Abstract
Complete utilization of every material mined seems to be a highly obvious strategy to save natural resources and avoid residues to be dumped. In fact, examples where this is practiced are very limited. Often residues out of metal production are not fully exploited and contain still considerable amounts of various valuable elements when being dumped. Using the full potential of mined material would significantly support the metal supply, while optimizing the CO2 balance, as much of the afford of winning a metal is already done, especially the mining. Not only the CO2 footprint, but also the avoidance of residues is an omnipresent topic nowadays. Pyrometallurgical treatment is unavoidably linked to slags. Regarding a zero-waste solution, these slags have high potential to be used as construction materials. Referring to this, zero-waste solutions for residues from primary zinc and lead production were tested, emphasizing the potential of this strategy.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Treating residues from metal producing industry for metal extraction is getting more and more attention [1]. However, such residues are not only of interest as secondary resources for metal winning, but also because of increasing challenges of landfilling due to the ever-growing environmental awareness. With this, costs for dumping are increasing and requirements are getting stricter.
Residues from nonferrous metal production often contain considerable amounts of various metals [2]. This is because the corresponding ores, in opposite to iron ores for example, usually contain many different metals. Whilst the main metal and in some cases a second or third one is won, many of the present metals are not recovered and end up in the waste material. The metal contents within these materials can be even higher than in primary ores, but can cause major problems for methods of mineral processing due to grain size, phase distribution, etc. However, a big part of the effort of winning metals, namely the mining, is already done for these materials, making it even more obvious to use the materials full potential and with this, to minimize the overall CO2 footprint.
Modern efforts of using metallurgical residues as secondary resources often face the problem of causing again large amounts of new residues. Even if a residue is treated successfully for metal winning, the handling of the non-metal fraction causes challenges again. The only possibility to avoid dumps is to use every fraction produced during a process. For some residues, like blast furnace slag, the use as construction material is very common, whereas in many cases, especially in nonferrous metallurgy, there is no possibility of valorization so far.
Natural sand is an often underestimated resource. It must fulfill different criteria such as grain size distribution and grain shape to be used in construction. Due to the low price of sand, gravel, etc., transport distances also have to be short. Sand is not yet being classified as critical resource, but following the exponentially growing building activities, it will most likely become critical in future. This, together with the avoidance of dumps in general, makes the use of all kinds of technical materials for construction a highly reasonable strategy.
Materials
The main part of research to date and presented here has been done on residues from hydrometallurgical zinc winning process. Main component of these residues is jarosite, a sulfate mineral which is being formed in order to remove iron out of sulphuric acid solution used to leach zinc from the roasted concentrate [3, 4]. Commonly, the whole residue is also called jarosite, even though it contains not only the precipitation product (jarosite phase), but also other residues formed during the overall metal winning process. Treatment of the jarosite residue is of interest due to different reasons. Because of its hazardous properties, dumping is an increasing problem for many plants. As existing dumps will be full in near future and there will be no permissions for new ones. From another point of view, the jarosite residue bears several valuable metals, especially zinc, lead, and silver, in some cases also copper, gold, indium, and germanium. Recovery of these elements using a roast-reduction process was tested extensively with promising success up to 1000 kg scale [5, 6]. Further trials in smaller scale were performed not only to win the valuable metals, but also to evaluate the usability of the produced slag as construction material. Initially, its suitability as substitute for natural sand in Portland cement-based concrete was tested.
A second residue, whose treatment for multi-metal recovery has been studied, is slag out of lead production. The slag was taken from a historical dump of a former lead and zinc smelter, which used the imperial smelter furnace technology [2]. The dump located in Macedonia is considered as potential source for pollution, mainly because of its lead and arsenic content [7]. To date, the work on this material focused on the pyrometallurgical treatment of this specific slag to recover zinc and minor amounts of lead. The process is based on selective reduction on a lead bath, producing a liquid slag which can be modified to meet requirements for further utilization. Trials on its applicability of the remaining slag as construction material will be part of upcoming research in near future. Also, in this case, the aim is to realize a zero-waste solution and to minimize the overall CO2 footprint of lead and zinc production.
Characterization of Jarosite Material
As the main part of the material is a precipitation product, the grain size is generally very small, with about 90 wt% passing 25 µm and 60 wt% passing 10 µm. However, due to agglomerations and the measurement by sieve analysis, the grains size distribution is suspected to be even smaller. Characterization of such small-grained material quickly reveals the limits of common methods such as light microscopy and even scanning electron microscopy [8]. Table 1 shows the main elements of interest measured by X-ray fluorescence analysis. Zinc, lead, and silver are the most valuable phases in jarosite residues. Copper is often present, but of lesser importance due to the low amount. Indium can be of interest in some specific jarosite residues.
The main components, identified mainly by scanning electron microscopy and X-ray diffraction analysis, are obviously jarosite phases. The typical endmember formed in the process is natrojarosite (NaFe3(SO4)2(OH)6) or ammoniojarosite ((NH4)Fe3(SO4)2(OH)6). To a minor extend also, hydroniumjarosite (H3O)Fe3(SO4)2(OH)6) and plumbojarosite (PbFe6(SO4)4(OH)12) can be present. Contrary to expectations out of the overall hydrometallurgical zinc winning process, the jarosite phase proved also to bear considerable amounts of zinc and lead, which are the two most important valuable elements. Silver can theoretically also be present, even though it was not clearly identified [9]. Other phases bearing these elements are zinc sulfide, zinc ferrite, lead oxide, sulfate, and sulfide. Silver is often present in inclusions together with copper and lead in various phases, especially in silicates (quartz and feldspar) [6].
Characterization of Lead Slag
The granulated slag is fine grained, with about 95 wt% passing 3 mm. Grains of agglomerated slag with diameters up to 20 mm can be found. The glassy and homogenous matrix consists of a CaO-SiO2-FeO phase. Main valuable elements are zinc (7.9 wt%) and lead (2.4 wt%) (Table 2). Beside the amorphous matrix, zinc oxide, iron zinc oxide, ferrous sphalerite, iron silicate, calcium arsenate, and pyrite were identified using X-ray diffraction analysis and scanning electron microscopy. These phases are present as irregular-shaped inclusions. Lead appears mainly as oxide or sulfide as droplets with diameters of up to 75 µm. Metallic lead is rare. Zinc is mainly bound to the matrix, where it makes up to 7 wt%. Other zinc phases, such as zinc sulfide may be found, but are very rare [2, 10, 11].
Pyrometallurgical Treatment of Jarosite Material
Due to the dominance of the jarosite phase, and very limited amount of other phases, results gathered by the detailed characterization do not allow any scope for concentration by means of mineral processing. Nevertheless, small scale trials in grain size and magnetic separation and flotation were performed in parallel. Despite smaller concentrations of single elements, the findings from the characterization were confirmed and no noteworthy concentration was achieved [8].
Prior to metallurgical treatment, the material was pelletized with a disc pelletizer. This was necessary, mainly for making sample handling easier and to avoid carryover of material with the off gas, but also to allow a better gas flow. Besides water, no additives were necessary to form stable pellets with a diameter of about 3–10 mm.
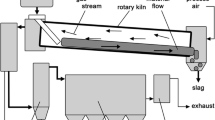
The investigated pyrometallurgical process for multi-metal recovery consists of two steps. First the material is calcinated, to remove volatile compounds, especially hydroxides and sulfur. This could be done in a top-blown-rotary-converter or short-drum-furnace. The second step is the reduction in an electric furnace where all valuable metals are won. Zinc evaporates and can be caught in the off gas after reoxidation as ZnO. Lead and silver (copper, gold) are collected in the liquid lead bath at the bottom. The slag is theoretically free of heavy metals and has a fayalitic composition, meaning that iron is in this case not part of the metal product but of the non-metal product (slag).
Use of Slag from Jarosite Treatment
The slag from pyrometallurgical jarosite treatment was tested for its potential in being used as sand replacer in concrete. During the reduction step, after the successful separation of slag and metal (Zn, Pb, Ag, Cu…), the fayalitic slag was quenched in water and sieved to three grain sizes, <1 mm, 1–2 mm, and 2–4 mm. Every grainsize was introduced to concrete (with Portland cement 32.5 and normalized sand EN196) in three different ratios, replacing natural, normalized sand. As reference, one concrete sample was produced only with Portland cement and sand (Table 3). Water was added until the result of the flow table test reached a value between 160 and 180 mm.
For the utilization as construction material, the mechanical strength (compression and flexural) and chemical resistance (leachability) were tested.
Compressive resistance determination was done on 12 cubic samples (5 × 5 cm) for each condition and flexural strength evaluation on 6 samples 16 × 4 × 4 cm per condition.
After 7 and 28 days of curing at 20–23 °C, the tests on flexural and compression strength were performed on the produced samples. For eluate testing, samples after 28 days of curing time were used.
Pyrometallurgical Treatment of Lead Slag
The proposed technology for multi-metal recovery is very similar to the treatment of jarosite material concerning the reduction step. However, due to the composition of the lead slag, calcination is not necessary. Often iron is already present as fayalite out of primary lead winning, forming ideal conditions for the re-melting on a lead bath [11].
After removing zinc and lead, the liquid slag can be optimized by additives to fulfil the requirements of a sand substitute.
Results
Results from Jarosite Material Treatment
The pyrometallurgical two-step process proved its applicability for jarosite material successfully in different scales. Using only the second step (reduction) is possible, but leads to a big loss of metal to the slag phase due to the sulfur content. Temperature is a critical parameter in both process steps. For calcination, it must be high enough for volatilizing SO2, water, and hydroxides but must not be too high to avoid the loss of valuable components, such as PbO.
For the reduction step, the temperature must be high enough for melting all the material, but again, unwanted vaporization of components must be avoided. Additionally, due to energetic and economic reasons, the temperature should be as low as possible. The success of the trials was measured by analyzing remaining contents of valuables, especially lead and zinc, in the slag. With remaining 2–3 wt% each, the values did not get as low as expected according to other similar trials (Table 4).
After detailed investigations of the slag, using a scanning electron microscope, it becomes clear that especially the remaining lead is present as metallic droplets in the slag (Fig. 1). This indicates problems in the settling behavior of the liquid metal. Therefore, the problem does not relate to the chemical process of separation, but to the technical implementation, respectively the furnace. Due to turbulent conditions, the separation of slag and metal was obviously not as clear as expected. In further optimized trials, it has been shown that remaining values of lead and zinc distinctly lower than 1 wt% each are feasible.
Use of Slag from Jarosite Treatment
The tests on slag–concrete samples showed very promising results, especially in mechanical properties. The samples containing slag were at least comparable, but in many cases even higher in their flexural and compressive strength compared to the slag-free reference sample. This touches especially the samples containing slag with grain sizes 1–2 mm and 2–4 mm. Also, the differences were higher after 7 days of curing time. Interestingly, the amount of slag in each sample did not show big differences for grainsizes < 1 mm and 2–4 mm, whereas the strength of samples with grainsizes 1–2 mm increased with higher amount of slag (Figs. 2 and 3).
For the leachability testing, one sample of each slag grain size containing 20 wt% of slag was crushed (<10 mm) and mixed with distilled water in a proportion solid:liquid 1:10. Referring to international standards for inert landfilling, only the leachability of lead did not match the limits, with 0.93 mg/kg exceeding the limit of 0.5 mg/kg nearly twice (Table 5: Results from leachability testing.). However, as mentioned above, the slag contained comparably high amounts of remaining lead present as metallic inclusions, which is due to technical reasons during the trial. However, this value does not exceed the limits for deposition of residues in non-hazardous wastes landfills. For some other elements, there is some limited leaching but not exceeding the respective limits.
Results from Lead Slag Treatment
Small-scale trials proved the pyrometallurgical technique being applicable for treatment of lead slag. Yields of zinc and lead were >85 wt% and >95 wt%. The slag shows more the 60 wt% of the material present as fayalite. Some optimizations regarding basicity have been done. In one case, sand was added to reach a fayalite percentage of more than 80 wt%. Minimizing the lead content in the slag allowed values after standard leaching which are below the official regulations. Further investigations will focus on optimizing the slag composition and with this the fayalitic part as well as the influence of cooling conditions after slag tapping.
The usability of this slag as construction material was not yet evaluated, but this will be a focus of further research, and investigations similar to those for the slags from jarosite treatment will follow.
Summary
The potential of jarosite material was clearly shown. Not only for the winning of valuable metals, but also in producing a slag, applicable as construction material. In both cases, it can contribute to save natural resources and to avoid waste material. Furthermore, also historical dumps could be treated to remove the dump or to make it available for other, even more problematic materials, which still cannot be recycled.
The applicability of the pyrometallurgical process is out of question. Critical in this case is of course the energy required for the process. However, together with the global efforts of developing and optimizing sources for green energy, electricity will become greener and get ever closer to CO2 neutrality. With this, technologies relying on electrical energy will do so as well.
Tests on using slag out of jarosite treatment as replacer for sand in concrete showed very satisfying results. In terms of mechanical properties, it is even slightly better than concrete using natural sand only. Leaching tests were not as successful in the first run. However, lower amounts of sand being replaced by slag will most likely also reduce the leaching of lead. Furthermore, in a second analysis with slag from another but similar test, only with a lower remaining lead content, the necessary requirements were fulfilled, even when replacing higher amounts of natural sand.
Further research will focus on increasing the lead and zinc yield of the pyrometallurgical process (and with this, smaller contents in the slag) and additional tests on the leachability of the produced concrete.
Multi metal recovery of lead slag showed also very promising results. However, concerning a zero-waste solution, the slag must also become a usable product. For this, test work on its usability as construction material will take place.
References
Höber L, Steinlechner S (2021) A comprehensive review of processing strategies for iron precipitation. Cleaner Eng Technol
Schatzmann W, Antrekowitsch J (2019) Assessment of by-products—from waste to values. Wastes: Solutions. Treat Opport III:506–511
Sinclair R (2005) The extractive metallurgy of zinc. Victoria: Australasian Institute of Mining and Metallurgy
Svens K, Kerstiens B, Runkel M (2003) recent experiences with modern zinc processing technology. Erzmetall 56(2):94–103
Steinlechner S, Antrekowitsch J (2018) Thermodynamic considerations for a pyrometallurgical extraction of indium and silver from a jarosite residue. Metals 8(5)
Antrekowitsch J, Hanke G (2020) Characterization and processing of residues from hydrometallurgical zinc smelters. In: PbZn 2020: 9th international symposium on lead and zinc processing, pp 437–445
Stafilov T, Sajn R, Pancevski Z, Boev B, Frontasyeva M, Strelkova L (2010) Heavy metal contamination of topsoils around a lead and zinc smelter in the Republic of Macedonia. J Hazard Mater 175:896–914
Hanke G, Bernhart W, Antrekowitsch J (2019) Treatment of precipitation residues from. World of metallurgy-Erzmetall: internationale Fachzeitschrift für Metallurgie 72(6)
Pappu A, Saxena M, Asolekar S (2006) Jarosite characteristics and its utilisation potentials. Sci Total Environ 359:232–243
Leuchtenmüller M, Schatzmann W, Steinlechner S (2020) A kinetic study to recover valuables from hazardous ISF slag. J Environ Chem Eng 8(4)
Unger A (2015) Entwicklung eines Recyclingprozesses zur simultanen Rückgewinnung von Wertmetallen aus Reststoffen der Blei- und Zinkindustrie. Ph.D. thesis, Montanuniversität Leoben
Acknowledgements
COMMBY is a FFG Austria COMET project. Thank you to the funders Österreichische Forschungsförderungsgesellschaft (FFG), Steirische Wirtschaftsförderungsgesellschaft (SFG), Wirtschaft Burgenland GmbH, Land Steiermark and Land Burgenland.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Hanke, G., Antrekowitsch, J., Castro, F., Krug, H. (2022). Maximizing the Efficiency of By-Product Treatment by Multi-metal Recovery and Slag Valorization. In: Lazou, A., Daehn, K., Fleuriault, C., Gökelma, M., Olivetti, E., Meskers, C. (eds) REWAS 2022: Developing Tomorrow’s Technical Cycles (Volume I). The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-92563-5_22
Download citation
DOI: https://doi.org/10.1007/978-3-030-92563-5_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92562-8
Online ISBN: 978-3-030-92563-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)