for SOX2 and NKX2.1 and, if passaged, will be SOX2+ SOX9+ [51]. Budded structures of these progenitor organoids undergo bifurcation and are positive for club cells, goblet cells, and pro-surfactant protein C.
Recently, a different protocol was described by Carvalho et al. to direct hPSC differentiation into mature lung and airway epithelial cells [69, 70]. By generating NKX2-1+ lung progenitors first in 2D cultures followed by embedding cells in collagen I without inhibiting glycogen synthase kinase 3, they could generate a more mature multilineage of alveolar and airway cells including AT1 and AT2 cells as well as basal, ciliated, club and neuroendocrine cells [69, 70]. Notably, KRT14+ NGFR+ (a mature basal cell marker) basal cells are formed following this protocol, which could also be easily isolated and expanded for subsequent basal cell culture. This protocol was built off of their previous work [61]. They used collagen I in place of Matrigel due to the fact that it could allow for a broader range of lung lineages but found that it produced similar lineages to protocols that use Matrigel. They found that NOTCH signalling induced a distal cell fate whereas WNT signalling induced a proximal cell fate. When cells that were placed in collagen I did not have GSK3 in culture, the cells matured into AT1 and AT2 cells. Better understanding signalling pathways involved in lung maturation has been crucial to better understanding lung development and the specification of different lung areas for experimentation.
Comparison Between hPSC- and Primary Stem Cell-Derived Lung Organoid Models
Several protocols have been developed using either primary stem cells, including adult stem cells (ASCs) and fetal stem cells or hPSCs to generate 3D lung organoids that can mimic the morphological and functional features of the human lung in vitro. Both organoid generation systems have their own advantages and limitations.
Human primary stem cell-derived lung organoids are usually generated from biopsies directly isolated from healthy or diseased patients' lungs. These organoids are often limited by the shortage of primary tissues and difficulty in accessing them. Heterogeneity among donors and unclear information of prior culture/preservation conditions of primary tissue present as limiting factors in primary stem cell-derived organoids. An advantage of using lung organoids from primary human tissues is that they are valuable for rare diseases such as CF to allow for drug modelling and better understand how the CFTR mutation will affect a particular patient [71]. On the other hand, hPSC-derived organoids can be used continuously to generate different models once a protocol is established. There is no concern as to where to obtain samples since they are derived from cells that can be purchased commercially. These organoids can be useful for a wide variety of diseases and have been found to recapitulate diseases successfully.
Another difference between primary stem cell- and hPSC-derived organoids is that primary stem cell-derived organoids are preserved to differentiate towards a certain lineage, such as proximal/airway or distal/alveolar lineage, and cannot transdifferentiate into others by just changing the culture environment. In contrast, the lineage determination of hPSC-derived organoids largely depends on the external manipulation of signalling pathways and components in the culture medium. Therefore, hPSC-derived lung organoids usually contain a mixture of proximal and distal cells with a greater cellular heterogeneity, while ASC-derived lung organoids are limited to certain lineages depending on the primary tissue used. Nevertheless, the flexibility and uncertainty in hPSC differentiation can raise issues of certain lineage specification and inclusion of unwanted cell types in the culture. Both methods are complementary and can be used depending on the end goal of the experiments. ASC-derived lung organoids are useful to study specific cell types impacted by a particular lung disease. hPSC-derived organoids offer a more versatile approach to studying a wide range of cells and how a wide variety of diseases impacts the proximal and distal cell types.
An important advantage regarding the use of hPSCs is their ability to be easily genetically modified: isogenic cell lines with specific mutations can be generated using CRISPR-Cas9 and these genetically modified hPSC-derived organoids can then be used to model multiple respiratory diseases such as CF [72] and idiopathic pulmonary fibrosis (IPF) [65]. CF is known to be caused by mutations in the CFTR [73]. A study using a CRISPR-mediated gene editing approach successfully targeted and corrected the endogenous CFTR locus in CF iPSCs [72]. The gene-corrected iPSCs could later be differentiated into mature airway epithelial cells with normal CFTR expression and function [72]. Using this method of genetically modifying hPSC-derived lung organoids, more studies of lung diseases can be done. An example of a potential disease to study could be Hermansky-Pudlak syndrome (HPS), a rare autosomal disorder. patients with mutations in HPS genes have been found to develop HPS, specifically those with a mutation in the gene HPS1 [74]. They found that the mutation in HPS1 showed a high incidence of developing pulmonary fibrosis [74]. Mutations in some HPS associated genes can cause HPS-associated interstitial pneumonia (HPSIP), which resembles IPF [74]. HPS-associated mutations could be introduced into hPSC-derived 3D lung organoids using CRISPR-Cas9 to study the potential pathogenesis of IPF caused by HPS mutations [65]. It would be more difficult to obtain primary tissue from patients with HPS, but if possible, the research could also be performed on primary tissue to better understand how this mutation impacts the lungs.
Currently, the maturation of hPSC-derived lung organoids to adult stages remains a challenge. Most organoids show a transcriptome profile similar to embryonic developmental stages. Adult airway-like structures can only be generated after in vivo xenotransplantation, and most in vitro hPSC-derived lung organoids cannot mature beyond the second trimester of human gestation. While hPSC-derived lung organoids are closer to the human fetal lung, primary stem cell-derived lung organoids recapitulate features of adult lungs better in terms of maturity. A combination of primary stem cell- and hPSC-derived organoid systems would provide a more comprehensive understanding in human lung development and regeneration. Both primary tissue and hPSC-derived lung organoids have their advantages and disadvantages, but ultimately both are crucial to the advancement of lung research.
Mouse and Human Lung Difference
Animal models, especially mouse models, have greatly improved our understanding in lung development and disease. Mouse genetic gain- and loss-of-function studies enable us to learn more about lung development and signalling pathways controlling morphogenesis [40]. Mice lung injury models can reproduce some key features in complex human pulmonary diseases such as ALI and pulmonary fibrosis [75]. Although mice have been widely used for studying human lung development, function, and various respiratory diseases, it is worth noting that there are significant interspecies differences between mouse and human lungs. Given the significant differences, mouse models cannot fully recapitulate human lung physiology nor be applied to human lung development and disease studies. Some promising findings from mouse models fail to translate into effective therapeutic targets in subsequent human studies [76].
Cellular Composition
In both mouse and human lungs, the trachea and proximal conducting airways are lined by pseudostratified columnar epithelium, and the peripheral conducting airways are lined by cuboidal epithelium. Despite the similar structure in airway epithelia, the relative proportions of different types of cells along this proximal-distal axis of the airway vary between human and mouse lungs. In addition, this complex pseudostratified epithelium structure extends to terminal bronchioles in humans, whereas this structure is limited to the trachea and more proximal airways in mice. In human lungs, the more proximal intrapulmonary airways are lined by tall, pseudostratified, columnar epithelium composed of basal, ciliated, club, serous, mucus, intermediate, and neuroendocrine cells. These airways also exhibit abundant submucosal glands. In mouse lungs, however, the more proximal intrapulmonary conducting airways are lined by low columnar epithelium composed mainly of ciliated and club cells with some clusters of neuroendocrine cells. No basal cells and only a few mucus cells are found in the mouse airways [77]. Basal cells marked by the expression of transcription factor TP63 are only found in the mouse trachea, while the distribution of these cells extends to the bronchi in human lungs [52].
Architectural Organization
Both human and mouse lungs consist of multiple lobes but vary in numbers and organization. Mouse lungs have one lobe on the left and four on the right, while human lungs have two lobes on the left and three on the right [78]. For each lobe in the human lung, extensive interlobular and segmental connective tissues are separated into individual lobules or segments, while no such subunits exist in the mouse lung [79]. The alveoli and blood-air barrier are smaller and thinner in mouse lungs compared to that of humans [80].
Molecular Characteristics
Differences in the expression of several marker genes have been observed during human and mouse lung development. SOX2 and SOX9 are two essential transcription factors in lung development. During the pseudoglandular stage in developing mouse lungs, there is a clear separation between Sox9+ tip and Sox2+ stalk cells. These cells are formed and regulated through multiple signalling mechanisms [81]. This tip-stalk demarcation can also be seen in human fetal lungs; however, some levels of SOX2 are also found to be expressed with SOX9 in human embryonic lung distal tip epithelium. This co-expression pattern is never found in mice while the SOX2+ SOX9+ progenitor population persists during the pseudoglandular stage up to 16 weeks' gestation during human lung development [82]. The maintenance of this SOX2+ SOX9+ progenitor population is also proposed for proper branching morphogenesis in the human lung [37].
Lung Organoids: Potential Applications in Lung Repair and Regeneration
Lung disease is a major cause of morbidity and mortality worldwide. For many patients with end-stage lung diseases, lung transplantation remains the only available therapy. However, the number of patients listed for lung transplantation surpasses the number of suitable organ donors. Understanding the cellular and molecular mechanisms driving lung regeneration and repair is crucial for the development of novel therapeutic approaches, with the ultimate goal to repair the damaged lung in situ or regenerate the damaged lung for transplantation. The lung is a highly quiescent organ, previously thought to have a relatively limited reparative and regenerative capacity [83]. It is now known that following injury, the lung has a robust ability to repair and regenerate through distinct cell types. The ability to replace defective cells with cells that can engraft, integrate, and restore lung functions could be the potential cure of a number of lung diseases (Fig. 2.3).
Lung organoids: potential applications. As mentioned earlier, lung organoids can be established directly from patients via fresh biopsies and resected lung tissues, blood samples, and skin samples. Lung biopsies and resections contain adult stem cells (ASCs), whereas blood and skin samples contain induced pluripotent stem cells (iPSCs), which can be reprogrammed into the desired cell type. Regardless of the type of sample obtained, all can be differentiated towards the desired lineage. Lung organoids can also be derived from embryonic stem cells (ESCs). Lung organoids provide unique opportunities for (1) basic research: including studies of lung developmental processes, responses to external stimuli and stress signals, cell-to-cell interactions and mechanisms of stem cell homeostasis; (2) drug screening: in which patient-derived organoids can be used to predict how patients will respond to drugs; (3) disease modelling: to understand the mechanisms of lung diseases such as infectious diseases, inheritable genetic disorders, and cancer; (4) regenerative medicine: their capacity to engraft and survive in vivo; their ability to self-organize to complex structures resembling mini-organs ex vivo; and their potential to generate bioengineered tissue makes them optimally suited for regenerative medicine.
-
1.
Proximal airway repair and regeneration
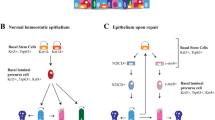
The proximal airways serve as the first line of defence in the respiratory system as they are exposed to frequent insults from the environment. They consist of the trachea: a pair of primary bronchi and many bronchioles of various sizes generated through the branching morphogenesis process [84]. The proximal airways are lined by a pseudostratified columnar epithelium consisting primarily of three types of epithelial cells, (basal cells, club cells and ciliated cells) that play crucial roles in tissue repair, mucociliary clearance (MCC), and host defence. They also contain a small number of neuroendocrine cells, goblet cells, ionocytes, and tuft cells [12, 85,86,87,88]. The primary method of defense against the external environment is mucociliary clearance which requires the cells involved to be working properly [89]. If MCC in the lungs is nonfunctional or damaged, the lungs become vulnerable to other infections, making treatment even more challenging. An example of the MCC in the lungs not functioning properly since birth is in primary ciliary dyskinesia (PCD), a genetic disorder that causes motile cilia dysfunction crucial for MCC [89, 90]. These patients are more likely to get respiratory infections and are at risk for severe lung damage to the point of needing a lung transplant [91]. Examples of illnesses that cause damage to the lungs are the previous outbreaks of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) that have shown to have long-term consequences on patients [92−94]. This raises concerns for the current SARS-CoV-2 pandemic since there is no way to obtain evidence for long-term consequences for patients at the moment. This concern supports the idea of utilizing all possible ways of studying lung disease and repair; lung organoids can be pivotal in helping the lung field answer important questions.
The mouse trachea closely resembles the structure of human proximal airways, providing a valuable tool to study airway regeneration. Pioneering studies from Rock et al. [52], have shown that basal cells function as proximal airway epithelial stem cells. During physiological cell turnover or after injury, basal cells can self-renew and differentiate into various airway epithelial cell types for maintaining the epithelial integrity of proximal airways [52, 95]. Basal cells in the proximal airways are the major stem cell population that self-renew and, when necessary, give rise to multiple cell types such as secretory, goblet, and multi-ciliated cells [52, 95,96,97]. However, striking differences are also found between mouse and human lungs, as we discussed in the previous section, with the most relevant being the presence of basal cells [98]. In mice, basal cells reside in the main trachea, whereas, in humans, this population extends for several airway generations. Unlike human lungs, the intrapulmonary airways of mouse lungs are not pseudostratified and lack basal cells, highlighting the interspecies difference [76]. Organoids derived from human cells could provide an in vitro model for regenerating the mucociliary epithelium from basal cells. Using human lung organoids would provide a better model and give researchers more confidence to translate their results to patient care.
To model proximal airway functions, lung organoids have been derived from human and mouse basal cells. Depending on their origins, basal organoids derived from tracheal cells are known as tracheosphere while those from from large airway cells are called brochospheres in humans [52, 99,100,101]. These organoids have been used to test regenerative mechanisms proposed from in vivo studies. They can also be used to screen for drugs, small molecules, and molecular pathways participating in pulmonary cellular plasticity and lineage outcomes, and regulating crucial epithelial cell functions. For instance, Gao et al., used human basal cells derived organoids to identify a central role for the transcription factor grainyhead-like 2 (GRHL2) in coordinating barrier function and differentiation. Using CRISPR/Cas9 genome editing, they further revealed the transcription factor ZNF750 as a new component of the ciliogenesis pathway in the human lung [102]. If more lung organoids are used to study human disease, they will be the main method to fill the gaps of knowledge that hold research back from finding cures to lung diseases.
While studies have shown that basal cells are essential in repairing the damaged airway epithelium, other epithelial cells also participate in tissue repair as facultative stem/progenitor cells. Studies in mice have shown that club cells that reside throughout the airway epithelium are facultative progenitor cells [103]. Studies have shown that club cells can directly differentiate into mucus-secreting goblet cells by IL13 stimulation in both mice and humans, especially in more proximal lung regions [104]. Another example is PNECs which are neurosensory cells that spread sparsely throughout the bronchial epithelium and studies have shown that PNECs can self-renew and differentiate into club cells and ciliated cells following lung injury [105, 106]. Lung organoid technology can be applied to investigate the functions of these cells. For instance, using a 3D co-culture organoid system, Lee et al., demonstrated that Lgr5 and Lgr6 are markers of mesenchymal cells in the adult lung. Moreover, these cells play important roles in direct airway differentiation of Scgb1a1+ progenitors and alveolar regeneration [107]. Organoid culture provides a model system for studying different airway epithelial stem/progenitor cells during repair, testing the effect of individual cytokines and growth factors on the proliferation and differentiation of secretory cells under pathological conditions, and identifying subpopulations of cells with enhanced regenerative potential.
-
2.
Alveolar repair and regeneration
The alveolar epithelium of the lung is composed of two distinct epithelial cell types. AT1 cells cover 95% of the surface area of the alveoli and perform the function of gas exchange [108]. AT2 cells are characterized by the production of pulmonary surfactant proteins, which are essential for reducing the surface tension of the alveolar surface area to prevent the lungs from collapsing upon every breath [23, 108,109,110]. The alveolar compartment remains largely quiescent in the uninjured lung, and most cells within this niche exhibit a relatively slow turnover [88]. After lung injury, multiple alveolar cell types are able to proliferate and, when the repair is effective, alveolar structure and function are both restored [88]. Although the function of AT2 cells involves repair, this may not be enough to treat certain lung diseases where the cells no longer perform their standard roles. This is why research is still being done to unlock how these cells fulfill their many duties as the progenitors of the lung and recent studies have determined that this mechanism may be due to verying cell populations.
AT2 cells are the alveolar epithelial stem cells: they can react to injury involving both activations of self-renewal and differentiation into more mature cell lineages [109]. AT2 cells can form alveolospheres and differentiate into organoid structures that contain both AT2 and AT1 cells [17]. Within the population of AT2 cells, there are subpopulations that play certain roles both in the human lung and organoids. A subset of AT2 cells that express the transcriptional target of Wnt signalling, Axin2, were identified by Zacharias et al. in the human lung and were found to be responsible for generating the majority of AT2 cell growth in human alveolar organoids [111]. Another group identified a population of adult distal lung epithelial progenitor cells with low Wnt/β-catenin activity with strong organoid-forming capacity, suggesting their role in the alveolar epithelial repair [112]. A recent study identified the damage-associated transient progenitors (DATPs) via lung organoid models [113]. The DATPs are distinct AT2-lineage populations that are required for AT2 cells to differentiate to mature AT1 cells [113]. Alveolar organoids have also been used to study trophic interactions between different cell populations in the distal airways. For instance, alveolar organoids have been recently used to provide functional evidence that multiple signalling pathways originate in Pdgfra+ lipofibroblasts to influence AT2 cell self-renewal and differentiation into AT1 cells through mediating multiple signalling, including BMP, FGF, and WNT signaling [114−116]. These discoveries are pivotal to better understanding how AT2 cells perform their endogenous functions. Once fully understood, these subpopulation roles could be manipulated in such a manner that could aid in repair and regeneration.
The contribution of AT1 cells to alveolar epithelial repair has not been studied extensively. A small subgroup of Hopx + AT1 cells can dedifferentiate into AT2 cells and thus participate in alveolar repair [117, 118]. Further in-depth characterization of these AT1 cells is needed to better understand the regulatory mechanisms guiding AT1 to AT2 transdifferentiation. Lung organoids can provide a useful model for identifying the cell types to increase insights into alveolar epithelial stem/progenitor cells during a repair. It is known now that cell populations such as immune cells are activated or recruited to the alveolar niche following lung injury. More complex lung organoid cultures incorporating immune cells will allow us to study the contribution of these niche cells that drive alveolar repair.
-
3.
Recapitulating lung damage, repair, and fibrosis with lung organoids
While lung organoids are still in the early stage of development compared to animal models or conventional cell lines, recent studies using lung organoid models have largely advanced our understanding of the underlying pathogenesis of distinct chronic lung diseases [119].
-
4.
Idiopathic pulmonary fibrosis
IPF is the most common and lethal form among interstitial lung diseases (ILDs) [120]. IPF is characterized by progressive fibrotic scarring in the lung tissue surrounding the air sacs, which ultimately leads to dyspnea. The etiology and pathogenesis of this disease are unclear [121, 122] and existing drugs can only slow disease progression [123, 124]. Lung transplantation is an option for IPF patients and has been found to extend their life but lung donors are limited, leading to extensive wait times which can cost the life of the patient [125, 126]. These limitations have motivated researchers to establish in vivo models to help mimic IPF in hopes of gaining insight to how to treat it. Models such as the bleomycin-induced mouse model and others have some gross similarities to human IPF but they fail to faithfully reproduce the pathophysiology of the disease [127]. Therefore, understanding the common pathways and pathogenetic mechanisms of lung fibrogenesis using representative models is critical for developing efficacious therapies [121].
Human pluripotent stem cells (hPSCs) have been shown to generate functional alveolar epithelial cells [67]. CRISPR genome editing has been used to introduce IPF-related genes in hPSC-derived lung organoid cultures which lead to the formation of abnormal cellular and morphological structures, including enhanced accumulation of mesenchymal cells and collagen, recapitulating important features of IPF [63, 65]. This provided a platform to identify pathogenic mechanisms of IPF that are likely clinically relevant in vitro. Using 3D pulmospheres from patients with IPF, Surolia et al. revealed the role of vimentin intermediate filaments in restricting the invasiveness of IPF fibroblasts [128]. The development of 3D organoid models can be adopted to model some forms of lung fibrosis, human distal lung structures, functions, and cell and matrix interactions opening the possibilities for high‐throughput in vitro drug efficacy and toxicity screening assays.
-
5.
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide. The pathogenesis of COPD has been linked to cigarette smoking and, more generally to environmental exposures, i.e. air pollution and toxicants. A genetic component, autoimmunity, and accelerated cell senescence are partly involved in the pathogenesis of COPD. COPD is a complex disease that can present with emphysema, chronic bronchitis, or both. In particular, the presence of mucus plugging associated with goblet cell metaplasia (GCM) contributes to cough, sputum production, and airway obstruction [129]. Most of the animal models employed for the study of COPD develop emphysema but not bronchitis and the knowledge we have on the mechanisms involved specifically in the origin of the overproduction of mucus is still limited.
Despite advances in the field, there is still much to be learned about cellular and molecular mediators uniquely involved in the onset of GCM in COPD, and the identity of stem/progenitor cells in the human lung and how deficient repair may contribute to COPD. Recent data suggests a significant amount of plasticity in the lung, and the source of cells contributing to the increased numbers of goblet cells in COPD is currently not clear. Lung organoids may be a useful model to explore these questions. For instance, bronchospheres have been used to show that NOTCH inhibition limits goblet cell metaplasia in vitro [101]. Using lung organoid cultures, a previous study showed that upregulated noncanonical WNT signalling, through increased WNT-5a and -5b contributes to emphysema by negatively regulating alveolar repair [130]. Using alveolar organoids, Jacob et al. demonstrated that temporal regulation of Wnt activity could promote maturation of iPSC-derived AT2 cells [67]. These studies provide evidence supporting the use of alveolar organoids to explore the regulation of Wnt signalling in alveolar epithelial progenitor cells of COPD patients and to discover new treatment strategies.
-
6.
Lung infection
Viral infections in the distal lung have been implicated in the progression of pneumonia to ARDS [131]. Respiratory viruses, including SARS-CoV-2, target lung epithelial cells, including AT2 cells [132]. Influenza viruses target AT2 and AT1 cells after intratracheal infection in mouse models [133]. However, there are currently no reliable models that recapitulate the phenotypes of lung infections in vitro. Lung organoids derived from hPSCs offer remarkable models to study the impact of different viruses including measles virus, respiratory syncytial virus (RSV), and the human parainfluenza virus type 3 (HPIV3) infections [63, 64]. RSV mainly causes respiratory tract infection in infants, and no vaccine or effective drugs have been developed yet [134]. RSV‐infected hPSC‐derived lung organoid cells led to detachment and shedding of infected cells into the lung organoid lumens recapitulating important features of the RSV-infected human infant lung [63, 64]. HPIV3 is a prevalent cause of lower respiratory tract disease in children. Consistent with clinical observations, HPIV3-infected lung organoids showed no detectable change in tissue integrity nor shedding of infected cells into the lumen [64]. Importantly, whole‐genome sequencing of HPIV3 in the lung organoids was found to be identical to the virus isolated in the clinical settings, suggesting that no selective pressure exists on the virus in the organoids [64]. The virus behaved similarly in the organoid models as it normally does in human infant lungs, indicating that the organoids are an optimal model for this particular infection. Lung organoids may be the key to generating a vaccine or treatment for HPIV3 which would be a significant accomplishment in the lung research field. Other viruses have been studied with lung organoids and are found to be as successful as well.
Influenza virus infection represents a major threat to public health worldwide. Zhou et al., developed human ASC-derived airway organoids (AOs) which can morphologically and functionally simulate human airway epithelium. These organoid cultures provide a reliable model to predict the infectivity of different human influenza virus can potentially provide a universal platform for studying the biology and pathology of the human airway [135].
Finally, the current COVID‐19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) targets lung epithelial cells, including AT2 cells [132, 136]. Lung organoids as well as alveolar and airway spheroids that strongly recapitulate the lung structure and cellular environment have been used to uncover the pathogenesis and screen efficient therapeutic agents for COVID-19 [55, 137,138,139]. These studies suggest lung organoids may serve as an authentic model for respiratory viral pathogenesis, providing a valuable tool to study host‐pathogen interaction, infection in the lung, and mechanisms of how viruses spread in the lung.
-
7.
Eyes to the future: Bioengineering of the whole lung
To date, the only available treatment for patients with a variety of end-stage lung disease remains lung transplantation. Approximately 5,000 lung transplants are performed annually worldwide, with equal numbers of patients on waiting lists [140]. However, there are not enough donor lungs to meet current or anticipated future needs. To meet the growing demand for transplants, a promising area of research is the regeneration of pulmonary tissue using ex vivo bioengineering methods.
Bioengineering of the whole lung ex vivo for transplantation is extremely complex due to the complexity of the lung. With the progress of regenerative medicine and stem cell biology, decellularized lungs have been used as native scaffolds for seeding cells to regenerate the lung. Early studies were mainly performed using mouse lungs and several methods were later developed to decellularize the lungs of rats, pigs, non-human primates, and humans to subsequently recellularize this scaffolds [141−146]. However, most of these strategies focus on epithelial cells without the endothelialization of decellularized lungs [147]. Thus, one of the major challenges in whole lung bioengineering remains the generation of functional pulmonary vasculature. Moreover, given the limit access of biological materials as scaffolds, an emerging idea is to create a hybrid lung scaffold that combines extracellular matrix (ECM) components with synthetic scaffolds. Finally, lung organoids combined with bioengineering could generate more complex and mature organoids that can be applied to developmental biology, personalized medicine and lung regeneration. We will discuss in more detail in the following section.
Lung Organoids and Personalized Repair
Lung diseases impose a great socioeconomic burden due to their high morbidity and mortality rate worldwide. With the limited effective treatments available in the last decade, developing novel therapies for pulmonary diseases is pressing. Lung organoids serve as one of the most promising modelling approaches to study patient-specific therapy and personalized medicine.
Bioengineered Lung
A growing number of tissue engineering techniques widens the potential of establishing more physiological relevant and functional human lungs ex vivo for transplantation. A common method to manufacture whole lung tissue ex vivo is to first derive decellularized 3D lung scaffolds from various species such as humans, pigs and rodents, and then reseed the scaffolds with patient-derived stem cells or primary lung progenitor cells [147]. The use of 3D scaffolds is beneficial as the native architecture of the lung is mostly retained following decellularization compared to de novo lung bioengineering [147]. A study done by Ghaedi et al. had shown the potential in human lung regeneration and lung transplantation via repopulated decellularized human and rat lungs with iPSC-derived epithelial progenitor cells [148]. The epithelial progenitor cells were found to perfuse in both airway and alveolar compartments of decellularized lung scaffolds to form a bioengineered ex vivo lung [148]. This lung regeneration approach could be applied in clinics by combining a native matrix scaffold with patient-derived cells to generate a personalized lung for lung transplantation therapies.
Wilkinson et al. developed another scaffold-based approach to generate self-assembled human lung organoids with the use of functionalized alginate beads under rotation in a bioreactor [149]. The engineered 3D lung organoids contained multiple cell types including pulmonary fibroblasts, small airway epithelial cells, and human umbilical vein endothelial cells [149]. By scaffolding mesenchymal cells into the interstitial space between hydrogel beads, this engineered lung organoid could recapitulate the anatomy of distal lung alveolar sacs [149]. They also showed the capability of this scalable iPSC-derived mesenchymal organoid culture approach to model IPF. Having the advantage of assembling organoids through different combinations of various types of cell-coated scaffold units, this organoid generation system could be personalized for patient-specific disease modelling and drug discovery [149].
3D-Printing Facilitated Precision Tissue Engineering
With the development of 3D printing technology, artificial organs with patient-specific spatial architecture have emerged as an attractive alternative for precision medicine. Customized implants with patient-specific size and shape can be accurately manufactured by 3D printing. These customized implants can perfectly fit the defect sites of patients to significantly reduce surgical operation time [150]. Grigoryan et al. have developed a bioinspired alveolar model using poly (ethylene glycol) diacrylate (PEGDA) and a stereolithographic printer, which contains regions reminiscent of native alveolar air sacs and alveolar buds [151]. This distal lung model contains functional intravascular oxygen transport through measuring blood oxygenation entering and leaving the model [151]. Another research group generated a bio-3D-printed artificial trachea using a Regenova bio-3D printer to create a scaffold-free tubular tissue from multicellular spheroids [152]. After generating multicellular spheroids from the mixture of rat chondrocytes, endothelial cells, and mesenchymal stem cells, the aggregated spheroids are then assembled into trachea constructs in a needle array, and the artificial trachea can become mature in terms of chondrogenesis and vasculogenesis in a bioreactor. Taniguchi et al. showed that the scaffold-free artificial tracheas remain functional with sufficient mechanical strength after transplantation into an isogenic rat for several weeks [152].
Lung Cancer Organoid Models
Many researchers work hard on generating patient-derived 3D lung tumour models, including spheroids and organoids, to study personalized medicine. Li et al. have established 12 patient-derived organoid lines from lung adenocarcinoma (LADC), which recapitulate the 3D structure and retain the genetic mutations of parental tumors [153]. Li et al. established these LADC organoids through dissociating tumour cells isolated from LADC samples and cultured them in Matrigel. These patient-derived organoid lines can be used for tumour biomarker identification and high-throughput drug screening. Together, this LADC organoid biobank serves as a good model to generate personalized therapy.
There is still much more to be learned about lung organoids, but their potential applications in personalized medicine is in the near future. As of late, there is no research involving the direct use of lung organoids on humans, but multiple studies are using them to advance human health. Researchers have used lung organoids generated from fetus and infant tissues diagnosed with Bochdalek congenital diaphragmatic hernia (CDH) to better understand the disease [154]. This study showed that the lung organoids could model CHD ex vivo and provide better ways of studying human diseases without using deceased human tissues. Other researchers have been able to model lung cancer using tumor tissues ex vivo and study potential patient-specific drug responses by comparing the lung organoid responses to patient responses [155]. Hu et al. determined the potential of a 1-week on-chip drug sensitivity test to predict patient responses to anti-lung cancer drugs [155]. Although this study found that the patient responses correlated 100% with only 11 of the 21 organoid samples, it gives hope to the idea of personalized medicine using lung organoid models.
Although many challenges need to be addressed before realising precision medicine treatment for lung diseases, such as the lack of a vascular system in most lung organoid models, the future of engineering functional lung and transplanting engineered lungs to patients is very promising.
Abbreviations
- PNECs:
-
Pulmonary neuroendocrine cells
- AT1:
-
Alveolar epithelial type 1
- AT2:
-
Alveolar epithelial type 2
- AT2-s:
-
AT2 signaling
- PAECs:
-
Pulmonary artery endothelial cells
- PVECs:
-
Pulmonary vein endothelial cells
- aCap:
-
Aerocyte capillary
- gCap:
-
General capillary
- BAECs:
-
Bronchial artery endothelial cells
- BMVECs:
-
Bronchial microvascular endothelial cells
- ARDS:
-
Acute respiratory distress syndrome
- ALI:
-
Acute lung injury
- E:
-
Embryonic
- P:
-
Postnatal day
- pcw:
-
Post-conception weeks
- SMCs:
-
Smooth muscle cells
- hPSCs:
-
Human pluripotent stem cells
- BME:
-
Basement membrane extract
- h3AC:
-
Human 3D alveolar type 2 cell culture
- hAT2:
-
Human AT2
- ESCs:
-
Embryonic stem cells
- iPSCs:
-
Induced pluripotent stem cells
- DE:
-
Definitive endoderm
- AFE:
-
Anterior foregut endoderm
- BMP:
-
Blocking bone morphogenetic protein
- TGFβ:
-
Transforming growth factor beta
- FGF:
-
Fibroblast growth factor
- RA:
-
Retinoic acid
- HH:
-
Hedgehog
- LBOs:
-
Lung bud organoids
- iAT2s:
-
Isolated alveolar epithelial type 2 cells
- SFTPC:
-
Surfactant protein C
- pro-SFTPC:
-
Pro-surfactant protein C
- HLOs:
-
Human lung organoids
- ASCsyy:
-
Adult stem cells
- CF:
-
Cystic fibrosis
- IPF:
-
Idiopathic pulmonary fibrosis
- CFTR:
-
Cystic fibrosis transmembrane regulator
- HPS:
-
Hermansky-Pudlak syndrome
- HPSIP:
-
HPS-associated interstitial pneumonia
- GRHL2:
-
Grainyhead-like 2
- Lgr:
-
Leucine-rich repeat-containing G protein-coupled receptor
- DATPs:
-
Damage-associated transient progenitors
- ILDs:
-
Interstitial lung diseases
- COPD:
-
Chronic obstructive pulmonary disease
- GCM:
-
Goblet cell metaplasia
- RSV:
-
Respiratory syncytial virus
- HPIV3:
-
Human parainfluenza virus type 3
- SARS‐CoV‐2:
-
Severe acute respiratory syndrome coronavirus 2
- ECM:
-
Extracellular matrix
- PEGDA:
-
Poly (ethylene glycol) diacrylate
- LADC:
-
Lung adenocarcinoma
- CDH:
-
Congenital diaphragmatic hernia
- PCD:
-
Primary ciliary dyskinesia
- SB:
-
SB-431542
- CHIR:
-
CHIR99021
- GSK:
-
Glycogen synthase kinase
- SAG:
-
Smoothened agonist
- SU:
-
SU5402
- MCC:
-
Mucociliary clearance
- SARS:
-
Severe acute respiratory syndrome
- MERS:
-
Middle East respiratory syndrome
References
Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER (1982) Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 126:332–337. https://doi.org/10.1164/arrd.1982.126.2.332
Travaglini KJ et al (2020) A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 587:619–625. https://doi.org/10.1038/s41586-020-2922-4
Kurn H, Daly DT (2021) Histology, epithelial cell. https://www.ncbi.nlm.nih.gov/books/NBK559063/
Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME (2008) Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc 5:772–777. https://doi.org/10.1513/pats.200805-041HR
Schittny JC (2017) Development of the lung. Cell Tissue Res 367:427–444. https://doi.org/10.1007/s00441-016-2545-0
Knight DA, Holgate ST (2003) The airway epithelium: structural and functional properties in health and disease. Respirology 8:432–446. https://doi.org/10.1046/j.1440-1843.2003.00493.x
Button B et al (2012) A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337:937–941. https://doi.org/10.1126/science.1223012
Dao DPD, Le PH (2021) Histology, goblet cells. https://www.ncbi.nlm.nih.gov/books/NBK553208/
Jeffery PK, Gaillard D, Moret S (1992) Human airway secretory cells during development and in mature airway epithelium. Eur Respir J 5:93–104
Evans MJ, Cox RA, Shami SG, Wilson B, Plopper CG (1989) The role of basal cells in attachment of columnar cells to the basal lamina of the trachea. Am J Respir Cell Mol Biol 1:463–469. https://doi.org/10.1165/ajrcmb/1.6.463
Harkema JR, Nikula KJ, Haschek WM (2013). In: Haschek WM, Rousseaux CG, Wallig MA (eds) Haschek and Rousseaux's handbook of toxicologic pathology, 3rd edn, pp 1935–2003. Academic Press
Montoro DT et al (2018) A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560:319–324. https://doi.org/10.1038/s41586-018-0393-7
Guillot L et al (2013) Alveolar epithelial cells: master regulators of lung homeostasis. Int J Biochem Cell Biol 45:2568–2573. https://doi.org/10.1016/j.biocel.2013.08.009
Weibel ER (1971) The mystery of “non-nucleated plates” in the alveolar epithelium of the lung explained. Acta Anat (Basel) 78:425–443. https://doi.org/10.1159/000143605
Weibel ER (2015) On the tricks alveolar epithelial cells play to make a good lung. Am J Respir Crit Care Med 191:504–513. https://doi.org/10.1164/rccm.201409-1663OE
Yang J et al (2016) The development and plasticity of alveolar type 1 cells. Development 143:54–65. https://doi.org/10.1242/dev.130005
Barkauskas CE et al (2013) Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123:3025–3036. https://doi.org/10.1172/JCI68782
Millar FR, Summers C, Griffiths MJ, Toshner MR, Proudfoot AG (2016) The pulmonary endothelium in acute respiratory distress syndrome: insights and therapeutic opportunities. Thorax 71:462–473. https://doi.org/10.1136/thoraxjnl-2015-207461
Comhair SA et al (2012) Human primary lung endothelial cells in culture. Am J Respir Cell Mol Biol 46:723–730. https://doi.org/10.1165/rcmb.2011-0416TE
King J et al (2004) Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67:139–151. https://doi.org/10.1016/j.mvr.2003.11.006
Gillich A et al (2020) Capillary cell-type specialization in the alveolus. Nature 586:785–789. https://doi.org/10.1038/s41586-020-2822-7
Moldobaeva A, Wagner EM (2002) Heterogeneity of bronchial endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol 283:L520-527. https://doi.org/10.1152/ajplung.00451.2001
Lorusso B et al (2015) Isolation and characterization of human lung lymphatic endothelial cells. Biomed Res Int 2015. https://doi.org/10.1155/2015/747864
Karoubi G, Cortes-Dericks L, Breyer I, Schmid RA, Dutly AE (2009) Identification of mesenchymal stromal cells in human lung parenchyma capable of differentiating into aquaporin 5-expressing cells. Lab Invest 89:1100–1114. https://doi.org/10.1038/labinvest.2009.73
Sadeghian Chaleshtori S, Mokhber Dezfouli MR, Jabbari Fakhr M (2020) Mesenchymal stem/stromal cells: the therapeutic effects in animal models of acute pulmonary diseases. Respir Res 21:110. https://doi.org/10.1186/s12931-020-01373-5
Hennrick KT et al (2007) Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med 175:1158–1164. https://doi.org/10.1164/rccm.200607-941OC
Agostini C, Chilosi M, Zambello R, Trentin L, Semenzato G (1993) Pulmonary immune cells in health and disease: lymphocytes. Eur Respir J 6:1378–1401
Patterson CE et al (1989) The role of activation of neutrophils and microvascular pressure in acute pulmonary edema. Am Rev Respir Dis 140:1052–1062. https://doi.org/10.1164/ajrccm/140.4.1052
Razavi HM et al (2004) Pulmonary neutrophil infiltration in murine sepsis: role of inducible nitric oxide synthase. Am J Respir Crit Care Med 170:227–233. https://doi.org/10.1164/rccm.200306-846OC
Pechous RD (2017) With friends like these: the complex role of neutrophils in the progression of severe pneumonia. Front Cell Infect Microbiol 7:160. https://doi.org/10.3389/fcimb.2017.00160
Tateda K et al (2001) Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol 166:3355–3361. https://doi.org/10.4049/jimmunol.166.5.3355
Semenzato G et al (1996) Lung lymphocytes: origin, biological functions, and laboratory techniques for their study in immune-mediated pulmonary disorders. Crit Rev Clin Lab Sci 33:423–455. https://doi.org/10.3109/10408369609084692
Venet F et al (2009) Lymphocytes in the development of lung inflammation: a role for regulatory CD4+ T cells in indirect pulmonary lung injury. J Immunol 183:3472–3480. https://doi.org/10.4049/jimmunol.0804119
Herriges M, Morrisey EE (2014) Lung development: orchestrating the generation and regeneration of a complex organ. Development 141:502–513. https://doi.org/10.1242/dev.098186
Faure S, de Santa Barbara P (2011) Molecular embryology of the foregut. J Pediatr Gastroenterol Nutr 52(1):S2–3. https://doi.org/10.1097/MPG.0b013e3182105a1a
Swarr DT, Morrisey EE (2015) Lung endoderm morphogenesis: gasping for form and function. Annu Rev Cell Dev Biol 31:553–573. https://doi.org/10.1146/annurev-cellbio-100814-125249
Danopoulos S et al (2018) Human lung branching morphogenesis is orchestrated by the spatiotemporal distribution of ACTA2, SOX2, and SOX9. Am J Physiol Lung Cell Mol Physiol 314:L144–L149. https://doi.org/10.1152/ajplung.00379.2017
Kitaoka H, Burri PH, Weibel ER (1996) Development of the human fetal airway tree: analysis of the numerical density of airway endtips. Anat Rec 244:207–213. https://doi.org/10.1002/(sici)1097-0185(199602)244:2%3c207::Aid-ar8%3e3.0.Co;2-y
Burri PH (1984) Fetal and postnatal development of the lung. Annu Rev Physiol 46:617–628. https://doi.org/10.1146/annurev.ph.46.030184.003153
Morrisey EE, Hogan BL (2010) Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 18:8–23. https://doi.org/10.1016/j.devcel.2009.12.010
Cooney TP, Thurlbeck WM (1982) The radial alveolar count method of Emery and Mithal: a reappraisal 1–postnatal lung growth. Thorax 37:572–579. https://doi.org/10.1136/thx.37.8.572
Herring MJ, Putney LF, Wyatt G, Finkbeiner WE, Hyde DM (2014) Growth of alveoli during postnatal development in humans based on stereological estimation. Am J Physiol Lung Cell Mol Physiol 307:L338-344. https://doi.org/10.1152/ajplung.00094.2014
Nikolic MZ, Sun D, Rawlins EL (2018) Human lung development: recent progress and new challenges. Development 145. https://doi.org/10.1242/dev.163485
Zeltner TB, Caduff JH, Gehr P, Pfenninger J, Burri PH (1987) The postnatal development and growth of the human lung I. Morphometry. Respir Physiol 67:247–267. https://doi.org/10.1016/0034-5687(87)90057-0
Folkman J, Long DM Jr, Becker FF (1963) Growth and metastasis of tumor in organ culture. Cancer 16:453–467. https://doi.org/10.1002/1097-0142(196304)16:4%3c453::aid-cncr2820160407%3e3.0.co;2-y
Gerrelli D, Lisgo S, Copp AJ, Lindsay S (2015) Enabling research with human embryonic and fetal tissue resources. Development 142:3073–3076. https://doi.org/10.1242/dev.122820
Kovar J, Sly PD, Willet KE (2002) Postnatal alveolar development of the rabbit. J Appl Physiol 1985(93):629–635. https://doi.org/10.1152/japplphysiol.01044.2001
Hyde DM et al (2007) Alveoli increase in number but not size from birth to adulthood in rhesus monkeys. Am J Physiol Lung Cell Mol Physiol 293:L570-579. https://doi.org/10.1152/ajplung.00467.2006
Mund SI, Stampanoni M, Schittny JC (2008) Developmental alveolarization of the mouse lung. Dev Dyn 237:2108–2116. https://doi.org/10.1002/dvdy.21633
Schittny JC, Mund SI, Stampanoni M (2008) Evidence and structural mechanism for late lung alveolarization. Am J Physiol Lung Cell Mol Physiol 294:L246-254. https://doi.org/10.1152/ajplung.00296.2007
Miller AJ et al (2019) Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc 14:518–540. https://doi.org/10.1038/s41596-018-0104-8
Rock JR et al (2009) Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 106:12771–12775. https://doi.org/10.1073/pnas.0906850106
Sachs N et al (2019) Long-term expanding human airway organoids for disease modeling. EMBO J 38. https://doi.org/10.15252/embj.2018100300
Tindle C et al (2020) Adult stem cell-derived complete lung organoid models emulate lung disease in COVID-19. bioRxiv. https://doi.org/10.1101/2020.10.17.344002
Salahudeen AA et al (2020) Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature 588:670–675. https://doi.org/10.1038/s41586-020-3014-1
Youk J et al (2020) Three-dimensional human alveolar stem cell culture models reveal infection response to SARS-CoV-2. Cell Stem Cell 27:905-919.e910. https://doi.org/10.1016/j.stem.2020.10.004
Wyler E et al. (2021) Transcriptomic profiling of SARS-CoV-2 infected human cell lines identifies HSP90 as target for COVID-19 therapy. iScience 24:102151. https://doi.org/10.1016/j.isci.2021.102151
Green MD et al (2011) Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol 29:267–272. https://doi.org/10.1038/nbt.1788
Yasunaga M et al (2005) Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol 23:1542–1550. https://doi.org/10.1038/nbt1167
Huang SX et al (2014) Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 32:84–91. https://doi.org/10.1038/nbt.2754
Huang SX et al (2015) The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat Protoc 10:413–425. https://doi.org/10.1038/nprot.2015.023
Dye BR et al (2015) In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4. https://doi.org/10.7554/eLife.05098
Chen YW et al (2017) A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19:542–549. https://doi.org/10.1038/ncb3510
Porotto M et al. (2019) Authentic modeling of human respiratory virus infection in human pluripotent stem cell-derived lung organoids. MBio 10. https://doi.org/10.1128/mBio.00723-19
Strikoudis A et al (2019) Modeling of fibrotic lung disease using 3D organoids derived from human pluripotent stem cells. Cell Rep 27:3709–3723 e3705. https://doi.org/10.1016/j.celrep.2019.05.077
McCauley KB, Hawkins F, Kotton DN (2018) Derivation of epithelial-only airway organoids from human pluripotent stem cells. Curr Protoc Stem Cell Biol 45. https://doi.org/10.1002/cpsc.51
Jacob A et al (2017) Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell 21:472–488 e410. https://doi.org/10.1016/j.stem.2017.08.014
Mao P et al (2015) Human alveolar epithelial type II cells in primary culture. Physiol Rep 3. https://doi.org/10.14814/phy2.12288
de Carvalho A et al (2019) Glycogen synthase kinase 3 induces multilineage maturation of human pluripotent stem cell-derived lung progenitors in 3D culture. Development 146. https://doi.org/10.1242/dev.171652
Rodrigues Toste de Carvalho AL et al (2021) The in vitro multilineage differentiation and maturation of lung and airway cells from human pluripotent stem cell-derived lung progenitors in 3D. Nat Protoc. https://doi.org/10.1038/s41596-020-00476-z
Mou H et al (2012) Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell 10:385–397. https://doi.org/10.1016/j.stem.2012.01.018
Firth AL et al (2015) Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep 12:1385–1390. https://doi.org/10.1016/j.celrep.2015.07.062
Bobadilla JL, Macek M Jr, Fine JP, Farrell PM (2002) Cystic fibrosis: a worldwide analysis of CFTR mutations–correlation with incidence data and application to screening. Hum Mutat 19:575–606. https://doi.org/10.1002/humu.10041
Vicary GW, Vergne Y, Santiago-Cornier A, Young LR, Roman J (2016) Pulmonary fibrosis in Hermansky-Pudlak syndrome. Ann Am Thorac Soc 13:1839–1846. https://doi.org/10.1513/AnnalsATS.201603-186FR
Moore BB, Hogaboam CM (2008) Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294:L152-160. https://doi.org/10.1152/ajplung.00313.2007
Rydell-Törmänen K, Johnson JR (1940) The applicability of mouse models to the study of human disease. Meth Mol Biol 3–22:2019. https://doi.org/10.1007/978-1-4939-9086-3_1
Plopper CG, Hyde DM (2008) The non-human primate as a model for studying COPD and asthma. Pulm Pharmacol Ther 21:755–766. https://doi.org/10.1016/j.pupt.2008.01.008
Pan H, Deutsch GH, Wert SE (2019) Comprehensive anatomic ontologies for lung development: a comparison of alveolar formation and maturation within mouse and human lung. J Biomed Semant 10:18. https://doi.org/10.1186/s13326-019-0209-1
Irvin CG, Bates JH (2003) Measuring the lung function in the mouse: the challenge of size. Respir Res 4:4. https://doi.org/10.1186/rr199
Matute-Bello G et al (2011) An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 44:725–738. https://doi.org/10.1165/rcmb.2009-0210ST
Nikolic MZ et al (2017) Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife 6. https://doi.org/10.7554/eLife.26575
Danopoulos S, Bellusci S, Warburton D, Al Alam D (2017) Identification of a SOX2/SOX9 double positive cells progenitor cell population required for branching morphogenesis in human lung. FASEB J 31:872.871–872.871. https://doi.org/10.1096/fasebj.31.1_supplement.872.1
Beers MF, Morrisey EE (2011) The three R’s of lung health and disease: repair, remodeling, and regeneration. J Clin Invest 121:2065–2073. https://doi.org/10.1172/jci45961
Metzger RJ, Krasnow MA (1999) Genetic control of branching morphogenesis. Science 284:1635–1639. https://doi.org/10.1126/science.284.5420.1635
Basil MC et al (2020) The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell 26:482–502. https://doi.org/10.1016/j.stem.2020.03.009
Hogan BL et al (2014) Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15:123–138. https://doi.org/10.1016/j.stem.2014.07.012
Plasschaert LW et al (2018) A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560:377–381. https://doi.org/10.1038/s41586-018-0394-6
Zepp JA, Morrisey EE (2019) Cellular crosstalk in the development and regeneration of the respiratory system. Nat Rev Mol Cell Biol 20:551–566. https://doi.org/10.1038/s41580-019-0141-3
Bustamante-Marin XM, Ostrowski LE (2017) Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol 9. https://doi.org/10.1101/cshperspect.a028241
Noone PG et al (2004) Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med 169:459–467. https://doi.org/10.1164/rccm.200303-365OC
Shapiro AJ et al (2016) Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr Pulmonol 51:115–132. https://doi.org/10.1002/ppul.23304
Leung TYM et al (2020) Short- and potential long-term adverse health outcomes of COVID-19: a rapid review. Emerg Microbes Infect 9:2190–2199. https://doi.org/10.1080/22221751.2020.1825914
Memish ZA, Perlman S, Van Kerkhove MD, Zumla A (2020) Middle East respiratory syndrome. Lancet 395:1063–1077. https://doi.org/10.1016/s0140-6736(19)33221-0
Drosten C et al (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348:1967–1976. https://doi.org/10.1056/NEJMoa030747
Rock JR et al (2011) Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 8:639–648. https://doi.org/10.1016/j.stem.2011.04.003
Hegab AE et al (2012) Isolation and in vitro characterization of basal and submucosal gland duct stem/progenitor cells from human proximal airways. Stem Cells Transl Med 1:719–724. https://doi.org/10.5966/sctm.2012-0056
Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR (2004) Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol 164:577–588. https://doi.org/10.1016/S0002-9440(10)63147-1
Rock JR, Randell SH, Hogan BL (2010) Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech 3:545–556. https://doi.org/10.1242/dmm.006031
Hild M, Jaffe AB (2016) Production of 3-D airway organoids from primary human airway basal cells and their use in high-throughput screening. Curr Protoc Stem Cell Biol 37:Ie.9.1–ie.9.15. https://doi.org/10.1002/cpsc.1
Butler CR et al (2016) Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med 194:156–168. https://doi.org/10.1164/rccm.201507-1414OC
Danahay H et al (2015) Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep 10:239–252. https://doi.org/10.1016/j.celrep.2014.12.017
Gao X, Bali AS, Randell SH, Hogan BL (2015) GRHL2 coordinates regeneration of a polarized mucociliary epithelium from basal stem cells. J Cell Biol 211:669–682. https://doi.org/10.1083/jcb.201506014
Rawlins EL et al (2009) The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4:525–534. https://doi.org/10.1016/j.stem.2009.04.002
Seibold MA (2018) Interleukin-13 stimulation reveals the cellular and functional plasticity of the airway epithelium. Ann Am Thorac Soc 15:S98-s102. https://doi.org/10.1513/AnnalsATS.201711-868MG
Garg A, Sui P, Verheyden JM, Young LR, Sun X (2019) Current topics in developmental biology, vol 132. In: Wellik DM (ed). Academic Press, pp 67–89
Ouadah Y et al (2019) Rare pulmonary neuroendocrine cells are stem cells regulated by Rb, p53, and notch. Cell 179:403-416.e423. https://doi.org/10.1016/j.cell.2019.09.010
Lee JH et al (2017) Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell 170:1149-1163.e1112. https://doi.org/10.1016/j.cell.2017.07.028
Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA (2007) A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci 104:4449–4454. https://doi.org/10.1073/pnas.0700052104
Mason RJ, Williams MC (1977) Type II alveolar cell. Defender of the alveolus. Am Rev Respir Dis 115:81–91. https://doi.org/10.1164/arrd.1977.115.S.81
Fehrenbach H (2001) Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res 2:33. https://doi.org/10.1186/rr36
Zacharias WJ et al (2018) Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555:251–255. https://doi.org/10.1038/nature25786
Hu Y et al (2020) Wnt/β-catenin signaling is critical for regenerative potential of distal lung epithelial progenitor cells in homeostasis and emphysema. Stem Cells 38:1467–1478. https://doi.org/10.1002/stem.3241
Choi J et al (2020) Inflammatory signals induce AT2 cell-derived damage-associated transient progenitors that mediate alveolar regeneration. Cell Stem Cell 27:366-382.e367. https://doi.org/10.1016/j.stem.2020.06.020
Chung MI, Bujnis M, Barkauskas CE, Kobayashi Y, Hogan BLM (2018) Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development 145. https://doi.org/10.1242/dev.163014
Green J, Endale M, Auer H, Perl AK (2016) Diversity of interstitial lung fibroblasts is regulated by platelet-derived growth factor receptor α kinase activity. Am J Respir Cell Mol Biol 54:532–545. https://doi.org/10.1165/rcmb.2015-0095OC
Zepp JA et al (2017) Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 170:1134–1148 e1110. https://doi.org/10.1016/j.cell.2017.07.034
Jain R et al (2015) Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nat Commun 6:6727. https://doi.org/10.1038/ncomms7727
Wang Y et al (2018) Pulmonary alveolar type I cell population consists of two distinct subtypes that differ in cell fate. Proc Natl Acad Sci USA 115:2407–2412. https://doi.org/10.1073/pnas.1719474115
Tian L et al (2020) Human pluripotent stem cell-derived lung organoids: Potential applications in development and disease modeling. Wiley Interdiscip Rev Dev Biol e399. https://doi.org/10.1002/wdev.399
American Thoracic Society (2000) Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 161:646–664. https://doi.org/10.1164/ajrccm.161.2.ats3-00
Noble PW, Barkauskas CE, Jiang D (2012) Pulmonary fibrosis: patterns and perpetrators. J Clin Invest 122:2756–2762. https://doi.org/10.1172/jci60323
Ryu JH et al (2014) Idiopathic pulmonary fibrosis: evolving concepts. Mayo Clin Proc 89:1130–1142. https://doi.org/10.1016/j.mayocp.2014.03.016
King TE Jr et al (2014) A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370:2083–2092. https://doi.org/10.1056/NEJMoa1402582
Richeldi L et al (2014) Efficacy and safety of Nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370:2071–2082. https://doi.org/10.1056/NEJMoa1402584
Laporta Hernandez R, Aguilar Perez M, Lázaro Carrasco MT, Ussetti Gil P (2018) Lung transplantation in idiopathic pulmonary fibrosis. Med Sci 6:68
Sharples L, Belcher C, Dennis C, Higenbottam T, Wallwork J (1994) Who waits longest for heart and lung transplantation? J Heart Lung Transplant 13:282–291
Chua F, Gauldie J, Laurent GJ (2005) Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol 33:9–13. https://doi.org/10.1165/rcmb.2005-0062TR
Surolia R et al (2019) Vimentin intermediate filament assembly regulates fibroblast invasion in fibrogenic lung injury. JCI Insight 4. https://doi.org/10.1172/jci.insight.123253
Burney PG, Patel J, Newson R, Minelli C, Naghavi M (2015) Global and regional trends in COPD mortality, 1990–2010. Eur Respir J 45:1239–1247. https://doi.org/10.1183/09031936.00142414
Wu X et al (2019) Mesenchymal WNT-5A/5B signaling represses lung alveolar epithelial progenitors. Cells 8. https://doi.org/10.3390/cells8101147
Fowler AA et al (1983) Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med 98:593–597. https://doi.org/10.7326/0003-4819-98-5-593
Chen N et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513. https://doi.org/10.1016/S0140-6736(20)30211-7
Quantius J et al (2016) Influenza virus infects epithelial stem/progenitor cells of the distal lung: impact on Fgfr2b-driven epithelial repair. PLoS Pathog 12. https://doi.org/10.1371/journal.ppat.1005544
Collins PL, Fearns R, Graham BS (2013) Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Curr Top Microbiol Immunol 372:3–38. https://doi.org/10.1007/978-3-642-38919-1_1
Zhou J et al (2018) Differentiated human airway organoids to assess infectivity of emerging influenza virus. Proc Natl Acad Sci USA 115:6822–6827. https://doi.org/10.1073/pnas.1806308115
Ziegler CGK et al (2020) SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181:1016-1035.e1019. https://doi.org/10.1016/j.cell.2020.04.035
Han Y et al (2021) Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 589:270–275. https://doi.org/10.1038/s41586-020-2901-9
Huang J et al (2020) SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell 27:962-973.e967. https://doi.org/10.1016/j.stem.2020.09.013
Katsura H et al (2020) Human lung stem cell-based alveolospheres provide insights into SARS-CoV-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell 27:890-904.e898. https://doi.org/10.1016/j.stem.2020.10.005
Yusen RD et al (2015) The registry of the international society for heart and lung transplantation: thirty-second official adult lung and heart-lung transplantation report–2015; focus theme: early graft failure. J Heart Lung Transplant 34:1264–1277. https://doi.org/10.1016/j.healun.2015.08.014
Dorrello NV et al (2017) Functional vascularized lung grafts for lung bioengineering. Sci Adv 3. https://doi.org/10.1126/sciadv.1700521
Ott HC et al (2010) Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16:927–933. https://doi.org/10.1038/nm.2193
Petersen TH et al (2010) Tissue-engineered lungs for in vivo implantation. Science 329:538–541. https://doi.org/10.1126/science.1189345
Rosen C et al (2015) Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat Med 21:869–879. https://doi.org/10.1038/nm.3889
Wagner DE et al (2013) Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology 18:895–911. https://doi.org/10.1111/resp.12102
Wobma H, Vunjak-Novakovic G (2016) Tissue engineering and regenerative medicine 2015: a year in review. Tissue Eng Part B Rev 22:101–113. https://doi.org/10.1089/ten.TEB.2015.0535
De Santis MM, Bölükbas DA, Lindstedt S, Wagner DE (2018) How to build a lung: latest advances and emerging themes in lung bioengineering. Eur Respir J 52. https://doi.org/10.1183/13993003.01355-2016
Ghaedi M et al (2018) Bioengineered lungs generated from human iPSCs-derived epithelial cells on native extracellular matrix. J Tissue Eng Regen Med 12:e1623–e1635. https://doi.org/10.1002/term.2589
Wilkinson DC et al (2017) Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl Med 6:622–633. https://doi.org/10.5966/sctm.2016-0192
Sun W et al (2019) Engineering Precision Medicine. Adv Sci (Weinh) 6:1801039. https://doi.org/10.1002/advs.201801039
Grigoryan B et al (2019) Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364:458–464. https://doi.org/10.1126/science.aav9750
Taniguchi D et al (2018) Scaffold-free trachea regeneration by tissue engineering with bio-3D printing†. Interact Cardiovasc Thorac Surg 26:745–752. https://doi.org/10.1093/icvts/ivx444
Li Z et al (2020) Human lung adenocarcinoma-derived organoid models for drug screening. iScience 23:101411. https://doi.org/10.1016/j.isci.2020.101411
Kunisaki SM et al (2021) Human induced pluripotent stem cell-derived lung organoids in an ex vivo model of the congenital diaphragmatic hernia fetal lung. Stem Cells Transl Med 10:98–114. https://doi.org/10.1002/sctm.20-0199
Hu Y et al (2021) Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat Commun 12:2581. https://doi.org/10.1038/s41467-021-22676-1
Acknowledgements
This work was supported by funding from the Hastings Foundation, Francis Foundation, Baxter Foundation, Keck Foundation, and the American Lung Association.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tian, L., Zhang, C.C., Rea, M.G., Chen, YW. (2022). Lung Organoids: A New Pathway into Lung Regeneration and Repair. In: Yahaya, B.H. (eds) Organoid Technology for Disease Modelling and Personalized Treatment . Stem Cell Biology and Regenerative Medicine, vol 71. Humana, Cham. https://doi.org/10.1007/978-3-030-93056-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-93056-1_2
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-93055-4
Online ISBN: 978-3-030-93056-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)