Abstract
Alzheimer’s disease (AD) is the most frequent cause of dementia due to neurodegeneration. It is stated that the most important risk factor for the late onset AD development is age. AD develops during decades and appears most of the time after 65 years of age. Even though its incidence is increasing with age but not all the centenarians are suffering from AD. The most important underlying age-related factor is immunosenescence/inflammaging. Indeed, aging is associated with immune changes which are thought to be the most prevalent cause of the age-related chronic inflammatory diseases. However, it is now postulated that the changes occurring with aging in the immune system may not be only detrimental but also adaptive. Therefore, in this review we will describe whether and how immunosenescence/inflammaging may contribute to the development of AD. We will also examine whether this can lead to novel treatment approaches different form the current.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Alzheimer’s disease (AD)
- Mild cognitive impairment (MCI)
- Immunosenescence
- Inflammaging neuroinflammation
- Monocytes
- Macrophages
- Phagocytosis
- Free radicals
- Cytokines
- Signaling
9.1 Introduction
Aging is considered by many scientists as the most important risk factor for the development of the so-called chronic age-related diseases (ARD) (Fülöp et al. 2016; Franceschi et al. 2018; Barbé-Tuana et al. 2020). What are these diseases and why it is so? This concept gained popularity mainly because most of these diseases such as diabetes, neurocognitive disorders (dementia), cancers and cardiovascular diseases have a common denominator which is chronic inflammation (Hansen 2018; Kurakim and Bredsen 2020). Therefore, as aging is associated with immunosenescence including its corollary the inflammaging, the slipping extension of this fact became a true overarching paradigm (Barbé-Tuana et al. 2020). Thus, as aging is characterised by immunosenescence/inflammaging and most of the ARD have inflammation as a common root, therefore age is the most important risk factor for these chronic inflammatory diseases, especially AD (Tam and Pasternak 2012; Kern and Behl 2009). However altogether only around 10% of older subjects over 65 of age are affected by AD (Solana et al. 2018). So, could age be the greatest risk factor for AD? Considering these data, the obvious conclusion is no, however it should be recognised that the age-related immune changes may contribute.
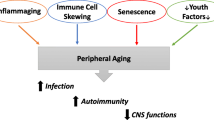
Among these diseases one of the most frequent and devastating is dementia and most specifically the Alzheimer’s disease (AD) (Prince et al. 2013). This disease was named after Alois Alzheimer who more than 100 years ago described the main pathological hallmarks of the disease from the pathological and clinical point of view (Hardy et al. 2002). This led to the extraordinary development of the research on AD but unfortunately this was not translated neither in the real understanding of the etiology of AD nor in even a minimal capacity of treatment (Mehta et al. 2017; Long et al. 2019). So, until now we struggle with a devastating disease that we do not know the cause and have no cure nor prevention. In this chapter we will briefly describe what is AD, what are the putative causes of its onset and how it may be considered as a consequence of the age-related immune changes and what could be the resulting interventions to mitigate the effects of this eventual role of immunosenescence (Fig. 9.1).
9.2 What is AD?
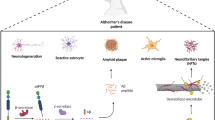
As a systemic inflammatory and clinical syndrome, AD is the most frequent type of dementia mainly affecting the brain (Solana et al. 2018; Paouri and Georgopoulos 2019; Walker et al. 2019). This starts in most of the cases with the loss of memory and speech capacity to continue until the complete disorganization of the whole cognitive sphere and even personality. However, the clinical presentation may be more nuanced and sometimes starting by other symptoms like frontal manifestations. From a pathological aspect, the AD is characterized by the deposition of the extracellular amyloid plaques composed by amyloid beta (Aβ) and by intracellular neurofibrillary tangles composed by hyperphosphorylated tau protein (pTau) (McGeer and McGeer 2013; Hardy and Allsop 1991). This was originally described by Alois Alzheimer, however he already noted that this was not the whole story, but some other alterations might be as important as these changes in the brain. This is even more important as the patient who served to describe the later named AD was most probably a familiar AD, and so her disease was genetically driven (causing around 5% of all cases of the disease).
It is now recognized that AD is a lifelong process which is revealed clinically as we age but the whole pathological process occurs well before old ages when we are only able to diagnose it (Shen et al. 2020). When the cognitive alterations become clinically noticeable the pathological process has been present for decades. Therefore, the pathological process whatever is its cause would not exist as a disease if some changes related to the physiological aging process would not reveal it. It could be strongly suggested that the immune changes manifesting as so called immunosenescence/inflammaging are the driver of this clinical manifestation of the lifelong process, but not of the disease as currently conceptualized (Constantini et al. 2018; Zhao et al. 2020).
9.3 What Could Be the Cause(s) of AD?
There are many theories to explain the causes of AD. The most prevalent theory is the amyloid cascade hypothesis. This hypothesis states that the depot of Aβ, forming amyloid plaques and initiating the Tau hyperphosphorylation, causes the AD by destroying first the synapses and later leading to a neurodegeneration. Moreover, according to this hypothesis, the deposition of amyloid-beta (Aβ) in senile plaques, leads to inflammation and ultimately to the death of neurons (McGeer and McGeer 2013; Hardy and Allsop 1991; Beyreuther and Masters 1991). However, attempts to decrease the Aβ load or to prevent its formation had no effect on AD (Sacks et al. 2017; Cummings et al. 2018), even if we consider the recent very controversial approval of Aducanumab by the FDA. No cure whatsoever exists or seems to be on the horizon (Mehta et al. 2017; Long et al. 2019). These facts seriously question the validity of this mainstream hypothesis (Itzhaki et al. 2016; Herrup et al. 2015; Ricciarelli and Fedele 2017).
Therefore, new research needs to be pursued to unravel new pathomechanisms which may include the Aβ cascade hypothesis. It is certain that AD is initiated decades before its clinical diagnosis, suggesting that the driving pathological processes occur well before the appearance of symptoms. It has been also observed that AD, as an irreversible condition, displays signs of a systemic inflammation (Paouri and Georgopoulos 2019; Walker et al. 2019; McManus and Heneka 2017; Bolós et al. 2017), suggesting that systemic inflammation could precede the well-established AD hallmarks i.e. deposit of Aβ plaques, neurofibrillary tangles and neuroinflammation (Akiyama et al. 2000; Giunta et al. 2008). This implies that AD results from the chronic progression of these noxious inflammatory events in the brain, notably via Aβ production and accumulation (Webers et al. 2020). This local neuroinflammation continues at a low level throughout life with little negative effect, but repeated stimulations by infections, dysbiosis, vascular (ischemia), metabolic (glucose, lipids) or other insults (free radicals) result each time in an acute inflammatory response which culminate and is particularly severe in the elderly (Maloney and Lahiri 2016; Whalley et al. 2006; Fülöp et al. 2013; Fülöp et al. 2016; Li et al. 2010). These insults gradually cause damage to the blood–brain-barrier (BBB) (Nation et al. 2019; Noe et al. 2020) allowing brain inflammatory mediators to reach the periphery and trigger peripheral innate and adaptive inflammatory responses (Le Page et al. 2018; Ellwardt et al. 2016; Šimić et al. 2019; Dionisio-Santos et al. 2019; Shad et al. 2013).
Recent studies also suggested that neuroinflammation and systemic inflammation could progress over decades following triggering events of infectious origin. Latent virus infections e.g. cytomegalovirus (CMV) have been described as major chronic innate immunity activators that contribute to inflammaging and therefore to AD development Bauer and Fuente 2016; Biagi et al. 2012; Thevaranjan et al. 2017). It could sound provocative to propose that AD may result from infection, but some major scientific discoveries were made from offensive hypotheses. Nobody would have bet few years ago that peptic ulcer diseases and cervical cancer were both caused by infections (respectively by Helicobacter pylori and human papillomavirus) (Kelly 1998; Caselli et al. 1997; Kessler 2017).
Infection by specific microorganisms as a plausible AD pathomechanism had been voiced several years ago but did not receive significant attention (Block 2019; Dominy et al. 2019; Osorio et al. 2019; Singhrao and Olsen 2019). The demonstration of the presence of HSV-1 viral DNA or spirochetes in AD brains was too instrumental to consider infections as contributors of AD pathogenesis (Wozniak et al. 2007, 2009; Miklossy 2016). Based on the most recent evidence, the infectious hypothesis provides a plausible stimulus for neuroinflammation. Local brain neuroinflammation may exist at a low level throughout life with little negative effect. However, when exacerbated by reactivation of infections or by the persistence of microbial metabolites, the ongoing inflammatory response combined with immunosenescence/inflammaging becomes difficult to control in order to repair the injury (Kritsilis et al. 2018; Blach-Olszewska et al. 2015; Busse et al. 2017). When it becomes chronic and a threshold of neuron death is surpassed, the disease manifests itself clinically in the brain with irreversible damages (Leszek et al. 2016; Di Benedetto et al. 2017; Goldeck et al. 2016).
It is of interest to mention that this infection hypothesis was strongly supported by the recent discovery that Aβ is an antimicrobial peptide able to protect the brain tissue from viral and bacterial infections (Bourgade et al. 2015, 2016; Soscia et al. 2010). The epidemiological data as well as the susceptibility genes (e.g. Apolipoprotein E4) also support the infection hypothesis (Lopatko et al. 2019). The fact that Aβ is an antimicrobial peptide explain also why these amyloid plaques may be found in normal brains as the so-called cemetery for microbes inside the biofilms (Miklossy 2016; Perl 2010; Fülöp et al. 2018a, b, c).
Besides this infection hypothesis since many years other hypotheses gain importance for the development of AD, the vascular hypothesis being one of the oldest. Even if there are plaques, AD is only clinically manifested when the vascular burden becomes overwhelming as it was shown in the Nunn study. Ischemia was also shown to be one of the triggers of Aβ production (Li et al. 2007; Han and Fukunaga 2009). Ischemia is a powerful trigger of the neuroinflammatory process by the production of the free radicals, pro-inflammatory cytokines and chemokines. All these processes initiated by ischemia contribute to AD development by inducing the preceding linflammation (Marchesi 2011). Recently, the cellular senescence was also favoured as the main source of the neuroinflammation (Walton et al. 2020).
Whatever is the exact cause of AD the main factors are age, genetics, epigenetics and environmental factors in its development (Kurakin and Bredesen 2020; Komleva et al. 2021; López-Otín et al. 2013). Besides central or peripheral infections, other peripheral stressors including gastrointestinal inflammation, dysbiosis, toxic products as well as metabolic disorders (diabetes, obesity and atherosclerosis) via innate immunity activation leads to neuroinflammation resulting in AD (Komleva et al. 2021; Kowalski and Mulak 2019; Cai et al. 2013). These environmental factors contribute for at least 40% to AD development, beside the well-established genetic markers (Kremen et al. 2019). This means that age could be a risk factor but in reality, lifelong environmental factors have much more influence in the development of the disease. All the envirobiographic changes, including nutrition, physical activity, cognitive stimulation/education, hand-in-hand with the lifelong immunobiography and genetic susceptibility, contribute to the development of AD (Ngandu et al. 2015). This envirobiography is the key to explain the heterogeneity of the pathological process, the clinical manifestations and the difficulty to find a single treatment. Therefore, it seems to be much more important that age but also an elusive health parameter to measure yet.
9.4 What is Immunosenescence/Inflammaging?
Aging is accompanied by many physiological changes which lead to the decrease of the body reserves ending up with a homeostenosis condition (i.e. a reduction in the ability to maintain homeostasis/homeodynamics). The immune system is not an exception from this dynamic change leading also, due to the lifelong immunobiography/envirobiography, to a homeostenosis state composed from adaptative and mal-adaptative parts depending on the hormesis capacity of the entire organism (Fülöp et al. 2020a, b). The preponderance of one or the other will determine the protective role or disease-favouring-role of the older immune system.
The constant internal and external challenges are shaping the immune system through the entire life. Immune cells are able to react to various challenges via the damage associated molecular patterns (DAMPs) generated by injured cells and pathogen associated molecular patterns (PAMPs) (Kumar et al. 2011; Magrone et al. 2020). This is occurring through patterns recognition receptors (PRR) including the Toll-like receptors (TLRs), NOD-like receptors (NLR) and retinoic acid-inducible gene-I-like receptors (RLRs). As the immune system is in a such central position via its fundamental role for life by fighting all noxious stresses that its changes during aging will likely influence the clinical manifestation of most of the age-related diseases (Fülöp et al. 2018). Therefore, it is conceivable that these changes in the immune system may also drive the clinical apparition of AD (Fülöp et al. 2018a).
We will briefly describe the most important changes which may affect the development of AD. The innate immune system is an ancestral immune response assuring the first line of defence against internal and external challenges such as pathogenic microorganisms and damaged cells. In its prime, the innate immune system is able to return to a quiescent state after neutralizing the aggressions, but with the time-dependent accumulation of stressors, the innate immune cells become more permanently activated even at its “resting” state constituting the “trained innate memory” (Fülöp et al. 2016; Kleinnijenhuis et al. 2012; van der Heijden et al. 2017; Arts et al. 2016; Domínguez-Andrés et al. 2020). This is an excessively adaptable and useful process based on epigenetic and metabolic changes in innate immune cells. This contributes at any moment of the organism life to protect earlier and stronger to each successive challenge (Ciarlo et al. 2019; Fransceschi et al. 2017).
However, constant challenges lead to an exhaustion and the system become tolerogenic and could result in detrimental effects (Salani et al. 2019). The pro-inflammatory and anti-inflammatory balance will determine the outcome of the aggression as being resolved with an innate memory or becoming chronic and harming the organism. Therefore, this permanent antigenic stimulation contributes to low but significant secretion of pro-inflammatory mediators creating an activation/inhibition disequilibrium and participating to inflammaging (Fransceschi et al. 2000, 2007, 2018a, b).
Non-infectious agents have been implicated in the spreading of inflammatory processes fueling inflammaging over time. The senescence-associated secretory phenotype (SASP) is considered as the main non-infectious trigger of inflammaging (Walton et al. 2020; Magrone et al. 2020; Coppé et al. 2018; Tchkonia et al. 2013; Birch et al. 2017). Senescent cells secrete pro-inflammatory molecules but also exosomes that can modulate immune system functions by transporting regulatory micro-RNAs and proteins through bodily fluids (Campisi 2016; Giuliani 2017; Terlecki-Zaniewicz et al. 2018). The appearance of senescent cells is primarily a protective mechanism against cancer development and reinforcing immunosurveillance, but their continuous formation is becoming detrimental along aging. Therefore, cellular senescence represents a dual process depending on its time of manifestation and the type of secretome induction (Walton et al. 2020).
The innate immune system as the main primary defense of the organism contributes to the immune changes and to inflammaging (Franceschi et al. 2000). All cells composing the innate immune response are impacted by aging but to different degrees (Müller et al. 2019; Goldberg et al. 2020; Bandaranayake and Shaw 2016). Their phenotypic and functional changes may all contribute to the development and progression of AD (Le Page et al. 2018). The monocytes are particularly changing in their phenotypes shifting towards the more inflammatory and senescent type of the so called intermediary and non-classical phenotype (Costantini et al. 2018; Zhao et al. 2020; Magrone et al. 2020). The monocytes at the periphery are also more activated than in physiological conditions. Paradoxically, essential defense functions such as chemotaxis and killing are decreased. These monocytes are able to infiltrate the brain because of the increased permeability of the BBB (Di Benedetto et al. 2017; Huang et al. 2020). Their differentiation in macrophages is skewed with mainly M2 subset with immunosuppressive functions (Nyugen et al. 2010). There is also alteration in Natural Killer (NK) cell phenotype and function with aging (Solana et al. 2018; Solana et al. 2012). Similarly, dendritic cell (DC) antigen presentation to T cells is altered with aging (Gupta 2014). Noteworthy, most of the changes are controversial as they were obtained in laboratory animal models. In humans as it was shown that controlled inflammation was a better predictor of longevity than any other previously thought biomarkers such as telomere shortening (Arai et al. 2015).
The adaptive immunity is taking over when the innate immunity may not cope efficiently with the aggression. Therefore, the adaptive immune system functioning is basically dependent on the efficiency of the innate immune system and its antigen presenting capacity (e.g. with DCs) and the controlled cytokine production (e.g. IL-12). The antigen presenting capacity but also the number of the naïve CD4+ and CD8+ T cells are decreased with aging (Wong et al. 2013). These data led to the common concept that the adaptive immune system is profoundly altered with aging being responsible for the well-known ARD (Castelo-Branco and Soveral 2014; Pawelec 2018). However, there are still many unsettled questions to really declare that the adaptive immunity is globally decreased. We do not know to what extent the antigen presentation is altered, to what extent the naïve T cells are decreased with aging and most importantly, how these changes may be part of an adaptative process occurring with aging (Fülöp et al. 2020a, b; Pawelec 2020; Pawelec et al. 2020). Therefore, it will be important to optimize the immune resources to assure a better survival and functionality inside the already known challenges. There should be also a balance between the proinflammatory Th17 T cells and regulatory T cells (Tregs) (Magrone et al. 2020). With aging this equilibrium is perturbed; while Th17 become more inflammatory the anti-inflammatory control of Tregs is decreasing as even if their number is increasing the secretion of IL-10 by Tregs is decreased (Schmitt et al. 2013; Salminen 2020; Churov et al. 2020).
Inflammaging was introduced by Franceschi et al. (2000) as a basic characteristic of the biology of aging. Since its original description, the concept of inflammaging evolved considerably. From the concept of disbalance between the innate and adaptive immunity, in favour of the innate part other factors have been discovered and the molecular bases were also described. Thus, the roles of cellular senescence (SASP), the microbiome, the mitochondrial metabolic changes have been associated (Fülöp et al. 2019). The central role of the NF-kB is now well recognized as it is activated via various receptors and transduction pathways, but also by ROS, cellular senescence and DNA damage, in the increased production of IL-6, TNFα as hallmark of inflammaging (Smale 2011; Salvioli et al. 2013). In the meantime, another way to produce pro-inflammatory cytokines (e.g. IL-1) is via the NLR mediated the inflammasome activation (Humphries and Fitzgerald 2019). Inflammasome activation has numerous similar mediators than the NF-kB activation and among them, particularly the ROS and Aβ (Hu et al. 2019). It is also to mention that the nature has foreseen many possible control mechanisms to avoid the overactivation of inflammasome such as the stimulation of AMPK, Sirtuins, the type I interferon and autophagy (Bae et al. 2016; Price et al. 2012; de Kreutzenberg et al. 2015).
Together, inflammaging is characterized by an inflammatory status that is chronic, systemic and low grade. The level of cytokines often remains within the normal range (Sanada et al. 2018; Frasca et al. 2017; Rubino et al. 2019), justifying why inflammaging is also referred as low-grade inflammation. Considering all these factors, aging, associated with inflammaging, may be the most important risk factor for the clinical manifestation of late onset AD (Tam et al. 2012; Kern and Behl 2009; Castellani et al. 2010). Thus, this progressive pro-inflammatory situation, exacerbated with age, creates local and systemic inflammatory responses that activate cytotoxic microglia, unbalanced cytokine production, Aβ accumulation and irreversible brain damage. Therefore, the innate immune system activation by some still unspecified triggers will result over time in a baseline inflammatory state. At younger age this is compensated by the anti-inflammatory environment however with the passage of time in susceptible individuals the ravages of the environmental damages would lead to AD as it is not anymore counterbalanced. It is of note that in many older subjects this scenario is either delayed or efficiently combatted. This once again means that aging is not the most important risk factors, but the conglomerates of pathological processes driven by lifelong bad habits/envirobiographie. Thus, the question is naturally occurring how immunosenescence/inflammaging may contribute to AD?
9.5 What is the Role of Inflammation in AD Development and Progression?
It took many years to the AD community to accept that inflammation is part of the pathogenesis of AD however it is still mainly considered as the direct result of the Aβ deposition (McGeer and McGeer 2013). In this concept the neuroinflammation is the consequence of AD and not the cause as Aβ produced under various situations would trigger the activation of microglia in the brain via TLR2 and TLR4 receptors resulting in pro-inflammatory cytokine and chemokine production (Selkoe and Hardy 2016; Kumar 2019). Assuming the antimicrobial role of Aβ as described above (Bourgade et al. 2015, 2016; Soscia et al. 2010), still this protective process becomes detrimental when the Aβ can not be anymore ingested and cleared by the cumulative exhausted/senescent microglia and become deposited in diffuse amyloid plaques which in turn continue to activate/stimulate/exhaust microglia (Costantini et al. 2018; Cunningham et al. 2013; Regen et al. 2017; Floden et al. 2011; Wyatt-Johnson and Brutkiewicz 2020). In the meantime, the peripheral immune system is also activated (as the Aβ is both an antigen and enhancer of the T cell reactivity) (Jóźwik et al. 2012) making AD a systemic disease and could be an earlier biomarker of brain inflammation. Therefore, changes in peripheral innate immune biomarkers may also precede by decades the clinical appearance of AD (Busse et al. 2017; Morgan et al. 2019).
Considering the recent progress, the picture is much more complicated than that. It is now well-recognised that the inflammation is preceding by decades the clinical manifestation of dementia. This inflammation may originate from the brain where it would be a reaction to local noxious agents of any sort which would initiate an inflammatory reaction and results in the activation of the local innate system by the stimulation of PAMP and DAMP (Paouri and Georgopoulos 2019; Walker et al. 2019; McGeer and McGeer 2013). One of the most important cytokines implicated is IL-1β which has also a dual role. IL-1β is beneficial at the beginning by activating the microglia to protect the brain, but at long-term becomes deleterious and causes microgliosis in AD (Licastro et al. 2000; Dansokho et al. 2016). The local innate system is composed mainly from microglia and astrocytes. These cells under continuous stimulation will secrete pro-inflammatory mediators via the activation of NF-kB and inflammasome which will reinforce the secretion of antimicrobial Aβ by neurons (Kurakin and Bredesen 2020; Costantini et al. 2018). This Aβ will initially fight the infections and may neutralize other toxic substances (Kumar et al. 2016a, b). Thus, the activated microglia will try to eliminate the aggressors and create dense core plaques which will be a sort of Aβ dense cemetery for the aggressors fueled and organized by the Aβ (Fyfe 2021). These new data shed light on the complex role of microglia which at the beginning of the process, by their inflammatory action, have a strongly protective activity (Wyatt-Johnson SK and Brutkiewicz 2020). Indeed, this is a very efficient way for decades to control both the aggressions/pathogens and the Aβ deposition by creating the dense-core plaques. Therefore, amyloid plaques may be seen in almost every aging brain (Rodrigue et al. 2009). However, as we will describe later the specific changes occurring concomitantly with aging may alter this equilibrium and AD may eventually develop. Indeed, meanwhile this neuroinflammation will have progressive systemic effects and induce a peripheral inflammation. The inflammatory mediators released from the brain will stimulate the peripheral innate immune response. Among these cells at a very early stages are monocytes and NK cells (Solana et al. 2018). This will result to the maintenance of the activation of the innate immune system which will in turn activate the adaptive immune response. We should mention that this phenomenon may also occur in another way, specifically a systemic inflammation by the mediation of the inflammatory molecules and the migration of peripheral innate cells to the brain (Kurakin and Bredesen 2020). This may increase the BBB permeability which is letting to penetrate the inflammatory mediators and cells to penetrate into the brain and leading to neuroinflammation. Thus, a vicious circle will develop either whether the inflammation originate from the brain and spread to the periphery or vice versa (Paouri and Georgopoulos 2018; Tejera et al. 2019; Yang et al. 2020).
The reactive permeability of the blood brain barrier (BBB) is an important aspect of neuroinflammatory process (Nzou et al. 2020; Kowalski and Mulak 2019; Festoff 2016). In normal situation this is a semi-impermeable entity between the periphery and the central nervous system. Either aging or any inflammatory processes will impair this impermeable status of BBB (Chen 2011). This results in the passage of pro-inflammatory mediators and cells from the periphery to the brain and vice versa. Not only the BBB becomes permeable, but also the transporters are also altered. Comorbidities occurring with aging such as obesity and diabetes (diabesity) favor the increased BBB permeability and are also risk factors for AD (Chiu et al. 2015). Another probable contribution of the periphery to control the brain inflammation and physiology is the gut-brain axis (Kowalski and Mulak 2019; Cattaneo et al. 2017). This was known for a while but recently it became a major way for the interaction and mutual control between the brain and the gut. The study of this crosstalk axis is of utmost importance to understand the role of immunity and inflammation in central nervous degenerative diseases.
Collectively, this data suggest that inflammation is the basic process fighting and later maintaining the development and progression of AD.
9.6 Immunosenescence/Inflammaging and AD
From what we have described it can be easily concluded that the are-associated immune changes may contribute to the development of AD and consequently, it is very tempting to blame immunosenescence/inflammaging for the development of AD. However, it would be very reductionist to think that age-related immune changes may play a fundamental role in the development of AD. Nevertheless, considering what are the main characteristics of immunosenescence/inflammaging it is more than plausible that they play a role in the clinical manifestation/appearance of AD.
Immunosenescence/inflammaging will result in a clinically imperceptible, chronic low-grade inflammatory state which will maintain the innate immune system stimulation, the production of more than normal pro-inflammatory cytokines and finally the stimulation of the adaptive immune system (Franceschi et al. 2000; Fülöp et al. 2019). This will manifest by the decrease in naïve cells and the increase in memory cells, further contributing to the inflammation (Müller et al. 2019). This is clearly occurring as a reaction to the lifelong (immunobiography) constant external and internal challenges (Fülöp et al. 2020; Franceschi et al. 2017). Therefore, this is primarily a defense mechanism that may lead to AD in the long run. In this way the age-related immune changes may contribute by several ways.
The constant inflammation may have detrimental role and induce the neuronal death. Furthermore, the constant stimulation will result in the sustained production of pro-inflammatory mediators preventing the action of anti-inflammatory processes. We should also mention that these pro-inflammatory challenges will result in exhaustion of the immune cells mainly that of the adaptive immune system by the increase of memory CD8+ T cells. In the brain this stimulation will result in the apparition of the senescent microglia which will contribute to the inflammation and neurodegeneration instead of fighting this.
However, if we consider that AD develops through decades before its clinical apparition, immunosenescence/inflammaging will have a very little role in the development of AD as the neuroinflammation is independent of the aging process. Nevertheless, when the process proceeds the immunosenescence/inflammaging through the exhaustion of the innate and adaptive immune response, it may contribute to the clinical appearance of AD (Solana et al. 2018; Costantini et al. 2018; Magrone et al. 2020).
As mentioned, under the constant stimulation, microglia will become exhausted and instead of being protector it becomes harmful. This has been shown through the progression of AD. It was a major progress to establish the dual role of microglia (Subhramanyam et al. 2019; Colonna and Butovsky 2017). The same process may also affect the astrocytes (Garwood et al. 2017).
Another way that the aging immune system may contribute is the deregulation of the immune suppressive system. The changes in Tregs and myeloid-derived suppressor cells (MDSC) cells will favor the appearance of higher specific immune reactions suppression but concomitantly the fueling of the chronic inflammatory process (Le Page et al. 2017).
Therefore, we should be very cautious before we attribute the major risk factor title to aging with its immunological attribute because this can have far reaching conceptual and treatment consequences. However instead if we integrate these changes in a complex systems biology view of the AD development we could perhaps improve the possibilities of prevention and slowing down the disease progression (Ardura-Fabregat et al. 2017).
9.7 Cues for Intervention Targeting the Inflammation and the Immune Changes Contributing to AD
So far, most of the anti-inflammatory drug trials were failures besides many epidemiological data demonstrating the use of non-steroidal anti-inflammatory drugs (especially in arthritis patients) reduced the risk of AD (Rivers-Auty et al. 2020). This may be explained by considering that these trials did not consider the complex role of inflammation in the pathogenesis of AD. At the beginning inflammation should be sustained to fight the aggression and later it should be downsized to avoid the aggravation and the ongoing nature of the neuroinflammation reinforced by the dysregulated immunosenescence/inflammaging.
Presently many trials are ongoing targeting different molecules, pathways and cells of the immune system to modulate the inflammation (Fülöp et al. 2020, 2021). None of them directly is addressing the altered immune system with aging, with the exception perhaps the tentative reduction of Tregs (Ballard et al. 2020). All of them targets the original neuroinflammation. This should lead either to the prevention or the decreasing/delaying of the progression of AD (Munafò et al. 2020).
Presently the only way to prevent and influence the immune changes with aging is the intervention in multimodal way on the envirobiography (Ngandu et al. 2015). This intervention would consist of a very early implementation of good nutritional habits, regular exercise, decrease of bad stress (hormetically negative), cognitive reserve increase implemented since the middle age (Komleva et al. 2021; Atri 2019; Eiser and Fülöp 2020; de Oliveira Silva et al. 2019). In this way the use of a ketogenic diet may increase some cognitive functions in MCI subjects by potentially acting as a better fuel for the brain and an inhibitor of the inflammasome (Fortier et al. 2021; Myette-Côté et al. 2021; Wissler et al. 2020).
Another popular anti-aging intervention is the use of senolytics which would eliminate one source of aging, namely the senescent cells and as such would alleviate inflammaging, hoping that the organism will be capable to better fight against the aggressions (Zhao et al. 2020; Schubert et al. 2018; Mannick et al. 2014; Kang 2019). We really at this stage do not know neither the short nor the long-term effects of such interventions.
9.8 Conclusion and Translational Perspectives
AD is clearly a neuroinflammation mediated neurodegenerative disease. Aging is considered as one of the most important risk factors mainly by its attribute influencing the immune system changes. The development of inflammation is preceding decades before the clinical apparition of AD. In this way immunosenescence/inflammaging may play some role. The clear conceptualization and better understanding of AD will tone down the role of aging and will bring viable treatment opportunities. However, considering the envirobiography and the genetic origins of AD it is hardly conceivable that a monotherapy may be available, even if the aducanumab (Aduhelm) was very recently approved by the FDA. We should aim to treat AD by a multimodal approach in a system biology perspective which could lead to a real personalized approach (Gauthier et al. 2018; Fülöp et al. 2021).
References
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging May–June 2021;21(3):383–421. https://doi.org/10.1016/s0197-4580(00)00124-x
Arai Y, Martin-Ruiz CM, Takayama M, Abe Y, Takebayashi T, Koyasu S, Suematsu M, Hirose N, von Zglinicki T (2015) Inflammation, but not telomere length, predicts successful ageing at extreme old age: a longitudinal study of semi-supercentenarians. EBioMedicine 2(10):1549–1558. https://doi.org/10.1016/j.ebiom.2015.07.029
Ardura-Fabregat A, Boddeke EWGM, Boza-Serrano A, Brioschi S, Castro-Gomez S, Ceyzériat K, Dansokho C, Dierkes T, Gelders G, Heneka MT, Hoeijmakers L, Hoffmann A, Iaccarino L, Jahnert S, Kuhbandner K, Landreth G, Lonnemann N, Löschmann PA, McManus RM, Paulus A, Reemst K, Sanchez-Caro JM, Tiberi A, Van der Perren A, Vautheny A, Venegas C, Webers A, Weydt P, Wijasa TS, Xiang X, Yang Y (2017) Targeting neuroinflammation to treat Alzheimer’s disease. CNS Drugs 31(12):1057–1082. https://doi.org/10.1007/s40263-017-0483-3
Arts RJ, Joosten LA, Netea MG (2016) Immunometabolic circuits in trained immunity. Semin Immunol 28:425–430
Atri A (2019) Current and future treatments in Alzheimer’s disease. Semin Neurol 39(2):227–240. https://doi.org/10.1055/s-0039-1678581
Bae HR, Kim DH, Park MH, Lee B, Kim MJ, Lee EK, Chung KW, Kim SM, Im DS, Chung HY (2016) β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget 7(41):66444–66454. https://doi.org/10.18632/oncotarget.12119
Ballard C, Aarsland D, Cummings J et al (2020) Drug repositioning and repurposing for Alzheimer disease. Nat Rev Neurol 16(12):661–673. https://doi.org/10.1038/s41582-020-0397-4
Bandaranayake T, Shaw AC (2016) Host resistance and immune aging. Clin Geriatr Med 32(3):415–432. https://doi.org/10.1016/j.cger.2016.02.007
Barbé-Tuana F, Funchal G, Schmitz CRR, Maurmann RM, Bauer ME (2020) The interplay between immunosenescence and age-related diseases. Semin Immunopathol 42(5):545–557. https://doi.org/10.1007/s00281-020-00806-z
Bauer ME, de la Fuente M (2016) The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech Ageing Dev. 158:27–37
Beyreuther K, Masters CL (1991) Amyloid precursor protein (APP) and beta A4 amyloid in the etiology of Alzheimer’s disease: precursor-product relationships in the derangement of neuronal function. Brain Pathol 1(4):241–251. https://doi.org/10.1111/j.1750-3639.1991.tb00667.x
Biagi E, Candela M, Fairweather-Tait S, Franceschi C, Brigidi P (2012) Aging of the human metaorganism: the microbial counterpart. Age (dordr) 34:247–267
Birch J, Passos JF (2017) Targeting the SASP to combat ageing: Mitochondria as possible intracellular allies? Bioessays 39(5)
Blach-Olszewska Z, Zaczynska E, Gustaw-Rothenberg K, Avila-Rodrigues M, Barreto GE, Leszek J, Aliev G (2015) The innate immunity in Alzheimer disease—relevance to pathogenesis and therapy. Curr Pharm Des 21:3582–3588
Block J (2019) Alzheimer’s disease might depend on enabling pathogens which do not necessarily cross the blood-brain barrier. Med Hypotheses 125:129–136
Bolós M, Perea JR, Avila J (2017). Alzheimer’s disease as an inflammatory disease. Biomol Concepts 8(1):37–43. https://doi.org/10.1515/bmc-2016-0029
Bourgade K, Garneau H, Giroux G, Le Page AY, Bocti C, Dupuis G, Frost EH, Fülöp T Jr (2015) β-Amyloid peptides display protective activity against the human Alzheimer’s disease-associated herpes simplex virus-1. Biogerontology 16:85–98
Bourgade K, Le Page A, Bocti C, Witkowski JM, Dupuis G, Frost EH, Fülöp T Jr (2016) Protective effect of amyloid-β peptides against herpes simplex virus-1 infection in a neuronal cell culture model. J Alzheimers Dis 50:1227–1241
Busse M, Michler E, von Hoff F, Dobrowolny H, Hartig R, Frodl T, Busse S (2017) Alterations in the peripheral immune system in dementia. J Alzheimers Dis 58:1303–1313
Cai Z, Yan Y, Wang Y (2013) Minocycline alleviates beta-amyloid protein and tau pathology via restraining neuroinflammation induced by diabetic metabolic disorder. Clin Interv Aging 8:1089–1095. https://doi.org/10.2147/CIA.S46536
Campisi J (2016) Cellular senescence and lung function during aging. Yin and Yang. Ann Am Thorac Soc. 13(Supplement_5):S402.
Caselli M, Trevisani L, Tursi A, Sartori S, Ruina M, Luzzi I, Gaudenzi P, Alvisi V, Gasbarrini G (1997) Short-term low-dose triple therapy with azithromycin, metronidazole and lansoprazole appears highly effective for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol 9(1):45–48
Castellani RJ, Rolston RK, Smith MA (2010) Alzheimer disease. Dis Mon 56:484–546
Castelo-Branco C, Soveral I (2014) The immune system and aging: a review. Gynecol Endocrinol 30(1):16–22. https://doi.org/10.3109/09513590.2013.852531
Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, Ferrari C, Guerra UP, Paghera B, Muscio C, Bianchetti A, Volta GD, Turla M, Cotelli MS, Gennuso M, Prelle A, Zanetti O, Lussignoli G, Mirabile D, Bellandi D, Gentile S, Belotti G, Villani D, Harach T, Bolmont T, Padovani A, Boccardi M, Frisoni GB; INDIA-FBP Group (2017) Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 49:60–68. https://doi.org/10.1016/j.neurobiolaging.2016.08.019
Chen RL (2011) Is it appropriate to use albumin CSF/plasma ratio to assess blood brain barrier permeability? Neurobiol Aging 32(7):1338–1339. https://doi.org/10.1016/j.neurobiolaging.2008.08.024
Chiu C, Miller MC, Monahan R, Osgood DP, Stopa EG, Silverberg GD (2015) P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: preliminary observations. Neurobiol Aging 36(9):2475–2482. https://doi.org/10.1016/j.neurobiolaging.2015.05.020
Churov AV, Mamashov KY, Novitskaia AV (2020) Homeostasis and the functional roles of CD4(+) Treg cells in aging. Immunol Lett 226:83–89. https://doi.org/10.1016/j.imlet.2020.07.004
Ciarlo E, Heinonen T, Théroude C, Asgari F, Le Roy D, Netea MG, Roger T (2019) Trained immunity confers broad-spectrum protection against bacterial infections. J Infect Dis pii:jiz692
Colonna M, Butovsky O (2017) Microglia function in the central nervous system during health and neurodegeneration. Ann Rev Immunol 26(35):441–468. https://doi.org/10.1146/annurev-immunol-051116-052358
Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J (2018) Senescence-associated secretory phenotypes reveal cell- nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6:2853–2868
Costantini E, D’Angelo C, Reale M (2018) The role of immunosenescence in neurodegenerative diseases. Mediators Inflamm 8(2018):6039171. https://doi.org/10.1155/2018/6039171
Cummings J, Ritter A, Zhong K (2018) Clinical trials for disease-modifying therapies in Alzheimer’s disease: a primer, lessons learned, and a blueprint for the future. J Alzheimers Dis 64(s1):S3–S22. https://doi.org/10.3233/JAD-179901
Cunningham C (2013) Microglia and neurodegeneration: the role of systemic inflammation. Glia 61(1):71–90. https://doi.org/10.1002/glia.22350
Dansokho C, Ait Ahmed D, Aid S, Toly-Ndour C, Chaigneau T, Calle V, Cagnard N, Holzenberger M, Piaggio E, Aucouturier P, Dorothée G (2016) Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 139(Pt 4):1237–1251. https://doi.org/10.1093/brain/awv408
de Kreutzenberg SV, Ceolotto G, Cattelan A, Pagnin E, Mazzucato M, Garagnani P, Borelli V, Bacalini MG, Franceschi C, Fadini GP, Avogaro A (2015) Metformin improves putative longevity effectors in peripheral mononuclear cells from subjects with prediabetes. A randomized controlled trial. Nutr Metab Cardiovasc Dis 25(7):686–693. https://doi.org/10.1016/j.numecd.2015.03.007
de Oliveira SF, Ferreira JV, Plácido J, Sant’Anna P, Araújo J, Marinho V, Laks J, Camaz DA (2019) Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with Alzheimer’s disease: a randomized controlled trial. Maturitas 126:28–33. https://doi.org/10.1016/j.maturitas.2019.04.217
Di Benedetto S, Müller L, Wenger E, Düzel S, Pawelec G (2017) Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev 75:114–128
Dionisio-Santos DA, Olschowka JA, O’Banion MK (2019) Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J Neuroinflammation 16(1):74. 48. https://doi.org/10.1186/s12974-019-1453-0
Domínguez-Andrés J, Fanucchi S, Joosten LAB, Mhlanga MM, Netea MG (2020) Advances in understanding molecular regulation of innate immune memory. Curr Opin Cell Biol 63:68–75
Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, Holsinger LJ, Arastu-Kapur S, Kaba S, Lee A, Ryder MI, Potempa B, Mydel P, Hellvard A, Adamowicz K, Hasturk H, Walker GD, Reynolds EC, Faull RLM, Curtis MA, Dragunow M, Potempa J (2019) Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 5(1):eaau3333
Eiser AR, Fulop T (2020) Extra-cranial factors in the development of Alzheimer’s disease. Brain Res 1(1748):147076. https://doi.org/10.1016/j.brainres.2020.147076
Ellwardt E, Walsh JT, Kipnis J, Zipp F (2016) Understanding the Role of T Cells in CNS Homeostasis. Trends Immunol 37:154–165
Festoff BW (2016) HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer’s disease. J Neuroinflam 13:194
Floden AM, Combs CK (2011) Microglia demonstrate age-dependent interaction with amyloid-β fibrils. J Alzheimers Dis 25(2):279–293. https://doi.org/10.3233/JAD-2011-101014
Fortier M, Castellano CA, St-Pierre V, Myette-Côté É, Langlois F, Roy M, Morin MC, Bocti C, Fulop T, Godin JP, Delannoy C, Cuenoud B, Cunnane SC (2021) A ketogenic drink improves cognition in mild cognitive impairment: results of a 6-month RCT. Alzheimers Dement 17(3):543–552. https://doi.org/10.1002/alz.12206
Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254
Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S (2007) Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128:92–105
Franceschi C, Salvioli S, Garagnani P, de Eguileor M, Monti D, Capri M (2017) Immunobiography and the heterogeneity of immune responses in the elderly: a focus on inflammaging and trained immunity. Front Immunol 8:982
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A (2018a) Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018 Oct;14(10):576–590. https://doi.org/10.1038/s41574-018-0059-4
Franceschi C, Zaikin A, Gordleeva S, Ivanchenko M, Bonifazi F, Storci G, Bonafè M (2018b) Inflammaging 2018: an update and a model. Semin Immunol 40:1–5
Frasca D, Blomberg BB, Paganelli R (2017) Aging, obesity, and inflammatory age-related diseases. Front Immunol 8:1745
Fülöp T, Lacombe G, Cunnane S, Le Page A, Dupuis G, Frost EH, Bourgade-Navarro K, Goldeck D, Larbi A, Pawelec G (2013) Elusive Alzheimer’s disease: can immune signatures help our understanding of this challenging disease? Part 2: new immune paradigm. Discov Med 15(80):33–42
Fülöp T, Dupuis G, Baehl S, Le Page A, Bourgade K, Frost E, Witkowski JM, Pawelec G, Larbi A, Cunnane S (2016) From inflamm-aging to immune-paralysis: a slippery slope during aging for immune-adaptation. Biogerontology 17(1):147–157. https://doi.org/10.1007/s10522-015-9615-7
Fülöp T, Witkowski JM, Olivieri F, Larbi A (2018a) The integration of inflammaging in age-related diseases. Semin Immunol 40:17–35. https://doi.org/10.1016/j.smim.2018.09.003
Fülöp T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C (2018b) Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol Jan 10;8:1960. https://doi.org/10.3389/fimmu.2017.01960
Fülöp T, Witkowski JM, Bourgade K, Khalil A, Zerif E, Larbi A, Hirokawa K, Pawelec G, Bocti C, Lacombe G, Dupuis G, Frost EH (2018c) Can an infection hypothesis explain the beta amyloid hypothesis of Alzheimer’s disease? Front Aging Neurosci Jul 24;10:224. https://doi.org/10.3389/fnagi.2018.00224
Fülöp T, Larbi A, Witkowski JM (2019) Human inflammaging. Gerontology 65(5):495–504. https://doi.org/10.1159/000497375
Fülöp T, Munawara U, Larbi A, Desroches M, Rodrigues S, Catanzaro M, Guidolin A, Khalil A, Bernier F, Barron AE, Hirokawa K, Beauregard PB, Dumoulin D, Bellenger JP, Witkowski JM, Frost E (2020a) Targeting infectious agents as a therapeutic strategy in Alzheimer’s disease. CNS Drugs 2020 Jul;34(7):673–695. https://doi.org/10.1007/s40263-020-00737-1
Fülöp T, Larbi A, Hirokawa K, Cohen AA, Witkowski JM (2020b) Immunosenescence is both functional/adaptive and dysfunctional/maladaptive. Semin Immunopathol 2020 Oct;42(5):521–536. https://doi.org/10.1007/s00281-020-00818-9
Fülöp T, Tripathi S, Rodrigues S, Desroches M, Bunt T, Eiser A, Bernier F, Beauregard PB, Barron AE, Khalil A, Plotka A, Hirokawa K, Larbi A, Bocti C, Laurent B, Frost EH, Witkowski JM (2021) Targeting impaired antimicrobial immunity in the brain for the treatment of Alzheimer’s disease. Neuropsychiatr Dis Treat 4(17):1311–1339. https://doi.org/10.2147/NDT.S264910
Fülöp T, Dupuis G, Witkowski JM, Larbi A (2016) The role of immunosenescence in the development of age-related diseases. Rev Invest Clin March–April 2016;68(2):84–91
Fyfe I (2021) Dense-core plaques could be beneficial in AD. Nat Rev Neurol 17(6):328. https://doi.org/10.1038/s41582-021-00513-9
Garwood CJ, Ratcliffe LE, Simpson JE, Heath PR, Ince PG, Wharton SB (2017) Review: astrocytes in Alzheimer’s disease and other age-associated dementias: a supporting player with a central role. Neuropathol Appl Neurobiol 43(4):281–298. https://doi.org/10.1111/nan.12338
Gauthier S, Ng KP, Pascoal TA, Zhang H, Rosa-Neto P (2018) Targeting Alzheimer’s disease at the right time and the right place: validation of a personalized approach to diagnosis and treatment. J Alzheimers Dis 64(s1):S23–S31. https://doi.org/10.3233/JAD-179924
Giuliani A, Prattichizzo F, Micolucci L, Ceriello A, Procopio AD, Rippo MR (2017) Mitochondrial (Dys) function in inflammaging: do mitomirs influence the energetic, oxidative, and inflammatory status of senescent cells? Mediators Inflamm 2017:2309034
Giunta B, Fernandez F, Nikolic WV, Obregon D, Rrapo E, Town T, Tan J (2008) Inflammaging as a prodrome to Alzheimer’s disease. J Neuroinflam 11(5):51. https://doi.org/10.1186/1742-2094-5-51
Goldberg EL, Shaw AC, Montgomery RR (2020) How inflammation blunts innate immunity in aging. Interdiscip Top Gerontol Geriatr 43:1–17. https://doi.org/10.1159/000504480 Epub 2020 Apr 9
Goldeck D, Witkowski JM, Fülop T, Pawelec G (2016) Peripheral immune signatures in Alzheimer disease. Curr Alzheimer Res 13(7):739–749
Gupta S (2014) Role of dendritic cells in innate and adaptive immune response in human aging. Exp Gerontol 54:47–52. https://doi.org/10.1016/j.exger.2013.12.009
Han F, Fukunaga K (2009) Beta-amyloid accumulation in neurovascular units following brain embolism. J Pharmacol Sci 111(2):101–109. https://doi.org/10.1254/jphs.09r02cp
Hansen PR (2018) Chronic inflammatory diseases and atherosclerotic cardiovascular disease: innocent bystanders or partners in crime? Curr Pharm Des 24(3):281–290. https://doi.org/10.2174/1381612824666180110102341
Hardy J, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12(10):383–388. https://doi.org/10.1016/0165-6147(91)90609-v
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356. https://doi.org/10.1126/science.1072994
Herrup K (2015) The case for rejecting the amyloid cascade hypothesis. Nat Neurosci 18(6):794–799. https://doi.org/10.1038/nn.4017
Hu MY, Lin YY, Zhang BJ, Lu DL, Lu ZQ, Cai W (2019) Update of inflammasome activation in microglia/macrophage in aging and aging-related disease. CNS Neurosci Ther 25(12):1299–1307. https://doi.org/10.1111/cns.13262
Huang Z, Wong LW, Su Y, Huang X, Wang N, Chen H, Yi C (2020) Blood-brain barrier integrity in the pathogenesis of Alzheimer’s disease. Front Neuroendocrinol 59:100857. https://doi.org/10.1016/j.yfrne.2020.100857
Humphries F, Fitzgerald KA (2019) Assembling the inflammasome Piece by Piece. J Immunol. 203(5):1093–1094. https://doi.org/10.4049/jimmunol.1900764
Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, Bullido MJ, Carter C, Clerici M, Cosby SL, Del Tredici K, Field H, Fulop T, Grassi C, Griffin WS, Haas J, Hudson AP, Kamer AR, Kell DB, Licastro F, Letenneur L, Lövheim H, Mancuso R, Miklossy J, Otth C, Palamara AT, Perry G, Preston C, Pretorius E, Strandberg T, Tabet N, Taylor-Robinson SD, Whittum-Hudson JA (2016) Microbes and Alzheimer’s disease. J Alzheimers Dis 51(4):979–984. https://doi.org/10.3233/JAD-160152
Jóźwik A, Landowski J, Bidzan L, Fülop T, Bryl E, Witkowski JM (2012) Beta-amyloid peptides enhance the proliferative response of activated CD4CD28 lymphocytes from Alzheimer disease patients and from healthy elderly. PLoS One 7(3):e33276. https://doi.org/10.1371/journal.pone.0033276
Kang C (2019) Senolytics and senostatics: a two-pronged approach to target cellular senescence for delaying aging and age-related diseases. Mol Cells 42(12):821–827. https://doi.org/10.14348/molcells.2019.0298
Kelly DJ (1998) The physiology and metabolism of the human gastric pathogen Helicobacter pylori. Adv Microb Physiol 40:137–189
Kern A, Behl C (2009) The unsolved relationship of brain aging and late-onset Alzheimer disease. Biochim Biophys Acta 10:1124–1132. https://doi.org/10.1016/j.bbagen.2009.07.016
Kessler TA (2017) Cervical cancer: prevention and early detection. Semin Oncol Nurs 33(2):172–183
Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JW, van Crevel R, Netea MG (2012) Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA 109:17537–17542
Komleva Y, Chernykh A, Lopatina O, Gorina Y, Lokteva I, Salmina A, Gollasch M (2021) Inflamm-aging and brain insulin resistance: new insights and role of life-style strategies on cognitive and social determinants in aging and neurodegeneration. Front Neurosci 14(14):618395. https://doi.org/10.3389/fnins.2020.618395
Kowalski K, Mulak A (2019) Brain-Gut-Microbiota axis in Alzheimer’s disease. J Neurogastroenterol Motil 25(1):48–60. https://doi.org/10.5056/jnm18087
Kremen WS, Beck A, Elman JA, Gustavson DE, Reynolds CA, Tu XM, Sanderson-Cimino ME, Panizzon MS, Vuoksimaa E, Toomey R, Fennema-Notestine C, Hagler DJ Jr, Fang B, Dale AM, Lyons MJ, Franz CE (2019) Influence of young adult cognitive ability and additional education on later-life cognition. Proc Natl Acad Sci USA 116(6):2021–2026. https://doi.org/10.1073/pnas.1811537116
Kritsilis M, Rizou SV, Koutsoudaki PN, Evangelou K, Gorgoulis VG, Papadopoulos D. (2018) Ageing, cellular senescence and neurodegenerative disease. Int J Mol Sci 19(10), pii:E2937.
Kumar V (2019) Toll-like receptors in the pathogenesis of neuroinflammation. J Neuroimmunol 15(332):16–30. https://doi.org/10.1016/j.jneuroim.2019.03.012
Kumar H, Kawai T, Akira S (2011) Pathogen recognition by the innate immune system. Int Rev Immunol 30(1):16–34. https://doi.org/10.3109/08830185.2010.529976
Kumar DK, Eimer WA, Tanzi RE, Moir RD (2016a) Alzheimer’s disease: the potential therapeutic role of the natural antibiotic amyloid-β peptide. Neurodegener Dis Manag 6(5):345–348
Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, Moir RD (2016b) Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med 8(340):340ra72
Kurakin A, Bredesen DE (2020) Alzheimer’s disease as a systems network disorder: chronic stress/dyshomeostasis, innate immunity, and genetics. Aging (Albany, NY) 12(18):17815–17844. https://doi.org/10.18632/aging.103883
Le Page A, Garneau H, Dupuis G, Frost EH, Larbi A, Witkowski JM, Pawelec G, Fülöp T (2017) Differential phenotypes of myeloid-derived suppressor and T regulatory cells and cytokine levels in amnestic mild cognitive impairment subjects compared to mild alzheimer diseased patients. Front Immunol 7(8):783. https://doi.org/10.3389/fimmu.2017.00783
Le Page A, Dupuis G, Frost EH, Larbi A, Pawelec G, Witkowski JM, Fulop T (2018) Role of the peripheral innate immune system in the development of Alzheimer’s disease. Exp Gerontol 107:59–66
Leszek J, Barreto GE, Gąsiorowski K, Koutsouraki E, Ávila-Rodrigues M, Aliev G (2016) Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: role of brain innate immune system. CNS Neurol Disord Drug Targets 15:329–336
Li L, Zhang X, Yang D, Luo G, Chen S, Le W (2019) Hypoxia increases abeta generation by altering betaand gamma-cleavage of APP. Neurobiol Aging. 30(7):1091–1098. https://doi.org/10.1016/j.neurobiolaging.2007.10.011
Li S, Wang W, Wang C, Tang YY (2010) Possible involvement of NO/NOS signaling in hippocampal amyloid-beta production induced by transient focal cerebral ischemia in aged rats. Neurosci Lett 470(2):106–110. https://doi.org/10.1016/j.neulet.2009.12.064
Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, Casadei V, Grimaldi LM (2000) Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? J Neuroimmunol 103(1):97–102. https://doi.org/10.1016/s0165-5728(99)00226-x
Long JM, Holtzman DM (2019) Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179(2):312–339. https://doi.org/10.1016/j.cell.2019.09.001
Lopatko Lindman K, Weidung B, Olsson J, Josefsson M, Kok E, Johansson A, Eriksson S, Hallmans G, Elgh F, Lövheim H (2019) A genetic signature including apolipoprotein Eε4 potentiates the risk of herpes simplex-associated Alzheimer’s disease. Alzheimers Dement (n y). 4(5):697–704. https://doi.org/10.1016/j.trci.2019.09.014
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Magrone T, Magrone M, Russo MA, Jirillo E (2020) Peripheral immunosenescence and central neuroinflammation: a dangerous liaison—a dietary approach. Endocr Metab Immune Disord Drug Targets 20(9):1391–1411. https://doi.org/10.2174/1871530320666200406123734
Maloney B, Lahiri DK (2016) Epigenetics of dementia: understanding the disease as a transformation rather than a state. Lancet Neurol 15(7):760–774. https://doi.org/10.1016/S1474-4422(16)00065-X
Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB (2014) mTOR inhibition improves immune function in the elderly. Sci Transl Med 6(268):268ra179. https://doi.org/10.1126/scitranslmed.3009892
Marchesi VT (2011) Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J 25(1):5–13. https://doi.org/10.1096/fj.11-0102ufm
McGeer PL, McGeer EG (2013) The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol 126(4):479–497. https://doi.org/10.1007/s00401-013-1177-7
McManus RM, Heneka MT (2017) Role of neuroinflammation in neurodegeneration: new insights. Alzheimers Res Ther. 9(1):14. https://doi.org/10.1186/s13195-017-0241-2
Mehta D, Jackson R, Paul G, Shi J, Sabbagh M (2017) Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert Opin Investig Drugs 26(6):735–739. https://doi.org/10.1080/13543784.2017.1323868
Miklossy J (2016) Bacterial amyloid and dna are important constituents of senile plaques: further evidence of the spirochetal and biofilm nature of senile plaques. J Alzheimers Dis 53:1459–1473
Morgan AR, Touchard S, Leckey C, O’Hagan C, Nevado-Holgado AJ, NIMA Consortium, Barkhof F, Bertram L, Blin O, Bos I, Dobricic V, Engelborghs S, Frisoni G, Frölich L, Gabel S, Johannsen P, Kettunen P, Kłoszewska I, Legido-Quigley C, Lleó A, Martinez-Lage P, Mecocci P, Meersmans K, Molinuevo JL, Peyratout G, Popp J, Richardson J, Sala I, Scheltens P, Streffer J, Soininen H, Tainta-Cuezva M, Teunissen C, Tsolaki M, Vandenberghe R, Visser PJ, Vos S, Wahlund LO, Wallin A, Westwood S, Zetterberg H, Lovestone S, Morgan BP (2019) Annex: NIMA–Wellcome trust consortium for neuroimmunology of mood disorders and Alzheimer’s disease. Inflammatory biomarkers in Alzheimer’s disease plasma. Alzheimers Dement 2019 Jun;15(6):776–787. https://doi.org/10.1016/j.jalz.2019.03.007
Müller L, Di Benedetto S, Pawelec G (2019) The immune system and its dysregulation with aging. Subcell Biochem 91:21–43. https://doi.org/10.1007/978-981-13-3681-2_2
Munafò A, Burgaletto C, Di Benedetto G et al (2020) Repositioning of immunomodulators: a ray of hope for Alzheimer’s disease? Front Neurosci 14:614643. https://doi.org/10.3389/fnins.2020.614643
Myette-Côté É, St-Pierre V, Beaulieu S, Castellano CA, Fortier M, Plourde M, Bocti C, Fulop T, Cunnane SC (2021) The effect of a 6-month ketogenic medium-chain triglyceride supplement on plasma cardiometabolic and inflammatory markers in mild cognitive impairment. Prostaglandins Leukot Essent Fatty Acids 169:102236. https://doi.org/10.1016/j.plefa.2020.102236
Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV (2019) Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med Feb 2019;25(2):270–276
Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M (2015) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385(9984):2255–2263. https://doi.org/10.1016/S0140-6736(15)60461-5
Noe CR, Noe-Letschnig M, Handschuh P, Noe CA, Lanzenberger R (2020) Dysfunction of the blood-brain barriers—a key step in neurodegeneration and dementia. Front Aging Neurosci 12:18539
Nyugen J, Agrawal S, Gollapudi S, Gupta S (2010) Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol 30(6):806–813. https://doi.org/10.1007/s10875-010-9448-8
Nzou G, Wicks RT, VanOstrand NR, Mekky GA, Seale SA, El-Taibany A, Wicks EE, Nechtman CM, Marrotte EJ, Makani VS, Murphy SV, Seeds MC, Jackson JD, Atala AJ (2020) Multicellular 3D neurovascular unit model for assessing hypoxia and neuroinflammation induced blood-brain barrier dysfunction. Sci Rep 10(1):9766. https://doi.org/10.1038/s41598-020-66487-8
Osorio C, Kanukuntla T, Diaz E, Jafri N, Cummings M, Sfera A (2019) The post-amyloid era in Alzheimer’s disease: trust your gut feeling. Front Aging Neurosci 11:143
Paouri E, Georgopoulos S (2019) Systemic and CNS inflammation crosstalk: implications for Alzheimer’s disease. Curr Alzheimer Res 16(6):559–574. https://doi.org/10.2174/1567205016666190321154618
Pawelec G (2018) Age and immunity: What is “immunosenescence”? Exp Gerontol 105:4–9. https://doi.org/10.1016/j.exger.2017.10.024
Pawelec G (2020) The human immunosenescence phenotype: does it exist? Semin Immunopathol 42(5):537–544. https://doi.org/10.1007/s00281-020-00810-3
Pawelec G, Bronikowski A, Cunnane SC, Ferrucci L, Franceschi C, Fülöp T, Gaudreau P, Gladyshev VN, Gonos ES, Gorbunova V, Kennedy BK, Larbi A, Lemaître JF, Liu GH, Maier AB, Morais JA, Nóbrega OT, Moskalev A, Rikkert MO, Seluanov A, Senior AM, Ukraintseva S, Vanhaelen Q, Witkowski J, Cohen AA (2020) The conundrum of human immune system “senescence.” Mech Ageing Dev 192:111357. https://doi.org/10.1016/j.mad.2020.111357
Perl DP (2010) Neuropathology of Alzheimer’s disease. Mt Sinai J Med January–February 2010; 77(1):32–42. https://doi.org/10.1002/msj.20157
Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15(5):675–690. https://doi.org/10.1016/j.cmet.2012.04.003
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013) The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9(1):63-75.e2. https://doi.org/10.1016/j.jalz.2012.11.007
Regen F, Hellmann-Regen J, Costantini E, Reale M (2017) Neuroinflammation and Alzheimer’s disease: implications for microglial activation. Curr Alzheimer Res 14(11):1140–1148. https://doi.org/10.2174/1567205014666170203141717
Ricciarelli R, Fedele E (2017) The amyloid cascade hypothesis in Alzheimer’s disease: it’s time to change our mind. Curr Neuropharmacol 15(6):926–935. https://doi.org/10.2174/1570159X15666170116143743
Rivers-Auty J, Mather AE, Peters R, Lawrence CB, Brough D (2020) Anti-inflammatories in Alzheimer’s disease-potential therapy or spurious correlate? Brain Commun 2020 Jul 24;2(2):fcaa109. https://doi.org/10.1093/braincomms/fcaa109
Rodrigue KM, Kennedy KM, Park DC (2009) Beta-amyloid deposition and the aging brain. Neuropsychol Rev 19(4):436–450. https://doi.org/10.1007/s11065-009-9118-x
Rubino G, Bulati M, Aiello A, Aprile S, Gambino CM, Gervasi F, Caruso C, Accardi G (2019) Sicilian centenarian offspring are more resistant to immune ageing. Aging Clin Exp Res 31(1):125–133
Sacks CA, Avorn J, Kesselheim AS (2017) The failure of Solanezumab—How the FDA saved taxpayers billions. N Engl J Med 376(18):1706–1708. https://doi.org/10.1056/NEJMp1701047
Salani F, Sterbini V, Sacchinelli E, Garramone M, Bossù P (2019) Is innate memory a double-edge sword in Alzheimer’s disease? A reappraisal of new concepts and old data. Front Immunol 7(10):1768. https://doi.org/10.3389/fimmu.2019.01768
Salminen A (2020) Activation of immunosuppressive network in the aging process. Ageing Res Rev 57:100998. https://doi.org/10.1016/j.arr.2019.100998
Salvioli S, Monti D, Lanzarini C, Conte M, Pirazzini C, Bacalini MG, Garagnani P, Giuliani C, Fontanesi E, Ostan R, Bucci L, Sevini F, Yani SL, Barbieri A, Lomartire L, Borelli V, Vianello D, Bellavista E, Martucci M, Cevenini E, Pini E, Scurti M, Biondi F, Santoro A, Capri M, Franceschi C (2013) Immune system, cell senescence, aging and longevity–inflamm-aging reappraised. Curr Pharm Des 19(9):1675–1679
Sanada F, Taniyama Y, Muratsu J, Otsu R, Shimizu H, Rakugi H, Morishita R (2018) Source of chronic inflammation in aging. Front Cardiovasc Med. 5:12
Schmitt V, Rink L, Uciechowski P (2013) The Th17/Treg balance is disturbed during aging. Exp Gerontol 48(12):1379–1386. https://doi.org/10.1016/j.exger.2013.09.003
Schubert D, Currais A, Goldberg J, Finley K, Petrascheck M, Maher P (2018) Geroneuroprotectors: effective geroprotectors for the brain. Trends Pharmacol Sci 39(12):1004–1007. https://doi.org/10.1016/j.tips.2018.09.008
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8(6):595–608. https://doi.org/10.15252/emmm.201606210
Shad KF, Aghazadeh Y, Ahmad S, Kress B (2013) Peripheral markers of Alzheimer’s disease: surveillance of white blood cells. Synapse 67(8):541–543
Shen H, Guan Q, Zhang X, Yuan C, Tan Z, Zhai L, Hao Y, Gu Y, Han C (2020) New mechanism of neuroinflammation in Alzheimer’s disease: the activation of NLRP3 inflammasome mediated by gut microbiota. Prog Neuropsychopharmacol Biol Psychiatry 8(100):109884. https://doi.org/10.1016/j.pnpbp.2020.109884
Šimić G, Španić E, Langer Horvat L, Hof PR (2019) Blood-brain barrier and innate immunity in the pathogenesis of Alzheimer’s disease. Prog Mol Biol Transl Sci 168:99–145
Singhrao SK, Olsen I (2019) Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J Oral Microbiol 11(1):1563405
Smale ST (2011) Hierarchies of NF-κB target-gene regulation. Nat Immunol 12(8):689–694. https://doi.org/10.1038/ni.2070
Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T (2012) Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol 24(5):331–341. https://doi.org/10.1016/j.smim.2012.04.008
Solana C, Tarazona R, Solana R (2018) Immunosenescence of natural killer cells, inflammation, and Alzheimer’s disease. Int J Alzheimers Dis 1(2018):3128758. https://doi.org/10.1155/2018/3128758
Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD (2010) The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One 5:e9505
Subhramanyam CS, Wang C, Hu Q, Dheen ST (2019) Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol 94:112–120. https://doi.org/10.1016/j.semcdb.2019.05.004
Tam JH, Pasternak SH (2012) Amyloid and Alzheimer’s disease: inside and out. Can J Neurol Sci 39:286–298. https://doi.org/10.1017/s0317167100013408
Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL (2013) Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123:966–972
Tejera D, Mercan D, Sanchez-Caro JM, Hanan M, Greenberg D, Soreq H, Latz E, Golenbock D, Heneka MT (2019) Systemic inflammation impairs microglial Abeta clearance through NLRP3 inflammasome. EMBO J 2019 Sep 2;38(17):e101064. https://doi.org/10.15252/embj.2018101064. Epub 2019 Jul 30
Terlecki-Zaniewicz L, Lämmermann I, Latreille J, Bobbili MR, Pils V, Schosserer M, Weinmüllner R, Dellago H, Skalicky S, Pum D, Almaraz JCH, Scheideler M, Morizot F, Hackl M, Gruber F, Grillari J (2018) Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging (Albany, NY) 10(5):1103–1132
Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, Schertzer JD, Larché MJ, Davidson DJ, Verdú EF, Surette MG, Bowdish DME (2017) Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21:455
van der Heijden CDCC, Noz MP, Joosten LAB, Netea MG, Riksen NP, Keating ST (2017) Epigenetics and trained immunity. Antioxid Redox Signal 29(11):1023–1040
Walker KA, Ficek BN, Westbrook R (2019) Understanding the role of systemic inflammation in Alzheimer’s disease. ACS Chem Neurosci 10(8):3340–3342. https://doi.org/10.1021/acschemneuro.9b00333
Walton CC, Begelman D, Nguyen W, Andersen JK (2020) Senescence as an amyloid cascade: the amyloid senescence hypothesis. Front Cell Neurosci 19(14):129. https://doi.org/10.3389/fncel.2020.00129
Webers A, Heneka MT, Gleeson PA (2020) The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol Cell Biol 98(1):28–41. https://doi.org/10.1111/imcb.12301
Whalley LJ, Dick FD, McNeill G (2006) A life-course approach to the aetiology of late-onset dementias. Lancet Neurol 5(1):87–96. https://doi.org/10.1016/S1474-4422(05)70286-6
Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL (2020) Discovery, development, and future application of senolytics: theories and predictions. FEBS J 287(12):2418–2427. https://doi.org/10.1111/febs.15264
Wong C, Goldstein DR (2013) Impact of aging on antigen presentation cell function of dendritic cells. Curr Opin Immunol 25(4):535–541. https://doi.org/10.1016/j.coi.2013.05.016
Wozniak MA, Itzhaki RF, Shipley SJ, Dobson CB (2007) Herpes simplex virus infection causes cellular beta-amyloid accumulation and secretase upregulation. Neurosci Lett 429:95–100
Wozniak MA, Mee AP, Itzhaki RF (2009) Herpes simplex virus type 1 DNA is located within Alzheimer’s disease amyloid plaques. J Pathol 217:131–138
Wyatt-Johnson SK, Brutkiewicz RR (2020) The complexity of microglial interactions with innate and adaptive immune cells in Alzheimer’s disease. Front Aging Neurosci 19(12):592359. https://doi.org/10.3389/fnagi.2020.592359
Yang J, Wise L, Fukuchi KI (2020) TLR4 cross-talk with NLRP3 inflammasome and complement signaling pathways in Alzheimer’s disease. Front Immunol 2020 Apr 23;11:724. https://doi.org/10.3389/fimmu.2020.00724. eCollection 2020
Zhao Y, Zhan JK, Liu Y (2020) A perspective on roles played by immunosenescence in the pathobiology of Alzheimer’s disease. Aging Dis 11(6):1594–1607. https://doi.org/10.14336/AD.2020.0205
Acknowledgements
This work was supported by grants from Canadian Institutes of Health Research (CIHR) (No. 106634) and No. PJT-162366) to AK and TF, the Société des médecins de l’Université de Sherbrooke and the Research Center on Aging of the CIUSSS-CHUS, Sherbrooke and the FRQS Audace grant to TF and EF; by the Polish Ministry of Science and Higher Education statutory grant 02-0058/07/262 to JMW; by Agency for Science Technology and Research (A*STAR) to AL.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest
Author declare no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by the author.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fulop, T. et al. (2022). Immunosenescence and Alzheimer’s Disease. In: Bueno, V., Pawelec, G. (eds) Healthy Longevity and Immune System. Healthy Ageing and Longevity, vol 16. Springer, Cham. https://doi.org/10.1007/978-3-030-87532-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-87532-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-87531-2
Online ISBN: 978-3-030-87532-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)