Abstract
Nanocelluloses are a very promising material that has been widely explored for the most diverse applications. The pursuit for sustainable and environmentally friendly materials is in line with the nature of nanocelluloses and therefore they have emerged as the perfect candidate for plastics substitution, food additive, rheology controller, 3D printing of diverse structures, among many other possibilities. This derives from their interesting characteristics, such as reduced size and high specific surface area, high tensile strength, crystallinity and transparency, and from the fact that, such as cellulose, they are obtained from renewable sources, with relative ease for functionalization in order to obtain desired specificities. Thus, the industry is trying to react and effectively respond to the exponential growth of published research in the last years, and therefore new facilities (not only lab and pilot plants but already industrial sites) have been producing nanocelluloses. This new fibrous materials can be obtained from different raw-materials by different methodologies, leading to different types of nanocelluloses with, obviously, different characteristics. Nonetheless, technical and economical constraints have been addressed, such as the high energy demand or the clogging of homogenizers/microfluidizers.
This chapter intends to present a review addressing the main features related to the production, characterization and market of nanocelluloses and providing additional information regarding the vast literature published in these domains.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nanocelluloses are defined as cellulosic materials with, at least, one dimension at the nanometer scale [1, 41, 66, 112, 138]. The interest in this material has increased exponentially due to its peculiar characteristics like high aspect ratio (AR), specific surface area (SSA), mechanical strength, low coefficient of thermal expansion and good optical properties [1, 16, 41, 112].
The number of publications regarding the production, characterization and/or utilization of nanocelluloses has increased exponentially (Fig. 6.1Footnote 1).
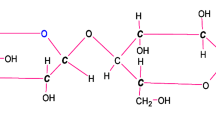
In the last years several terms have arisen for the classification of nanocelluloses. According to ISO standard TS 20477 [65] and standard proposal TAPPI WI 3021 [141], terms like cellulose nanofibrils (CNF), cellulose microfibrils (CMF), cellulose nanocrystals (CNC), cellulose microcrystals and bacterial nanocellulose (BNC) can be found, depending on the production process (raw material used and process conditions) and on the final dimensions. The process of production may be top-down, in which the nanocelluloses are obtained through fibrillation of lignocellulosic biomass, such as wood (types CNF, CMF, CNC) or bottom-up, in which they are created from glucose monomer units, using for example cellulose-producing bacteria (type BNC). Figure 6.2 presents the hierarchical structure of cellulose and the isolation of the cellulose nanomaterials from wood (top-down method).
2 Nanocelluloses – Sources and Types
2.1 Cellulose Nanofibrils (CNF) and Cellulose Microfibrils (CMF)
Cellulose nanofibrils (also called nanofibrillar cellulose or cellulose nanofibers) and microfibrils (or cellulose microfibres) are a type of nanocellulose that possesses amorphous and crystalline parts (Fig. 6.2). With aspect ratio usually greater than 10, their lengths are found to be up to 100 μm and, in the case of CNF, the width is usually 3-100 nm. CMF have a size distribution with not only cellulose fibrils at the nanoscale but also a significant amount of fibrils with non-nanometric dimensions, being sometimes difficult to distinguish between CNF and CMF. The dimensions referred to above are specified in ISO standard TS 20477 [65]. Nonetheless, it must be taken into account that many other dimensions can be found in the literature: e.g. distinction between CNF and CMF by the cross sections of 3–20 nm and 10–30 nm, respectively [36] or even more specific, distinguishing also the length between CNF (diameter 2–10 nm, length > 10 μm, aspect-ratio > 1000) and CMF (diameter 10–100 nm, length 0,5–10 μm, aspect-ratio 50–100) [132]. Cellulose filaments (CF) can be considered as a variant of CMF with a greater aspect ratio than the latter, i.e., 1000 or more, with diameter of 80 to 300 nm and length of 100 to 2000 μm [77].
CNF consist in a bundle of stretched cellulose molecules chains, very flexible and long, and thus tend to become entangled, which is one of the reasons why they are so valued as they are good for strength, reinforcement, and rheology modification [1, 66, 74]. The typical sources for their production are wood, hemp, flax, sugar beet, potato tuber, among others (Table 6.1).
In recent years, considerable research has arisen on lignocellulosic nanofibers (LCNF), in order to value residual biomass, reduce raw material costs and environmental impact [40]. The raw materials studied by various authors include residues of the primary industrialization of wood and straw wastes [39, 43, 122, 143, 144]. Some authors have even drawn attention to the best production performance of LCNF when compared to CNF [60, 131].
2.2 Cellulose Nanocrystals (CNC) and Cellulose Microcrystals
CNC (also known as nanowhiskers or nanorods) have an elongated rod-like shape and are highly crystalline, presenting low flexibility and an aspect ratio smaller than that of CNF (CNC AR = 5–50, according to ISO standard TS 20477 [65]), usually with diameters of 3–50 nm and lengths as low as 100 nm. In its turn, cellulose microcrystals (also known as microcrystalline cellulose) contain 90% of the material with diameters superior to 5 nm and aspect ratio higher than 2. They exhibit a high degree of crystallinity (50–90%) [18, 166] with limited flexibility compared to CNFs. The degree of crystallinity and their morphology depend on the cellulosic material used for their production (usually wood, cotton, wheat and rice straw, tunicin, bacteria and algae), as well as on the preparation conditions and on the techniques used. Besides being good for strength, reinforcement and rheology modification, CNC are also good for the enhancement of optical, electrical, and chemical properties.
2.3 Bacterial Nanocellulose (BNC)
Finally, BNC (also called biocellulose or microbial cellulose) are produced from the glucose units of a genus of bacteria: Gluconacetobacter [1, 41, 54]. The bacteria are cultivated in common aqueous nutrient media and the BNC are excreted to the air resulting in a highly swollen network (diameters between 10 and 40 nm) with a distinct tunnel and pore structure [73]. This type of nanocellulose possesses high molecular weight, crystallinity and good mechanical stability. Besides, it is free of lignin, hemicellulose and pectin, being a source of very pure cellulose (≥98%) [131].
3 Production
For ease of understanding, the available state of the art on the nanocellulose production will be divided into the top-down and bottom-up methodologies. A recent report produced by TAPPI (Technical Association of the Pulp and Paper Industry), summarized the state of the industry regarding the production of cellulose nanomaterials, and the numbers revealed that a) nanocelluloses are produced not only in laboratory and pilot facilities, but already at industrial scale and that b) CMF is produced in greater quantities when compared to CNF and CNC [93]. These nanomaterials are being produced worldwide, and therefore a topic related to their commercialization will be addressed in sect. 4.
3.1 Mechanical Treatments
CNF and CMF can be produced by mechanical, chemical or enzymatic treatments or by a combination of the aforementioned. The defibration of the fibers involves an intensive mechanical treatment and for that refining, homogenization, microfluidization, high intensity ultrasonication, milling or cryocrushing can be used. The most common mechanical treatments used to produce CNF are refining and high pressure homogenization (HPH), being commonly used together. In the first, the fibres are forced through a gap between two surfaces fitted (one or both) with bars and grooves, which damages the microfibril structure promoting external fibrillation by gradually peeling off the external cell wall layers (primary and secondary S1) and exposing the secondary S2 layer (Fig. 6.2). In this way, the fibers are ready for treatment in the homogenizer in which the fiber suspension is submitted to high pressures in order to pass through a small nozzle at high velocity so that the impact and shear rates suffered by the suspension result in the reduction of the fibers size to the nanoscale (Fig. 6.3). This process, although very efficient and simple, presents some drawbacks, namely the frequent obstruction of the small nozzle and the high energy consumption [1]. The microfluidization process is very similar to the HPH: the fiber undergoes high-pressure treatments as the slurry is accelerated and sent out of the equipment, passing through a chamber with a Z-shape structure that promotes an intense collision between particles so that the high impact splits the fibers into fibrils. In this equipment, the smaller the Z-shape constriction, the higher is the pressure and therefore the higher the fibrillation degree [104].
Scheme of the valve of a high-pressure homogenizer (reproduced entirely from Wikimedia https://commons.wikimedia.org/wiki/File:Homogenizing_valve.svg)
In the high intensity ultrasonication (HIU) the fibrils are isolated by ultrasound hydrodynamic forces created by a powerful mechanical oscillation that promotes intense waves [155]. According to Wang and Cheng [155] several factors (temperature, power, time) may affect the efficiency of fibrillation and a mixture of micro and nanofibrillar material is obtained and therefore the authors claimed that by combining HIU and HPH a more uniform fibrillar suspension is obtained. The grinding treatment is based on a static and a rotating grind stones system generating shear forces that individualize the nanofibers from the pulp wall structure. However, in this process, the pulp fibers can become highly degraded which may affect their reinforcing potential [129, 158]. Both in HPH and grinding, it is common to repeat the process several times, by increasing the number of cycles, in order to increase the degree of fibrillation. Another alternative to produce nanocellulose is cryocrushing. In this method the water swollen cellulosic fibers are immersed in liquid nitrogen and submitted to high shear forces, which leads to the rupture of the cell wall by the pressure exerted by the ice crystals. The grinding and cryocrushing processes are usually accompanied by high pressure treatments [56, 68, 156].
As expected, the average particle size decreases with increasing energy consumption [42]. This is most important when considering the potential of nanocelluloses to be used at an industrial scale. Despite the many efforts to reduce the energy consumption while producing nanocelluloses with controlled sizes, it is legitimate to say that the process is still not economically feasible for smaller added-value applications, such as paper and paperboard products [104]. In fact, large amounts of energy were reported with values exceeding 30.000 kW h/t [85].
3.2 Chemical and Enzymatic Treatments
Since the aforementioned treatments are not 100% efficient in producing nanofibrils and the energy costs necessary to perform them are high, it has become usual to pre-treat the fibers, before the mechanical step. In fact, it is stated that, for cellulosic fibers, the pre-treatment helps reducing the energy consumption by 91–98% [11, 129].
The pre-treatments can be of enzymatic or chemical nature. In the first, the enzyme is used to modify or degrade the lignin and the hemicellulose, besides helping to hydrolyze cellulosic fiber specific components [1, 59]. The most conventional enzyme applied for the modification of pulp fibers in order to produce CNF is endo-1,4-β-D-glucanase, which requires some disordered structure in cellulose to disrupt it [1]. Pääkko et al. [105] and Henriksson et al. [58] applied a mild enzymatic hydrolysis (using endoglucanase) combined with refining and passes in a HPH to produce a CMF gel with diameters in the nanometer range and high aspect ratio. In these studies they also compared the enzymatic treatment with a chemical one (acid hydrolysis), concluding that the CMF produced by the former method possessed a more favorable structure with a more homogeneous distribution of nanofiber geometries and higher aspect ratio than the CMF produced by the latter method. The enzymatic hydrolysis is already being studied as a cost-effective approach to produce CMF to be used as a paper reinforcement: in a quite recent article Tarrés et al. [142] concluded that the pulp consistency, pH of the suspension, the treatment time and enzyme dosage have a key role during the production of CMF with high specific surface. In this article the authors used an enzyme cocktail which contains endo-β-1,4-glucanases but the same authors also produced CNF with a commercial enzyme obtained from genetically modified Trichoderma reesei [55]. Since cellulosic fibers contain different organic compounds it is usual to apply a cocktail of cellulase enzymes in order to disrupt the fibers, which is hardly done by a single enzyme [113].
Another approach for the nanocellulose production is by chemical treatment. The most commonly used process for the extraction of CNCs from native cellulose is based on a strong acid hydrolysis under strictly controlled conditions of temperature, agitation, and time. Hydrochloric and sulfuric acid have been mostly used in the extraction process [115, 161]. An acidic attack dissolves the amorphous portions of cellulose, resulting in the formation of a nanocrystal structure [72]. During this process, negatively charged sulfate groups are introduced on the cellulose chain, leading to intermolecular repulsive forces that confer electrostatic stability to CNCs in polar aqueous suspensions [30, 90, 115].
For the cellulose nanofibrils production there are several possibilities, such as the use of ionic liquids to dissolve cellulose or the introduction of carboxyl groups on the fibers to facilitate the fiber wall delamination. Li et al. [80] pretreated sugarcane bagasse with an ionic liquid in order to dissolve the cellulose and stated that this facilitated the mechanical treatment in a HPH. Besides, other chemical pre-treatments such as acetylation, silylation, or treatments with isocyanate have been used to generate CNF hydrophobic surfaces in order to reduce the agglomeration of these materials. Nonetheless the most effective and used pre-treatments are based on the modification of the fibers in order to introduce ionic groups. One approach that fits this purpose is carboxymethylation that negatively charges the cellulosic fibers surface and increases the breakup of lignocellulosic fibers to nanosize by adding carboxymethyl groups to the cellulose chains of the fibers. The most cited author regarding this pre-treatment is J. A. Walecka [153] and his work is based on the etherification of the cellulose hydroxyl groups with monochloroacetic acid (MCA) in its sodium salt form, in the presence of sodium hydroxide (Fig. 6.4). Wagberg et al. [152] used this method followed by HPH to produce carboxymethylated CNF with cross section diameters of 5–15 nm. In this study it was shown that very high concentrations of the salt or too low pH would cause agglomeration of the fibers. The same authors studied the accessibility of polyelectrolytes to carboxymethylated cellulose microfibrils and found that high molecular weight polyelectrolytes were accessible to all carboxyl groups [151], which can be very important when considering the additives used in papermaking. Carboxymethylated CNF are known to increase the water retention value [22], reducing hornification during drying [44], and to limit aggregation of particles [130].
Carboxymethylation reaction with sodium monochloroacetate. R depends on the progress of the reaction [88]
Another common approach is oxidation. The most reliable method is based on the use of TEMPO (2,2,6,6-Tetramethylpiperidine-1-oxyl) to mediate the oxidation, in which carboxylic groups are introduced at the C6 position of the glucose unit [66, 117,118,119,120]. Figure 6.5 shows the scheme of the oxidation in which the primary oxidant (NaClO) is added to a cellulose suspension in the presence of catalytic amounts of TEMPO and NaBr at pH 9–11. In this reaction, the C6 primary hydroxyl groups of cellulose are converted to carboxylic groups via C6 aldehyde groups, at the expense of NaClO and NaOH consumption as the oxidation proceeds [118].
Some side reactions can occur in this reaction under alkaline conditions, such as the depolymerization or discoloration of the oxidized cellulose due to the presence of residual aldehyde groups and therefore some authors applied a different system consisting of TEMPO/NaClO/NaClO2 under neutral or slightly acidic conditions [121, 140, 163]. Several studies have proven that, by pre-treating the cellulosic fibers with TEMPO, it is possible to reduce the number of passes in the homogenizer required to produce CNF [16, 17, 31]. Other oxidation pre-treatment commonly used is periodate-chlorite oxidation since it improves the fibrillation efficiency of CNF. In this pre-treatment a sequential oxidation of the cellulose fibers with periodate and chlorite occurs, in which firstly the vicinal hydroxyl groups of cellulose at C2 and C3 positions are oxidized to the corresponding aldehyde groups, and then these aldehyde groups are further oxidized to carboxyl. Liimatainen et al. [82] stated that, by using this pre-treatment, oxidized celluloses with carboxyl contents ranging from 0.38 to 1.75 mmol/g could nanofibrillate to highly viscous and transparent gels with yields of 85–100% without clogging the homogenizer.
3.3 Biosynthesis of Bacterial Cellulose
As stated, bacterial nanocellulose is manufactured by a bottom-up method, contrary to the processes aforementioned. The most studied species of bacteria for production of cellulose is Glunoacetobacter xylinus. These bacteria produce an extracellular, chemically pure-glucan, supporting their survival in the natural environment since the cells are kept at the surface of culture media, being entrapped inside gelatinous, skin-like membranes, consisting of entangled cellulose fibers [50]. The advantage of bacterial derived cellulose microfibrils is that it is possible to adjust culturing conditions to alter the microfibril formation and crystallization [96].
The G. xylinus species are usually cultivated at 30 °C for 7–14 days in a Hestrin-Schramm medium (composed of a carbon source, enriched nitrogen source and a small amount of citric acid) with pH adjusted to 5.7. Several authors have modified the composition of this medium in order to optimize BNC production [95, 159, 164]. Besides, and according to Gama et al. [50], there is the need to optimize separately the conditions of cellulose biosynthesis from diverse carbon sources for each BNC producer. In most cases glucose, glycerol, sucrose, and mannitol were found to be the most suitable carbon sources for cellulose production (here mentioned in the order from the most to the least efficient source).
The culture can be performed under static or agitated conditions. In the static culture, the microbiological medium is placed in shallow trays and inoculated with bacteria, being therefore a more expensive method and characterized by low productivity [50, 95]. As for the agitated culture, a higher power supply is needed, but it has the main advantage of high cell concentration and productivity [168].
Since several authors have considered that the industrial scale production of BNC is still not efficient or cost effective in static cultures, some research has been carried out to produce BNC in a large scale at a low cost by using culture medium composed of agroindustrial sources or wastes [10].
4 Properties and Characterization
The production methods abovementioned usually generate an aqueous suspension/dispersion with low amounts of solids (CNC 1–2 wt% and CMF 0.5–3 wt%). Besides, the pre-treatments including functionalization of the cellulose structure can also give rise to a gel (Fig. 6.6), which is stable and transparent, also at very low solids concentration (such as 1–2 wt% for oxidized CNF) [45]. For their characterization, but mainly for their commercialization, there may be the need to dry them, and therefore nanocelluloses can be manipulated as a film, an aerogel or a foam. The mechanisms for drying will be discussed in Sect. 3.7.
As stated, nanocelluloses have many unique properties that make them attractive for several applications. According to a previous review, the main points that should be addressed are the amount of produced nanomaterial, the rheology of the dispersion, the average particle size and size distribution, crystallinity, specific surface area, surface chemistry, and mechanical properties [71]. Obviously, taking into account the foreseen applications, some properties can have more importance than others. An accurate, consistent and reliable characterization of the nanocelluloses is essential, not only for their application, but also to evaluate the interaction with the local environment, which is fundamental for their commercialization. With this regard, mention is due to the following publications: i) the review article by Foster et al. [45] which establishes the details of the best practices, methods and techniques for characterizing CNC and CNF and ii) the ISO standard TS 21346 [64] that defines the characterization techniques to be used in elementary fibrils, or individualized cellulose nanofibrils.
In the present text the state of the art of the nanocelluloses characterization is divided by the properties considered as more important and will be focused mainly in CNF, CMF and BNC. Other types of nanocelluloses or even other methods for their production will remain out of scope.
4.1 Amount of Nanomaterial
As stated in sect. 1, there are several types of nanocelluloses with different characteristics and the amount of nanomaterial is an important property to be determined since the samples are not usually entirely composed of nano-sized material. The most common technique used for the estimation of this property is ultracentrifugation. By this method the nanofibrils are separated from the large size particles that remain concentrated at the bottom of the sample holder and, by weight difference, the nano-sized material content is determined. The centrifugation conditions to be used are much dependent on the type of sample and on the degree of fibrillation: Ahola et al. [4] applied 10,400 rpm to nanocellulose dispersions for 2 h while Taipale et al. [138] used only 45 min with the same speed and Gamelas et al. [51] used only 9000 rpm for 30 min (ca. 9000 g) since the nanofibers were more fibrillated. ISO standard TS 21346 [64] states that the suspensions should be at 0.1% consistency and the centrifugal separation performed at more than 12,000 g for longer than 20 min.
4.2 Morphology and Fibril Dimensions
The assessment of the fibrils appearance, morphology, shape and size has been performed using different techniques, being the most common those based in microscopy, although some indirect methods, such as turbidimetry or light scattering, are becoming common [45].
Among the microscopic methods, it is usual to start by performing optical microscopy (OM) to get an overview of the sample and of its homogeneity. After, higher resolutions are needed in order to analyze the fibrils details and for that field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM) and atomic force microscopy (AFM) are used. These techniques have the advantage of allowing the visualization of the nanocelluloses and, when combined with image analysis, measuring their dimensions [4, 24, 27, 59]. However, typically, the size distribution is limited to the width distribution since the aspect-ratio is too high to obtain the length-distribution values. Figure 6.7 shows an example of FE-SEM and AFM images taken on nanocelluloses. Nonetheless, it is worth mention that these techniques may require a careful preparation of the samples (particularly TEM) and are laborious, time consuming and very user-dependent. Besides, the observation field is limited and therefore the results are not always representative of the entire sample. The shape of the nanofibers may appear different depending on the method used: e.g., when using AFM, tip-broadening effects make it difficult to understand if the morphology observed is due to individual particles or to agglomerates. Therefore, the techniques mentioned provide different but complementary information about the morphology and dimensions of the nanocellulose fibrils and, in order to obtain a good and accurate analysis, one most use a combination of the microscopic methods [24].
FE-SEM image of a mechanically-produced CMF (left) and AFM image of TEMPO-oxidized nanocellulose from wood (right) [87]
Indirect measurements such as turbidimetry have also been used. For suspensions of TEMPO-oxidized CNF, the visible spectra in the transmittance mode evidenced higher transmittance for more fibrillated samples, corresponding to a clearer suspension with higher amount of nanosized material [51, 119]. On the other hand, techniques based on light scattering and diffraction, such as dynamic light scattering (DLS) or laser diffraction spectrometry (LDS), can overcome some of the drawbacks mentioned for the microscopy-based techniques [97, 112]. However, the particles should be spherical, and since cellulose nanofibrils are a fibrillar-like material with high aspect ratio, the values obtained from DLS cannot be directly linked to the particle length or cross-section dimensions and cannot be directly correlated with particle size distributions. It should therefore be taken as a hydrodynamic “apparent particle size” that can be used as an internally consistent method to assess the dispersion quality or state of aggregation. Notwithstanding, it was reported for cellulose nanocrystals that the equivalent hydrodynamic radius, measured by DLS, did not differ much from the theoretical hydrodynamic radius, calculated for cylinder-shaped particles based on the dimensions of length and width assessed by FE-SEM [46, 51] Thus, microscopy and light scattering methods are considered complementary. In fact, Gamelas et al. [51] analyzed different CNF obtained by NaClO/NaBr/TEMPO pre-oxidation and mechanical treatment and calculated the nanofibrils length based on the width measured by AFM and the hydrodynamic diameter assessed by DLS.
4.3 Physical Properties
Some important physical properties to consider when characterizing nanocelluloses are the crystallinity, specific surface area (SSA) and the degree of polymerization (DP). The crystallinity can be determined by X-ray diffraction (XRD), Raman spectroscopy, infrared spectroscopy (FT-IR) and 13C nuclear magnetic resonance (NMR) but it strongly depends on the source and processes used to produce the nanocelluloses. Alemdar and Sain [6] determined by XRD the crystallinity of wheat straw and of soy hull nanofibers produced by a chemical-mechanical technique and concluded that an increase of the crystallinity of 35% and of 16%, respectively, occurred because the treatment removed non-cellulosic components such as lignin and hemicelluloses. The same conclusions were stated by Jonoobi et al. [70] with nanofibers extracted from kenaf core. However, it is difficult to compare results from the literature since they depend on the calculation methods used to obtain the values (peak height/intensity, peak area/deconvolution, amorphous subtraction) [71].
Regarding the specific surface area determination, different methods have been used. One of the most common is the Brunauer−Emmett−Teller (BET) method by N2 adsorption for nanopapers or freeze-dried nanocelluloses. The drawback of this technique is the sample preparation (drying) that highly affects the surface area due to aggregation. According to Sehaqui et al. [125], after direct water evaporation, the specific surface area can be as low as 10−2 m2g−1 corresponding to a nanopaper with ca. 20% porosity. However, if a water exchange to methanol or acetone prior to drying is performed, the porosity increases to 28% and 40%, respectively [59]. Sehaqui et al. [125] produced a CNF nanopaper by supercritical CO2 drying with exceptionally high specific surface area (up to 480 m2 g−1). Other method often used to determine the specific surface area is the Congo red adsorption. Spence et al. [132] determined the specific surface area of freeze-dried bleached and unbleached fibers/microfibrils and concluded that the unbleached samples adsorbed about 1.8 times more Congo red per unit of BET surface area than the bleached samples since, contrary to the BET method, the Congo red adsorption method is considered to depend on the chemical composition of the fibers. Specifically, there is more rapid adsorption of the dye to hydrophobic lignin than to hydrophilic cellulose.
The degree of polymerization has been reported to strongly depend on the aspect ratio of the nanofibers [79]. Shinoda et al. [126] found a linear relation between DP and length of TEMPO-oxidized CNF. It is common to apply the ISO standard 5351 [63] that calculates the average DP by applying the Staudinger–Mark–Houwink equation through the determination of the limiting viscosity number with a solution of cupriethylenediamine (CED) [59, 167]. When considering TEMPO-oxidized CNF, Shinoda et al. [126] stated that only CED could completely dissolve this type of nanofibrils consisting of both partially oxidized and unoxidized cellulose molecules. According to Zimmerman et al. [167], the production of CMF from softwood sulfite pulp led to a decrease in viscosities/DPs between 15% and 63%. The authors also referred that the strength properties of films or composites containing CMF decreased with the decrease of the DP which makes it a valuable tool for evaluation of the CMF performance.
4.4 Chemical Properties
Concerning the chemical properties of nanocelluloses, perhaps the most important issue to consider is their surface chemistry. As stated, nanocelluloses can be modified by different methods which, in consequence, will inevitably modify their surface chemistry. In this matter, it is usual to measure the surface charge, for example by the identification of the functional groups present at the surface. Stenstad et al. [135] and Taipale et al. [138] analyzed the charge of CMF samples by zeta potential measurements. In the first study, the authors produced CMF by homogenization and modified its surface with different chemicals, changing the surface charge from negative to positive, while in the second study, CMF produced by carboxymethylation revealed to possess twice the surface charge than CMF produced by only mechanical treatments. Gamelas et al. [51, 52] also determined the zeta potential of TEMPO-oxidized CNF by measuring the electrophoretic mobility concluding that this treatment leads to strongly negatively charged fibers, in accordance with other authors [15, 97, 165]. The production of CMF from the same source, but with enzymatic treatments, did not alter the charge of the initial fibers [89].
To determine the content of functional groups at the surface of nanofibers it is common to use titrimetic methods. For CNF produced by TEMPO-mediated oxidation several authors determined the content of aldehyde and carboxylic groups using conductometric titrations [12, 87, 117]. For instance, Saito and Isogai [117] determined carboxyl and aldehyde contents of 0.67 and 0.21 mmol/g, respectively, for nanocellulose produced from cotton linter. Other related methods such as potentiometric or polyelectrolyte titrations can be used. Syverud et al. [137] used both the conductometric and potentiometric titrations and obtained similar results for the carboxyl’s content of TEMPO-oxidized cellulose (0.52 and 0.51 mmol/g, respectively).
Characterization techniques such as FT-IR to determine the oxidation level during the TEMPO-mediated oxidation (through the measurement of the intensity of the band at 1738 cm−1 due to the carbonyl stretching [119]), or X-ray photoelectron spectroscopy (XPS) to determine the surface chemical composition regarding the surface modification of nanofibers are also usually applied to nanocellulose.
4.5 Rheology
As abovementioned, nanocelluloses can form a gel even at very low concentrations in water – usually 1 to 5%, but for values as low as 0.125% a gel can also be found [105]. This is one of the reasons why they are a suitable material for diverse applications. CNF suspensions also appear as a rheology modifier to be applied in cosmetics, paints, food, as mineral suspending agent, among other applications [9]. Therefore, it becomes essential to assess the rheological behavior of this gel, e.g., for paper surface treatments in which the dosage and coating must be well controlled. Several authors have studied the rheological behavior of nanocelluloses. Hubbe et al. [62] dedicated a 100 pages review article to the subject, containing issues such as flow, fluid layers, entanglement of cellulose fibrils and effect of pH or salt addition, among others.
Most publications evidence their pseudoplastic behavior [16, 78, 99, 105], meaning that the increase of the shear stress or of the shear strain leads to a decrease of the viscosity. However, these authors also claim that this behavior is noticed above the critical concentration – e.g., for Lasseuguette et al. [78] this value is of 0.23% – while below this the behavior can be similar to that of a Newtonian fluid. According to Kangas et al. [71] this is due to the fact that, at this concentration, the fibrils form a strong entangled network. Also, the gel point – the lowest fibrous volume at which all the flocs are interconnected forming a self-supporting network [81] – is claimed to be reduced with the addition of cationic polymers [147]. This is related to the compressive yield stress of the flocs, which is affected by the strength of the interparticle bridging forces. In papermaking, it is important that the nanocellulose sample possesses a low gel point in order to improve drainage [81]. Alves et al. [9] also studied the fibrils aggregation as a major factor in the suspensions rheology, stating that as the pH is decreased and carboxylic groups is protonated, the suspensions viscosity increases.
Thixotropy, i.e. reversible shear-thinning behavior, is also referred to as a property of nanocelluloses [32, 62], with recovery times depending on the type of material assessed – e.g., higher fibrillation reduces the recovery time [33]. Regarding temperature, it was found that heating a cellulose nanofibrils suspension did not affect significantly its viscosity, since the nanocellulose had a dominant effect over the aqueous medium [3, 57]. Finally, it was also demonstrated that the introduction of charged groups to the nanocellulose structure is responsible for the decrease of viscosity, due to strong repulsive forces between surfaces, which act as kind of lubricant [62]. In this sense, several authors have introduced salts, dispersants or surfactants to the nanocellulose suspension in order to control the dispersion stability and reduce the viscosity [9]. Sodium chloride and carboxymethylcellulose are the most reported additives when studying rheology of nanocellulose [32, 100].
In papermaking, nanocelluloses have been found to be a great rheology-controller for coating formulations, as not only viscosity is enhanced but also water-binding [32, 62].
4.6 Toxicity
The production of nanocellulose at an industrial scale and its application in a multiplicity of products and biomedical devices can represent a potential hazard to workers along the lifecycle as well as to consumers [149]. Vartiainen et al. [148] concluded that workers’ exposure to particles in the air during grinding and spray drying of birch cellulose was low or non-existent with the implementation of appropriate protection equipment and proper handling. However, the high aspect ratio of CNF and its biodurability in the human lungs [133] resembles the fiber paradigm that has been associated to the adverse effects of other fibrous nanomaterials (e.g., carbon nanotubes). Therefore, to ensure the safety of CNF to humans prior to their largescale commercialization, it is of utmost importance to investigate their potential toxicological properties, particularly their genotoxicity that is closely associated to carcinogenicity. Cytotoxicity deals with the effect of the CNF on cell viability, while immunotoxicity regards the effects on the functioning of local and systemic immune systems and finally, the genotoxicity is related with the direct or indirect damaging effects on DNA or chromosomes. Most toxicological studies have focused on nanocellulose types with morphological and surface chemical characteristics different from the above-mentioned CNF. These include BNC [69, 83, 98, 108, 123, 124] and CNC [20, 28, 35, 76, 127, 160]. These nanocellulose types are generally considered as nontoxic, although CNC could induce low cytotoxicity and immunotoxicity in vitro and in vivo [28, 160]. Regarding CNF, the few published studies mainly indicate no relevant cytotoxic, genotoxic or immunotoxic effects [7, 29, 103, 109, 148]. Nevertheless, a recent study by Catalán et al. [21] showed that mice exposure by pharyngeal aspiration to CNF produced through TEMPO oxidation led to an acute lung inflammatory response and induced DNA damage in lung cells. Moreover, Lopes et al. [86] reported that an unmodified CNF induced a pro-inflammatory effect in THP-1 macrophages that could be moderated by the introduction of surface modifications.
4.7 CNF/CMF Drying and Films
Nanocelluloses are usually processed in their aqueous suspension form because of their hydrophilic nature and of the propensity to agglomerate during drying. In fact, the hydrogen bonds between water and the cellulose particles enable the system to remain thermally and kinetically stable even at different moisture contents [107]. However, if properly dried, nanocellulose can be used to produce composites or form films and aerogels with excellent properties. Films made entirely of nanocelluloses are usually called “nanopapers” and reported to be transparent rigid films with high strength, flexibility, low thermal expansion coefficient and good barrier properties [1, 41, 79, 158], which make them excellent materials to be used as substrates in several applications. However, removing water from the CNF suspensions can be a delicate process and some authors tried to propose viable solutions to the problem, being already in operation some pilot plants to the production of nanopaper [139]. Peng et al. [107] studied the effect of several techniques to dry cellulose nanocrystals and nanofibrillated cellulose: oven drying, freeze-drying, supercritical drying and spray-drying. The authors concluded that spray-drying was the most suitable technique to dry CNF without affecting the particles nano-scale, while the other techniques created a highly networked structure with cellulose agglomerates. Pääkkö et al. [105] produced aerogels with strong mechanical properties by applying two different freeze-drying techniques (cryogenic and vacuum) to a CNF suspension, stating that these are advantageous and cheaper than the usual technique: supercritical drying. Fig. 6.8a shows a freeze-dried TEMPO-nanocellulose.
Regarding the formation of nanopapers, several techniques can be used, namely vacuum filtration, spraying, solvent casting, solvent exchange, spin-coating, among others [27, 45, 59, 91]. The fastest method to produce nanopapers is vacuum filtration by using a dynamic sheet former. Some authors stated that with the use of an appropriate wire, usually membranes or polyamide cloths, it is possible to obtain transparent and strong nanopapers [47, 59, 114]. The main objective of the production of nanopapers has been the study of their mechanical properties, but also, in a minor degree, the analysis of the optical and barrier properties. Syverud and Stenius [136] produced nanopapers with thickness values of 20–33 μm that possessed strength properties comparable to, or higher than, those of cellophane. Besides, they stated that the dense structure formed by the fibrils gave superior barrier properties and the films were comparable, in terms of oxygen transmission, to the best synthetic polymers used for packaging, like polyvinylidene chloride or polyester. It must be stressed out that the properties of the films strongly depend on the raw material used for their production and values of tensile strength around 130 MPa for nanopapers produced from sulfite pulp [59] or as high as 233 MPa with TEMPO-oxidized softwood pulps [47] can be found. The classical method to produce nanopapers is solvent casting in which the solvent is evaporated with controlled temperature, relative humidity and time. However, it is a time consuming method that can take up to five days if, for example, room temperature is used [13, 24, 132]. Figure 6.8b shows a nanopaper made by solvent casting of a TEMPO-oxidized BEKP. Other processes such as solvent exchange are commonly found in the literature. With these methods it is also possible to produce porous films. According to Sehaqui et al. [125] a water exchange to methanol or acetone prior to drying increases the porosity from 20% to 28% and 40%, respectively, which is due to the less hydrophilic character of the solvents that reduce the capillary effects during the drying process. In this work, the authors produced nanopapers using three different methods, namely liquid CO2 evaporation, supercritical CO2 drying and tert-butanol freeze-drying, obtaining nanopapers with high specific surface area and with mechanical properties comparable to those of typical commodity thermoplastics but with much lower density (640 kg m−3). Finally, Ahola et al. [5] produced a thin and smooth film by another strategy: the authors spin-coated cellulose nanofibril dispersions on silica substrates. This method differs from the previous in the sense that the nanopaper is formed directly on a suitable substrate.
Chinga-Carrasco et al. [24,25,26,27] have researched thoroughly the micro-structure of nanopapers surfaces by the use of image analysis techniques. In their works, novel microscopy techniques and automatic computerized image analysis have shown to be preferable to the common visual and subjective evaluations. The effect of residual fibers in the roughness of nanopapers was studied in detail, concluding that without a proper treatment, such as fractionation, the nanopapers had an extreme rough surface structure, which to some applications such as printing, is very detrimental [27]. By using laser profilometry topography, it was possible to distinguish size differences in the top and bottom sides of a TEMPO-oxidized nanopaper, and therefore a quantification of the amount of nanofibrils present in different samples was performed [51].
Regarding the optical properties, it is well known that films made entirely of nanocellulose are transparent since the size of the nanofibrils is much inferior to the wavelength of visible light [61, 129]. Indeed, Fukuzumi et al. [47] produced TEMPO-oxidized nanopapers with 20 μm thickness and stated that at 600 nm a 90% transmittance, when using softwood, and a 78% transmittance, when using hardwood, was found. Similar values were obtained by Wang et al. [158], with nanopapers derived from waste corrugated paper, and by Nogi et al. [102], that evaluated the influence of the surface roughness in transmittance, concluding that the light transmittance could be increased to ca. 90% if the films were polished or impregnated with an optical transparent polymer layer (acrylic resin).
As already stated, nanopapers have unique properties that make them an outstanding material for diverse applications: transparent films for food packaging [92], antimicrobial films [116], water treatment [91], electronic devices [145], conductive papers [61], coating technologies, among others. However, some problems remain associated with the nanopapers production/use that still need a solution, such as their hydrophilic nature, preservation and the fact that nanoceluloses do not redisperse, among others.
5 Market
5.1 Commercialization
In order to effectively commercialize the nanocellulose products, several aspects have to be taken into account. A report from Miller [93] identifies the main producers at large scale (Table 6.2). It must be taken into account that FiberLean Technologies produces a hybrid material as CMF are mixed with mineral fillers at a 1:1 ratio [53]. International Paper and Stora Enso companies are also reported to be producing CMF, largely for use in their own paper and paperboard products [94].
Some technical challenges, related to the aforementioned specific characteristics of the nanomaterials, are identified within this topic [2, 93, 146] and can be synthesized as follows:
-
Drying and dispersion
-
Compatibilization
-
Cost
-
Consistent quality from batch to batch
-
Safety and regulatory issues
If by the one hand the cost of production is the bottleneck of nanocellulose usage at industrial scale, by the other hand, drying is considering one of the most important issues. Due to the high hydrophilic character, and to the tendency to irreversibly aggregate while drying, one significant challenge is to produce dry CNF powder with a preserved nanoscale structure and re-dispersion capacity, which would provide advantages in CNFs storage and transportation. However, according to Miller [94], when considering the papermaking industry, 75% of all nanocellulose is produced by mills and used in their own production, which minimizes the challenges identified. Overall, there is the need to select the best material for a given application, define the optimal loading, and consider a learning curve for the end-user. Besides, the development of applications to use the product, with the research and development associated, and the scale up necessary, are still assumed to be a challenge for commercialization [94].
A variety of market reports and guides for end users have been published forecasting the nanocellulose market, including companies such as Future Markets Inc., RISI, Market Intel, LLC and TAPPI [75]. A report from Future Markets Inc. predicted the global market for nanocellulose until 2030, stating that, overall, the production costs of these nanomaterials should be reduced (as example, TEMPO-CNF should decrease from 50 USD/kg to ≈ 2 USD/kg) [48]. The cost of producing nanocellulose is primarily dependent on the type of pre-treatment applied, with the cheapest process being probably the enzymatic pre-treatment, where the cost for making CMF from the pulp integrated in a pulp mill is 0.4 €/kg, which today is in operation in large-scale papermaking applications [75]. For non-integrated use of CNF/CMF in papermaking applications, the cost including pulp cost and profits should be lower than 2.5 €/kg [75].
5.2 Applications
Due to the amazing properties presented by nanocelluloses, and considering the opportunity to produce a functional material with specific characteristics directed to the desired requests, several applications have been arising and the nanocellulose use is almost endless. In this sense nanocelluloses have been applied, as said, in the most diverse fields such as papermaking, textiles, medicine, cosmetics, pharmaceuticals, food industry and technology. Table 6.3 presents the applications with higher potential volume for nanocelluloses, as depicted by BioBased Markets in their 2018 annual report. Examples of applications are the water treatment [91, 150, 154], printed electronics [145, 162], tissue engineering and drug delivery [14, 83, 110].
According to Klemm et al. [75], there is an agreement that high value and/or high volume applications should be pursuit in order to reduce the nanocellulose production cost. Considering the aforementioned, the major potential use of nanocelluloses is in papermaking.
Nonetheless, despite the high potential of use in papermaking, textiles or coatings, these are low-value products, and it is noticed that the research available has been primarily focused on high-value products, especially in composite materials. According to Siró and Placket [129] nanocomposites are two-phase materials in which one of the phases has at least one dimension in the nanometer range (1–100 nm). Besides their excellent mechanical properties, nanocelluloses present many advantages in the production of composites, such as biocompatibility, transparency and high reactivity due to the presence of hydroxyl groups within a high surface area. Composites with nanocelluloses have been produced with petroleum-derived non-biodegradable polymers such as polyethylene (PE) or polypropylene (PP) and also with biodegradable polymers such as polylactic acid (PLA), polyvinyl alcohol (PVOH) and starch [129], and several works have arisen with inorganic fillers [8, 145]. The main purpose of the works published in this field is to improve the strength properties of the composites [49, 101, 106]. Besides, exceptionally smooth surfaces are reported under specific conditions, which make these composites a promising material for printed electronics. However, it is noteworthy that all of the research performed strongly depends on the nature and preparation method of the nanocelluloses used [167]. Some disadvantages of using nanocelluloses in composites for reinforcement applications should also be referred to, namely the high moisture absorption and the incompatibility with most of the polymeric matrices and of course the temperature limitation because lignocellulosic materials start to degrade near 220 °C [128], which can restrict the type of composite that can be produced. Examples of applications are the CNC composite filter papers for rapid removal of bacteria from aqueous solutions [23], the electrically conductive composites (Zhang et al. 2019) or even the CMF films with acetic anhydride that possess barrier properties similar to the common packaging materials [114], among several others.
6 Final Remarks
Nanocelluloses, in their varied denominations, shapes and properties, have been widely explored in the last decades. The state of the art regarding these interesting and promising materials is very extensive and covers the research and development based on the possible sources, production, properties and characterization but also on the proposed usages.
In this sense, nanocelluloses can have very distinct characteristics, depending on the raw material used, as well as on the treatments applied for their production. The different characteristics will have distinct impacts on their final applications, which make it very important to always perform a complete analysis of the intrinsic properties of these new materials.
Their use in the most diverse applications has been widely explored, as nanocelluloses may be used for plastics substitution, as food additive, rheology controller, 3D printing of diverse structures, among many other possibilities, which reinforces the idea that the behavior of nanocelluloses in the presence of other components should be carefully studied, with all of its specificities.
Notes
- 1.
The following keywords were used for the search in the Web of Science database: “nanocellulose” OR “cellul*” NEAR/1 (“microfib*” OR “nanofib*” OR “bact*” OR “microb*” OR “nanocryst*” OR “microcryst*” OR “whisk*”).
References
Abdul Khalil HPS, Davoudpour Y, Nazrul Islam M, Mustapha A, Sudesh K, Dungani R, Jawaid M (2014) Production and modification of nanofibrillated cellulose using various mechanical processes: a review. Carbohydr Polym 99:649–665
Agenda 2020 Technology Alliance (2016) Cellulose nanomaterials – research roadmap
Agoda-Tandjawa G, Durand S, Berot S, Blassel C, Gaillard C, Garnier C, Doublier J-L (2010) Rheological characterization of microfibrillated cellulose suspensions after freezing. Carbohydr Polym 80(3):677–686
Ahola S, Österberg M, Laine J (2008a) Cellulose nanofibrils – adsorption with poly(amideamine) epichlorohydrin studied by QCM-D and application as a paper strength additive. Cellulose 15(2):303–314
Ahola S, Salmi J, Johansson L, Laine J, Österberg M (2008b) Model films from native cellulose Nanofibrils. Preparation, swelling, and surface interactions. Biomacromolecules 9:1273–1282
Alemdar A, Sain M (2008) Isolation and characterization of nanofibers from agricultural residues-wheat straw and soy hulls. Bioresour Technol 99:1664–1671
Alexandrescu L, Syverud K, Gatti A, Chinga-Carrasco G (2013) Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 20:1765–1775
Alves L, Ferraz E, Gamelas JAF (2019) Composites of nanofibrillated cellulose with clay minerals: a review. Adv Colloid Interf Sci 272:101994
Alves L, Ferraz E, Lourenço AF, Ferreira PJ, Rasteiro MG, Gamelas JAF (2020) Tuning rheology and aggregation behaviour of TEMPO oxidized cellulose nanofibrils aqueous suspensions by addition of different acids. Carbohydr Polym 237:116109. https://doi.org/10.1016/j.carbpol.2020.116109
Andrade FK, Morais JPS, Muniz CR, Nascimento JHO, Vieira RS, Gama FMP, Rosa MF (2019) Stable microfluidized bacterial cellulose suspension. Cellulose 26:5851–5864
Ankerfors M (2012) Microfibrillated cellulose: Energy-efficient preparation techniques and key properties. Litentiate thesis presented to Innventia AB and KTH Royal Institute of Technology
Araki J, Wada M, Kuga S (2001) Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir 17:21–27
Aulin C, Gällstedt M, Lindström T (2010) Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 17:559–574
Bacakova L, Pajorova J, Bacakova M, Skogberg A, Kallio P, Kolarova K, Svorcik V (2019) Versatile application of Nanocellulose: from industry to skin tissue engineering and wound healing. Nano 9(2):164
Benhamou K, Dufresne A, Magnin A, Mortha G, Kaddami H (2014) Control of size and viscoelastic properties of nanofibrillated cellulose from palm tree by varying the TEMPO-mediated oxidation time. Carbohydr Polym 99:74–83
Besbes I, Alila S, Boufi S (2011a) Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: effect of the carboxyl content. Carbohydr Polym 84:975–983
Besbes I, Vilar MR, Boufi S (2011b) Nanofibrillated cellulose from alfa, eucalyptus and pine fibres: preparation, characteristics and reinforcing potential. Carbohydr Polym 86:1198–1206
Bras J, Viet D, Bruzzese C, Dufresne A (2011) Correlation between stiffness of sheets prepared from cellulose whiskers and nanoparticles dimensions. Carbohydr Polym 84:211–215
Brodin F, Eriksen Ø (2015) Preparation of individualised lignocellulose microfibrils based on thermomechanical pulp and their effect on paper properties. Nordic Pulp Paper Res J 30:443–451
Catalán J, Ilves M, Järventaus H, Hannukainen KS, Kontturi E, Vanhala E, Alenius H, Savolainen KM, Norppa H (2015) Genotoxic and immunotoxic effects of cellulose nanocrystals in vitro. Environ Moecular Mutagenesis 56:171–182
Catalán J, Rydman E, Aimonen K, Hannukainen KS, Suhonen S, Vanhala E, Moreno C, Meyer V, Perez DD, Sneck A, Forsström U, Højgaard C, Willemoes M, Winther JR, Vogel U, Wolff H, Alenius H, Savolainen KM, Norppa H (2017) Genotoxic and inflammatory effects of nanofibrillated cellulose in murine lungs. Mutagenesis 32(1):23–31
Chen Y, Wan J, Dong X, Ma Y (2013) Fiber properties of eucalyptus Kraft pulp with different carboxyl group contents. Cellulose 20:2839–2846
Chen W, Chen F, Zhang G, Liu X, Kong S, Cai W, Wang J, Du L, Wu C (2019) Fabrication of cellulose nanocrystal composite filter papers for rapid and highly efficient removal of bacteria from aqueous solutions. Cellulose 26:7027–7035
Chinga-Carrasco G, Yu Y, Diserud O (2011a) Quantitative electron microscopy of cellulose nanofibril structures from eucalyptus and Pinus radiata Kraft pulp fibers. Microsc Microanal 17:563–571
Chinga-Carrasco G, Miettinen A, Hendriks CLL, Gamstedt EK, Kataja M (2011b) Structural characterisation of Kraft pulp Fibres and their Nanofibrillated materials for biodegradable composite applications. In nanocomposites and polymers with analytical methods (ed J Cuppoletti). INTECH:243–260
Chinga-Carrasco G, Tobjörk D, Österbacka R (2012) Inkjet-printed silver nanoparticles on nano-engineered cellulose films for electrically conducting structures and organic transistors: concept and challenges. J Nanopart Res 14:1213
Chinga-Carrasco G, Averianova N, Kondalenko O, Garaeva M, Petrov V, Leinsvang B, Karlsen T (2014) The effect of residual fibres on the micro-topography of cellulose nanopaper. Micron 56:80–84
Clift MJ, Foster EJ, Vanhecke D, Studer D, Wick P, Gehr P, Rothen-Rutishauser B, Weder C (2011) Investigating the interaction of cellulose nanofibers derived from cotton with a sophisticated 3D human lung cell coculture. Biomacromolecules 12:3666–3673
Colić M, Mihajlovic D, Mathew A, Naseri N, Kokol V (2015) Cytocompatibility and immunomodulatory properties of wood based nanofibrillated cellulose. Cellulose 22:763–778
Das K, Ray D, Bandyopadhyay NR, Ghosh T, Mohanty AK, Misra M (2009) A study of the mechanical, thermal and morphological properties of microcrystalline cellulose particles prepared from cotton slivers using different acid concentrations. Cellulose 16:783–793
Delgado-Aguilar M, González I, Tarrés Q, Alcalà M, Pèlach MÀ (2015) Approaching a low-cost production of cellulose nanofibers for papermaking applications. Bioresources 10:5345–5355
Dimic-Misic K, Salo T, Paltakari J, Gane P (2014) Comparing the rheological properties of novel nanofibrillar cellulose-formulated pigment coating colours with those using traditional thickener. Nordic Pulp Paper Res J 29(2):253–270
Dimic-Misic K, Vanhatalo K, Dahl O, Gane P (2018) Rheological properties comparison of aqueous dispersed nanocellulose derived from a novel pathway-produced microcrystalline cellulose or by conventional methods. Appl Rheol 28:64474
Dinand E, Chanzy H., Vignon M. R. (1999) Suspensions of cellulose microfibrils from sugar beet pulp. Food Hydrocoll 13: 275–283
Dong S, Hirani AA, Colacino KR, Lee YW, Roman M (2012) Cytotoxicity and cellular uptake of cellulose nanocrystals. Nano Life 2(3):1241006
Dufresne A (2012) Nanocellulose – from nature to high performance tailored materials. De Gruyter, Germany. ISBN 978-3-11-025456-3
Dufresne A, Cavaille J, Vignon MR (1997) Mechanical behavior of sheets prepared from sugar beet cellulose microfibrils. J Appl Polym Sci 64:1185–1194
Dufresne A, Dupeyre D, Vignon MR (2000) Cellulose microfibrils from potato tuber cells : processing and characterization of starch – cellulose microfibril composites. J Appl Polym Sci 76:2080–2092
Ehman N, Tarrés Q, Delgado Aguilar M, Vallejos ME, Felissia F, Area MC, Mutjé P (2016) From pine sawdust to cellulose nanofibres. Cellul Chem Technol 50(3–4):361–367
Ehman NV, Lourenço AF, McDonagh BH, Vallejos ME, Felissia FE, Ferreira PJT, Chinga-Carrasco G, Area MC (2020) Influence of initial chemical composition and characteristics of pulps on the production and properties of lignocellulosic nanofibers. Int J Biol Macromol 143:453–461
Eichhorn SJ, Dufresne A, Aranguren M, Marcovich NE, Capadona JR, Rowan SJ, Weder C, Thielemans W, Roman M, Renneckar S, Gindl W, Veigel S, Keckes J, Yano H, Abe K, Nogi M, Nakagaito AN, Mangalam A, Simonsen J, Benight AS, Bismarck A, Berglund LA, Peijs T (2010) Review: current international research into cellulose nanofibres and nanocomposites. J Mater Sci 45:1–33
Eriksen Ø, Syverud K, Gregersen Ø (2008) The use of microfibrillated cellulose produced from Kraft pulp as strength enhancer in TMP paper. Nordic Pulp Paper Res J 23:299–304
Espinosa E, Tarrés Q, Delgado-Aguilar M, González I, Mutjé P, Rodríguez A (2016) Suitability of wheat straw semichemical pulp for the fabrication of lignocellulosic nanofibres and their application to papermaking slurries. Cellulose 23:837–852
Eyholzer C, Bordeanu N, Lopez-Suevos F, Rentsch D, Zimmermann T, Oksman K (2010) Preparation and characterization of water-redispersible nanofibrillated cellulose in powder form. Cellulose 17:19–30
Foster EJ, Moon RJ, Agarwal UP, Bortner MJ, Bras J, Camarero-Espinosa S, Chan KJ, Clift MJD, Cranston ED, Eichhorn SJ, Fox DM, Hamad WY, Heux L, Jean B, Korey M, Nieh W, Ong KJ, Reid MS, Renneckar S, Roberts R, Shatkin JA, Simonsen J, Stinson-Bagby K, Wanasekara N, Youngblood J (2018) Current characterization methods for cellulose nanomaterials. Chem Soc Rev 47(8):2609–2679
Fraschini C, Chauve G, Le Berre J-F, Ellis S, Méthot M, Connor BO, Bouchard J (2014) Critical discussion of light scattering and microscopy techniques for CNC particle sizing. Nordic Pulp Paper Res J 29:31–40
Fukuzumi H, Saito T, Iwata T, Kumamoto Y, Isogai A (2009) Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 10:162–165
Future Markets Inc (2014) The global market for nanocellulose
Gabr MH, Phong NT, Abdelkareem MA, Okubo K, Uzawa K, Kimpara I, Fujii T (2013) Mechanical, thermal, and moisture absorption properties of nano-clay reinforced nano-cellulose biocomposites. Cellulose 20:819–826
Gama M, Dourado F, Bielecki S (2016) Bacterial Nanocellulose: From Biotechnology to Bio-Economy. Elsevier ISBN: 978–0–444-63458-0
Gamelas JAF, Pedrosa J, Lourenço AF, Mutjé P, González I, Chinga-Carrasco G, Singh G, Erreira PJT (2015a) On the morphology of cellulose nanofibrils obtained by TEMPO-mediated oxidation and mechanical treatment. Micron 72:28–33
Gamelas JAF, Pedrosa J, Lourenço AF, Ferreira PJ (2015b) Surface properties of distinct nanofibrillated celluloses assessed by inverse gas chromatography. Colloids Surf A Physicochem Eng Asp 469:36–41
Gane PAC, Schenker M, Subramanian R, Schoelkopf J (2018) Process for the production of gel-based composite materials. Patent EP 3:266–931
Gardner DJ, Oporto GS, Mills R, Azizi Samir MAS (2008) Adhesion and surface issues in cellulose and nanocellulose. J Adhes Sci Technol 22:545–567
González I, Vilaseca F, Alcalá M, Pèlach MA, Boufi S, Mutjé P (2013) Effect of the combination of biobeating and NFC on the physico-mechanical properties of paper. Cellulose 20:1425–1435
Hassan ML, Mathew AP, Hassan EA, El-wakil NA, Oksman K (2012) Nanofibers from bagasse and rice straw: process optimization and properties. Wood Sci Technol 46:193–205
Heggset EB, Chinga-Carrasco G, Syverud K (2017) Temperature stability of nanocellulose dispersions. Carbohydr Polym 157:114–121
Henriksson M, Henriksson G, Berglund LA, Lindström T (2007) An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose ( MFC ) nanofibers. Eur Polym J 43:3434–3441
Henriksson M, Berglund LA, Isaksson P, Lindstro T, Nishino T (2008) Cellulose Nanopaper structures of high toughness. Biomacromolecules 9:1579–1585
Horseman T, Tajvidi M, Diop CIK, Gardner DJ (2017) Preparation and property assessment of neat lignocellulose nanofibrils (LCNF) and their composite films. Cellulose 24(6):2455–2468
Hu L, Zheng G, Yao J, Liu N, Weil B, Eskilsson M, Karabulut E, Ruan Z, Fan S, Bloking JT, McGehee MD, Wågberg L, Cui Y (2013) Transparent and conductive paper from nanocellulose fibers. Energy Environ Sci 6:513
Hubbe MA, Tayeb P, Joyce M, Tyagi P, Kehoe M, Dimic-Misic K, Pal L (2017) Rheology of nanocellulose-rich aqueous suspensions: a review. Bioresources 12(4):9556–9661
ISO 5351:2010 Pulps — Determination of limiting viscosity number in cupri-ethylenediamine (CED) solution
ISO/TS 21346:2021 Nanotechnologies – Characterization of individualized cellulose nanofibril samples
ISO/TS 20477:2017 Nanotechnologies — Standard terms and their definition for cellulose nanomaterial
Isogai A (2013) Wood nanocelluloses: fundamentals and applications as new bio-based nanomaterials. J Wood Sci 59:449–459
Isogai A, Saito T, Fukuzimi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3:71–85
Iwamoto S, Nakagaito AN, Yano H, Nogi M (2005) Optically transparent composites reinforced with plant fiber-based nanofibers. Appl Phys A Mater Sci Process 81:1109–1112
Jeong SI, Lee SE, Yang H, Jin YH, Park CS, Park YS (2010) Toxicologic evaluation of bacterial synthesized cellulose in endothelial cells and animals. Mol Cellular Toxicol 6:373–380
Jonoobi M, Harun J, Tahir PM, Zaini LH, Azry SS, Makinejad MD (2010) Characteristics of nanofibers extracted from kenaf core. Bioresources 5:2556–2566
Kangas H, Lahtinen P, Sneck A, Saariaho A-M, Laitinen O, Hellén E (2014) Characterization of fibrillated celluloses. A short review and evaluation of characteristics with a combination of methods. Nordic Pulp Paper Res J 29:129–143
Kargarzadeh H, Mariano M, Gopakumar D, Ahmad I, Thomas S, Dufresne A, Huang J, Lin N (2018) Advances in cellulose nanomaterials. Cellulose 25:2151–2189
Klemm D, Schumann D, Kramer F, Heßler N, Hornung M, Schmauder HP, Marsch S (2006) Nanocelluloses as innovative polymers in research and application. Polysaccharides II in Adv Polymer Sci 205:49–96
Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50:5438–5466
Klemm D, Cranston ED, Fischer D, Gama FM, Kedzior SA, Kralisch D, Kramer F, Kondo T, Lindström T, Nietzsche S, Petzold-Welcke K, Rauchfuß F (2018) Nanocellulose as a natural source for groundbreaking applications in materials science: todays state. Mater Today 21(7):720–748
Kovacs T, Naish V, O’Connor B, Blaise C, Gagné F, Hall L, Trudeau V, Martel P (2010) An ecotoxicological characterization of nanocrystalline cellulose (NCC). Nanotoxicology 4:255–270
Kruger Inc (2019) The FiloCell Advantage. Available at https://biomaterials.kruger.com/products/the-filocell-advantage/ [consulted 12.2019]
Lasseuguette E, Roux D, Nishiyama Y (2008) Rheological properties of microfibrillar suspension of TEMPO-oxidized pulp. Cellulose 15:425–433
Lavoine N, Desloges I, Dufresne A, Bras J (2012) Microfibrillated cellulose – its barrier properties and applications in cellulosic materials: a review. Carbohydr Polym 90:735–764
Li J, Wei X, Wang Q, Chen J, Chang G, Kong L, Su J (2012) Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr Polym 90:1609–1613
Li Q, Raj P, Abbas Husain F, Varanasi S, Rainey T, Garnier G, Batchelor W (2016) Engineering cellulose nanofibre suspensions to control filtration resistance and sheet permeability. Cellulose 23:391–402
Liimatainen H, Visanko M, Sirviö JA, Hormi OEO, Niinimaki J (2012) Enhancement of the nanofibrillation of wood cellulose through sequential periodate-chlorite oxidation. Biomacromolecules 13:1592–1597
Lin N, Dufresne A (2014) Nanocellulose in biomedicine: current status and future prospect. Eur Polym J 59:302–325
Lin N, Huang J, Chang PR, Feng J, Yu J (2011) Surface acetylation of cellulose nanocrystal and its reinforcing function in poly ( lactic acid ). Carbohydr Polym 83:1834–1842
Lindström T, Naderi A, Wiberg A (2015) Large scale applications of Nanocellulosic materials – a comprehensive review. J Korea TAPPI 47:5–21
Lopes VR, Sanchez-Martine C, Strømme M, Ferraz N (2018) In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: surface chemistry aspect. Part Fibre Toxicol 14:1
Lourenço AF, Gamelas JAF, Nunes T, Amaral J, Mutjé P, Ferreira PJ (2017) Influence of TEMPO-oxidised cellulose nanofibrils on the properties of filler-containing papers. Cellulose 24(1):349–362
Lourenço AF, Godinho D, Gamelas JAF, Sarmento P, Ferreira PJ (2019a) Carboxymethylated cellulose nanofibrils in papermaking: influence on filler retention and paper properties. Cellulose 26:3489–3502
Lourenço AF, Gamelas JAF, Sarmento P, Ferreira PJ (2019b) Enzymatic nanocellulose in papermaking – the key role as filler flocculant and strengthening agent. Carbohydr Polym 224:115200
Lu P, Hsieh Y (2010) Preparation and properties of cellulose nanocrystals: rods , spheres , and network. Carbohydr Polym 82:329–336
Mautner A, Maples HA, Sehaqui H, Zimmermann T, de Larraya UP, Mathew AP, Lai C, Li K, Bismarck A (2016) Nitrate removal from water using a nanopaper ion-exchanger. Environ Sci Water Res Technol 2:117–124
Mikkonen KS, Tenkanen M (2012) Sustainable food-packaging materials based on future biorefinery products: Xylans and mannans. Trends Food Sci Technol 28:90–102
Miller J (2018) Nanocellulose: producers, products, and applications. A Guide for End Users, Biobased Markets
Miller J (2019) Nanocellulose: Packaging Applications and Commercial Development. Presentation at International Conference on Nanotechnology for Renewable Materials, Chiba, Japan
Mohammadkazemi F, Azin M, Ashori A (2015) Production of bacterial cellulose using different carbon sources andculture media. Carbohydr Polym 117:518–523
Moon RJ, Martini A, Nairn J, Simonsenf J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994
Morais JPS, Rosa MDF, Filho MDSM, Nascimento LD, do Nascimento DM, Cassales AR (2013) Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr Polym 91:229–235
Moreira S, Silva NB, Almeida-Lima J, Rocha HA, Medeiros SR, Alves C Jr, Gama FM (2009) BC nanofibres: in vitro study of genotoxicity and cell proliferation. Toxicol Lett 189:235–241
Naderi A, Lindström T (2016) A comparative study of the rheological properties of three different nanofibrillated cellulose systems. Nordic Pulp Paper Res J 31(3):354–363
Naderi A, Lindström T, Torbjörn T (2014) The state of carboxymethylated nanofibrils after homogenization-aided dilution from concentrated suspensions: a rheological perspective. Cellulose 21:2357–2368
Nakagaito AN, Fujimura A, Sakai T, Hama Y, Yano H (2009) Production of microfibrillated cellulose (MFC)-reinforced polylactic acid (PLA) nanocomposites from sheets obtained by a papermaking-like process. Compos Sci Technol 69:1293–1297
Nogi BM, Iwamoto S, Nakagaito AN (2009) Optically transparent nanofiber paper. Adv Mater 21:1595–1598
Nordli HR, Chinga-Carrasco G, Rokstad AM, Pukstad B (2016) Producing ultrapure wood cellulose nanofibrils and evaluating the cytotoxicity using human skin cells. Carbohydr Polym 150:65–73
Osong SH, Norgren S, Engstrand P (2016) Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose , and applications relating to papermaking: a review. Cellulose 23:93–123
Pääkko M, Ankerfors M, Kosonen H, Nykänen A, Ahola S, Österberg M, Ruokolainen J, Laine J, Larsson PT, Ikkala O, Lindström T (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 8:1934–1941
Pahimanolis N, Salminen A, Penttilä PA, Korhonen JT, Johansson LS, Ruokolainen J, Serimaa R, Seppälä J (2013) Nanofibrillated cellulose/carboxymethyl cellulose composite with improved wet strength. Cellulose 20:1459–1468
Peng Y, Gardner DJ, Han Y (2012) Drying cellulose nanofibrils: in search of a suitable method. Cellulose 19:91–102
Pertile RAN, Moreira S, da Costa RMG, Correia A, Guardao L, Gartner F, Vilanova M, Gama M (2012) Bacterial cellulose: long-term biocompatibility studies. J Biomat Sci 23:1339–1354
Pitkänen M, Kangas H, Laitinen O, Sneck A, Lahtinen P, Peresin MS, Niinimäki J (2014) Characteristics and safety of nano-sized cellulose fibrils. Cellulose 21:3871–3886
Plackett D. V., Letchford K., Jackso, J. K., Burt H. M. (2014) A review of nanocellulose as a novel vehicle for drug delivery. Nordic Pulp and Paper Research Journal 29: 105–118
Pommet M, Juntaro J, Heng JYY, Mantalaris A, Lee AF, Wilson K, Kalinka G, Shaffer MSP, Bismarck A (2008) Surface modification of natural fibers using bacteria: depositing bacterial cellulose onto natural fibers to create hierarchical fiber reinforced nanocomposites. Biomacromolecules 9:1643–1651
Qua EH, Hornsby PR, Sharma HSS, Lyons G (2011) Preparation and characterisation of cellulose nanofibres. J Mater Sci 46:6029–6045
Ribeiro RSA, Pohlmann BC, Calado V, Bojorge N, Pereira N (2019) Production of nanocellulose by enzymatic hydrolysis: trends and challenges. Eng Life Sci 19:279–291
Rodionova G, Lenes M, Eriksen Ø, Gregersen Ø (2011) Surface chemical modification of microfibrillated cellulose: improvement of barrier properties for packaging applications. Cellulose 18:127–134
Roman M, Winter WT (2004) Effect of sulfate groups from sulfuric acid hydrolysis 1194 on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 5:1671–1677
Saini S, Falco CY, Belgacem MN, Bras J (2016) Surface cationized cellulose nanofibrils for the production of contact active antimicrobial surfaces. Carbohydr Polym 135:239–247
Saito T, Isogai A (2004) TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 5:1983–1989
Saito T, Isogai A (2005) Ion-exchange behavior of carboxylate groups in fibrous cellulose oxidized by the TEMPO-mediated system. Carbohydr Polym 61:183–190
Saito T, Isogai A (2006) Introduction of aldehyde groups on surfaces of native cellulose fibers by TEMPO-mediated oxidation. Colloids Surfaces A Physicochem Eng Asp 289:219–225
Saito T, Isogai A (2007) Wet strength improvement of TEMPO-oxidized cellulose sheets prepared with cationic polymers. Ind Eng Chem Res 46:773–780
Saito T, Hirota M, Tamura N, Kimura S, Fukuzumi H, Heux L, Isogai A (2009) Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 10:1992–1996
Sánchez R, Espinosa E, Domínguez-Robles J, Mauricio Loaiza J, Rodríguez A (2016) Isolation and characterization of lignocellulose nanofibers from different wheat straw pulps. Int J Biol Macromol 92:102–1033
Saska S, Scarel-Caminaga RM, Teixeira LN, Franchi LP, dos Santos RA, Gaspar AM, de Oliveira PT, Rosa AL, Takahashi CS, Messaddeq Y, Ribeiro SJ, Marchetto R (2012) Characterization and in vitro evaluation of bacterial cellulose membranes functionalized with osteogenic growth peptide for bone tissue engineering. J Mater Sci Mater Med 23:2253–2266
Scarel-Caminaga RM, Saska S, Franchi LP, Santos RA, Gaspar AMM, Capote TSO, Ribeiro SJL, Messaddeq Y, Marchetto R, Takahashi CS (2014) Nanocomposites based on bacterial cellulose in combination with osteogenic growth peptide for bone repair: cytotoxic, genotoxic and mutagenic evaluations. J Appl Biol Biotechnol 2:1–8
Sehaqui H, Zhou Q, Ikkala O, Berglund LA (2011) Strong and tough cellulose nanopaper with high specific surface area and porosity. Biomacromolecules 12:3638–3644
Shinoda R, Saito T, Okita Y, Isogai A (2012) Relationship between length and degree of polymerization of TEMPO-oxidized cellulose Nanofibrils. Biomacromolecules 13:842–849
Shvedova AA, Kisin ER, Yanamala N, Farcas MT, Menas AL, Williams A, Fournier PM, Reynolds JS, Gutkin DW, Star A, Reiner RS, Halappanavar S, Kagan VE (2016) Gender differences in murine pulmonary responses elicited by cellulose nanocrystals. Part Fibre Toxicol 13:28
Siqueira G, Bras J, Dufresne A (2010) Cellulosic bionanocomposites: a review of preparation, properties and applications. Polymers (Basel) 2:728–765
Siró I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17:459–494
Siró I, Plackett D, Hedenqvist M, Ankerfors M, Lindström T (2011) Highly transparent films from Carboxymethylated microfibrillated cellulose – the effect of multiple homogenization steps on key properties. J Appl Polym Sci 119:2652–2660
Skočaj M (2019) Bacterial nanocellulose in papermaking. Cellulose 26:6477–6488
Spence KL, Venditti RA, Rojas OJ, Habibi Y, Pawlak JJ (2011) A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 18:1097–1111
Stefaniak AB, Seehra MS, Fix NR, Leonard SS (2014) Lung biodurability and free radical production of cellulose nanomaterials. Inhal Toxicol 26(12):733–749
Stenius P (2014) Nanocellulose technology – conclusions and perspectives. In. Proc. Recent advances in cellulose nanotechnology research – Production, characterization and applications. Seminar, PFI, Trondheim (Norway)
Stenstad P, Andresen M, Tanem BS, Stenius P (2008) Chemical surface modifications of microfibrillated cellulose. Cellulose 15:35–45
Syverud K, Stenius P (2009) Strength and barrier properties of MFC films. Cellulose 16:75–85
Syverud K, Xhanari K, Chinga-Carrasco G, Yu Y, Stenius P (2011) Films made of cellulose nanofibrils : surface modification by adsorption of a cationic surfactant and characterization by computer-assisted electron microscopy. J Nanoparticle Res 13:773–782
Taipale T, Österberg M, Nykänen A, Ruokolainen J, Laine J (2010) Effect of microfibrillated cellulose and fines on the drainage of Kraft pulp suspension and paper strength. Cellulose 17:1005–1020
Tammelin T., Hippi U., Salminen A. (2013) Method for the preparation of nfc films on supports. WO patent 2013/060934 A2
Tanaka R, Saito T, Isogai A (2012) Cellulose nanofibrils prepared from softwood cellulose by TEMPO/NaClO/NaClO2 systems in water at pH 4.8 or 6.8. Int J Biol Macromol 51:228–234
TAPPI standard proposal WI 3021. Standard terms and their definition for cellulose nanomaterials, draft. Available at: http://www.tappi.org/content/hide/draft3.pdf
Tarrés Q, Saguer E, Pèlach MA, Alcalà M, Delgado-Aguilar M, Mutjé P (2016) The feasibility of incorporating cellulose micro / nanofibers in papermaking processes : the relevance of enzymatic hydrolysis. Cellulose 23:1433–1445
Tarrés Q, Pellicer N, Balea A, Merayo N, Negro C, Blanco A, Delgado-Aguilar M, Mutjé P (2017a) Lignocellulosic micro/nanofibers from wood sawdust applied to recycled fibers for the production of paper bags. Int J Biol Macromol 105:664–670
Tarrés Q, Ehman NV, Vallejos ME, Area MC, Delgado-Aguilar M, Mutjé P (2017b) Lignocellulosic nanofibers from triticale straw: the influence of hemicelluloses and lignin in their production and properties. Carbohydr Polym 163:20–27
Torvinen K, Sievänen J, Hjelt T, Hellén E (2012) Smooth and flexible filler-nanocellulose composite structure for printed electronics applications. Cellulose 19:821–829
USDA (2014) Cellulose nanomaterials — a path towards commercialization. Workshop Report, USDA Forest Service, Washington D.C., USA
Varanasi S, Batchelor W (2014) Superior non-woven sheet forming characteristics of low-density cationic polymer-cellulose nanofibre colloids. Cellulose 21:3541–3550
Vartiainen J, Pöhler T, Sirola K, Pylkkänen L, Alenius H, Hokkinen J, Tapper U, Lahtinen P, Kapanen A, Putkisto K, Hiekkataipale P, Eronen P, Ruokolainen J, Laukkanen A (2011) Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 18:775–786
Ventura C, Lourenço AF, Sousa-Uva A, Ferreira PJT, Silva MJ (2018) Evaluating the genotoxicity of cellulose Nanofibrils in a co-culture of human lung epithelial cells and monocyte-derived macrophages. Toxicol Lett 291:173–183
Voisin H, Bergström L, Liu P, Mathew A (2017) Nanocellulose-based materials for water purification. Nano 7(3):57
Wågberg L, Winter L, Ödberg L, Lindström T (1987) On the charge stoichiometry upon adsorption of a cationic polyelectrolyte on cellulosic materials. Colloids Surf 27:163–173
Wågberg L, Decher G, Norgren M, Lindström T, Ankerfors M, Axnäs K (2008) The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 24:784–795
Walecka JA (1956) An Investigation of Low Degree of Subsitution Carboxymethylcelluloses. Doctor’s Dissertation presented to The Institute of Paper Chemistry, Wisconsin
Wang D (2019) A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 26(2):687–701
Wang S, Cheng Q (2009) A novel process to isolate fibrils from cellulose fibers by high-intensity Ultrasonication , part 1 : process optimization. J Appl Polym Sci 113:1270–1275
Wang B, Sain M (2007) Dispersion of soybean stock-based nanofiber in a plastic matrix. Polym Int 56:538–546
Wang B, Sain M, Oksman K (2007) Study of structural morphology of hemp fiber from the micro to the nanoscale. Appl Compos Mater 14:89–103
Wang H, Li D, Zhang R (2013) Preparation of ultralong cellulose nanofibers and optically transparent nanopapers derived from waste corrugated paper pulp. Bioresources 8:1374–1384
Yamanaka S, Watanabe K, Kitamura N, Iguchi M, Mitsuhashi S, Nishi Y, Uryu M (1989) The structure and mechanical properties of sheets prepared from bacterial cellulose. J Mater Sci 24:3141–3145
Yanamala N, Farcas MT, Hatfield MK, Kisin ER, Kagan VE, Geraci CL, Shvedova AA (2014) In vivo evaluation of the pulmonary toxicity of cellulose nanocrystals: a renewable and sustainable nanomaterial of the future. ACS Sustain Chem Eng 2:1691–1698
Yu H, Qin Z, Liang B, Liu N, Zhou Z, Chen L (2013) Facile extraction 1305 of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J Mater Chem A 1:3938–3944
Zhang H., Dou C., Pal L., Hubbe M. A. (2019) Review of Electrically Conductive Composites and Films. BioResources 14: 1–49.
Zhao M, Li J, Mano E, Song Z, Tschaen DM, Gravowski EJJ, Reider PJ (1999) Oxidation of primary alcohols to carboxylic acids with sodium chlorite catalyzed by TEMPO and bleach. J Org Chem 64:2564–2566
Zhou L, Sun D, Hu L, Li Y, Yang J (2007) Effect of addition of sodium alginate on bacterial cellulose production by Acetobacter xylinum. J Ind Microbiol Biotechnol 34:483–489
Zhou YM, Fu SY, Zheng LM, Zhan HY (2012) Effect of nanocellulose isolation techniques on the formation of reinforced poly(vinyl alcohol) nanocomposite films. Express Polym Lett 6:794–804
Zhu H, Luo W, Ciesielki PN, Fang Z, Zhu JY, Henriksson G, Himmel ME, Hu L (2016) Wood-derived materials for green electronics, biological devices, and energy applications. Chem Rev 116:9305–9374
Zimmermann T, Bordeanu N, Strub E (2010) Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohydr Polym 79:1086–1093
Zywicka A, Peitler D, Rakoczy R, Konopacki M, Kordas M, Fijałkowski K (2015) The effect of different agitation modes on bacterial cellulose synthesis by Gluconacetobacter Xylinus strains. Acta Scientiarum Polonorum Zootechnica 14(1):137–150
Acknowledgments
The authors acknowledge FCT Project ToxApp4NanoCELFI (PTDC/SAU-PUB/32587/2017), cofunded by ToxOmics – Center for Toxicogenomics and Human Health (UIDB/00009/2020;UIDP/00009/2020), CIEPQPF – Strategic Research Centre Project (UIDB/00102/2020), FCT PhD. Grant SFRH/BDE/108095/2015.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ferreira, P.J.T., Lourenço, A.F. (2022). Nanocelluloses: Production, Characterization and Market. In: Louro, H., Silva, M.J. (eds) Nanotoxicology in Safety Assessment of Nanomaterials. Advances in Experimental Medicine and Biology, vol 1357. Springer, Cham. https://doi.org/10.1007/978-3-030-88071-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-88071-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-88070-5
Online ISBN: 978-3-030-88071-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)