Abstract
Today, advances in science and technology may contribute to the resolution of medical devices for pediatric. This research focused on the development of the InfaWrap device; a tool to monitor neonate's heart rate and SpO2. InfaWrap is designed to help the clinicians and parents to observe the baby's heart rate and oxygen saturation. The InfaWrap device uses a pro mini Arduino as a microcontroller, a MAX30100 oximeter sensor to measure SpO2 and heart rate, and an LM35 to measure body temperature. Besides, we focus on the design and convenience wear criteria, including design characteristics, and structures to ensure the device is lightweight and more comfortable. The proposed InfaWrap device embedded an advanced wireless network sensor system. The data will be appeared in the mobile application installed on the doctor's or parent's mobile phone via Bluetooth module. Overall, based on three different babies as a subject in this study, we obtained that the InfaWrap device accuracy results reach the average of 96% for SpO2, 81 bpm for baby heart rate, and 36.4 °C for baby body temperature.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Newborns are monitored for oxygen saturation from the first life until six months of age because of its possible adverse effects on brain development [1,2,3,4]. The American Academy of Pediatrics (AAP) and the American Heart Association (AHA) in 2010 [5] determined the SpO2 objectives for the first 10 min after birth, establishing the arterial oxygen saturation that a baby must-have in the first minutes after birth [5]. The SpO2 objectives table has assisted in the assessment of recovery and in avoiding the unnecessary administration of oxygen in newborns [5].
A study was performed in which 90% of babies with congenital cyanotic heart disease [6, 7] were detected using a pulse oximeter for use of screening within a few hours of birth. According to the evidence, infants with cyanotic heart disease is a critical sudden infant death syndrome (SIDS) may also be linked genetically to congenital coronary cyanotic diseases. It may be difficult to identify the major causes of SIDS [8, 9] most parents are making an extraordinary effort for their baby's health. By using the wireless healthcare technology, People who use sensing devices can move freely openly without being hindered by complicated wires [10, 11], and doctors at a remote care center can keep a close watch on the patient's health and thus offer patient recovery advice and long-term care in real time [12, 13].
This paper discusses the intelligent baby wrap model framework, InfaWrap, developed together with an accuracy test. One of the special aspects of the InfaWrap device features uses a wireless sensor to reduce clinicians and parents’ load. This system is assembled and bundled in a compact, micro and Android smartphone using Bluetooth connectivity. This device is often fitted with battery charging cards, so that it can be refilled without changing the battery once the power runs out. It is easy to use this measurement device by connecting one of the foot sole to the specified sensor, then the three parameters of the scale would conveniently show on the LCD and Android mobile.
2 Methodology
2.1 Design Specification
The InfaWrap prototypes were designed using SolidWorks 2019 software and then shaped directly using 3D printing technology. For convenient wear of the InfaWrap device, the structure's design consequently had to be smaller, more comfortable to wear, and user-friendly. InfaWrap device is designed to monitor SpO2, heart rate, and neonatal temperature through a cable-free wrapping concept.
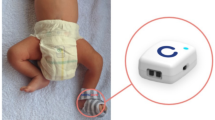
This device is designed to have a small screen to prevent too many electronic parts inside the device. InfaWrap device is so lightweight with maximum total weight is 44.36 g. Figure 1 shows the final product of the InfaWrap device. By using the Arduino board, all the measured values were monitored, analyzed, and displayed on the LCD screen. The results will be sent to an android application.
2.2 Device Components
Two sensors were used to measure three different conditions using this device. Figure 2 shows the complete system and electronic components of InfaWrap. The Arduino microcontroller is utilized to control the whole system. The SpO2 and heart rate are measured using the MAX30100 sensor. Meanwhile, for LM35 sensor is used to measure of temperature [14, 15]. The HC-05 Bluetooth module is used to transfers measurement data from device to MyI-Wrap application or mobile app.
Figure 3b shows the development of the MyI-Wrap mobile app, which allows parents or doctors to monitor their baby's health status from anywhere, in a quick and efficient manner. This software is available free through Google Play. Parents may also monitor their children's health while they sleep with this MyI-Wrap. As illustrated in Fig. 3a, the monitoring process is split into two steps; (1) data from the sensor was gathered; (2) data will be shown on the device and mobile phone via Bluetooth connection [16].
3 Results and Discussions
The InfaWrap device is specifically designed for newborns to assist clinicians and parents in monitoring their baby's heart rate, SpO2 and temperature. Besides, this device needs several criteria requirements, such as small [17], light-weight, ergonomics, and low power consumption (or long battery life) that shows in Fig. 4. This section discusses the functionality of the InfaWrap device [18], standard operation how to wear the InfaWrap, and the sensor's accuracy based on three participants with differences in demographic data [19].
3.1 Accuracy Test
The participants involved in this study were two baby girls and one baby boy. All of them are the Malaysian citizens. The participants were volunteer for their contribution. Details about the participants as shows in Table 1.
A reliability test is conducted to measure the InfaWrap device performances by repeated the measurement in 10 times. Table 2 shows the heart rate values obtained in 10 min’ duration. Figure 5 clearly shows the trend of heart rate in 10 min reading. In the starting evaluation, subject 3 shows higher in heart rate. This is due to the situation where this baby a bit afraid and he cried during wearing the InfaWrap device.
Table 3 shows the SpO2 of the babies in 10-time reading. Figure 6 shows the normal percentage of the SpO2 for the baby is around 98–100%. We observed the trend of the SpO2 for the three babies are good and acceptable which is around 97–99% [20]. For the resuscitation, stabilization and continuing treatment of the extremely low birth weight baby the optimum oxygen saturation values remain mostly undefined. We examined existing evidence for the usage of clinical oxygen in newborns. Median SpO2 in babies delivered vaginally was 3% greater than for those born in the first 10 min of life in the previous research than in infants born with cesarean delivery. Term infants had considerably greater saturation and saturation than preterm infants amounted to 90% quicker namely 4.7 min versus 6.5 min.
Table 4 shows the temperature level of the baby. Temperature is essentially important to know the condition of the baby especially during daily activities. Figure 7 shows the three babies’ healthy condition without fever. The temperature value also presented good in detected the baby body temperature. A normal temperature for your baby is defined as a rectal reading between 36.5 and 37.0 °C; a temperature of 37.7 °C or higher is called a fever.
When a baby's temperature is abnormally high, it can indicate infection and it is better to meet a pediatrician, particularly if other symptoms such as a stuffy nose, sore throat, or cough continue. Based on the observation of the heart rate, SpO2 and temperature values, the InfaWrap device's performance shows good and acceptable value especially in the accuracy of the data analysis.
4 Conclusion
This paper presented the product of the InfaWrap device that has successfully developed. The studies were focused on the functionality of the InfaWrap device, standard operation of wearing the InfaWrap device, and the sensor's accuracy based on three participants with differences in demographic data. The InfaWrap device is designed to monitor oxygen saturation SpO2, heart rate, and temperature neonatal through a cable-free wrapping concept. Besides the measured value, it can analyze the health status for warning indicators are sent through mobile applications via Bluetooth to be stored and shared with the parents and clinicians. Besides, the InfaWrap device also has been developed to provide patients with the necessary support.
References
Saugstad, O.: Oxygen saturations immediately after birth. J. Pediatr. 148, 569–570 (2006)
Dawson, J.A., Davis, P.G., O’Donnell, C.F., Kamlin, C.O., Morley, C.J.: Pulse oximetry for monitoring infants in the delivery room: a review. Arch. Dis. Child Fetal Neonatal Ed. 92, F4–F7 (2007)
Kamlin, O., Colm, P., O’Donnell, C., Davis, P.G., Morley, C.J.: Oxygen saturation in healthy infants immediately after birth. J. Pediatr. 148, 585–589 (2006)
Toth, B., Becker, A., Seelbach-Gobel, B.: Oxygen saturation in healthy newborn infants immediately after birth measured by pulse oximetry. Arch. Gynecol. Obstet. 266, 105–107 (2002)
Kattwinkel, J., Perlmann, J.M., Aziz, K.: Neonatal “Resuscitation 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 122, S909–S919 (2010)
Limaye, D.: Development of medical devices. Curr. Ther. Res. 78, S9 (2016)
Mathur, N.B., Gupta, A., Kurien, S.: Pulse oximetry screening to detect cyanotic congenital heart disease in sick neonates in a neonatal intensive care unit. Indian Pediatr. 52(9) (2015)
Jortveit, J., et al.: Sudden unexpected death in children with congenital heart defects. Eur. Heart J. 37(7), 621–626 (2016). https://doi.org/10.1093/eurheartj/ehv478
Miladinia, M., Baraz, S., Nouri, E.M.: Sudden infant death syndrome: risk factors and the relationship between them. Int. J. Pediatr. 3(6), 1103–1110 (2015)
Sola, A., et al.: Safe oxygen saturation targeting and monitoring in preterm infants: can we avoid hypoxia and hyperoxia? Acta Paediatr. Int. J. Paediatr. 103(10), 1009–1018 (2014). https://doi.org/10.1111/apa.12692
Seifi, S., Khatony, A., Moradi, G., Abdi, A., Najafi, F.: Accuracy of pulse oximetry in detection of oxygen saturation in patients admitted to the intensive care unit of heart surgery: comparison of finger, toe, forehead and earlobe probes. BMC Nurs. 1–7 (2018)
Chen, B., Varkey, J.P., Pompili, D., Li, J.K., Marsic, I.: Patient vital signs monitoring using wireless body area networks. In: Proceedings of the 2010 IEEE 36th Annual Northeast Bioengineering Conference (NEBEC) 2010, April 2010. https://doi.org/10.1109/NEBC.2010.5458139
Ajith, S., Praveen, K., Ibrahim, S.I., Nagaraj, M.: Wireless monitoring of patients by IoT through pulse-OX & heart rate sensor. Int. J. Pure Appl. Math. 119(15), 973–979 (2018)
Adib, M.A.H.M., Rashid, M.A.A., Hasni, N.H.M.: Development of smart Infant-Wrap (InfaWrap) device for neonates. Proc. Mech. Eng. Res. Day 2019, 186–187 (2019)
Suprayitno, E.A., Marlianto, M.R., Mauliana, M.I.: Measurement device for detecting oxygen saturation in blood, heart rate, and temperature of human body. J. Phys. Conf. Ser. 1402(3), 6 (2019). https://doi.org/10.1088/1742-6596/1402/3/033110
Nakib Ul Hasan, M., Negulescu, I.I.: Wearable technology for baby monitoring: a review. J. Text. Eng. Fash. Technol. 6(4), 112–120 (2020). https://doi.org/10.15406/jteft.2020.06.00239
Rahim, M.H.A., Adib, M.A.H.M., Hasni, N.H.M.: The comprehensive study of product criteria on Infant-Wrap (InfaWrap) device: engineering perspective. J. Phys. Conf. Ser. 1529(5) (2020). https://doi.org/10.1088/1742-6596/1529/5/052082
Dangerfield, M.I., Ward, K., Davidson, L., Adamian, M.: Initial experience and usage patterns with the owlet smart sock monitor in 47,495 newborns. Glob. Pediatr. Health 4, 2333794X1774275 (2017). https://doi.org/10.1177/2333794x17742751
Rahim, M.H.A., Adib, M.A.H.M., Baharom, M.Z., Hasni, N.H.M.: Improving the Infant-Wrap (InfaWrap) device for neonates using MyI-Wrap mobile application. In: The 3rd Symposium on Intelligent Manufacturing & Mechatronics (SIMM 2020). The Lecture Notes in Mechanical Engineering (2020)
Rahim, M.H.A., Adib, M.A.H.M., Baharom, M.Z., Sahat, I.M., Hasni, N.H.M.: Non-invasive study: monitoring the heart rate and SpO2 of the new born using InfaWrap device. In: 2020 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), 2021, pp. 212–217.https://doi.org/10.1109/IECBES48179.2021.9398749
Acknowledgements
Thank you to Universiti Malaysia Pahang (UMP) and Medical Engineering & Health Intervention Team (MedEHiT) for providing us with excellent facilities to complete these research activities under grant PDU203205 and PGR2003200.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this paper
Cite this paper
Rahim, M.H.A., Adib, M.A.H.M., Baharom, M.Z., Hasni, N.H.M. (2022). Infant-Wrap (InfaWrap) Device as Pediatric Technology Tool: The Heart Rate and SpO2 Monitoring for Neonates. In: Usman, J., Liew, Y.M., Ahmad, M.Y., Ibrahim, F. (eds) 6th Kuala Lumpur International Conference on Biomedical Engineering 2021. BIOMED 2021. IFMBE Proceedings, vol 86 . Springer, Cham. https://doi.org/10.1007/978-3-030-90724-2_36
Download citation
DOI: https://doi.org/10.1007/978-3-030-90724-2_36
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-90723-5
Online ISBN: 978-3-030-90724-2
eBook Packages: EngineeringEngineering (R0)