Abstract
Solid-state anaerobic digestion (SS-AD) is commonly used to treat feedstocks with high solid content such as municipal solid waste and lignocellulosic biomass. Compared to liquid state anaerobic digestion (LS-AD), SS-AD has multiple advantages including high organic loading, minimal digestate generated, and low energy requirement for heating. However, the main disadvantages limiting the efficiency of SS-AD are long solid retention time, incomplete mixing, and an accumulation of inhibitors. For a successful and efficient SS-AD, it is important to control operation parameters such as nutrient levels, C/N ratio, feedstock-to-inoculum ratio, pH, temperature, and mixing. Biogas production in SS-AD performance can be enhanced by feedstock pretreatment, co-digestion, and supplement of additives such as biochar. The aim of this chapter is to provide a comprehensive summary of the current development in SS-AD as an effective way for treating solid waste materials.

Graphical Abstract
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
With the growth of world population and economics, the production of solid wastes is increasing tremendously. A large quantity of these waste materials is biodegradable agricultural residues and municipal solid wastes (MSW). It is estimated that the annual production of MSW will reach 2.2 billion tons by 2025 [1]. These abundant materials can be used as a feedstock for anaerobic digestion (AD) to produce energy while solving waste disposal problems.
AD is a process in which microorganisms decompose organic matters to produce biogas in the absence of oxygen. An AD process typically consists of four stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. In the hydrolysis stage, macromolecules such as cellulose, starch, proteins, and lipids are decomposed into monomers such as sugars, amino acids, and fatty acids. Those monomers are then converted into C2–C5-based volatile fatty acids (VFAs) and alcohols, as well as H2 and CO2 in the acidogenesis stage. In the acetogenesis stage, VFAs and alcohols are converted into acetate. In the methanogenesis stage, methane (CH4) is produced through the conversion of acetate to CH4 and CO2 (acetoclastic methanogenesis) or the reduction of formate or CO2 to CH4 (hydrogenotrophic methanogenesis). Among these four steps, hydrolysis is commonly the rate-limiting step particularly when the feedstock is the complex organic substrates. When easily digestible organic matters are used as a feedstock, methanogenesis becomes the limiting step [2]. The biogas produced from an AD process usually contains 60–70% CH4 and 20–30% CO2 with trace amounts of ammonia, hydrogen sulfide, and hydrogen. The biogas can be combusted to generate heat and/or electricity or upgraded and refined into transportation fuels. Meanwhile, the digestate rich in nutrients, such as nitrogen and phosphorus, can be recycled as fertilizers or processed into biochar that can be used as soil amendment [3].
Based on the total solid (TS) content, AD can be defined as liquid state AD (LS-AD) with TS less than 15% or solid-state AD (SS-AD) with TS greater than 15% [4]. LS-AD is used to treat high moisture substrates such as animal manures and sewage. However, the large amount of water used in LS-AD process leads to a decreased volumetric CH4 productivity and creates the problem of disposing large amount of digestate [5]. On the contrary, SS-AD can handle feedstocks with high organic loading with minimal water demand and results in a high volumetric CH4 productivity. The wastewater generated and heating energy required in SS-AD are also reduced. However, due to inadequate mass transfer, SS-AD has disadvantages such as longer retention time, high cost, and a tendency to accumulate inhibitors [6]. In the past decade, a steady increase of publications in SS-AD indicates a great interest in this area (Fig. 1). The aim of this chapter is to provide a comprehensive review of the recent advances of SS-AD including feedstock, inoculum, factors affecting SS-AD performance, operation mode, and digestion process enhancement.
2 Feedstocks
Feedstocks with high moisture content, such as animal manure or municipal sewage, have been traditionally treated with LS-AD. Recent development of AD has expanded to feedstocks with high solid content such as agricultural residues (e.g., corn stover, wheat straw, and rice straw), industrial wastes, and municipal solid wastes; SS-AD has been increasingly used to treat these feedstocks. Corn stover, with a 1:1 weight ratio of residue to grain [7], is the most abundant agricultural residue in the United States, with approximately 80 million dry tons of corn stover produced each year [8]. It has been reported that SS-AD (18% TS) treatment of corn stover produced a higher CH4 yield than LS-AD (5% TS) [9, 10]. SS-AD of wheat straw, another abundant agriculture residue, also resulted in a much higher CH4 yield than LS-AD [8, 11].
The microstructure of the fibrous feedstock significantly affects SS-AD performance. Cui et al. [10] examined the fiber structure in wheat straw by scanning it with an electron microscope (SEM). Compared to the raw wheat straw with long and smooth intact fibers, the spent wheat straw with rough fiber and serrations at the edge was more digestible. Similarly, corn stover treated by sodium hydroxide was more digestible than the raw corn stover [11].
The organic municipal solid wastes (OMSW), such as food and yard wastes, have also been commonly used as feedstock for SS-AD. It is estimated that 1.3 billion tons per year of food wastes are produced worldwide [12]. In the United States, food waste accounts for 12% of total municipal solid waste [13]. Food waste composition varies widely depending on geographical locations and the eating habits of local populations. In general, food wastes containing soluble organic matters can be easily converted into VFAs, which may inhibit the subsequent CH4 formation if VFAs are overproduced. A two-phase SS-AD can successfully overcome this problem [14]. Among the 31 million dry tons per year of yard wastes generated in the United States, more than 60% were treated through composting, during which energy was wasted as respiration heat [5]. SS-AD as an alternative to composting can recover energy. However, the types of yard wastes affect the methane yield due to different TS, VS, and C/N in those materials [15, 16].
3 Inoculum
Inoculum brings the microbes, nutrients, and water to SS-AD reactors. Typical inoculum in SS-AD includes sewage sludge, ruminant cultures, and digested manure [17]. Since most solid feedstock does not naturally contain methanogens, methanogens-rich inoculum is essential to a SS-AD process [15]. Characterization of the microbial community in inoculum is important for an insightful understanding, particularly the functional partitioning of a SS-AD process. Shi et al. [16] studied the microbial community in SS-AD of corn stover using denaturing gradient gel electrophoresis and found enriched archaeal and bacterial communities in the system. In SS-AD of rice straw, a high-throughput sequencing analysis revealed that Methanobacteria, Bacteroidia, Clostridia, Betaproteobacteria, and Gammaproteobacteria were the primary species [18]. The acetoclastic Methanosarcina and hydrogenotrophic Methanoculleus coexisted in this system. For example, in the first 20 days of AD, Methanosarcina accounted around 86.5% of microbial population, while Methanoculleus accounted 32.1% of microbial population from days 7 to 45 [18]. Bacteria producing low temperature-adaptive lipases, Psychrobacter, was identified in SS-AD of a mixed kitchen waste, pig manure, and the sludge [19]. In SS-AD of fruit waste, a three-stage system was developed to accommodate the favorable conditions for hydrolysis, acidogenesis, and methanogenesis [20]. Lactobacillaceae and Pseudomonadaceae were predominant in the hydrolysis of carbohydrate into lactate and biomic acids, respectively. In the acidogenesis stage, the most abundant bacteria were switched to Porphyromonadaceae and Enterobacteriaceae, while methanogens were the dominant species in the methanogenic stage [20].
4 Factors Affecting Solid-State AD
4.1 Nutrients
Anaerobic microbes need balanced nutrients such as carbon, nitrogen, phosphorous, and minerals for their growth. Carbon, the primary energy source for cell growth, is usually rich in organic materials. Nitrogen and phosphorous are also essential for anaerobic microbes to synthesize proteins and nucleic acids, respectively. Ammonium is the nitrogen form methanogens can utilize [21] but will inhibit the microbial growth at high levels. The C/N ratio of the feedstock also plays an important role in digestion process. C/N ratio ranging from 20:1 to 30:1 with an optimal C/N ratio of 25:1 is recommended [22]. Too low C/N ratios increase the risk of ammonia inhibition, resulting in insufficient utilization of carbon sources, while an excessively high C/N ratio results in insufficient nitrogen to maintain microbial growth and biogas production. The demand of phosphorus is usually 15% of that of nitrogen [23].
Trace elements such as iron, cobalt, nickel, and sulfur are essential for methane fermentation [24,25,26,27]. Iron is often supplemented in AD systems to activate enzymes such as ATPase, PEP carboxylase, and serine transhydroxymethylase [28, 29]. Due to its reduction capacity, iron often reacts with sulfur to form FeS precipitant, reducing H2S generation and alleviating odor problem [30]. Nickel is an essential element in coenzyme F430, hydrogenase, and CO dehydrogenase in methanogenic microbes [31,32,33]. Cobalt is involved in the activity of methyl transferase and CO dehydrogenase (CODH) in acidogenesis [31]. The addition of cobalt has been reported to stimulate CH4 productivity in methanol LS-AD process [25]. Molybdenum is present in CO2 reductase, a molybdoprotein that is responsible for reducing CO2 to formate and subsequently reducing to CH4 [34].
4.2 Feedstock-to-Inoculum Ratio
Feedstock-to-inoculum ratio (F/I) is another important factor in SS-AD. Too high F/I ratio could result in overproduction of VFAs due to excess organic loads, which eventually leads to an acidic pH and inhibition on methanogens. Zhou et al. [35] reported that the CH4 yield of rice straw SS-AD was inversely proportional to F/I due to the VFAs accumulation and poor mass transfer. On the contrary, SS-AD of palm oil mill residues achieved the highest CH4 production rates at the lowest F/I ratio within the range of 2:1–5:1, while a rapid hydrolysis at F/I ratio of 4:1–5:1 resulted in a VFAs accumulation and low CH4 yield [36].
4.3 pH
The pH of a SS-AD system also affects the digestion performance. The ideal pH for a SS-AD process is within a narrow range of 6.8–7.2 [37]. However, different groups of microbes in SS-AD have different optimal pH requirements. For example, the optimal pH for acidogens is between 5.5 and 6.5, while methanogens are most active at pH 6.5–8.2 with an optimum at pH 7.0 [38]. Due to this discrepancy of pH requirement, two-stage SS-AD, i.e., separating acidogenesis and methanogenesis into two reactors, is usually used [37].
During an AD process, pH is affected by many parameters. In a SS-AD of OMSW with liquid digestate recirculation, the pH was low (<6.5) initially due to high VFAs concentration and then gradually increased to 8 after VFAs decreased from 12,000 to 1,000 mg/L within 1 week [39]. The buffer capacity of an AD system to resist pH fluctuation is evaluated through alkalinity. For example, in a corn stover SS-AD system with a less alkalinity (1,036 mg CaCO3/kg), pH dropped from nine to below six rapidly with a decreased CH4 yield [16]. When the alkalinity of the system was increased (>1,700 mg CaCO3/kg) through adjusting the F/I ratio, pH of the same system was stabilized with only slight a decrease from 9 to 8.4 [16]. In order to maintain a stable pH during SS-AD process, it is essential to balance VFAs concentration and bicarbonate. In general, reducing organic loading, adding bases or bicarbonates, and modifying F/I ratio are used to increase alkalinity in SS-AD systems [37].
4.4 Temperature
SS-AD is commonly operated at mesophilic (~37°C) or thermophilic (~55°C) conditions. Compared to the mesophilic AD, thermophilic AD has a shorter start-up time and hydraulic retention time (HRT) due to accelerated feedstock hydrolysis. The CH4 yield in thermophilic SS-AD is also higher as methanogenic microbes have an optimal growth at 55°C [40]. Thermophilic AD can also produce pathogen-free digestate. Pohl et al. [41] compared the performance of wheat straw SS-AD under 37°C and 55°C. The CH4 yield from the thermophilic AD was 36% higher than that in mesophilic AD due to a faster disintegration and hydrolysis of the feedstock.
However, compared to mesophilic SS-AD, poor stability and reliability often represent obstacles in thermophilic SS-AD. In general, microbes in thermophilic conditions are more sensitive to environmental changes, exhibiting poor stability and less diversity and richness in microbial community [38]. Also, the fast hydrolysis of feedstock in thermophilic processes often results in a rapid VFAs production, causing an imbalance between acidogenesis and methanogenesis. The higher temperature also shifts NH3/NH4 + equilibrium toward the cytotoxic ammonia [40]. Heating energy in theomorphic AD is also higher [42]. Due to those reasons, theomorphic digesters are still not commonly used in commercial SS-AD.
4.5 Inhibitors
A variety of compounds have been reported inhibitory to SS-AD, causing an adverse shift in microbial population, an instability of the process, and a decreased CH4 yield [43]. In LS-AD, the inhibitor concentrations can be diluted, while inhibitory effects in SS-AD cannot be alleviated and often cause severe inhibition to the system. The easily digestible feedstock often leads to a rapid hydrolysis and acidification, producing excessive VFAs which inhibit methanogens. For example, in SS-AD of tomato residues, VFAs concertation (12.48 g/L) was much higher than the threshold level (6 g/L) and caused CH4 production inhibition [44]. Compounds derived from phenolic degradation, such as p-cresol, inhibit acetogenesis, resulting in accumulation of VFAs [45].
The partial pressures of CO2 and H2 in SS-AD system also affect the CH4 production. Increasing CO2 partial pressure results in an increased dissolved CO2, which causes acidification and inhibition of methanogenesis. An elevated H2 partial pressure leads to an accumulation of dissolved H2, which inhibits the degradation of VFAs [46]. In SS-AD of wheat straw, high H2 partial pressure also led to a strong inhibition on the initial hydrolysis step [47]. Since both CO2 and H2 are needed to produce acetate/CH4, a balanced CO2/H2 pressure in the headspace is essential to prevent inhibition.
Ammonia is produced from the degradation of nitrogenous compounds (e.g., protein and urea) during AD process. A moderate amount of ammonia is essential for bacterial growth and neutralizing VFAs to maintain a stable pH; however, excessive ammonia can inhibit methanogenesis. Ammonia exists as an equilibrium between ammonium ion (NH4 +) and free ammonia (NH3) [43]. Free ammonia can permeate cell membrane and cause proton imbalance and thus is inhibitory to microbial cells. Animal manure usually contains excessive ammonia, resulting in process inhibition. For example, in SS-AD of chicken manure, the digester was completely inhibited when influent total Kjeldahl nitrogen (TKN) (mainly ammonia) was 8.2 g/L [48]. After ammonia was removed from influent, the digester achieved a much higher CH4 yield.
4.6 Mixing
A certain degree of mixing in SS-AD is necessary to enhance the transfer of organic substrates to microbes, prevent the sedimentation of denser particles or floating lighter materials, and facilitate the release of gas bubbles trapped in the solid feedstock. In SS-AD of rice straw, intermittent mixing with a 5/25 min on/off cycle at 160 rpm resulted in a good mass transfer while saving energy compared to a continuous mixing [35]. Premixing of the feedstock with inoculum is also needed before loading into SS-AD reactor [49, 50].
The methods of mixing in SS-AD can be liquid (leachate) recirculation, solid mixing using augers, and biogas recirculation [4], among which the leachate recirculation is commonly used. Leachate recirculation in SS-AD facilitates the nutrient diffusion from substrates to microbial cells [51] and also reduces the amount of inoculum as the microbe-containing leachate collected from the reactor can be reapplied to the digestion systems [52].
In addition to mixing, leachate recirculation also provides other benefits to SS-AD. For example, when leachate recirculation was used in the acidogenic reactor of a two-stage hybrid solid-liquid AD system, the extraction of organic matters from the feedstock was facilitated, and the pH was buffered [53]. In the SS-AD of hay and soybean processing wastes, leachate recirculation accelerated the daily CH4 production to the peak value due to the enhancement of VFAs mass transfer from acidogenic to methanogenic pockets [54]. However, leachate recirculation may also lead to accumulation of VFAs and other inhibitors compounds; therefore, dilution of leachate with fresh water may be needed [15]. A leachate recirculation rate also needs to be carefully controlled to avoid irreversible acidification of the system [55].
5 Process Operations of SS-AD
5.1 Batch vs. Continuous Operations
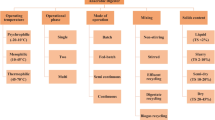
Batch and continuous operations are two operation modes commonly used in SS-AD. Table 1 compares the performance of batch and continuous operation of SS-AD. Compared to continuous operation, batch operation is easier to maintain because it needs less capital and operating costs with less process control requirements. However, the biogas production in batch SS-AD is variable with time, and the majority of biogas is produced only at peak production time. For example, it was reported that in a 55-day batch SS-AD of corn stover, more than 80% of biogas was produced only at 36-day period of methanogenic phase [22]. Another limitation in batch SS-AD is the requirement of a large amount of inoculum (i.e., low F/I ratio). For example, Capson-Tojo et al. [56] reported that a batch SS-AD of food waste and cardboard mixture can only produce biogas at a F/I lower than 0.25; the biogas production had completely ceased when the F/I ratio was above this ratio due to overproduction of VFAs. Similar results were obtained for a batch operation of yard trimmings SS-AD process in which the highest CH4 yield (244 L/kg VS) was obtained at the lowest level of the F/I ratio ranging from 0.2 to 2 [57]. The inoculum sources also significantly affected the batch SS-AD process. Guendouz et al. [58] studied three successive batches of MSW SS-AD and found that the second and third batches inoculated with the residue from the previous batch shortened the lag phase and accelerated reaction, which was due to the adaptation of the microbes to the digestion system.
Contrary to the batch operation, continuous SS-AD can consistently produce CH4 at steady state. Organic loading rate (OLR), CH4 production, and solid retention time (SRT) are the three main parameters in determining the interaction between microorganisms and substrates and thus are used in designing and evaluating a continuous SS-AD performance [51]. OLR represents the conversion capacity of an AD system; a maximum OLR level in SS-AD depends on various parameters such as reactor design, feedstock characteristics, microbial activity, temperature, pH, and toxicity level [59]. A high OLR is always preferred as it means an improved utilization efficiency and reduced digester size. However, high OLR can also lead to VFAs overproduction, causing an imbalance between acidogens and methanogens. For example, in a batch SS-AD process of rice straw, increasing TS loading from 20% to 24% prolonged the lag phase from 15 days to 20 days [35]. Similarly, increasing OLR from 2.3 to 9.2 kg VS/m3 day in semicontinuous SS-AD of food waste slowed down bacteria acclimatization in the new environment, resulting in a prolonged adaptation time from 2 days to 31 days [60]. In a co-digestion of chicken manure and poplar leaf, CH4 yield decreased when OLR increased from 4.0 to 8.0 g VS/L day [61].
One important operational parameter in continuous SS-AD is solid retention time (SRT); the time organic compounds stay in the digester. Due to slower mass transfer, the SRT in SS-AD is usually longer than the HRT commonly used in LS-AD [54]. The retention time needed for a complete degradation of solid feedstock can be determined through biomethane potential (BMP) assay [62]. An optimal SRT depends on many factors such as the feedstock, OLR, and TS. Decreasing SRT leads to washing out of microorganisms and insufficient substrate utilization. A longer SRT is usually not economical because it would require larger reactor volumes and higher costs for maintenance. SRT has a considerable impact on CH4 production. In SS-AD of organic waste containing vegetable, fruit, and green waste, increasing SRT from 15 days to 35 days increased methane yield from 360 to 454 mL/kg VS [63].
5.2 Single-Stage vs. Multistage Operations
SS-AD can be operated in a single stage or multiple stages. In a single-stage system, the multiple steps of the conversion of organic substrates into biogas are implemented in one reactor vessel. In a multiple-stage operation, different conversion steps are implemented into different reactor vessels. A two-stage AD is commonly used as a multiple-stage operation during which the hydrolysis/acidogenesis is in the first reactor and the methanogenesis is in the second reactor [64].
Compared to a two-stage operation, a single-stage reactor is easier to design and build with less operating costs. However, the OLR in a single-stage digester is often limited in order to avoid VFAs overproduction and rapid pH drop [4]. Unlike the single-stage digester, two-stage systems can accommodate each conversion step, such as acidogenesis and methanogenesis, at their own optimal conditions (pH, temperature, OLR, and SRT). Two-stage systems generally perform better than a single-stage system. For instance, SS-AD of brewery spent grain (BSG) in a single-stage reactor was limited by the inhibitors, such as weak acids, furan derivatives, and phenolic substances, generated in the degradation of lignocellulose in BSG [65]. While in a two-stage SS-AD system, separating hydrolysis in one reactor and acidogenesis and methanogenesis in another granular-based reactor, both biogas production and feedstock biodegradation were improved (Table 2).
In some occasions, AD systems with more than two stages, such as three stages, are designed to create different favorable conditions for hydrolyzing bacteria, acidogenic bacteria, and methanogens, with each group of microbes performing a particular role. A three-stage system was used in the co-digestion of food waste and horse manure in which the first-stage hydrolysis and second-stage acidogenesis were operated as a solid state, while the methanogenesis was operated as a liquid state. This hybrid system increased CH4 yield by 11.2–22.7% and the abundance of methanogenic archaea by 0.8–1.28 times compared to the single-stage reactor.
It should be noted that despite the fact that multistage AD systems are advantageous in improving AD performance, high capital and operating costs are the main hurdles for implementing this type of systems at a commercial scale. As a result, single-stage AD is still dominantly used. In Europe, for example, about 90% of the installed AD capacity is from single-stage systems, and only about 10% is from multistage systems [4].
6 Enhancement of Digestion Performance in SS-AD
6.1 Feedstock Pretreatment
As hydrolysis is the rate-limiting step for SS-AD of most solid feedstocks, various treatment technologies have been developed to accelerate the feedstock hydrolysis so overall biogas yield can be enhanced. Those pretreatment methods are summarized in Table 3.
6.1.1 Physical Treatment
Physical treatment such as milling and grinding reduces the particle size of the feedstock and thus provides a greater surface area for microorganisms to access. Tian et al. [66] reported a 29% increase in CH4 yield in SS-AD of rape straw when the feedstock size was reduced from 2–2.5 cm to 0.5 mm. However, a too fine particle size may negatively affect the AD performance. For example, Motte et al. [82] compared the SS-AD of straw at three particle sizes (0.25, 1, and 10 mm) and found that the coarse particles (10 mm) resulted in the highest CH4 yield followed with the medium size particles (1 mm) and the finest size (0.25 mm). The reason for this phenomenon was due to rapid acidification of the substrate at smaller particle sizes, which resulted in an overproduction of VFAs and rapid pH drop.
Thermal treatment is an effective treatment method for industrial SS-AD [83]. In addition to enhancing the reaction rate, thermal treatment removes pathogens, improves dewaterability, and decreases viscosity of the digestion system. In the SS-AD of steam autoclaved MSW, the digestate passed all the criteria for biosolids land application in the United States [69]. In the thermal treatment, an appropriate combination of temperature and time is needed as the high energy consumption often offsets the overall benefits. Liao et al. [70] studied the effect of treatment temperature (60, 70, and 80°C) on the SS-AD of sewage sludge and found that 70°C for 30 min was optimal for SS-AD. Under this condition, biogas yield increased by 11% and SRT reduced from 22 to 15 days.
Other physically based treatments were also reported. For example, ultrasound treatment generates both mechanical effects through cavitation and chemical effects through formation of free radicals. OMSW treated with low-frequency ultrasound released more soluble organic matters, resulting in a 16% increase in biogas production in SS-AD [67]. Microwave treatment is related to structure modification as well as thermal effects, contributing to increased sludge solubility [84], shortened initial lag phase [85], and improved CH4 yield [68].
6.1.2 Chemical Treatment
Chemicals such as acids, alkaline, or oxidants can facilitate the breaking down of recalcitrant structures of feedstock. The effectiveness of chemical treatment relies on the feedstock characteristics and the reagents used. Feedstocks with easily digestible carbohydrates such as starch are typically not suited for chemical treatment because it accelerates starch degradation leading to VFAs overproduction and accumulation [86].
Alkaline treatment is usually carried out at ambient temperature with lime, sodium hydroxide, potassium hydroxide, and ammonium hydroxide as agents. The mechanism of alkaline treatment is to remove lignin from lignocellulose, improving the accessibility of hemicellulose and cellulose by the microbes and enzymes [87]. Additionally, the presence of residue alkali neutralizes carboxylic acids resulted from lignocellulose degradation in subsequent acidogenesis stage and prevents pH drop [71]. Zhu et al. [71] reported a 37% increase in biogas yield in SS-AD of corn stover treated with 5.0% NaOH compared to that of untreated corn stover. Liew et al. [88] achieved a 24-fold higher CH4 yield in SS-AD of fallen leaves treated with 3.5% NaOH. However, excessive alkali loading may inhibit AD due to high pH or ion toxicity [89]. For instance, in SS-AD of corn stover, although the lignin degradation of corn stover increased with NaOH loadings from 1.0% to 7.5%, the biogas yield was not improved correspondingly; 7.5% NaOH loading actually inhibited biogas production due to VFAs accumulation and acidification [71].
Compared to alkali treatment, acid treatment is more effective to break down the recalcitrant lignocellulosic structure and produce reducing sugars [83]. However, compounds such as furfural and hydroxymethylfurfural (HMF) can be produced during acid treatment which inhibit the AD process [86]. Acid treatment also requires additional bases to neutralize pH before starting SS-AD. Overall, acid treatment is less preferable than alkaline treatment in SS-AD.
Ozonation is another chemically based treatment in SS-AD with no chemical residues left in the system. As a strong oxidant, ozone decomposes into radicals and reacts with the soluble and insoluble fractions of the substrates [90]. The optimal ozone dosage is reported in the range of 0.05–0.5 g O3/g TS [86]. In SS-AD of OMSW, a 37% increase in biogas yield was achieved with feedstock treated with ozone at 0.16 g O3/g TS; however, higher ozone dosages (0.4 and 1.2 g O3/g TS) led to a lower biogas yield, probably due to the formation of intermediate compounds that are difficult to be digested [67].
Organic solvent is another chemical used in the treatment of lignocellulose-based feedstock by removing lignin and thus improve degradability of lignocelluloses. For example, in SS-AD of elm, pine, and rice straw, treating the feedstock with ethanol prior to SS-AD enhanced CH4 production by 73%, 84%, and 32%, respectively [73].
6.1.3 Biological Treatment
Biological treatment relies on microorganisms and/or enzymes to break down the recalcitrant structure of the feedstock. Enzymes such as peptidase, carbohydrase, and lipase [86] are commonly added to the LS-AD system to speed up the digestion. However, the practices of adding external enzymes to the SS-AD process have not been widely reported. Microorganisms, such as white-rot fungi, capable of decomposing lignin and altering the linkage between lignin and polysaccharides are commonly used in SS-AD [91]. The fungi Pleurotus ostreatus and Trichoderma reesei were used to decompose rice straw as an effective way to enhance CH4 yield in SS-AD of this feedstock [74]. The white-rot fungus Ceriporiopsis subvermispora is considered one of the most effective species to degrade lignin while preserving cellulose [76]. Due to its selective degradation feature, C. subvermispora-treated SS-AD led to a 20.9% lignin degradation of yard trimming and only 7.4% cellulose degradation, achieving a 154% increase in CH4 yield in the subsequent SS-AD [75]. When C. subvermispora was used to treat albizia chips, CH4 yield increased 3.7-fold compared to the untreated feedstock [76].
Composting, an aerobic process facilitated by bacteria and fungi, is another biological treatment for SS-AD. Yan et al. [18] reported that composting rice straw resulted in a decrease of 63.6% TS, while the total carbon did not reduce significantly, proving that composting can effectively improve the biodegradability of rice straw. In order to improve the composting efficiency, pre-aeration is often used to generate enough self-heating to increase the temperature of OMSW for the start-up of thermophilic AD without external heating [92]. Composting with pre-aeration can also reduce the excessive organic compounds in feedstocks and thus reduce the risk of VFA overproduction and acidification in the following SS-AD [92]. However, excessive pre-aeration may cause toxic effect on methanogens by introducing oxygen. For example, Zhou et al. [35] reported that rice straw aerated for 2 days achieved the highest CH4 yield, while the CH4 yield gradually decreased when the aeration times increased from 4 days to 8 days.
6.2 Co-digestion
Co-digestion of different feedstocks is commonly used to adjust carbon-to-nitrogen (C/N) ratio of the substrates in SS-AD. Other advantages of co-digestion include improved nutrient profiles, a more balanced microbial community, obtaining a desired moisture content, and economic advantages by sharing equipment. However, there are several drawbacks of co-digestion such as the extra logistical cost of the different feedstock, premixing requirement, varied policy to control different waste materials, and increased effluent COD [93].
The optimal C/N ratio for an AD process is in the range of 20:1–30:1. Most lignocellulose has a higher C/N ratio than 30; therefore, blending lignocellulosic feedstock with animal manures (with a C/N ratio less than 20) is a good approach to balance C/N ratio of SS-AD system. Li et al. [61] reported co-digestion of poplar leaf (C/N = 35.4), and chicken manure (C/N = 8.09) brought C/N ratio to the optimal range (Table 4) and produced 15.28% more CH4 than digestion of poplar leaf only.
In addition to adjusting C/N ratio, co-digestion of different feedstocks also provides other benefits such as better nutrients, diverse microorganism consortium, and stable pH and higher buffering capacity [61]. Khairuddin et al. [95] reported that the co-digestion of household organic waste and cow manure, even with a low C/N ratio of 11.1, still increased CH4 yield by 10.7% compared to digestion of household organic waste only. Similarly, co-digestion of spent mushroom substrate and yard trimmings (with a C/N ratio of 74.6) produced 16-fold higher CH4 yield than digestion of spent mushroom only [99].
The ratio of the co-digested substrates is important for a successful SS-AD. Li et al. [44] conducted a SS-AD of tomato residues, corn stover, and dairy manure with eight mixing ratios. The authors reported that a mixing ratio of tomato residues, corn stover, and dairy manure at 13:33:54 (TS based) achieved the highest CH4 yield, while digestion of tomato residues failed due to ammonia inhibition. Similarly, co-digestion of food waste with distiller’s grains under four ratios (1:4, 1:6, 1:8, 1:10) showed that food waste vs. distiller’s grains ratio at 1:8 resulted in the highest CH4 yield [98].
6.3 Additives
Various additives have been used to supplement AD systems to improve digestion performance [102]. Biochar, a charcoal-like product of incomplete combustion (pyrolysis) of organic materials, has been used as an additive in AD with multiple functions. In a study of chicken manure AD, Liang et al. [103] found that adding biochar reduced the lag phase by 41% and increased CH4 production rate by 18% with reduced H2S. In another study of AD of sludge amended with biochar, average CH4 content in biogas was up to 92.3%, corresponding to a CO2 sequestration by 66.2% [104]. A biochar addition also enhanced process stability through increasing the alkalinity and alleviated free ammonia inhibition [104]. Qin et al. [105] used magnetic biochar (a composite of biochar and magnetic medium) as an additive in sludge AD and recorded 11.69% increase in CH4 production. The authors attributed the enhancement to the selective enrichment of functional bacteria and methanogens absorbed on magnetic biochar.
Materials promoting direct interspecies electron transfer (DIET) are also used as additives to accelerate the conversion of organic substrate to CH4 [106]. For instance, carbon cloth and granular activated carbon were used to stimulate CH4 production in AD of dog food, tolerate high OLR, and recover from soured digester faster [106]. Conductive materials were also effective in stimulating the syntrophic conversion of ethanol to CH4 in upflow anaerobic sludge blanket reactor [107]. The CH4 production rates increased by 30–45% with the addition of conductive materials at each OLR [107].
It should be noted that although various additives have been shown to be beneficial to AD systems, few studies have been done to apply those additives to SS-AD. Further studies are needed to evaluate the technical and economic feasibility of using additive in SS-AD systems.
7 Conclusion and Perspectives
SS-AD has become a popular approach to digest organic wastes with high solid content due to its inherent advantages such as high OLR, reduced reactor size, and minimal amount of digestate generated. A variety of materials, from municipal solid wastes to agricultural residues, can be used as feedstock for SS-AD. To ensure a successful SS-AD, operation conditions such as nutrient levels, feedstock-to-inoculum ratio, pH, temperature, and mixing need to be carefully controlled. Moreover, reactor systems configured with different operation modes (batch vs. continuous; one stage vs. multiple stage) have been applied based on diverse characteristics of the feedstocks. To enhance SS-AD performance, pretreatment is needed to make lignocellulosic feedstock more amenable for microorganism to degrade. Co-digestion of different feedstocks and supplement external additives such as biochar are also effective to improve biogas production.
Further studies on SS-AD should focus on several issues in order to develop an effective commercial-scale process. First, feedstock pretreatment should be carefully selected to address the operational costs, treatment effectiveness, and inhibitors. Second, mass transfer limitation needs to be effectively overcome. Finally, understanding the microbial consortium and metabolic pathways involved in SS-AD processes is crucial to provide potential guidance to improve the digestion performance. Solving these hurdles will facilitate the application of SS-AD as a promising alternative to the traditional waste disposal process.
References
Hoornweg D, Bhada P (2012) What a waste. A global review of solid waste management. Urban Dev Ser Knowl Pap 281(19):44
Tomei MC, Braguglia CM, Cento G, Mininni G (2009) Modeling of anaerobic digestion of sludge. Crit Rev Environ Sci Technol 39(12):1003–1051
Inyang M, Gao B, Pullammanappallil P, Ding W, Zimmerman AR (2010) Biochar from anaerobically digested sugarcane bagasse. Bioresour Technol 101(22):8868–8872
Rapport RB, Zhang J, Jenkins R, Williams BM (2008) Current anaerobic digestion technologies used for treatment of municipal organic solid waste. Calif Integr Waste Manag Board:90
Ge X, Xu F, Li Y (2016) Solid-state anaerobic digestion of lignocellulosic biomass: recent progress and perspectives. Bioresour Technol 205:239–249
Shi J, Wang Z, Stiverson JA, Yu Z, Li Y (2013) Reactor performance and microbial community dynamics during solid-state anaerobic digestion of corn stover at mesophilic and thermophilic conditions. Bioresour Technol 136:574–581
Langholtz MH, Stokes BJ, Eaton LM (2016) 2016 Billion-ton report advancing domestic resources for a thriving bioeconomy. Oak Ridge Natl Lab 1160(July):448–2172
Kadam K, McMillan J (2003) Availability of corn stover as a sustainable feedstock for bioethanol production. Bioresour Technol 88(1):17–25
Brown D, Shi J, Li Y (2012) Comparison of solid-state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production. Bioresour Technol 124:379–386
Cui Z, Shi J, Li Y (2011) Solid-state anaerobic digestion of spent wheat straw from horse stall. Bioresour Technol 102(20):9432–9437
Li Y, Zhang R, He Y, Liu X, Chen C, Liu G (2014) Thermophilic solid-state anaerobic digestion of alkaline-pretreated corn stover. Energy Fuels 28(6):3759–3765
Food and Agriculture Organization and of the United Nations (2012) Towards the future we want: end hunger and make the transition to sustainable agricultural and food systems. FAO, pp 1–42
Zhang C, Su H, Baeyens J, Tan T (2014) Reviewing the anaerobic digestion of food waste for biogas production. Renew Sust Energ Rev 38:383–392
Cho JK, Park SC, Chang HN (1995) Biochemical methane potential and solid state anaerobic digestion of Korean food wastes. Bioresour Technol 52(3):245–253
Yang L, Xu F, Ge X, Li Y (2015) Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass. Renew Sust Energ Rev 44:824–834
Shi J, Xu F, Wang Z, Stiverson JA, Yu Z, Li Y (2014) Effects of microbial and non-microbial factors of liquid anaerobic digestion effluent as inoculum on solid-state anaerobic digestion of corn stover. Bioresour Technol 157:188–196
Karthikeyan OP, Visvanathan C (2013) Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: a review. Rev Environ Sci Biotechnol 12(3):257–284
Yan Z, Song Z, Li D, Yuan Y, Liu X, Zheng T (2015) The effects of initial substrate concentration, C/N ratio, and temperature on solid-state anaerobic digestion from composting rice straw. Bioresour Technol 177:266–273
Li A et al (2013) A pyrosequencing-based metagenomic study of methane-producing microbial community in solid-state biogas reactor. Biotechnol Biofuels 6(3):17
Zhang J, Loh KC, Lee J, Wang CH, Dai Y, Wah Tong Y (2017) Three-stage anaerobic co-digestion of food waste and horse manure. Sci Rep 7(1):1–10
Takashima M, Speece RE, Parkin GF (1990) Mineral requirements for methane fermentation. Crit Rev Environ Control 19(5):465–479
Liu CM et al (2018) Evaluation of methane yield using acidogenic effluent of NaOH pretreated corn stover in anaerobic digestion. Renew Energy 116:224–233
Speece RE, McCarty PL (1964) Nutrient requirements and biological solids accumulation in anaerobic digestion. Pergamon Press, Oxford
Hoban DJ, van den Berg L (1979) Effect of iron on conversion of acetic acid to methane during methanogenic fermentations. J Appl Bacteriol 47(1):153–159
Florencio L, Jeniček P, Field JA, Lettinga G (1993) Effect of cobalt on the anaerobic degradation of methanol. J Ferment Bioeng 75(5):368–374
Scherer P, Sahm H (1981) Effect of trace elements and vitamins on the growth of Methanosarcina barkeri. Acta Biotechnol 1:57–65
Diekert G, Konheiser U, Piechulla K, Thauer RK (1981) Nickel requirement and factor F430 content of methanogenic bacteria. J Bacteriol 148(2):459–464
Kiener A, Husain I, Sancar A, Walsh C (1989) Purification and properties of Methanobacterium thermoautotrophicum DNA photolyase. J Biol Chem 264(23):13880–13887
Nessim SJ, Nisenbaum R, Bargman JM, Jassal SV (1986) Tetrahydromethanopterin-dependent serine transhydroxymethylase from Methanobacterium thermoautotrophicum. Perit Dial Int 32(3):316–321
Park CM, Novak JT (2013) The effect of direct addition of iron(III) on anaerobic digestion efficiency and odor causing compounds. Water Sci Technol 68(11):2391–2396
Kayhanian M, Rich D (1995) Pilot-scale high solids thermophilic anaerobic digestion of municipal solid waste with an emphasis on nutrient requirements. Biomass Bioenergy 8(6):433–444
Diekert RK, Graf GB, Thauer EG (1979) Nickel requirement for carbon monoxide dehydrogenase formation in Clostridium pasteurianum. Arch Microbiol 122:117–120
Daniels L, Fuchs G, Thauer RK, Zeikus JG (1977) Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol 132(1):118–126
Schönheit P, Moll J, Thauer RK (1979) Nickel, cobalt, and molybdenum requirement for growth of Methanobacterium thermoautotrophicum. Arch Microbiol 123(1):105–107
Zhou Y, Li C, Achu I, Liu J (2017) The effects of pre-aeration and inoculation on solid-state anaerobic digestion of rice straw. Bioresour Technol 224:78–86
Suksong W et al (2017) Thermophilic solid-state anaerobic digestion of solid waste residues from palm oil mill industry for biogas production. Ind Crop Prod 95:502–511
Ward AJ, Hobbs PJ, Holliman PJ, Jones DL (2008) Optimisation of the anaerobic digestion of agricultural resources. Bioresour Technol 99(17):7928–7940
Mao C, Feng Y, Wang X, Ren G (2015) Review on research achievements of biogas from anaerobic digestion. Renew Sust Energ Rev 45:540–555
Di Maria F, Barratta M, Bianconi F, Placidi P, Passeri D (2017) Solid anaerobic digestion batch with liquid digestate recirculation and wet anaerobic digestion of organic waste: comparison of system performances and identification of microbial guilds. Waste Manag 59:172–180
Croce S, Wei Q, D’Imporzano G, Dong R, Adani F (2016) Anaerobic digestion of straw and corn stover: the effect of biological process optimization and pre-treatment on total bio-methane yield and energy performance. Biotechnol Adv 34(8):1289–1304
Pohl M, Mumme J, Heeg K, Nettmann E (2012) Thermo- and mesophilic anaerobic digestion of wheat straw by the upflow anaerobic solid-state (UASS) process. Bioresour Technol 124:321–327
Sheets JP, Ge X, Li Y (2015) Effect of limited air exposure and comparative performance between thermophilic and mesophilic solid-state anaerobic digestion of switchgrass. Bioresour Technol 180:296–303
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99(10):4044–4064
Li Y, Li Y, Zhang D, Li G, Lu J, Li S (2016) Solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover for biogas production. Bioresour Technol 217:50–55
Panjičko M, Zupančič GD, Fanedl L, Logar RM, Tišma M, Zelić B (2017) Biogas production from brewery spent grain as a mono-substrate in a two-stage process composed of solid-state anaerobic digestion and granular biomass reactors. J Clean Prod 166:519–529
Abbassi-Guendouz A et al (2012) Total solids content drives high solid anaerobic digestion via mass transfer limitation. Bioresour Technol 111:55–61
Cazier EA, Trably E, Steyer JP, Escudie R (2015) Biomass hydrolysis inhibition at high hydrogen partial pressure in solid-state anaerobic digestion. Bioresour Technol 190:106–113
Bayrakdar A, Sürmeli RÖ, Çalli B (2017) Dry anaerobic digestion of chicken manure coupled with membrane separation of ammonia. Bioresour Technol 244(June):816–823
Zhu J, Yang L, Li Y (2015) Comparison of premixing methods for solid-state anaerobic digestion of corn stover. Bioresour Technol 175:430–435
Li Y et al (2018) High-solid anaerobic digestion of corn straw for methane production and pretreatment of bio-briquette. Bioresour Technol 250:741–749
Fagbohungbe MO, Dodd IC, Herbert BMJ, Li H, Ricketts L, Semple KT (2015) High solid anaerobic digestion: operational challenges and possibilities. Environ Technol Innov 4:268–284
André L, Pauss A, Ribeiro T (2018) Solid anaerobic digestion: state-of-art, scientific and technological hurdles. Bioresour Technol 247:1027–1037
Stabnikova O, Liu XY, Wang JY (2008) Anaerobic digestion of food waste in a hybrid anaerobic solid-liquid system with leachate recirculation in an acidogenic reactor. Biochem Eng J 41(2):198–201
Zhu J, Zheng Y, Xu F, Li Y (2014) Solid-state anaerobic co-digestion of hay and soybean processing waste for biogas production. Bioresour Technol 154:240–247
Veeken AHM, Hamelers BVM (2000) Effect of substrate-seed mixing and leachate recirculation on solid state digestion of biowaste. Water Sci Technol 41(3):255–262
Capson-Tojo G et al (2017) Dry anaerobic digestion of food waste and cardboard at different substrate loads, solid contents and co-digestion proportions. Bioresour Technol 233:166–175
Xu F, Wang F, Lin L, Li Y (2016) Comparison of digestate from solid anaerobic digesters and dewatered effluent from liquid anaerobic digesters as inocula for solid state anaerobic digestion of yard trimmings. Bioresour Technol 200:753–760
Guendouz J, Buffière P, Cacho J, Carrère M, Delgenes JP (2010) Dry anaerobic digestion in batch mode: design and operation of a laboratory-scale, completely mixed reactor. Waste Manag 30(10):1768–1771
Amani T, Nosrati M, Sreekrishnan TR (2010) Anaerobic digestion from the viewpoint of microbiological, chemical, and operational aspects – a review. Environ Rev 18:255–278
Nguyen DD et al (2016) Dry thermophilic semi-continuous anaerobic digestion of food waste: performance evaluation, modified Gompertz model analysis, and energy balance. Energy Convers Manag 128:203–210
Li W, Lu C, An G, Chang S (2017) Comparison of alkali-buffering effects and co-digestion on high-solid anaerobic digestion of horticultural waste. Energy Fuel 31(10):10990–10997
Kondusamy D, Kalamdhad AS (2014) Pre-treatment and anaerobic digestion of food waste for high rate methane production – a review. J Environ Chem Eng 2(3):1821–1830
Wu D, Lü F, Shao L, He P (2017) Effect of cycle digestion time and solid-liquid separation on digestate recirculated one-stage dry anaerobic digestion: use of intact polar lipid analysis for microbes monitoring to enhance process evaluation. Renew Energy 103:38–48
Fox P, Pohland FG (1994) Anaerobic treatment applications and fundamentals: substrate specificity during phase separation. Water Environ Res 66(5):716–724
Panjičko M, Zupančič GD, Zelić B (2015) Anaerobic biodegradation of raw and pre-treated brewery spent grain utilizing solid state anaerobic digestion. Acta Chim Slov 62(4):818–827
Tian JH, Pourcher AM, Bureau C, Peu P (2017) Cellulose accessibility and microbial community in solid state anaerobic digestion of rape straw. Bioresour Technol 223:192–201
Cesaro A, Belgiorno V (2013) Sonolysis and ozonation as pretreatment for anaerobic digestion of solid organic waste. Ultrason Sonochem 20(3):931–936
Jackowiak D, Bassard D, Pauss A, Ribeiro T (2011) Optimisation of a microwave pretreatment of wheat straw for methane production. Bioresour Technol 102(12):6750–6756
Holtman KM, Bozzi DV, Franqui-Villanueva D, Offeman RD, Orts WJ (2017) Pilot scale high solids anaerobic digestion of steam autoclaved municipal solid waste (MSW) pulp. Renew Energy 113:257–265
Liao X, Li H, Zhang Y, Liu C, Chen Q (2016) Accelerated high-solids anaerobic digestion of sewage sludge using low-temperature thermal pretreatment. Int Biodeterior Biodegrad 106:141–149
Zhu J, Wan C, Li Y (2010) Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment. Bioresour Technol 101(19):7523–7528
Appels L, van Assche A, Willems K, Degrève J, van Impe J, Dewil R (2011) Peracetic acid oxidation as an alternative pre-treatment for the anaerobic digestion of waste activated sludge. Bioresour Technol 102(5):4124–4130
Mirmohamadsadeghi S, Karimi K, Zamani A, Amiri H, Horváth IS (2014) Enhanced solid-state biogas production from lignocellulosic biomass by organosolv pretreatment. Biomed Res Int 2014:6
Mustafa AM, Poulsen TG, Sheng K (2016) Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. Appl Energy 180:661–671
Zhao J, Zheng Y, Li Y (2014) Fungal pretreatment of yard trimmings for enhancement of methane yield from solid-state anaerobic digestion. Bioresour Technol 156:176–181
Ge X, Matsumoto T, Keith L, Li Y (2015) Fungal pretreatment of albizia chips for enhanced biogas production by solid-state anaerobic digestion. Energy Fuels 29(1):200–204
Vasco-Correa J, Li Y (2015) Solid-state anaerobic digestion of fungal pretreated Miscanthus sinensis harvested in two different seasons. Bioresour Technol 185:211–217
Srilatha HR, Nand K, Babu KS, Madhukara K (1995) Fungal pretreatment of orange processing waste by solid-state fermentation for improved production of methane. Process Biochem 30(4):327–331
Zhou S, Zhang Y, Dong Y (2012) Pretreatment for biogas production by anaerobic fermentation of mixed corn stover and cow dung. Energy 46(1):644–648
Liang YG, Yin SS, Si YB, Zheng Z, Yuan SJ, Nie E, Luo XZ (2014) Effect of pretreatment and total solid content on thermophilic dry anaerobic digestion of Spartina alterniflora. Chem Eng J 237:209–216
Kalyani DC, Zamanzadeh M, Müller G, Horn SJ (2017) Biofuel production from birch wood by combining high solid loading simultaneous saccharification and fermentation and anaerobic digestion. Appl Energy 193:210–219
Motte JC et al (2015) Substrate milling pretreatment as a key parameter for solid-state anaerobic digestion optimization. Bioresour Technol 173:185–192
Jain S, Jain S, Wolf IT, Lee J, Tong YW (2015) A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew Sust Energ Rev 52:142–154
Ahn J, Gu S, Hwang S (2009) Effect of microwave irradiation on the disintegration and acidogenesis of municipal secondary sludge. Chem Eng J 153:145–150
Beszédes S, László Z, Horváth ZH, Szabó G, Hodúr C (2011) Bioresource technology comparison of the effects of microwave irradiation with different intensities on the biodegradability of sludge from the dairy- and meat-industry. Bioresour Technol 102:814–821
Ariunbaatar J, Panico A, Esposito G, Pirozzi F, Lens PNL (2014) Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl Energy 123:143–156
Mosier N et al (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686
Liew LN, Shi J, Li Y (2011) Enhancing the solid-state anaerobic digestion of fallen leaves through simultaneous alkaline treatment. Bioresour Technol 102(19):8828–8834
Rinzema A, van Lier J, Lettinga G (1988) Sodium inhibition of acetoclastic methanogens in granular sludge from a UASB reactor. Enzym Microb Technol 10(1):24–32
Braguglia CM, Gianico A, Mininni G (2012) Comparison between ozone and ultrasound disintegration on sludge anaerobic digestion. J Environ Manag 95:S139–S143
Wan C, Li Y (2011) Effectiveness of microbial pretreatment by Ceriporiopsis subvermispora on different biomass feedstocks. Bioresour Technol 102(16):7507–7512
Charles W, Walker L, Cord-Ruwisch R (2009) Effect of pre-aeration and inoculum on the start-up of batch thermophilic anaerobic digestion of municipal solid waste. Bioresour Technol 100(8):2329–2335
Kangle KM, Kore SV, Kore VS, Kulkarni GS (2012) Recent trends in anaerobic codigestion: a review. Univers J Environ Res Technol 2(4):210–219
Pezzolla D, Di Maria F, Zadra C, Massaccesi L, Sordi A, Gigliotti G (2017) Optimization of solid-state anaerobic digestion through the percolate recirculation. Biomass Bioenergy 96:112–118
Khairuddin N, Manaf LA, Hassan MA, Halimoon N, Wan Ab WA, Ghani K (2016) High solid anaerobic co-digestion of household organic waste with cow manure for mass and energy recovery. Pol J Environ Stud 25(4):1549–1554
Riya S, Suzuki K, Terada A, Hosomi M, Zhou S (2016) Influence of C/N ratio on performance and microbial community structure of dry-thermophilic anaerobic co-digestion of swine manure and rice straw. J Med Bioeng 5(1):11–14
Brown D, Li Y (2013) Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour Technol 127:275–280
Wang LH, Wang Q, Cai W, Sun X (2012) Influence of mixing proportion on the solid-state anaerobic co-digestion of distiller’s grains and food waste. Biosyst Eng 112(2):130–137
Lin Y, Ge X, Li Y (2014) Solid-state anaerobic co-digestion of spent mushroom substrate with yard trimmings and wheat straw for biogas production. Bioresour Technol 169:468–474
Xu F, Li Y (2012) Solid-state co-digestion of expired dog food and corn stover for methane production. Bioresour Technol 118:219–226
Nielfa A, Cano R, Fdz-Polanco M (2015) Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol Rep 5(1):14–21
Romero-Güiza MS, Vila J, Mata-Alvarez J, Chimenos JM, Astals S (2016) The role of additives on anaerobic digestion: a review. Renew Sust Energ Rev 58:1486–1499
Liang Y, Qiu L, Guo X, Pan J, Lu W, Ge Y (2017) Start-up performance of chicken manure anaerobic digesters amended with biochar and operated at different temperatures. Nat Environ Pollut Technol 16:615–621
Shen Y, Linville JL, Ignacio-de Leon PAA, Schoene RP, Urgun-Demirtas M (2016) Towards a sustainable paradigm of waste-to-energy process: enhanced anaerobic digestion of sludge with woody biochar. J Clean Prod 135:1054–1064
Qin Y, Wang H, Li X, Cheng JJ, Wu W (2017) Improving methane yield from organic fraction of municipal solid waste (OMSW) with magnetic rice-straw biochar. Bioresour Technol 245:1058–1066
Dang Y et al (2016) Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour Technol 220:516–522
Zhao Z, Zhang Y, Woodard TL, Nevin KP, Lovley DR (2015) Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour Technol 191:140–145
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zhou, H., Wen, Z. (2019). Solid-State Anaerobic Digestion for Waste Management and Biogas Production. In: Steudler, S., Werner, A., Cheng, J. (eds) Solid State Fermentation. Advances in Biochemical Engineering/Biotechnology, vol 169. Springer, Cham. https://doi.org/10.1007/10_2019_86
Download citation
DOI: https://doi.org/10.1007/10_2019_86
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23674-8
Online ISBN: 978-3-030-23675-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)