Abstract
The treatment of wastewater occurring at separate stages of technological processes is one of the relevant problems for metallurgical plants, in particular, pipe and tube rolling ones. Acid wastewater with a high content of heavy metals occurs at the stage of etching and thermal treatment. To neutralize them, it is reasonable to use local sorption purification with cheap sorption materials. The purpose of the paper is to test charcoal and high-moor peat as sorbents for local treatment facilities in the pipe industry. The experimental research proved that high-moor peat from the deposits in the Chelyabinsk region and charcoal can be used as sorbents to extract heavy metals from acid wastewater of the tube and pipe facilities. The technology can be implemented both by means of sorbent addition into wastewater with a subsequent separation and in dynamic conditions by means of filtration through the sorption material layer. At the building of treatment facilities it is reasonable to use composite sorbents including charcoal and high-moor peat. The article also presents the analysis for the most promising methods of wastewater treatment, such as: phytoremediation; adsorption; ion exchange; electrodialysis; floatation; electrocoagulation and other. The authors highlighted the advantages and flaws of the methods enumerated.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The wastewater treatment in industrial facilities is a relevant contemporary problem due to a high potential hazard of environmental problems’ occurrence. Industrial wastewater are peculiar and, in certain cases, contain complex multi-component mixtures dangerous for the human and environment. To remove them, it is necessary to develop complex treatment facilities. When iron-bearing acid water gets into reservoirs the ferrous hydroxide contained there absorbs dissolved oxygen and gradually evolves into ferrous hydroxide during oxydation. Ferrous hydroxide precipitates on the reservoir bottom and banks forming a large amount of rust-colored precipitation. In small reservoirs such drains can fully absorb dissolved oxygen which causes the elimination of organic life [1].
In most cases the reservoirs contaminated with iron-bearing acid drain water become inappropriate as the sources of household and technical water supply; that is why water disposal and wastewater treatment should take a special place in the work of each industrial plant.
The most wide-spread methods applied today for industrial wastewater treatment are provided in Table 1. Popular methods have their advantages and drawbacks, thus, to select the treatment technology for particular wastewater basing upon their chemical composition, pollutant concentration, equipment complexity, its energy consumption and treatment efficiency.
Each of the method enumerated has its application area. For example, ion exchange is used for the wastewater treatment with the hourly consumption of up to 500 m3/h and metal concentration of up to 50 mg/dm3; electrodyalisis—for wastewater with metal concentration from 2500 to 15,000 mg/l; floatation—to eliminate colloid suspensoids, small hard particles and dissolved susbstances; electrofloatation—to treat wastewater from weighed substances, heavy metals, resinous substances, suspended matters; electrocoagulationis applied for water drains with the consumption of up to 80 m3/h and metal concentration up to 30 mg/l.
Today one of the most relevant methods of deep wastewater treatment and purification from heavy metals is a sorption method which allows a significant decrease of heavy metals concentration in wastewater with a possible use of purified drains in the closed systems of plant water circulation [2]. This method is covered in numerous scientific papers [3,4,5,6,7,8,9,10,11,12]. In addition, sorbents should meet the following requirements: efficient metal sorption in acid or weak acid environments characteristic for metallurgic wastewater drains, good filtration characteristics, comparatively low cost.
Today more and more attention is being paid to natural sorbents as their virtually unlimited reserves, low cost, wide spread of deposits, quite high adsorption properties make them economically feasible in terms of wastewater treatment.
The choice of this or that sorbent as a sorption-filtering material is based on the research of sorption characteristics, in particular: optimal pH of metal sorption, sorption capacity in static and dynamic conditions, sorbent fraction composition, its filtration properties, regenerating capacity and the specific surface area.
This paper studies the opportunity to develop a local sorption technology for purifying acid wastewater occurring during pipe production where their acid treatment in the etching and thermal workshop is one of the technological stages; it is based upon the application of natural sorbents, such as charcoal and high-moor peat.
2 Research Objects and Methods
The research object is washwater of the etching and thermal workshops. Wastewater at this stage is characterized by the unstable composition largely depending on the facility workload, the steel composition and grade of the pipes processed as well as the process peculiarities as it stipulates for DC component and a recurring washwater release. After pipe treatment with working mixtures (including in the etching tanks) the technological process stipulates for the transition of remaining solutions into washwater (rinse tanks and other tanks). These remaining solutions also take into a washwater drain by means of overflows, spills or tank leakage [1]. The aggregate compositions of wastewater formed during the technological process are provided in Table 2.
The paper studied wastewater occurring in the facility (Table 3).
Today peat is widely applied for wastewater purification from heavy metals and oil products [13,14,15,16,17]. The authors selected and tested the materials easily found in the Chelyabinsk region of all the known sorption materials.
The sorption process was studied in static and dynamic conditions by known methods [18,19,20].
In the static mode the sorption process was studied by means of the limited volume method at the ratio hard phase—liquid equal to 1:30. The sorbent was placed into a beaker, added the studied water sample and left for 7, 14 and 28 days without mixing at the ambient temperature of 283.15; 293.15 and 303.15 К. After the sorption process completion the authors took the solution sample over the sorbent to conduct chemical analysis. The analysis was conducted by means of spectrometry with the inductively coupled plasma at the atomic emission spectrometer with an inductively coupled plasma OPTIMA 2100DV.
Under dynamic conditions the research of the system “sorbent-water” with technogenic contaminants was conducted at a specially built unit allowing one to change the dynamic mode characteristics enumerated above. The rate of water motion in the system was set by the experiment conditions (0.3; 0.6 and 1.2 l/h) and supported by the pump constant parameters. At the experiment conduct the authors used sorbents with the fraction composition of 0.5–1.25 mm. The working layer thickness made 80 mm. Before the start of tests the authors defined the sorbent mass. The water volume with technogenic contaminants, contacted with the sorbent, was measured by a volumetric cylinder.
The method of experiment conduct included the following stages: setting the rate of the simulated solution feed into the research plant; measurement of the simulated solution pH and heavy metal cations content in the filtrate in certain periods.
The pH index was measured by the ion-meter I160MI. The content of admixtures in water was measured by the atomic emission spectrometer OPTIMA 2100DV (Perkin Elmer, USA). As the base solution the authors used water with a particular treatment degree obtained at the device “Simplicity UV” (France).
3 Results and Discussion
The study of metal sorption extraction in static conditions with the sorbents under research showed that the sorption of virtually all the metal ions studied reaches the maximum value in 1.5–2 h since the start of phase mixture while the completeness of the sorbate ion exchange with sorbent superficial groups significantly depends on the solution pH and temperature.
Table 4 provides data showing the dependence of metal cations adsorption on the sorbent type and the time contact with the sorbent under various temperatures.
The authors established that the temperature increase improves the sorption efficiency by the sorption studied. The pH solution with the charcoal increased to 7.29, with peat—to 5.52.
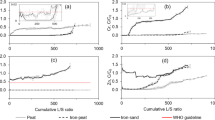
At the dynamic mode the sorption treatment of water with technogenic contaminants the following parameters are important: solution movement rate; sorbent fraction composition; thickness of the sorbent layer; ratio of the volume of the solution purified to the granule mass. The research results are provided in Figs. 1 and 2.
The experimental data by the efficiency of heavy metals sorption with high-moor peat and charcoal provided in Fig. 1 showed that, given the filtration rate of 0.3 l/h, the efficiency of iron-bearing wastewater and, correspondingly, the amount of metal cations occluded by the sorbent mass unit are higher than at the filtration rate of 1.2 l/h. The exception can be titanium sorption which demonstrated 100% purification at all the filtration rates under study. One should point that high-moor peat has the highest aluminium sorption at the filtration rate of 1.2 l/h. Therefore, at the development of the wastewater treatment technology it is necessary to recommend the range of filtration rates from 0.3 to 0.6 l/h or the use of peat and charcoal as a composite sorbent component.
The research proved that the maximum change of the drain pH with peat use is observed at the filtration rate of 0.3 l/h and is 5.28 while at the charcoal application the solution remains acid under various filtration rates.
4 Conclusion
The experimental research proved that high-moor peat from the deposits in the Chelyabinsk region and charcoal can be used as sorbents to extract heavy metals from acid wastewater of the tube and pipe facilities. The technology can be implemented both by means of sorbent addition into wastewater with a subsequent separation and in dynamic conditions by means of filtration through the sorption material layer. When building local treatment facilities it is reasonable to use composite sorbents including charcoal and peat.
References
Khvang ST, Kammermeyer K (1981) Membrane separation processes/translated from English. Khimiya, Moscow
Laptedulche NK, Dremicheva ES (2014) Water: Chem Ecol 12:81
Gelfman MI, Tarasova YuV (2002) Research of sorption characteristics of natural and modified sorbent on the alumosilicate material basis. Khimicheskaya Promyshlennost 8:119–125
Egorova EY, Mitrofanov RY, Lebedeva AA (2007) Sorbent obtaining from pine nut shuck by low-temperature treatment. Polzunov News Bull 3:35–39
Zhukova IL, Khmylko LI (2009) Sorbents on the basis of cellulose-containing materials and their utilization. Ecol Indus Russia 7:30–33
Ulrich DV, Bryukhov MN, Zhbankov GO, Denisov SE, Timofeeva SS (2013) Measurements for advanced neutralized wastewater treatment applying the sorption method. Scienceand society: 4rd international scientific and practical conference, London, 141–147
Lesmana SO, Febriana N, Soetaredjo FE, Sunarso J, Ismadji S (2009) Studies on potential applications of biomass for the separation of heavy metals from water and wastewater. Biochem Eng J 44(1):19–41
Domracheva VA (2005) Wasterwater purification from heavy metals at application of sorbents from brown coals of Irkutsk coal field. Civil Protection 6:11–14
Zhang SJ, Shao T, Karanfil T (2011) The effects of dissolved natural organic matter on the adsorption of synthetic organic chemicals by activated carbons and carbon nanotubes. Water Res 45(3):1378–1386
Adsobent obtaining method on the peat basis. Patent RU 2102319, published on January 20, 1998
Epshtein SA, Meidel IM, Nesterova VG, Minaev VI (2012) Melik-Gaikazov Ya.I. Industrial wastewater treatment with peat-based reagents. Mining Inf Anal Bull 5:307–311
Epshtein SA, Titorova YuA, Meidel IM (2012) Recovery of precipitation from industrial wastewater treatment with peat-based reagents. Mining Inf Anal Bull 9:303–311
Cojocaru C, Macoveanu M, Cretescu I (2011) Peat-based sorbents for the removal of oil spills from water surface: application of artificial neural network modeling. Colloids Surf A 384(1):675–684. https://doi.org/10.1016/j.colsurfa.2011.05.036
Pandey S, Alam A(2019) Peat moss: A hyper-sorbent for oil spill cleanup - a review. Plant Science Today 6(4):416. doi:https://doi.org/10.14719/pst.2019.6.4.586
Heiderscheidt E, Leiviskä T, Lopez FC, Tesfamariam A, Postila H (2020) Suitability of natural and chemically modified peat as a sorbent material for mining water purification in small-scale pilot systems. Environ Technol Sep 2:1–12. https://doi.org/10.1080/09593330.2020.1812007
Brown PA, GillS SA, Allen J (2000) Metal removal from wastewater using peat. Water Res 34(16):3907–3916. https://doi.org/10.1016/S0043-1354(00)00152-4
Goher ME, Hassan AM, Abdel-Moniem IA, Fahmy AH, Abdo MH, El-sayed SM (2015) Removal of aluminum, iron and manganese ions from industrial wastes using granular activated carbon and Amberlite IR-120H. Egyptian J Aquatic Res 41(2):155–164
Timofeeva SS, Lykova OV (1986) Metal extraction from plating industry wastewater by industrial wastes of wood-processing and paper and pulp plants. Ore cleaning, Irkutsk, p 87–92
Timofeeva SS, Lykova OV (1990) Sorption metal extraction from plating plant wastewater. Chem Water Technol 5:440–443
Timofeeva SS, Lykova OV, Kukharev BF (1990) Sorbent use to extract metals from wastewater. Chem Water Technol 12(6):505–508
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Bryukhov, M., Ulrikh, D., Timofeeva, S. (2022). Peat and Charcoal in Treatment of Iron-Containing Production Wastewater in Pipe Industry. In: Radionov, A.A., Ulrikh, D.V., Timofeeva, S.S., Alekhin, V.N., Gasiyarov, V.R. (eds) Proceedings of the 5th International Conference on Construction, Architecture and Technosphere Safety. Lecture Notes in Civil Engineering, vol 168. Springer, Cham. https://doi.org/10.1007/978-3-030-91145-4_51
Download citation
DOI: https://doi.org/10.1007/978-3-030-91145-4_51
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91144-7
Online ISBN: 978-3-030-91145-4
eBook Packages: EngineeringEngineering (R0)