Abstract
Aged-related changes in the spine are initiated in the discovertebral complex and will extend to the adjacent vertebral bone marrow, cervical uncovertebral and facet joints and spinal ligaments. These occur in response to a wide range of insults, repetitive low-level mechanical trauma being the most important. Degenerative spinal stenosis represents a significant cause of pain and disability in the elderly population. Age-related changes and disc herniation may narrow the central canal and neuroforamina, causing cord compression and myelopathy, cauda equina compromise or individual radiculopathy.
Accurate and comprehensive interpretation of age-related spinal imaging findings can be challenging because often there is poor correlation with symptoms. Imaging may confirm the diagnosis of spinal canal stenosis and identify exactly the affected levels and anatomic structures involved, therefore enabling clinical correlation and treatment planning.
Plain film imaging remains the first line technique in the evaluation of spinal degenerative changes, being useful in assessing the morphology and alignment of the vertebrae, including the assessment of instability where needed using flexion and extension lateral radiographs and the degree of disc/facet joint degeneration and reactive osteophytosis. Computer tomography (CT) imaging provides more detailed information on the bony components of the spinal degenerative process but suboptimally assesses protrusive disc disease. CT myelography is an invasive procedure mainly restricted to cases in which MR is contraindicated. Magnetic resonance imaging (MRI) is the preferred imaging technique for spinal pathologies as it can clearly delineate the soft tissue contents of the spinal canal and neuroforamina to define both annuloprotrusive and neural pathology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Degenerative spine
- Age-related changes of the spine
- Disc herniation
- Spinal canal stenosis
- Degenerative cervical myelopathy (DCM)
- Cauda equina
1 Introduction
The main functions of the osseoligamentous and discogenic complexes of the spinal column are to give structural support, allow trunk mobility, shock absorb compressive axial forces and protect the neuraxis (Oxland 2016). The cervical spine is designed to allow a great range of movement for the cranium, the thoracic spine to aid respiration via its costovertebral articulations, whereas the lumbar spine is primarily designed for weight bearing (Bland and Boushey 1990).
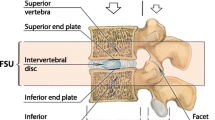
The basic biomechanical element of the spine is the functional spinal unit (FSU) composed by two contiguous vertebrae, the intervertebral disc, the facet joints and the spinal ligaments.
1.1 Discovertebral Complex
The discovertebral complex is an amphiarthrosis composed by the adjacent vertebral body endplates and the intervertebral disc. Its function is to transmit mechanical loads to the underlying vertebra and facilitate the motion of the spine.
The intervertebral disc is formed by the central nucleus pulposus constrained by the peripheral anulus fibrosus. The nucleus pulposus, a remnant of the notochord, is a gelatinous structure rich in proteoglycans and water. Aggrecan is the most abundant proteoglycan delivering osmotic properties essential to resist compression. The anulus fibrosus surrounds the nucleus pulposus and is composed by collagen fibres arranged in concentric rings called lamellae, with an inner fibrocartilage portion which blends with the nucleus pulposus. The external lamellae are connected to the longitudinal ligaments and vertebral bodies (Sharpey fibres). The lamellae of the anulus fibrosus are thinner posteriorly than anteriorly, and this anatomic disposition may explain the propensity for posterior disc herniation. It provides reinforcement during axial loading, bending and torsion, as well as maintaining the nucleus pulposus osmotic pressure.
The vertebral endplates are composed of a central bony disc covered by hyaline cartilage and an elevated bony rim called the ring apophysis. The cartilaginous endplate is strongly fixed to the intervertebral disc, more loosely bound to the underlying subchondral vertebral bone (Hukins 1988).
The intervertebral discs are the largest non-vascularised structures in the body, and their nutrition is based largely on diffusion across the vertebral endplates (Holm et al. 1981).
Only the outer third of the anulus fibrosus is innervated (Ashton et al. 1994), more abundantly in the posterior and posterolateral margins of the intervertebral disc.
Spinal degeneration occurs in response to a wide range of insults, mechanical trauma being the most important aetiologic factor, including chronic overuse and sequelae of vertebral fracture or surgery (Gallucci et al. 2005; Bogduk 2012). Other causes include metabolic disease, e.g. diabetes mellitus and ochronosis (Aufdermaur et al. 1980; Robinson et al. 1998; Millucci et al. 2017), nutritional and genetic factors.
2 Aging of the Intervertebral Disc
Increasing age is associated with disc degeneration in the cervical and lumbar spine (Boden et al. 1990a, b; Cheung et al. 2009). The process of intervertebral disc aging is called intervertebral chondrosis, and when affects the adjacent vertebral body, osteochondrosis.
Notochordal cells disappear before maturity and are substituted by chondrocyte-like cells that synthetize a collagen-rich and less hydrated matrix (Trout et al. 1982; Hunter et al. 2003). The aging cartilaginous endplate sustains calcification followed by resorption and bony replacement (Bernick and Caillet 1982), disrupting nutrient exchange (Roberts et al. 1997).
A majority of the studies of the aging intervertebral disc relate to the lumbar spine, but the morphology and functions of the intervertebral discs differ by spinal level (Mercer and Bogduk 1994) and so does their response to aging.
The cervical intervertebral discs show progressive disappearance of the nucleus pulposus, being replaced by fibrocartilage and dense fibrous tissue, known as “dry” disc (Bland and Boushey 1990). During early adulthood, the cervical discs develop cracks and fissures that are believed to be physiological (Bland and Boushey 1990) and thought to allow rotational movement (Bogduk and Mercer 2000).
The aging lumbar intervertebral discs undergo biochemical changes with loss of water and proteoglycans in the nucleus pulposus (Gower and Pedrini 1969) accompanied by an increase in collagen within the disc (Olczyk 1992). On MRI, these biochemical changes translate into loss of intranuclear hyperintensity on T2 WI and subsequent loss of disc height. A number of morphological grading systems to evaluate degeneration of cervical (Matsumoto et al. 1998; Miyazaki et al. 2008; Nakashima et al. 2015) and lumbar (Pfirrmann et al. 2001; Griffith et al. 2007) intervertebral discs have been proposed.
These changes lead to altered biomechanics of the disc resulting in mechanical load transfer into the anulus fibrosus (Adams et al. 1996) and facet joints. Hence age-related changes comprise disc desiccation and fibrosis, disc space loss, diffuse annular bulging and fissuring, intradiscal fluid and vacuum phenomena (see below), ligamentous hypertrophic changes, bone marrow changes, disc herniation, marginal vertebral osteophyte formation, malalignment and stenosis (Modic and Ross 2007).
3 Anular Fissures
Anular fissures are separations between the anular fibres or separations of anular fibres from their insertions to the vertebrae. According to their orientation, they are classified into concentric, radial and transverse fissures. These lesions are present in the majority of degenerated discs (Yu et al. 1988). T2 WI demonstrates localised high signal intensity zones (HIZ) within the anulus (Modic et al. 1988a, b; Carragee et al. 2000; Schellhas et al. 1996) representing fluid and granulation tissue and may show enhancement on post-gadolinium T1 WI. Its appearances may remain stable over years (Munter et al. 2002) and therefore they do not denote acute injury.
As disc degeneration evolves, vacuum phenomenon, intradiscal fluid accumulation and intradiscal calcification may happen. Vacuum phenomenon refers to gas collection, mainly nitrogen within clefts in aged discs with intradiscal negative pressure.
4 Disc Displacement
4.1 Disc Bulging
Disc bulging describes the contour of the outer anulus extending beyond the edges of the ring apophyses symmetrically throughout the disc circumference, usually by less than 3 mm as seen on axial imaging—it is not considered a form of herniation. There are two types of disc bulging—diffuse and asymmetric. Asymmetric disc bulging describes the contour of the outer anulus extending beyond the edges of the ring apophyses greater than 25% of the disc circumference (Fardon et al. 2014) (Fig. 1). It is believed that disc bulging is an asymptomatic lesion (Jensen et al. 1994).
4.2 Disc Herniation
Disc herniation is defined as a localized or focal displacement of disc material beyond the limits of the intervertebral disc space (Fardon et al. 2014). Acute disc herniation may happen within age-related discogenic changes in trauma or heavy lifting settings due to acute increase in intradiscal pressure (Kushchayev et al. 2018). In subacute disc herniations, there is fluctuating displacement of disc material according to intradiscal pressure variations with patient position. In chronic disc herniations, there is a static displacement of the disc material (Kushchayev et al. 2018). There are no specific imaging criteria to differentiate between acute, subacute or chronic herniations (Fardon et al. 2014).
Based on the shape of the herniated disc material, it can be categorised into protrusion, extrusion or extrusion with sequestration. Protrusion is a focal displacement of disc material involving less than 25% of the disc circumference; the greatest diameter of the herniated disc is less than its base at the site of herniation (Fardon et al. 2014). Extrusion is present when at least in one plane the maximum diameter of the herniated disc is greater than its base. Extrusion with sequestration is present if there is no connection of the herniated disc material to the parent disc (Fig. 2). The term ‘migration’ implies displacement of disc material away from the site of extrusion craniocaudally or mediolaterally. Disc herniations may be further categorised by containment as well as referenced by size, location and neural relationships. Those covered by outer anulus fibres and/or the posterior longitudinal ligament are named contained or subligamentous disc herniations. If this covering is absent, they are called uncontained or transligamentous.
The location of the herniated disc may be expressed using anatomic landmarks in the axial (zones) and sagittal (levels) planes (Wiltse et al. 1997). Within the transverse plane, the disc is divided into five zones—posterocentral, paracentral (subarticular), foraminal (lateral), extraforaminal (far lateral) and anterior (Fig. 3). The location of the disc herniation foresees which nerve is most likely to be affected; e.g. foraminal and extraforaminal disc herniations may impinge the exiting/exited nerve root whereas posterocentral and paracentral disc herniations may affect the traversing nerve root (Fig. 4). On the sagittal plane, the herniated disc may be located at the disc level, suprapedicular level, pedicular level or infrapedicular level (Fig. 5).
A 34-year-old woman with lower back pain and new urinary incontinence. Sagittal midline (a) and axial (b) T2 WI demonstrates a L4–5 posterocentral disc extrusion (a, white arrow) (b, star), causing left L5 nerve root compression (b, white arrowhead). Anular fissures and disc bulging at L2–3 and L3–4 are also seen (a, small white arrowheads). The patient underwent lumbar L4–5 discectomy
According to central canal compromise, the herniation may be classified as mild (less than one-third reduction of the spinal canal), moderate (between one-third and two-thirds reduction of the spinal canal) and severe (more than two-thirds reduction of the spinal canal).
To describe the effect of the disc herniation on the lumbar nerves, a grading system of four categories can be used, ranging from grades 0 to 3 (Pfirrmann et al. 2004). Grade 0 where there is no herniated disc neural contact; grade 1 where there is contact between the undisplaced nerve root and the herniated disc; grade 2 where there is dorsal displacement of the nerve root by the herniated disc; grade 3 where the nerve root is compressed between the herniated disc and other elements of the spinal canal.
To describe the compromise of the exiting nerve roots within their neuroforamina, a dichotomous system of no root compression and root compression (obliteration of the periradicular fat) can be used (van Rijn et al. 2005).
Disc herniations may cause neurological, vascular or focal complications. Disc herniation is the most common cause of radicular pain (Izzo et al. 2015). Neurological complications are secondary to spinal cord, cauda equina or more peripheral nerve root impingement potentially warranting surgical intervention. Vascular complications occur after the compression of the spinal arteries and veins. Focal complications refer to epidural scarring developed in subacute or chronic disc herniations due to ongoing inflammation.
Disc herniations can regress spontaneously over time (Bozzao et al. 1992; Borota et al. 2008; Bush et al. 1992) with sequestered disc herniations usually demonstrating greater morphologic decrease in size (Ahn et al. 2000). It is hypothesized that there is an absorption process with neovascularisation, inflammatory cell infiltration and phagocytosis of the disc herniated material (Ikeda et al. 1996).
5 Osteophyte Formation
Spinal osteophyte formation (spondylosis deformans) is believed to represent an attempt to increase joint surface and reduce the pressure within an aging spinal joint (Bogduk 2012). Osteophytes arising from the anterior aspect of the lumbar vertebral bodies are classified into traction and claw osteophytes. Traction osteophytes are more common than claw osteophytes, but both may be part of the same process. Traction osteophytes are a marker of spinal instability (Pate et al. 1988).
Osteophytes arising from the posterior endplates of the vertebrae contribute to central canal stenosis. Uncovertebral spurs may impinge upon the V2 segment of the vertebral artery, radicular artery and spinal nerve roots. Osteophytes arising from the facet joints are more prominent in the lumbar spine, and they may cause compression of the lateral recess and neuroforamen.
In addition to impingement of the spinal canal and neuroforamina, osteophytes may be compress neighbouring structures along the spine, causing dysphagia, compression of main bronchus and neural or vascular complications (Klaassen et al. 2011; Ullman et al. 2016). Endplate osteophytes and herniated discs have been associated with ventral dural sac tears resulting in spontaneous intracranial hypotension (Thielen et al. 2015; Hoxworth et al. 2012; Eross et al. 2002).
6 Vertebral Endplate Changes
MRI classification of degenerative changes of the vertebral endplates uses a six-stage system from normal appearances to extensive changes (Rajasekaran et al. 2008). The initial aging changes include endplate thinning and focal defect without subchondral bone marrow changes. Associated with Modic endplate changes, more extensive endplate damage can be seen with less than 25%, up to 50% or complete destruction of the endplate surface. Intravertebral herniation (Schmorl’s node) occurs when the disc herniates through a fracture in the endplate into the vertebral body with MRI demonstrating bone marrow oedema in the acute setting—such acute intravertebral herniation may be an underestimated cause of back pain (Takahashi et al. 1995).
7 Degenerative Marrow Changes
Three forms of degenerative change involving the bone marrow adjacent to the vertebral endplates have been described (Modic et al. 1988a, b). Type 1 consists of fibrovascular tissue, type 2 fatty bone marrow and type 3 bone sclerosis. Mixed types 1/2 and 2/3 may also be seen.
Modic type 1 changes are strongly associated with non-specific low back pain (Modic et al. 1988a, b) with characteristic low signal on T1 WI and high signal on T2 WI with enhancement on postcontrast imaging. These appearances may mimic infective spondylodiscitis or rarely malignancy—characteristic morphological changes of the disc, endplates and adjacent soft tissues may help in differentiating between these conditions.
In Modic type 1 change, the disc is often normal or exhibits low signal on T2 W2 whereas in infectious spondylodiscitis it returns high signal (James and Davies 2006; Diehn 2012). Prominent destruction of the endplates is often characteristic of infective spondylodiscitis (James and Davies 2006, Diehn 2012). Modic type 1 changes often reveal the claw sign on DWI, well-defined usually symmetrical high signal areas around the affected disc, whereas this sign is absent in infectious spondylodiscitis (Patel et al. 2014). The presence of perivertebral/epidural soft tissue thickening or collection should suggest infective spondylodiscitis (James and Davies 2006, Diehn 2012). Modic type 2 endplate change correlates with high signal on T1 WI and isointense to high signal on T2 WI, without enhancement after contrast administration. Modic type 3 endplate change demonstrates low signal on both T1 and T2 WI.
The proposed theories to explain Modic vertebral endplate changes are based on both biomechanical and biochemical mechanisms. The temporal evolution of Modic vertebral endplate changes is uncertain, and conversion of type 1 into type 2 or vice versa in cases of spinal instability is possible, with mixed type changes probably representing intermediate stages.
8 Facet Joint Age-Related Changes
Facet joint osteoarthritis comprises a complex of joint space narrowing, erosive subchondral sclerosis, cartilage thinning, joint effusion, joint capsule calcification and osteophytic hypertrophy of the articular processes with possible vacuum joint phenomenon and spondylolisthesis. It is associated with ligamentum flavum thickening.
There is a high prevalence of facet joint osteoarthritis in the population (Kalichman et al. 2008), and as L4–5 is the level of maximum mobility, it is the most affected level (Eubanks et al. 2007). This may cause stenosis of the central spinal canal, lateral recesses and neuroforamina (Modic and Ross 2007).
8.1 Facet Joint Syndrome
Facet joint syndrome is defined as unilateral or bilateral back pain radiating to one or both buttocks, groin and thighs stopping above the knee (Manchikanti et al. 2001). The prevalence of facet joint pain is unknown, but it has been estimated from 27 to 40% in patients with chronic lumbar back pain (Datta et al. 2009). The diagnosis of facet joint syndrome based on history and clinical examination is difficult (Jackson 1992). The most common cause of facet joint pain is osteoarthritis (Kalichman et al. 2008); other causes include septic and inflammatory arthropathies. Pain is thought to be caused by inflammation from degeneration of the facet joints and adjacent tissues. In the differential diagnosis, sciatica and hip or sacroiliac pathology need to be considered (Perolat et al. 2018). The initial radiographic assessment should include AP, lateral and oblique views of the lumbar spine (Varlotta et al. 2011) and may be useful to detect facet joint osteoarthritis, spondylolisthesis and defects of the pars interarticularis. CT and MRI are more efficient in demonstrating facet osteoarthritis (Weishaupt et al. 1999). CT better depicts bony anatomy (Varlotta et al. 2011) but MRI is able to show synovitis and oedema of the articular processes of the facet joints (D’Aprile et al. 2006) (Fig. 6). Facet joint synovitis is demonstrated after the enhancement of the capsule on postcontrast imaging.
Bone scans, particularly SPECT-CT, shows good agreement in detecting symptomatic facet or disc disease prior to surgery or facet injection (Malham et al. 2015).
8.2 Synovial Cysts of the Facet Joints
Synovial cysts are herniations of the synovium through the capsule of a degenerated facet joint, and they represent the most common cause of juxta-articular cysts. Their development is associated with degenerative changes of the facet joints (Banning et al. 2001; Hsu et al. 1995; Pirotte et al. 2003), spinal instability and spondylolisthesis (Reust et al. 1988; Parlier-Cuau et al. 1999).
A majority of the synovial cysts are incidental findings (Hemminghytt et al. 1982). They have been reported anywhere in the spine, but there is a marked lumbar predominance; the most common location is L4–5 level, followed by L5-S1 level. They may be lateral or medial to the facet joint, and if there is intracanalicular growth of synovial cyst, it may cause compression of neural structures leading to back or radicular pain (Howington et al. 1999) and neurological deficits.
On imaging, synovial cysts appear as a well-defined mass adjacent to a degenerated facet joint. MRI is the preferred imaging technique and typically will demonstrate low signal on T1WI and high signal intensity on T2WI. However, cysts with high proteinaceous content fluid as a result of internal debris or haemorrhage may exhibit heterogeneously high T1 and low T2 signal (Khan and Girardi 2006). Rarely they may present with acute haemorrhage (Cicuendez et al. 2010). Peripheral rim enhancement may be seen on postcontrast imaging. CT may reveal dense rim or calcification of the wall or gas within the cyst (Wang et al. 1987) (Fig. 7).
Juxta-articular cysts. Axial T2 WI (a) demonstrates an epidural cystic lesion (a, white arrow) adjacent to an osteoarthritic facet joint. The traversing right nerve root (a, white arrowhead) is mildly displaced. CT image (b, different patient) demonstrates facet joints osteoarthritis and a synovial cyst arising from the right facet joint with a hyperdense rim and a punctate calcification (b, black arrow) causing stenosis of the spinal canal
9 Posterior Longitudinal Ligament and Ligament Flavum Changes
The spinal canal ligaments, posterior longitudinal ligament (PLL) and ligamentum flavum (LF) fill a small space in the healthy spine. However, abnormally thickened spinal ligaments may contribute to decreasing the spinal canal size.
9.1 Posterior Longitudinal Ligament Changes
PLL ossification may narrow the spinal canal anteriorly, particularly in the cervical spine leading to radiculopathy and myelopathy, more commonly affecting men in their 50s and 60s, especially of Japanese descent. There are four types of PLL ossification—continuous, segmental, mixed or localised. Multilevel effacement of the anterior CSF space and spinal cord compression may be observed with a low signal intensity band between the posterior surface of the vertebral bodies and the thecal sac on both T1 and T2 WI (Luetkehans et al. 1987). Sometimes T1 WI demonstrates high or intermediate signal within the ossified lesions secondary to its fatty marrow content (Yamashita et al. 1990). Thin ossification of the PLL is difficult to identify on MRI, and plain X-rays and CT are more sensitive (Wong et al. 2011). It has been associated with diffuse idiopathic skeletal hyperostosis (DISH) and ligamentum flavum (LF) calcification (referred to as tandem ossification, Guo et al. 2009) and ankylosing spondylitis.
DISH (also known as Forestier’s disease) is a systemic noninflammatory entity causing ossification and calcifications of entheses and ligaments. It characteristically mainly involves the thoracic spine and consists of flowing ossification along the anterior or right anterolateral aspect of at least four contiguous vertebrae in the absence of intervertebral disc age–related changes and sacroiliitis (Resnick and Niwayama 1976). The estimated prevalence is roughly 10% of those aged 50 years and over with male predominance and is largely asymptomatic. Spinal involvement in DISH may lead to dysphagia, dyspnoea, stridor, myelopathy, atlantoaxial pseudoarthrosis or subluxation and low-energy vertebral fractures (Mader 2002; Westerveld et al. 2009; Caron et al. 2010) (Fig. 8).
9.2 Ligamentum Flavum Hypertrophy and Cysts
Disc degeneration and facet joint arthrosis cause mechanical stress and spinal instability that correlate with LF infolding, hypertrophy and fibrosis (Fukuyama et al. 1995; Okuda et al. 2004). LF thickness increases with aging (Okuda et al. 2004; Altinkaya et al. 2011; Twomey and Taylor 1988) and it plays a key role in the development of lumbar spinal canal stenosis. The normal LF is 2–4 mm thick. In the lower lumbar spine, the LF is said to be hypertrophic if it is thicker than 5 mm (Grenier et al. 1987). As the LF is thinnest at midline, the reduction of canal size is most prominent on parasagittal images. The LF may suffer from ossification and calcification, and it may rarely lead to myelopathy (Miyasaka et al. 1983) (Fig. 9).
Reformatted sagittal CT (a) and T2-weighted (b) images showing spinal age-related changes of an elderly lady resulting in stenosis of the cervical canal at C5–6 due to degenerative listhesis and calcification of the ligamentum flavum (a, white arrowhead). There is subtle degenerative cervical myelopathy at C6 level (b, white arrow)
LF cysts are an uncommon cause of juxta-articular ganglion cysts (Chimento et al. 1995) arising from an aged LF, mostly within the lumbar spine, particularly at L4–5 level. Rarely symptomatic, they may represent an unusual cause of age-related spinal stenosis. It may be difficult to differentiate from synovial facet joint cysts on imaging, but there is no communication with the facet joint.
10 Baastrup’s Disease
Baastrup’s disease (‘kissing spine’) is characterized by excessive abnormal contact between adjacent spinous processes resulting in their enlargement, flattening and sclerosis often with interspinous bursitis. Clinically there is focal pain alleviated during flexion and worsening during extension (Hazlett 1964). Large interspinous collections may project into the posterior epidural space (Chen et al. 2004).
11 Degenerative Intervertebral Instability
Degenerative intervertebral instability is due to the combined failure of the intervertebral complex, facet joints and ligaments in maintaining the alignment of the involved functional spinal unit; and it will extend to adjacent levels, evolving from a segmental into a regional pathology. Patients with degenerative instability present with back pain exacerbated by movement, affecting the cervical and lumbar spine (the thoracic spine is almost always spared).
The cascade of degenerative instability is classified into dysfunction, instability and restabilisation (Kirkaldy-Willis and Farfan 1982). During the initial dysfunction phase, age-related changes of the intervertebral disc and facet joints occur. This is followed by the instability phase, characterised by abnormal motion leading to anterolisthesis or retrolisthesis. On imaging Modic type 1 endplate changes, pedicle and isthmus bone marrow oedema, traction osteophytes, discal vacuum phenomenon, facet joint effusion or intraarticular vacuum phenomenon (Chaput et al. 2007), synovial cysts, anular fissure, and listhesis can be found (Kirkaldy-Willis and Farfan 1982). During the final restabilisation phase, there is decreasing mobility with rigidity development. On imaging marked loss of height of the disc, Modic type 3 end plate changes, ‘claw’ osteophytes of the vertebral bodies, ‘wrap around bumper’ osteophytes of the facet joints, and Baastrup’s disease.
11.1 Degenerative Spondylolisthesis
Degenerative spondylolisthesis is the displacement of one vertebra to another in the sagittal plane, as a result of subluxation of osteoarthritic facet joints (Cavanaugh et al. 1996). It is classified into two categories, dynamic and static, according to radiological evidence of instability on flexion/extension radiographs (Even et al. 2014), the diagnostic technique of choice. Ancillary findings of instability are facet joint effusion or osteoarthritis, facet synovial cysts and intradiscal vacuum phenomenon. Aged-related changes of the intervertebral disc can result in vertebral displacement in the coronal plane—lateral listhesis—which together with lateral wedging of the vertebral body and asymmetric facet joint osteoarthrosis may cause degenerative scoliosis.
Cervical degenerative spondylolisthesis most commonly occurs at C3–4 and C4–5 levels and may be more frequent than previously recognised (Jiang et al. 2011). Different classifications have been proposed to describe cervical degenerative spondylolisthesis. One of these systems is based on the degree of disc degeneration and spondylosis at adjacent levels, type I or adjacent spondylolisthesis at the transition between a more mobile segment to a more rigid one and type II or spondylotic spondylolisthesis related to advanced disc degeneration (Dean et al. 2009). Another system employs maximum slippage on flexion/extension radiographs (Kawasaki et al. 2007) classified as mild (less than 2.0 mm), moderate (2.0–3.4 mm) and severe (3.5 mm or more). Degenerative retrolisthesis is more commonly seen in the cervical and upper lumbar levels (Gallucci et al. 2007).
Degenerative lumbar spondylolisthesis most often occurs at the L4–5 level (Kalichman et al. 2008), and it is significantly more frequent in women (Kauppila et al. 1998). Lumbar spondylolisthesis is described employing the Meyerding classification (Meyerding 1932) based on the position of the posterior margin of the displaced superior vertebral body to the superior endplate of the vertebra below which is divided into four quarters—grade I, displacement less than 25%; grade II, 25–50%; grade III, 50–75%; grade IV, 75–100%; grade V or spondyloptosis. Degenerative spondylolisthesis is usually a grade 1. Sagittal planes are useful to assess the resultant degree of central canal and neuroforaminal stenosis.
12 Degenerative Stenosis of the Spine
Spinal stenosis is the consequence of congenital or acquired narrowing of the spinal canal. The former type includes achondroplastic syndromes and developmentally narrowed spinal canal. Acquired narrowing of the spine comprises degenerative changes, iatrogeny, systemic processes and trauma. Mixed stenosis refers to degenerative stenosis in patients with developmentally narrowed spinal canal (Gallucci et al. 2005), and in these cases, the contribution of age-related changes on a pre-existing developmentally narrowed canal may cause stenosis at an earlier age. The aging spine may be narrowed anteriorly by disc bulge or herniation, osteophytes or PLL ossification; posterolaterally by facet joint osteoarthritis and ligamentum flavum hypertrophy; anterolaterally in the cervical spine by uncovertebral joint hypertrophy which may compromise the cervical neuroforamina. In addition to the combination of these factors, degenerative spondylolisthesis may further reduce the spinal canal and neuroforaminal dimensions.
Stenosis of the spinal canal is a frequent observation in asymptomatic population with prevalence increasing with age (Boden et al. 1990a, b), and a distinction between morphological stenosis and clinically stenosis must be drawn. The role of imaging techniques is to assist the clinician in confirming the diagnosis of spinal canal stenosis, identifying the affected levels and anatomic structures involved, and to aid in treatment planning.
The symptoms are related to the location of stenosis—vague manifestations and ill-defined pain are present in central spinal stenosis whereas well-defined pain pattern is common in neuroforaminal stenosis. Cervicothoracic spinal stenosis can lead to spinal cord and radicular compression, resulting in pain, myelopathy, radiculopathy or myeloradiculopathy. Lumbar spinal stenosis causes nerve compression, resulting in neurogenic claudication or radicular leg pain. Neurogenic claudication refers to pain and weakness in the legs and calves when walking and may lessen when sitting, bending forward or lying down. Cauda equina syndrome is caused by the dysfunction of multiple sacral and lumbar nerve roots in the lumbar spinal canal with impairment of bladder, bowel, or sexual function, and perianal or “saddle” numbness. The most common aetiology of cauda equina syndrome is spinal canal stenosis secondary to large central disc herniation at the L4/5 and L5/S1 level (Ahn et al. 2000) (Fig. 10).
Sagittal midline T2- (a) and T1 (b)-weighted images of a young man with cauda equina syndrome on presentation after lifting weights. A L4–5 disc extrusion (white arrowhead) causing severe stenosis of the thecal sac (star) is demonstrated. Magnetic susceptibility artefact due to prior L5-S1 discectomy noted (b, white arrow)
12.1 Degenerative Cervical Spinal Canal Stenosis
Degenerative stenosis of the cervical canal is secondary to disc degeneration, uncovertebral and facet joint osteoarthritis, thickening of the ligamentum flavum, and ossification of the posterior longitudinal ligament.
The normal developmental segmental sagittal diameter is the distance from the posterior vertebral body to the nearest point of the corresponding spinolaminar line. Individuals with developmentally narrowed mid-cervical sagittal diameters smaller than 10 mm frequently have degenerative cervical myelopathy (DCM) and individuals with central canals from 13 to 17 mm often have symptomatic degenerative change often without myelopathy (Edwards and LaRocca 1983). In patients with developmentally narrowed cervical canal in addition to the restricted available space, there is more segmental mobility from C4–5 to C6–7 compared to individuals with normal cervical diameters, with more marked age-related changes at these levels (Morishita et al. 2009).
The pathophysiology of DCM encompasses static factors leading to stenosis of the cervical canal and dynamic factors causing repetitive cord damage due to spinal instability. Mechanical cord compression causes direct damage to the cord; and in addition to this ischemia, inflammation, and apoptosis are secondary causes of DCM (Baptiste and Fehlings 2006).
Clinically degenerative changes in the cervical spine may result in neck pain, myelopathy and radiculopathy. DCM is a clinical syndrome characterised by gait imbalance, loss of hand dexterity and sphincter dysfunction (Tetreault et al. 2015) with a progressive course. It represents the most common cause of spinal cord dysfunction in adults (Kalsi-Ryan et al. 2013; Fehlings et al. 2013).
Imaging in cervical degenerative stenosis is indicated to demonstrate the cause and level of stenosis and presence or absence of myelopathy. Previously lateral X-ray of the cervical spine has been employed to measure the developmental segmental sagittal diameter (distance from the posterior vertebral body to the nearest point of the corresponding spinolaminar line) and the vertebral body ratio method or Pavlov ratio (the sagittal diameter of the spinal canal is divided by the sagittal diameter of the corresponding vertebral body, stenotic canal if ≤0.82) (Pavlov et al. 1987). Flexion-extension lateral X-rays are useful in demonstrating spinal instability.
MRI is the gold standard imaging method to assess spinal canal stenosis (Al-Mefty et al. 1988) and along with CT is the preferred imaging method to assess the dimensions of the cervical canal. Grading systems to describe central spinal canal have been proposed (Kang et al. 2011). Sagittal T1 WI and FSE T2 WI, and axial T2 gradient recalled echo (GRE) sequences are routinely used. Axial T2 GRE sequences can differentiate between disc-osteophyte complex and disc herniation and are useful in assessing the morphology of the neuroforamina. However, T2 GRE sequences may overestimate the degree of neuroforaminal narrowing because of magnetic susceptibility artefacts (Czervionke et al. 1988).
Intramedullary signal changes may reflect a wide spectrum of pathological changes from reversible oedema to established structural changes (Mizuno et al. 2003; Al-Mefty et al. 1988; Ohshio et al. 1993) (Fig. 11). Faint and ill-defined intramedullary T2 high signal more likely reflects reversible oedema. Strong and well-demarcated intramedullary T2 high signal is suggestive irreversible gliosis or cystic necrotic change correlating with hypointensity on T1 WI. Usually no postcontrast imaging in patients with suspected DCM is obtained; however, in a minority of patients (7.3%), intramedullary enhancement can be seen, and it has been associated with worse prognosis after decompressive surgery. Spinal cord enhancement is located between the intervertebral disc at the level of the maximal stenosis and the superior half of the lower vertebral body. Peripheral or scattered enhancing areas are the most prevalent pattern of enhancement seen on axial images. After surgery, the enhancement of the spinal cord may disappear or decrease (Ozawa et al. 2010). Some cases of DCM may be misdiagnosed as inflammatory or neoplastic diseases, as MRI demonstrates a long fusiform T2-signal abnormality and enlargement of the spinal cord, with enhancement just caudal to the point of maximal narrowing of the thecal sac (Flanagan et al. 2014). There is increasing interest in the evaluation of DCM with advanced MRI techniques—diffusion tensor imaging (DTI), MR spectroscopy and magnetization transfer—and the strongest correlation with symptoms has been found with DTI metric fractional anisotropy (Martin et al. 2015).
An 86-year-old woman with acute deterioration, subacute urinary retention and high clinical suspicion of cervical myelopathy. Age-related changes causing spinal canal stenosis and focal high signal of the spinal cord on T2 WI (a, arrowhead). Axial GRE T2 images at C4–5 level (b) shows posterior disc osteophyte complex, myelopathy and bilateral neural foraminal stenosis
CT scanning better depicts bone anatomy, osteophytes and ossification of the posterior longitudinal ligament and flavum ligament. Myelogram and CT myelogram are used in selected cases when MR is contraindicated and the cause or the site of neural impingement is not depicted on conventional CT. As well it has an important role in showing the site of osteophyte-related CSF leaks (Yoshida et al. 2014).
12.2 Foraminal Stenosis in the Cervical Spine
Osteophytes at the uncovertebral joints (joints of Lushka) or posterior vertebral body, osteoarthritis of the facet joints or lateral disc herniations may lead to neuroforaminal stenosis (Wainner and Gill 2000; Yousem et al. 1991; Abbed and Coumans 2007). Uncovertebral joint hypertrophic spurs are the most common cause of neuroforaminal stenosis usually narrowing the superior aspect of the foramen—the nerve root sits in the lower aspect of the neuroforamen. Many papers have evaluated neuroforaminal stenosis (Park et al. 2013; Kim et al. 2015) but there is no universally accepted classification.
12.3 Degenerative Thoracic Spinal Canal Stenosis
Degenerative stenosis of the thoracic spine is less common than cervical or lumbar stenosis, as the thoracic spine is attached to the rib cage, thus limiting its motion range (Girard et al. 2004). It can occur associated with lumbar canal stenosis (Palumbo et al. 2001). Degenerative stenosis and disc herniations may impinge upon the spinal cord, causing localised back pain, myelopathy and sensory level. Symptomatic disc herniations are less frequently encountered in the thoracic spine than in other spinal levels (Arce and Dohrmann 1985). The majority of the thoracic herniations requiring surgery are located in the lower half of the thoracic spine (Oppenheim et al. 1993; Stillerman et al. 1998; Gille et al. 2006; Cornips et al. 2011) and are typically large and exhibit calcification (Stillerman et al. 1998; Cornips et al. 2011; Gille et al. 2006) (Fig. 12).
An 80-year-old woman with a pacemaker and clinical suspicion of thoracic myelopathy. Sagittal midline reformatted (a) and axial (b) CT images of the spine showing a calcified lesion posterior to T11 vertebral body (a, black arrow) and ossification of the ligamentum flavum (a, black star). There is gas within the calcified material (b, white arrow) consistent with calcified thoracic disc herniation. Multilevel intervertebral vacuum gas phenomena noted, including T10–11 disc. Lumbar degenerative listhesis, central canal stenosis and Baastrup’s disease (a, white arrowhead) are also seen
12.4 Lumbar Spinal Canal Stenosis
Degenerative stenosis of the lumbar spinal canal is secondary to bulging or herniated disc, osteophytes, hypertrophy of the facet joints and flavum ligament. In patients with vertebral instability and listhesis, the severity of stenosis may increase in upright position.
As a general rule, sagittal diameter of the central canal less than 12 mm in the lumbar spine is considered stenotic (Mamisch et al. 2012), and less than 10 mm is considered absolute stenosis.
Lateral recess stenosis is usually secondary to hypertrophy of the superior articular facet, although any other degenerative changes may coexist. The lateral recess height is the distance between the anterior superior articular facet and the posterior vertebral body. The cut-off value for stenosis of the lateral recess height is 3 mm (Mamisch et al. 2012).
Foraminal stenosis is secondary to osteophytes, disc bulging or herniation or spondylolisthesis. The cut-off value for stenosis of the lumbar neural foramen is 3 mm (Mamisch et al. 2012).
The role of imaging in patients with acute back pain is to exclude ‘red flag indications’—suspicion of aortic pathology, neoplasm, infection, cauda equina syndrome, fracture or motor weakness (Lateef and Patel 2009). Imaging studies are to be considered in those patients who had no improvement of acute low back pain or radiculopathy after 4–6 weeks after conservative management.
12.5 Redundant Nerve Roots of the Cauda Equina
Redundant nerve roots of the cauda equina is a frequent finding in patients with lumbar spinal canal stenosis. Elongated, enlarged and serpiginous nerve roots within the thecal sac are seen on sagittal T2 WI (Fig. 13). Its main differential diagnosis on MRI must done with spinal dural arteriovenous fistula or spinal extradural arteriovenous fistulas, in which spinal cord oedema or serpentine flow voids on the dorsal surface of the spinal cord are seen (Jeng et al. 2015). Patients with redundant nerve roots on imaging have more severe clinical symptoms than patients without this imaging feature (Ono et al. 2007). After decompressive surgery, the outcome of this group of patients may also be worse in comparison with no redundant nerve roots (Min et al. 2008).
Sagittal T2 WI (a) shows age-related changes in the lumbar spine with multilevel stenosis of the central spinal canal, listhesis, hypertrophy of the ligamentum flavum (a, white arrows) and redundant nerve roots of the cauda equina (a, white arrowhead). Axial T2 WI (b) at L3–4 level shows severe stenosis of the central spinal canal and lateral recesses with facet joint osteoarthritis and hypertrophy of the ligamentum flavum
13 Epidural Lipomatosis
Epidural lipomatosis is characterized by excessive presence of fat in the spinal epidural space reducing the size of the thecal sac. The symptomatology depends on the spinal levels affected, with spinal cord, conus medullaris or radicular compression. Epidural lipomatosis may aggravate spinal canal narrowing in patients with degenerative spinal canal stenosis. It is most commonly found in patients on long-term steroid treatment but can be found in obese patients, patients with endocrinopathies and some idiopathic cases (Fogel et al. 2005). Thoracic epidural lipomatosis is slightly more common than lumbar involvement (Fessler et al. 1992; Stern et al. 1994). In patients with thoracic epidural lipomatosis, the posterior epidural fatty tissue impinges upon the thecal sac, with effacement of the peri medullary CSF and flattening of the spinal cord. At the lumbar spine, the excessive fat tissue causes geometric thecal sac deformation, with polygonal, stellate or ‘Y’ sign (Kuhn et al. 1994) on axial MR or CT images. The cause for this anatomical configuration can be explained due to mass effect of the hypertrophic epidural fat on the thecal sac and meningovertebral ligaments (Geers et al. 2003).
14 Conclusion
Spinal degeneration occurs naturally with age and is exacerbated in response to different aetiologies, mainly mechanical insults. They are routinely encountered in radiological examinations and are a common cause of pain and disability. Back pain is the second most common reason for a doctor’s visit, second only to common cold. The degenerative process will be initiated in the discovertebral complex and will spread to the adjacent vertebral bone marrow, cervical uncovertebral and facet joints and spinal ligaments. Age-related changes and disc herniation may cause stenosis of the spinal canal and neural foramina with subsequent cord compression and myelopathy, cauda equina syndrome or radiculopathy. Other spinal conditions, some age-related such as facet joint syndrome, Baastrup’s disease and others such as DISH and epidural lipomatosis have also been described. Imaging examinations are to be interpreted cautiously in relation with the clinical picture, as often there is poor correlation between symptoms and radiological findings. Plain films, CT and MR exams are routinely employed in the assessment of these patients while more specialised exams like myelogram or bone scans are used in selected patients. MRI is the preferred imaging technique due to its superior soft tissue resolution; it is particularly useful in confirming suspected disc herniations, nerve root entrapment, cord compression and spinal canal stenosis.
Abbreviations
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- DCM:
-
Degenerative cervical myelopathy
- DISH:
-
Diffuse idiopathic skeletal hyperostosis
- DTI:
-
Diffusion tensor imaging
- FSU:
-
Functional spinal unit
- LF:
-
Ligamentum flavum
- MR:
-
Magnetic resonance
- PLL:
-
Posterior longitudinal ligament
References
Abbed KM, Coumans JV (2007) Cervical radiculopathy: pathophysiology, presentation, and clinical evaluation. Neurosurgery 60(1 Supp1 1):S28–S34
Adams MA, McNally DS, Dolan P (1996) ‘Stress’ distributions inside inter-vertebral discs. The effects of age and degeneration. J Bone Joint Surg Br 78:965–972
Ahn UM, Ahn NU, Buchowski JM et al (2000) Cauda equina syndrome secondary to lumbar disc herniation: a meta-analysis of surgical outcomes. Spine 25:1515–1522
Al-Mefty O, Harkey LH, Middleton TH et al (1988) Myelopathic cervical spondylotic lesions demonstrated by magnetic resonance imaging. J Neurosurg 68:217–222
Altinkaya N, Yildirim T, Demir S et al (2011) Factors associated with the thickness of the ligamentum flavum: is ligamentum flavum thickening due to hypertrophy or buckling? Spine (Phila Pa 1976) 36(16):E1093–E1097
Arce CA, Dohrmann GJ (1985) Herniated thoracic disks. Neurol Clin 3(2):383–392
Ashton IK, Roberts S, Jaffray DC et al (1994) Neuropeptides in the human intervertebral disc. J Orthop Res 12(2):186–192
Aufdermaur M, Fehr K, Lesker P et al (1980) Quantitative histochemical changes in intervertebral discs in diabetes. Exp Cell Biol 48:89–94
Banning CS, Thorell WE, Leibrock LG (2001) Patient outcome after resection of lumbar juxtafacet cysts. Spine 26(8):969–972
Baptiste DC, Fehlings MG (2006) Pathophysiology of cervical myelopathy. Spine J 6(6 Suppl):190S–197S
Bernick S, Caillet R (1982) Vertebral end-plate changes with aging of human vertebrae. Spine 7:97–102
Bland JH, Boushey DR (1990) Anatomy and physiology of the cervical spine. Semin Arthritis Rheum 20:1–20
Boden SD, Davis DO, Dina TS et al (1990a) Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 72(3):403–408
Boden SD, McCowin PR, Davis DO et al (1990b) Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 72(8):1178–1184
Bogduk N (2012) Degenerative joint disease of the spine. Radiol Clin N Am 50(4):613–628
Bogduk N, Mercer SR (2000) Biomechanics of the cervical spine. I: Normal kinematics. Clin Biomech 15:633–648
Borota L, Jonasson P, Agolli A (2008) Spontaneous resorption of intradural lumbar disc fragments. Spine J 8(2):397–403
Bozzao A, Gallucci M, Masciocchi C et al (1992) Lumbar disk herniation: MR imaging assessment of natural history in patients treated without surgery. Radiology 185(1):135–141
Bush K, Cowan N, Katz DE et al (1992) The natural history of sciatica associated with disc pathology. A prospective study with clinical and independent radiologic follow-up. Spine 17(10):1205–1212
Caron T, Bransford R, Nguyen Q et al (2010) Spine fractures in patients with ankylosing spinal disorders. Spine 35:E458–E464
Carragee EJ, Paragioudakis SJ, Khurana S (2000) Lumbar high-intensity zone and discography in subject without low back problems. Spine 25:2987–2992
Cavanaugh JM, Ozaktay AC, Yamashita HT et al (1996) Lumbar facet pain: biomechanics, neuroanatomy and neurophysiology. J Biomech 29(9):1117–1129
Chaput C, Padon D, Rush J et al (2007) The significance of increased fluid signal on magnetic resonance imaging in lumbar facets in relationship to degenerative spondylolisthesis. Spine 32(17):1883–1887
Chen CK, Yeh L, Resnick D et al (2004) Intraspinal posterior epidural cysts associated with Baastrup’s disease: report of 10 patients. AJR 182:191–194
Cheung KM, Karppinen J, Chan D et al (2009) Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thou-sand forty-three individuals. Spine (Phila Pa 1976) 34(9):934–940
Chimento GF, Ricciardi JE, Whitecloud TS 3rd (1995) Intraspinal extradural ganglion cyst. J Spinal Disord 8:82–85
Cicuendez M, Alen JF, Ramos A et al (2010) Spontaneous hemorrhage into a lumbar synovial cyst. Eur Spine J 19(Suppl 2):190–192
Cornips EM, Janssen ML, Beuls EA (2011) Thoracic disc herniation and acute myelopathy: clinical presentation, neuroimaging findings, surgical considerations, and outcome. J Neurosurg Spine 14(4):520–528
Czervionke L, Daniels D, Wehrli F et al (1988) Magnetic susceptibility artefacts in gradient-recalled echo MR imaging. AJNR Am J Neuroradiol 9:1149–1155
D’Aprile P, Tarantino A, Lorusso V et al (2006) Fat saturation technique and gadolinium in MRI of lumbar spinal degenerative disease. Neuroradiol J 19(5):654–671
Datta S, Lee M, Falco FJ et al (2009) Systematic assessment of diagnostic accuracy and therapeutic utility of lumbar facet joint interventions. Pain Physician 12(2):437–460
Dean CL, Gabriel JP, Cassinelli EH et al (2009) Degenerative spondylolisthesis of the cervical spine: analysis of 58 patients treated with anterior cervical de-compression and fusion. Spine J 9:439–446
Diehn FE (2012) Imaging of spinal infection. Radiol Clin N Am 50(4):777–798
Edwards WC, LaRocca H (1983) The developmental segmental sagittal diameter of the cervical spinal canal in patients with cervical spondylosis. Spine 8(1):20–27
Eross EJ, Dodick DW, Nelson KD et al (2002) Orthostatic headache syndrome with CSF leak secondary to bony pathology of the cervical spine. Cephalalgia 22:439–443
Eubanks JD, Lee MJ, Cassinelli E et al (2007) Prevalence of lumbar facet arthrosis and its relationship to age, sex, and race: an anatomic study of cadaveric specimens. Spine (Phila Pa 1976) 32(19):2058–2062
Even JL, Chen AF, Lee JY (2014) Imaging characteristics of “dynamic” versus “static” spondylolisthesis: analysis using magnetic resonance imaging and flex-ion/extension films. Spine J 14(9):1965–1969
Fardon DF, Williams AL, Dohring EJ et al (2014) Lumbar disc nomenclature: Version 2.0 Recommendations of the combined task forces of the North American Spine Society, the American Society of Spine Radiology and the American Society of Neuroradiology. Spine J 14(11):2525–2545
Fehlings MG, Tetreault LA, Wilson JR et al (2013) Cervical spondylotic myelopathy: current state of the art and future directions. Spine (Phila Pa 1976) 38(22 suppl 1):S1–S8
Fessler RG, Johnson DL, Brown FD et al (1992) Epidural lipomatosis in steroid-treated patients. Spine 17:183–188
Flanagan EP, Krecke KN, Marsh RW et al (2014) Specific pattern of gadolinium enhancement in spondylotic myelopathy. Ann Neurol 76:54–65
Fogel GR, Cunningham PY 3rd, Esses SI (2005) Spinal epidural lipomatosis: case reports, literature review and meta-analysis. Spine J 5(2):202–211
Fukuyama S, Nakamura T, Ikeda T et al (1995) The effect of mechanical stress on hypertrophy of the lumbar ligamentum flavum. J Spinal Disord 8(2):126–130
Gallucci M, Puglielli E, Splendiani A et al (2005) Degenerative disorders of the spine. Eur Radiol 15:591–598
Gallucci M, Limbucci N, Paonessa A et al (2007) Degenerative disease of the spine. Neuroimaging Clin N Am Feb 17(1):87–103
Geers C, Lecouvet FE, Begets C et al (2003) Polygonal deformation of the dural sac in lumbar epidural lipomatosis: anatomic explanation by the presence of meningovertebral ligaments. AJNR Am J Neuroradiol 24(7):1276–1282
Gille O, Soderlund C, Razafimahandri HJC et al (2006) Analysis of hard thoracic herniated discs: review of 18 cases operated by thoracoscopy. Eur Spine J 15(5):537–542
Girard CJ, Schweitzer ME, Morrison WB et al (2004) Thoracic spine disc-related abnormalities: longitudinal MR imaging assessment. Skeletal Radiol 33(4):216–222
Gower WE, Pedrini V (1969) Age-related variations in protein polysaccharides from human nucleus pulposus, annulus fibrosus, and costal cartilage. J Bone Joint Surg Am 51(6):1154–1162
Grenier N, Kressel HY, Schiebler ML et al (1987) Normal and degenerative posterior spinal structures: MR imaging. Radiology 165(2):517–525
Griffith JF, Wang YX, Antonio GE et al (2007) Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine 32(24):E708–E712
Guo JJ, Yang HL, Cheung KM et al (2009) Classification and management of the tandem ossification of the posterior longitudinal ligament and flaval ligament. Chin Med J (Engl) 122(2):219–224
Hazlett J (1964) Kissing spines. J Bone Joint Surg Br 46:1368–1369
Hemminghytt S, Daniels DL, Williams AL et al (1982) Intraspinal synovial cysts: natural history and diagnosis by CT. Radiology 145:375–376
Holm S, Maroudas A, Urban JPG et al (1981) Nutrition of the intervertebral disc. Solute transport and metabolism. Connect Tissue Res 8:101–119
Howington JU, Connolly ES, Voorhies RM (1999) Intraspinal synovial cysts: 10-year experience at the Ochsner Clinic. J Neurosurg 91.2(Suppl):193–199
Hoxworth JM, Trentman TL, Kotsenas AL et al (2012) The role of digital subtraction myelography in the diagnosis and localization of spontaneous spinal CSF leaks. AJR 99:649–653
Hsu KY, Zucherman JF, Shea WJ et al (1995) Lumbar intraspinal synovial and ganglion cysts (facet cysts). Ten-year experience in evaluation and treatment. Spine (Phila Pa 1976) 20(1):80–89
Hukins DWL (1988) Disc structure and function. In: Ghosh P (ed) The biology of the intervertebral disc, vol 1. CRC Press Inc, Boca Raton, FL, pp l–37
Hunter CJ, Matyas JR, Duncan NA (2003) The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng 9:667–677
Ikeda T, Nakamura T, Kikuchi TJ et al (1996) Pathomechanism of spontaneous regression of the herniated lumbar disc: histologic and immunohistochemical study. Spinal Disord 9(2):136–140
Izzo R, Popolizio T, D'Aprile P et al (2015) Spinal pain. Eur J Radiol 84(5):746–756
Jackson RP (1992) The facet syndrome. Myth or reality? Clin Orthop Relat Res 279:110–121
James SL, Davies AM (2006) Imaging of infectious spinal disorders in children and adults. Eur J Radiol 58:27–40
Jeng Y, Chen DY, Hsu HL et al (2015) Spinal dural arteriovenous fistula: imaging features and its mimics. Korean J Radiol 16(5):1119–1131
Jensen MC, Brant-Zawadski MN, Obuchowski N et al (1994) Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med 331:69–73
Jiang SD, Jiang LS, Dai LY (2011) Degenerative cervical spondylolisthesis: a systematic review. Int Orthop 35(6):869–875
Kalichman L, Li L, Kim DH et al (2008) Facet joint osteoarthritis and low back pain in the community-based population. Spine (Phila Pa 1976) 33(23):2560–2565
Kalsi-Ryan S, Karadimas SK, Fehlings MG (2013) Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 19:409–421
Kang Y, Lee JW, Koh YH et al (2011) New MRI grading system for the cervical canal stenosis. Am J Roentgenol 197(1):W134–W140
Kauppila LI, Eustace S, Kiel D et al (1998) Degenerative displacement of lumbar vertebrae: a 25-year follow up study in Framingham. Spine 23:1868–1873
Kawasaki M, Tani T, Ushida T et al (2007) Anterolisthesis and retrolisthesis of the cervical spine in cervical spondylotic myelopathy in the elderly. J Orthop Sci 12:207–213
Khan AM, Girardi F (2006) Spinal lumbar synovial cysts. Diagnosis and management challenge. Eur Spine J 15(8):1176–1182
Kim S, Lee JW, Chai JW et al (2015) A new MRI grading system for cervical foraminal stenosis based on axial T2-weighted images. Korean J Radiol 16(6):1294–1302
Kirkaldy-Willis WH, Farfan HF (1982) Instability of the lumbar spine. Clin Orthop Relat Res 165:110–123
Klaassen Z, Tubbs RS, Apaydin N et al (2011) Vertebral spinal osteophytes. Anat Sci Int 86(1):1–9
Kuhn MJ, Youssef HT, Swan TL et al (1994) Lumbar epidural lipomatosis: the “Y” sign of thecal sac compression. Comput Med Imaging Graph 18(5):367–372
Kushchayev SV, Glushko T, Jarraya M et al (2018) ABCs of the degenerative spine. Insights Imaging 9(2):253–274
Lateef H, Patel D (2009) What is the role of imaging in acute low back pain? Curr Rev Musculoskelet Med 2(2):69–73jeng
Luetkehans TJ, Coughlin BF, Weinstein MA (1987) Ossification of the posterior longitudinal ligament diagnosed by MR. AJNR Am J Neuroradiol 8:924–925
Mader R (2002) Clinical manifestations of diffuse idiopathic skeletal hyperostosis of the cervical spine. Semin Arthritis Rheum 32:130–135
Malham GM, Parker RM, Ballock ZE et al (2015) Bone scans are reliable for the identification of lumbar disk and facet pathology. Global Spine J 5(1):23–30
Mamisch N, Brumann M, Hodler J et al (2012) Radiologic criteria for the diagnosis of spinal stenosis: results of a Delphi survey. Radiology 264(1):174–179
Manchikanti L, Singh V, Pampati V et al (2001) Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician 4(4):308–316
Martin AR, Aleksanderek I, Cohen-Adad J et al (2015) Translating state-of-the-art spinal cord MRI techniques to clinical use: a systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. Neuroimage Clin 10:192–238
Matsumoto M, Fujimura Y, Suzuki N et al (1998) MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br 80:19–24
Mercer S, Bogduk N (1994) The ligaments and anulus fibrosus of human adult cervical intervertebral discs. Spine 24:619–626
Meyerding HW (1932) Spondylolisthesis. Surg Gynecol Obstet 54:371–377
Millucci L, Bernardini G, Spreafico A et al (2017) Histological and ultra-structural characterization of alkaptonuric tissues. Calcif Tissue Int 101:50–64
Min JH, Jang JS, Lee SH (2008) Clinical significance of redundant nerve roots of the cauda equina in lumbar spinal stenosis. Clin Neurol Neurosurg 110:14–18
Miyasaka K, Kaneda K, Sato S et al (1983) Myelopathy due to ossification or calcification of the ligamentum flavum: radiologic and histologic evaluations. Am J Neuroradiol 4:629–632
Miyazaki M, Hong SW, Yoon SH et al (2008) Reliability of a magnetic resonance imaging-based grading system for cervical intervertebral disc degeneration. J Spinal Disord Tech 21:288–292
Mizuno J, Nakagawa H, Inoue T et al (2003) Clinicopathological study of “snake-eye appearance” in compressive myelopathy of the cervical spinal cord. J Neurosurg Spine 99(2):162–168
Modic MT, Ross JS (2007) Lumbar degenerative disc disease. Radiology 245(1):43–61
Modic MT, Masaryk TJ, Ross JS et al (1988a) Imaging of degenerative disc disease. Radiology 168:177–186
Modic MT, Steinberg PM, Ross JS et al (1988b) Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 166:193–199
Morishita Y, Naito M, Hymanson H et al (2009) The relationship between the cervical spinal canal diameter and the pathological changes in the cervical spine. Eur Spine J 18(6):877–883
Munter FM, Wasserman BA, Wu HM et al (2002) Serial MR imaging of annular tears in lumbar intervertebral disks. AJNR Am J Neuroradiol 23(7):1105–1109
Nakashima H, Yukawa Y, Suda K et al (2015) Cervical disc protrusion correlates with the severity of cervical disc degeneration: a cross-sectional study of 1,211 relatively healthy volunteers. Spine (Phila Pa 1976) 40:E774–E779
Ohshio I, Hatayama A, Kaneda K et al (1993) Correlation between histopathologic features and magnetic resonance images of spinal cord lesions. Spine (Phila Pa 1976) 18(9):1140–1149
Okuda T, Baba I, Fujimoto Y et al (2004) The pathology of ligamentum flavum in degenerative lumbar disease. Spine 29(15):1689–1697
Olczyk K (1992) Age related changes in collagen of human intervertebral discs. Gerontology 38:196–204
Ono A, Suetsuna F, Irie T et al (2007) Clinical significance of the redundant nerve roots of the cauda equina documented on magnetic resonance imaging. J Neurosurg Spine 7(1):27–32
Oppenheim JS, Rothman AS, Sachdev VP (1993) Thoracic herniated discs: review of the literature and 12 cases. Mt Sinai J Med 60(4):321–326
Oxland TR (2016) Fundamental biomechanics of the spine – what we have learned in the past 25 years and future directions. J Biomech 49(6):817–832
Ozawa H, Sato T, Hyodo H et al (2010) Clinical significance of intramedullary Gd-DTPA enhancement in cervical myelopathy. Spinal Cord 48(5):415–422
Palumbo MA, Hilibrand AS, Hart RA et al (2001) Surgical treatment of thoracic spinal stenosis: a 2- to 9-year follow-up. Spine 26(5):558–566
Park H-J, Kim SS, Lee S-Y et al (2013) A practical MRI grading system for cervical foraminal stenosis based on oblique sagittal images. Br J Radiol 86(1025):20120515
Parlier-Cuau C, Wybier M, Nizard R et al (1999) Symptomatic lumbar facet joint synovial cysts: clinical assessment of facet joint steroid injection after 1 and 6 months and long-term follow-up in 30 patients. Radiology 210(2):509–513
Pate D, Goobar J, Resnick D et al (1988) Traction osteophytes of the lumbar spine: Radiographic-pathologic correlation. Radiology 166(3):843–846
Patel KB, Poplawski MM, Pawha PS et al (2014) Diffusion-weighted MRI "claw sign" improves differentiation of infectious from degenerative Modic type 1 signal changes of the spine. AJNR Am J Neuroradiol 35(8):1647–1652
Pavlov H, Torg JS, Robie B et al (1987) Cervical spinal stenosis: determination with vertebral body ratio method. Radiology 164(3):771–775
Perolat R, Kastler A, Nicot B et al (2018) Facet joint syndrome: from diagnosis to interventional management. Insights Imaging 9(5):773–789
Pfirrmann CW, Metzdorf A, Zanetti M et al (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 26:1873–1878
Pfirrmann C, Dora C, Schmid M et al (2004) MR image based grading of lumbar nerve root compromise due to disk herniation: reliability study with surgical correlation. Radiology 230(2):583–588
Pirotte B, Gabrovsky N, Massager N et al (2003) Synovial cysts of the lumbar spine: surgery-related results and outcome. J Neurosurg 99(1 Suppl):14–19
Rajasekaran S, Venkatadass K, Naresh Babu J et al (2008) Pharmacological enhancement of disc diffusion and differentiating of healthy, ageing and degenerated discs: results from in-vivo serial post-contrast MRI studies in 365 human lumbar discs. Eur Spine J 17(5):626–643
Resnick D, Niwayama G (1976) Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology 119:559–568
Reust P, Wendling D, Lagier R, Pageaut G et al (1988) Degenerative spondylolisthesis, synovial cyst of the zygapophyseal joints, and sciatic syndrome: re-port of two cases and review of the literature. Arthritis Rheum 31(2):288–294
Roberts S, McCall IW, Menage J et al (1997) Does the thickness of the vertebral subchondral bone reflect the composition of the intervertebral disc? Eur Spine J 6:385–389
Robinson D, Mirovsky Y, Halperin N et al (1998) Changes in proteoglycans of intervertebral disc in diabetic patients: a possible cause of increased back pain. Spine 23:849–856
Schellhas KP, Pollei SR, Gundry CR et al (1996) Lumbar disc high intensity zone. Correlation of magnetic resonance imaging and discography. Spine 21:79–86
Stern JD, Quint DJ, Sweasey TA et al (1994) Spinal epidural lipomatosis: two new idiopathic cases and a review of the literature. J Spinal Disord 7:343–349
Stillerman CB, Chen TC, Couldwell WT et al (1998) Experience in the surgical management of 82 symptomatic herniated thoracic discs and review of the literature. J Neurosurg 88(4):623–633
Takahashi K, Miyazaki T, Ohnari H et al (1995) Schmorl’s nodes and low-back pain. Analysis of magnetic resonance imaging findings in symptomatic and asymptomatic individuals. Eur Spine J 4:56–59
Tetreault L, Goldstein CL, Arnold P et al (2015) Degenerative cervical myelopathy: a spectrum of related disorders affecting the aging spine. Neurosurgery 77(suppl 4):S51–S67
Thielen KR, Sillery JC, Morris JM et al (2015) Ultrafast dynamic computed tomography myelography for the precise identification of high-flow cerebrospinal fluid leaks caused by spiculated spinal osteophytes. J Neurosurg Spine 22:324–331
Trout JJ, Buckwalter JA, Moore KC et al (1982) Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell 14:359–369
Twomey L, Taylor J (1988) Age changes in the lumbar spinal and intervertebral canals. Paraplegia 26(4):238–249
Ullman N, Gregg L, Becker D et al (2016) Anterior disco-osteo-arterial conflict as a cause of intersegmental arterial flow impairment and spinal cord ischemia. Neuroradiology 58:1109–1115
van Rijn JC, Klemetso N, Reitsma JB et al (2005) Observer variation in MRI evaluation of patients suspected of lumbar disk herniation. AJR Am J Roentgenol 184:299–303
Varlotta GP, Lefkowitz TR, Schweitzer M et al (2011) The lumbar facet joint: a review of current knowledge: part 1: anatomy, biomechanics and grading. Skelet Radiol 40(1):13–23
Wainner RS, Gill H (2000) Diagnosis and nonoperative management of cervical radiculopathy. J Orthop Sports Phys Ther 30:728–744
Wang AM, Haykal HA et al (1987) Synovial cysts of the lumbar spine: CT evaluation. Comput Radiol 11(5–6):253–257
Weishaupt D, Zanetti M, Boos N et al (1999) MR imaging and CT in osteoarthritis of the lumbar facet joints. Skelet Radiol 28(4):215–219
Westerveld LA, Verlaan JJ, Oner FC (2009) Spinal fractures in patients with ankylosing spinal disorders: a systematic review of the literature on treatment, neurological status and complications. Eur Spine J 18:145–156
Wiltse LL, Berger PE, McCulloch JA (1997) A system for reporting the size and location of lesions in the spine. Spine 22:1534–1537
Wong JJ, Leung OC, Yuen MK (2011) Questionable adequacy of magnetic resonance for the detection of ossification of the posterior longitudinal ligament of the cervical spine. Hong Kong J Radiol 14:78–83
Yamashita Y, Takahashi M, Matsuno Y et al (1990) Spinal cord compression due to ossification of ligaments: MR imaging. Radiology 175(3):843–848
Yoshida H, Takai K, Taniguchi M (2014) Leakage detection on CT myelography for targeted epidural blood patch in spontaneous cerebrospinal fluid leaks: calcified or ossified spinal lesions ventral to the thecal sac. J Neurosurg Spine 21(3):432–441
Yousem DM, Atlas SW, Goldberg HI et al (1991) Degenerative narrowing of the cervical spine neural foramina: evaluation with high-resolution 3DFT gradient-echo MR imaging. AJNR Am J Neuroradiol 12:229–236
Yu S, Haughton VM, Sether LA et al (1988) Anulus fibrosus in bulging inter-vertebral disks. Radiology 169:761–763
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Santamaria, N., Polidura, M.d.C., Bunea, G., Spratt, J. (2022). Emergent Degenerative and Disc Diseases. In: Scaglione, M., Çalli, C., Muto, M., Wirth, S. (eds) Emergency Radiology of the Head and Spine. Medical Radiology(). Springer, Cham. https://doi.org/10.1007/978-3-030-91047-1_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-91047-1_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91046-4
Online ISBN: 978-3-030-91047-1
eBook Packages: MedicineMedicine (R0)