Abstract
A better understanding of normal lymphatic anatomy and how it changes in lymphoedema is crucially important in planning effective surgical interventions. The seminal works of the lymphatic anatomy were published by notable anatomists in the early twentieth century, but their focus was limited to the normal condition. However, the development of clinical imaging techniques since then has enabled us to investigate anatomical changes in the lymphatics that arise in patients with lymphedema. The lymphatics are structured in two layers, comprising superficial and deep systems separated by the deep fascia. The superficial lymphatic system is particularly significant for understanding the pathology of lymphedema. This chapter describes both the normal anatomy of the superficial lymphatic system in the extremities and also how it alters in lymphedema.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Lymphosome; Dermal backflow
- ICG fluorescence lymphography
- Lymphoscintigraphy
- Lymphangiography
- Manual lymphatic drainage
- Breast cancer-related lymphedema
Current Understanding of Lymphatic Anatomy
Hippocrates described ‘white blood’ in the body in the year 5 BCE, and this is considered to be the oldest account of the lymphatics. The first discovery of the lymphatics in academia is credited to Gaspare Aselli and his canine study in 1622 was published posthumously in 1627 [1]. The word ‘lymphatics’ was coined by Thomas Bartholin in 1653 in his book Vasa Lymphatica , in which he stated that the lymphatics were a vascular system independent to the blood system [2]. Anton Nuck (1691) developed a new technique to visualize the lymphatics in cadavers using mercury, and his technique enabled anatomists to investigate the lymphatics for the next three centuries [3]. Anatomical study of the lymphatic system reached its pinnacle around the early twentieth century with several notable publications: Sappey (1874), Delamere et al. (1903), Bartels (1909) and Rouviere (1932) [4,5,6,7]. These seminal works provided us with fundamental knowledge about normal lymphatic anatomy. However, their anatomical descriptions did not include any morphological changes that occur in pathological conditions such as lymphedema.
Kubik conducted a review of lymphatic anatomical studies and collated them in a chapter in Foldi’s book for physicians and lymphedema therapists [8]. One of his achievements was a body chart of skin lymphatic territories. His chart has become a popular educational resource to guide lymphedema therapists in applying manual lymphatic drainage (MLD) for lymphedema patients. The author (HS) coined the term ‘lymphosome’ to describe a skin lymphatic territory divided by their corresponding node group and created a lymphosome chart (Fig. 2.1) [9, 10]. Lymphosomes provide an overview of normal lymphatic anatomy and are also a useful way of comparing and contrasting the lymphatics between species in animal research.
Lymphosomes of the body. The lymphatic territories are demarcated according to their corresponding lymphatic basins: 1. temporal, 2. occipital, 3. submental, 4. subclavicular, 5. subscapular, 6. lateral axillary, 7. pectoral, 8. superior inguinal, 9. lateral inguinal, 10. inferior inguinal, 11. popliteal. (Reproduced with permission of Hiroo Suami)
The lymphatics are described as a two-layer system, consisting of superficial and deep systems separated by the deep fascia. Each system is independent from the other except at a few sites, but they unite in the deep axillary or intrapelvic regions. The superficial lymphatic system transfers lymph fluid from the skin and subcutaneous tissue, and the deep lymphatic system carries lymph fluid from the musculoskeletal tissue. The tissue changes that occur in lymphedema represent an accumulation of fluid and adipose tissue and fibrosis, but these changes are identified predominantly in the superficial soft tissue above the deep fascia. Thus, the superficial lymphatic system has a special significance for understanding the pathology of lymphedema. As a result, this chapter focuses primarily on the anatomy of the superficial lymphatics in both the normal condition and lymphedema.
Imaging Options for the Diagnosis of Lymphedema
Lymphedema is a chronic swelling of soft tissue caused by lymph stasis. The pathophysiology of lymphedema is not yet fully understood. Damage to the lymphatic system following lymph node dissection, radiotherapy or filariasis provokes lymphatic dysfunction and retention of lymph fluid in the affected limbs. Lymph stasis triggers structural damage in the lymphatic vessels, giving rise to progressive change such as fibrosis of vessel walls, a narrowing lumen and a reduction in smooth muscle cells which are a feature of lymphedema [11].
There are several imaging techniques that can identify anatomical change in the lymphatic system and aid the development of diagnostic criteria for lymphedema. The first of these, and the current gold standard for diagnostic imaging for lymphedema, is lymphoscintigraphy. It is a form of nuclear medicine imaging developed in the 1950s and demonstrates lymph nodes as hot spots [12]. The reduction or absence of nuclear tracer in the lymph nodes is a criterion of lymphedema. Although lymphoscintigraphy has been used in lymphedema diagnosis for several decades, the poor-resolution, two-dimensional images produced are not ideal for evaluating the condition of the lymphatic vessels. In advanced lymphedema, the nuclear tracer commonly moves only a short distance from the injection site, and no imaging information can be obtained in the proximal body regions.
Lymphangiography is an imaging technique developed by Kinmonth in 1952 [13]. It is no longer used for lymphedema diagnosis because one of its side effects was to make lymphedema worse. However, the high-resolution images produced by this technique provided the most detailed information about the lymphatic vessels in lymphedema.
Indocyanine green (ICG) fluorescence lymphography has been widely adopted as a new way to conduct lymphatic imaging. The camera system uses near-infrared technology and it was first applied to the lymphatics in 2005 [14]. ICG dye injected into dermal or subcutaneous tissue is spontaneously absorbed into the lymphatic capillaries and fluoresces when excited by near-infrared light. The camera and filter system selectively picks up the near-infrared rays and identifies lymphatic structures within a depth of 2 centimetres from the surface of the skin. The use of photoacoustic imaging with ICG dye has the advantage of demonstrating the lymphatics as a three-dimensional image [15].
Lymphatic imaging technology continues to develop, with each development providing further detailed images of the lymphatics. Although significant advances have been made in lymphatic imaging techniques, the relationship between the tracer injection site and the lymphatic pathway has not been much discussed. When different techniques use different injection sites, it is difficult to compare the images obtained. To address this issue, we undertook anatomical studies of the lymphatic system in cadaver legs using both CT lymphography and ICG lymphography [16]. We found that standard injection sites at the web spaces between the toes did not help visualize some lymph nodes of the leg. Additional injection sites in the medial, lateral and posterior aspect of the foot were required for evaluating the whole lymphatic pathways. We would like to stress the importance of developing a precise knowledge of normal lymphatic anatomy because this knowledge enables us to distinguish the structural changes that occur in lymphedema.
Normal Lymphatic Anatomy in the Lower Extremity
To understand the normal anatomy of the lymphatics in the lower extremity, a knowledge of embryological development of the cutaneous veins and lymphatic vessels is crucial. The superficial lymphatic vessels run alongside the superficial veins, and the superficial lymph nodes are located near the junction of the great saphenous vein (GSV) and the common femoral vein. Recent articles have revealed that the peripheral lymphatic vessels develop before lymph nodes [17, 18]. In the foetus, lymph node anlages emerge at the junction of the GSV and the common femoral vein and fuse to the lymphatic vessels. Thus, lymph nodes in the lower extremity are concentrated in the inguinal region, and the superficial lymphatic vessels along the GSV connect to these nodes.
The superficial lymphatic vessels are distributed circumferentially around the foot. Our anatomical studies using fresh cadaver specimens indicate that the superficial lymphatic vessels in the lower leg are classified into four subgroups according to their anatomical relationship with the cutaneous veins (Fig. 2.2) [16, 19, 20]. These four distinct subgroups are the posteromedial, anteromedial, anterolateral and posterolateral groups. In our study, three of the four – the anteromedial, anterolateral, and posteromedial – connected to the inguinal nodes. Both posteromedial and anteromedial groups of vessels connected to the same superficial lymph nodes in the medial inguinal region, but the anterolateral group connected to different inguinal lymph nodes located in the lateral inguinal region (Fig. 2.3). The posteromedial group of vessels ran along the main trunk of the GSV, while the vessels in the anteromedial and anterolateral groups ran along branches of the GSV. The posterolateral group of vessels alone ran along the lesser saphenous vein (LSV) and connected to the popliteal lymph nodes. The posterolateral and posteromedial groups of vessels were composed of only a few lymphatic vessels. Their diameter was larger and they ran deeper than the vessels in the other two groups. The lymphatic vessels in the anteromedial group originated in the dorsum of the foot and were greater in number than the other groups in the lower leg. The lymphatic vessels in the anterolateral group originated in the lateral foot. They ran along a lateral branch of the GSV in the lower leg and then along a lateral accessory branch of the GSV in the thigh.
A schematic diagram of detailed lymphosomes in the lower extremity and the correlation between a lymphosome and the location of the first-tier lymph node. Lymphatic groups are color-coded using the same scheme as in Fig. 2.2. Three regional lymph nodes received most of the lymphatic fluid in the lower limbs: inferior lateral (IL) 1 and 2 and superficial popliteal (SP). IM = inferior medial, SL = superior lateral, SM = superior medial
To provide a comprehensive imaging examination, it is important that all four subgroups can be identified. The ICG dye must be injected into four specific sites: below the medial and lateral malleolus, at the first toe web and at the midpoint between the head of the fifth metatarsal bone and below the lateral malleolus.
Anatomical Changes in Lower Extremity Lymphedema
The pathology of cancer-related lymphedema is explained as the obstruction of superficial lymphatic vessels at different levels in the lower extremity that causes collateral pathways to form to maintain lymph flow. Lymph fluid in the affected vessels spills backwards into the dermal lymphatics at the obstructed site in a phenomenon known as ‘dermal backflow’ (Fig. 2.4). Dermal backflow is a specific criterion in diagnosing lymphedema and enables a connection to be made between the obstructed vessel and a nearby patent vessel. However, dermal backflow is not the only mechanism to maintain lymph flow. An alternative type of collateral pathway creation is lymphangiogenesis, whereby a new lymphatic vessel develops from the stump of an obstructed vessel and extends towards the remaining lymph nodes [21].
Imaging studies of the lymphatics have reported anatomical changes in lymphedema. Kinmonth performed lymphangiography in primary leg lymphedema patients and classified the cases into two categories, ‘proliferative’ and ‘aplastic’, according to the number of lymphatic vessels identified [22]. Maegawa et al. classified the severity of leg lymphedema through lymphoscintigraphy [23]. Their findings indicated that deterioration of the lymphatic vessels commenced in the inguinal region and extended distally as lymphedema progressed. All lymphatic vessels eventually disappeared, and the tracer did not move beyond the injection site in the most advanced stage.
Conservative management known as comprehensive decongestive therapy (CDT) or complex lymphedema therapy (CLT) has been the mainstay of lymphedema treatment. The axillo-inguinal pathway has routinely been used in the MLD sequence for leg lymphedema to move extra-cellular fluid from the affected leg to the axillary region. However, lymphatic imaging in leg lymphedema rarely demonstrated this pathway, but often only demonstrated the pathway to the contralateral inguinal region. This suggests that there is a discrepancy between the general principle of conservative management and imaging findings. Further imaging investigations into the altered anatomy in lymphedema will shed light on the pathophysiology of lymphedema and help develop an evidence-based management plan.
Normal Lymphatic Anatomy in the Upper Extremity
The lymphatics in the upper extremity originate in the lymphatic capillaries in the dermis of the fingertips and palm. Those vessels in the fingertips converge at the level of the distal interphalangeal joint to form one or two lymphatic vessels on each side. All lymphatic vessels from the fingers run in the dorsum of the hand. Those in the palm converge to form several lymphatic vessels at the anterior wrist. These superficial lymphatic vessels are arranged circumferentially around the wrist. The lymphatic vessels originating at the anterior wrist run straight towards the axilla. The lymphatic vessels originating at the dorsal hand run along the posterior forearm and divide into two courses distally from the olecranon (Fig. 2.5). They gradually change their course to the medial upper arm en route to the axilla.
The superficial lymphatic pathway connecting to the axillary lymph nodes is the dominant pathway, but an alternative pathway to the clavicular nodes exists as an anatomical variation. The lymphatic vessels running along the cephalic vein pass through an interval lymph node named the deltopectoral lymph node at the deltopectoral groove. The vessels run below the head of the pectoralis major and connect to the supraclavicular nodes. This lymphatic pathway was described by Sappey and Mascagni [4, 24]. Anatomical studies were conducted by Kubik and LeDuc [25, 26]. As this lymphatic pathway bypasses the axillary nodes, knowledge about it is important in skin cancer management to help identify cancer metastatic sites.
The deep lymphatic system is located below the deep fascia. The deep vessels run along the major arteries, including the ulnar, radial and humeral arteries. The superficial and deep vessels are generally independent of each other without any direct connection between them, but they are very close to each other at the anterior elbow. The superficial lymphatic vessels located along the basilic vein sometimes run together with the vein and merge with the deep lymphatic vessels.
In order to identify all lymphatic vessels running towards the axilla, the tracer injection needs to be given at multiple sites circumferentially around the hand. If the tracer is injected into the finger webs alone, only the lymphatic vessels in the posterior forearm are revealed, missing those in the anterior forearm.
Anatomical Changes in Upper Extremity Lymphedema
Lymphedema in the lower extremity is representative of various causes, including congenital maldevelopment and primary idiopathic, traumatic or cancer-related disorders. However, upper extremity lymphedema is predominantly caused by breast cancer treatment. Axillary surgery is the major factor in lymphedema development, and radiation given in addition to surgery increases lymphedema risk [27]. The pathology of upper extremity lymphedema has conventionally been explained as the blockage of arm lymphatic drainage provoked by surgical intervention that subsequently causes swelling of the arm. The current principle of conservative management is based on this theory, and MLD for arm lymphedema is performed to shift excess lymph fluid from the affected arm to other intact nodal regions by massaging it downwards to the ipsilateral inguinal region and horizontally to the contralateral axilla. However, our recent ICG lymphography study in breast cancer-related lymphedema (BCRL) revealed that more than two-thirds of arm lymphedema still drained to the ipsilateral axilla, the site of the breast surgery [28]. These results suggest that axillary node dissection does not always block the lymphatic drainage pathway to the axilla. Therefore, it is reasonable to reconsider that is the limitation of lymph flow to the axilla, rather than total blockage, that causes arm lymphedema.
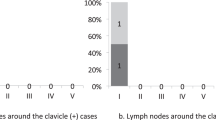
In normal anatomy, there are two bypassing lymphatic pathways – one to the supraclavicular nodes via the deltopectoral groove and the other to the deep lymphatic system at the anterior elbow – that play a key role in maintaining lymph flow in BCRL and help prevent lymphedema progression [21]. Lymph fluid in lymphedema is often diverted through these pathways when the superficial lymphatic pathway to the axilla is damaged or obstructed. Lymphedema is caused by damage to the superficial lymphatic vessels followed by identification of dermal backflow at the site. As a definite imaging criterion for lymphedema diagnosis, dermal backflow is often considered to be a negative sign. However, dermal backflow enables lymph fluid in the affected lymphatic vessels to be transported to the unaffected region, so it should be considered to be a positive reaction by the body to maintain lymph fluid drainage. The patterns of lymphatic drainage in BCRL found in our study are summarized in Fig. 2.6 [29].
Schematic diagrams show the patterns of lymphatic drainage in upper extremity lymphedema. (a) The ipsilateral axillary region; (b) the clavicular region; (c) the parasternal region; (d) the contralateral axillary region. (Reproduced from Ref. 29 with permission)
Summary
This chapter describes both the normal anatomy of the superficial lymphatic system in the extremities and the altered anatomy in lymphedema. We have demonstrated that the human body has the flexibility to maintain lymph drainage via anatomical structural changes even when lymphedema has developed. While surgical procedures for lymphedema are being continually refined, conservative management strategies must also be updated to reflect recent imaging findings.
References
Aselli G. De Lactibus Sive Lacteis Venis. J.B. Bidellius: Milan; 1627.
Bartholin T. Vasa lymphatica nuper Hafniae in animalibus inventa et hepatis exsequiae. Petrus Hakius: Hafniae (Copenhagen); 1653.
Nuck A. Adenographia curiosa et uteri foeminei anatome nova. Jordan Luchtmans: Leyden; 1691.
Sappey MPC. Anatomie, Physiologie, Pathologie des Vaisseaux Lymphatiques consideres chez L’Homme et les Vertebres. Paris: Adrien Delahaye; 1874.
Delamere G, Poirier P, Cuneo B. The lymphatics. In: Charpy PP, editor. A treatise of human anatomy. Westminster: Archibald Constable and Co Ltd; 1903.
Bartels P. Das Lymphgefäßsystem. Handb. d. Anat. Verlag von gustav fischer: Jena; 1909.
Rouviére H. Anatomie des lymphatiques de l'homme. Paris: Masson; 1932.
Foldi M, Foldi E, Kubik S. Textbook of lymphology for physicians and lymphedema therapists. Urban & Fischer: Munchen; 2003.
Suami H. Lymphosome concept: anatomical study of the lymphatic system. J Surg Oncol. 2017;115(1):13–7.
Suami H, Scaglioni M. Anatomy of the lymphatic system and the lymphosome concept with reference to lymphoedema. Semin Plast Surg. 2018;32:5–11.
Koshima I, Kawada S, Moriguchi T, Kajiwara Y. Ultrastructural observations of lymphatic vessels in lymphedema in human extremities. Plast Reconstr Surg. 1996;97:397–405.
Sherman AI, Ter-Pogossian M. Lymph-node concentration of radioactive colloidal gold following interstitial injection. Cancer. 1953;6:1238–40.
Kinmonth JB. Lymphangiography in man; a method of outlining lymphatic trunks at operation. Clin Sci. 1952;11:13–20.
Unno N, Inuzuka K, Suzuki M, et al. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J Vasc Surg. 2007;45:1016–21.
Suzuki Y, Kajita H, Konishi N, et al. Subcutaneous lymphatic vessels in the lower extremities: comparison between photoacoustic lymphangiography and near-infrared fluorescence lymphangiography. Radiology. 2020;295:469–74.
Shinaoka A, Koshimune S, Yamada K, et al. Correlations between tracer injection sites and lymphatic pathways in the leg: a near-infrared fluorescence Lymphography study. Plast Reconstr Surg. 2019;144:634–42.
Petrova TV, Koh GY. Organ-specific lymphatic vasculature: from development to pathophysiology. J Exp Med. 2018;215:35–49.
Bovay E, Sabine A, Prat-Luri B, et al. Multiple roles of lymphatic vessels in peripheral lymph node development. J Exp Med. 2018;215:2760–77.
Shinaoka AA, Koshimune S, Yamada K, et al. A fresh cadaver study on indocyanine green fluorescence lymphography: a new whole body imaging technique for investigating the superficial lymphatics. Plast Reconstr Surg. 2018;141:1161–4.
Shinaoka A, Koshimune S, Suami H, et al. Lower-limb lymphatic drainage pathways and lymph nodes: a CT lymphangiography cadaver study. Radiology. 2020;294(1):223–9.
Suami H. Anatomical theories of the pathophysiology of cancer-related lymphoedema. Cancers (Basel). 2020;12:1338.
Kinmonth JB. Primary lymphedema: classification and other studies based on oleo-lymphography and clinical features. J Cardiovasc Surg. 1969;10(suppl):65–77.
Maegawa J, Mikami T, Yamamoto Y, et al. Types of lymphoscintigraphy and indications for lymphaticovenous anastomosis. Microsurgery. 2010;30:437–42.
Mascagni P. Vasorum Lymphaticorum Corporis Humani Historia et Ichonographia. P. Carli: Sienne;1787.
Kubik S. The role of the lateral upper arm bundle and the lymphatic watersheds in the formation of collateral pathways in lymphedema. Acta Biol Acad Sci Hung. 1980;31:191–200.
Leduc A, Caplan I, Leduc O. Lymphatic drainage of the upper limb. Substitution lymphatic pathways. Eur J Lymphol. 1993;4:11–8.
Naoum GE, Roberts S, Brunelle CL, et al. Quantifying the impact of axillary surgery and nodal irradiation on breast cancer-related lymphedema and local tumor control: long-term results from a prospective screening trial [published online ahead of print, 2020 Jul 30]. J Clin Oncol. 2020:JCO2000459.
Suami H, Heydon-White A, Mackie H, Czerniec S, Koelmeyer L, Boyages J. A new indocyanine green fluorescence lymphography protocol for identification of the lymphatic drainage pathway for patients with breast cancer-related lymphoedema. BMC Cancer. 2019;19(1):985.
Suami H, Koelmeyer L, Mackie H, Boyages J. Patterns of lymphatic drainage after axillary node dissection impact arm lymphoedema severity: a review of animal and clinical imaging studies. Surg Oncol. 2018;27:743–50.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shinaoka, A., Suami, H. (2022). Anatomy of the Lymphatic System and Structural Changes in Lymphedema of the Extremities. In: Schaverien, M.V., Dayan, J.H. (eds) Multimodal Management of Upper and Lower Extremity Lymphedema. Springer, Cham. https://doi.org/10.1007/978-3-030-93039-4_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-93039-4_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93038-7
Online ISBN: 978-3-030-93039-4
eBook Packages: MedicineMedicine (R0)