Abstract
The care of a tracheostomy patient spans a continuum of time beginning with the inpatient setting and often continues to wean from the mechanical ventilator, downsizing of the device, and eventual decannulation. Care is provided by distinct members of the healthcare team at various stages. However, the healthcare provider-whether an intensivist or a surgeon-act as leader of the team, and their expertise will be sought, and their input valued. Hence, an understanding of the aspects of tracheostomies and tracheostomy care is encouraged. There is a plethora of published guidelines on tracheostomies and tracheostomy care; however, most are based on anecdotes and retrospective studies with a dearth of moderate to high-quality trials on which to rely.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Tracheostomy placement is an important step for ventilator weaning in those with prolonged mechanical ventilation needs. Like other invasive procedures and chronic tubes, care must be taken to ensure delivery of the intended benefits and to guard against complications from such a chronic foreign body. The presence of the tracheostomy assists weaning from the mechanical ventilator and potentially allows those who need life-long mechanical ventilation to live at home. However, the chronicity of the foreign tube could lead to stomal injury, tracheal stenosis, hemoptysis, and tracheal infections.
In this chapter, we discuss the care of the tracheostomy tube in the immediate post-placement period, weaning, speaking valve placement, capping trials, decannulation, and chronic long-term care of the tracheostomy tube for those who are dependent on one.
2 Tracheostomy Care
The procedure for insertion of a tracheostomy tube is discussed in detail in chapter Airway Access.
It is worth noting that tracheostomy tubes have different characteristics (Table 1). They are available in assorted sizes, based on either the International Standard Organization (ISO) or Jackson sizing. Each size has a different internal diameter (ID) and outer diameter (OD) with varying lengths (specific lengths and diameters of the same size may also differ from company to company). It is very important to familiarize one’s self with the products available to him or her, given the difference in measurements between manufacturers. A tracheostomy tube could come as a single- or dual-cannula tube depending on the presence of the inner cannula (Fig. 1). Most tracheostomy tubes come with a dual cannula which allows for seamless cleaning and care of the tube. This minimizes tube clogging by allowing the removal of the inner cannula when needed. Tracheostomy tubes made from silicone often come as a single cannula tube given the inherent resistance of silicone to microbial growth. The tracheostomy tube shaft could be angled or curved as it enters the airway. The curvature should allow for minimal pressure to be placed on the posterior wall of the trachea. Most tracheostomy tubes used are non-fenestrated. The presence of a single, or multiple openings along the shaft’s angulation or curvature makes the tube a fenestrated tube. Fenestrated tracheostomy tubes are designed to channel more airflow to the larynx and vocal cords and hasten phonation when the cuff is deflated and a speaking valve is present. However, given the location of the fenestrations, they are often occluded by the posterior wall of the trachea and could lead to granulation tissue formation and the potential for tracheal stenosis. The tracheostomy tube comes in standard lengths. However, an extended length (XLT) tracheostomy tube is also commercially available. The extended length portion of the tube could either be proximal (i.e., horizontal) or distal (i.e., vertical). The proximal XLT tracheostomy tubes are often useful in obese patients since they take into consideration increased soft tissue mass at the neck. The distal XLT may be helpful in patients when the distal end of the tracheostomy tube is rocking against the tracheal wall as it angles into the airway. The distal extra-length tube also allows it to be parallel to the tracheal lumen and minimizes obstruction. Additionally, custom-made tracheostomy tubes are an option offered by most manufacturers for those who require specific sizes and/or lengths.
The presence of a ‘balloon’ cuff differentiates a cuffed tracheostomy tube from a cuffless one. Cuffless tubes traditionally have a better profile and are preferred for patients who are no longer dependent on ventilatory support and have progressed to a speaking valve or capping trial. The balloon cuff is often filled with air, although some manufacturers recommend water. The most frequent type of cuff is a high-volume, low-pressure cuff. This allows it to conform to the airway diameter without significant transluminal pressure; thus, mitigating the risk of tracheal mucosal ischemia and injury. However, low-volume, high-pressure cuffs are also available, known as tight-to-shaft cuffed tubes. The slim profile, without the significant ridges associated with traditional tracheostomy cuffs when deflated, encourages the passage of air around the tracheostomy tube. This provides an enhanced opportunity for phonation and is intended for patients who require infrequent cuff inflation and mechanical ventilatory support. Foam cuffed tracheostomy tubes are also marketed. Made of polyurethane foam covered with a silicone sheath, they have a high residual volume and hence tend to conform to the airway better. The pilot balloon (cuff) is deflated during insertion then opened to ambient air once it is placed into the airway. The balloon expands and contracts with the respiratory cycle which promotes a favorable seal to the tracheal wall. It is important to place the correct-sized foam-cuffed tracheostomy tube as too small of the tube may not provide an adequate seal.
Tracheostomy tubes are manufactured from a variety of materials including stainless steel, sterling silver, silicone, polyvinyl chloride (PVC), and polyurethane. Metal tracheostomy tubes have a slim profile and are resistant to microbial growth. They are also easy to clean and sterilize if needed. However, they lack a balloon cuff and the 15-mm connector to attach to the mechanical ventilator. Non-metal tracheostomy tubes are softer and tend to better conform to the patient’s anatomy. However, despite the resistance to biofilm formation, their presence in the trachea leads to surface changes and degradation of the polymeric chain.
The obturator (Fig. 2), which accompanies all types of tracheostomy tubes, facilitates insertion. They should be used during tracheostomy changes and be readily accessible-preferably at the head of the bed in hospitalized patients.
3 Post-placement Care
Immediately after tracheostomy placement, the cuff should be inflated, and mechanical ventilation continued. The tracheostomy tube is secured with commercially available tracheostomy ties, most of which are made of cushioned material and fastened to each side of the tracheostomy flange with Velcro. Suturing the tracheostomy to the skin is optional in the author’s experience. To guard against pressure injury or ulceration, padded foam is routinely placed between the tracheostomy flange and the sternal notch. A pressure injury is most likely encountered at that location. Pre-cut drain sponges suffice in patients with longer neck anatomy. However, a heavier padded barrier might be helpful with obese patients and those with shorter necks.
The tracheostomy tube size and type should be documented, and the tubes kept clean. Dual- cannula tubes come with an inner cannula that allows for cleaning and ensures the patency of the artificial airway. Inner cannulas may be either disposable or non-disposable. Routine daily changing of the disposable cannula is standard. For non-disposable cannulas, two inner cannulas work better than one. The usable non-disposable cannula is removed and replaced with the second, pre-cleaned non-disposable cannula. This process allows for easy cleaning of the recently removed equipment in preparation for the next change.
For tracheostomy tubes without inner cannulas, care must be taken to educate personnel on removing and replacing the entire tracheostomy tube in case of obstruction. Regardless of the presence or absence of an inner cannula, a replacement tracheostomy tube of the same brand and size, and possibly one sized smaller, should be available for immediate use.
Skin in the peristomal area should be kept dry and clean to decrease the risk of maceration and infection. The peristomal site should be evaluated for signs of redness, tenderness, and firmness. It is equally important to evaluate for signs of pressure injury, especially under the flange. Adequate lighting is an important prerequisite when providing tracheostomy tube care.
If the tracheostomy tube is cuffed, pressure in the pilot balloon should be evaluated when the patient is receiving mechanical ventilation. Cuff pressure should be kept between 20–25 mmHg when inflated to guard against tracheal mucosal ischemia and injury. Pressures over 30 mmHg should be documented and reported to the team. High cuff pressure requirements to maintain a seal may indicate a less than adequate tracheostomy tube size and the tube should be considered for an exchange. For patients with cuffed tracheostomy tubes undergoing speaking valve or capping trials, the importance of fully deflating the cuff before these trials can’t be overstated as significant morbidity and even death have been reported. When a cuffed tube is no longer required changing to a cuffless one should be considered.
Adequate humidification of the tracheostomy tube and the distal airway is also essential. Since air flows in and out of the tracheostomy tube, normal anatomic structures that provide heat and humidity are bypassed. Thick, tenacious secretions may slowly accumulate that increase the risk of clogging the tube with inspissated respiratory secretions. Heated (32–34 °C) and humidified (100% relative humidity) air or oxygen-enriched gas can be delivered using a heated humidifier (Fig. 3a, b) or a disposable heat moisture exchanger (Fig. 4), ensuring adequate secretion management and patient comfort.
The tracheostomy tube is designed to be suctioned as needed although scheduled suctioning is usually unnecessary. Suction catheters should be advanced to the tip of the tracheostomy tube before applying suction. Deeper suctioning practices are associated with mucosal injury of the trachea and distal bronchi. Some reports showed that repeated suctioning was found to decrease the rate of tube clogging in the general ward. Despite this, there is a body of evidence showing repeated suctioning or deep suctioning may lead to tracheitis. Additionally, the routine use of saline bullets to aid in thinning respiratory secretions may lead to hypoxia and could dislodge secretions and microbes from the tracheostomy tube and displace them into the lower respiratory tract.
Protocolized tracheostomy care and specialized healthcare teams to manage inpatient tracheostomy patients are imperative. Studies evaluating the implementation of protocolized care sets have shown a decrease in median time to evaluation by occupational, physical, and speech therapy, and a decrease in tracheostomy days and length of stay. Minimizing the time to decannulation also resulted in shorter hospital stays. Multidisciplinary teams led to fewer tracheostomy-related complications and higher use of speaking valves. One could postulate that combining those two strategies is essential for improving patient outcomes.
The team must beware that accidental tube dislodgement can occur at any point of the care continuum. Management depends on the timing of the event and the maturity state of the stomal tract. If the tract is mature, replacing the tracheostomy tube can usually be done promptly. The presence of a second provider or a member of the healthcare team, if available, is favored. In the case of a less mature tract, replacement of the dislodged tracheostomy may still be attempted. However, if this step proves difficult or there are questions regarding proper tube placement and the resting position of the distal tip of the tube, it is usually safer to proceed with trans laryngeal intubation to secure the airway. A thorough, investigative evaluation of the stoma could then be performed. Colorimetric capnometry and end-tidal CO2 capnography are helpful adjuncts to ensure adequate placement.
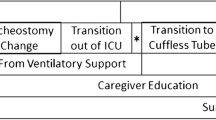
4 Tracheostomy Weaning to Decannulation
4.1 Downsizing
If the patient is successfully weaned from the mechanical ventilator and no longer requires ventilatory support, the determination to continue care with a life-long tracheostomy tube becomes the providers’ decision. This ultimately depends on the patient’s condition, underlying clinical diagnosis, and whether the clinical indication for placing the tracheostomy tube still exists.
If it is determined that the tracheostomy tube is not going to be needed for the ongoing treatment of the patient, and the patient has successfully been liberated from the ventilator, consideration to ‘downsize’ the tracheostomy tube is a strategy that is routinely employed. Downsizing is commonly the first step in the process of weaning from the tracheostomy tube and is performed before decannulation. For downsizing, it is customary to decrease the size of the airway, one step at a time, e.g. if the patient has a size 8.0 tracheostomy tube, downsizing to a 6.0 would be considered standard. Whether it is necessary to downsize further before decannulation is another decision made by the provider. If it is determined that further downsizing is appropriate, the final step would customarily be to change to a 4.0 tracheostomy tube. The 4.0 is generally considered to be the smallest adult-sized airway (in the author’s experience, the decision to downsize to a #4.0 tracheostomy tube is mostly unnecessary, and decannulation can be considered when a patient is tolerating a size 6.0 tracheostomy tube). After successful downsizing, speaking valve and/or capping trials are often helpful in determining whether decannulation is likely to be successful.
4.2 Passy-Muir Valve or Speaking Valve
David Muir is the inventor of the Passy-Muir speaking valve (Fig. 5). As a muscular dystrophy patient with quadriplegia, David suffered a respiratory arrest and endured a prolonged course before he eventually underwent a tracheostomy placement procedure. His frustration with the inability to communicate after the tracheostomy prompted him to invent the one-way ‘speaking’ valve. The Passy-Muir (P-M) valve is a device attached to the 15-mm connector on the protruding end of the tracheostomy tube. During inspiration, the valve opens and air (or oxygen-enriched gas if needed) enters through the tracheostomy tube into the tracheobronchial tree. During exhalation, however, the valve closes, and the exhaled air exits through the larynx, oropharynx, and nasopharynx. The movement of exhaled air is what allows phonation to take place. Added benefits of the P-M valve include the potential to increase end-expiratory pressure and improve swallowing and the sense of smell. But more importantly, it aids with weaning while allowing for phonation and may hasten decannulation.
The use of a P-M valve is recommended and encouraged after being liberated from mechanical ventilation. Speaking valve trials are customarily performed under supervision, especially with the initial placement. This can be done by the speech and language pathologist and/or by a respiratory therapist. Certain criteria should be met before this process is left to nursing staff to supervise. When first attempted, the patient must be awake. Patient position is not necessarily important-whether laying down or sitting up-however, in the author’s opinion, the head of the bed should be elevated at least 30° for the initial trials. Communicating with the patient on what to expect once the P-M valve is applied helps alleviate the anxiety that often accompanies its’ placement since the initial sensation is often overwhelming. Once the valve is applied, the patient’s condition is monitored for signs of respiratory distress. It is our practice to ensure that the patient is awake and following commands and can personally remove the speaking valve or call for assistance if respiratory distress develops before placement trials are transitioned to nursing staff supervision only.
The duration of the initial speaking valve trials depends on multiple factors, usually related to the patient, e.g., lack of active respiratory infections and/or the presence of manageable secretions, length of mechanical respiratory support, and the ability to move the upper extremities without limitations (patient overall strength).
As part of the weaning process to eventual decannulation, tolerating the P-M valve trials is essential.
4.3 Capping Trials
An alternative to a P-M valve, capping the tracheostomy also simulates decannulation. The device used is called a tracheostomy cap or a tracheostomy plug and is usually packaged along with the cuffless tracheostomy tube (Fig. 6). However, unlike a P-M valve, capping the tube directs air movement in and out of the respiratory tract during both inhalation and expiration exclusively through the normal anatomical pathway. As such, the patient is less dependent on the tracheostomy, but with a higher imposed work of breathing due to the increase in resistance encountered by the presence of the tracheostomy tube within the lumen of the airway.
Ideally, if the patient is tolerating the smallest desired tracheostomy tube, capping trials would be initiated after successful use of a P-M valve. However, at institutions without access to a P-M valve, capping trials can begin in the place of the valve. The duration of capping trials depends on patient tolerance. As with the P-M valve, successful capping trials depend on patient strength, manageable secretions, and the absence of uncontrolled respiratory infections. If the patient is tolerant of the initial trial, a full day of capping could be attempted.
Once the patient tolerates a 48-hour continuous capping trial, the patient is considered eligible for decannulation in the author’s practice and experience.
4.4 Decannulation
Decannulation, the process of removing the tracheostomy tube, is a rather simple procedure and may be performed in the inpatient or outpatient setting.
At the time of decannulation, the patient is placed in the semi-recumbent position, with the head of the bed elevated to at least 30°. The neck is kept slightly hyperextended. Occasionally, a sitting position with neck hyperextension is preferred. The tracheostomy tube is then removed completely (for cuffed tubes, ensure the cuff is fully deflated before removal). The stoma site is covered with gauze and a pressure dressing. The patient should be instructed to finger-occlude the stoma site whenever she or he phonates. This minimizes air movement through the stoma. The stoma will normally close within 5–7 days of decannulation. If it fails to close within 10–14 days, surgical approximation and closure may be necessary.
Long-term Care
There are patients, who will require a tracheostomy tube indefinitely. Those patients and their caregivers often experience a great deal of anxiety regarding the presence of a tracheostomy tube and its care. Adequate education before discharge from the hospital is crucial to alleviate any associated distress. To ascertain adequate learning of the required steps in tracheostomy care, patients or their caregivers should be taught about the device and then be able to demonstrate proper care, cleaning, and changing of the tracheostomy tube. Repeated evaluation and demonstration of their technique at set intervals ensures adequate retention and solidifies their competence. Formal education for family and caregivers has led to a decrease in the anxiety associated with tracheostomy care as well as the rate of hospital readmissions.
Tracheostomy tubes should be changed as per manufacturer recommendations and not be kept in place longer than suggested. Scanning electron microscopy images of some silicone, PVC, and polyurethane tracheostomy tubes demonstrate evidence of surface changes and degradation of the polymeric chains in as early as 30 days. The risk of infection also increases at 3 months.
Guidelines
Helpful consensus statements and guidelines are published from two, well-recognized professional organizations: The American Academy of Otolaryngology – Head and Neck Surgery and The American Association for Respiratory Care.
We encourage providers taking care of tracheostomy patients to review those regularly.
-
Clinical Consensus Statement: Tracheostomy Care.
-
AARC Clinical Practice Guidelines: Management of Adult Patients with Tracheostomy in the Acute Care Setting.
Bibliography
Perry A, Mallah MD, Cunningham KW, Christmas AB, Marrero JJ, Gombar MA, et al. PATHway to success: Implementation of a multiprofessional acute trauma health care team decreased length of stay and cost in patients with neurological injury requiring tracheostomy. J Trauma Acute Care Surg. 2020;88(1):176–9.
Masood MM, Farquhar DR, Biancaniello C, Hackman TG. Association of standardized tracheostomy care protocol implementation and reinforcement with the prevention of life-threatening respiratory events. JAMA Otolaryngol Head Neck Surg. 2018;144(6):527–32.
Jung YJ, Kim Y, Kyoung K, Keum M, Kim T, Ma DS, et al. The effect of systematic approach to tracheostomy care in patients transferred from the surgical intensive care unit to general ward. Acute Crit Care. 2018;33(4):252–9.
O'Toole TR, Jacobs N, Hondorp B, Crawford L, Boudreau LR, Jeffe J, et al. Prevention of tracheostomy-related hospital-acquired pressure ulcers. Otolaryngol Head Neck Surg. 2017;156(4):642–51.
Mah JW, Staff II, Fisher SR, Butler KL. Improving decannulation and swallowing function: a comprehensive, multidisciplinary approach to post-tracheostomy care. Respir Care. 2017;62(2):137–43.
Sutt AL, Caruana LR, Dunster KR, Cornwell PL, Anstey CM, Fraser JF. Speaking valves in tracheostomised ICU patients weaning off mechanical ventilation--do they facilitate lung recruitment? Crit Care. 2016;20:91.
Colandrea M, Eckardt P. Improving tracheostomy care delivery: instituting clinical care pathways and nursing education to improve patient outcomes. ORL Head Neck Nurs. 2016;34(1):7–16.
Sutt AL, Cornwell P, Mullany D, Kinneally T, Fraser JF. The use of tracheostomy speaking valves in mechanically ventilated patients results in improved communication and does not prolong ventilation time in cardiothoracic intensive care unit patients. J Crit Care. 2015;30(3):491–4.
Schreiber ML. Tracheostomy: site care, suctioning, and readiness. Medsurg Nurs. 2015;24(2):121–4.
Morris LL, McIntosh E, Whitmer A. The importance of tracheostomy progression in the intensive care unit. Crit Care Nurse. 2014;34(1):40–8; quiz 50.
Hess DR, Altobelli NP. Tracheostomy tubes. Respir Care. 2014;59(6):956–71; discussion 71-3.
Morris LL, Whitmer A, McIntosh E. Tracheostomy care and complications in the intensive care unit. Crit Care Nurse. 2013;33(5):18–30.
Mitchell RB, Hussey HM, Setzen G, Jacobs IN, Nussenbaum B, Dawson C, et al. Clinical consensus statement: tracheostomy care. Otolaryngol Head Neck Surg. 2013;148(1):6–20.
de Mestral C, Iqbal S, Fong N, LeBlanc J, Fata P, Razek T, et al. Impact of a specialized multidisciplinary tracheostomy team on tracheostomy care in critically ill patients. Can J Surg. 2011;54(3):167–72.
Cetto R, Arora A, Hettige R, Nel M, Benjamin L, Gomez CM, et al. Improving tracheostomy care: a prospective study of the multidisciplinary approach. Clin Otolaryngol. 2011;36(5):482–8.
Paul F. Tracheostomy care and management in general wards and community settings: literature review. Nurs Crit Care. 2010;15(2):76–85.
Parker V, Giles M, Shylan G, Austin N, Smith K, Morison J, et al. Tracheostomy management in acute care facilities–a matter of teamwork. J Clin Nurs. 2010;19(9–10):1275–83.
Garrubba M, Turner T, Grieveson C. Multidisciplinary care for tracheostomy patients: a systematic review. Crit Care. 2009;13(6):R177.
Backman S, Bjorling G, Johansson UB, Lysdahl M, Markstrom A, Schedin U, et al. Material wear of polymeric tracheostomy tubes: a six-month study. Laryngoscope. 2009;119(4):657–64.
Dennis-Rouse MD, Davidson JE. An evidence-based evaluation of tracheostomy care practices. Crit Care Nurs Q. 2008;31(2):150–60.
Arora A, Hettige R, Ifeacho S, Narula A. Driving standards in tracheostomy care: a preliminary communication of the St Mary’s ENT-led multi disciplinary team approach. Clin Otolaryngol. 2008;33(6):596–9.
Bjorling G, Axelsson S, Johansson UB, Lysdahl M, Markstrom A, Schedin U, et al. Clinical use and material wear of polymeric tracheostomy tubes. Laryngoscope. 2007;117(9):1552–9.
Eber E, Oberwaldner B. Tracheostomy care in the hospital. Paediatr Respir Rev. 2006;7(3):175–84.
Dhand R, Johnson JC. Care of the chronic tracheostomy. Respir Care. 2006;51(9):984–1001; discussion 2–4.
Ackerman MH, Mick DJ. Instillation of normal saline before suctioning in patients with pulmonary infections: a prospective randomized controlled trial. Am J Crit Care. 1998;7(4):261–6.
Hagler DA, Traver GA. Endotracheal saline and suction catheters: sources of lower airway contamination. Am J Crit Care. 1994;3(6):444–7.
Kleiber C, Krutzfield N, Rose EF. Acute histologic changes in the tracheobronchial tree associated with different suction catheter insertion techniques. Heart Lung. 1988;17(1):10–4.
Martin KA, Cole TDK, Percha CM, Asanuma N, Mattare K, Hager DN, et al. Standard versus accelerated speaking valve placement after percutaneous tracheostomy: a randomized-controlled feasibility study. Ann Am Thorac Soc. 2021;18(10):1693.
Heimer J, Eggert S, Fliss B, Meixner E. Fatal bilateral pneumothorax and generalized emphysema following contraindicated speaking-valve application. Forensic Sci Med Pathol. 2019;15(2):239–42.
Barraza GY, Fernandez C, Halaby C, Ambrosio S, Simpser EF, Pirzada MB, et al. The safety of tracheostomy speaking valve use during sleep in children: a pilot study. Am J Otolaryngol. 2014;35(5):636–40.
Selleng S, Antal M, Hansen T, Meissner K, Usichenko TI. Pneumothorax and cardiac arrest caused by speaking valve mistaken as moisture exchanger: an incident report. Br J Anaesth. 2013;111(2):297–8.
Elpern EH, Borkgren Okonek M, Bacon M, Gerstung C, Skrzynski M. Effect of the Passy-Muir tracheostomy speaking valve on pulmonary aspiration in adults. Heart Lung. 2000;29(4):287–93.
Leder SB. Effect of a one-way tracheotomy speaking valve on the incidence of aspiration in previously aspirating patients with tracheotomy. Dysphagia. 1999;14(2):73–7.
Lichtman SW, Birnbaum IL, Sanfilippo MR, Pellicone JT, Damon WJ, King ML. Effect of a tracheostomy speaking valve on secretions, arterial oxygenation, and olfaction: a quantitative evaluation. J Speech Hear Res. 1995;38(3):549–55.
Passy V, Baydur A, Prentice W, Darnell-Neal R. Passy-Muir tracheostomy speaking valve on ventilator-dependent patients. Laryngoscope. 1993;103(6):653–8.
Lewarski JS. Long-term care of the patient with a tracheostomy. Respir Care. 2005;50(4):534–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alnijoumi, M., Whitacre, T. (2022). Tracheostomy Care. In: Arora, N. (eds) Procedures and Protocols in the Neurocritical Care Unit. Springer, Cham. https://doi.org/10.1007/978-3-030-90225-4_20
Download citation
DOI: https://doi.org/10.1007/978-3-030-90225-4_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-90224-7
Online ISBN: 978-3-030-90225-4
eBook Packages: MedicineMedicine (R0)