Abstract
Huge quantities of agricultural residues and stubbles are mainly disposed by burning on site causing air pollution. The organic matter present in the residues and stubble can be utilized in a planned manner, subsequently reducing the emission (greenhouse gases) caused by burning. These agricultural stubbles are an attractive feedstock for clean energy production through anaerobic digestion (AD). Conventional liquid anaerobic digestion systems may be profitable but have a high-water footprint. Solid-state anaerobic digestion (SSAD) not only helps to reduce water consumption, but it also allows for a high organic loading rate and prevent nutrient loss in the digestate. Nevertheless, process stability of an anaerobic digestion system running on high solid concentrations may have several constraints such as limited mass transfer and process inhibitors like ammonia, p-cresol and D-limonene if present in the feedstock for SSAD. In the case of lignocellulosic biomass, its recalcitrant nature may hinder the methane production under the SSAD. Apart from these, the high total solid (TS) content may inhibit the process stability by producing excess total volatile fatty acids (TVFAs) during SSAD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The modern world’s economic status and growth rate are based on energy and its consumption (Cantarero 2020; Paritosh et al. 2020a). Depleting fossil fuel reserves, geo-political issues over crude oil reserves, greenhouse gas (GHG) emissions and its socio-environmental impacts are detrimental factors for a sustainable world. In the last decades, global energy demand has increased manifold and mankind has been forced to explore other alternative forms of energy from sustainable sources. Renewable sources like wind, solar, ocean, small hydro, geothermal and biomass have been accepted as major players for ensuring energy supply under a sustainable development goal (MNRE 2019). Keeping this in mind, many countries including the developing ones are investing in renewable energy sources. For example, total installed grid connected renewable energy capacity in India has increased to 85.9 GW at the end of 2019 as per Ministry of New and Renewable Energy, Government of India (MNRE 2019).
Bioenergy derived from biomass, i.e. biofuel, can be classified as liquid or gaseous biofuels. Liquid biofuels encompass bioethanol, biodiesel and biobutanol while example of gaseous biofuels is biomethane, biohydrogen and syngas. The biofuels are also classified as first, second, third and fourth generation biofuels based on the substrate used for their production. In first generation biofuels, food crops and grains are used for biofuel production while for second generation, crop residues such as rice straw, wheat straw, corn stover and millet straw are used. The third generation biofuels are derived from algae and fourth generation biofuels are those obtained from genetically modified microorganism. First generation biofuel is less desirables as it competes with food whereas the other three biofuels are attractive to the investors and stakeholder as they utilise renewable and waste biomass.
India produces around 634 million tonnes of agricultural stubbles on yearly basis (Kumar et al. 2018). Organic carbon present in the agricultural stubbles and residues may be processed for fuels and energy production. Due to the lack of effective and efficient technology, farmers are compelled to burn these stubbles on site as to clean it before the next crop season. This direct burning of the agricultural stubble may produce around 1600 kg of CO2, 112 kg of CO, 9.2 kg of CH4, 5 kg of particulate matter and 6 kg of hydrocarbons per ha land (Guo et al. 2020). Theoretically, burning of these stubbles not only contributes to high GHG emissions, but also causes immediate problems in the surrounding areas in the form of severe deterioration of air quality (smog formation), and hence crop burning is not at all a sustainable approach for its disposal.
Agricultural stubbles have huge energy potential and may substitute fossils for fuel or electricity and are a promising alternative to meet future energy demands (Hansen et al. 2020). Presently, bio-based energy has approximately 15% share in the Worlds’ total energy use which is almost 45 EJ. Numerous studies have suggested that the potential market for bioenergy may increase up to 50% of the total energy use by the year 2050 (Perea-Moreno et al. 2019). As per a study, 220 billion tonnes of dry biomass are produced worldwide annually (Dahunsi and Enyinnaya 2019). This biomass is equivalent to 4500 EJ of solar energy obtained every year and has the ability to support an annual market of 270 EJ. Because of the huge energy potential and sustainable nature of it, biomass seems to be an attractive substitute to fossil fuels (Maletta and Díaz-Ambrona 2020).
2 Anaerobic Digestion
Anaerobic digestion (AD) is a biological process, practiced extensively for conversion of biodegradable waste to renewable bioenergy (biomethane) using anaerobic microorganisms (Caposciutti et al. 2020). This method has capability of utilizing different organic wastes such as forest woods, lignocellulosic materials, agricultural crops, food waste and municipal solid waste with high efficiency and minimum by-product generation. Biogas produced from the AD process can be utilized as fuel having a high calorific value of 30–35 MJ/m3 and has the potential of replacing other fuel sources like liquid petroleum gas and natural gas (Sheets et al. 2015). Other high energy requiring technologies and methods such as landfilling, pyrolysis and incineration are utilized to handle biodegradable waste, but AD is preferred due to its biological nature which is a lost cost and low energy operation. Also, AD can utilize various feedstocks at large or small scale and further provide help in the reduction of waste sludge, killing of pathogens, and provide essential nutrients in the form of digestate (Xu et al. 2018).
AD is a microbe driven, multi-phase and complex bio-chemical process. The AD process comprises of mainly four different biochemical phases namely hydrolysis, acidogenesis, acetogenesis and lastly, methanogenesis. These biological phases include application of microorganisms in order to decompose organic matter and produce biogas consisting of primarily methane (CH4) and carbon dioxide (CO2). However, the efficiency of AD process depends on different factors such as type of biomass feedstock provided and operational parameters such as temperature, pH, alkalinity, mass transfer rate, volatile fatty acid accumulation, carbon to nitrogen (C/N) ratio, recalcitrant nature of lignocellulosic residues, low concentration of micronutrient and ammonia inhibition. Operating bioreactor under non-optimal condition or imbalance of any of these factors can cause inhibition to the microorganisms and that can result in the deterioration of the methanogenesis performance (Thanh et al. 2016).
Various methods have been developed to resolve these issues such as solid concentration optimization in anaerobic digester for better mass transfer, buffering agent addition in the reactor to balance pH, substrate co-digestion to stabilize the C/N ratio, pre-treatment of biomass (particularly the lignocellosic ones) to disrupt the lignin complex for enhancement of methanogenesis (Jain et al. 2015). Other than that, to achieve good process stability and performance, many material supplementation such as carbon-based additives are added to anaerobic reactors for improving its performance and to enhance its economic feasibility (Paritosh et al. 2021). The carbon-based additives reinforce direct interspecies electron transfer in the system and improve syntrophic relations in the reactor. Moreover, the presence of materials such as biochar or activated carbon accelerate the utilization of volatile fatty acids (VFA) and ensure availability of substrate to methanogens. Whereas, addition of elements such as cobalt (Co), nickel (Ni), iron (Fe) and zinc (Zn). in the AD acts as micronutrients and accelerate the metabolic activities of methanogens which provides a better yield of methane (Paritosh et al. 2020b). Nanoparticles of the above mentioned trace elements have also been supplied by various researches to enhance anaerobic digestion of biomass and biogas production (Lee and Lee 2019).
3 Solid State Anaerobic Digestion
Anaerobic digestion can be categorized into two distinct forms based on their total solid (TS) content in the reactor medium. The first one is liquid state anaerobic digestion with a solid content <15%, whereas the other one being solid state anaerobic digestion (SSAD) with a TS content >15%. SSAD has the following advantages over liquid anaerobic digestion (LAD): feasibility of using higher organic loading rate (OLR), less energy requirement, smaller reactor volume and increased volumetric methane yield (Brown et al. 2012; Rico et al. 2015; Panjičko et al. 2017). Beside, pathogen inactivation may also be achieved in SSAD of biodegradable waste (Jiang et al. 2018).
However, SSAD has a few challenges which include slow mass transfer, process instability, end product needs additional treatment and lower biogas production (Karthikeyan and Visvanathan 2013; Carlos-Pinedo et al. 2019). These issues need to be addressed in order to enhance process efficiency, and to further ensure its feasibility at a larger scale for successful commercialization of this technology.
4 Feedstock Identification for SS–AD
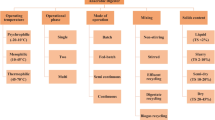
Several types of feedstocks including various wastes such as the organic fraction of municipal solid waste (OFMSW), food waste, forest waste, agricultural waste, animal waste, solid manure, energy crops, industrial waste, residual lignocellulosic biomass, paper and pulp waste have been established as good substrates for biogas generation using the SSAD process (Fig. 7.1). The physical and chemical composition of the substrate are very important and directly affect the process efficiency of AD system. For example, the presence of a high amount of recalcitrant compounds (such as lignin) in the biomass lower the biogas production whereas a high amount of easily utilizable compounds (such as sugars) enhances methanogenic activity (Paritosh et al. 2019). Hence, even before starting the process the suitability of any substrate, including lignocellulosic biomass for SSAD, should be determined by biomass characterization.
4.1 Organic Fraction of Municipal Solid Waste (OFMSW)
The organic fraction of municipal solid waste (OFMSW) comprises of yard trimmings, grass clippings, vegetable wastes, food wastes and fruit peel wastes (Kesharwani and Bajpai 2020). The approach for waste collection and transportation plays a major role in the SSAD process performance. Also, the seasonal variations and environment conditions can affect the composition of the OFMSW which in turn affects the SSAD process performance (Forster-Carneiro et al. 2007). Several studies have demonstrated that both physical as well as chemical properties of OFMSW have significant impact on biogas production.
Michele et al. (2015) performed SSAD of OFMSW by recirculation of the digestate. This liquid digestate flushing helped in removing fermentative products (such as volatile fatty acids (VFA)) inhibiting methanogenesis. The ratio of solid waste to digestate was in between 1:1.18–1:0.9 on w/w basis. The total solids removal was 36.9%, however the loss of organic matter was attributed to the washout with the percolate from the reactor. Hence, the percolate which was high in organic content was subjected to AD for biogas production in a second AD reactor (LAD). The mass balance showed that the methane content from the dry AD and the percolate were 18.4% and 49.7%, respectively, at a 21 d hydraulic retention time (HRT). However, only 20.4% and 25.7% of potential producible methane was generated by adopting 15 and 20 d of HRT using LAD of the same waste.
Food waste is also considered a part of OFMSW and contains organic materials which are transformed into simple molecules that are readily digested in the AD process. However, accumulation of VFAs caused by high soluble organic contents act as inhibitor by decreasing the pH of the system leading to reduction in methane yield of the AD process (Micolucci et al. 2018). Co-digestion of OFMSW with lignocellulosic biomass can be a beneficial approach for enhancing the process efficiency. Brown and Li (2013) examined the effect of feedstock to inoculum (F/I) ratio (1, 2 and 3) and substrate concentration (0, 10 and 20%) on co-digestion of food waste (FW) with yard waste on biogas production using SSAD. A high volumetric biogas production rate (8.6 L per L reactor volume) was achieved with 10% FW concentration and a F/I value of 2.
In another study conducted by Wang et al. (2012), the effect of different ratios of FW to distiller’s grain on biogas production using SSAD was investigated. A 75.7% increase in the biogas production was observed with co-digestion compared with mono-digestion. Favourable synergistic effects were shown on the VFA/alkalinity ratio and propionate/acetate ratio when, distiller’s grain and FW were co-digested. The optimum ratio for FW to distiller’s grain was 8:1 with 20% TS in this study.
Zhu et al. (2014) examined co-digestion of soybean processing waste with addition of hay through SSAD for methane production. The authors studied the effect of the F/I ratio, leachate recirculation and pre-mixing of inoculum with substrate on biogas production. Maximum methane production was achieved at a F/I ratio of 3 (256 L/kg VS) and soybean processing waste and hay ratio of 75:25. The methane production during co-digestion was 148% and 50% higher as compared to mono-digestion of soybean processing waste and hay individually. The leachate recirculation accelerated the SSAD process, however no effect of premixing on the biogas production was observed.
Million tonnes of yard trimmings, grasses and leaves waste are generated in urban centres, and can be considered as a major component of OFMSW. These green wastes largely consist of hemicellulose and cellulose which are beneficial substrates for higher biogas production in the AD process. Xu et al. (2016) conducted research on yard trimmings by comparing SSAD digestate and dewatered LAD finished material as inoculum. The F/I ratio was varied from 0.2 to 2 whereas the TS content selected for the study was in between 20 and 35%. The highest methane production of 244 L/kg VS was obtained at a F/I ratio of 0.2 and TS content of 20%. The dewatered effluent at 24% TS and F/I ratio of 0.6 showed an increased volumetric methane yield compared with other experimental conditions.
4.2 Lignocellulosic Biomass and Residues
Lignocellulosic biomass is derived from plant based wastes such as agricultural residues, wastes generated from municipal parks and forests, and is one of the main sources of renewable energy production. Lignocellulosic material mainly constitutes of three main complex components which are cellulose, hemicelluloses and lignin. The carbohydrate part, i.e. cellulose (9–80%) and hemicelluloses (10–50%), is fermentable, whereas lignin presence is 5–35% in the biomass and is considered as inhibitory compound in the AD process (Fig. 7.2) (Yadav et al. 2019). The characteristic of lignocellulosic materials such as structural and chemical properties vary greatly depending on its source (biomass type). These properties are the main deciding factor for successful microbial degradation of the biomass and sometimes can cause complications for biogas production due to the higher presence of inhibitory substances.
Cellulose is a linear polysaccharide polymer of cellobiose which is connected by β-1, 4-glycosidic bonds. When the cellulose chain is linked by hydrogen bonds or van der Waals forces, high tensile strength microfibrils are produced. Cellulose is further comprised of two components, the first one is amorphous cellulose which is readily digestible and the other is crystalline form which is difficult to hydrolyse. Hemicellulose is more amorphous in nature and constitutes of pentoses (e.g. arabinose, xylose), hexoses (e.g. glucose, rhamnose) and acids (e.g. galacturonic acid). Lignin is a complex polymer consisting of sinapyl, coumaryl and coniferyl alcohol which is inert and insoluble in nature. These features of lignin make it recalcitrant and difficult parts of biomass to digest during AD process.
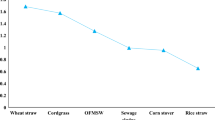
Brown et al. (2012) conducted a comparative study of a variety of lignocellulosic materials namely switch grass, yard waste, leaves, waste paper, wheat straw, corn stover, maple and pine biomass for biogas production using LAD and SSAD methods. For most of the biomass, there was no significant difference in methane production by SSAD and LAD method, except for pine and waste paper. However, due to the small volume used in SSAD systems, the volumetric methane productivity was higher in the SSAD reactor for all the feedstocks. Corn stover, wheat straw and switch grass produced comparatively more methane (2–5 times) than yard waste, maple and pine biomass.
Xu et al. (2013) studied biogas production by the SSAD process using corn stover as feedstock along with digestate of LAD from three distinct anaerobic systems under mesophilic conditions. Anaerobic digestate was collected from three LAD reactors that were fed with dairy waste, FW and sewage sludge, respectively. The anaerobic digestate to corn stover was mixed in such a manner that the F/I ratios will vary in between 2 and 6. The SSAD reactor at the F/I ratio of 2 showed the highest methane yield (238.5 L/kg VS) with digestate from a LAD reactor treating dairy waste as the inoculum. In case of the SSAD reactor inoculated with digested FW, the maximum methane production of 199.6 L/kg VS was at F/I ratio of 4. In a similar study, Liew et al. (2012) compared the biogas production potential of corn stover with yard waste, leaves and wheat straw using SSAD at F/I ratio of 2. The maximum methane yield of 81.2 L/kg VS was for corn stover as feedstock, while yard waste, leaves and wheat straw yielded 40.8, 55.4 and 66.9 L/kg VS of methane, respectively.
Methane production from albizia plant biomass was examined in two different anaerobic digestion systems, i.e. SSAD and LAD rectors (Ge et al. 2014). The study found higher methane production using LAD where the methane yield from albizia leaves and wood chips were 161 and 113 L/kg VS, respectively. The methane production from albizia leaves using SSAD was comparable (156.8 L/kg VS) to the LAD system, however, it was much lower (59.6 L/kg VS) in case of albizia wood chips using SSAD. Similar to other previously mentioned studies, the volumetric methane production was much higher (five times more) in the SSAD system in comparison to the LAD reactor.
Cui et al. (2011) compared raw wheat straw and spent wheat straw from horse stall for biogas production in a SSAD system. The experimental conditions used during the study were 20% TS, F/I ratio of 2, 4 and 6, and the inoculum used was digestate collected from a LAD reactor. The maximum daily methane yield from spent wheat straw was observed 8 and 3 days earlier in comparison to raw wheat straw with a F/I ratio of 2 and 4, respectively, indicating improved degradation rate for spent wheat straw. The maximum methane generation of 150 L/kg was with spent wheat straw when the F/I ratio was 4 and it was 56.2% higher than that of raw straw. Cellulose and hemicellulose digestibility was also, respectively, 24.1 and 49.4% higher in spent wheat straw compared with raw straw.
Yan et al. (2015) investigated the effects of different parameters such as solid concentration, temperature and C/N ratio on the digestion of rice straw employed for biogas production using a SSAD system. Maximum biogas production (447.4 mL/g VS) was observed with an initial TS of 20% and C/N ratio of 29.6 at 35.6 oC.
Sheets et al. (2015) investigated the influence of different factors, namely TS concentration (20 and 30%), temperature (36 and 55 °C) and controlled air exposure, on biogas production using switch grass as the substrate in a SSAD system. The air exposure did not show any positive effect on the methane production from switch grass. The biogas generation increased from 102 and 145 L CH4/kg of VS with increase in TS concentration from 20 and 30% in mesophilic conditions. Under thermophilic conditions, the methane yields were 88 and 113 L CH4/kg VS for 20 and 30% of solid concentrations, respectively.
Contrary to this previous study, biogas production decreased with increase in initial TS concentration from 8 to 38% during biogas production from giant reed biomass (Yang and Li 2014). The inhibitory effect was attributed to the high accumulation of VFA at high solids concentration. The maximum methane production (129.7 L CH4/kg VS) was at a F/I ratio of 2 and TS content of 20–23% using the SSAD process (Table 7.1).
5 Factors Affecting SSAD Process
5.1 Solid Concentration
Solid concentration is one of the most important parameters for the SSAD process and significantly affects the process efficiency. Hence, many of the previous studies have focused on optimizing the solids concentration in the digester. A very high solid concentration in the SSAD process contributes to reduced biogas production by limiting microbial access to the substrate (Bollon et al. 2013). The water content in the system is also relevant in this regard as it facilitates mass transfer and low water content can suppress the digestion process in the SSAD system (Le Hyaric et al. 2012).
Anaerobic digestion of municipal solid waste (MSW) was studied at two different solid concentrations of 20% and 30% under mesophilic conditions (Fernández et al. 2010). The dissolved organic carbon and VFAs removal was higher at low TS concentration of 20%, whereas at high TS concentration (30%) digestion of organic waste compounds decreased by 17%. Abbassi-Guendouz et al. (2012) investigated digestion of cardboard at various solid concentrations (10–30%). The results demonstrated that increase in the solid concentration was detrimental to the methane production rate. The threshold value for TS was 30% in this study, and beyond this methanogenic activity gets inhibited. In another similar study on methane production from organic wastes obtained from the palm oil industry (oil farm fronds, oil palm trunks and empty fruit branches) at three different solid contents (16, 25 and 35%) observed a negative correlation with increase in solid concentration in the AD process (Suksong et al. 2016). The maximum methane production (72 L/kg biomass) and total solids removal was at 16% solid concentration, whereas the methane yield decreased for the other two solid contents.
Hence, from the above studies it can be summarized that methane yield and methanogenic activity tends to decrease with an increase in solid concentration. The reason behind this trend is mainly related to the dysfunction of mass transfer at high solid content (Abbassi-Guendouz et al. 2012; Fernández et al. 2010). For example, Bollon et al. (2013) found that when solid concentration increased from 10% to 25%, the medium solutes diffusion coefficient reduced by 3.7 times.
5.2 Inoculum
Inoculum is another important factor as it provides the microbes, the main catalyst in the AD process (Cui et al. 2011; Shi et al. 2014). LAD effluents and digestate from the SSAD process are generally better inocula than activated sludge, rumen fluid and manure because the digestate from anaerobic processes provides high numbers of active methanogens that are more suited to the AD process. For example, Xu et al. (2016) established in their study that effluent from the LAD process is a better inoculum source than manure, rumen fluid, lake sediments and sewage sludge for initiating the SSAD process. In another study, Forster-Carneiro et al. (2007) noted that the lag phase in the SSAD process reduced from 20–30 days to 2–5 days when LAD effluent was used as inoculum instead of fresh manure. Suksong et al. (2019) reported a twofold increase in methane yield using LAD effluent as inoculum in comparison to SSAD finished materials. The LAD effluent used had high alkalinity (5.9 g/kg) and low VFA concentration (0.05 g/kg) which may have contributed to the better performance of the system (Suksong et al. 2019).
Often recalcitrant components in biomass prevent efficient utilization of the biomass for biogas production. In such cases, different process improvement strategies are applied, one among them is the use of hydrolytic microorganisms. Weiß et al. (2010) used enriched hydrolytic microbes for enhanced degradation of lignocellulosic biomass rich in hemicellulose. The study found an increase in xylanase activity by 1.62% as well as 53% increase in methane yield with supplementation of hemicellulolytic bacteria to the AD process. According to Ma et al. (2013) the optimal ratio of hydrolytic microbes to methanogens was recommended to be 24 in AD process, the hydrolysis process becomes the rate limiting step at a ratio below 24, while a ratio higher than 24 makes methanogenesis the rate limiting step. Similarly, enhancement in biogas production from corn stover due to the addition of dairy manure as inocula was attribute to the activity of hydrolytic microbes in the AD process (Xu et al. 2013). The biogas yield from corn stover using dairy manure was 30% and 100% higher than those using sewage sludge finished material and food waste as inoculum.
Gu et al. (2014) compared different inoculum sources such as digestates from dairy manure, chicken manure, municipal sludge, swine manure, paper mill sludge and anaerobic granular sludge for biogas production with rice straw as the substrate. Compared to sludge, digested manure as inoculum demonstrated significantly improved lignocellulose degradation and methane production due to the high enzyme activity (mainly cellulase and xylanase) in animal manure digestates.
The inoculation size in SSAD is another aspect which has the ability to increase methanogenic activity. The optimized concentration of inoculum can give a good start to the SSAD process and may as well reduce the lag phase of the AD process significantly (Yang et al. 2015). The inoculum size in AD is often described as food to inoculum (F/I) ratio. At mesophilic conditions, inoculation size as F/I ratio of 2–3 on VS basis is recommended for the AD process of lignocellulosic biomass (Zhu et al. 2014; Liew et al. 2012; Ma et al. 2013). Under thermophilic range, the optimal F/I ratio should be in the range of 4–6 when the experiment was performed on corn stover. This difference in optimum F/I ratio under different temperature conditions was also confirmed by Li et al., where the maximum methane yield for mesophilic and thermophilic conditions was at F/I ratio of 2.43 and 4.58, respectively. Lin et al. (2015) investigated SSAD of yard trimmings comprised of wood chips, maple leaves and lawn grass as substrate for biogas production and found a F/I ratio of 4–6 to be better for the digestion process under thermophilic conditions (55 oC). In another study, the F/I ratio of 1 showed best results for methane production under mesophilic temperature (Brown and Li 2013).
Mixing of inoculum with the substrate is another important aspect of the SSAD process. In this regard, mixing of inoculum with the substrate is required prior to the loading in the SSAD reactors. This pre-mixing is particularly needed in case of processes with high solid content. In large or pilot scale SSAD bioreactors, the interaction between microbes and feedstock sometimes fails due to improper mixing. Two different scenarios were created by Zhu et al. (2014) for analysing the effect of premixing and partial mixing on SSAD process stability and net methane yield. In the first scenario, the whole inoculum was completely mixed with the substrate at the start of the process. In the second scenario, half of the inoculum was mixed with substrate, following which the rest of the 50% inoculum was poured onto the top. Although, the methane yield was the same in both scenarios, the start-up time was less in the premixed SSAD reactor. In another study, three premixing strategies were employed to digest corn stover anaerobically in a SSAD reactor (Zhu et al. 2014). Comparison of the completely mixed scenario with partially mixed in one layer and two layers was performed. The reactor with two layered partial mixing of inoculum yielded the highest methane at F/I ratio of 4 to 6.
5.3 Temperature
Temperature is one of the most important determining factors for the growth and survival of microbes in the AD process at both laboratory and industrial scale systems (de Diego-Díaz et al. 2018). Reactor temperature can selectively enrich microbes and has the capacity to enhance the rate of biochemical reactions in the bioreactor. The temperature ranges used for the AD process are as follows: thermophilic (55–70 oC), mesophilic (20–45 oC) and psychrophilic (0–20 °C). Among these temperature ranges thermophilic and mesophilic conditions have been extensively practiced for the degradation process of lignocellulosic biomass (LCB) and OFMSW in SSAD. The mesophilic temperature range is more preferred when compared to thermophilic temperatures due to greater process stability as well as better growth of methanogens. Although the thermophilic temperature zone has its own benefits in the AD process, it requires more energy input in the process, making the process economics unsustainable. However, Sheets et al. (2015) during SSAD of switch grass concluded that under thermophilic conditions, net energy input can be decreased with the increase in methane production rate.
Furthermore, thermophilic temperature accelerates the process at initial level and drives the hydrolysis faster, but often methanogenic conversion is not satisfactory (Yang et al. 2015). Hydrolysis of substrate can be accelerated in thermophilic conditions due to enrichment of hydrolytic microorganisms inside the SSAD bioreactor. But faster hydrolysis of biomass often results in volatile fatty acids (VFAs) accumulation in the system, causing acidification of the reactor (Shi et al. 2014). This acidification further reduces methanogenesis, decreasing biogas production and also reducing stability of the SSAD system (Yan et al. 2015).
Shi et al. (2014) reported that the degradation rate of cellulose and hemicellulose was higher under thermophilic conditions in contrast to the mesophilic temperature range. In another study, a total 6–41% of cellulose and 2–34% of hemicelluloses digestion was observed during thermophilic SSAD of lignocellulosic biomass. These improved results were attributed to the increased (10–50 times) presence of cellulolytic and xylanolytic microorganisms in the thermophilic SSAD bioreactor (Fernández-Rodríguez et al. 2013).
5.4 Inhibition
There are many factors that can cause inhibition in the methanogenesis process in SSAD. For example, excess VFA accumulation can greatly affect methanogens, causing instability in the bioreactor (Carlos-Pinedo et al. 2019). Acidification results in decreased pH values, thus inhibiting methanogens which are most susceptible to the environmental conditions (Rocamora et al. 2020). The significant reason behind the increment in VFA accumulation in anaerobic digestion reactors is feedstock overloading (Eko and Chaiprasert 2020). Zhang et al. demonstrated that the use of alternative feedstock can avoid VFA accumulation for better stability of the AD process. The addition of packaging waste along with FW can avoid VFA accumulation during the SSAD process. The study suggested that choice of heterogenous waste as feedstock may permit high loading of substrate during the digestion. The ratio of VFA to alkalinity can assist to regulate digester stability. A VFA/alkalinity ratio within 0.3–0.4 is generally observed in AD plants, but a ratio in the range 0.4–0.6 can provide a stable and safe operation when high organic containing substrates are used (Lossie and Pütz 2008).
Besides VFA accumulation and alkalinity, the ammonia nitrogen content can also bring instability in the AD process. A study conducted by Duan et al. (2012) on sewage sludge found reduced methane generation even at a VFA/alkalinity ratio of 0.2 due to excessive ammonia nitrogen concentrations. This demonstrates that measuring the VFA/alkalinity ratio to monitor reactor condition could be deceptive in the long term operation of SSAD. A suitable knowledge of ammonium inhibition is required to predict the process steadiness.
Free ammonia (NH3) and ammonium ion (NH4+) are available during the digestion of nitrogenous matter and feedstocks rich in protein (FW and OFMSW). The concentration of the ionic form as well as the non-ionic form of ammonia is influenced by both temperature and pH of the SSAD system as described by the following equations (7.1) and (7.2) (Calli et al. 2005).
where, pKa is the dissociation constant of ammonium ions, T is temperature (oC), FAN is free ammonia nitrogen and TAN is total ammonia nitrogen.
During the ammonification process, about 60–80% of nitrogen in the substrate gets transformed into ammonium or ammonia ion (Yabu et al. 2011). Among these, free ammonia (FAN) is the major reason behind inhibition: when present in higher concentrations, it can cause potassium deficiency and proton imbalance within cells (Yang et al. 2015). Threshold values of the non-ionic form of ammonia is suggested in the range of 300–800 mg/L (Duan et al. 2012; Yabu et al. 2011).
Production of inhibitory compounds due to pretreatment of substrate is another concern, which has negative impact on the biogas production. During pretreatment of lignocellulosic feedstocks, furan derived compounds such as 5-hydroxyl methyl furfural and furfurals are produced which negatively affects the AD process (Barakat et al. 2012). According to Atelge et al. (2020), the inhibitory concentration of furan and 5-hydroxymethylfurfural on anaerobic digestion process is 1 mg/L and 3 mg/L, respectively, beyond which they can reduce the methane production rate.
Apart from these inhibitory substances, certain compounds present in specific substrates are also reported in the literature for their negative effect on the AD process. For example, a compound named D-limonene, found in citrus fruits peelings and processed fruits waste, has been described to be inhibitory to the methanogenesis process (Ruiz and Flotats 2014). D-limonene is a colourless and aqueous secondary plant metabolite that contains cyclic terpenes. It is inhibitory to methanogens and can destroy the microbial cell membrane. Hence, this compound needs to be removed in order to successfully utilize citrus fruit waste for methane production in SSAD. D-limonene can be removed by steam distillation and solvent extraction methods, but this will increase the process step and can make the process more energy and cost intensive (Calabrò et al. 2020). Another such inhibitory compound is p-cresol, present as degradation product in brewery spent grains. However, two stage SSAD utilizing granular biomass has shown capability to reduce the negative effect of p-cresol in the methanogenic reactor (Panjičko et al. 2017).
6 Approaches for Enhancing SSAD Performance
Lignin present in lignocellulosic biomass is inhibitory to the SSAD process due to its recalcitrant nature. In order to increase the production of biogas and reduce inhibition, different pretreatment methods can be applied (Kumar et al. 2018; Saha et al. 2018). Chemical pretreatment involves acid, alkali, ionic liquids (ILs) and organic solvents to disrupt linkage between complexes in the lignocellulosic matrix (Kumar et al. 2018). Whereas, physiochemical pretreatment involves usage of carbon dioxide explosion, ammonia fibre explosion (AFEX) and wet oxidation. AFEX treatment includes pressurized ammonia given to biomass with rapid decompression (Stoklosa et al. 2017). As a result, hydrolysis and ammonolysis reactions break the ester cross links in the cell wall biopolymers. With the help of biomass pretreatment, various advantages can be achieved such as lignin removal, decrystallization of cellulose, increase accessible surface area, alteration of inter-linkage of hemicelluloses and cellulose in biomass structure (Rouches et al. 2019). The cellulose decrystallization causes cellulose to become more porous and readily available to the microbes, which enhances its bioconversion efficiency (Paritosh et al. 2021; Yadav et al. 2019).
Pretreatment for decrystallization of cellulose before digestion can be carried out with the help of acids. Inorganic acids such as hydrochloric acid (HCl), sulphuric acids (H2SO4) and phosphoric acid (H3PO4) are commonly employed for this purpose. However, in the recent times ionic liquids (ILs) have also been used for biomass pretreatment. ILs are less corrosive in nature, connect with the hydroxyl group of cellulose by breaking hydrogen bonds and this ensures dissolution of cellulose (Han et al. 2020). The pretreatment process using ionic liquids is efficient in recovering decrystallized cellulose with the help of anti-solvents such as methanol, acetone, ethanol or water and also, the ILs can be recovered to a very high extent (even 100% in some cases) (Han et al. 2020).
The most preferred ionic liquid used for pretreatment of lignin containing biomass is N-methyl morpholine-N-oxide monohydrate (NMMO). Akhand and Méndez Blancas (2012) reported a total of 47% increase in methane yield when rice straw biomass was subjected to NMMO pretreatment. The pretreatment increased the substrate surface area which facilitated increased microbial degradation of the feedstock to produce biogas.
Physical pretreatment such as size reduction was applied for methane production from napier grass with three sizes of 6, 10 and 20 mm (biomass passing through respective size sieves) (Surendra and Khanal 2015). A higher methane yield was found for the smallest biomass size of 6 mm as compared to the two other biomass sizes (10 and 20 mm). This improved results is again attributed to the increase in specific surface area for microbial degradation of biomass.
Various pretreatment methods such as steam explosion, irradiation, dilute acid application and liquid hot water have been developed to enhance biogas production and reduce inhibition (Kumar et al. 2018). In addition, other methods such as wet oxidation, alkaline treatment and biological methods (fungal or enzymatic) can be applied for lignin removal (Kumar et al. 2018). Zhao et al. (2014) investigated pretreatment of yard trimmings using white rot fungi (Ceriporiopsis subvermispora) for improving the SSAD process. Ceriporiopsis subvermispora pretreatment at 40% solid concentration showed the highest methane production (44.6 L/kg VS) which was 154% higher than methane produced from raw yard trimmings. Similarly, when albizia chips were pretreated with the same fungal strain of Ceriporiopsis subvermispora, 370% increase in biogas yield was reported (Ge et al. 2015). Pretreatment of rice straw with combined physical (milling) and biological (fungal) methods for improved biodegradability of feedstock in the SSAD system was studied (Mustafa et al. 2017). A 1 month long incubation with Pleurotus ostreatus and subsequent milling of the rice straw achieved 30.4% lignin removal and 165% higher methane production in comparison to the experiments with untreated rice straw.
However, to degrade a higher lignin content in feedstocks such as spruce (29% lignin content), the alkaline pretreatment method is more suited. In a study by Mohsenzadeh et al. (2012), birch and spruce biomass was pretreated with different alkaline reagent combinations (NaOH/urea, NaOH/thiourea, NaOH/urea/thiourea, and NaOH/polyethylene glycol) at four different temperatures (−15, 0, 22 and 80 oC). The pretreament with combinations of NaOH/thiourea at −15 oC showed the best results in terms of 59.9% and 45.3% increase in yield using birch and spruce biomass, respectively. Although lignin removal was not maximum at this pretreatment condition, product yield was the highest, indicating other factors such as crystallinity of sugars in the biomass have more significance. According to the authors, a decrease in crystallinity index has positive correlation with the hydrolysis rate.
Zhu et al. (2010) studied alkali (NaOH) pretreatment of corn stover at different concentrations (1–7.5% w/w) in order to increase methane production. The lignin removal increased from 9.1 to 46.2% by increasing the NaOH concentration from 1 to 7.5% and at optimum condition, a high biogas production of 372.4 L/kg VS was realised. Pretreatment of poplar waste with NaOH showed improved lignin reduction by 19.2% and a high methane production (98.2 L/kg VS) from the resulting biomass by SSAD process (Yao et al. 2017).
References
Abbassi-Guendouz A, Brockmann D, Trably E, Dumas C, Delgenès JP, Steyer JP, Escudié R (2012) Total solids content drives high solid anaerobic digestion via mass transfer limitation. Bioresour Technol 111:55–61
Akhand M, Méndez Blancas A (2012) Optimization of NMMO pre-treatment of straw for enhanced biogas production. Master’s thesis. https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A1308820&dswid=-9346
Atelge MR, Atabani AE, Banu JR, Krisa D, Kaya M, Eskicioglu C, Kumar G, Lee C, Yildiz YŞ, Unalan SE, Mohanasundaram R, Duman F (2020) A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 270:117494
Barakat A, Monlau F, Steyer JP, Carrere H (2012) Effect of lignin-derived and furan compounds found in lignocellulosic hydrolysates on biomethane production. Bioresour Technol 104:90–99
Bollon J, Benbelkacem H, Gourdon R, Buffière P (2013) Measurement of diffusion coefficients in dry anaerobic digestion media. Chem Eng Sci 89:115–119
Brown D, Li Y (2013) Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour Technol 127:275–280
Brown D, Shi J, Li Y (2012) Comparison of solid-state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production. Bioresour Technol 124:379–386
Calabrò PS, Fazzino F, Sidari R, Zema DA (2020) Optimization of orange peel waste ensiling for sustainable anaerobic digestion. Renew Energy 154:849–862
Calli B, Mertoglu B, Inanc B, Yenigun O (2005) Effects of high free ammonia concentrations on the performances of anaerobic bioreactors. Process Biochem 40(3–4):1285–1292
Cantarero MMV (2020) Of renewable energy, energy democracy, and sustainable development: a roadmap to accelerate the energy transition in developing countries. Energy Res Soc Sci 70:101716
Caposciutti G, Baccioli A, Ferrari L, Desideri U (2020) Biogas from anaerobic digestion: power generation or biomethane production? Energies 13(3):743
Carlos-Pinedo S, Wang Z, Eriksson O (2019) Methane yield from SS-AD: experiences to learn by a full spectrum analysis at laboratory-, pilot-and full-scale. Biomass Bioenergy 127:105270
Cui Z, Shi J, Li Y (2011) Solid-state anaerobic digestion of spent wheat straw from horse stall. Bioresour Technol 102(20):9432–9437
Dahunsi OS, Enyinnaya M (2019) The bioenergy potentials of lignocelluloses. Energy Convers Curr Technol Future Trends 93
de Diego-Díaz B, Cerdán JMA, Peñas FJ, Fernández-Rodríguez J (2018) Impact of supplementary nutrients on codigestion of agricultural waste: study of temperatures. Food Bioprod Process 110:120–125
Duan N, Dong B, Wu B, Dai X (2012) High-solid anaerobic digestion of sewage sludge under mesophilic conditions: feasibility study. Bioresour Technol 104:150–156
Eko H, Chaiprasert P (2020) Enhancement of methane production from high solid anaerobic digestion of pretreated palm oil decanter cake using a modified solid inclined reactor. J Chem Technol Biotechnol 95(3):781–790
Fernández J, Pérez M, Romero LI (2010) Kinetics of mesophilic anaerobic digestion of the organic fraction of municipal solid waste: influence of initial total solid concentration. Bioresour Technol 101(16):6322–6328
Fernández-Rodríguez J, Pérez M, Romero LI (2013) Comparison of mesophilic and thermophilic dry anaerobic digestion of OFMSW: kinetic analysis. Chem Eng J 232:59–64
Forster-Carneiro T, Pérez M, Romero LI, Sales D (2007) Dry-thermophilic anaerobic digestion of organic fraction of the municipal solid waste: focusing on the inoculum sources. Bioresour Technol 98(17):3195–3203
Ge X, Matsumoto T, Keith L, Li Y (2014) Biogas energy production from tropical biomass wastes by anaerobic digestion. Bioresour Technol 169:38–44
Ge X, Matsumoto T, Keith L, Li Y (2015) Fungal pretreatment of Albizia chips for enhanced biogas production by solid-state anaerobic digestion. Energy Fuel 29(1):200–204
Gu Y, Chen X, Liu Z, Zhou X, Zhang Y (2014) Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour Technol 158:149–155
Guo L, Ma Y, Tigabu M, Guo X, Zheng W, Guo F (2020) Emission of atmospheric pollutants during forest fire in boreal region of China. Environ Pollut 264:114709
Han SY, Park CW, Kwon GJ, Kim NH, Kim JC, Lee SH (2020) Ionic liquid pretreatment of lignocellulosic biomass. J For Environ Sci 36(2):69–77
Hansen JH, Hamelin L, Taghizadeh-Toosi A, Olesen JE, Wenzel H (2020) Agricultural residues bioenergy potential that sustain soil carbon depends on energy conversion pathways. GCB Bioenergy 12(11):1002–1013. https://mnre.gov.in/img/documents/uploads/file_f-1597797108502.pdf
Jain S, Jain S, Wolf IT, Lee J, Tong YW (2015) A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew Sustain Energy Rev 52:142–154
Jiang Y, Dennehy C, Lawlor PG, Hu Z, Zhan X, Gardiner GE (2018) Inactivation of enteric indicator bacteria and system stability during dry co-digestion of food waste and pig manure. Sci Total Environ 612:293–302
Karthikeyan OP, Visvanathan C (2013) Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: a review. Rev Environ Sci Biotechnol 12(3):257–284
Kesharwani N, Bajpai S (2020) Batch anaerobic co-digestion of food waste and sludge: a multi criteria decision modelling (MCDM) approach. SN Appl Sci 2(8):1–11
Kumar S, Paritosh K, Pareek N, Chawade A, Vivekanand V (2018) De-construction of major Indian cereal crop residues through chemical pretreatment for improved biogas production: an overview. Renew Sustain Energy Rev 90:160–170
Le Hyaric R, Benbelkacem H, Bollon J, Bayard R, Escudié R, Buffière P (2012) Influence of moisture content on the specific methanogenic activity of dry mesophilic municipal solid waste digestate. J Chem Technol Biotechnol 87(7):1032–1035
Lee YJ, Lee DJ (2019) Impact of adding metal nanoparticles on anaerobic digestion performance–a review. Bioresour Technol 292:121926
Liang YG, Cheng B, Si YB, Cao DJ, Li DL, Chen JF (2016) Effect of solid-state NaOH pretreatment on methane production from thermophilic semi-dry anaerobic digestion of rose stalk. Water Sci Technol 73(12):2913–2920
Liew LN, Shi J, Li Y (2012) Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass Bioenergy 46:125–132
Lima DRS, Adarme OFH, Baêta BEL, Gurgel LVA, de Aquino SF (2018) Influence of different thermal pretreatments and inoculum selection on the biomethanation of sugarcane bagasse by solid-state anaerobic digestion: a kinetic analysis. Ind Crop Prod 111:684–693
Lin Y, Ge X, Liu Z, Li Y (2015) Integration of Shiitake cultivation and solid-state anaerobic digestion for utilization of woody biomass. Bioresour Technol 182:128–135
Lossie U, Pütz P (2008) Targeted control of biogas plants with the help of FOS/TAC. Practice Report Hach-Lange
Ma J, Frear C, Wang ZW, Yu L, Zhao Q, Li X, Chen S (2013) A simple methodology for rate-limiting step determination for anaerobic digestion of complex substrates and effect of microbial community ratio. Bioresour Technol 134:391–395
Maletta E, Díaz-Ambrona CH (2020) Lignocellulosic crops as sustainable raw materials for bioenergy. Green Energy Sustain Strateg Global Ind:489–514
Mamimin C, Chanthong S, Leamdum C, Sompong O, Prasertsan P (2021) Improvement of empty palm fruit bunches biodegradability and biogas production by integrating the straw mushroom cultivation as a pretreatment in the solid-state anaerobic digestion. Bioresour Technol 319:124227
Michele P, Carlo M, Sergio S, Fabrizio A (2015) Optimization of solid state anaerobic digestion of the OFMSW by digestate recirculation: a new approach. Waste Manag 35:111–118
Micolucci F, Gottardo M, Pavan P, Cavinato C, Bolzonella D (2018) Pilot scale comparison of single and double-stage thermophilic anaerobic digestion of food waste. J Clean Prod 171:1376–1385
MNRE (2019) Annual report 2019–20. Ministry of New and Renewable Energy. Government of India. www.mnre.gov.in. Accessed 10 Dec 2020
Mohsenzadeh A, Jeihanipour A, Karimi K, Taherzadeh MJ (2012) Alkali pretreatment of softwood spruce and hardwood birch by NaOH/thiourea, NaOH/urea, NaOH/urea/thiourea, and NaOH/PEG to improve ethanol and biogas production. J Chem Technol Biotechnol 87(8):1209–1214
Momayez F, Karimi K, Horváth IS (2018) Enhancing ethanol and methane production from rice straw by pretreatment with liquid waste from biogas plant. Energ Conver Manage 178:290–298
Mustafa AM, Poulsen TG, Sheng K (2016) Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. Appl Energy 180:661–671
Mustafa AM, Poulsen TG, Xia Y, Sheng K (2017) Combinations of fungal and milling pretreatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Bioresour Technol 224:174–182
Nugraha WD, Keumala CF, Matin HHA (2018) The effect of acid pre-treatment using acetic acid and nitric acid in the production of biogas from rice husk during solid state anaerobic digestion (SS-AD). In E3S web of conferences (Vol 31), p 01006. EDP Sciences
Panjičko M, Zupančič GD, Fanedl L, Logar RM, Tišma M, Zelić B (2017) Biogas production from brewery spent grain as a mono-substrate in a two-stage process composed of solid-state anaerobic digestion and granular biomass reactors. J Clean Prod 166:519–529
Paritosh K, Pareek N, Chawade A, Vivekanand V (2019) Prioritization of solid concentration and temperature for solid state anaerobic digestion of pearl millet straw employing multi-criteria assessment tool. Sci Rep 9(1):1–11
Paritosh K, Balan V, Vijay VK, Vivekanand V (2020a) Simultaneous alkaline treatment of pearl millet straw for enhanced solid state anaerobic digestion: experimental investigation and energy analysis. J Clean Prod 252:119798
Paritosh K, Yadav M, Chawade A, Sahoo D, Kesharwani N, Pareek N, Vivekanand V (2020b) Additives as a support structure for specific biochemical activity boosts in anaerobic digestion: a review. Front Energy Res 8:88
Paritosh K, Yadav M, Kesharwani N, Pareek N, Karthyikeyan OP, Balan V, Vivekanand V (2021) Strategies to improve solid state anaerobic bioconversion of lignocellulosic biomass an overview. Bioresour Technol 125036
Perea-Moreno MA, Samerón-Manzano E, Perea-Moreno AJ (2019) Biomass as renewable energy: worldwide research trends. Sustainability 11(3):863
Qian X, Shen G, Wang Z, Zhang X, Chen X, Tang Z, Lei Z, Zhang Z (2019) Enhancement of high solid anaerobic co-digestion of swine manure with rice straw pretreated by microwave and alkaline. Bioresour Technol Rep 7:100208
Rico C, Montes JA, Muñoz N, Rico JL (2015) Thermophilic anaerobic digestion of the screened solid fraction of dairy manure in a solid-phase percolating reactor system. J Clean Prod 102:512–520
Rocamora I, Wagland ST, Villa R, Simpson EW, Fernández O, Bajón-Fernández Y (2020) Dry anaerobic digestion of organic waste: a review of operational parameters and their impact on process performance. Bioresour Technol 299:122681
Rouches E, Escudié R, Latrille E, Carrère H (2019) Solid-state anaerobic digestion of wheat straw: impact of S/I ratio and pilot-scale fungal pretreatment. Waste Manag 85:464–476
Ruiz B, Flotats X (2014) Citrus essential oils and their influence on the anaerobic digestion process: an overview. Waste Manag 34(11):2063–2079
Saha S, Jeon BH, Kurade MB, Jadhav SB, Chatterjee PK, Chang SW, Govindwar SP, Kim SJ (2018) Optimization of dilute acetic acid pretreatment of mixed fruit waste for increased methane production. J Clean Prod 190:411–421
Sheets JP, Ge X, Li Y (2015) Effect of limited air exposure and comparative performance between thermophilic and mesophilic solid-state anaerobic digestion of switchgrass. Bioresour Technol 180:296–303
Shi J, Xu F, Wang Z, Stiverson JA, Yu Z, Li Y (2014) Effects of microbial and non-microbial factors of liquid anaerobic digestion effluent as inoculum on solid-state anaerobic digestion of corn stover. Bioresour Technol 157:188–196
Stoklosa RJ, del Pilar Orjuela A, da Costa Sousa L, Uppugundla N, Williams DL, Dale BE, Hodge DB, Balan V (2017) Techno-economic comparison of centralized versus decentralized biorefineries for two alkaline pretreatment processes. Bioresour Technol 226:9–17
Suksong W, Kongjan P, Prasertsan P, Imai T, Sompong O (2016) Optimization and microbial community analysis for production of biogas from solid waste residues of palm oil mill industry by solid-state anaerobic digestion. Bioresour Technol 214:166–174
Suksong W, Mamimin C, Prasertsan P, Kongjan P, Sompong O (2019) Effect of inoculum types and microbial community on thermophilic and mesophilic solid-state anaerobic digestion of empty fruit bunches for biogas production. Ind Crop Prod 133:193–202
Surendra KC, Khanal SK (2015) Effects of crop maturity and size reduction on digestibility and methane yield of dedicated energy crop. Bioresour Technol 178:187–193
Thanh PM, Ketheesan B, Yan Z, Stuckey D (2016) Trace metal speciation and bioavailability in anaerobic digestion: a review. Biotechnol Adv 34(2):122–136
Wang LH, Wang Q, Cai W, Sun X (2012) Influence of mixing proportion on the solid-state anaerobic co-digestion of distiller’s grains and food waste. Biosyst Eng 112(2):130–137
Wang TT, Sun ZY, Huang YL, Tan L, Tang YQ, Kida K (2018) Biogas production from distilled grain waste by thermophilic dry anaerobic digestion: pretreatment of feedstock and dynamics of microbial community. Appl Biochem Biotechnol 184(2):685–702
Weiß S, Tauber M, Somitsch W, Meincke R, Müller H, Berg G, Guebitz GM (2010) Enhancement of biogas production by addition of hemicellulolytic bacteria immobilised on activated zeolite. Water Res 44(6):1970–1980
Xu F, Shi J, Lv W, Yu Z, Li Y (2013) Comparison of different liquid anaerobic digestion effluents as inocula and nitrogen sources for solid-state batch anaerobic digestion of corn stover. Waste Manag 33(1):26–32
Xu F, Wang F, Lin L, Li Y (2016) Comparison of digestate from solid anaerobic digesters and dewatered effluent from liquid anaerobic digesters as inocula for solid state anaerobic digestion of yard trimmings. Bioresour Technol 200:753–760
Xu F, Li Y, Ge X, Yang L, Li Y (2018) Anaerobic digestion of food waste—challenges and opportunities. Bioresour Technol 247:1047–1058
Yabu H, Sakai C, Fujiwara T, Nishio N, Nakashimada Y (2011) Thermophilic two-stage dry anaerobic digestion of model garbage with ammonia stripping. J Biosci Bioeng 111(3):312–319
Yadav M, Paritosh K, Pareek N, Vivekanand V (2019) Coupled treatment of lignocellulosic agricultural residues for augmented biomethanation. J Clean Prod 213:75–88
Yan Z, Song Z, Li D, Yuan Y, Liu X, Zheng T (2015) The effects of initial substrate concentration, C/N ratio, and temperature on solid-state anaerobic digestion from composting rice straw. Bioresour Technol 177:266–273
Yang L, Li Y (2014) Anaerobic digestion of giant reed for methane production. Bioresour Technol 171:233–239
Yang L, Xu F, Ge X, Li Y (2015) Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass. Renew Sustain Energy Rev 44:824–834
Yao Y, Chen S, Kafle GK (2017) Importance of “weak-base” poplar wastes to process performance and methane yield in solid-state anaerobic digestion. J Environ Manage 193:423–429
Zhao J, Zheng Y, Li Y (2014) Fungal pretreatment of yard trimmings for enhancement of methane yield from solid-state anaerobic digestion. Bioresour Technol 156:176–181
Zhu J, Wan C, Li Y (2010) Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment. Bioresour Technol 101(19):7523–7528
Zhu J, Zheng Y, Xu F, Li Y (2014) Solid-state anaerobic co-digestion of hay and soybean processing waste for biogas production. Bioresour Technol 154:240–247
Zhu Q, Li X, Li G, Li J, Li C, Che L, Zhang L (2020) Enhanced bioenergy production in rural areas: synthetic urine as a pre-treatment for dry anaerobic fermentation of wheat straw. J Clean Prod 260:121164
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Paritosh, K., Singh, H., Kesharwani, N., Pareek, N., Vivekanand, V. (2022). Solid State Anaerobic Digestion of Agricultural Waste for Bioenergy Production. In: Sinharoy, A., Lens, P.N.L. (eds) Renewable Energy Technologies for Energy Efficient Sustainable Development. Applied Environmental Science and Engineering for a Sustainable Future. Springer, Cham. https://doi.org/10.1007/978-3-030-87633-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-87633-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-87632-6
Online ISBN: 978-3-030-87633-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)