Abstract
Physical plasma is considered as the fourth state of matter and forms from a gas by further energy input. Cold plasmas, where only a small percentage of the atoms or molecules are ionized, are highly non-equilibrium systems, where most of the energy couple to the free electrons. In dependence on the discharge conditions, ultraviolet, visible and infrared radiation, and additional electrical fields are formed. Due to their controllability by electrical operation parameters, cold plasmas have found entrance in almost all areas of research, production, and conversion processes. Most recently, the field of plasma medicine was established both in biomedical research and in clinical application. Here, cold plasmas are used to investigate and treat conditions connected to increased microbial burdens or inflammatory processes such as chronic and acute wounds, cancers and precancerous lesions, and other disorders with involvement of the immune system. In the chapter at hand, the history of cold physical plasmas in medicine, the principles of their generation, and their composition are described. Finally, relevant applications and research efforts beyond the medical themes covered in the textbook are introduced briefly.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cold physical plasma
- Violet wand
- Plasma medicine
- Gas discharge
- Breakdown voltage
- Reactive oxygen species
- Reactive nitrogen spe cies
-
Cold plasmas are gaseous multicomponent phenomena comprising chemical entities, light, and electrical fields that can be generated and controlled by various concepts.
-
Plasma phenomena have been tested for medical use since the second half of the nineteenth century.
-
The term and field of plasma medicine developed since the early twenty-first century.
-
In medicine, cold plasmas are investigated and applied in inflammatory processes.
-
Reactive species and UV light are the main contributors of biomedical plasma effects.
-
Plasmas are used in a wide range of production and conversion processes, especially to treat surfaces, liquids, and exhaust gases.
1 Introduction
Plasmas are considered as the fourth state of matter (besides solid, liquid, gaseous) and are natural to the world we live in – the sun, stars, northern lights, or lightings are examples of matter in the plasma state. Many other plasmas are not so obvious, like the solar wind, a stream of charged particles released from the sun’s atmosphere, or the rare St. Elmo’s fire, a bright blue glow at sharp and tall objects at ships antedating severe thunderstorms. Altogether, 99% of the known and visible matter are in the plasma state. Consequently, plasmas found entrance into human myth, art, science, and technology (◘ Fig. 2.1). The term plasma is of ancient Greek origin (πλάσμα) meaning a moldable substance. Its use to describe a gas discharge goes back to Irving Langmuir, a chemist, physicist, and engineer, working on gas discharges in the 1920s, as a then colleague remembered later: “…the equilibrium part of the discharge … reminded him of the way the blood plasma carries around red and white corpuscles and germs. So he proposed to call our uniform discharge a plasma” [1]. This term poses a risk for disambiguation, since in life science, blood plasma plays a fundamental role in both research and diagnostics. To circumvent this issue, a number of amendments have been introduced for plasma discharges used in the biomedical research or application that point at the physical origin of the appearance: non-thermal plasma, tissue-tolerable plasma, atmospheric pressure plasma, cold atmospheric plasma (CAP), cold physical plasma, and many more. Most terms are still in use, and when seeking for information on plasmas, this must be borne in mind. Physical plasmas denominate a physical phenomenon where the atoms or molecules of a gas, e.g., air, become ionized by the input of energy. In contrast to thermal plasmas, which are very hot (up to millions of Kelvins background gas and/or ion temperature), cold plasmas can be operated at room temperature or at least far below 1000 K. To achieve this at atmospheric pressure, cold plasma devices have a special design. The key aspect is that only a small number of atoms or molecules is actually ionized – the lion’s share of the gas remains in the normal ground state. Furthermore, most of the energy is coupled to the free electrons; thus, its mean energy or temperature is higher than the temperature of the ions and the neutral background gas as mentioned above. Such non-equilibrium state allows a precise control of the plasma activity. Consequently, cold plasmas are tools allowing the manipulation of delicate targets. For many decades, cold plasmas have been investigated and developed for technical and industrial applications. It serves for illumination purposes (“neon tubes,” fluorescent tubes), provides the ability to produce nanometer-sized high aspect ratio structures for CPUs, allows the anti-reflexive, touch-sensitive coatings of their displays, and much more. Over the past 15 years, backed by an extensive biomedical research, cold plasmas made their way from the technical to the living world and subsequently medical application. Starting out from chronic wound management – where cold plasmas now represent a pari passu choice to classical interventions, the range of actual and potential applications covers numerous diseases that possess an inflammatory component. This includes cancer and precancerous lesions or other application fields, e.g., in dentistry or ophthalmology. This chapter will address some fundamental and scientific aspects of cold plasmas: (1) a brief history emphasizing their medical use; (2) how cold plasmas are generated; (3) which principal components cold plasmas have; and (4) a brief survey of its current applications.
Box 3 Important Notes
-
Cold plasmas are a natural phenomenon.
-
Cold plasmas comprise in part ionized (noble) gases.
-
Cold plasmas are utilized by humankind for industry, consumers, and healthcare.
2 A Brief History of Cold Plasmas
Electricity for medical purposes fascinated men since antiquity. In the first half of the nineteenth century, methods and devices were established for “franklinization” (pulsed static electricity), “galvanization” (direct currents), or “faradization” (alternating currents) [2]. The resulting effects remained mysterious, and the application was, from the medical point of view, more regarded as fraud and quackery than earnest therapy. Later in the same century (1860–70), legitimate scientific studies appeared, conducted by various European scientists. In the late nineteenth and the beginning of the twentieth centuries, pioneering work by Tesla, d’Arsonval, and Oudin led to devices that allowed the application of a cold plasma to the human body [3,4,5]. Using high voltage (10–300 kV) and high frequency (≈10 kHz), stronger spark-like discharges or milder dielectric barrier discharges were applied to stimulate body functions or pain reduction during teeth pulling. In all cases, significant electrical power is delivered to larger areas of the human body, leading to non-specific generalized effects. In principle, the setup consisted of the energy source (a battery), an induction coil (Tesla coil), two capacitors (Leyden jars), spark gaps for wave formation, and an applicator – a coil (d’Arsonval), brush electrodes (Oudin), or plate electrodes (Tesla). The noisy devices were in use until the 1920s. The achieved medical effects were described as “creating … intense skin irritations and erythema by secondary capillary dilation, leading to decreased arterial blood pressure, among other things. Depending on the disposition of the patient, erythema lasting for hours was reported…” ([6] and citations therein). Microscopic alterations such as pyknosis and leucocytic infiltrations that can be interpreted as local cell death by apoptosis and inflammatory processes were described. The stage of the early research did not allow full understanding and attributed these effects to a de novo generation of proteins (“protein therapy”) or simple “anionic” effects. Underlying the poorly understood medical effects, chemical, mechanical, and optical processes were discussed that tell of an early recognition of the plasma compositions. Especially the splitting of electrons from nitrogen (N2) and oxygen (O2) molecules and the formation of molecules like ozone, nitric, and nitrous acid as well as the UV radiation were described and come close to modern interpretation of cold plasmas [7] (◘ Fig. 2.2).
Early application of discharges for medical application. Effluviation using an Oudin resonator system, around 1900 (left) and early violet ray device (vacuum electrode) by Monell, around 1910 (right). (© IEEE, reprinted with permission from [2])
The role of the assumed mechanical effects – the formation of ion winds by acceleration of air molecules – might be discussed controversially. The technology was early on further developed by the German physician and scientist Nagelschmidt around 1908 into the diathermy, a principle exploiting the production of heat by high frequency alternating currents flowing through a tissue [8] (◘ Fig. 2.3).
Violet ray plasma device, assortment of electrodes, hand-held wand, and control unit. (Reproduced with permission from [6]/CC BY 4.0)
This therapy option is still open and used for pain reduction and suppression of inflammatory peaks in various rheumatoid disorders, but the modern devices lack the plasma aspect their ancestors had.
Box 3 Important Notes
-
Cold plasmas are investigated for medical purposes since mid-nineteenth century.
-
Experimental spark discharges yielded general effects, encouraging further work.
-
Electrosurgery was invented as a spin-off in 1926, and since is in use.
-
Violet ray devices (small light-emitting plasmas) were in use until the mid-1940s.
-
Research on cold plasmas for medical purposes restarted in the late 1990s.
Starting from 1926, surgical diathermy (electrosurgery) was established. The procedure involved the use of high-frequency electric current in surgery as either a cutting modality or else to cauterize small blood vessels to stop bleeding (cauterization). The devices possess characteristics of cold plasma devices; however, they are considerably hotter than most of the cold plasma sources under research today. The American physicians Strong and Monell promoted the use of vacuum electrodes with different shapes around 1910 [9]. Under reduced pressure, a glow discharge was ignited that functioned as a transmitter between the higher voltage electrode and the human body, reducing the energy densities significantly and allowing patients self-treatment without the physician’s on-hand supervision. Emitting X-rays and/or UV light, these devices were used to “promote circulation, increase metabolism, optimize body function” (Strong), various skin diseases (Monell), and as disinfecting device due to its ozone formation. The UV emitting vacuum electrodes (termed “violet wand” or “violet ray” devices) were kept in service in the United States for a vast variety of applications, including lower back pain, carbuncles, or nasal catarrh. Due to limited – if not completely missing – evidence, legislation stopped its use from the 1940s. Approaches that are more recent strive to rehabilitate these devices [6]. Cold plasma devices started to reoccur in the interests of scientists and physicians in the mid-1990s, initially for “sterilization” procedures of tools and infectious waste [10]. Quickly, interest turned to human cells and tissues, with pioneering work from Stoffels and co-workers [11,12,13,14,15,16,17], Fridman and colleagues [18,19,20,21,22,23,24], Pouvesle and Robert [25,26,27], Weltmann/von Woedtke and colleagues [28,29,30,31,32,33,34,35], Lademann [36,37,38,39,40], and more (reviewed in part in [41,42,43]).
3 How to Generate a Cold Plasma?
Creating a plasma sounds easy: add energy to gaseous matter – et voilà – a spark or glow can be observed. Among chemical processes, heating, or compression, the generation via electric fields in electrical gas discharges or electromagnetic radiation are the most prominent methods for the generation of technical plasmas. In the simplest setup, two metal plate electrodes with a certain distance between each other and connected to a high voltage source are placed in a glass tube that is filled with a gas of desired composition and pressure. Air and all other gas mixtures or pure gases are electrical insulators at standard conditions. Its background ionization (by cosmic rays or radioactive radiation, or maybe, previous discharge cycles) is not sufficient to allow formation of an electrical gas discharge. However, if these initial electrons and ions are accelerated by outer electric or magnetic fields, they gain higher mean energies. If the energy of the electrons exceeds the threshold for ionization of the gas particles, electron avalanches are generated and the breakdown occurs. The voltage threshold or breakdown voltage is specific on the gas composition (electron shell configuration, purity of the gas) and solely depends on the product of gas pressure and electrode distance [44]. As shown in ◘ Fig. 2.6, the so-called Paschen-curves show a gas-specific minimum, i.e., the number of collisions between energetic electrons and background gas species has an optimum at which a so-called self-sustained gas discharge is formed. Counter-intuitively, at some distance and pressure settings, the breakdown voltages rises. At short inter-electrode distances, the number of collisions is too low to generate sufficient secondary species at a given voltage. Thus, a higher voltage is needed to ensure that the electrons gain enough energy between two ionizing collisions. Of note, the noble gases, especially neon and neon-mixtures with argon or helium are most easily ignited (see neon tubes). Molecular gases, such as hydrogen, nitrogen, or oxygen need far higher voltages to form a plasma under these conditions. As an example to interpret the graph, take the atmospheric pressure (1021 mbar or 760 mm Hg) [45].
A vertical line corresponding to 76 on the x-axis gives the breakdown voltage for a gas between a pair of flat electrodes separated by 1 mm. For air, this gives a breakdown voltage of about 5 kV DC. However, electrode design such as surface roughness and sharp edges can reduce the breakdown voltage considerably. Of note, electrodes are not essential for gas breakdown – any method producing a strong electric field will also cause the gas tube (or regions therein) to glow: discharge tubes light up when placed near resonant radio antennas and coils. Once a glow discharge has been ignited, a low resistance current-path is formed that sustains the discharge. The glow will continue, until the current falls below a value called the “extinction point.” The voltage at the extinction point is usually considerably lower than the breakdown voltage, i.e., the discharge is difficult to get going, and then difficult to stop once started. In a cold, non-equilibrium plasma, most of the present atoms or molecules remain in the lowest energy ground states. Beside ionization, atoms and molecules are electronically excited to higher states. The spontaneous de-excitation of these unstable excited states lead to emission of photons and generates the typical appearance of a plasma by the emission of light.
Box 3 Important Notes
-
To ionize a gas, energy is needed – often, electrical energy is applied by an electrode.
-
Most gases, including air, can be ionized, but noble gases (argon, helium) are ideal.
-
Localization and movement of electrons are most important to generate/maintain a discharge.
-
Cold plasmas can assume any shape; jets and sheet-like discharges dominate in medical applications.
-
Modern devices are designed to deliver a stable, cold, and safe plasma discharge.
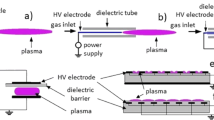
In ◘ Fig. 2.4, a typical current vs. voltage curve of a DC-operated discharge tube is shown. The voltages, currents, and relative length of the regions depend on the plasma sources design, but the general discharge events are universal. At low voltages and currents, a Townsend discharge (or dark discharge as it emits almost no light) is generated. Irradiating the electrode (more precisely the cathode) produces additional initial electrons by the photo-effect and thus, increases the current. Increasing the inter-electrode voltage enhances the number of ionizing collisions, and consequently leads to a point where the current increases significantly for minute increases in voltage – an unstable voltage plateau is reached. Upon further increase of the current, more and more ions – which are by orders of magnitude less mobile than the electrons – will remain in the discharge gap and form a space charge. The maximum of the space charge potential shifts towards the cathode with increasing current. In this situation, the electrons will gain most of its energy in the region between the cathode surface and the space charge potential maximum, the so-called cathode fall. This is the most important feature of the so-called glow-discharge, which is characterized by alternating dark spaces and light emitting areas (“glows” and “columns”). The glow discharge regime is stable over a wide range of currents. With a further increase of the current, the electrode area will be covered more and more and finally the full electrode will be glowing. Then, a further increase of the current leads to an increase of the conductivity, accompanied by an increase of the running voltage, and the glow discharge is called anomalous. This phase transits almost abruptly to the arc discharge if the current is further increased. In this mode, high currents pass through the plasma, while the potential between the plates falls. In arcing mode, the particle temperatures increase and the discharge is constricted to a small volume, resulting in potential damage of the device. Furthermore, thermionic emission of electrons becomes important. Thus, heating the electrode externally or by bombardment with particle beams favors arcing at lower currents. In clinical application, some surgery tools (e.g., coagulators) use this regime. Due to the atmospheric operational pressure, a rapid thermalization between electrons and the background gas takes place; thus, such plasmas are hot plasmas (some 1000 K gas temperature). In all other biomedical applications, gas discharges are operated as cold, non-equilibrium plasmas similar to the glow discharge mode. The main challenge devices have to take is to control, sustain, and modulate the discharge in a way that is safe, reliable, and reproducible. To achieve this, cold plasma devices operate typically at low energy input, have a special electrode configuration, and may use a gas flow for cooling, and limit power dissipation [46,47,48,49]. An extremely rich variety of non-equilibrium atmospheric pressure plasmas exists ranging from Townsend discharges to spark discharges that span a range of ionization degrees of ten orders of magnitude. The most important in the scope of biomedical applications are dielectric barrier discharges (DBDs) and plasma jets (◘ Fig. 2.5). As the name suggests, the main feature of the DBD is the presence of a dielectric barrier in the discharge path, e.g., by covering at least one of the electrodes. This can be any insulating material, but quartz, glass, or ceramics are favorable. Polymers, which would allow lightweight and sturdy plasma sources, must be carefully tested for suitability since plasmas can etch them, leading to a loss of function or impurities in the discharge [50].
Idealized current vs. voltage characteristic for a gas discharge tube. (Reprinted with friendly permission of David Knight from ► http://g3ynh.info/disch_tube/intro.html)
Principle setup of a dielectric barrier discharge (left), a plasma jet (center), and recent flexible dielectric barrier discharges (silicone, fabric). Notice the presence of a powered electrode and a counter electrode, an insulator, and a high voltage source. (Figure adapted by K. Wende basing on drawings from R. Brandenburg)
The dielectric surface is charged during the breakdown. This reduces the local electric field that leads to the extinction of the discharge activity within nano- to microseconds. Consequently, it limits the current density and local energy dissipation. Due to the capacitive character of the discharge arrangement, DBDs are operated with alternating voltage, usually in the kHz-range or by short high-voltage pulses. In most molecular gases, but also in argon or mixtures of noble gases with molecular gases, the streamer mechanism leads to so-called microdischarges that visually appear as filaments. Usually, several discharge channels are ignited and decay within each half-cycle of the high-voltage period. In case of higher frequencies in the MHz range, current limitation by the dielectric barriers is less effective and breakdown voltage is lower [51]. The discharge operation changes significantly since charge carriers in the volume do not completely diminish between two subsequent high voltage half-periods. The mobility of the ions is too small to follow the rapid changes of the applied electric field. The ions will be trapped at the discharge barriers and be deposited as surface charges there. In other words, the role of barrier is not to induce the self-pulsing character described above. The discharge operates in steady state regime and it can be characterized as a capacitively coupled plasma. The role of the barrier is mainly the protection of the electrode material. Plasma jets are gas discharges operated in a non-sealed electrode arrangement and projected outside the electrode arrangement into the environment [48]. The plasma region outside the inner electrode configuration (“effluent” or “plume”) is generated from the core plasma by a gas flow. Gas flow contributes to the removal of heat away from the discharge and, at the same time, the enhancement of the transport of plasma species away from the discharge and onto the substrate. In many cases, noble gas (Ar, He) is used as a carrier gas because of the much lower breakdown voltage compared with air or other molecular gases (see the Paschen curves, ◘ Fig. 2.6). With the noble gas flow, a channel in which ionization takes place preferentially is generated. This effluent is most often composed of so-called guided streamers confined in the gas channel. Plasma jets are well suited for the delivery of localized treatments, e.g., wounds. Plasma jets have been realized with different electrode configurations and can be operated with DC and AC high voltage of different frequencies (from Hz to GHz).
Breakdown voltage of various gases in relation to electrode distance and pressure (Paschen curve). Observe bathtub shape of the curves (see text). (Adapted based on Wittenberg [52] by Dave Knight (► http://g3ynh.info/disch_tube/intro.html). Reprinted with permission)
4 Principal Composition of a Cold Plasma
Cold physical plasmas are multi-component systems, similar to its namesake, the blood plasma and inherently not in equilibrium. The principal components are ultraviolet, visible, and infrared light, electrical fields, free electrons, ions, radicals, and excited gas atoms/molecules, dispersed in the bulk of slow gas atoms or molecules in ground state. These slow particles keep the average temperature of the system low. Although the electrons have, in certain areas of the plasma, energies of a few electron volts (eV), relating to a temperature of several 10,000 K, the whole plasma remains significantly cooler (Te >> Tgas). Depending on the intended application and the design of the plasma source, temperatures of the visible plume of plasma jet devices range close to room or body temperature. In normal application mode, plasma sources for the biomedical application are designed in a way that thermal damage of the tissue can be excluded. In addition, thermal load to the skin can be minimized assuming a brush-type treatment regimen with a velocity of about 10 mm/s [39] (◘ Fig. 2.7).
4.1 Radiation
The radiation emitted by cold physical plasmas is classified as vacuum ultraviolet (VUV) light (<200 nm), UVC, UVB, and UVA light (200–280 nm, 280–320 nm, and 320–400 nm, respectively), visible light (mainly below 450 nm), and infrared light of the NIR range (700–1000 nm) [53,54,55]. The spectrum of the plasma and, thus the contributions to the different spectral ranges strongly depends on the gas composition and the energy density of the plasma. An example is given below (◘ Fig. 2.8).
Qualitative emission spectrum of an argon plasma jet (200–1000 nm). Below 200 nm (vacuum-UV) argon excimers radiate (small insert). Note the significant emission in the near-infrared range above 700 nm and in the UVB/UVA range (300–400 nm). (Figure adapted by K. Wende from Mahdikia and Jablonowski [54])
By the ultraviolet radiation, photochemical processes can be initiated. Depending on the wavelength, photon energies range between 12.4 eV or 1200 kJ mol−1 (100 nm) and 3.1 eV or 300 kJ mol−1 (400 nm). Accordingly, the fission of chemical bonds is possible: many single bonds in organic molecules have bond energies starting from 305 kJ mol−1 (C–N) and 347 kJ mol−1 (C–C).Footnote 1 Chemical modifications by plasma-derived photons have been described but are more of scientific than of clinical interest [56, 57]. The penetration of the energy-rich VUV and UVC into cells or tissues is limited, as the photons are absorbed immediately, e.g., by the Stratum corneum’s keratohyalin and urocaninic acid, interstitial liquids, or epithelial cells. When adhering to the suggested maximum treatment times, the overall radiation energy delivered does not exceed the accepted limits of the International Commission on Non-Ionizing Radiation Protection (ICNIRP) recommendations (30 Jeff m−2)Footnote 2 [58]. For the argon plasma jet kINPen, only 1/30 of this dose is delivered [59]. In other applications, predominantly when using low-pressure plasmas, e.g., the inactivation of bacteria and spores, UV light contribute significantly to the effect [60,61,62]. The infrared light emitted by the discharge can penetrate tissues and cells significantly better than the short-wavelength counterparts. The observed increase in blood circulation and tissue oxygenation after plasma treatment of chronic wounds may in part be induced by the near-infrared radiation, as observations using plasma treatment and research focusing infrared light in wound management suggest [26, 63,64,65].
Box 3 Important Notes
-
Cold plasmas are cocktails of reactive components.
-
Small reactive oxygen and nitrogen species (ROS/RNS), ultraviolet light, and electrical fields are most important in medical use.
-
Generated ROS/RNS mimic those occurring naturally in cells, allowing interference/modulation.
-
Species in gas phase and liquid phase differ due to short lifetimes.
4.2 Electrical Fields
The role of the electric fields is discussed ambivalently in the recent years. It is generally accepted, that cold physical plasma discharges create electrical fields around them. The intensity and temporal and spatial changes along with the ignition process and/or the development in the discharge are under investigation [66,67,68]. It may be assumed that the electrical field may reach three to ten times higher strength at condensed (and short-lived) phenomena, such as streamers and bullets, in the discharge than the field necessary to create the discharge in the first place. Although these high electric fields can exert significant impact in biological systems, their role in plasma medicine is not fully elucidated yet. Due to the applied alternating currents to ignite the plasma, the generated electric fields fluctuate in the millisecond (kHz plasmas), microsecond (MHz plasmas), or nanosecond range (ns-pulsed plasmas). The fields can be extremely inhomogeneous, and their strength change with time, direction, surrounding gas, and discharge cycle, making the investigations difficult. Theoretically, they can be strong enough to allow the manipulation of cell membranes (kHz, MHz plasmas) or cell organelle membranes (ns-pulsed plasmas), as research using pulsed electric fields suggest [69]. However, to form a pore in a cell membrane, a potential of 1 kV cm−1 is needed, for a mitochondrion at least 20 kV cm−1. Whether standard plasma sources can deliver such high electrical fields outside the electrode area, e.g., at the tip of an effluent, is under debate, but a number of recent publications foster this notion [70,71,72]. Some substantial hypotheses assume a synergistic effect of the electric fields with small reactive species that are a major component of cold physical plasmas [73, 74]. An oxidation of the cell membrane lipids would yield in an increased membrane fluidity and fragility, facilitating pore formation [75].
4.3 Reactive Species
Small chemical entities like neutrals, ions, and radicals are a major fraction of the plasma. They are often referred to as species, circumscribing a (dynamic) mixture of short-lived and fast-reacting small chemicals, predominantly composed of single atoms or ions thereof (e.g., O*, O+), radicals (e.g., hydroxyl radicals OH, nitric oxide NO), or neutrals (e.g., hydrogen peroxide, H2O2).
4.3.1 In the Gas Phase
Composition and proportions of these species vary massively in dependence of plasma source design, working gas, presence of modifiers (e.g., molecular gas admixtures such as oxygen or nitrogen), power consumption, location in respect to the electrodes, and time. Numerous diagnostic methods like two-photon absorption laser-induced fluorescence (TALIF), Fourier-transformed infrared spectroscopy (FTIR), cavity-enhanced ring-down spectroscopy (CRDS), some offering spatio-temporal resolution in combination with computational models have been developed and deployed to investigate gas phase composition of cold physical plasmas. With time, an extensive hoard of knowledge has been compiled that cannot be reflected in full in this textbook chapter. The interested reader is referred to primary knowledge sources such as the following reviews [46, 76,77,78,79,80].
In the active plasma zone, close to the igniting electrode, primary species are formed by collisions and electron impact and collision with other reactive species. In jet plasmas driven by a noble gas (argon, helium, neon, or mixes thereof) excited states, ions, and excimers (excited dimers) or molecular ions of the respective noble gas atoms form. In the case of an argon jet (kINPen), argon excimers (Ar2 (a3\( {\Sigma}_u^{+}\left)\right) \) and various excited argon states are present in the active plasma zone. These species have high energies and are mostly short-lived, and their presence strictly correlates with the electric field providing the energy for the discharge. In ◘ Fig. 2.9, the spike-like appearance of higher energy states of argon (e.g., Ar+, Ar(4s, 3P1)) corresponds to the plasma sources alternating current delivering the necessary energy in a megahertz frequency. The de-excitation processes are almost as quick as the formation (e.g., for the Ar+ ions) and in the order of one microsecond and even faster. De-excitation can be linked with the emission of (UV) light (argon excimer radiation –126 nm), the collision with walls, or the inelastic collision with atomic or molecular species (quenching). If energy supply is cut, the plasma extinguishes.
Densities of argon species in the kINPen (model). (Reproduced with permission from [81]/CC BY 4.0)
In addition, primary reactive oxygen or nitrogen species are generated by electron impact if suitable molecules such as oxygen (O2), nitrogen (N2), or water are present in the active plasma zone. For example, oxygen molecules are cleaved by fast electrons (3.9 eV) to form atomic oxygen:
Nitrogen molecules are fragmented to atomic nitrogen; water molecules yield hydroxyl radicals (OH) and hydrogen atoms (H). In addition, excited molecule states are formed, e.g., singlet oxygen, a molecule where in contrast to the ground state oxygen the electrons in antibonding molecular π-orbitals flip spin and orbital occupation. This results in an unstable molecule that has a higher oxidative potential than oxygen in the ground state. It, e.g., quickly reacts with the essential amino acid histidine, leading to a loss or gain of function of proteins in dependence of the histidine position. In the photodynamic therapy (PDT), where a photosensitizer and intense light are used to produce singlet oxygen, similar effects are exploited [82, 83].
The short-lived primary species, especially the noble gas excited states form in collision with molecular gases in the plasma core, but also at the intersection between the active plasma zone or an effluent with ambient gas molecules, secondary species:
This example uses molecular oxygen, but all other gas molecules can be attacked to form radicals or excited states. The extent and location of the reaction between excited noble gas species and ambient gas molecules (e.g., O2, N2, H2O of the air) are determined by the design of a plasma source and the respective gas flow dynamics. In the kINPen plasma jet, using argon with a higher gas flow velocity, and turbulences at the effluent, ambient air interface result in its significant enrichment with molecular gas species. As a result, this plasma source shows a desired high basal production of reactive oxygen and nitrogen species (ROs/RNS). ◘ Figure 2.10 illustrates the different reactive species present in the discharge, and their respective origin (either via electron impact or reaction with argon ions; also see ◘ Fig. 2.9). In the case of the argon-driven plasma jet kINPen (see ► Chap. 16), the generation of reactive species via secondary reactions of excited argon species dominate the profile.
Density of primary ROS/RNS generated by a single discharge event at different dry synthetic air mole fractions (10−4, 10−3 and 10−2). Contributions from direct electron impact reactions (green) and reactions involving excited argon species (pale rose) are distinguished. Note the tenfold higher production of singlet oxygen (O21\( {\Sigma}_g^{+} \) and O21Δg) and hydroxyl radicals/hydrogen atoms (H/OH). (Reproduced with permission from [81]/CC BY 4.0)
In the case of a helium jet plasma source that has been designed specifically for research purposes (μAPPJ or COST-Jet) [84,85,86,87], species output is low when no molecular gas has been added to the working gas flow. The lower gas flow rate and significantly smaller specific weight of helium cause laminar gas flow dynamics and reduce the mixing with ambient air and with that the formation of secondary reactive species. Secondly, the very high energy of excited helium states (e.g., He*(23S1), He2* helium excimers, He+ ions) is less well transferred to molecular gases as in the case of the excited argon species. When molecular gases, especially oxygen, are added to the helium gas flow, this source is very effective in the production of atomic oxygen [88]. Cell experiments using this condition revealed a high toxicity towards cancer cells [89]. Atomic oxygen is also a major component in other (experimental) plasma sources [90,91,92].
By further elastic and inelastic collision of primary and secondary species with ambient molecules and themselves, downstream (tertiary) species are created. By the reaction of higher energy nitrogen species (N2 (a)) with molecular oxygen, a number of nitrogen oxides occur (dinitrogen oxide N2O, nitric oxide NO, nitrogen dioxide NO2, nitrogen trioxide NO3, and dinitrogen pentoxide N2O5). Having a different lifetime and reactivity, interconversion or reaction with other species present yield in highly dynamic non-equilibrium chemistry. The long-lived nitrogen species nitric and nitrous acid (HNO2, HNO3), and N2O5 are the major products. By tertiary reaction of (excited) oxygen species among themselves or with hydrogen atoms (H), long-lived reactive oxygen species like ozone (O3) or hydrogen peroxide (H2O2) are formed along the metastable hydroperoxide radical (HO2•).
4.3.2 In the Liquid Phase
The interaction between a plasma and a liquid became a target of interest with the increasing use of cold plasmas in biomedical applications in the recent 20 years only. The liquid phase is by far less well investigated than the gas phase. A number of reasons contributed to this imbalance: most plasmas are single-phase gaseous phenomena, and fundamental research concentrated on its description. The investigation of chemical and mass flow processes in liquids is hampered by the physics of itself: a gas-liquid interface confines the body of the liquid (the bulk). This interface, a few nanometers in width, is an extremely inhomogeneous environment. Local concentrations and species lifetimes are significantly different compared to the liquid bulk due to the molecular structures at the interface that affects how solute molecules are adsorbed or solvated into the liquid. Due to high dynamics, the investigation of the interface is demanding and limited by the current technical development of scientific instrumentation. At the gas-liquid interphase, the generation of tertiary species is assumed. Among these are nitric oxide radicals (•NO) [93], peroxynitrite ions (ONOO-) [94,95,96], and hypochlorite (from atomic oxygen reacting with chloride ions) [89, 92, 97]. While NO is also generated in the gas phase of cold plasmas, its transport through the interface into the liquid bulk is not favored due to its bad solubility in water. Data indicate that it is formed at the gas-liquid interface by the reaction of nitrogen dioxide radicals and atomic oxygen. Peroxynitrite can be formed by different reactions, e.g., between nitrite and hydrogen peroxide. This reaction takes place only in rather acidic conditions below pH 3.5 (blood pH is 7.4). The necessary low pH is reached at the thin interface between the gas phase and the liquid phase, in the bulk of distilled water treated for moderate to long times, but not in physiologic liquids that typically contain buffer systems (e.g., phosphate buffered saline, cell culture media, or blood). Atomic oxygen is a primary species, formed in the active plasma zone. It is short-lived, and penetration into the bulk of a liquid has not been observed so far. At the interface, it may be scavenged by chloride ions (Cl−), common to all biological systems yielding hypochlorite (OCl-). Hypochlorite is a moderate oxidant, and preserves the chemical activity of atomic oxygen that otherwise would be lost. It undergoes an aging process, with stable chlorate (ClO3−) as the final product. When deionized water is treated, atomic oxygen reacts with water to give hydroxyl radicals that recombine to form H2O2 and are quenched by the reaction with impurities and walls.
The liquid bulk, acting as a reservoir for the plasma-generated species, is a 1000-fold denser material than a gas at normal pressure. While 1 L of water contains ≈3.3 × 1025 H2O molecules, 1 L of oxygen (at normal pressure) contains only ≈2.7 × 1022 molecules (1 mol of gas ≈ 22.4 L). This reduces species lifetimes due to a significantly higher number of collision than in gas phase, and the water molecule, a dipol, is very reactive.
4.3.3 In the Solid Phase
For many decades, targets of plasmas were solids – metals, glass, or similar materials [98,99,100]. The interaction with such surfaces has been investigated thoroughly, but the transferability of the results to human or animal tissue is very limited.
5 Applications of Cold Plasmas
Along with the versatility of cold plasmas, its spectrum of uses is too broad to be covered in this chapter except for a brief outlook predominantly introducing literature for further reading (e.g., compiled in [101, 102]). The following paragraph will focus only on applications outside plasma medicine to provide an overview on relevant application fields of cold plasma technology. Besides the direct use of an open or hidden plasma source like in plasma medicine or in illumination technology, plasmas are predominantly applied in numerous production processes. In automotive, surfaces, especially of the window glass, are refined to increase surface tension with the effect that water droplets run off the glass without the need for wiping. Dominating percentages of the facades of recent buildings are made of glass – yielding to the need of special glass properties such as heat and light reflection, insulation, or endurance against harsh conditions. Thin surface coatings made of metal oxides and metal nitrides are generated on the glass by industrial cold plasma processes (plasma sputtering). In addition, etching of hard and/or delicate surfaces can be achieved by plasma guns, yielding ultra-precise optical surfaces. The heart piece of the world’s largest optical telescope to be, the extremely large telescope (ELT) built by the European Southern Observatory on Cerro Amazones in the Atacama Desert of northern Chile (Antofagasta, Chile), is a 39 m diameter segmented primary mirror. Each of its 798 pieces with 1.45 m edge length will be refined by a plasma ion beam polishing process to reduce surface roughness down to a few nanometers [103]. Polymers and plastics, inevitable items of modern technology, are etched by plasma to improve bonding strength of glues or to facilitate imprinting. Here, the surface of the respective material is chemically modified, e.g., to contain polar chemical groups such as amino or carboxyl groups. Surplus to the general improvement of the polymers wettability, these groups can be used as chemical anchors to immobilize proteins or other biomolecules, opening the field of biosensors and other functional surfaces [104]. Further, metals and ceramics intended for implantation into the human or animal body may be modified to foster osseointegration, both in dentistry (dental implants) [105,106,107] and in trauma or salvage surgery (e.g., joint replacements) [108, 109]. Plasma sputter processes achieve a surface suitable for cell attachment, and release antimicrobial agents such as silver ions to reduce the risk of infections [110, 111]. Also in the energy industry, plasmas are of relevance. Switching a few hundred thousand volts is a demanding task needed in power plants – the simple disconnection of two pieces of conducting metal that works well to switch off the light at home does not yield the desired result: a plasma arc forms between the two pieces, and since the plasma is a decent conductor, the current is not stopped. A whole research field has formed around this phenomenon, and modern high circuit breakers use a combination of suitable enduring materials, electrode shapes, hard to ionize gas fillings, and timing to disconnect the energy supply safely [112]. Research is needed in the case of direct current circuit breakers that are of interest in terms of photovoltaic energy generation and direct current energy grids [113]. This also applies towards the use of plasma processes for the production of new materials, for hydrogen technologies, energy conversion and storage, where efficiency increases and upscaling are required in addition to providing unique material properties [101]. Welding is still the most important technique to join metals and, more recently, used on thermoplastics. (Electrical) welding processes make use of the same phenomenon with inverted algebraic signs; research on electrode material, supply gases, and current waveforms is performed to improve the bonding between the welded materials and to reduce energy consumption. Plasma discharges are used for cleaning processes – both in liquids (wastewater treatments) or gas phase (exhaust gases, air conditioning, and deodorization), combating environmental pollution [114]. Large-scale plasma-reactors can remove NOx, SOx, and dust in the flue gas emissions of industrial incinerators (50,000 m3/h) [115]. More recently, the conversion of greenhouse gases mainly carbon dioxide (CO2) and methane (CH4) into value-added chemicals and liquid fuels gained increasing attention, and research targets the efficacy of the process [116]. In agriculture, also the fixation of nitrogen from air for soil fertilization is an emerging plasma process that is of interest, where transport is the limiting factor and energy can be generated locally from renewable energies [117]. Overall, optimization of pre- and post-harvesting processes in agriculture and food industry is an emerging field of plasma technology [102]. The disinfection of food containers and fresh produce is a major, near-to-market application in this respect [118, 119].
Box 3 Important Notes
-
Cold plasmas are versatile:
-
Main fields of cold plasma application are: illumination technology, surface editing and refinement, cleaning purposes (including liquids and gases), welding, chemical conversions.
Conclusion
Cold physical plasmas of natural and artificial origin are common to the environment we live in. They represent an enabling technology that can be adapted by engineering to a multitude of applications, both in the technical and the biomedical world. Being an attractive research target, cold plasmas entered biomedical application in the later nineteenth century. Their use subsided due to the lack of scientific background in the middle of the twentieth century, and since the beginning of the current millennium, a substantial increase in knowledge supported their successful return to clinical application. Cold plasmas comprise a mix of potentially bioactive components, with reactive oxygen and nitrogen species, ultraviolet light, and electrical fields as major effectors. Acting jointly, biological processes relevant in various inflammatory events such as cell-cell-communication, cell proliferation, cell migration, and others are modulated. Subsequently, a precise stimulation of tissue functions allows the application of plasmas in conditions like acute and chronic wounds, precancerous and cancerous lesions, and to stop bleeding (e.g., after surgical interventions).
Literature
Mott-Smith HM. History of plasmas. Nature. 1971;233(5316):219.
Graves DB. Lessons from tesla for plasma medicine. IEEE Trans Radiat Plasma Med Sci. 2018;2(6):594–607.
Brenni P. Les courants à haute-fréquence apprivoisés à travers la darsonvalisation et les spectacles publics (1890-1930). Annales historiques de l’électricité. 2010;8(1):53.
Collins AF. An easily-made high-frequency apparatus. Sci Am. 1907;63(1618supp):25929.
Reif-Acherman S. Jacques Arsene d’Arsonval: his life and contributions to electrical instrumentation in physics and medicine. Part iii: high-frequency experiences and the beginnings of diathermy [scanning our past]. Proc IEEE. 2017;105(2):394–404.
Napp J, Daeschlein G, Napp M, von Podewils S, Gumbel D, Spitzmueller R, et al. On the history of plasma treatment and comparison of microbiostatic efficacy of a historical high-frequency plasma device with two modern devices. GMS Hyg Infect Control. 2015;10:Doc08.
Schnee A. Kompendium der Hochfrequenz in ihren verschiedenen Anwendungsformen einschliesslich der Diathermie. O. Nemnich; 1920.
Rhees DJ. Electricity – “the greatest of all doctors”: an introduction to “high frequency oscillators for electro-therapeutic and other purposes”. Proc IEEE. 1999;87(7):1277–81.
Monell SH. High frequency electric currents in medicine and dentistry: their nature and actions and simplified uses in external treatments. New York: William R. Jenkins Company; 1910.
Laroussi M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans Plasma Sci. 1996;24(3):1188–91.
Stoffels E, Flikweert AJ, Stoffels WW, Kroesen GMW. Plasma needle: a non-destructive atmospheric plasma source for fine surface treatment of (bio)materials. Plasma Sources Sci Technol. 2002;11(4):383–8.
Stoffels E, Kieft IE, Sladek REJ. Superficial treatment of mammalian cells using plasma needle. J Phys D Appl Phys. 2003;36(23):2908–13.
Kieft IE, Broers JL, Caubet-Hilloutou V, Slaaf DW, Ramaekers FC, Stoffels E. Electric discharge plasmas influence attachment of cultured CHO K1 cells. Bioelectromagnetics. 2004;25(5):362–8.
Sosnin EA, Stoffels E, Erofeev MV, Kieft IE, Kunts SE. The effects of UV irradiation and gas plasma treatment on living mammalian cells and bacteria: a comparative approach. IEEE Trans Plasma Sci. 2004;32(4):1544–50.
Stoffels E, Sladek REJ, Kieft IE. Gas plasma effects on living cells. Phys Scr. 2004;T107(5):79–82.
Kieft IE, Kurdi M, Stoffels E. Reattachment and apoptosis after plasma-needle treatment of cultured cells. IEEE Trans Plasma Sci. 2006;34(4):1331–6.
Stoffels E, Gonzalvo YA, Whitmore TD, Seymour DL, Rees JA. A plasma needle generates nitric oxide. Plasma Sources Sci Technol. 2006;15(3):501–6.
Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Applied plasma medicine. Plasma Process Polym. 2008;5(6):503–33.
Kalghatgi SU, Fridman G, Fridman A, Friedman G, Clyne AM. Non-thermal dielectric barrier discharge plasma treatment of endothelial cells. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:3578–81.
Dobrynin D, Fridman G, Friedman G, Fridman A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J Phys. 2009;11(11):115020.
Kalghatgi SU, Fridman A, Friedman G, Clyne AM. Cell proliferation following non-thermal plasma is related to reactive oxygen species induced fibroblast growth factor-2 release. Conf Proc IEEE Eng Med Biol Soc. 2009;1:6030–3.
Kalghatgi S, Friedman G, Fridman A, Clyne AM. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth Factor-2 release. Ann Biomed Eng. 2010;38(3):748–57.
Dobrynin D, Fridman G, Friedman G, Fridman A, editors. Physical mechanisms of plasma assisted wound healing: production and delivery of active species. Slovakia: Demanovska dolina; 2011.
Kalghatgi S, Kelly CM, Cerchar E, Torabi B, Alekseev O, Fridman A, et al. Effects of non-thermal plasma on mammalian cells. PLoS One. 2011;6(1):e16270.
Robert E, Sarron V, Ries D, Dozias S, Vandamme M, Pouvesle JM. Characterization of pulsed atmospheric-pressure plasma streams (PAPS) generated by a plasma gun. Plasma Sources Sci Technol. 2012;21(3):34017.
Collet G, Robert E, Lenoir A, Vandamme M, Darny T, Dozias S, et al. Plasma jet-induced tissue oxygenation: potentialities for new therapeutic strategies. Plasma Sources Sci Technol. 2014;23(1):012005.
Pouvesle JM, Robert E, editors. Non thermal atmospheric plasma jets: a new way for cancer treatment? GD2008 Conference Proceedings; 2014.
von Woedtke T, Kramer A, Weltmann K-D. Plasma sterilization: what are the conditions to meet this claim? Plasma Process Polym. GD2008 Conference Proceedings. 2008;5(6):534–9.
Bender C, Matthes R, Kindel E, Kramer A, Lademann J, Weltmann KD, et al. The irritation potential of nonthermal atmospheric pressure plasma in the HET-CAM. Plasma Process Polym. 2010;7(3–4):318–26.
Wende K, Landsberg K, Lindequist U, Weltmann KD, von Woedtke T. Distinctive activity of a nonthermal atmospheric-pressure plasma jet on eukaryotic and prokaryotic cells in a cocultivation approach of keratinocytes and microorganisms. IEEE Trans Plasma Sci. 2010;38(9):2479–85.
Haertel B, Wende K, von Woedtke T, Weltmann KD, Lindequist U. Non-thermal atmospheric-pressure plasma can influence cell adhesion molecules on HaCaT-keratinocytes. Exp Dermatol. 2011;20(3):282–4.
Bekeschus S, von Woedtke T, Kramer A, Weltmann K-D, Masur K. Cold physical plasma treatment alters redox balance in human immune cells. Plasma Med. 2013;3(4):267–78.
Bundscherer L, Bekeschus S, Tresp H, Hasse S, Reuter S, Weltmann K-D, et al. Viability of human blood leukocytes compared with their respective cell lines after plasma treatment. Plasma Med. 2013;3(1–2):71–80.
Schmidt A, Wende K, Bekeschus S, Bundscherer L, Barton A, Ottmuller K, et al. Non-thermal plasma treatment is associated with changes in transcriptome of human epithelial skin cells. Free Radic Res. 2013;47(8):577–92.
Winter J, Wende K, Masur K, Iseni S, Dunnbier M, Hammer MU, et al. Feed gas humidity: a vital parameter affecting a cold atmospheric-pressure plasma jet and plasma-treated human skin cells. J Phys D Appl Phys. 2013;46(29):295401.
Lademann O, Richter H, Meinke MC, Patzelt A, Kramer A, Hinz P, et al. Drug delivery through the skin barrier enhanced by treatment with tissue-tolerable plasma. Exp Dermatol. 2011;20(6):488–90.
Lademann O, Richter H, Kramer A, Patzelt A, Meinke MC, Graf C, et al. Stimulation of the penetration of particles into the skin by plasma tissue interaction. Laser Phys Lett. 2011;8(10):758–64.
Lademann O, Richter H, Patzelt A, Alborova A, Humme D, Weltmann KD, et al. Application of a plasma-jet for skin antisepsis: analysis of the thermal action of the plasma by laser scanning microscopy. Laser Phys Lett. 2010;7(6):458–62.
Lademann J, Richter H, Alborova A, Humme D, Patzelt A, Kramer A, et al. Risk assessment of the application of a plasma jet in dermatology. J Biomed Opt. 2009;14(5):054025.
Teichmann A, Heuschkel S, Jacobi U, Presse G, Neubert RH, Sterry W, et al. Comparison of stratum corneum penetration and localization of a lipophilic model drug applied in an o/w microemulsion and an amphiphilic cream. Eur J Pharm Biopharm. 2007;67(3):699–706.
von Woedtke T, Schmidt A, Bekeschus S, Wende K, Weltmann KD. Plasma medicine: a field of applied redox biology. In Vivo. 2019;33(4):1011–26.
Weltmann KD, von Woedtke T. Plasma medicine-current state of research and medical application. Plasma Phys Controlled Fusion. 2017;59(1):014031.
Graves DB. Mechanisms of plasma medicine: coupling plasma physics, biochemistry, and biology. IEEE Trans Radiat Plasma Med Sci. 2017;1(4):281–92.
Meichsner J, Schmidt M, Schneider R, Wagner HE. Nonthermal plasma chemistry and physics. Boca Raton: CRC Press; 2013.
Loveless AM, Garner AL. A universal theory for gas breakdown from microscale to the classical Paschen law. Phys Plasmas. 2017;24(11):113522.
Reuter S, von Woedtke T, Weltmann KD. The kINPen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J Phys D Appl Phys. 2018;51(23):233001.
Weltmann KD, von Woedtke T. Basic requirements for plasma sources in medicine. Eur Phys J Appl Phys. 2011;55(1):13807.
Winter J, Brandenburg R, Weltmann KD. Atmospheric pressure plasma jets: an overview of devices and new directions. Plasma Sources Sci Technol. 2015;24(6):064001.
Bruggeman PJ, Iza F, Brandenburg R. Foundations of atmospheric pressure non-equilibrium plasmas. Plasma Sources Sci Technol. 2017;26(12):123002.
Winter J, Nishime TMC, Bansemer R, Balazinski M, Wende K, Weltmann KD. Enhanced atmospheric pressure plasma jet setup for endoscopic applications. J Phys D Appl Phys. 2019;52(2):024005.
Kunhardt EE. Generation of large-volume, atmospheric-pressure, nonequilibrium plasmas. IEEE Trans Plasma Sci. 2000;28(1):189–200.
Wittenberg HH. Gas tube design. Electron tube design. Harrison: Radio Corporation of America; 1962. p. 792–817.
Wattieaux G, Yousfi M, Merbahi N. Optical emission spectroscopy for quantification of ultraviolet radiations and biocide active species in microwave argon plasma jet at atmospheric pressure. Spectrochim Acta B At Spectrosc. 2013;89:66–76.
Jablonowski H, Bussiahn R, Hammer MU, Weltmann KD, von Woedtke T, Reuter S. Impact of plasma jet vacuum ultraviolet radiation on reactive oxygen species generation in bio-relevant liquids. Phys Plasmas. 2015;22(12):122008.
Lange H, Foest R, Schafer J, Weltmann KD. Vacuum UV radiation of a plasma jet operated with rare gases at atmospheric pressure. IEEE Trans Plasma Sci. 2009;37(6):859–65.
Fingerhut BP, Herzog TT, Ryseck G, Haiser K, Graupner FF, Heil K, et al. Dynamics of ultraviolet-induced DNA lesions: Dewar formation guided by pre-tension induced by the backbone. New J Phys. 2012;14(6):065006.
Stinson CA, Xia Y. Radical induced disulfide bond cleavage within peptides via ultraviolet irradiation of an electrospray plume. Analyst. 2013;138(10):2840–6.
Protection ICoN-IR. Guidelines on limits of exposure to ultraviolet radiation of wavelengths between 180 nm and 400 nm (incoherent optical radiation). Health Phys. 2004;87(2):171–86.
Jablonowski H, Bussiahn R, Hammer MU, Weltmann K-D, von Woedtke T, Reuter S. Impact of plasma jet vacuum ultraviolet radiation on reactive oxygen species generation in bio-relevant liquids. Phys Plasmas. 2015;22(12):122008.
Lackmann JW, Schneider S, Edengeiser E, Jarzina F, Brinckmann S, Steinborn E, et al. Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. J R Soc Interface. 2013;10(89):20130591.
von Woedtke T, Julich WD, Thal S, Diederich M, Stieber M, Kindel E. Antimicrobial efficacy and potential application of a newly developed plasma-based ultraviolet irradiation facility. J Hosp Infect. 2003;55(3):204–11.
Judee F, Wattieaux G, Merbahi N, Mansour M, Castanie-Cornet MP. The antibacterial activity of a microwave argon plasma jet at atmospheric pressure relies mainly on UV-C radiations. J Phys D Appl Phys. 2014;47(40):405201.
Hoffmann G, Hartel M, Mercer JB. Heat for wounds - water-filtered infrared-a (wIRA) for wound healing - a review. Ger Med Sci. 2016;14:Doc08.
Daeschlein G, Rutkowski R, Lutze S, von Podewils S, Sicher C, Wild T, et al. Hyperspectral imaging: innovative diagnostics to visualize hemodynamic effects of cold plasma in wound therapy. Biomed Tech (Berl). 2018;63(5):603–8.
Kisch T, Schleusser S, Helmke A, Mauss KL, Wenzel ET, Hasemann B, et al. The repetitive use of non-thermal dielectric barrier discharge plasma boosts cutaneous microcirculatory effects. Microvasc Res. 2016;106:8–13.
Darny T, Pouvesle JM, Puech V, Douat C, Dozias S, Robert E. Analysis of conductive target influence in plasma jet experiments through helium metastable and electric field measurements. Plasma Sources Sci Technol. 2017;26(4):045008.
Schmidt-Bleker A, Norberg SA, Winter J, Johnsen E, Reuter S, Weltmann KD, et al. Propagation mechanisms of guided streamers in plasma jets: the influence of electronegativity of the surrounding gas. Plasma Sources Sci Technol. 2015;24(3):035022.
Gaborit G, Jarrige P, Lecoche F, Dahdah J, Duraz E, Volat C, et al. Single shot and vectorial characterization of intense electric field in various environments with pigtailed electrooptic probe. IEEE Trans Plasma Sci. 2014;42(5):1265–73.
Nuccitelli R. Application of pulsed electric fields to cancer therapy. Bioelectricity. 2019;1(1):30–4.
Griseti E, Kolosnjaj-Tabi J, Gibot L, Fourquaux I, Rols MP, Yousfi M, et al. Pulsed electric field treatment enhances the cytotoxicity of plasma-activated liquids in a three-dimensional human colorectal cancer cell model. Sci Rep. 2019;9(1):7583.
Steuer A, Wolff CM, von Woedtke T, Weltmann KD, Kolb JF. Cell stimulation versus cell death induced by sequential treatments with pulsed electric fields and cold atmospheric pressure plasma. PLoS One. 2018;13(10):e0204916.
Keidar M, Shashurin A, Volotskova O, Stepp MA, Srinivasan P, Sandler A, et al. Cold atmospheric plasma in cancer therapy. Phys Plasmas. 2013;20(5):057101.
Yusupov M, Van der Paal J, Neyts EC, Bogaerts A. Synergistic effect of electric field and lipid oxidation on the permeability of cell membranes. Biochim Biophys Acta. 2017;1861(4):839–47.
Wolff CM, Kolb JF, Weltmann KD, von Woedtke T, Bekeschus S. Combination treatment with cold physical plasma and pulsed electric fields augments ROS production and cytotoxicity in lymphoma. Cancers (Basel). 2020;12(4):845.
Azan A, Gailliègue F, Mir LM, Breton M. Cell membrane Electropulsation: chemical analysis of cell membrane modifications and associated transport mechanisms. In: Transport across natural and modified biological membranes and its implications in physiology and therapy. Cham: Springer; 2017. p. 59–71.
Golda J, Held J, Redeker B, Konkowski M, Beijer P, Sobota A, et al. Concepts and characteristics of the ‘COST reference microplasma jet’. J Phys D Appl Phys. 2016;49(8):084003.
Adamovich I, Baalrud SD, Bogaerts A, Bruggeman PJ, Cappelli M, Colombo V, et al. The 2017 plasma roadmap: low temperature plasma science and technology. J Phys D Appl Phys. 2017;50(32):323001.
Bruggeman PJ, Kushner MJ, Locke BR, Gardeniers JGE, Graham WG, Graves DB, et al. Plasma-liquid interactions: a review and roadmap. Plasma Sources Sci Technol. 2016;25(5):053002.
Graves DB. Reactive species from cold atmospheric plasma: implications for cancer therapy. Plasma Process Polym. 2014;11(12):1120–7.
Graves DB. Low temperature plasma biomedicine: a tutorial review. Phys Plasmas. 2014;21(8):080901.
Schmidt-Bleker A, Winter J, Bosel A, Reuter S, Weltmann KD. On the plasma chemistry of a cold atmospheric argon plasma jet with shielding gas device. Plasma Sources Sci Technol. 2016;25(1):015005.
Callaghan S, Senge MO. The good, the bad, and the ugly – controlling singlet oxygen through design of photosensitizers and delivery systems for photodynamic therapy. Photochem Photobiol Sci. 2018;17(11):1490–514.
Fan W, Huang P, Chen X. Overcoming the Achilles’ heel of photodynamic therapy. Chem Soc Rev. 2016;45(23):6488–519.
Reuter S, Niemi K, Schulz-von der Gathen V, Dobele HF. Generation of atomic oxygen in the effluent of an atmospheric pressure plasma jet. Plasma Sources Sci Technol. 2009;18(1):015006.
Schulz-von der Gathen V, Schaper L, Knake N, Reuter S, Niemi K, Gans T, et al. Spatially resolved diagnostics on a microscale atmospheric pressure plasma jet. J Phys D Appl Phys. 2008;41(19):194004.
Knake N, Reuter S, Niemi K, Schulz-von der Gathen V, Winter J. Absolute atomic oxygen density distributions in the effluent of a microscale atmospheric pressure plasma jet. J Phys D Appl Phys. 2008;41(19):6.
Schulz-von der Gathen V, Buck V, Gans T, Knake N, Niemi K, Reuter S, et al. Optical diagnostics of micro discharge jets. Contrib Plasma Physics. 2007;47(7):510–9.
Waskoenig J, Niemi K, Knake N, Graham LM, Reuter S, Schulz-von der Gathen V, et al. Atomic oxygen formation in a radio-frequency driven micro-atmospheric pressure plasma jet. Plasma Sources Sci Technol. 2010;19(4):045018.
Bekeschus S, Wende K, Hefny MM, Rodder K, Jablonowski H, Schmidt A, et al. Oxygen atoms are critical in rendering THP-1 leukaemia cells susceptible to cold physical plasma-induced apoptosis. Sci Rep. 2017;7(1):2791.
Park GY, Hong YJ, Lee HW, Sim JY, Lee JK. A global model for the identification of the dominant reactions for atomic oxygen in He/O-2 atmospheric-pressure plasmas. Plasma Process Polym. 2010;7(3–4):281–7.
Georgescu N, Lungu CP, Lupu AR, Osiac M. Atomic oxygen maximization in high-voltage pulsed cold atmospheric plasma jets. IEEE Trans Plasma Sci. 2010;38(11):3156–62.
Wende K, Williams P, Dalluge J, Gaens WV, Aboubakr H, Bischof J, et al. Identification of the biologically active liquid chemistry induced by a nonthermal atmospheric pressure plasma jet. Biointerphases. 2015;10(2):029518.
Jablonowski H, Schmidt-Bleker A, Weltmann KD, von Woedtke T, Wende K. Non-touching plasma-liquid interaction - where is aqueous nitric oxide generated? Phys Chem Chem Phys. 2018;20(39):25387–98.
Breen C, Pal R, Elsegood MRJ, Teat SJ, Iza F, Wende K, et al. Time-resolved luminescence detection of peroxynitrite using a reactivity-based lanthanide probe. Chem Sci. 2020;11(12):3164–70.
Girard F, Badets V, Blanc S, Gazeli K, Marlin L, Authier L, et al. Formation of reactive nitrogen species including peroxynitrite in physiological buffer exposed to cold atmospheric plasma. RSC Adv. 2016;6(82):78457–67.
Lukes P, Dolezalova E, Sisrova I, Clupek M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2and HNO2. Plasma Sources Sci Technol. 2014;23(1):015019.
Jirásek V, Lukeš P. Formation of reactive chlorine species in saline solution treated by non-equilibrium atmospheric pressure He/O2 plasma jet. Plasma Sources Sci Technol. 2019;28(3):035015.
d’Agostino R, Favia P, Oehr C, Wertheimer MR. Low-temperature plasma processing of materials: past, present, and future. Plasma Process Polym. 2005;2(1):7–15.
Foest R, Kindel E, Ohl A, Stieber M, Weltmann KD. Non-thermal atmospheric pressure discharges for surface modification. Plasma Phys Control Fusion. 2005;47(12B):B525–B36.
Cvelbar U, Walsh JL, Černák M, de Vries HW, Reuter S, Belmonte T, et al. White paper on the future of plasma science and technology in plastics and textiles. Plasma Process Polym. 2019;16(1):1700228.
Weltmann KD, Kolb JF, Holub M, Uhrlandt D, Šimek M, Ostrikov K, et al. The future for plasma science and technology. Plasma Process Polym. 2018;16(1):1800118.
Brandenburg R, Bogaerts A, Bongers W, Fridman A, Fridman G, Locke BR, et al. White paper on the future of plasma science in environment, for gas conversion and agriculture. Plasma Process Polym. 2018;16(1):1–18.
Schulze C, Nestler M, Zeuner M. Ion-beam figuring of x-ray mirrors. SPIE; 2019.
Makhneva E, Barillas L, Farka Z, Pastucha M, Skládal P, Weltmann K-D, et al. Functional plasma polymerized surfaces for biosensing. ACS Appl Mater Inter. 2020;12(14):17100–12.
Duske K, Koban I, Kindel E, Schroder K, Nebe B, Holtfreter B, et al. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J Clin Periodontol. 2012;39(4):400–7.
Le Guehennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23(7):844–54.
Naujokat H, Harder S, Schulz LY, Wiltfang J, Florke C, Acil Y. Surface conditioning with cold argon plasma and its effect on the osseointegration of dental implants in miniature pigs. J Craniomaxillofac Surg. 2019;47(3):484–90.
Liang H, Shi B, Fairchild A, Cale T. Applications of plasma coatings in artificial joints: an overview. Vacuum. 2004;73(3–4):317–26.
Naresh Kumar N, Yap SL, Bt Samsudin FND, Khan MZ, Pattela Srinivasa RS. Effect of argon plasma treatment on tribological properties of UHMWPE/MWCNT nanocomposites. Polymers. 2016;8(8):295.
Chen Y-H, Hsu C-C, He J-L. Antibacterial silver coating on poly(ethylene terephthalate) fabric by using high power impulse magnetron sputtering. Surf Coat Technol. 2013;232:868–75.
Kratochvíl J, Kuzminova A, Kylián O. State-of-the-art, and perspectives of, silver/plasma polymer antibacterial nanocomposites. Antibiotics. 2018;7(3):78.
Schade E. Physics of high-current interruption of vacuum circuit breakers. IEEE Trans Plasma Sci. 2005;33(5):1564–75.
Tahata K, Oukaili SE, Kamei K, Yoshida D, Kono Y, Yamamoto R, et al. HVDC circuit breakers for HVDC grid applications. IET Conference Proceedings [Internet]. 2015:[044(9.)-(9.) pp.]. Available from: https://digital-library.theiet.org/content/conferences/10.1049/cp.2015.0018.
Timmermann E, Prehn F, Schmidt M, Hoft H, Brandenburg R, Kettlitz M. Indoor air purification by dielectric barrier discharge combined with ionic wind: physical and microbiological investigations. J Phys D Appl Phys. 2018;51(16):164003.
Kim HH. Nonthermal plasma processing for air-pollution control: a historical review, current issues, and future prospects. Plasma Process Polym. 2004;1(2):91–110.
Bogaerts A, Kozak T, van Laer K, Snoeckx R. Plasma-based conversion of CO2: current status and future challenges. Faraday Discuss. 2015;183:217–32.
Graves DB, Bakken LB, Jensen MB, Ingels R. Plasma activated organic fertilizer. Plasma Chem Plasma Process. 2018;39(1):1–19.
Bourke P, Ziuzina D, Boehm D, Cullen PJ, Keener K. The potential of cold plasma for safe and sustainable food production. Trends Biotechnol. 2018;36(6):615–26.
Schnabel U, Niquet R, Schlüter O, Gniffke H, Ehlbeck J. Decontamination and sensory properties of microbiologically contaminated fresh fruits and vegetables by microwave plasma processed air (PPA). J Food Process Preserv. 2015;39(6):653–62.
Further Reading
Adamovich I, et al. The 2017 plasma roadmap: low temperature plasma science and technology. J Phys D Appl Phys. 2017;50(32):323001.
Becker KH, et al. Non-equilibrium air plasmas at atmospheric pressure. Boca Raton: CRC press; 2004.
Brandenburg R. Dielectric barrier discharges: progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci Technol. 2017;26(5):053001.
Fridman A. Plasma chemistry. Cambridge, Cambridge university press; 2008.
Graves DB. Low temperature plasma biomedicine: a tutorial review. Phys Plasmas. 2014;21(8):080901.
Graves DB. Lessons from tesla for plasma medicine. IEEE Trans Radiat Plasma Med Sci. 2018;2(6):594–607.
Laroussi M, et al. Perspective: the physics, diagnostics, and applications of atmospheric pressure low temperature plasma sources used in plasma medicine. J Appl Phys. 2017;122(2):020901.
Lu X, et al. On atmospheric-pressure non-equilibrium plasma jets and plasma bullets. Plasma Sources Sci Technol. 2012;21(3):034005.
Meichsner J, et al. Nonthermal plasma chemistry and physics. Boca Raton: CRC Press; 2012.
Metelmann H-R, et al. Plasmamedizin. Berlin/Heidelberg: Springer; 2016. (in German)
Metelmann H-R, et al. Comprehensive clinical plasma medicine: cold physical plasma for medical application. Cham: Springer; 2018.
Privat-Maldonado A, et al. ROS from physical plasmas: redox chemistry for biomedical therapy. Oxidative Med Cell Longev. 2019;2019:9062098.
Reuter S, et al. The kINPen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J Phys D Appl Phys. 2018;51(23):233001.
Winter J, et al. Atmospheric pressure plasma jets: an overview of devices and new directions. Plasma Sources Sci Technol. 2015;24(6):064001.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wende, K., Brandenburg, R. (2022). Cold Physical Plasma: A Short Introduction. In: Metelmann, HR., von Woedtke, T., Weltmann, KD., Emmert, S. (eds) Textbook of Good Clinical Practice in Cold Plasma Therapy. Springer, Cham. https://doi.org/10.1007/978-3-030-87857-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-87857-3_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-87856-6
Online ISBN: 978-3-030-87857-3
eBook Packages: MedicineMedicine (R0)