Abstract
Osteoarthritis is a common complication in the elderly and is often associated with osteophyte growth on vertebral bodies. The clinical presentation of vertebral osteophytes is related to anatomical structures adjacent to the spinal column. For instance, cervical osteophytes potentially involve the pharynx and esophagus, leading to dysphagic symptoms that may be accompanied by food aspiration, vocal fold paralysis and obstructive sleep apnea. In addition to anterior cervical osteophytes, posterior and uncinate process osteophytes may form, compressing the spinal cord and vertebral artery blood supply, respectively. Cervical osteophytes have also been shown to form an accessory median atlanto-occipital joint when the relationship between the atlas, dens and basiocciput is involved. In the thorax, the esophagus is often affected by osteophytes and may result in dysphagia. Traumatic and non-traumatic thoracic aorta pseudoaneurysm formation has been attributed to sharp osteophytes lacerating the aorta, a direct complication of the relationship between the aorta anterior vertebral column. Additionally, aspiration pneumonia was reported in patients with compression of a main stem bronchus, due to mechanical compression by thoracic osteophytes. In the lumbar spinal region, the two major structures in close proximity to the spine are the inferior vena cava and abdominal aorta, both of which have been reported to be affected by osteophytes. Treatment of osteophytes is initially conservative with anti-inflammatory medications, followed by surgical removal. Increasing obesity and geriatric populations will continue to result in an array of osteoarthritic degenerative changes such as osteophyte formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background Information
Osteoarthritis is complicated by many types of degenerative changes including osteophyte formation on spinal vertebral bodies. Osteophytes (osteo-osteo = bone and phyte-futon = plant) are defined as abnormal bony growths or bone spurs that are bony projections formed along joints. While osteophytes may form on any degenerating joint, such as the hip, knee and distal interphalangeal joints (Heberden’s nodes), vertebral osteophytes present with clinical symptoms unique to affected adjacent structures. Interestingly, vertebral osteophytes are a common finding in the gross anatomy laboratory, and their clinical significance is rarely discussed in anatomy and embryology books. More importantly, vertebral osteophytes are a common radiological finding affecting 20–30% of the elderly population (Brown and Neumann 2004). The aim of this review therefore is to provide a comprehensive picture on the possible etiologies, presentation and treatment of this common condition in relationship to the gross anatomy and imaging.
A number of etiological factors result in vertebral osteophyte formation; however, the main factor is obesity, which is often accompanied by non-insulin dependent diabetes mellitus. Extra body mass increases weight on the vertebral column, contributing to mechanical stress associated with degenerative changes of osteoarthritis. Additional etiological factors are hypervitaminosis A (Seawright et al. 1965; Yee et al. 1985) and secretion of bone morphogenetic factor for stimulation of bony tissue growth (Hanamura et al. 1980; von Ludinghausen et al. 2005; Urist et al. 1979; Yee et al. 1985). Recent studies suggest excessive mechanical stress, particularly at a young age, may predispose one to osteophyte formation later in life (O’Neill et al. 1999; Schmitt et al. 2004).
The pathogenesis of osteophytes begins with degeneration of the spinal column, specifically, the nucleus pulposus. As one ages, the nucleus pulposus loses water content and turgor, resulting in decreased height of the intervertebral disc and less resiliency to load-bearing weight. Concomitantly, the annulus fibrosus also begins to lose elasticity. In order to maintain maximal load-bearing potential, the margins of the zygapophyseal joint form bony projections to increase the surface area for weight distribution. This adaptation does not adequately relieve joint pressure and results in increased bony protrusion from the vertebral body.
The epidemiology of the osteophyte formation with regards to population difference, gender, occupation, subsistence and other causes that may effect osteophytes formation has been reported in several physical anthropology studies (Sofaer-Derevenski 2000; Bridges 1994; Knusel et al. 1997). Sofaer-Derevenski (2000) compared vertebral degenerative changes from the sixteenth to nineteenth century site of Ensay, the Outer Hebrides and the medieval site of Wharram Percy, the Yorkshire Wolds, noting gender-specific facet remodeling and osteophytic changes in Ensay females related to load-bearing using creels. Furthermore, Knusel et al. (1997) analyzed 81 skeletons from social-class-specific plots from the thirteenth to fourteenth century medieval priory cemetery of St. Andrew, Fishergate, York, reporting no significant difference between the groups in regards to vertebral column degenerative joint disease (DJD). The authors postulated that differences between individuals were produced as a response to erect posture during bipedal locomotion rather than differences in occupational stress (Knusel et al. 1997).
Isolated data regarding specific regions of the body, such as cervical, thoracic and lumbar, are reported in this review (Table 1). In addition, there are no specific data regarding the frequency of osteophyte formation and its age-related changes. We know that osteophytes are one of the main characteristics of osteoarthritis. Bearing this in mind, we can assume that osteophytes will have similar presentation as osteoarthritis. By the age of 40 years, 90% of all persons have some degenerative changes in their weight-bearing joints, even though clinical symptoms are generally absent. In one study 85% of the patients aged 55–64 years had some degree of osteoarthritis in one or more joints (Brown and Neumann 2004). We can only hypothesize that osteophytes follow the same pattern of presentation.
Cervical osteophytes
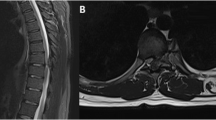
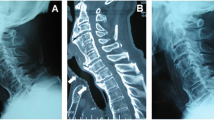
The sequela of cervical osteophytes is well documented, with cases implicating all vertebrae in circumstances related to their anatomic location. The anatomical relationship of the atlas, dens and basiocciput is affected by cervical osteophyte formation, as is C2–C3 with involvement of the posterior pharynx. Additionally, the relationship of C4–C7 to the larynx and esophagus is also important, as cervical osteophytes diminish the retropharyngeal space and compress these structures (Figs. 1, 2, 3, 4).
Even though osteophytes may form on all of the cervical vertebrae, there is a predilection for the lower vertebrae. Cervical vertebrae C5–C6 and C6–C7 have the greatest frequency of osteophyte formation, attributed to their mobility and load-bearing nature (Schmidek 1986; Yee et al. 1985; Yoskovitch and Kantor 2001). Also, these three vertebrae are at greatest risk for lordosis, adding to the likelihood of vertebral degeneration and subsequent osteophyte formation (Yoskovitch and Kantor 2001). With the esophagus resting on the anterior border of the cervical vertebrae from C4–C7, anterior osteophytes may hinder peristalsis of food through this segment of the esophagus (Fig. 5).
Dysphagia is a common complication in the elderly population and is often underreported. The underlying causes of dysphagia are numerous, including esophagitis and esophageal cancer, with cervical osteophyte compression of the esophagus being a less common but important etiological factor. Historically, dysphagia due to cervical osteophytes has been well reported in the literature (Aronowitz and Cobarrubias 2003; Di Vito 1998; Fuerderer et al. 2004; Goel et al. 1999; Kanbay and Selcuk 2006; Kodama et al. 1995; Ng et al. 2005; Rosen 1985; Stancampiano et al. 2002; Strasser et al. 2000; Stuart 1989; Srinivas and George 1999; Yee et al. 1985; Yutan et al. 2001), dating back to 1905 when Zahn (1905) reported the first case (Idem 1906). This was followed in 1926 by Mosher (1926) who reported two cases and in 1938 by Iglauer (1938) who performed the first surgical excision of cervical osteophytes in an attempt to relieve dysphagia. Additionally, Hilding and Tachdjian (1960) found 36 cases in a 1960 review of the literature, followed in 1973 by Meeks and Renshaw (1973), who studied 60 cases of dysphagia in which 10 were surgically repaired.
In general, there are five characteristics of osteophyte-induced dysphagia that are found in the majority of cases reported: (1) large osteophytes may mechanically cause blockage of the esophagus (Lambert et al. 1981; Resnick et al. 1978; Sobol and Rigual 1984; Strasser et al. 2000), (2) small osteophytes may cause obstruction by compressing the esophageal segment attached to the cricoid cartilage, most commonly at C5–C6 (Crowther and Ardran 1985; Davies et al. 1989; Strasser et al. 2000), (3) esophagus-compressing osteophytes coexisting with decreased closure of the larynx may additively increase dysphagia (Di Vito 1998), (4) osteophytes at C3–C4 may impair tilting of the epiglottis over the laryngeal inlet (Crowther and Ardran 1985; Strasser et al. 2000) and (5) inflammation around the osteophyte may lead to esophagitis or pharyngitis, ultimately worsening dysphagia (Deutsch et al. 1985; Di Vito 1998).
There are several unique and important cervical osteophyte-induced dysphagia cases. A case by Di Vito (1998) reported dysphagia due to a stroke complicated by a preexisting osteophyte complex bridging C3–C5. The stroke had reduced laryngeal elevation and epiglottic tilt, and this coupled with the osteophytic bridge indented the cricopharyngeal opening to deflect food boluses into the open larynx (Di Vito 1998). A similar osteophyte bridging mechanism was reported by Yoskovitch and Kantor (2001), with massive osteophytes on C3–C6 that displaced the esophagus and resulted in dysphagia and compression of the left tracheoesophageal groove. This compression of the tracheoesophageal groove compressed the recurrent laryngeal nerve and thus paralyzed the vocal fold. The concept of an osteophytic shelf was first introduced by Davies et al. (1989), but the hypothesis of the shelf acting as a deflection mechanism or being involved in vocal paralysis was not mentioned.
Complimenting the case introduced by Di Vito (1998) was a study by Strasser et al. (2000) who explored the concept of cervical osteophytes causing dysphagia and concomitant aspiration. Through their analysis of 55 patients with dysphagia, aspiration problems were present in 75% of patients with osteophytes larger than 10 mm and in 34% of patients with osteophytes smaller than 10 mm (Strasser et al. 2000). These results suggest that size of the osteophyte may correlate with the degree of compression and subsequent likelihood of difficulty with aspiration.
Obstructive sleep apnea may also accompany osteophyte-induced dysphagia. In a case series of five patients suffering from dysphagia, Fuerderer et al. (2004) noted that two patients also suffered from obstructive sleep apnea. Additionally, in one of these patients, a multi-level connection of osteophytes from C3–T1 perforated the pharynx, resulting in an emergency tracheostomy. Fuerderer et al. (2004) proposed that the retropharyngeal osteophytes compressed the airway in the supine position, explaining the apneic symptoms.
Whereas dysphagia is a result of anterior cervical osteophytes, there are situations in which osteophytes occur at other positions on the vertebral body. Posterior osteophytes are thought to form when mechanical stresses increase tension on Sharpey’s fibers inserting into vertebral bodies adjacent to degenerating intervertebral discs (Pate et al. 1988; Schmorl and Junghanns 1971). Osteophyte projections on the posterior region of the vertebral body directly implicate the spinal cord. At one point, it was hypothesized that correction of anterior bony protuberances would lead to resorption of posterior osteophytes (Cloward 1987; Robinson et al. 1962). This notion was refuted by Stevens et al. (1993) when they found that in 26 postoperative cases no posterior osteophytes showed resorption for up to 12 years following anterior osteophyte correction.
Two independent studies by von Ludinghausen et al. (2005, 2006) have shown important osteophytic relationships between the basiocciput, atlas and dens. In 2005, they noted osteophytes on the articular surfaces of the median atlanto-axial joint, coupled with large osteophytes of the anterior arch of the atlas contacting the basiocciput (von Ludinghausen et al. 2005). These additional contact points between the osteophyte and basiocciput were histologically confirmed to be real joints, designated accessory median atlanto-occipital joints (von Ludinghausen et al. 2005). Recently, von Ludinghausen et al. (2006) investigated 100 cadavers, of which 42 showed osteophytes of the articular surfaces of the median atlantoaxial joint. In five cases, a large osteophyte on the anterior arch of the atlas made contact with the basiocciput in a similar manner as described in their 2005 study (von Ludinghausen et al. 2005).
Osteophytes of the atlanto-occipital and atlanto-axial joints have important clinical manifestations. Physiotherapy and/or iatrogenic movement of the joints may lead to inadvertent fracture of the osteophyte, potentially causing pain and crepitation in the region (von Ludinghausen et al. 2006). Additionally, osteoarthritic degeneration of these two joints may cause immobility and stiffness of the cervical spine, as well as headaches secondary to occipital and cervical involvement (Aufdermauer 1979; Bovim et al. 1994; Chouret 1967; Edmeads 1978; Fournier and Rathelot 1959; Jackson 1967; Kamieth 1959; von Ludinghausen et al. 2006).
Osteophytes derived from vertebral uncinate processes may interfere with the transverse foramen, leading to compression of the vertebral artery. This mechanism was first recognized in 1924 when Barre (1924) noted that cervical deformities may compress the vasculature leading to the medulla, resulting in motor dysfunction. In 1940, Krogdahl and Torgersen (1940) studied 12 cadavers with uncovertebral arthritis, and concluded that vertebral deformities compressed both the vertebral artery and the sympathetic nerve plexus. Recently, Cagnie et al. (2005) observed 60% (n = 111) of dry vertebrae had osteophytes on the uncinate process of C5 and C6. Half of these osteophytes partially effaced the transverse foramen, forcing the vertebral artery to course around these projections, potentially compressing or dissecting the artery. Vertebral artery compression from osteophytes may occur anteriorly from the uncinate process or posteriorly from the superior or inferior articular facets (Cagnie et al. 2005; Ebraheim et al. 1997; Sheehan et al. 1960). In most cases, compression is likely to occur from the uncinate process rather than the articular facets, as osteophytes are present in 48 and 30% of cases, respectively (Cagnie et al. 2005).
Compression of the vertebral artery leads to a myriad of clinical symptoms, especially if blood supply to the brain stem is compromised. Impairment of blood flow may result in headaches and loss of strength in the legs (Dutton and Riley 1969) or in serious syncopal attacks (Schmidek 1986). Even with the presence of uncinate and articular facet osteophytes, vascular compression is not common. In most cases, the neck must be in a certain position to inflict compression, particularly hyperextension, flexion or head rotation (Dutton and Riley 1969; Schmidek 1986).
Thoracic osteophytes
Anatomically, the spatial relationship of structures in the thorax in relation to the spinal column is important when analyzing morbidity associated with osteophytes of thoracic vertebrae. Most structures located in close proximity to the spinal column (esophagus, descending thoracic aorta and lungs) may be implicated in damage caused by osteophytes projecting into the thorax. Of note, the tracheal bifurcation at T4–T5 and the esophagus piercing the diaphragm at T10 are important anatomical landmarks for thoracic osteophyte morbidity. Similar to the cervical vertebrae, the majority of symptomatic cases in the literature deal with osteophytes present on lower vertebral bodies. As reported by O’Neill et al. (1999), 681 women and 499 men over 50 years of age have thoracic osteophytes most frequently on T9 and T10. Additionally, O’Neill et al. (1999) postulated that heavy physical activity particularly in young adult life is important in osteophyte development on thoracic vertebrae.
Osteophytes on the ninth and tenth thoracic vertebra have been associated with cases of dysphagia (Fig. 6). Cai et al. (2003) reported a 73-year-old man with dysphagia in the supine position, leading to osteophytes extrinsically compressing the esophagus. Anatomically, this may be explained by easier compressibility of the esophagus as it passes through the diaphragm at T10, as well as the weight of organs in the thorax fixating the esophagus onto osteophytes in the supine position. Esophageal compression leading to dysphagia in the thoracic region is not entirely restricted to T9–T10, as osteophyte compression as high as T4 has been reported (Cai et al. 2003; Willing and El Gammal 1983).
The sympathetic trunk and the descending thoracic aorta may also be affected by thoracic osteophytes. At the level of T8–T10, the sympathetic trunk and deviating splanchnic nerves may be involved, as Nathan (1987) reported that the right greater splanchnic nerve is compressed most frequently at this position. Additionally, in his study of 1,000 cadavers, Nathan (1987) observed compression of sympathetic structures in 655 specimens, noting that the sympathetic trunk is only affected by osteophytes at lower thoracic levels. Presumably, the sympathetic fibers on the left side of the vertebral column were less involved as a result of the descending thoracic aorta shielding the sympathetic trunk from potential osteophyte damage.
Chtata et al. (2005) reported a case of osteophytes causing trauma to the descending thoracic aorta. In their study, the tip of an osteophyte on T11–T12 pierced the aorta of a 50-year-old man following a head on car collision. They speculated that the osteophyte was not irritating the aorta until the impact of the car collision compressed the point of the osteophyte into the vessel wall, leading to the pseudoaneurysm. Recently, Dregelid et al. (2007) reported the first non-traumatic osteophyte-induced pseudoaneurysm occurring at the level of L1. The pseudoaneurysm occurred adjacent to the root of the superior mesenteric artery, due to perforation from a needle-sharp osteophyte.
Thoracic vertebrae osteophytes have also implicated the respiratory system. A case was reported in which an anterior thoracic osteophyte on T6–T7 obstructed the right main stem bronchus, resulting in chronic obstructive pneumonia (Leon et al. 2000). The patient, with a 120-pack-year history of cigarette smoking, had been coughing up purulent sputum for 3 years. Following removal of the osteophyte and antibiotic treatment, the obstruction diminished and the pneumonia dissipated. In another case, Otake et al. (2002) reported focal pulmonary interstitial opacities adjacent to thoracic spinal osteophytes. A CT scan displayed the opacities in the subpleural region of the lower lobe of the right lung, suggesting that mechanical compression of the osteophyte on the adjacent pulmonary tissue leads to a fibrotic reaction (Otake et al. 2002). In concordance with the observation that sympathetic trunk fibers were more frequently implicated on the right side, the descending thoracic aorta may protect adjacent structures from being affected by osteophytes, as the left lung was not fibrotic in this case (Nathan 1987).
Lumbar and sacral osteophytes
The spinal column’s decent into the lumbar region to form vertebral bodies L1–L5 is linked to a similar course followed by the inferior vena cava and abdominal aorta. The abdominal aorta emerges into the abdominal cavity at T12 through the aortic hiatus, borders the left anterior portion of the vertebral body until L4, at which time it bifurcates into the common iliac arteries. Furthermore, following the formation of the inferior vena cava at approximately L5, this vessel demonstrates a similar course as the abdominal aorta on the right side of the spinal column. A result of these structures occupying the anterior border of the lumbar vertebral bodies is the likelihood of complications due to anteriorly projecting osteophytes.
While the size and strength of the lumbar vertebra are important in load bearing, their functional requirements subject them to degenerative changes. In a study looking at the vertebral osteophytes in the elderly population, Cvijetic et al. (2000) found that 21.3% of men (n = 263) and 23.9% of women (n = 280) over 45 years of age had osteophytes on the lumbar spine. The observations of Pye et al. (2007) depicted that when the mean age is 65 years of age, 73% of men (n = 286) and women (n = 299) had evidence of lumbar osteophytes. In addition, they noted that with increasing severity of osteophyte formation, there was concomitant vertebral disc space narrowing, suggesting a potential relationship between worsening osteoarthritic changes and degree of osteophyte severity (Pye et al. 2007).
Similar to the etiology of thoracic vertebral osteophytes, lumbar vertebral osteophytes are a result of mechanical factors and heavy physical activity even from a young age (O’Neill et al. 1999) (Fig. 7). An interesting study on former elite track and field athletes demonstrated these two criteria. In their study, Schmitt et al. (2004) analyzed the lumbar vertebrae of 159 athletes looking for degenerative changes, namely the presence of osteophytes. They concluded that athletes in throwing disciplines, such as shot put and discus, and high-jumpers had a greater propensity for developing osteophytes in comparison to running athletes (Brandenberg and Leibrock 1986). Arguably, the increased rotation and load on the lower spine during these activities predisposes one to osteophyte formation.
Osteophyte formation occurs in conjunction with many other degenerative changes of the lumbar vertebrae. As mentioned (Pye et al. 2007), increasing osteophyte formation correlates with decreased disc space, which may also be associated with intervertebral disc calcification (Chanchairujira et al. 2004). Spinal stenosis is another degenerative change implicating the lumbar region, causing compression symptoms characteristic of the nerve root affected (Leon et al. 2000; Leroux et al. 1992). Thus, lumbar osteophytes may be seen as part of a general degenerative process accompanied by other degenerative changes occurring in the lumbar region.
A report from the Framingham Heart Study suggested that lumbar vertebral osteophytes may lead to complications involving the cardiovascular system. Karasik et al. (2006) analyzed lateral lumbar radiographs from 777 men and 1,241 women (age range 47–80 years old), comparing anterior lumbar osteophytes and abdominal aortic calcification. A correlation was found between these two variables, with a significant association in both men and women (Karasik et al. 2006). Additionally, lumbar osteophytes have been associated with inferior vena cava obstruction (Leon et al. 2000; Scapinelli 1997). The close proximity of the abdominal aorta and inferior vena cava to the spinal column is attributed to the pathological findings in such cases.
Osteophyte-induced nerve root compression is a common clinical scenario. The L5 nerve is occasionally compressed in the lumbosacral tunnel, consisting of the fifth lumbar vertebral body, the lumbosacral ligament and the sacral ala. In a 2002 study, Matsumoto et al. (2002) found 6 out of 7 cadavers with osteophytes on the vertebral bodies of L5 and S1, which entrapped the L5 nerve in the lumbosacral tunnel, compared to L5 compression in only 1 out of 22 cadavers without osteophytes. Interestingly, recent studies have shown a dual, bidirectional blood supply to the lumbar nerve roots (Gilchrist et al. 2002). This blood supply may allow provisional blood flow to a compressed nerve, particularly in the case of L5 compression in the lumbosacral tunnel. It is conceivable that cases of compressed L5 nerves are underreported as a result of the dual blood supply nourishing the nerve, with symptomatic cases being reported when blood supply is drastically diminished.
Treatment and conclusions
Vertebral osteophyte-induced complications of adjacent structures are a model for spatial anatomy. From the cervical to lumbar region, osteophytes growing from vertebral bodies directly influence the physiologic function of adjacent structures. Traditionally, the first line of treatment in symptom correction is a conservative approach, usually involving a non-steroidal anti-inflammatory drug to minimize the inflammatory reaction accompanying osteophyte formation. If a conservative treatment is unsuccessful, a surgical approach may be taken to access the osteophyte from an anterior or anterolateral direction, complimenting the most common direction of osteophyte orientation. In the majority of diagnosed cases, removal of the offending osteophyte will lead to resolution of the overlying symptoms (Uppal and Wheatley 1999).
A number of studies have used the anterior approach (Brandenberg and Leibrock 1986; Calisaneller et al. 2005; Hasegawa et al. 1984; Snodgrass 2004; Stevens et al. 1993) by excising the osteophytes and using a high-speed drill to smooth the surface of the vertebral body (Calisaneller et al. 2005). When osteophytes encompass C1–C2, a peri-oral-transpharyngeal approach is taken to access the vertebrae (Snodgrass 2004). Particularly when the osteophyte is present on maximal load-bearing vertebrae (C5–C6 and L1–L5), interbody fusion of the vertebrae is performed in order to decrease the probability of osteophytes recurring.
Compounding these factors with the obesity epidemic has lead to osteophyte formation secondary to osteoarthritis occurring more frequently, producing unique complications. Given that males and females show remarkably similar patterns of age-related changes in osteophyte development (Snodgrass 2004), osteophytes may eventually be considered part of the natural physiological aging process.
References
Aronowitz P, Cobarrubias F (2003) Anterior cervical osteophytes causing airway compromise. N Engl J Med 349:26
Aufdermauer M (1979) Bewegungsapparat. In: Buchner F, Grundmann E (eds) Lehrbuch der speziellen Pathologie, 6th edn. Urban & Schwarzenberg, München, pp 568–629
Barre JA (1924) Troubles pyramidaux et arthrite vertebrale chronique. Médecine 5:358
Bovim G, Schrader H, Sand T (1994) Neck pain in the general population. Spine 19:1307–1309
Brandenberg G, Leibrock LG (1986) Dysphagia and dysphonia secondary to anterior cervical osteophytes. Neurosurgery 18:90–93
Bridges PS (1994) Vertebral arthrits and physical activities in the prehistoric southeastern United States. Am J Phys Anthropol 92:83–93
Brown DE, Neumann RD (2004) Orthopedic secrets. In: Osteoarthritis, Chap I, 3rd edn. Elsevier, Philadelphia, pp 1–3
Cagnie B, Barbaix E, Vinck E, D’Herde K, Cambier D (2005) Extrinsic risk factors for compromised blood flow in the vertebral artery: anatomical observations of the transverse foramina from C3 to C7. Surg Radiol Anat 27:312–316
Cai FZJ, Rischmueller M, Pile K, Brady SJ (2003) Dysphagia associated with lower thoracic spondylosis. Rheumatology 42:1575–1576
Calisaneller T, Ozdemir O, Tosun E, Altinors N (2005) Dysphagia due to diffuse idiopathic skeletal hyperostosis. Acta Neurochir 147:1203–1206
Chanchairujira K, Chung CB, Kim JL, Papakonstantinou O, Lee MH, Clopton P, Resnick D (2004) Intervertebral disk calcification of the spine in an elderly population: radiographic prevalence, location, and distribution and correlation with spinal degeneration. Radiology 230:499–503
Chouret EE (1967) The greater occipital neuralgia headache. Headache 7:33–34
Chtata H, Koskas F, Cluzel P, Kieffer E (2005) Traumatic pseudoaneurysm of the descending thoracic aorta inflicted by a spinal osteophyte. Ann Vasc Surg 19:263–266
Cloward RB (1987) Spinal stenosis: treatment by posterior lumbar interbody fusion. Spine 3:457–516
Crowther A, Ardran GM (1985) Dysphagia due to cervical spondylosis. J Laryngol Otol 99:1167–1169
Cvijetic S, McCloskey E, Korsic M (2000) Vertebral osteophytosis and vertebral deformities in an elderly population sample. Wien Klin Wochenschr 112:407–412
Davies R, Sage MR, Brophy B (1989) Cervical osteophyte-induced dysphagia. Australas Radiol 33:3
Deutsch E, Schild J, Mafee M (1985) Dysphagia and Foresteir’s disease. Arch Otolaryngol 111:400–403
Di Vito J (1998) Cervical osteophytic dysphagia: single and combined mechanisms. Dysphagia 13:58–61
Dregelid E, Jenssen G, Jonung T, Braaten A (2007) Pseudoaneurysm of the abdominal aorta due to a needle-like osteophyte on the first lumbar vertebra. J Vasc Surg 45:1059–1061
Dutton CB, Riley LH (1969) Cervical migraine. Am J Med 47:141–148
Ebraheim N, Lu J, Hao Y, Biyani A, Yeasting RA (1997) Anatomic considerations for uncovertebral involvement in cervical spondylosis. Clin Orthop 334:200–206
Edmeads J (1978) Headaches and head pains associated with diseases of the cervical spine. Med Clin North Am 62:533–544
Fournier AM, Rathelot J (1959) L’arthrose atlanto-odontoidienne: Sa frequence comme cause d’algies nucales. J Radiol d’Electrol Med Nucl 22:812–813
Fuerderer S, Eysel-Gosepath K, Schroder U, Delank KS, Eysel P (2004) Retro-pharyngeal obstruction in association with osteophytes of the cervical spine. J Bone Joint Surg (Br) 86-B:837–840
Gilchrist RV, Slipman CW, Isaac Z, Lenrow DA, Chou LH (2002) Vascular supply to the lumbar spine: an intimate look at the lumbosacral nerve roots. Pain Phys 5:288–293
Goel R, Sampath P, Mikaelian DO (1999) Dysphagia caused by cervical osteophytes: three cases treated successfully by surgery. Otolaryngol Head Neck Surg 120:92–96
Hanamura H, Higuchi Y, Nakagawa M, Iwata H, Urist MR (1980) Solubilized bone morphogenetic protein (BMP) from mouse osteosarcoma and rat demineralized bone matrix. Clin Orthop 148:281–290
Hasegawa H, Bitoh S, Ohtsuki H, Obashi J, Furukawa H, Yamamoto T (1984) A case of Forestier’s disease causing dysphagia. No Shinkei Geka 12:1379–1383
Hilding DA, Tachdjian MO (1960) Dysphagia and hypertrophic spurring of the cervical spine. N Engl J Med 263:11–14
Idem K (1906) Ein zweiter Fall von Abkinckung des Speiserohre durch vertebrale Ekchondrose. Munch Med Wochenschr 53:906–907
Iglauer S (1938) A case of dysphagia due to an osteochondroma of the cervical spine–osteotomy–recovery. Ann Otol Rhinol Laryngol 47:799–803
Jackson R (1967) Headaches associated with disorders of the cervical spine. Headache 6:175–179
Kamieth H (1959) Ein nicht sicher einzuordnender Knochenkeil am Unterrand des Clivus. Fortshr Rontgenstr 91:334–339
Kanbay M, Selcuk H (2006) Dysphagia caused by cervical osteophytes: a rare case. J Am Geriatr 54:1147–1148
Karasik D, Kiel DP, Kiely DK, Cupples LA, Wilson PW, O’Donnell CJ, Felson DT (2006) Abdominal aortic calcification and exostoses at the hand and lumbar spine: the Framingham Study. Calcif Tissue Int 78:1–8
Knusel CJ, Goggel S, Lucy D (1997) Comparative degenerative joint disease of the vertebral column in the medieval monastic cemetery of the Gilbertine Priory of St. Andrew, Fishergate, York England. Am J Phys Anthropol 103:481–495
Kodama M, Sawada H, Udaka F, Kameyama M, Koyama T (1995) Dysphagia caused by an anterior cervical osteophyte: case report. Neuroradiology 37:58–59
Krogdahl T, Torgersen O (1940) Die “Unco-vertebral Gelenke” und die “Arthrosis Deformans Unco-vertebralis”. Acta Radiol 21:231
Lambert JR, Teppermann PS, Jimenez J, Newman A (1981) Cervical spine disease and dysphagia. Am J Gastroenterol 76:35–40
Leon JA, Calamia KT, Leventhal JP (2000) Chronic obstructive pneumonia caused by a vertebral body osteophyte. Mayo Clin Proc 75:185–188
Leroux JL, Legeron P, Moulinier L, Laroche M, Mazieres B, Blotman F, Arletet J (1992) Stenosis of the lumbar spinal cord in vertebral ankylosing hyperostosis. Spine 17:1213–1218
Matsumoto M, Chiba K, Nojiri K, Ishikawa M, Toyama Y, Nishikawa Y (2002) Extraforaminal entrapment of the fifth lumbar spinal nerve by osteophytes of the lumbosacral spine: anatomic study and a report of four cases. Spine 27:E169–E173
Meeks LW, Renshaw TS (1973) Vertebral osteophytosis and dysphagia. J Bone Joint Surg 55:197–201
Mosher HP (1926) Exostoses of the cervical osteophytes as a cause for difficulty in swallowing. Laryngoscope 36:181–182
Nathan H (1987) Osteophytes of the spine compressing the sympathetic trunk and splanchnic nerves in the thorax. Spine 12:527–532
Ng J, Gnanalingham KK, Stokes O, Singh A, Casey A (2005) Anterior cervico-thoracic osteophytes: an unusual cause of dysphagia. Br J Neurosurg 19:173–197
O’Neill TW, McCloskey EV, Kanis JA, Bhalla AK, Reeve J, Reid DM, Todd C, Woolf AD, Silman AJ (1999) The distribution, determinants, and clinical correlates of vertebral osteophytosis: a population based survey. J Rheumatol 26:842–848
Otake S, Takahashi M, Ishigaki T (2002) Focal pulmonary interstitial opacities adjacent to thoracic spine osteophytes. Am J Roentgenol 179:893–896
Pate D, Goobar J, Resnick D, Haghighi P, Sartoris DJ, Pathria MN (1988) Traction osteophytes of the lumbar spine: radiographic-pathologic correlation. Radiology 166:843–846
Pye SR, Reid DM, Lunt M, Adams JE, Silman AJ, O’Neill TW (2007) Lumbar disc degeneration: association between osteophytes, end-plate sclerosis and disc space narrowing. Ann Rheum Dis 66:330–333
Resnick DE, Shapiro RF, Wiesner KB, Niwayama G, Utsinger PD, Shaul SR (1978) Diffuse idiopathic skeletal hyperostosis (DISH). Semin Arthritis Rheum 17:153–187
Robinson RA, Walker AE, Ferlic DC, Wiecking DK (1962) The results of anterior interbody fusion of the cervical spine. J Bone Joint Surg A 44:1569–1587
Rosen HJ (1985) Dysphagia due to cervical spine osteophytes. Can Med Assoc J 133:15
Scapinelli R (1997) Compression of the inferior vena cava due to diffuse idiopathic skeletal hyperostosis. Rev Rhum Engl Ed 64:198–201
Schmidek HH (1986) Cervical spondylosis. Am Fam Phys 33:89–99
Schmitt H, Dubljanin E, Schneider S, Schiltenwolf M (2004) Radiographic changes in the lumbar spine in former elite athletes. Spine 29:2554–2559
Schmorl G, Junghanns H (1971) The human spine in health and disease, 2nd edn. Grune and Stratton, New York, pp 158–198
Seawright AA, English PB, Gartner RJW (1965) Hypervitaminosis A and hyperostosis of the cat. Nature (Lond) 206:1171–1172
Sheehan S, Bauer R, Meyer J (1960) Vertebral artery compression in cervical spondylosis. Arteriographic demonstration during life of vertebral artery insufficiency due to rotation and extension of the neck. Neurology 10:386–396
Snodgrass JJ (2004) Sex differences and aging of the vertebral column. J Forensic Sci 49:458–463
Sobol SM, Rigual NR (1984) Anterolateral extrapharyngeal approach for cervical osteophyte-induced dysphagia: literature review. Ann Otol Rhinol Laryngol 93:498–504
Sofaer-Derevenski JR (2000) Sex differences in activity-related osseous change in the spine and the gendered division of labor at Ensay and Wharram Percy, UK. Am J Phys Anthropol 111:333–354
Srinivas P, George J (1999) Cervical osteoarthropathy: an unusual cause of dysphagia. Age Aging 28:321–322
Stancampiano FF, Zavelta EG, Astor FC (2002) Anterior cervical osteophytes: a rare cause of dysphagia and upper airway obstruction in older patients. J Am Geriatr Soc 50:1910
Stevens JM, Clifton AG, Whitear P (1993) Appearances of posterior osteophytes after sound anterior interbody fusion in the cervical spine: a high definition computed myelographic study. Neuroradiology 35:227–228
Strasser G, Schima W, Schober E, Pokieser P, Kaider A, Denk DM (2000) Cervical osteophytes impinging on the pharynx: importance of size and concurrent disorders for development of aspiration. Am J Roentgenol 174:449–453
Stuart D (1989) Dysphagia due to cervical osteophytes. Int Orthop 13:95–99
Uppal S, Wheatley AH (1999) Transpharyngeal approach for the treatment of dysphagia due to Forestier’s disease. J Laryngol Otol 113:366–368
Urist MR, Mikulski A, Lietze A (1979) Solubilized and insolubilized bone morphogenetic protein (BMP). Proc Natl Acad Sci 76:1828–1832
von Ludinghausen M, Fahr M, Prescher A, Schindler G, Kenn W, Weiglein A, Yoshimura K, Kageyama I, Kobayashi K, Tsuchimochi M (2005) Accessory joints between basiocciput and atlas/axis in the median plane. Clin Anat 18:558–571
von Ludinghausen M, Prescher A, Kageya I, Yoshimura K (2006) The median atlanto-occipital joint in advanced age. Spine 31:E430–E436
Willing S, El Gammal T (1983) Thoracic osteophyte producing dysphagia in a case of diffuse idiopathic skeletal hypertrophy. Am J Gastroenterol 78:381–383
Yee C, Wong HY, Fewer HD, Rogers AG (1985) Two cases of dysphagia due to cervical spine osteophytes successfully treated surgically. Can Med Assoc J 132:810–812
Yoskovitch A, Kantor S (2001) Cervical osteophytes presenting as unilateral vocal fold paralysis and dysphagia. J Laryngol Otol 115:422–424
Yutan E, Daras M, Koppel BS (2001) Dysphagia due to cervical osteophytes. J Clin Imag 25:262–264
Zahn H (1905) Ein Fall von Abknickung der Speiserohre durch vertebrale Ekchondrose. Munch Med Wochenschr 52:1680–1682
Acknowledgments
The authors would like to thank Miss Kate Kryger for obtaining the pictures from the anatomy laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klaassen, Z., Tubbs, R.S., Apaydin, N. et al. Vertebral spinal osteophytes. Anat Sci Int 86, 1–9 (2011). https://doi.org/10.1007/s12565-010-0080-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-010-0080-8