Abstract
In this paper cellulose nanofibrils were used together with a cationic polylelectrolyte, poly(amideamine) epichlorohydrin (PAE), to enhance the wet and the dry strength of paper. The adsorption of nanofibrils and PAE on cellulose model surfaces was studied using quartz crystal microbalance with dissipation (QCM-D) and atomic force microscopy (AFM). The differences in fibril and polyelectrolyte adding strategies onto cellulose fibres were studied by comparing layer-structures and nano-aggregates formed by the nanofibrils and PAE. The results showed that when PAE was first adsorbed on the model fibre surface a uniform and viscous layer of nanofibrils could be adsorbed. When PAE and nanofibrils were adsorbed as cationic aggregates a non-uniform and more rigid layer was adsorbed. Paper sheets were prepared using both the bi-layer and nano-aggregate adding strategy of the nanofibrils and PAE. When PAE and nanofibrils were adsorbed on pulp fibres as a bi-layer system significant increase in both wet and dry tensile strength of paper could be achieved even at low added amounts of PAE. When the substances were added as nano-aggregates the improvements in paper strength properties were not as significant. Bulk and surface nitrogen content analyses of the paper samples showed that the adding strategy does not affect the total adsorbed amount of PAE but it has a strong effect on distribution of substances in the paper matrix which has a crucial effect on paper wet and dry strength development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose fibers can be mechanically divided into their substructural nano-scale units, microfibrils. Today there is an increasing interest in using cellulose microfibrils in different materials and industries, especially in nanotechnology applications, due to e.g. the size and good mechanical properties of the microfibrils (Berglund 2005). Several methods to prepare cellulose microfibrils have been introduced, all of them leading to a different type of fibril material, depending on the cellulose raw material used, the pre-treatment of the raw material and the disintegration process itself (Turbak et al. 1983; Herrick et al. 1983; Pääkkö et al. 2007; Saito et al. 2006; Tatsumi et al. 2002; Heux et al. 1999; Lowys et al. 2001; Nakagaito et al. 2005; Andresen et al. 2006). Recently, novel methods to prepare nano-scale fibrils from wood pulp, which combine either an enzymatic (Pääkkö et al. 2007) or a chemical (Saito et al. 2006) pretreatment of delignified pulp fibres with a mechanical homogenization process have been reported. They lead to less energy consumption and/or smaller and more uniform dimensions of the fibrils. The width of these fibrils has been reported to be 3–20 nm, depending on the material studied, and the homogeneity of the material was also rather good. Earlier attempts to produce microfibrillated cellulose lead to materials that were very inhomogeneous and the dimensions were in the range of one hundred nanometers and larger (Herrick et al. 1983). To distinguish the different kinds of cellulose microfibril products introduced, we thereby term these novel materials cellulose nanofibrils instead of the term microfibril which refers to the micrometer scale of the earlier materials.
When cellulose nanofibrils are mechanically disintegrated from fibres the chemical composition of the fibres is preserved. Therefore, cellulose nanofibrils—as well as cellulose fibres—have anionic charges on the surfaces. The most important anionic groups on both cellulose fibres and nanofibrils are carboxyl groups originating mostly from hemicelluloses. The anionic surface charges both affect the adsorption properties of cationic polyelectrolytes onto the fibres and fibrils, and can also participate in chemical reactions with some additives. In the paper making process different types of cationic polyelectrolytes are adsorbed on cellulose fibres in order to improve the quality of the end product and the efficiency of the process. Poly(amideamine) epichlorohydrin (PAE) is a cationic polyelectrolyte, which is used as a wet strength agent for paper (Fig. 1). In the paper making process PAE is adsorbed onto the fibres in the aqueous pulp slurry followed by a chemical reaction during or after paper drying. The reaction between cellulose fibres and PAE is a hetero-crosslinking reaction which is suggested to occur between the 3-hydroxy-azetidinium groups of PAE and the carboxyl groups of cellulose (Espy 1995; Wågberg and Björklund 1993; Lindström et al. 2005).
Wågberg and Björklund showed that the effect of PAE can be boosted by introducing new carboxyl groups on fibres through carboxymethylation. PAE has also been used together with carboxymethyl cellulose (CMC), an anionic cellulose derivative that contains carboxyl groups. It has been shown that significant improvements in paper wet strength can be achieved by pre-adsorbing CMC onto the cellulose fibres prior to PAE-adsorption (Laine et al. 2002) or by adsorbing CMC–PAE complexes (Gernandt et al. 2003; Gärdlund 2003) onto the fibres.
In order to understand the adsorption process and properties of the adsorbed layer on a molecular level, methods like quartz crystal microbalance with dissipation (QCM-D) or ellipsometry are used. The QCM-D gives in-situ information about the adsorption kinetics and the adsorbed amount at the solid/liquid interface as well as the visco-elastic properties of the formed layer (Rodahl et al. 1995). However, this technique requires that the studied material is rigidly attached on the substrate and it is oscillating with the crystal. Cellulosic fibres are too heavy to be used in QCM-studies and therefore, model cellulose films are needed. In a recent review (Kontturi et al. 2006) cellulose model surfaces for different purposes were discussed. QCM-D has been successfully used together with cellulose model surfaces to study the adsorption of wood components (Paananen et al. 2004; Tammelin et al. 2007) and cationic polyelectrolytes (Tammelin et al. 2006; Salmi et al. 2003) on cellulose. In addition, QCM-D has been utilized to study swelling of cellulose model surfaces (Fält et al. 2003; Tammelin et al. 2006) and dynamics of enzymatic activity on cellulose (Jeong et al. 2005).
Due to environmental reasons there is an interest to decrease the amount of PAE used in paper making industry. The objective of the present paper was to study the ability of cellulose nanofibrils to improve the wet-strengthening effect of PAE in order to reduce the amount of PAE used in papermaking. The cellulose nanofibrils used in this study contain significantly more carboxyl groups than reference pulp fibres due to a chemical pre-treatment prior to the disintegration process of the fibrils. Thus, the carboxyl group content influences the adsorption properties, the covalent bond formation with PAE and hence the wet strength of paper. Therefore, we expect that cellulose nanofibrils, having high amount of carboxyl groups and extremely good mechanical properties, could have an enhancing effect together with PAE on both wet and dry strength of paper. The adsorption of PAE and nanofibrils on LS-cellulose model surfaces was studied by QCM-D to gain deeper understanding about the interactions between nanofibrils, PAE and pulp fibres. In addition, the differences in fibril and polyelectrolyte adding strategies on to cellulose fibres were studied by comparing layer-structures and nano-aggregates formed by the nanofibrils and PAE.
Experimental
Materials
PAE
The cationic polyelectrolyte used in this study was a commercial sample of PAE (Wetres PA 13) kindly donated by Kemira Oyj in the form of a 13% water solution. The solution was used as delivered and fresh solutions were prepared every day by dilution with deionised water. All the other chemicals used were of analytical grade.
Cellulose nanofibrils
Cellulose nanofibrils were disintegrated from bleached sulphite pulp at STFI-Packforsk, Stockholm, Sweden using a high-pressure fluidizer (Microfluidizer M-110EH, Microfluidics Corp., Newton, U.S.) similarly to the procedure described by Pääkkö et al. (2007). Due to carboxymethylation pre-treatment of the pulp fibres, the nanofibrils contain significantly more carboxyl groups than the reference pulp. In the pre-treatment step the pulp fibres were dispersed in deionized water and solvent-changed to ethanol. The fibres were impregnated with a solution of monochloroacetic acid in iso-propanol for 30 min. The fibres were added to a NaOH solution in methanol and iso-propanol. The carboxymethylation reaction time was 1 h. After the carboxymethylation step the fibres were filtered and washed with water and acetic acid. The fibres were impregnated with NaHCO3-solution for 60 min, washed with deionized water and drained in a Büchner funnel. The pre-treatment procedure is explained more detailed by Wågberg et al. (2007). After the pre-treatment the fibres were homogenized in the fluidizer. Figure 2 shows AFM height and phase contrast images of cellulose nanofibrils on silica substrate having height of 2–5 nm and length up to several micrometers. The nanofibrils were obtained as a 2% gel suspension. The gel was diluted with Millipore water and efficiently stirred using ultrasound microtip (Sonifier S-450 D from Branson, Danbury, USA) at 25% amplitude setting. The total charge of the fibrils was determined to be 515 μeq/g by conductometric titration (Wågberg et al. 2007).
Pulp
The pulp used in the paper sheet preparation was a bleached pine kraft pulp from Metsä-Botnia, Finland. The pulp was refined according to SCAN 25:76 for 5 min to gently fibrillate the fibre surfaces. The fines material was removed using a 80-mesh wire. The pulp was washed to its sodium-form prior to the sheet preparation.
Water
In sheet preparation only deionised water and in QCM-D measurements Millipore water was used.
Methods
QCM-D
Polyelectrolyte and nanofibril adsorption on cellulose model surfaces was studied using the quartz crystal microbalance with dissipation (QCM-D) from Q-sense, Gothenburg, Sweden. The experiments were performed using the Q-Sense E4-instrument which is designed for controlled flow measurements. The QCM-D measures simultaneously changes in frequency and dissipation (frictional losses due to viscoelastic properties of the adsorbed layer) at the fundamental resonance frequency, 5 MHz, and its overtones 15, 25, 35, 45, 55 and 75 MHz. The interpretation of the data is described elsewhere (Sauerbrey 1959; Höök et al. 1998).
The sensor crystals used in QCM-D adsorption experiments were AT-cut quartz crystals, which were spin-coated with polystyrene by the supplier (Q-sense AB, Gothenburg, Sweden). Trimethylsilyl cellulose (TMSC) was deposited on the polystyrene-coated crystals using the horizontal Langmuir-Schaefer deposition technique. The preparation of the model cellulose films is presented in ref. (Tammelin 2006). Prior to use in QCM-D, TMSC deposited on the polystyrene crystal was converted to cellulose by desilylation (Schaub et al. 1993). The cellulose surfaces were allowed to swell in a 1 mM NaHCO3/0.1 mM NaCl solution overnight.
For QCM-D measurements, diluted nanofibril dispersion (1.67 g/L) was centrifuged at 10,400 rpm for 2 h to remove remaining fibril aggregates using a Optima L-90K ultracentrifuge from Beckman Coulter, USA. The supernatant was diluted and used in QCM-D. Fresh dispersions were prepared every day.
AFM
To characterize the nanofibrils and to verify the adsorption of PAE and nanofibrils on the cellulose model surfaces after QCM-D-experiments, AFM imaging was performed using a Nanoscope IIIa Multimode scanning probe microscope from Digital Instruments Inc., Santa Barbara, CA, USA. The nanofibril samples for characterization were prepared by pipetting a 0.12 g/L nanofibril suspension onto a clean silica surface. The sample was allowed to dry in room temperature overnight. The samples from QCM-D adsorption measurements were rinsed with Millipore water, taken out of the QCM-D-chamber, dried with nitrogen gas and allowed to stabilize in room temperature overnight before the AFM imaging. The images were scanned in tapping mode in air using silicon cantilevers (NSC15/AIBS) delivered by MicroMash, Estonia. The drive frequency of the cantilever was about 300–330 kHz. The size of the images was 2 × 2 μm or 5 × 5 μm and images were scanned on at least five different areas of the sample. No image processing except flattening was made.
Sheet preparation
Hand sheets were prepared according to the standard SCAN-C 26:76. Prior to the sheet preparation, PAE and nanofibrils were added to the pulp slurry. In the case of a bi-layer system, PAE was first added as a 1% aqueous solution and stirred for 5 min. The nanofibrils were then added as a 0.014–0.14% aqueous suspension (depending on the added amount) and stirred for 5 min. In the case of PAE-nanofibril-aggregates, PAE and nanofibrils were first mixed together, stirred for 5 min, added to the pulp slurry and stirred for 5 min. The salt concentration in all the systems was 1 × 10−4 M NaCl and the pH was 8. The grammage of the sheets was 60 g/m2. After drying the sheets were cured in oven at 105 °C for 10 min. The wet and dry tensile strengths of the sheets were determined according to the standards SCAN-P 20:95 and SCAN-P 67:93, respectively.
Other analyses
The surface nitrogen content of paper hand sheets was measured by X-ray Photoelectron Spectroscopy (XPS) to determine the surface content of PAE. A Kratos Analytical AXIS 165 electron spectrometer with a monochromated A1 Kα X-ray source was used. Spectra were recorded on at least three spots on each sample. For the quantitative analysis of nitrogen, the N 1s region was measured separately, using extended acquisition times (32 or 50 times the dwell time utilised in wides). The resolution in trace nitrogen measurements was as in wide spectra in order to further enhance the signal, but also because this way the data could be incorporated directly into the quantifications calculated from the wide spectra. Bulk nitrogen analysis of paper hand sheets was performed to determine the adsorbed amount of PAE using the Antek 7000 chemiluminiscence equipment at STFI-Packforsk, Stockholm, Sweden.
The zeta potential of the nanofibril-PAE aggregate liquid dispersions was measured using Doppler Electrophoretic Light Scattering Analyzer (DELSA) 440 from Coulter Electronics, USA. The concentration of the aggregate-dispersions was 0.8 –2.5 g/L depending on the amount of nanofibrils in the aggregates and all the dispersions contained 1 × 10−4 M NaCl and 1 × 10−3 M NaHCO3. The aggregates were allowed to stabilize and sediment 1 h before the measurements. Due to flockiness of the dispersions the samples were taken from the supernatant of the sedimented dispersion.
Results and discussion
Adsorption of PAE and cellulose nanofibrils on cellulose model surfaces
Adsorption of the nanofibrils on cellulose model surfaces was measured by QCM-D at the third overtone (15 MHz). Figure 3 shows that no adsorption was observed when the anionic nanofibrils (100 mg/L in 1 × 10−4 M NaCl and 1 × 10−3 M NaHCO3) were added on the anionic cellulose model surface at (t) = 10 min. This result indicates that there is no attraction between cellulose model surfaces and nanofibrils in aqueous environment. The very small increase in frequency may be due to bulk changes in fluid viscosity or very small desorption of cellulose surface.
When cationic PAE was first adsorbed on the cellulose model surface, the nanofibrils could be adsorbed on the PAE-layer. Figure 4 shows the adsorption of PAE and cellulose nanofibrils on the cellulose model surface. The PAE and the nanofibrils were both adsorbed from solutions of 1 × 10−4 M NaCl and 1 × 10−3 M NaHCO3. At time (t) = 22 min 100 mg/L PAE is added to the system. A rapid adsorption is observed as a decrease in frequency (∼18 Hz), which is proportional to the adsorbed mass. A small increase in dissipation indicates that the adsorbed PAE film is rather flat and rigid. After the PAE adsorption has stabilized, at time (t) = 93 min, cellulose nanofibrils (100 mg/L) are added to the system. Again, a decrease in frequency (∼43 Hz) is observed as the nanofibrils adsorb on the surface. Simultaneously, a considerable increase in dissipation (17 · 10−6) is observed. This indicates that the adsorbed fibril layer is very loose and viscous, containing a large amount of coupled water. At (t) = 170 min a rinsing step with a solution of 1 × 10−4 M NaCl and 1 × 10−3 M NaHCO3 is performed. No change in frequency and a minute increase in dissipation are observed after the rinsing, indicating that no desorption occurs.
It is noteworthy that the adsorbed nanofibril film is extremely loose. The ΔD/Δf change of the nanofibril film (D 17 · 10−6/43 Hz) is dramatically larger than the ΔD/Δf of the adsorbed PAE film (D 2 · 10−6/18 Hz). The ΔD/Δf change of the nanofibrils is also significantly larger than has been reported for polyelectrolyte films such as C-PAM and PDADMAC (Tammelin et al. 2006) on LS-cellulose model surfaces.
Adsorption of PAE-nanofibril-aggregates on the cellulose model surfaces
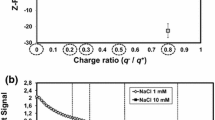
The effect of the addition strategy on the adsorption and the layer properties of the adsorbed substances were investigated by comparing the results from sequential addition discussed above with the adsorption of nano-aggregates formed by the nanofibrils and PAE. The PAE-nanofibril aggregates were mixed in different charge ratios and the zeta potential was measured to characterize the dispersions. Figure 5 shows that when there is an excess of the nanofibrils in the dispersion the charge of the aggregates is anionic and when there is an excess of PAE the charge is cationic.
The adsorption of PAE-nanofibril aggregates was studied by QCM-D. Figure 6 shows the adsorption of 3:1 (charge ratio) PAE-nanofibril aggregate dispersion on LS-cellulose model surface. The charge of the aggregates is cationic, as shown in Fig. 5. At time (t) = 24 min 100 mg/L aggregate dispersion is added to the system. The adsorption is observed as a decrease in frequency (∼22 Hz). An increase in dissipation is also observed. The frequency curve stabilizes rather rapidly, whereas the energy dissipation slowly increases throughout the experiment, until at (t) = 165 min a rinsing step with a solution of 1 × 10−4 M NaCl and 1 × 10−3 M NaHCO3 is performed. The increase in energy dissipation may be due to conformation changes in the aggregates. Both frequency and dissipation decrease during the rinsing step which indicates that something adsorbs on the surface but the layer becomes denser. This may be due to breaking up of the aggregates in the solution due to concentration changes in the beginning of the rinsing step. Some free PAE chains may then adsorb on the nanofibrils on the surface causing a stiffening of the layer. The total adsorbed amount of the cationic aggregate is rather small (∼22 Hz) compared to the adsorbed amount of PAE-nanofibril-bilayer system (∼60 Hz). The energy dissipation of the adsorbed layer is also much lower than in the bilayer system indicating that the film is more flat and dense (∼D 5.5 · 10−6). The QCM-D detects not only the dry mass of the adsorbent but also the mass of the coupled water in the adsorbed layer. One can assume that the larger change in frequency in the bi-layer system is largely due to the coupled water in the nanofibril layer which can also be observed as the higher energy dissipation values. Obviously we can only guess the actual conformation of the fibrils in the layer. However, a possible theory is that the nanofibrils adsorb as a brush like interface where the long fibrils are anchored to the PAE layer through electrostatic attraction but the electrostatic repulsion between the fibrils inside the layer inhibits a close packing of the material and this orientation causes the high dissipation values. As expected, anionically charged PAE-nanofibril aggregates did not show adsorption on cellulose. The QCM-D-curves were very similar to the curves showed in Fig. 3 where anionic nanofibrils did not adsorb on the cellulose surface and, therefore, are not presented here.
Morphology of the adsorbed PAE-nanofibril films on cellulose model surfaces
AFM imaging of the QCM-D crystals used in the adsorption studies was performed to verify the adsorption of PAE and nanofibrils on the LS-cellulose model surfaces after the experiments. Figure 7 shows AFM height images of the crystals after (a) the nanofibril addition, (b) the PAE and nanofibril bi-layer adsorption and (c) the PAE-nanofibril cationic aggregate adsorption. After the nanofibril addition no nanofibrils can be seen on the LS-cellulose substrate, as shown in Fig. 7(a). Only LS-cellulose can be seen on the surface. Hence, the image shows a very typical LS-cellulose model surface structure which is very similar to the images shown by Tammelin et al. (2006). Figure 7(b) shows an open film of nanofibrils on the LS-cellulose substrate after the PAE-nanofibril bi-layer adsorption. At least five different places on the sample were measured and all the images showed a uniformly distributed nanofibril layer on the substrate. PAE is more difficult to distinguish due to the small size of the polyelectrolyte. From the QCM-D and AFM experiments it can be concluded that by pre-adsorbing PAE on cellulose fibers one can attach a uniform and viscous layer of nanofibrils on fiber surfaces. It is also noticeable that the dissipation values from the QCM-D-experiments are extremely high compared to the very open network of the adsorbed nanofibrils, which can be seen in the AFM images. This further confirms that the layer in aqueous environment contains much coupled water.
AFM height images of LS-cellulose model films on QCM-D crystals after QCM-D experiments. The scan size is 5 × 5 μm and Z-range 20 nm. (a) LS-cellulose model film after the adsorption of nanofibrils alone. (b) LS-cellulose model film after the adsorption of PAE and nanofibrils on the surface. (c) LS-cellulose model film after the adsorption of PAE-nanofibril 3:1 aggregate
Figure 7(c) shows an AFM image of the QCM-D crystal from the PAE-nanofibril aggregate adsorption experiment. It can be seen that the adsorbed film is not uniform. Large aggregate layers can be seen on the LS-cellulose surface and on the other hand there are naked spots where nothing visible has been adsorbed on the LS-cellulose surface. Compared to the bi-layer adsorption strategy of PAE and nanofibrils, the nano-aggregate adsorption strategy has its limitations. The structure of the adsorbed film is less controlled and the film is not uniform. Only cationic PAE-nanofibril aggregates can be adsorbed on the cellulose surfaces and, therefore, only limited amount of anionic nanofibrils can be used with certain amount of PAE in order to get an adsorption of PAE-nanofibril aggregates. The ratio is naturally dependent on the charge densities of PAE and nanofibrils.
Wet and dry strength of paper sheets made from fibres treated with PAE and nanofibrils
Paper sheets were prepared from delignified softwood pulp fibres which had been treated with PAE and cellulose nanofibrils using different addition strategies. PAE and nanofibrils were adsorbed on the fibres either as a bi-layer-system, where PAE was first adsorbed on the fibres followed by the nanofibril adsorption or PAE and nanofibrils were first mixed and these nano-aggregates were added to the pulp. Figure 8(a, b) shows the wet and dry tensile index of paper sheets as a function of added amount of the nanofibrils at a constant amount of PAE. In the case of the bi-layer system both the wet and dry tensile strength increase significantly as the added amount of the nanofibrils increases. Compared to the reference, where only 5 mg/g PAE and no nanofibrils are added, the wet strength can be increased more than three times when nanofibrils are used. The dry tensile strength can be increased 2.5 times compared to the reference.
When the substances are adsorbed as aggregates the wet and dry tensile indices are not increased as much as in the bi-layer system. This can be partly due to the fact that when the amount of the nanofibrils is greater than 20 mg/g the aggregates are anionic and hence due to lack of electrostatic attraction the aggregates do not adsorb on to the fibres. Another reason for the lower values is that the aggregates are nonuniformly distributed on the fibre surfaces as was also suggested by the AFM measurements (Fig. 7c). The unexpected increase in wet and dry strength at the highest fibril addition may be due to precipitation or mechanical attachment of large aggregate flocs into the paper during sheet formation. These results are well in accordance with results of Maximova et al. (2001) who showed that by pre-adsorbing PDADMAC onto fibre surfaces a homogeneous lignin layer could be adsorbed and strength properties of paper made of these fibres were noticeably increased. In comparison, when PDADMAC and lignin were adsorbed as complexes, the adsorbed layer was very heterogenic and the aggregates adsorbed as large blobs.
From Fig. 8(a, b), it can be concluded that there is a synergistic effect to both wet and dry strength when PAE and cellulose nanofibrils are used together. We suggest that the increase in wet strength is partly due to better retention of the substances to the paper sheet but also due to chemical reactions between PAE and carboxyl groups of the nanofibrils. The increase in dry strength is most likely due to the good bonding properties of the nanofibrils. PAE alone did not have an effect on the dry strength but cationic PAE is needed to retain the anionic nanofibrils to the paper sheet.
Figure 9(a, b) shows the wet and dry tensile index of paper sheets as a function of added amount of PAE while the added amount of the nanofibrils is held constant (60 mg/1 g pulp). When PAE and the fibrils are adsorbed as a bi-layer system both wet and dry tensile index increase significantly as a function of PAE concentration, up to 5 mg/g PAE addition. After that, the wet and the dry tensile indices start to decrease. For the reference samples where only PAE is used, the wet and dry tensile indices are almost constant as a function of PAE addition. When PAE and the nanofibrils are added as nano-aggregates no linear effect on wet or dry strength can be seen. The values are rather close to the values of the reference PAE samples.
The amount of PAE retained in the paper sheets
In order to clarify more detailed the effect of PAE and nanofibrils on the wet and dry strength of paper, the amount of PAE in the sheets was determined by measuring the nitrogen content of the sheets. Bulk nitrogen content from paper sheets was measured by chemiluminiscence and surface nitrogen content was measured by XPS. The bulk nitrogen content results in Fig. 10(a) show that the nitrogen content of the sheets increases as a function of PAE addition. When only PAE is added to the system the increase is smaller than in the systems where nanofibrils are used together with PAE. The reason for this may be that only a certain amount is adsorbing to the fibres and the rest remains in the surrounding aqueous phase. When nanofibrils are added to the system, the fibrils adsorb on the adsorbed layer of PAE on the fibre surfaces and may also form complexes with unadsorbed PAE. In addition, the adsorbed nanofibrils offer new adsorption sites for the PAE which is remaining in the aqueous phase and hence more PAE can be adsorbed on the surfaces.
Surprisingly, the addition strategy of PAE and nanofibrils did not affect the attached amount of PAE even though the paper strength values were strongly affected by the addition strategy. The nitrogen content is equal for both bi-layer and nano-aggregate strategies. However, the surface nitrogen content results show a different trend than the bulk nitrogen content, Fig. 10(b). The surface nitrogen content is higher when PAE and nanofibrils are adsorbed as a bi-layer system compared to adding PAE alone. When only PAE is added the surface nitrogen content does not increase as a function of PAE addition which correlates with the paper strength results. In the bi-layer system the surface nitrogen content increases up to 5 mg/g PAE addition after which the nitrogen content first decreases and then increases again. The larger scatter in the results above 5 mg/g PAE addition may be due to aggregation of the nanofibrils and PAE before they are adsorbed on the pulp fibres, which affects the distribution of the substances on the fibre surfaces. This result also correlates with the paper strength results in Fig. 9(a, b) where the optimal addition of PAE was at 5 mg/g. Surface nitrogen content results of nano-aggregate system are not presented in the graph due to the scatter of the results (0.1−1.3% for one sample), originating from the inhomogeneous adsorption of the aggregates on the surfaces. This result is well in accordance with the AFM images where the inhomogeneity of the adsorbed film could be seen.
The bulk nitrogen analyses showed that when PAE and nanofibrils are used together, the adding strategy does not affect the total adsorbed amount of PAE. However, the distribution of substances in the paper sheet is crucial for the wet and dry strength development and the distribution is strongly dependent on the adding strategy. Figure 11 shows the wet strength of the paper sheets as a function of the bulk nitrogen content. It is evident that when PAE and nanofibrils are added as a bi-layer system, the wet strength for a certain nitrogen content value is significantly higher than for the nano-aggregate system or for the reference system where PAE is used alone.
In the case of the bi-layer addition strategy of PAE and nanofibrils, the substances are uniformly distributed on the fibre surfaces in the paper matrix and hence the wet and dry strengths of paper are improved. In the case of the nano-aggregate system the substances are very inhomogeneously distributed on the fibres and, hence, the strength properties are not improved. Compared to the reference system we observe that the nanofibrils offer more adsorption sites to PAE. Yet more importantly the carboxyl groups in the nanofibrils offer reaction sites for PAE to form covalent bonds which are needed for the wet strength development in the paper. This cannot, however, be the only reason for the significant improvement in wet strength for the bi-layer system. The nanofibrils probably have a strength enhancing effect by themselves. In addition the PAE is more concentrated to the surface in the bi-layer system. The possible brush like orientation of the adsorbed nanofibrils on the pulp fibre surfaces also provides a mechanism of increasing the amount of fibrils on the fibre surface which is similar to beating of pulp in order to increase paper strength. Hence, it is only natural that the bonding properties are improved by adsorbing nanofibrils on fibre surfaces.
At high PAE concentrations the nanofibrils and PAE start to aggregate before the fibrils adsorb on to the fibres. These aggregates may adsorb to the fibres but the adsorbed layer becomes more inhomogeneous and the strength properties are not improved anymore.
In addition, it is noteworthy that since the adsorbed nanofibril layer contains much water, this naturally will affect dewatering in the paper making process. However, during the hand sheet preparation slight changes in dewatering times were observed only with the highest added amounts (100 mg/g) of the nanofibrils. At the optimum dose of 60 mg/g the dewatering was not affected by the nanofibrils.
Similar improvements in wet and dry strength of paper as we found using nanofibrils have also been achieved by chemically modifying pulp fibres with CMC prior to PAE addition (Laine et al. 2002) and by adding CMC-PAE complexes to the fibres which had first been saturated with PAE (Gärdlund et al. 2003). The drawback of the CMC pre-adsorption approach is that the pulp treatment is rather time-consuming and high temperatures are needed. The CMC-PAE complex approach is simpler but the optimal charge ratio of the aggregates limits the added amount of PAE and CMC, and the added amount of PAE is rather high. Flocculation may also cause problems when complexes are used. Using nanofibrils together with PAE is a superior combination to improve both wet and dry strength of paper, as soon as the industrial scale production of the nanofibrils is possible. However, the surface chemical phenomena concerning adsorption and aggregate formation have to be well understood in order to achieve optimal improvements in product quality and process efficiency.
Conclusions
We have shown the high potential of cellulose nanofibrils as a biodegradable additive in papermaking. By using nanofibrils together with PAE as a bi-layer system, both wet and dry strength can be increased even at low added amounts of PAE. Hence, the amount of PAE needed to achieve a certain wet strength could be substantially decreased in paper making by using nanofibrils. In addition, due to the high dry strength values achieved, the nanofibrils could also replace some dry strength additives that are commonly used in the paper industry. The addition strategy of the nanofibrils and PAE did not affect the total adsorbed amount of PAE, but it had a strong effect on the distribution of substances in the paper matrix which had a crucial effect on paper wet and dry strength properties. By combining hand sheet testing with fundamental studies of adsorption and layer properties using model systems, a rather detailed picture of the properties of the adsorbed layer of PAE and nanofibrils on cellulose surfaces was achieved.
References
Andresen M, Johansson L-S, Steinar B et al (2006) Properties and characterization of hydrophobized microfibrillated cellulose. Cellulose 13:665–677
Berglund L (2005) Cellulose-based nanocomposites. In: Mohanty A, Misra M, Drzal L (eds) Natural fibers, biopolymers and biocomposites. CRC Press, Boca Raton
Espy H (1995) The mechanism of wet-strength development in paper: a review. Tappi J 78:90–99
Fält S, Wågberg L, Vesterlind E-L (2003) Swelling of model films of cellulose having different charge densities and comparison to the swelling behavior of corresponding fibers. Langmuir 19:7895–7903
Gernandt R, Wågberg L, Gärdlund L et al (2003) Polyelectrolyte complexes for surface modification of wood fibres I. Preparation and characterization of complexes for dry and wet strength improvement of paper. Coll Surf A 213:15–25
Gärdlund L, Wågberg L, Gernandt R (2003) Polyelectrolyte complexes for surface modification of wood fibres II. Influence of complexes on wet and dry strength of paper. Coll Surf A 218:137–149
Herrick F, Casebier R, Hamilton J et al (1983) Microfibrillated cellulose: morphology and accessibility. J Appl Polym Sci: Appl Polym Symp 37:797–813
Heux L, Dinand E, Vignon M (1999) Structural aspects in ultrathin cellulose microfibrils followed by 13C CP-MAS NMR. Carbohydr Polym 40:115–124
Höök F, Rodahl M, Brzezinski P et al (1998) Energy dissipation kinetics for protein and antibody-antigen adsorption under shear oscillation on a quartz crystal microbalance. Langmuir 14:729–734
Jeong C, Maciel A, Pawlak J et al (2005) Following cellulose activity by the quartz crystal microbalance technique. In: Proceedings of the 13th ISWFPC Symposium, Auckland, 2005, pp 495–502
Kontturi E, Tammelin T, Österberg M (2006) Cellulose-model films and the fundamental approach. Chem Soc Rev 35:1287–1304
Laine J, Lindström T, Glad Nordmark G et al (2002) Studies on topochemical modification of cellulosic fibres. Part 3. The effect of Carboxymethyl cellulose attachment on wet-strength development by alkaline-curing polyamide-amine epichlorohydrin resins. Nord Pulp Pap Res J 17:57–60
Lindström T, Wågberg L, Larsson T (2005) On the nature of joint strength in paper- a review of dry and wet strength resins used in paper manufacturing. Adv Pap Sci Tech 13th Fund Res Symp 1:457–562
Lowys M-P, Desbrieres J, Rinaudo M (2001) Rheological characterization of cellulosic microfibril suspensions. Role of polymeric additives. Food Hydrocolloids 15:25–32
Maximova N, Österberg M, Koljonen K et al (2001) Lignin adsorption on cellulose fibre surfaces: effect on surface chemistry, surface morphology and paper strength. Cellulose 8:113–125
Nakagaito A, Iwamoto S, Yano H (2005) Bacterial cellulose: the ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl Phys A 80:93–97
Paananen A, Österberg M, Rutland M et al (2004), Interaction between cellulose and xylan: an atomic force microscope and quartz crystal microbalance study. In: Gatenholm P, Tenkanen M (eds) 864 Hemicelluloses: Science and Technology, American Chemical Society. ACS Symp. Ser., Washington, pp 269–290
Pääkkö M, Ankerfors M, Kosonen H et al (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 8(6):1934–1941
Rodahl M, Höök F, Krozer A et al (1995) Quartz crystal microbalance setup for frequency and Q-factor measurements in gaseous and liquid environments. Rev Sci Instrum 66:3924–3930
Saito T, Nishiyama Y, Putaux J-L et al (2006) Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 7(6):1687–1691
Salmi J, Saarinen T, Laine J et al (2003) The effect of cationic polyelectrolytes on surface forces and structure of cellulose-polyelectrolyte interface. In: Proceedings of the 5th International Paper and Coating Chemistry Symposium, Montreal, pp 157–160
Sauerbrey G (1959) Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Zeitschrift für Physik 155:206–222
Schaub M, Wenz G, Wegner G et al (1993) Ultrathin films of cellulose silicon wafers. Adv Mater 5:919–922
Tammelin T, Saarinen T, Österberg M et al (2006) Preparation of Langmuir/Blodgett-cellulose surfaces by using horizontal dipping procedure. Application for polyelectrolyte adsorption studies performed with QCM-D. Cellulose 13:519–535
Tammelin T, Johnsen I, Österberg M et al (2007) Adsorption of colloidal extractives and dissolved hemicellulose on thermomechanical pulp fiber components studied by QCM-D. Nord Pulp Pap Res J 22:93–101
Tatsumi D, Ishioka S, Matsumoto T (2002) Effect of fiber concentration and axial ratio on the rheological properties of cellulose fiber suspensions. J Soc Rheol Japan 30:27–32
Turbak A, Snyder F, Sandberg K (1983) Microfibrillated cellulose, a new cellulose product: properties, uses, and commercial potential. J Appl Polym Sci: Appl Polym Symp 37:815–827
Wågberg L, Björklund M (1993) On the mechanism behind wet strength development in papers containing wet strength resins. Nord Pulp Pap Res J 1:53–58
Wågberg L, Decher G, Norgren M et al (2007) The build-up of polyelectrolyte multilayers of microfibrillated cellulose (MFC) and cationic polyelectrolytes. Langmuir, submitted
Acknowledgments
This work has been performed as a part of “Nanostructured cellulose products”-project in the Finnish-Swedish Wood Material Science Research Program. Prof. Tom Lindström and M.Sc. Mikael Ankerfors at STFI-Packforsk are acknowledged for providing the nanofibril samples. Dr. Leena-Sisko Johansson is greatly acknowledged for performing the XPS analysis and helping in the analyzing process. Mrs. Gunborg Glad Nordmark at STFI-Packforsk is acknowledged for performing the bulk nitrogen analysis. The experimental assistance of Mrs Marja Kärkkäinen and Mrs Aila Rahkola is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahola, S., Österberg, M. & Laine, J. Cellulose nanofibrils—adsorption with poly(amideamine) epichlorohydrin studied by QCM-D and application as a paper strength additive. Cellulose 15, 303–314 (2008). https://doi.org/10.1007/s10570-007-9167-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-007-9167-3