Abstract
Hemorrhage with subsequent anemia is of particular relevance in the elderly. In a geriatric patient with frequent comorbidities like already preexisting anemia or cardiovascular disease, knowledge about the optimal anemia treatment is important. While the risk of death increases with the severity of anemia, a liberal treatment with allogenic red blood cell (RBC) transfusions entails more disadvantages than benefits. An evidence-based approach to transfusion strategies determined a transfusion threshold of 7–8 g/dL to be the standard of care also in geriatric patients. Allogenic RBC transfusions are associated with a variety of adverse events and are of no proven benefit in hemodynamically stable patients with a hemoglobin concentration above 7 g/dL. Therefore, RBC transfusions should be avoided above this threshold, and generally not be used to treat anemias that can be corrected with specific hematinic medications such as iron, vitamin B12, folic acid, and erythropoietin. Patients benefit from these alternate anemia treatments without significant side effects. They can be used in a goal-directed isolated approach or in combination, if a situation warrants a quick and efficient therapy—as is often the case after trauma with relevant blood loss.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Exsanguination is one of the leading causes of death in trauma patients [1]. In a geriatric patient, physiological reserves decline and comorbidities become more frequent. For example, in addition to traumatic blood loss, in more than a third of all elderly patients, anemia is already prevalent before the injury. Other comorbidities, like cardiovascular disease, put crucial tissues at greater risk of hypoxia. This renders the management of posttraumatic anemia within the elderly population a challenging topic [2].
1 Allogenic Blood Transfusions
While the risk of death increases with the severity of anemia, a liberal treatment with allogenic red blood cell (RBC) transfusions, does more harm than good [3]. Still, RBC transfusions remain one of the most common medical interventions. However, their use is steadily declining with growing evidence regarding adverse events of transfusions and the advantages of restrictive transfusion thresholds. RCBs, like all biological blood components, are living human tissues, making every transfusion a liquid organ transplantation. Thus, they are associated with a number of short- and long-term immunomodulatory adverse events, as well as a variety of serious non-immunological complications. Protecting patients from these risks requires the prevention of inappropriate transfusions [4].
An RBC transfusion is appropriate when oxygen demand exceeds supply in a way that can only be adequately restored by the immediate administration of oxygen carriers. While this is basically a pathophysiologic condition, the threshold for an RBC transfusion is usually defined by a hemoglobin level, also called a transfusion trigger. Based on basic physiological models, these thresholds (triggers) used to be rather liberal and encouraged premature intervention. In the last two decades, however, supported by substantial scientific evidence, transfusion triggers became more restrictive. Most commonly, the restrictive transfusion threshold now is set at a hemoglobin level of 7 g/dL or 8 g/dL to trigger transfusion, while the liberal transfusion threshold implies a higher hemoglobin level of 9–10 g/dL.
In the general surgical population, definitive evidence has been provided that a restrictive approach to RBC transfusion not only reduces blood use but also does not cause harm. With mounting evidence, in 2016, a large meta-analysis by the Cochrane Collaboration analyzed more than 12,000 patients from 31 trials investigating liberal vs. restrictive transfusion triggers [5]. Of these trials, six were conducted in orthopedic surgery. Primarily, the restrictive transfusion strategy more than halved the risk of receiving an RBC transfusion. Further, overall as well as in the orthopedic surgery subgroup, there was no significant difference between the two triggers regarding clinical outcomes. Most importantly, 30-day mortality did not differ between the two approaches. Additionally, restrictive transfusion thresholds did not affect any of the other assessed outcomes including myocardial infarction or other cardiac events, stroke, thromboembolism, or infection. If these patients do not benefit clinically from a liberal transfusion threshold, there is no indication for transfusion above a hemoglobin concentration of 7–8 g/dL. Thus, applying restrictive triggers does not mean withholding treatment, but reasonably avoiding an unnecessary intervention with many adverse events.
Moreover, restrictive transfusion triggers may even be beneficial in certain situations or populations. In the meta-analysis, three studies cumulating more than 1500 patients suffering acute blood loss, were analyzed as a separate group. Here, a restrictive approach to transfusion triggers reduced 30-day mortality with a risk ratio of 0.65 (95% confidence interval (CI) 0.43–0.97). Importantly, in the largest trial from this subgroup the mean patient age was 65 years, which is a frequent cut-off value for geriatric research. This study in itself also favored a restrictive approach [6].
It has been argued—but wrongfully so—that due to limited physiological reserves, an elderly patient may require a liberal transfusion threshold. In a large prospective trial, over 2000 geriatric patients at high cardiovascular risk after hip replacement surgery were randomly assigned to either a restrictive (8 g/dL) or a liberal (10 g/dL) transfusion regimen. The mean patient age was an astonishing 82 years and all patients had either clinical evidence of or risk factors for cardiovascular disease. The first results were reported after a 60-day follow-up, where the liberal transfusion strategy, as compared with a restrictive strategy, did not reduce rates of death or inability to walk independently on 60-day follow-up [7]. Later, after a long-term follow-up of 3 years, no difference in mortality or cause of death was found [8]. This may be because the original idea that reduced physiological reserves would compromise the ability to tolerate acute anemia was incorrect. In fact, an increase in cardiac output due to anemia does not decrease with increasing age and consequently, neither does the ability to deliver oxygen [9]. While the present evidence already clearly favors a restrictive approach, in order to dispel last doubts, the Liberal trial (clinicalTrials.gov identifier: NCT03369210) is currently recruiting patients over 70, assigning them to either a 9 g/dL or 7.5 g/dL transfusion threshold, and evaluating mortality as well as anemia associated ischemic events (e.g., acute myocardial infarction or acute ischemic stroke) in a 90-day follow-up.
A transfusion threshold of 7–8 g/dL has become the standard of care in a geriatric patient. Allogenic RBC transfusions are of no benefit in hemodynamically stable patients with a hemoglobin concentration above 7 g/dL and should be avoided to minimize adverse events. If a normovolemic patient is hemodynamically unstable or shows signs of inadequate oxygenation despite exhausted respiratory and circulatory support, a new transfusion trigger may be set at 8 g/dL. Importantly, a low hemoglobin value on itself does not require a transfusion. The patient’s clinical state and individual needs should always be considered in the decision.
2 Alternative Treatments
Allogenic transfusions should be applied very restrictively. However, because of the risks associated with anemia, physicians should be familiar with alternative treatments. For this reason, the U.S. Food and Drug Administration container label extension for RBC units states the following contraindication: “Red-cell-containing components should not be used to treat anemias that can be corrected with specific hematinic medications such as iron, vitamin B12, folic acid, or erythropoietin” [10]. In the geriatric population, two scenarios must be considered.
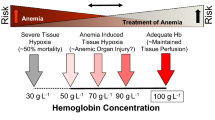
First, next to traumatic blood loss, other etiologies of anemia may have already been prevalent before the injury. Often, laboratory investigation can lead to the correct diagnosis, making management largely dependent on the underlying etiology. In these cases, anemia can be corrected by a goal-directed treatment with, for example, parenteral iron substitution in case of iron deficiency or vitamin B12 and folate. An erythropoietin deficiency with or without exocrine kidney insufficiency, or chronic inflammation, is also quite prevalent in older persons and may be treated with erythropoiesis-stimulating agents, depending on local regulations (Fig. 27.1).
Secondly, in the case of acute traumatic blood loss, a fast combination treatment may be the key—regardless of underlying conditions. A landmark study in cardiac surgery patients with preoperative anemia or iron deficiency was able to show that combination treatment with intravenous iron, subcutaneous erythropoietin alpha, vitamin B12, and oral folic acid only on the day before surgery, reduced allogeneic blood product transfusions without significant side effects [11]. If this ultra-short-term treatment succeeds in such a high-risk group of patients, it may very well also improve transfusion regimens for geriatric trauma patients.
References
Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019 Mar 27;23(1):98.
Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood. 2018;131(5):505–14.
LaPar DJ, Hawkins RB, McMurry TL, Isbell JM, Rich JB, Speir AM, et al. Preoperative anemia versus blood transfusion: which is the culprit for worse outcomes in cardiac surgery? J Thorac Cardiovasc Surg. 2018 Jul;156(1):66–74.e2.
Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med. 2017;377(13):1261–72.
Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 2016;10:CD002042.
Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21.
Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–62.
Carson JL, Sieber F, Cook DR, Hoover DR, Noveck H, Chaitman BR, et al. Liberal versus restrictive blood transfusion strategy: 3-year survival and cause of death results from the FOCUS randomised controlled trial. Lancet. 2015;385(9974):1183–9.
Casutt M, Seifert B, Pasch T, Schmid ER, Turina MI, Spahn DR. Factors influencing the individual effects of blood transfusions on oxygen delivery and oxygen consumption. Crit Care Med. 1999;27(10):2194–200.
AABB. Circular of Information for the Use of Human Blood and Blood Components [Internet]. 2017 [cited 2019 Jul 31]. Available from: http://www.aabb.org/tm/coi/Documents/coi1017.pdf
Spahn DR, Schoenrath F, Spahn GH, Seifert B, Stein P, Theusinger OM, et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet. 2019;393(10187):2201–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rössler, J., Breckwoldt, J., Spahn, D.R. (2022). Management of Anemia. In: Pape, HC., Kates, S.L., Hierholzer, C., Bischoff-Ferrari, H.A. (eds) Senior Trauma Patients . Springer, Cham. https://doi.org/10.1007/978-3-030-91483-7_27
Download citation
DOI: https://doi.org/10.1007/978-3-030-91483-7_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91482-0
Online ISBN: 978-3-030-91483-7
eBook Packages: MedicineMedicine (R0)