Abstract
This chapter aims to provide an overview of natural and synthetic organic lakes and pigments and of how their analysis by chromatography and mass spectrometry can provide useful information in the examination of works of art. The characteristics of natural organic colourants are reviewed, including information on their provenance and uses in the production of lakes; information on the date of production, availability as paint products and application in artworks of the most important synthetic organic pigments is also provided. This chapter specifically illustrates the state-of-the-art of organic pigments analysis by high performance liquid chromatography with different detectors, and by gas chromatography – mass spectrometry also coupled to analytical pyrolysis. Different mass spectrometry based techniques used both as detector and directly applied on a sample or its extract are also presented. Particular attention is paid to sample preparation; further issues related to difficulties in finding relevant standards or reference materials and understanding photo-degradation processes occurring in degraded organic pigments are also discussed. Several case studies are presented, showing the potentialities of liquid chromatography interfaced with mass spectrometry in determining properties of the samples which can be of assistance for assessing the provenance, dating or even the authenticity of a painting.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- High performance liquid chromatography
- Mass spectrometry
- Organic lakes
- Synthetic organic pigments
- Paint materials
1 Introduction
Organic lakes and pigments have been used in paintings and in ink formulations since pre-history. Their composition is, in some cases, specific to a time period or a geographical area, thus their identification may provide data for dating purposes or even for establishing authorship. Unfortunately, the analysis of organic lakes and pigments in artworks and in painting matrices is particularly arduous for the chemist. In addition to the analytical issues that must be overcome when we deal with the analysis of any microsample from a work of art, such as the small amount and representativeness of the sample available and the low concentration in which analytes are present, several other factors have to be considered, such as the lack of reliable recipes for lake preparation, or the uninformative product names provided by modern manufacturers due to patent wars. Finally, organic colourants are amongst the most fugitive species in works-of-art, and thus their composition as determined today strongly depends on the condition of the painting, which may have undergone irreversible degradation.
Consequently, the study of the composition in terms of colouring molecules of organic dyes and pigments can provide important information on the origin and dating of an object, given that a thorough comparison with reference materials, possibly also subjected to natural and artificial ageing, is correctly performed.
This chapter presents the main natural and synthetic organic lakes along with their most important characteristics for establishing provenance and dating. State-of-the-art methods of detection and identification of organic pigments and lakes based on chromatography and mass spectrometry will be reviewed, and specific issues related to sample treatment and photo-ageing processes will be presented. The level of information achievable by the analysis of organic pigments will be illustrated through selected case studies.
1.1 Natural Organic Lakes
In antiquity, organic coloured paint materials were obtained from natural sources derived from plant extracts or from the metabolism of insects or molluscs. Ancient Egyptians were the first to develop dye extraction procedures and to document them accurately in hieroglyphics (Zollinger 2003). The introduction of indigo, Tyrian purple, and the reds madder and cochineal were main milestones in the artistic and economical history of natural dyes (Zollinger 2003). Notably, different cultures around the globe developed methods to extract such colours, even if from different autochthonous plants or animal species. These dyes were used for colouring textiles, and for the preparation of organic pigments. Organic dyes and pigments differ in their solubility in water and in other binding media: dyes are soluble in binding media while pigments are insoluble (Colombini and Modugno 2009), and lakes are generally obtained by precipitation as salts, absorption on inert and colourless substrates and/or complexed as metal salts (Herbst et al. 2004; Kirby et al. 2005a, 2014). By this procedure, coloured insoluble particles are formed (through complexation or absorption phenomena), and can be separated by precipitation and filtration, washed and dried to obtain a solid lake pigment. The most commonly salt used in lake production was alum, and in particular hydrated potassium aluminium sulphate (KAl(SO4)2·12H2O), which was also employed as a mordant for textile dyeing. Other salts used in lake production were chalk (calcium sulphate, CaSO4) and gypsum (calcium sulphate dihydrate, CaSO4·2H2O). The metallic cation chosen may change and influence the colour shades that are produced.
Over the centuries, artists have applied lakes with different painting techniques and on several supports. Lakes have performed a less important role in classic painting compared to inorganic pigments because most of them exhibit lower lightfastness and great sensitivity to atmospheric agents and to pH variations. As a consequence, lakes were applied in a great extent for miniatures in illuminated manuscripts, and in a less extent in mural paintings and in easel or canvas painting. Notwithstanding this, evidence of the use for millennia of madder lake, purple and indigo pigments in wall painting and paint decorations on ceramics, and in codices, has been found by scientific examinations. Madder was identified on funerary vessels and in Pompei’s pigments (Andreotti et al. 2017; Colombini et al. 2017) while purple was used in Thera wall paintings (Karapanagiotis et al. 2017), in Macedonia and Magna Grecia funerary paintings (Andreotti et al. 2017), and indigo is the main ingredient of Maya blue (Grazia et al. 2020). Finally, yellow organic dyestuffs such as saffron (Crocus sativus), Buckthorn/Persian berries (Rhamnus spp.), and weld (Reseda luteola L.) have been used to produce lake pigments in works of art since antiquity (Ciatti and Marini 2009; Mayhew et al. 2013; Saunders and Kirby 1994).
If a survey is made of the recipes used in Europe between the fifteenth and the nineteenth centuries, the dyestuffs most frequently mentioned are those extracted from redwoods (brazilwood), madder, and the scale insects kermes, cochineal and Indian lac (Kirby and White 1996). Most of these natural sources were available in Europe since antiquity, while some others have been imported since early times from India and South and Central America (Kirby et al. 2014). In Fig. 9.1, the geographical locations of some natural sources of red dyes used to produce lake pigments are depicted. Lakes produced with these materials are characterized by a low colouring power given by the dye and translucency provided by substrate (Kirby and White 1996). From the study of recipes from the European and Mediterranean area and the material analysis of paintings, a timeline can be identified in the use of these materials: up to the early seventeenth century, brazilwood, lac and shearings of cloth dyed with kermes (“cimatura de grana”) were the most common sources for lake production. It is worth noting that some confusion can be generated by the inconsistent nomenclature of raw materials and final products reported in ancient treatises and recipes, and the paucity of details provided, which sometimes refer to undefined “common practices”. Fake pigments (e.g. “fake purple”) are attested since medieval times, and guild regulations were often disregarded by dyers and manufacturers (Saez et al. 2019). From the late sixteenth century onwards, cochineal became more common, because of the increased availability of scale insects from South America, and by the nineteenth century the most important sources have been cochineal and madder, while lac and kermes are barely mentioned. For their low covering propriety, these lakes have been widely employed in glazing on canvas, or to modify the hues of other inorganic red pigments. This technique, exhibited by several European painters of fifteenth and sixteenth century, consisted in the overlapping of coloured paint layers on already dried paint layers in order to achieve different nuances (Kirby et al. 2005a, 2014). Moreover, anthraquinoid lakes were used in illuminating manuscripts, where colours were protected from degradation processes induced by light and external agents. Different species were used to produce organic lakes in the East, such as safflower (Wouters et al. 2010).
Map showing the geographical location of natural sources of red dyes. (Adapted from Kirby et al. 2014)
Starting from the end of the eighteenth century, thanks to the advances in knowledge of the chemistry of organic substance and the availability of strong acid and bases, progress led to the production of higher quality organic dyes, pigments, and lake pigments. The distinction between natural and synthetic materials becomes blurred due to manufacturers experimentation of new approaches such as the exploitation of natural dyes as pigments, by precipitating the natural extracts as salts in alkaline conditions (Degano et al. 2017). In particular, several methods to extract the colouring materials from madder were already tested at the end of the eighteenth century, yielding products with slightly different names, such as Brown Madder. Later, the availability of sulphuric and sulphurous acids led to the isolation of alizarin and purpurin in 1826. Different modified madder-based products, e.g. garancine (1828), enriched in alizarin and purpurin, Kopp’s purpurin (also known in France as “purpurine commerciale”), enriched in both purpurin and pseudopurpurin, or “carmine de garance”, supposedly pure madder dyestuff without the addition of an inorganic salt, were then commercialized (Kirby et al. 2007). Several “carmines” based on cochineal are also reported in nineteenth – twentieth century catalogues, in a wide range of hues and prices, prepared using different recipes and inorganic salts (Kirby et al. 2007). New lakes based on brazilwood were also introduced, produced by varying the pH of the reagents used or employing tin in the precipitating agent (Doherty et al. 2021).
Natural organic lakes can be classified based on their colour. The main organic lakes and pigments prepared with natural dyes are listed in Table 9.1.
1.2 Synthetic Organic Pigments (SOPs)
The successful synthesis of molecules responsible for the coloration of natural extracts was performed in 1868 during the synthesis of alizarin by the German chemists Carl Gräbe and Carl Lieberman (Kirby et al. 2005b), leading to the gradual decrease of the production of natural dyes and organic pigments. In an early stage, synthetic and natural pigments were often admixed, but after the 1930s, synthetic pigments totally dominated the market. The following step in the history of organic pigments was the synthesis of completely new formulations from the late nineteenth century, which could be tuned for the desired hue and chemical properties. The first synthetic organic pigments produced was Picric acid. In 1742, the German alchemist Johann Glauber prepared this dye by treating wool or animal horn and resins with nitric acid (Read 2014). Picric acid was rarely used due to its high intense yellow hue and thus the history of synthetic organic dyes officially started in 1856, when William Henry Perkin first synthesized mauveine. He was experimenting under the direction of August von Hofmann at the Royal College of Chemistry in London, and he was trying to produce a synthetic alternative to quinine from coal tar, when he accidentally obtained a purple compound, characterize by a high wash- and lightfastness, that he called mauveine (Lomax et al. 2006). Notably, the advances in synthetic chemistry and the discovery of mauveine occurred during the industrial revolution, a period of great change and expansion for Europe, particularly in the textile trade. The availability of cheaper synthetic dyes made coloured textiles more accessible to the wider population. Mauveine rapidly became the most fashionable colour for Victorian ladies, and Perkin became a rich entrepreneur (Garfield 2011). The commercial success of mauveine, the potential wide variety of hues achievable by slightly modifying the synthetic route, the cheapness, and the availability of the reagents, pushed chemists to experiment with new colorant formulations.
In 1863, the first azo dye, a bright yellow colour named “Field’s yellow”, was synthesized by Fredrick Field (Skelton 1999). Several aniline based dyes, obtained by diazotization reaction and differing by the kind of substituents (Ball 2001), were produced: they are the so called coal-tar colors (Lomax et al. 2006). Many of these products were precipitated as lakes (“laked”) to offer new pigments for artists: the availability of synthetic organic pigments in different hues, the possibility to admix them with binding media and additives in paint tubes and the relatively low costs of production, all made these new materials more appealing for manufactures not only of natural organic lakes, but also of traditional inorganic pigments. Unfortunately, many of these products were commercialized without testing for fastness properties and fugitivity in oils and severely faded few years after their application. The experimental trial-and-error strategy showed severe limits and a more reliable approach based on understanding the chemistry of the colorants and extraction processes was developed for producing high performing formulations and adequately meeting market demands. In this regard, the discovery of the benzene structure by Kekulé (Herndon 1974) and the following elucidation of alizarin structure by Adolf Bayer, Carl Grabe and Carl Lieberman in 1868 (Graebe and Liebermann 1879) were fundamental in laying the foundation for the comprehension of colour chemistry.
In 1910, the production of Pigment Yellow 1, alternatively named “Hansa Yellow G”, by Meister Lucius & Brüning, introduced the important class of Monoazo or Hansa pigments on the market (Meister Lucius and Brüning 1909). This very bright yellow pigment was the first of many to be produced with great commercial success (Schulte et al. 2008).
In 1936, phthalocyanines were the first class of organic colorants introduced directly into the market as ‘true’ pigments without prior use as dyes or lakes (De Keijzer 2002). This new class of pigments quickly conquered the blue and green pigment markets in all ranges of applications due to their incredibly high performance in terms of tinting strength, fastness and chemical resistance (Skelton 1999). Phthalocyanines replaced synthetic indigo lake pigments as a popular artists’ pigments. Quinacridones (1950’s) and diketopyrrolopyrroles (1983) were later introduced and used in high-quality artists’ materials due their excellent fastness and resistance properties (Lomax et al. 2006). By the 1990s more pigment classes of synthetic organic origin, such as perylenes and pyrrols, were synthesized and are now sold as artist’s materials.
Synthetic organic pigments now dominate the colorant market and have almost completely replaced traditional natural organic colorants.
Keeping track of the huge variety of currently available SOPs and those used in the pastis nearly impossible and an attempt to define the most important classes, based on literature (Herbst et al. 2004; Lutzenberger and Stege 2009), is summarized in the diagram below (Fig. 9.2).
Synthetic pigments can be divided in two main groups: the azo pigments and the polycyclic ones. Amongst each group, pigments are further classified on the basis of their chemical structure. The azo pigments are characterized by the azo functional group (-N=N-): monoazo pigments contain one azo group, while disazo two. The synthetic strategy to produce azo pigments is easy and economical, and based on the reaction of a diazonium compound with a coupling component. The kind of coupling agent selected allows the grouping of monoazo and disazo pigments in subclasses. Conversely, polycyclic pigments can be distinguished by the number or type of rings constituting the aromatic structure (Lutzenberger and Stege 2009). The pigments which cannot be included in one of these two big groups are generally classified as miscellaneous.
Throughout the last 150 years, creative and not informative names have been ascribed to formulations of synthetic organic dyes and pigments by manufacturers and resellers. The total absence of scientific rigour in the naming has led to a general confusion which still does not easily allow the understanding of the composition of the commercialized formulations. Patent wars between manufacturers have compounded such confusion. Indeed, in 1924, the Society of Dyers and Colourists (SDC) and the American Association of Textile Chemists and Colorists (AATCC) recorded the most extensive compendium of dyes and pigments: Colour Index™ (C.I.) (Colour index n.d.). A “Colour Index generic name” (e.g. PY1 for Pigment yellow 1) and a “Colour Index constitution number” (e.g. C.I. 11680 for Pigment yellow 1) was assigned to every coloured compound. The first is referred to the application field/coloration method, the latter to the chemical structure (Evans 1990).
A summary of the most important synthetic pigments, along with the year of synthesis and introduction on the market, their use as paint materials and some case studies (paintings and paint fabrics only) in which they were detected are reported in Table 9.2. However, in consulting patents and manufacturers’ logbooks, it should be kept in mind that the commercial nomenclature of synthetic dyes and pigments is often uninformative or undeclared due to patent restrictions, and the recipes of paint tubes often contain mixtures of more than one pigment belonging to the same or different classes. Moreover, the several by-products present in different ratios, as a consequence of the various synthetic strategies adopted to produce the same pigment, have contributed to generate confusion regarding the composition of the starting materials (Lech et al. 2013). Additionally, artists themselves further complicate the composition of the final paint by mixing colours, as well as through the addition of media, thinners, driers, waxes, diluents and extra oils to obtain the desired effects in their artworks (Townsend 1994). Furthermore, artists have experimented with new and old materials, blending highly heterogeneous components, including those that were low cost and not specifically suitable for artistic purposes (Learner et al. 2006).
2 Analysis: Methods, Instrumentation, and Specific Issues
2.1 High Performance Liquid Chromatography (HPLC) and Ultra Performance Liquid Chromatography (UPLC)
Liquid Chromatography has been widely employed in the analysis of organic materials in Heritage Science objects. In particular, it can be considered the method of choice because of its reliability and performance for the analysis of organic colorants whenever sampling is allowed. The first applications were based on Thin Layer Chromatography (TLC) that was gradually supplanted by High Performance Liquid Chromatography (HPLC) due to higher selectivity and sensitivity, great chromatographic efficiency, and the possibility of performing quantitative analysis (if analytical standards are available), or semiquantitative evaluations (Degano 2019). Reversed-phase chromatography (RP-HPLC) has been applied for the last 30 years for the characterization of organic dyes and pigments, which are most often complex mixtures of organic molecules that are mostly aromatic and polar, generally water soluble and characterized by a strong absorption in the UV-Vis range (Degano and La Nasa 2016). While natural colorants are inherently composed by mixture of different compounds, synthetic dyes were often admixed to obtain specific hues, or contained synthesis by-products whose identification can provide interesting information on the manufacturer. Thus, the identification of materials is usually achieved by comparing the chromatographic profiles of extracts of unknown samples to those obtained for known reference materials.
The analytical column is the core of the chromatographic separation. Amongst the several columns available, the most commonly used in the analysis of organic pigments and lakes are constituted by C8 or C18 stationary phase, with sizes of 150 × 4.6 or 150 × 2 mm, with 3 or 5 μm particle size. UHPLC (Ultra High-Pressure Liquid Chromatography) allows the use of sub-2-μm particle size, bearing relatively higher back-pressures. While HPLC can operate continuously at high pressure, up to 6000 psi, UHPLC can operate up to 10,000 psi. The recent success of UHPLC is given by its higher chromatographic efficiency and sensitivity, shorter runtimes and thus faster analysis, saving solvent cost and waste (Serrano et al. 2013; Taujenis and Olšauskaite 2012; Troalen et al. 2014). The solvent flow generally ranges between 0.2 and 10 mL/min, while for nano-LC setups a flow 0.002 mL/min is required. For an efficient separation of mixtures of differently polar compounds, the fine-tuning of a suitable elution program in gradient mode is fundamental. Binary systems of water and methanol or acetonitrile acidified with formic acid or trifluoroacetic or acetic acids are generally selected (Halpine 1996). For the analysis of some classes of synthetic pigments, counter ion chromatography making use of ion pair reagents such as tetrabutylammonium hydroxide may be more efficient in achieving a better separation and improved peak symmetry (van Bommel et al. 2007). When dealing with strongly polar dyes or uncoloured degradation compounds, the use of a RP-amide i.e. a polar embedded reversed-phase column, may be effective (Restivo et al. 2014). Finally, 2D-HPLC, entailing the use of two columns exploiting different retention mechanisms, can be employed as a universal system to separate both extremely polar and relatively less polar organic dyes (Pirok Bob et al. 2016, 2019a, b).
For the detection of separated compounds, the most commonly employed detectors are spectrophotometric (UV-Vis, Diode Array and Fluorescence Detectors) and mass spectrometric (ESI-MS, APCI-MS) detectors. Whenever possible, the use of both spectrophotometric and mass spectrometric detectors in series is advisable, to exploit the ability of the first to assess the colour of the eluted components through the acquisition of the UV/Visible spectrum, and the sensitivity and selectivity of the latter. Fluorimetric detection is extremely sensitive and selective (van Bommel 2005; Colombini et al. 2004; Surowiec et al. 2003) due to the high sensitivity of fluorescence in low concentrations. On the other hand, the fact that only a few chromophores exhibit fluorescence can be a drawback in the application of this method of detection. Thus, in most cases, pre- or post-column derivatisation is applied; particularly using aluminium and gallium salts in post-column derivatisation (van Bommel 2005; Szostek et al. 2003). In recent years, the application of high resolution mass spectrometry has aided the resolution of complex mixtures containing unknown species (including degradation products and synthetic by-products) in replicas, in atelier materials and in paint samples (Chieli et al. 2019; La Nasa et al. 2020; Sabatini et al. 2018, 2020b, c).
2.2 Gas Chromatography – Mass Spectrometry (GC/MS) and Analytical Pyrolysis (Py-GC/MS)
In the study of natural dyes, gas chromatographic techniques have not been exploited extensively, due to the relatively high-molecular mass and polarity of target compounds. Derivatisation of coloured compounds is thus mandatory. Although uncommon, GC/MS based methods have been proposed mainly for dyed textiles and objects, based on the extraction of colouring molecules followed by their derivatization with a silylating agent, both by Colombini (Colombini et al. 2007) and Degani (Degani et al. 2014). Poulin instead developed a procedure for the simultaneous extraction and derivatization of molecules by TMTFTH (Poulin 2018). To bypass any sample pre-treatment, pyrolysis coupled with gas chromatography has been used. While for natural colours research is limited (Andreotti et al. 2004; Casas-Catalán and Doménech-Carbó 2005; Fabbri et al. 2000), several studies tackled the analysis by Py-GC/MS of synthetic organic pigments (SOPs), which are often insoluble or bound in synthetic binders that prove difficult to solubilize. Synthetic pigments are usually analysed without any derivatising reagent; recently, several libraries, listing the characteristic pyrolysis products of several classes of SOPs, have been created (Germinario et al. 2017; Ghelardi et al. 2015b; Learner 2005; Rehorek and Plum 2007; Russell et al. 2011; Sonoda 1999).
In spite of its ability to detect pigments which are not soluble in any extraction solvent, pyrolysis does not provide information on the chemically and thermally stable pigments. Moreover, many families of pigments have a common skeletal structure, differing only in the nature or position of the substituents on the aromatic rings, and thus they often yield common pyrolysis fragments. Notwithstanding this, differences between pyrograms of unaged and artificially aged paint model systems have been detected and have allowed the hypothesis of degradation pathways (Ghelardi et al. 2015a).
2.3 Mass Spectrometry Based Techniques
Mass spectrometry based techniques can be applied as detectors for liquid or gas chromatography, or as self-standing analytical tools. For liquid chromatography, the most widely used systems are based on electrospray ionization (ESI) tandem mass spectrometry. ESI allows the interfacing of a high pressure system such as HPLC with a mass spectrometer, which requires high vacuum, and performs the ionization of the analytes. Being a soft ionization technique, for small molecules as dyes ESI mostly produces monocharged molecular ions, whose mass to charge ratio corresponds to [M+H]+ or [M-H]− ions, for positive and negative analysis modes, respectively. A second stage of analysis after a further fragmentation of the molecular ion in a collision chamber is thus required to perform qualitative analysis and ultimately to improve sensitivity. Consequently, tandem mass spectrometry is employed in most applications, including ion traps, triple quadrupoles and quadrupole-time of flight analysers.
Other mass spectrometry based techniques used to detect organic dyes and pigments without any prior separation of the sample components are: direct temperature mass spectrometry (DTMS); laser-desorption/ionization (LDI) and matrix assisted laser desorption/ionization (MALDI) mass spectrometry; direct analysis in real time (DART) and surface acoustic wave nebulization (SAWN). Through the rapid acquisition and observation of positive and negative ion spectra, acidic, basic and neutral natural and synthetic colorants can be distinguished and characterized by their m/z values. The absence of preliminary separation means that the detection of minor components is quite difficult, but the features of these techniques make them suitable for the analysis of poorly soluble organic pigments and for the screening of large collections of samples.
DTMS was pioneered by Boon and Learner, but has essentially been abandoned in the last 10 years (Boon and Learner 2002; Lomax et al. 2007; Menke et al. 2009) in favour of more robust and more widely available techniques. LDI and MALDI-MS have been widely used for the analysis of synthetic organic pigments (Boon and Learner 2002; Kirby et al. 2009), but also applied to the detection of natural organic lakes (Boon et al. 2007; Sabatini et al. 2016; Soltzberg et al. 2007).
DART has been tested on organic dyes, even directly on textiles (Armitage et al. 2015, 2019), while it has only been applied to the detection of organic lakes on paint mock-ups in the recent publication by Alvarez-Martin on eosin lake (Alvarez-Martin et al. 2019).
SAWN has been employed by Astefanei for the identification of synthetic dyes in reference powder materials and historical textiles (Astefanei et al. 2017).
2.4 Sample Preparation
The application of liquid chromatography, gas chromatography and some of the direct MS techniques requires the preliminary extraction of the chromophore containing molecules from the matrix. The selection of the most suitable extraction method is crucial, considering the uniqueness of samples and the tiny amount of colorant they often contain (Bacci et al. 2006). The effectiveness of the pretreatment is strongly dependent on the solubility and stability of the colorants in solvents and the nature and the ageing of the matrix (Padfield n.d.). Thus, there is as yet no universal method of extraction applicable to all the many dye classes in every kind of heterogenous matrix. The most commonly used extraction methods for the analysis of organic pigments are listed below:
-
Boiling mixture of organic solvent and strong acid: this is the strongest and more aggressive extraction method, the organic solvent usually used is methanol (MeOH) and the acid is hydrochloric (HCl) or sulfuric acid (H2SO4) (Abrahart 1977; Halpine 1996). HCl 0.5 M and MeOH (1:1) (Halpine 1996) solution or 2 HCl: 1 MeOH: 1 H2O (v/v/v) (Wouters 1985) are the most effective and used methods. Nevertheless, some undesired effects such as the cleavage of labile bonds, esterification of carboxylic compounds and oxidation of moieties limit its utilization (Ferreira et al. 2002). Moreover, HCl is corrosive and difficult to evaporate;
-
Transmethylation with boron trifluoride and methanol (BF3/MeOH): a suitable method for disrupting highly polymerized painting films and extracting dyes from lake pigments. If BF3 is highly concentrated, interactions among compounds in the matrix and esterification may occur (Kirby and White 1996);
-
Hydrofluoric acid (HF): a weak acid and, in its dissociated form, also a strong aluminium-complexing agent as efficient as a strong acid (Sanyova 2008). This is a mild extraction preferrable for unstable colorants and applicable to a wide range of colorant classes and mixtures precipitated with different metal cations, applied on different supports and in various matrices (Padfield n.d.; Sanyova and Reisse 2006). It allows working at much milder pH (c.a. 1.5) than with HCl (Sanyova 2008) obtaining high yields for many colorants. The drawbacks of this method consist in the precautions for manipulation and treatment of HF. Its toxicity both for contact and for inhalation requires protections and its corrosive power, especially toward glass, requires the use of Teflon laboratory ware;
-
Formic acid (HCOOH): a weak acid which is used in an extracting solution of 5 HCOOH: 95 MeOH (v/v) which enable a mild extraction of the chromophore containing molecules (Zhang and Laursen 2005);
-
Complexation agents: ethylenediaminetetracetic acid (EDTA) is used for its capability as a strong chelator of aluminium and of other ions, enabling the release of dyes molecule without decomposing them. An efficient extracting solution used is 2 H2EDTA: 10 ACN: 88 MeOH (v/v/v) (Zhang and Laursen 2005). Finally, in order to improve the extraction yields of complexation agents, it is also possible to use an organic solvent such as dimethylformamide (DMF) (Surowiec 2008). Another method exploiting a complexing agent entails the use of oxalic acid in a mixture of oxalic acid/MeOH/acetone/H2O (1:30:30:40, v/v/v/v) (Guinot and Andary 2006; Manhita et al. 2011);
-
Organic solvent: pyridine, dimethylsulfoxide, or dimethylformamide (Kirby and White 1996; Wouters 1991) are commonly used, heating at maximum 100 °C for short times (Manhita et al. 2011).
2.5 Data Interpretation
The interpretation of chromatographic and mass spectrometric data relies on the identification of specific molecules, typical of organic dyes, based on their retention time, mass spectra and possibly spectroscopic properties (e.g. UV-Vis or fluorescence spectra). The unambiguous identification of the source of colour is a much more complex task, since minor components and in some cases even the ratio between different molecules can enable the distinction between different species, or synthetic routes. Thus, data can only be interpreted by comparing both the qualitative and semi-quantitative profile of the extract of an unknown sample with that of reference materials, possibly subjected to artificial ageing. Comparison with published data should be performed by keeping in mind that different extraction protocols may lead to slightly different results, thus extra caution is needed whenever using novel sample treatments. Finally, multivariate statistical data analysis can be successfully employed to discriminate between closely related materials, such as the dyes and organic pigments extracted from scale insects, as demonstrated by Serrano et al. in extensive research on cochineal (Serrano et al. 2011).
3 A Further Analytical Issue: Photo-Degradation of Coloured Compounds
Organic lakes and pigments are among the most labile materials used in works-of-art. They are coloured, which means that they absorb light in the UV-Visible range and are susceptible to photochemical reactions i.e. photo-oxidation processes that lead to a change in colour or even to bleaching. The photo-oxidation of synthetic organic pigments already heavily affects the appearance of modern and contemporary paintings; therefore, specific storage and display conditions need to be designed to minimize reactions leading to the degradation of chromophore-containing molecules. Thus, improving our comprehension of photooxidation chemical pathways and fading mechanism is the only way to develop suitable preventive conservation strategies.
The stability of materials to photo-oxidation and damages induced by light is defined as photostability (Anghelone et al. 2018). The absorption of photons of suitable energy by an organic molecule provides an electronically excited state, which is the starting point for the subsequent reactions (Weyermann et al. 2009). The presence of oxygen frequently accelerates the degradation via radical-initiated oxidation and only in a few cases it can retard dye-fading by re-oxidizing reduced molecules.
Artificial accelerated ageing of reference materials is commonly used to mimic and study the degradation process undergone by colorants and of other materials present in artworks. In most cases, accelerated ageing procedures are performed in order to assess rates of fading and identify the factors that lead to lightfastness. Accelerated ageing is based on the reciprocity principle: degradation is assumed to be proportional to net exposure calculated as the product of intensity of illuminance and exposition time (Feller 1994). There are many examples of deviations from the reciprocity principle in natural materials. Furthermore artificial accelerated ageing is not able to accurately reproduce natural ageing due to the impossibility to recreate the variety of different conditions an artwork might be exposed during its life (Weyermann and Spengler 2008). Nonetheless, artificial ageing is the only tool available to simulate photo-oxidation. The cross-checking between results collected from the analysis of artificially aged reference materials with historical samples is mandatory to validate the reliability of accelerated ageing procedures.
With regard to natural lake pigments, the most studied chromophores in terms of photo-stability are flavonoids (Colombini et al. 2007; Ferreira et al. 1999, 2002; Saunders and Kirby 1994); degradation products have been identified in aged specimens that were prepared in the laboratory and sampled from works of art. Hydroxybenzoic acids were detected in the extracts from aged specimens, the presence of which may be due to the photo-oxidation of the double bond C2-C3 of the flavonoids, leading to the formation of a depside. This latter is formed by condensation of two or more hydroxybenzoic acids whereby the carboxyl group of one molecule is esterified with a phenolic hydroxyl group of a second molecule. Cleavage also yields low-molecular mass products, such as dihydroxybenzoic acids and trihydroxybenzoic acids (Fig. 9.3). Some of these degradation products were identified in historical and archaeological samples as well (Ferreira et al. 2003; Zhang et al. 2007).
Suggested degradation pathway for morin: (a) oxidation catalysed by a metallic ion (Mn+) and (b) oxidation by atmospheric oxygen activated by light (O2, hν). The square and the circle evidence the fates of the two aromatic rings (Colombini et al. 2007)
Madder degradation has been extensively investigated, due to its wide occurrence in works of art (Ahn and Obendorf 2004; Clementi et al. 2007; Saunders and Kirby 1994). Ahn and co-workers worked on alizarin in water solution and identified possible degradation products such as phthalic acid and phtalic anhydride, and benzoic acid. However, Clementi and co-workers did not identify any degradation products after accelerated ageing of wool yarns dyed with madder.
Many studies have been carried out on the synthetic dyes and pigments characterization while few have been performed on their artificial ageing and in particular on the identification of the relative degradation compounds in paintings, drawings or historical textiles (Alvarez-Martin and Janssens 2018; Anghelone et al. 2018; Burnstock et al. 2005; Confortin et al. 2010, n.d.; Crews 1987; Dunn et al. 2003; Favaro et al. 2012; Ghelardi et al. 2015a; Weyermann et al. 2006, 2009). Extensive publications describe the detection of synthetic dyes in wastewaters along with remediation strategies, whereas only few discuss in detail the photodegradation pathways in solution (Pirok Bob et al. 2019a, b; Sabatini et al. 2021). Thus, the database of reference systems and case studies analysed needs to be enlarged in order to reveal degradation processes of SOPs.
4 Case Studies
In the previous section, the potential of chromatography-mass spectrometry and several of the anlytical issues involving the analysis of synthetic organic pigments were discussed. In this section, some case studies will be described to hightlight how chromatography-mass spectrometry allows the analyst to overcome analytical challenges and to produce data that can be used for authentication purposes.
4.1 Commercial Paint Materials: Database of Manufacturers
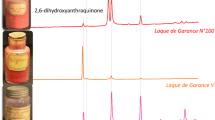
One of the main causes of confusion on the effective composition of commercial paint materials is the use of creative and non-informative names by the manufacturers, who even used the same name for defining formualtions with different compositions. An explicative example of this latter case is constituted by three red historical colorants from LeFranc Archive in Le Mans (France) and one paint tube, dated to the early twentieth century. The three powders were all labelled as Laque de Garance, while the paint tube was named Lac Fine. The containers of the powders and the paint tube are shown in Fig. 9.4, along with the chromatograms acquired on the extracts of microsamples (Degano et al. 2017).
HPLC-DAD chromatograms (extracted at 480 nm) of the extracts of one Lefranc paint tube and three powders (from the bottom to the top): Lac Fine (Gar. Andr.); Laque de Garance N°100; Laque de Garance N°101; Laque de Garance V (Degano et al. 2017)
In all the chromatograms a peak ascribable to alizarin was detected but, while in the paint tube alizarin was the main colorant, in the three powders it was present in variable amounts and together with other abundant coloured compounds. These species were identified by chromatography with high resolution tandem mass spectrometry (HPLC-ESI-Q-ToF) as 2,6-dihydroxyanthraquinone, flavopurpurin and a hydroxyanthraquinone (possibly 2-hydroxyanthraquinone), which are synthetic species and plausible by-products in the synthesis alizarin, as suggested on the basis of the process reported in (Degano et al. 2017; Fieser 1930). “Laque de Garance Number 100” also contains alizarin-9-imine, possibly formed by treating synthetic alizarin with ammonia. The qualitative and quantitative differences in composition of the three powders from the same period, manufacture and labelled with the same name highlight how important it is to characterise reference materials in order to build rich databases. At the same time, this example clarifies how extensive knowledge of commercial paint formulations may allow the scientist to trace specific manufacture on the basis of the profile collected for a sample from a disputed work of art. Therefore, this information can be correlated with the dating of the artwork and the information related to paint suppliers.
4.2 Commercial Paint Materials: Comparison of Yellow Formulations
Four case studies have reported the detection of Hansa Yellow pigments by chromatography with spectrophotometric and mass spectrometric detection (HPLC-DAD and HPLC-ESI-Q-ToF), in materials and works-of-art dating from the beginning of the twentieth century to 2003. The chromatographic profiles obtained for the extracts of a LeFranc green paint tube labelled as Vert Anglais N°3 (Degano et al. 2017), a yellow powder from Fernando Melani’s archive (1907–1985, yellow 3_Melani) (Carlesi et al. 2013), a red sample taken from the pop-art sculpture Disgelo by Piero Gilardi (1942) (La Nasa et al. 2019) and a yellow sample taken from the painting Quartet by Alex Harding (2003) (La Nasa et al. 2020) are provided in Fig. 9.5.
HPLC-DAD chromatograms (extracted at 400 nm) on the left, HPLC-ESI-Q-ToF Extract Ion Chromatograms (EIC) (acquired in negative ionization mode) of C17H16N4O4, C16H10N4O5, C16H11N3O3 and C16H12Cl2N4O4 of the extracts of atelier materials and artworks (from the bottom to the top): Vert Anglais N°3 (Degano et al. 2017), yellow 3_Melani (Carlesi et al. 2013), the painting Quartet (Alex Harding 2003) (La Nasa et al. 2020); the pop-art sculpture Disgelo (Piero Gilardi, 1942) (La Nasa et al. 2019)
The combined information provided by the two detectors disclosed the composition of these four formulations. Even if the four samples are coloured in different hues, the yellow base for all of them is a Hansa Yellows (PY1, PY1 isomer and PY3) suggesting a common trend to use these pigments together. Nevertheless, the relative amount of PY1 and PY3 differs in the samples suggesting different recipes that were possibly adapted on the basis of the other organic and/or inorganic pigments present in the formulations. In particular, the LeFranc tube, Quartet and Disgelo chromatograms are richer in PY3 while the yellow 3_Melani in PY1. Considering the good lightfastness of Hansa Yellow pigments and their stability in time, the ratio between these two pigments may be used to discriminate formulations of different paint manufactures detected in artworks. If the painter’s atelier materials are available, the evaluation of the authenticity of artworks may be performed by comparing not only the qualitative but also the semi-quantitative composition of the pigments identified.
4.3 Ageing and a Cautionary Tale
Unfortunately, the evaluation of the relative pigment ratio cannot be used as a reliable criterion for all classes of pigments. Many of them, such as triarylmethines and xanthenes, exhibit poor lightfastness, resulting in degradation and consequent modification of the chromatographic profiles. Methyl violet, a triarylmethine pigment, was one of the colours employed by Giulio Turcato to create the effect of a lunar surface in his painting Composizione-Superficie Lunare (1965) (Sabatini et al. 2020c). The artwork was overheated and damaged by fire. The HPLC-ESI-Q-ToF analysis of two samples collected from two areas of the painting suggested the use of methyl violet, even though different profiles were obtained: sample 1 shows lower amount of hexa-N-methyl pararosaniline (hexa MP) and an increase in intensity of the respective demethylated compounds (Fig. 9.6).
HPLC-ESI-Q-ToF Extract Ion Chromatograms (EIC) (acquired in positive ionization mode) of C19H18N3+, C20H20N3+, C21H22N3+, C22H24N3+, C23H26N3+, C24H28N3+ and C25H30N3+ of the extracts of two samples (1 and 2) taken from the painting Composizione-Superficie Lunare (Giulio Turcato, 1965) (Sabatini et al. 2020c)
Methyl violet is a complex mixture generally constituted by tetra, penta and hexa-N-methyl pararosaniline (tetra MP, penta MP, hexa MP, respectively) but many formulations were available that vary based on the synthetic strategy adopted. Moreover, the composition of methyl violet formulations might be altered by degradation, proceeding via demetylation (Confortin et al. 2010). Thus, the relative ratio of the different N-methyl pararosaniline products is not distinctive for a specific formulation. Finally, the only conclusion to be drawn for the present case study is that sample 1 is more degraded than sample 2.
4.4 Ageing and a Tale of Success
Artificial ageing of reference materials may be a reliable simulation of the natural ageing possibly occurring in an artwork, as demonstrated in the following case study. The drawing Montmajour by Vincent Van Gogh (1888) appears brownish but purple shades are present on the edges where the ink has been protected from light by the frame (Confortin et al. 2010). The purple ink resembled that used for drawings and letters produced in the same period by the artist, in which methyl violet had been already found. The positive matching between the chromatographic (HPLC-PDA) profile collected for aged model samples of historical crystal violet (mainly constituted by hexa-N-methyl pararosaniline) faded on paper (irradiated by UV light for 340 h) and a sample from a purple ink area of Montmajour (Fig. 9.7) gave evidence of the reliability of the model samples prepared, at the same time, validating the authenticity of the drawing.
HPLC-PDA chromatograms (extracted at 590 nm) of model sample of crystal violet faded on paper and of purple ink from the drawing Montmajour (Vincent Van Gogh 1888). Adapted from (Confortin et al. 2010)
5 Conclusions
The review of scientific publications and the case studies presented demonstrate the ability of chromatographic and mass spectrometric techniques to provide qualitative and even semi-quantitative information on the complex mixtures constituting organic lakes and pigments. Such detailed information, if corroborated by a comparison with a suitable set of reference materials, can be used to supplement other scientific data to refute a specific historical period, or identify paint materials or additions that may be consistent with a particular period or artist’s practice.
References
Abrahart EN. Dyes and Their Intermediates 1977
Ahn, C., Obendorf, S.K.: Dyes on archaeological textiles: analyzing alizarin and its degradation products. Text. Res. J. 74, 949–954 (2004). https://doi.org/10.1177/004051750407401102
Alvarez-Martin, A., Janssens, K.: Protecting and stimulating effect on the degradation of eosin lakes. Part 1: Lead white and cobalt blue. Microchem. J. 141, 51–63 (2018). https://doi.org/10.1016/j.microc.2018.05.005
Alvarez-Martin, A., Cleland, T.P., Kavich, G.M., Janssens, K., Newsome, G.A.: Rapid evaluation of the debromination mechanism of eosin in oil paint by direct analysis in real time and direct infusion-electrospray ionization mass spectrometry. Anal. Chem. 91, 10856–10863 (2019). https://doi.org/10.1021/acs.analchem.9b02568
Andreotti, A., Bonaduce, I., Colombini, M.P., Ribechini, E.: Characterisation of natural indigo and shellfish purple by mass spectrometric techniques. Rapid Commun. Mass Spectrom. (2004). https://doi.org/10.1002/rcm.1464
Andreotti, A., Colombini, M.P., Ribechini, E., D’Alessio, A., Frezzato, F.: The characterisation of red-violet organic colours in ancient samples. In: The Diversity of Dyes in History & Archaeology, pp. 97–106 (2017)
Anghelone, M., Stoytschew, V., Jembrih-Simbürger, D., Schreiner, M.: Spectroscopic methods for the identification and photostability study of red synthetic organic pigments in alkyd and acrylic paints. Microchem. J. 139, 155–163 (2018). https://doi.org/10.1016/j.microc.2018.02.029
Armitage, R.A., Jakes, K., Day, C.: Direct analysis in real time-mass spectroscopy for identification of red dye colourants in Paracas Necropolis Textiles. Sci. Technol. Archaeol. Res. 1, 60–69 (2015). https://doi.org/10.1179/2054892315Y.0000000009
Armitage, R.A., Fraser, D., Degano, I., Colombini, M.P.: The analysis of the Saltzman Collection of Peruvian dyes by high performance liquid chromatography and ambient ionisation mass spectrometry. Herit. Sci. 7, 81 (2019). https://doi.org/10.1186/s40494-019-0319-1
Astefanei, A., van Bommel, M., Corthals, G.L.: Surface acoustic wave nebulisation mass spectrometry for the fast and highly sensitive characterisation of synthetic dyes in textile samples. J. Am. Soc. Mass Spectrom. 28, 2108–2116 (2017). https://doi.org/10.1007/s13361-017-1716-x
Bacci, M., Casini, A., Picollo, M., Radicati, B., Stefani, L.: Integrated non-invasive technologies for the diagnosis and conservation of the cultural heritage. J. Neutron. Res. 14, 11–16 (2006). https://doi.org/10.1080/10238160600672930
Ball, P.: Bright Earth: Art and the Invention of Color. Straus, New York (2001)
Boon, J.J., Learner, T.: Analytical mass spectrometry of artists’ acrylic emulsion paints by direct temperature resolved mass spectrometry and laser desorption ionisation mass spectrometry. J. Anal. Appl. Pyrolysis. 64, 327–344 (2002). https://doi.org/10.1016/S0165-2370(02)00045-1
Boon, J.J., Hoogland, F.G., van der Horst, J.: Mass spectrometry of modern paints. In: Modern Paints Uncovered: Proceedings from the Modern Paints Uncovered Symposium (2007)
Bosi, A., Ciccola, A., Serafini, I., Guiso, M., Ripanti, F., Postorino, P., et al.: Street art graffiti: discovering their composition and alteration by FTIR and micro-Raman spectroscopy. Spectrochim Acta Part A Mol. Biomol. Spectrosc. 225, 117474 (2020). https://doi.org/10.1016/j.saa.2019.117474
Burnstock, A., Lanfear, I., van den Berg, K.J., Carlyle, L., Clarke, M., Hendriks, E., et al.: Comparison of the fading and surface deterioration of red lake pigments in six paintings by Vincent van Gogh with artificially aged paint reconstructions. In: ICOM Committee for Conservation 14th Triennial Meeting. Den Haag, p. 4569. James and James, London (2005)
Carlesi, S., Bartolozzi, G., Cucci, C., Marchiafava, V., Picollo, M.: The artists’ materials of Fernando Melani: a precursor of the Poor Art artistic movement in Italy. Spectrochim Acta Part A Mol. Biomol. Spectrosc. 104, 527–537 (2013). https://doi.org/10.1016/j.saa.2012.11.094
Carlesi, S., Bartolozzi, G., Cucci, C., Marchiafava, V., Picollo, M., La Nasa, J., et al.: Discovering “the Italian Flag” by Fernando Melani (1907-1985). Spectrochim Acta – Part A Mol. Biomol. Spectrosc. 168, 52–59 (2016). https://doi.org/10.1016/j.saa.2016.05.027
Casas-Catalán, M.J., Doménech-Carbó, M.T.: Identification of natural dyes used in works of art by pyrolysis-gas chromatography/mass spectrometry combined with in situ trimethylsilylation. In: Analytical and Bioanalytical Chemistry, vol. 382, pp. 259–268. Springer (2005). https://doi.org/10.1007/s00216-005-3064-0
Chieli, A., Romani, A., Degano, I., Sabatini, F., Tognotti, P., Miliani, C.: New insights into the fading mechanism of Geranium lake in painting matrix. New J. Chem. Della RCS. (2019) submitted
Christiansen, M.B., Baadsgaard, E., Sanyova, J., Simonsen, K.P.: The artists’ materials of P. S. Krøyer: an analytical study of the artist’s paintings and tube colours by Raman, SEM--EDS and HPLC. Herit. Sci. 5, 39 (2017). https://doi.org/10.1186/s40494-017-0153-2
Ciatti, M., Marini, P. (eds.): Andrea Mantegna. La Pala di San Zeno: studio e conservazione. Edifir, Firenze (2009)
Claro, A., Melo, M.J., de Melo, J.S.S., van den Berg, K.J., Burnstock, A., Montague, M., et al.: Identification of red colorants in van Gogh paintings and ancient Andean textiles by microspectrofluorimetry. J. Cult. Herit. 11, 27–34 (2010). https://doi.org/10.1016/j.culher.2009.03.006
Clementi, C., Nowik, W., Romani, A., Cibin, F., Favaro, G.: A spectrometric and chromatographic approach to the study of ageing of madder (Rubia tinctorum L.) dyestuff on wool. Anal. Chim. Acta. 596, 46–54 (2007). https://doi.org/10.1016/j.aca.2007.05.036
Colombini, M.P., Modugno, F.: Organic materials in art and archaeology. In: Colombini, M.P., Modugno, F. (eds.) Organic Mass Spectrometry in Art and Archaeology, pp. 1–36. Wiley (2009). https://doi.org/10.1002/9780470741917.ch1
Colombini, M.P., Carmignani, A., Modugno, F., Frezzato, F., Olchini, A., Brecoulaki, H., et al.: Integrated analytical techniques for the study of ancient Greek polychromy. Talanta. 63, 839–848 (2004). https://doi.org/10.1016/j.talanta.2003.12.043
Colombini, M.P.M.P., Andreotti, A., Baraldi, C., Degano, I., Łucejko, J.J.: Colour fading in textiles: a model study on the decomposition of natural dyes. Microchem. J. 85, 174–182 (2007). https://doi.org/10.1016/j.microc.2006.04.002
Colombini, M.P., Degano, I., Ribechini, E.: A multi analytical approach to determine Madder lake in a funerary clay vessel found in a chamber tomb in Taranto. In: Kirby (ed.) The Diversity of Dyes in History & Archaeology. Archetype, London (2017)
Colour index. https://colour-index.com/
Confortin, D., Neevel, H., Brustolon, M., Franco, L., Kettelarij, A.J., Williams, R.M., et al.: Crystal violet: study of the photo-fading of an early synthetic dye in aqueous solution and on paper with HPLC-PDA, LCMS and FORS. J. Phys. Conf. Ser. 231, 1–9 (2010)
Confortin, D., Neevel, H., Van Bommel, M., Reissland, B.: Study of the degradation of an early synthetic dye. Crystal Violet, 197–201 (n.d.)
Crews, P.C.: The fading rates of some natural dyes. Stud. Conserv. 32, 65–72 (1987). https://doi.org/10.1179/sic.1987.32.2.65
Cucci, C., Bartolozzi, G., De Vita, M., Marchiafava, V., Picollo, M., Casadio, F.: The colors of Keith Haring: a spectroscopic study on the materials of the mural painting Tuttomondo and on reference contemporary outdoor paints. Appl. Spectrosc. 70, 186–196 (2016). https://doi.org/10.1177/0003702815615346
De Keijzer, M.: The history of modern synthetic inorganic and organic artists’ pigments. In: Contributions to Conservation: Research in Conservation at the Netherlands Institute for Cultural Heritage (ICN Instituut Collectie Nederland), pp. 42–54. James & James, London (2002)
de Keijzer, M.: The delight of modern organic pigment creations. In: van den Berg, K.J., Burnstock, A., de Keijzer, M., Krueger, J., Learner, T., Tagle, A., et al. (eds.) Issues in Contemporary Oil Paint, pp. 45–73. Springer, Cham (2014). https://doi.org/10.1007/978-3-319-10100-2_4
Degani, L., Riedo, C., Gulmini, M., Chiantore, O.: From plant extracts to historical textiles: characterization of dyestuffs by GC–MS. Chromatographia. 77, 1683–1696 (2014). https://doi.org/10.1007/s10337-014-2772-z
Degano, I.: Liquid chromatography: current applications in heritage science and recent developments. Phys. Sci. Rev. 4, 20180009 (2019)
Degano, I., La Nasa, J.: Trends in high performance liquid chromatography for cultural heritage. Top. Curr. Chem. 374, 20 (2016). https://doi.org/10.1007/s41061-016-0020-8
Degano, I., Tognotti, P., Kunzelman, D., Modugno, F.: HPLC-DAD and HPLC-ESI-Q-ToF characterisation of early 20th century lake and organic pigments from Lefranc archives. Herit. Sci. 5, 7 (2017). https://doi.org/10.1186/s40494-017-0120-y
Doherty, B., Degano, I., Romani, A., Higgitt, C., Peggie, D., Colombini, M.P., et al.: Identifying Brazilwood’s marker component, Urolithin C, in historical textiles by surface-enhanced Raman spectroscopy. Heritage. 4, 1415–1428 (2021). https://doi.org/10.3390/heritage4030078
Dunn, J., Siegel, J., Allison, J.: Photodegradation and laser desorption mass spectrometry for the characterization of dyes used in red pen inks. Sci. J. Foren. 48, 652 (2003)
Evans, D.G.: The Color Index international papers for 1992. J. Soc. Dye. Colour. 106, 192–193 (1990)
Fabbri, D., Chiavari, G., Ling, H.: Analysis of anthraquinoid and indigoid dyes used in ancient artistic works by thermally assisted hydrolysis and methylation in the presence of tetramethylammonium hydroxide. J. Anal. Appl. Pyrolysis. 56, 167–178 (2000). https://doi.org/10.1016/S0165-2370(00)00092-9
Favaro, G., Confortin, D., Pastore, P., Brustolon, M.: Application of LC-MS and LC-MS-MS to the analysis of photo-decomposed crystal violet in the investigation of cultural heritage materials aging. J. Mass Spectrom. 47, 1660–1670 (2012). https://doi.org/10.1002/jms.3110
Feller, R.L.: Accelerated Aging-Photochemical and Thermal Aspects. The Getty Conservation Institute (1994)
Ferreira, E., Quye, A., McNab, H., Hulme, A., Wouters, J., Boon, J.J.: The analytical characterisation of flavonoid photodegradation products: a novel approach to identifying natural yellow dyes in ancient textiles. ICOM-CC 12th Trienn. Meet. Lyon. 1, 221–227 (1999)
Ferreira, E.S., Quye, A., McNab, H., Hulme, A.N.: Photo-oxidation products of quercetin and morin as markers for the characterisation of natural flavonoid yellow dyes in ancient textiles. Dye Hist. Archaeol. 18, 63–72 (2002)
Ferreira, E., Quye, A., Hulme, A., McNab, H.: LC-Ion trap MS and PDA HPLC complementary techniques in the analysis of flavonoid dyes in historical textiles: the case study of an 18th century herald’s tabard. Dye. 19, 13–18 (2003)
Fieser, L.: The dicovery of synthetic alizarin. J. Chem. Educ. 7, 2609–2633 (1930)
Garfield, S.: Purple Patch. Faber, London (2011)
Gautier, G., Bezur, A., Muir, K., Casadio, F., Fiedler, I.: Chemical fingerprinting of ready-mixed house paints of relevance to artistic production in the first half of the twentieth century. Part I: inorganic and organic pigments. Appl. Spectrosc. 63, 597–603 (2009). https://doi.org/10.1366/000370209788559584
Geldof, M., de Keijzer, M., van Bommel, M.R., Pilz, K., Salvant, J., van Keulen, H., et al.: Van Gogh’s geranium lake. In: Vellekoop, M., Muriel, G., Hendriks, E., Jansen, L., de Tagle, A. (eds.) Van Gogh’s Studio Practice, pp. 268–289. Yale University/Mercatorfonds, Amsterdam/Brussels (2013)
Germinario, G., Rigante, E.C.L.L., van der Werf, I.D., Sabbatini, L.: Pyrolysis gas chromatography–mass spectrometry of triarylmethane dyes. J. Anal. Appl. Pyrolysis. 127, 229–239 (2017). https://doi.org/10.1016/j.jaap.2017.08.001
Ghelardi, E., Degano, I., Modugno, F., Colombini, M.P.: An integrated approach to the study of Ri de pomme, a painting by Julian Schnabel. Int. J. Conserv. Sci. 6, 51–62 (2015)
Ghelardi, E., Degano, I., Colombini, M.P., Mazurek, J., Schilling, M., Khanjian, H., et al.: A multi-analytical study on the photochemical degradation of synthetic organic pigments. Dyes Pigments. 123, 396–403 (2015a). https://doi.org/10.1016/j.dyepig.2015.07.029
Ghelardi, E., Degano, I., Colombini, M.P., Mazurek, J., Schilling, M., Learner, T.: Py-GC/MS applied to the analysis of synthetic organic pigments: characterization and identification in paint samples. Anal. Bioanal. Chem. 407, 1415–1431 (2015b). https://doi.org/10.1007/s00216-014-8370-y
Graebe, G., Liebermann, C.: L’alizarine artificielle. Montieur. Sci. 21, 394 (1879)
Grazia, C., Buti, D., Amat, A., Rosi, F., Romani, A., Domenici, D., et al.: Shades of blue: non-invasive spectroscopic investigations of Maya blue pigments. From laboratory mock-ups to Mesoamerican codices. Herit. Sci. 8, 1 (2020). https://doi.org/10.1186/s40494-019-0345-z
Guinot, P., Andary, C.: Molecules involved in the dyeing process with flavonoids. Dye Hist. Archaeol. 25, 21–22 (2006)
Halpine, S.M.: An improved dye and lake pigment analysis method for high-performance liquid chromatography and diode-array detector. Stud. Conserv. 41, 76–94 (1996). https://doi.org/10.2307/1506519
Herbst, W., Hunger, K., Wilker, G., Ohleier, H., Winter, R.: Industrial Organic Pigments, 3rd edn. Wiley (2004). https://doi.org/10.1002/3527602429
Herndon, W.C.: Resonance theory and the enumeration of Kekule structures. J. Chem. Educ. 51, 10 (1974). https://doi.org/10.1021/ed051p10
Karapanagiotis, I., Sotiropoulou, S., Chryssikopoulou, E., Magiatis, P., Andrikopoulos, K.S., Chryssoulakis, Y.: Investigation of Tyrian purple occurring in prehistoric wall paintings of Thera. In: The Diversity of Dyes in History & Archaeology, pp. 82–89 (2017)
Kirby, J., White, R.: The identification of red lake pigment dyestuffs and a discussion of their use. Natl. Gall. Tech. Bull. 17, 56–80 (1996)
Kirby, J., Spring, M., Higgitt, C.: The technology of red lake pigment manufacture: study of the dyestuff substrate. Natl. Gall. Tech. Bull. 26, 71–87 (2005a)
Kirby, J., Townsend, K.H., Stijnman, A.: The reconstruction of late 19th-century French red lake pigments. In: Archetype Publications (ed.) Art of the Past – Sources and Reconstructions, p. 69. Archetype Publications, London (2005b)
Kirby, J., Spring, M., Higgitt, C.: The technology of eighteenth- and nineteenth-century red lake pigments. Natl. Gall. Tech. Bull. 28, 69–87 (2007)
Kirby, D.P., Khandekar, N., Sutherland, K., Price, B.A.: Applications of laser desorption mass spectrometry for the study of synthetic organic pigments in works of art. Int. J. Mass Spectrom. 284, 115–122 (2009). https://doi.org/10.1016/j.ijms.2008.08.011
Kirby, J., van Bommel, M.R., Verhecken, A.: Natural Colorants for Dyeing and Lake Pigments: Practical Recipes and Their Historical Sources. Archetype Publication, London (2014)
La Nasa, J., Biale, G., Sabatini, F., Degano, I., Colombini, M.P., Modugno, F.: Synthetic materials in art: a new comprehensive approach for the characterization of multi-material artworks by analytical pyrolysis. Herit. Sci. 7, 8 (2019). https://doi.org/10.1186/s40494-019-0251-4
La Nasa, J., Nodari, L., Nardella, F., Sabatini, F., Degano, I., Modugno, F., et al.: Chemistry of modern paint media: the strained and collapsed painting by Alexis Harding. Microchem. J. 155, 104659 (2020). https://doi.org/10.1016/J.MICROC.2020.104659
La Nasa, J., Campanella, B., Sabatini, F., Rava, A., Shank, W., Lucero-Gomez, P., et al.: 60 years of street art: a comparative study of the artists’ materials through spectroscopic and mass spectrometric approaches. J. Cult. Herit. 48, 129–140 (2021). https://doi.org/10.1016/j.culher.2020.11.016
Learner, T.J.S.: Analysis of Modern Paints. The Getty (2005)
Learner, T.J.S., Smithen, P., Krueger, J.W., Schilling, M.R.: Modern Paints Uncovered Symposium. Tate Modern, London (2006)
Lech, K., Wilicka, E., Witowska-Jarosz, J., Jarosz, M.: Early synthetic dyes – a challenge for tandem mass spectrometry. J. Mass Spectrom. 48, 141–147 (2013). https://doi.org/10.1002/jms.3090
Lomax, S.Q.L.T., Lomax, S.Q., Learner, T.: A review of the classes, structures, and methods of analysis of synthetic organic pigments. J. Am. Inst. Conserv. 45, 107–125 (2006). https://doi.org/10.1179/019713606806112540
Lomax, S., Schilling, M., Learner, T.: The Identification of Synthetic Organic Pigments by FTIR and DTMS. Mod. Paint. Uncovered (2007)
Lomax, S.Q., Lomax, J.F., De Luca-Westrate, A.: The use of Raman microscopy and laser desorption ionization mass spectrometry in the examination of synthetic organic pigments in modern works of art. J. Raman Spectrosc. 45, 448–455 (2014). https://doi.org/10.1002/jrs.4480
Lutzenberger, Stege, H.: From Beckmann to Baselitz-towards an improved micro-identification of organic pigments in paintings of 20th century art. Preserv. Sci. 6, 89–100 (2009)
Magrini, D., Bracci, S., Cantisani, E., Conti, C., Rava, A., Sansonetti, A., et al.: A multi-analytical approach for the characterization of wall painting materials on contemporary buildings. Spectrochim Acta Part A Mol. Biomol. Spectrosc. 173, 39–45 (2017). https://doi.org/10.1016/j.saa.2016.08.017
Manhita, A., Ferreira, T., Candeias, A., Barrocas, D.C.: Extracting natural dyes from wool-an evaluation of extraction methods. Anal. Bioanal. Chem. 400, 1501–1514 (2011). https://doi.org/10.1007/s00216-011-4858-x
Mayhew, H.E., Fabian, D.M., Svoboda, S.A., Wustholz, K.L.: Surface-enhanced Raman spectroscopy studies of yellow organic dyestuffs and lake pigments in oil paint. Analyst. 138, 4493–4499 (2013). https://doi.org/10.1039/c3an00611e
Meister Lucius & Brüning. DRP 257 488 1909
Menke, C.A., Rivenc, R., Learner, T.: The use of direct temperature-resolved mass spectrometry (DTMS) in the detection of organic pigments found in acrylic paints used by Sam Francis. Int. J. Mass Spectrom. 284, 2–11 (2009). https://doi.org/10.1016/j.ijms.2008.12.006
Moretti, P., Germinario, G., Doherty, B., van der Werf, I.D., Sabbatini, L., Mirabile, A., et al.: Disclosing the composition of historical commercial felt-tip pens used in art by integrated vibrational spectroscopy and pyrolysis-gas chromatography/mass spectrometry. J. Cult. Herit. 35, 242–253 (2019). https://doi.org/10.1016/j.culher.2018.03.018
Padfield, J.: Mild Extraction Methods for Organic Colorant Analysis, A Bibliographic Reference Database. Charisma Project Website, Natl Gall Sci Dep (http://research.ng-london.org.uk/scientific/colourant/)
Pirok Bob, W.J., Moro, G., Meekel, N., SVJ, B., Schoenmakers, P.J., van Bommel, M.R.: Mapping degradation pathways of natural and synthetic dyes with LC-MS: influence of solvent on degradation mechanisms. J. Cult. Herit. (2019a). https://doi.org/10.1016/j.culher.2019.01.003
Pirok Bob, W.J., den Uijl, M.J., Moro, G., Berbers, S.V.J., Croes, C.J.M., van Bommel, M.R., et al.: Characterization of dye extracts from historical cultural-heritage objects using state-of-the-art comprehensive two-dimensional liquid chromatography and mass spectrometry with active modulation and optimized shifting gradients. Anal. Chem. 91, 3062–3069 (2019b). https://doi.org/10.1021/acs.analchem.8b05469
Pirok, B.W.J.J., Knip, J., van Bommel, M.R., Schoenmakers, P.J.: Characterization of synthetic dyes by comprehensive two-dimensional liquid chromatography combining ion-exchange chromatography and fast ion-pair reversed-phase chromatography. J. Chromatogr. A. 1436, 141–146 (2016). https://doi.org/10.1016/j.chroma.2016.01.070
Poulin, J.: A new methodology for the characterisation of natural dyes on museum objects using gas chromatography–mass spectrometry. Stud. Conserv. 63, 36–61 (2018). https://doi.org/10.1080/00393630.2016.1271097
Pozzi, F., Basso, E., Centeno, S.A., Smieska, L.M., Shibayama, N., Berns, R., et al.: Altered identity: fleeting colors and obscured surfaces in Van Gogh’s Landscapes in Paris, Arles, and Saint-Rémy. Herit. Sci. 9, 15 (2021). https://doi.org/10.1186/s40494-021-00489-1
Read, R.: Q and A: picric acid. Chem. Aust. (2014)
Rehorek, A., Plum, A.: Characterization of sulfonated azo dyes and aromatic amines by pyrolysis gas chromatography/mass spectrometry. Anal. Bioanal. Chem. 388, 1653–1662 (2007). https://doi.org/10.1007/s00216-007-1390-0
Restivo, A., Degano, I., Ribechini, E., Colombini, M.P.: Development and optimisation of an HPLC-DAD-ESI-Q-ToF method for the determination of phenolic acids and derivatives. PLoS One. 9, e88762 (2014). https://doi.org/10.1371/journal.pone.0088762
Russell, J., Singer, B.W., Perry, J.J., Bacon, A.: The identification of synthetic organic pigments in modern paints and modern paintings using pyrolysis-gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 400, 1473–1491 (2011). https://doi.org/10.1007/s00216-011-4822-9
Sabatini, F., Lluveras-Tenorio, A., Degano, I., Kuckova, S., Krizova, I., Colombini, M.P.: A matrix-assisted laser desorption/ionization time-of-flight mass spectrometry method for the identification of anthraquinones: the case of historical lakes. J. Am. Soc. Mass Spectrom. 27 (2016). https://doi.org/10.1007/s13361-016-1471-4
Sabatini, F., Giugliano, R., Degano, I.: Photo-oxidation processes of Rhodamine B: a chromatographic and mass spectrometric approach. Microchem. J. 140, 114–122 (2018). https://doi.org/10.1016/j.microc.2018.04.018
Sabatini, F., Degano, I., Colombini, M.P.: Development of a method based on high performance liquid chromatography coupled with diode array, fluorescence and mass spectrometric detectors for the analysis of eosin at trace levels. Sep. Sci. Plus. 3, 207–215 (2020a). https://doi.org/10.1002/sscp.202000002
Sabatini, F., Eis, E., Degano, I., Thoury, M., Bonaduce, I., Lluveras-Tenorio, A.: The issue of eosin fading: a combined spectroscopic and mass spectrometric approach applied to historical lakes. Dyes Pigments. 180, 108436 (2020b). https://doi.org/10.1016/j.dyepig.2020.108436
Sabatini, F., Manariti, A., Di Girolamo, F., Bonaduce, I., Tozzi, L., Rava, A., et al.: Painting on polyurethane foam: “Composizione-Superficie Lunare” by Giulio Turcato. Microchem. J. 156, 104872 (2020c). https://doi.org/10.1016/j.microc.2020.104872
Sabatini, F., Degano, I., Bommel, M.: Investigating the in-solution photodegradation pathway of Diamond Green G by chromatography and mass spectrometry. Color. Technol., cote.12538 (2021). https://doi.org/10.1111/cote.12538
Saez, N.O., Vanden, B.I., Schalm, O., De Munck, B., Caen, J.: Material analysis versus historical dye recipes: ingredients found in black dyed wool from five Belgian archives (1650-1850). Conserv. Patrim. 31, 115–132 (2019). https://doi.org/10.14568/cp2018025
Sanyova, J.: Mild extraction of dyes by hydrofluoric acid in routine analysis of historical paint micro-samples. Microchim. Acta. 162, 361–370 (2008). https://doi.org/10.1007/s00604-007-0867-z
Sanyova, J., Reisse, J.: Development of a mild method for the extraction of anthraquinones from their aluminum complexes in madder lakes prior to HPLC analysis. J. Cult. Herit. 7, 229–235 (2006)
Saunders, D., Kirby, J.: Light-induced colour changes in red and yellow lake pigments. Natl. Gall. Tech. Bull. 15, 79–97 (1994). https://doi.org/10.1017/CBO9781107415324.004
Schulte, F., Brzezinka, K.W., Lutzenberger, K., Stege, H., Panne, U.: Raman spectroscopy of synthetic organic pigments used in 20th century works of art. J. Raman Spectrosc. 39, 1455–1463 (2008). https://doi.org/10.1002/jrs.2021
Serrano, A., Sousa, M.M., Hallett, J., Lopes, J.A., Oliveira, M.C.: Analysis of natural red dyes (cochineal) in textiles of historical importance using HPLC and multivariate data analysis. Anal. Bioanal. Chem. 401, 735–743 (2011). https://doi.org/10.1007/s00216-011-5094-0
Serrano, A., Van Bommel, M., Hallett, J.: Evaluation between ultrahigh pressure liquid chromatography and high-performance liquid chromatography analytical methods for characterizing natural dyestuffs. J. Chromatogr. A. 1318, 102–111 (2013). https://doi.org/10.1016/j.chroma.2013.09.062
Skelton, H.: A colour chemist’s history of Western art. Rev. Prog. Color. Relat. Top. 29, 43–64 (1999). https://doi.org/10.1111/j.1478-4408.1999.tb00127.x
Soltzberg, L.J., Hagar, A., Kridaratikorn, S., Mattson, A., Newman, R.: MALDI-TOF mass spectrometric identification of dyes and pigments. J. Am. Soc. Mass Spectrom. 18, 2001–2006 (2007). https://doi.org/10.1016/j.jasms.2007.08.008
Sonoda, N.: Characterization of organic azo-pigments by pyrolysis–gas chromatography. Stud. Conserv. 44, 195–208 (1999). https://doi.org/10.1179/sic.1999.44.3.195
Stenger, J., Kwan, E.E., Eremin, K., Speakman, S., Kirby, D., Stewart, H., et al.: Lithol red salts: characterization and deterioration. E-PreservationScience. 7, 147–157 (2010)
Surowiec, I.: Application of high-performance separation techniques in archaeometry. Microchim. Acta. 162, 289–302 (2008). https://doi.org/10.1007/s00604-007-0911-z
Surowiec, I., Orska-Gawryś, J., Biesaga, M., Trojanowicz, M., Hutta, M., Halko, R., et al.: Identification of natural dyestuff in archeological Coptic textiles by HPLC with fluorescence detection. Anal. Lett. 36, 1211–1229 (2003). https://doi.org/10.1081/AL-120020154
Szostek, B., Orska-Gawrys, J., Surowiec, I., Trojanowicz, M.: Investigation of natural dyes occurring in historical Coptic textiles by high-performance liquid chromatography with UV-Vis and mass spectrometric detection. J. Chromatogr. A. 1012, 179–192 (2003). https://doi.org/10.1016/S0021-9673(03)01170-1
Taujenis, L., Olšauskaite, V.: Identification of main constituents of historical textile dyes by ultra performance liquid chromatography with photodiode array detection. Chemija. 23, 210–215 (2012)
Townsend, J.H.: Whistler’s oil painting materials. Burlingt. Mag. 136, 690–695 (1994)
Troalen, L.G., Phillips, A.S., Peggie, D.A., Barran, P.E., Hulme, A.N.: Historical textile dyeing with Genista tinctoria L.: a comprehensive study by UPLC-MS/MS analysis. Anal. Methods. 6, 8915–8923 (2014). https://doi.org/10.1039/C4AY01509F
van Bommel, M.R.: The analysis of dyes with HPLC coupled to photodiode array and fluorescence detection. Dye. Hist. Archaeol. 20, 30–38 (2005)
Van Bommel, M.R., Gedolf, M., Hendriks, E.: An investigation of organic red pigments used in paintings by Vincent Van Gogh. ArtMatters. 3, 111–137 (2005)
van Bommel, M.R., Vanden Berghe, I., Wallert, A.M., Boitelle, R., Wouters, J.: High-performance liquid chromatography and non-destructive three-dimensional fluorescence analysis of early synthetic dyes. J. Chromatogr. A. 1157, 260–272 (2007). https://doi.org/10.1016/j.chroma.2007.05.017
Weyermann, C., Spengler, B.: The potential of artificial aging for modelling of natural aging processes of ballpoint ink. Forensic Sci. Int. 180, 23–31 (2008). https://doi.org/10.1016/j.forsciint.2008.06.012
Weyermann, C., Kirsch, D., Costa-Vera, C., Spengler, B.: Photofading of ballpoint dyes studied on paper by LDI and MALDI MS. J. Am. Soc. Mass Spectrom. 17, 297–306 (2006). https://doi.org/10.1016/j.jasms.2005.11.010
Weyermann, C., Kirsch, D., Vera, C.C., Spengler, B.: Evaluation of the photodegradation of crystal violet upon light exposure by mass spectrometric and spectroscopic methods. J. Forensic Sci. 54, 339–345 (2009). https://doi.org/10.1111/j.1556-4029.2008.00975.x
Wouters, J.: High performance liquid chromatography of anthraquinones: analysis of plant and insect extracts and dyed textiles. Stud. Conserv. 30, 119–128 (1985). https://doi.org/10.1179/sic.1985.30.3.119
Wouters, J.: A new method for the analysis of blue and purple dyes in textiles. Dye Hist. Archaeol. 10, 17–21 (1991)
Wouters, J., Grzywacz, C.M., Claro, A.: Markers for identification of faded safflower (Carthamus tinctorius L.) colorants by HPLC-PDA-MS – ancient fibres, pigments, paints and cosmetics derived from antique recipes. Stud. Conserv. 55, 186–203 (2010). https://doi.org/10.1179/sic.2010.55.3.186
Zaffino, C., Passaretti, A., Poldi, G., Fratelli, M., Tibiletti, A., Bestetti, R., et al.: A multi-technique approach to the chemical characterization of colored inks in contemporary art: the materials of Lucio Fontana. J. Cult. Herit. 23, 87–97 (2017). https://doi.org/10.1016/j.culher.2016.09.006
Zhang, X., Laursen, R.A.: Development of mild extraction methods for the analysis of natural dyes in textiles of historical interest using LC-diode array detector-MS. Anal. Chem. 77, 2022–2025 (2005). https://doi.org/10.1021/ac048380k
Zhang, X., Boytner, R., Cabrera, J.L., Laursen, R.: Identification of yellow dye types in Pre-Columbian Andean Textiles. Anal. Chem. 79, 1575–1582 (2007). https://doi.org/10.1021/ac061618f
Zollinger, H.: Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments. Wiley (2003)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sabatini, F., Degano, I. (2022). Analysis of Natural and Synthetic Organic Lakes and Pigments by Chromatographic and Mass Spectrometric Techniques. In: Colombini, M.P., Degano, I., Nevin, A. (eds) Analytical Chemistry for the Study of Paintings and the Detection of Forgeries. Cultural Heritage Science. Springer, Cham. https://doi.org/10.1007/978-3-030-86865-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-86865-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86864-2
Online ISBN: 978-3-030-86865-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)