Abstract
Sixteen sulfonated and unsulfonated azo dyes as well as eleven sulfonated and unsulfonated aromatic amines were analyzed and qualitatively characterized by means of pyrolysis gas chromatography/mass spectrometry at different temperatures. Aniline and aminonaphthalene were found to be the dominant pyrolysis products of sulfonated aromatic amines and dyes. Azo dye and dye class specific key compounds such as benzidine, vinyl-p-base and 4-aminoazobenzene could be identified by pyrolysis gas chromatography/mass spectrometry of commercial acid, cationic, direct, reactive and solvent dyes. 500 °C was the optimal pyrolysis temperature for most of the pyrolyzed compounds. The method was applied to a dried sample of a textile wastewater concentrate from a dyeing process. Reactive azo dyes of the group of Remazol dyes and anthraquinone dyes could be identified as the major compounds of the sample. The finding of caprolactam (a printing additive) suggests that the wastewater contained effluent from a process of heat-activated printing with reactive dyes. p-Chloraniline, a banned aromatic amine, was identified. Chemical reduction of the wastewater sample prior to pyrolysis resulted in the release of volatile aromatic amines and aided the classification of several products of pyrolysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More than 10,000 dyes with different chemical properties are listed in the Color Index [1]. 112 azo dyes that can be cleaved to release carcinogenic amines as defined by Directive 2002/61/EC of the European Parliament are still on the market around the world [2, 3]. The global production of textile dyes was believed to exceed 1 million tons in 2003 [4]. Colored wastewater resulting from the loss of up to 40% of the dye during the dyeing process is passed to wastewater treatment plants or into the environment [5]. A universal analytical method is required that is able to classify unknown dye samples—a complicated task due to the large number of chemically different dyes in several dye classes. Liquid chromatographic techniques like HPLC and TLC are commonly used to identify sulfonated azo dyes in production and environmental studies because sulfonated compounds are known to be nonvolatile. However, the identification of sulfonated azo dyes requires extensive data libraries and a series of chromatographic separation methods due to the wide range of chemical properties of azo dyes. Standard analysis methods that could be used to assign unknown dye samples are required in production analysis, environmental research, archeometry and forensic science but are yet to be developed. Pyrolysis gas chromatography/mass spectrometry (Py-GC–MS) is employed for the identification and characterization of nonvolatile organic materials in industrial production, environmental and artistic object samples [6, 7]. In 1995, DeFelippis et al. were the first to pyrolyze pure azo dyes at 750 °C on a pyro-probe ribbon [8]. It was found that thermal cleavage of the azo bonds and scission of nonvolatile substituents releases volatile aromatic amines from the azo dye under pyrolysis. Extensive characterization of synthetic azo-pigments by Py-GC–MS was performed by Sonoda in 1999 [9]. Pyrolysates of monoazo pigments contained both the coupling and diazo components. Diazo pigments decomposed in the same way but did not yield any fragments characteristic of the diazo component. Detection of all pyrolysis products from real paint samples was very difficult because of their extremely low concentrations in the paint formulation [9]. Anthraquinoid and indigoid dyes can be analyzed by thermally assisted hydrolysis and methylation in real samples [10]. In [11] we described the identification of banned aromatic amines released from azo dyes on different textiles by Py-GC–MS.
Another class of nonvolatile sulfonated aromatics, linear alkylbenzenesulfonates, was studied by GC–MS via the formation of sulfonyl chlorides [12–15]. Extraction, cleaning and derivatization require a time-consuming sample preparation procedure. Py-GC–MS was used for the quantitative determination of chloroligno sulfonic acids in water samples for environmental analysis purposes via identification of the thermally released chloroguaiacyl isomers [16, 17]. Most azo dyes are made nonvolatile by giving them large molecular weights—up to 1,000 Da—and by sulfonate substitution. Most azo dyes are made water-soluble by adding one to four sulfonic acid groups. The degree of sulfonation depends upon the technical properties of the dye. Most basic and solvent azo dyes do not contain sulfonic acid groups.
In this work, attempts were made to release volatile compounds such as aromatic amines from twenty-seven nonvolatile sulfonated azo dyes and aromatic amines as well as unsulfonated volatile reactants in dye synthesis using pyrolysis (see Table 1, Fig. 1). The pyrolysate was separated by GC and analytes were detected by mass spectrometry. Mass spectra of primary pyrolysis products were analyzed by commercial MS data libraries. The investigation of pyrolysis products from azo dyes was carried out in order to identify typical key compounds of such dyes, so that these compounds could be used as identifiers for dyes of several dye classes. We focused on the pyrolysis of pure azo dyes, including banned aromatic amines such as Direct Brown 1, Direct Blue 151, Solvent Red 23 and Solvent Red 26. The method was applied to dried wastewater samples from an unknown dyeing process in order to screen the pyrolysate for dye identifiers.
A limited selection of figures are shown below due to the fact that an in-depth description of the complete data investigation on the pyrolysis of azo dyes, sulfonated and unsulfonated aromatic amines would make for an excessively long paper. Additional data can be obtained upon request from the authors.
Experimental data
Chemicals and equipment
Acid, basic, direct and solvent dyes were purchased from Sigma–Aldrich (Munich, Germany). Reactive dyes and reactants were kindly provided by DyStar (Leverkusen, Germany). A complete list of all of the compounds investigated is presented in Table 1, and Fig. 1 illustrates their structures. Unfortunately, information on the purities of most of the dyes used was rather poor: they were simply described as . A 20-fold concentrate of unknown content comprising a mixture of several dyebaths from a textile dyeing process was kindly provided by Enviro Chemie (Rossdorf, Germany).
The pyrolyser, chromatograph and detection system, all from Shimadzu (Duisburg, Germany), comprised a Pyr-4A continuous mode furnace pyrolyser, a GC-17A capillary gas chromatograph with AFC-17 flow controller, and a QP5050 quadrupole mass spectrometer detector. An HT8 (L = 25 m, 0.22 mm i.d., d f = 0.25 μm) capillary film column from SGE (Ringwood, VIC, Australia) and helium was used for chromatographic separation. All data procedures were performed by GCMSsolution software from Shimadzu, run on a personal computer connected to the analytical system.
Measuring conditions and analytical procedure
The temperature of the pyrolyser varied between 300 and 800 °C during the experiments, which were performed with a split ratio of 1:106. The liquid standard injection and pyrolysis were performed at a column flow of 0.7 mL min−1, which corresponds to a linear velocity of 33.2 cm s−1 (at initial temperature) at a column inlet pressure of p i = 65.3 kPa. The column oven temperature was programmed from 100 °C (1 min hold) to 320 °C at a heating rate of 30 °C min−1 (4 min hold). The column inlet pressure was programmed from 65.3 kPa (1 min hold) to 120 kPa (4 min hold) at a rate of 7.4 kPa min−1. Mass spectra were recorded at a frequency of 125 scans min−1 under electron impact ionization of 70 eV, with a scan range of m/z 40–305. The interface (end of the capillary end:ion source) was kept at 320 °C. Samples were weighed (0.5 mg) within the pyrolysis crucible, which consisted of platinum.
Results and discussion
Sulfonated aromatic amines
Aminohydroxynaphthalene sulfonic acids are better known as letter acids in the dye industry. A selection of aminohydroxynaphthalene sulfonic acids and aromatic sulfonic acids, which are used as reactants in several azo dye syntheses, were pyrolyzed (for the structures of these compounds, see Fig. 1). Aniline, aminonaphthalene and sulfur (S8) were found to be major volatile pyrolysis products. The sulfonated aromatic amines which were subjected to pyrolysis are listed in Table 1 (#17, #19–22, #26–27).
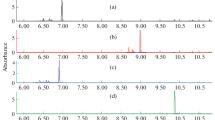
The release and formation of volatile compounds from sulfonated aromatics can be observed provided that desulfonation has taken place as a first step. Figure 2 shows the structures of the molecules released by the pyrolysis of sulfonated as well as unsulfonated aromatic amines. The radical formed should abstract a hydrogen atom to produce volatile aromatic amines such as aniline. When interpreting the processes, it should be noted that some of the radicals may be lost due to irreversible adsorption on surfaces or on the solid residue of the material. Moreover, when more than one hydrogen atom is abstracted from several molecules, they could combine into a solid residue of high molecular mass, which remains in the pyrolyser. The chromatogram of sulfanilic acid in Fig. 2 indicates that this recombination of two radicals was negligible.
The aminobenzene isomers sulfanilic acid, metanilic acid and orthanilic acid yielded the most aniline under pyrolysis [Fig. 2 presents a pyrogram of sulfanilic acid (SA), other data not shown]. While sulfanilic acid exhibited a maximum at 500 °C, metanilic acid and orthanilic acid required a pyrolysis temperature of 800 °C to release the maximum amounts of aniline. The desulfonation and release of aniline depends on the pyrolysis temperature, due to the position of the sulfonic acid group (ortho, meta, para). Due to a lower optimal pyrolysis temperature, desulfonation at the para position seems to take place at a lower energetic level than in the ortho or meta positions. This can be explained by the fact that the weakest bond breaks first under pyrolysis [6]. However, the highest absolute yield of aniline in relation to the weighted amount of sample was observed for orthanilic acid.
The amount of aminonaphthalene from letter acids such as H-acid, C-acid and I-acid released depends less on the degree of sulfonation than on the degree of substitution with other functional groups, such as amino and hydroxy groups (data not shown). Even disulfonated aminonaphthalenes such as C-acid show characteristic amounts of aminonaphthalene. It was observed that the release of aminonaphthalene diminished with an additional hydroxyl group (I-acid), and was not detectable from the highly substituted H-acid.
Sulfur (S8) was detected under pyrolysis at temperatures of >650 °C from H-acid and I-acid. The observed formation of aminonaphthalene from I-acid was negligible in relation to the release of other volatile compounds (data not shown); this may be due to the presence of impurities, or it may happen at >650 °C, when evolution of CO begins to occur because the OH-bearing aromatic ring is broken. This CO can reduce other hydroxyl groups [18]. It seems that the desulfonation product (sulfur dioxide) is also reduced to elemental sulfur, yielding S8. The observation that S8 was detected only when the compound contained a phenolic hydroxyl group (H-acid, I-acid) confirms the supposition that CO was the reducing agent. It was not detected upon the pyrolysis of any azo dye comprising phenolic hydroxyl and sulfonic groups.
A comparison of C-acid, H-acid and I-acid showed that the formation of volatile pyrolysates from letter acids is strongly influenced by further substitutions. Desulfonation and abstraction of a hydrogen atom to produce a volatile aromatic amine seems to be enhanced if the molecule does not comprise hydroxyl groups. Investigations on azo dyes which contain pyrolyzed sulfonated aromatic amines show that azo bond substitution of letter acids leads to a further reduction in the amount of characteristic volatile products as well as a reduction in the absolute intensity in relation to the weighted amount of sample. The number of nonspecific compounds formed upon the pyrolysis of aminohydroxynaphthalenes is larger than the number released upon the pyroloysis of aminonaphthalene (for instance CO2, SO2, benzene, styrene, naphthalene).
Acid dyes
Both acid dyes investigated were monosulfonated and had molecular weights of <400 Da (see Fig. 1, Table 1, #1–2). Due to the use of sulfanilic acid as the diazonium component, the dyes were nonvolatile. Aniline, N-phenyl-benzene-1,4-diamine (PBDA) and diphenylamine (DPA) were identified as the characteristic pyrolysis products of the textile dye Acid Orange 5 (see Fig. 2 for pryrograms). The thermally induced release of aromatic amines from Acid Orange 5 started at a pyrolysis temperature of 500 °C, where aniline may be formed from Acid Orange 5 by C–N cleavage at the right hand side of the molecule, while PBDA is the result of azo bond cleavage in this dye. PBDA could be detected at temperatures >650 °C and it contributed >50% of the total peak area, which makes it a primary key compound. The maximum release of all three products of pyrolysis was observed at 800 °C. The release of DPA is caused by cleavage of a C–N bond, whereas PBDA came from the cleavage of a N=N bond. The intensities of both peaks increased with the temperature (data not shown). The intensity of PDBA from the pyrolysis of pure PBDA was almost constant between 300 and 800 °C.
The key compound N,N-dimethyl-benzene-1,4-diamine (DMBDA) released from Acid Orange 52 could be identified between 300 and 650 °C. It is very improbable that the azo bond could be cleaved at temperatures as low as 300 °C. Thus, it may be that Acid Orange 52 contains some DMBDA as an impurity. Maximum release was found to occur at between 500 °C and 650 °C, while 1,4-benzenediamine (BDA) reached its maximum at 650 °C (due to the loss of two methyl groups from DMBDA). The content of aniline steadily increased with the temperature.
Both dyes comprise the same diazonium component, sulfanilic acid. Aniline, the key compound of sulfanilic acid, exhibited different detection intensities depending on whether it came from Acid Orange 5, Acid Orange 52 or sulfanilic acid. Aniline derived from sulfanilic acid exhibited maximum release at 500 °C. The maximum amount of aniline derived from both Acid Orange 5 and Acid Orange 5 occurred at 800 °C. This suggests that the dyes are cleaved at additional sites to those producing smaller volatile molecules when exposed to higher temperatures. Therefore, every molecule released by pyrolysis reaches an maximum intensity at a certain temperature [19].
Detection of higher amounts of SO2 and CO2 is believed to occur due to thermal degradation of organic compounds above 650 °C. Figure 2 shows pyrograms of the pyrolyzed acid dyes Acid Orange 5, Acid Orange 52 and sulfanilic acid at 650 °C. The characteristic pyrograms of similar compounds enable pyrolysis fingerprint comparisons of pure samples as an analytical method.
Cationic dye
Basic Red 46 is a monoazo dye used to dye polyacrylnitrile fibres. The dye does not have a sulfonic acid substituent (see Fig. 1, Table 1, #3). It acquires its water solubility through the positive charge located on the triazole ring. Thermal degradation by pyrolysis releases a derivative of the dye, typically triazole 4-methyl-4H-[1,2,4]triazol-3-ylamine (MTAA, data not shown). Other characteristic dye compounds are N-benzyl-N-methyl-benzene-1,4-diamine (BMBDA), and benzyl-methyl-phenyl-amine (BMPA). The presence of both of these compounds again indicates that the cleavage of C–N bonds competes with the cleavage of N=N bonds, as observed for the DPA and PDBA released from acid dyes. The optimal intensity—i.e., the ratio of peak area to mM sample—was found at a pyrolysis temperature of 500 °C for all three products of pyrolysis.
Direct dyes
One commonly used tetraazo dye, Direct Blue 71, and two diazobenzidine-based dyes, Direct Brown 1 and Direct Blue 151, were investigated. In addition to Direct Blue 71, three characteristic sulfonated and unsulfonated aromatic amines corresponding to the reactants in the dye synthesis and metabolites of the chemical reduction of the dye respectively [naphthalene-1,4-diamine (14NDA), 5-acetylamino-8-amino-naphthalene-2-sulfonic acid (5A8A), C-acid] were also pyrolyzed. Examination of the pyrogram of Direct Brown 1 revealed that thermal degradation of the azo bonds released the banned aromatic amines benzidine and p-aminodiphenyl. It is assumed that p-aminodiphenyl is the product of the thermal degradation of Direct Brown 1 via the cleavage of a N=N bond and a C–N bond. Another pyrolysis product was aniline from sulfanilic acid, which was connected to the rest of the dye molecule by azo bonding. Thermally initiated cleavage of one of the two azo bonds and the loss of the carboxylic group was observed due to the detection of 4-aminophenol. The expected release of a 1,2,4,5-tetraaminobenzene could not be confirmed by detection with GC–MS. Benzidine shows the highest peak area of any compound found after the pyrolysis of Direct Brown 1 (see Fig. 3). 500 °C is presumed to be the optimal temperature for pyrolysis.
Direct Blue 151 is based on the banned diazotated aromatic amine dimethoxybenzidine, which is coupled with I-acid and Neville–Winther acid. Dimethoxybenzidine was not found as a volatile pyrolysis product at any temperature. The continuous presence of a compound in the pyrograms at any temperature from 300 to 800 °C indicates that it simply evaporates from the sample. It must be a contamination, as there is no realistic thermal reaction that could produce such a compound from Direct Blue 151. However, 2,5-dimethoxybenzenamine was detected continuously between 300 and 600 °C at a low intensity compared to the benzidine released from Direct Brown 1. Aminonaphthalene, a possible pyrolysis product of I-acid and Neville–Winther acid, was not detected. Substitution with a hydroxyl group and azo bonding is believed to suppress the release of aminonaphthalene, just as observed for I-acid and H-acid.
In contrast to Direct Blue 151, pyrolysis of the tetraazo dye Direct Blue 71 produced two characteristic components which could be identified as aminonaphthalene and 1,4-diaminonaphthalene. It was not possible to distinguish between 1-aminonaphthalene and 2-aminonaphthalene by analysis with data libraries. Pyrolysis of the reactants (I-acid, C-acid) and products of the reductive cleavage of the azo bonds (5A8A, 14NDA) yielded the origins of the key compounds detected for the dyes. 14NDA is the most characteristic product of the pyrolysis of Direct Blue 71, as it is released from both the 14NDA and 1,4-diaminonaphthalene-6-sulfonic acid parts of the dye molecule, which are linked via azo bonds. The intensity of 14NDA remained stable between 500 and 800 °C, whether it was released from the Direct Blue 71 itself or from single molecules of 5A8A or 14NDA (data not shown).
Reactive dyes
Reactive azo dyes comprise over 30% of the global market for textile dyes. High water solubility, excellent coloring properties and covalent bonding with both natural and synthetic textile fibers are the major advantages of this dye class. Three of the reactive azo dyes most intensively produced and used globally and four additional reactants from dye synthesis (RB–OH, RB–NH2, p-Base, vinyl-p-Base) were pyrolyzed or evaporated, respectively (for structures, see Fig. 1). The reactivity of so-called Remazol dyes (vinylsulfone dyes) is due to the 3-sulfato-ethylsulfonyl-group, which forms a reactive vinylsulfon group through the loss of sodium hydrogen sulfate under alkaline dyeing conditions. A stable chemical bond connecting the dye to the fiber is generated by the reacting a vinyl group (in the dye) with a hydroxyl group (cellulose) under alkaline conditions. The reactive dyeing process is accompanied by the loss of 10–50% of the dye due to a hydrolysis reaction involving the vinyl group (dye) under alkaline dyeing conditions. Both forms of the dye, the sulfuric acid ester and the hydrolyzed form, were investigated by pyrolysis.
Vinyl-p-Base was found to be the major pyrolysis product and the key compound of all pyrolyzed reactive dyes and dye reactants. The highest amounts of vinyl-p-Base released from reactive dyes were obtained at 500 °C. However, pure vinyl-p-Base reached its maximum at 650 °C (data not shown).
Sulfonic acid ester groups (nonhydrolyzed dye) have an strong inhibiting influence on the release of vinyl-p-Base from dye molecules by pyrolysis. This was discovered by comparing nonhydrolyzed and hydrolyzed dyes under the same experimental conditions (see Fig. 4). Loss of sulfonic acid by hydrolysis enhanced the release of the key compound vinyl-p-Base from RB5-H and RO16-H. The reverse effect was observed upon the pyrolysis of RO107-H. Hence, RO107-H yielded the most vinyl-p-Base at 300 °C, which may be due to contamination of the sample with unbonded p-Base or vinyl-p-Base. The release of vinyl-p-Base from RB–OH was three times greater than that from the isomer RB–NH2. The tautomerized form of RB–OH, which is observed in aqueous solution, could prompt the thermally initiated cleavage of the azo bond upon pyrolysis.
The highest amounts of vinyl-p-Base were released upon the evaporation of p-Base and vinyl-p-Base in the pyrolysis furnace. An evaluation of the data showed that the release of vinyl-p-Base upon the pyrolysis of dye molecules (containing the structure of vinyl-p-Base) was far less intense than that observed upon the evaporation of equimolar amounts of p-Base or vinyl-p-Base itself. Vinyl-p-Base could not be detected from p-Base at 300 °C. A comparison of peak areas in relation to the molar amount is shown in Fig. 4. It suggests that the release of vinyl-p-Base from the dye is influenced by the bonding via an azo group, the thermally initiated neutral loss of H2O from the alkanoic alcohol group, as well as by the loss of disodium sulfate from the sulfuric ester derivative. The intensity of the vinyl-p-Base released upon the pyrolysis of p-Base in relation to the molar concentration was about 2/3 of that observed from vinyl-p-Base. Investigations on pure p-Base showed that it could not be detected by Py-GC–MS without the loss of H2O. Detection was achieved by GC–MS via liquid injection of p-Base dissolved in methanol.
Solvent dyes
Poorly water-soluble solvent dyes are used to dye synthetic fibers such as polyester, PVC and waxes. The release of pyrolysis products is strongly enhanced, as nonvolatile sulfonic groups are not present in the dye molecules. Even compounds with an azo bond were released from the dye molecules, which was the first time that this phenomenon had been observed. Cleavage of one of the azo bonds from Solvent Red 23 released 4-aminoazobenzene (AAB) and 1-amino-naphthalen-2-ol (NOL), which were two key compounds of the dye. NOL was most intense at 500 °C, whereas AAB had a first maximum at 350 °C and a second one at 500 °C (see Fig. 5) Aniline was liberated upon cleavage of the second azo bond. Increasing the pyrolysis temperature caused a decrease in the intensity of the aniline signal. This agrees with the results of DeFellipis et al. for Solvent Red 23 [8].
The decrease of AAB as a function of the pyrolysis temperature is shown in Fig. 5. Thermally initiated loss of an amino group from AAB was not found to affect the intensity of azobenzene (AB). The release of AB was connected with the cleavage of a C–N bond, just as described earlier in this work. In addition to the pyrolysis products described, benzene was detected above 700 °C as a final thermal degradation product.
Another azo compound, o-aminoazotoluene (AAT), was found upon the pyrolysis of Solvent Red 26 between 300 and 600 °C (see Fig. 4). The product intensities of NOL and AAT released by the thermally initiated cleavage of one azo bond were more stable over 300–600 °C than those of the products of Solvent Red 23. In addition to 2-methyl-aniline (2MA), which resulted from the cleavage of the second azo bond, 2,4-dimethylaniline and 4-methyl-1,2-diaminobenzene were observed over the whole temperature range (data not shown). This is due to the partial cleavage of the second azo bond, or it may be due to the contamination of the dye.
Unknown dye mixture from a dried textile wastewater concentrate
A 10-mL sample of textile wastewater concentrate from a dyeing process was dried under air. 0.5 mg of the residue were pyrolyzed without further sample preparation. Aniline, caprolactam, 2-aminobenzothiol, 4-(methylsulfonyl)-aniline and vinyl-p-Base were found to be the major products of pyrolysis at 500 °C. Afterwards, the same sample was treated with sodium dithionite in order to reduce the azo bonds chemically, then dried and pyrolyzed under the same conditions. The analysis showed that the chemical reduction of the dye mixture prompted the release of volatile aromatic amines. N-Ethylbenzene, p-chloroaniline, 1-methylethylester-benzenesulfinic acid, sulfanilamide, p-Base, 2-amino-9,10-anthracenedione and 1-amino-4-hydroxy-9,10-anthracendione were found in addition to the components mentioned above. p-Base and vinyl-p-Base were found to be identifiers for Remazol dyes (vinylsulfone dyes) from the class of reactive dyes. The detection of sulfanilamide could indicate the presence of a further group of reactive dyes (i.e., Basazol dyes) in the residue of the dried wastewater.
1-Amino-4-hydroxy-9,10-anthraquinone and 2-amino-9,10-anthraquinone were identified as the chromophore parts of the dyes. Several anthraquinone-based dyes are used for vat or reactive dyeing and belong to the classes of indantherene and reactive dyes.
The discovery of p-chloraniline suggests that a dye containing the banned aromatic amine as part of its structure was used. p-Chloraniline is known to be a synthetic by-product of p-Base, which is an reactant used in Remazol dye synthesis. It should have been detectable in the untreated wastewater sample if it was not covalently bonded to a dye.
2-Aminobenzothiol and N-ethyl-aminobenzene were further products of the pyrolysis, but they could not be used to identify a particular class of dyes, whereas caprolactam was also found, which is used as an intensifier in disperse dyeing processes and as an additive during heat-activated printing with reactive dyes [20].
The textile wastewater concentrate was therefore assigned to a reactive textile dyeing process because of the presence of several identifiers which are indicative of certain reactive dyes, namely Remazol dyes, Basazol dyes and anthraquinone dyes. Investigations by HPLC–DAD on the same sample confirmed the presence of Remazol dyes due to the identification of RB5, RO16 and RO107 (data not shown). The presence of caprolactam indicates that the sample originates from a printing process with reactive dyes.
Conclusion
The maximum release of volatile aromatic amines was observed at temperatures above 500 °C for most of the compounds investigated. Sulfonated aromatics are subject to partial desulfonation upon pyrolysis at temperatures of between 300 and 800 °C. Provided that desulfonation takes place as a first step, the radical formed should abstract a hydrogen atom to produce a volatile aromatic amine such as aniline or aminonaphthalene. The release of volatile aromatic amines follows a substitute-dependent mechanism. Substitution with hydroxy groups suppressed the release of aminonaphthalene from the monosulfonated and disulfonated aminonaphthalenes. Evolution of CO starts when the OH-bearing aromatic ring breaks at temperatures above 650 °C. CO can reduce other hydroxyl groups and it supports the formation of S8 by reducing the SO2 released during desulfonation. Pyrolysis of aminobenzene sulfonic acids with amino groups at different positions showed that the ortho position required higher pyrolysis temperatures to achieve maximum release of aniline (800 °C). In the para position, maximum release was detected at 500 °C.
Unsulfonated as well as sulfonated hydroxyaminonaphthalenes and aminobenzenes that were connected to dye-structures via azo bonds released significantly decreased amounts of volatile aromatic amines than the pyrolyzed or evaporated compound itself. Investigations on reactive dyes revealed that the key compound vinyl-p-Base was released from the original and the hydrolyzed dyes at 300 °C. Aromatic amines can be released by the sample itself, which contains small amounts of p-Base or vinyl-p-Base as a contaminant from the synthesis or hydrolyzation process. It is suggested that the thermally initiated cleavage of an azo bond competes with the cleavage of a C–N bond, as observed on DPA/PBDA (Acid Orange 52), BMPA/BMBDA (Basic Red 46), benzidine/ADP (Direct Brown 1), NA/14NA (Direct Blue 71) and AB/AAB (Solvent Red 23). Evidence for the release of diazotated compounds through the cleavage of C–N bonds was not found. However, intact azo bonds could be identified by the pyrolysis of low-substituted unsulfonated azo dyes, such as Solvent Red 23 and Solvent Red 26. Complete azo dyes were not detected.
Nevertheless, the pyrolysis of unsulfonated dyes such as Solvent Red 23, Solvent Red 26 and Basic Red 46 showed that these dyes release aromatic amines in a way that allows the reconstruction of the original dye structure by reconnecting the amino groups to the azo bonds. This is a promising method for the structural elucidation of unsulfonated dyes. The identification of key dye-pyrolysis identifier compounds allows the assignment of unknown sulfonated dye samples to certain dye classes, as was demonstrated for a sample of textile wastewater. A list of identifiers is given in Table 2.
Investigations on dyes based on banned aromatic amines showed that pyrolysis can be used as a tool for fast thermal extraction of these compounds from wastewater instead of extensive physicochemical sample preparation (e.g., liquid–liquid extraction of aromatic amines [11]). Chemical reduction of the sample containing azo dyes was found to enhance the release of volatile aromatic amines. Pyrolysis of the pure dye (e.g., Direct Brown 1) showed that the banned aromatic amine (benzidine) is released from the dye by thermal degradation of the azo bonds without chemical reduction treatment. In the case of Direct Blue 151, the release of dimethylbenzidine as a banned aromatic amine was not observed. Further investigations could show whether 2,5-dimethoxybenzeneamine could be used as a key dye-pyrolysis identifier compound for dimethylbenzidine. The utilization of pyrogram-fingerprint databases would help to identify certain dyes that produce characteristic pyrograms, but it would requires extensive work in compiling the databases. Quantification by means of pyrolysis requires qualified reference materials and more investigations into the influence of the individual dye sample (e.g., fibers), as well as statistical data evaluation.
References
Zollinger H (1991) Color chemistry, 2nd edn. VCH, New York, p 1
DyStar (2000) German legislation on azo dyes, 2nd issue. DyStar GmbH, Leverkusen, Germany
EC (2006) Directive 2002/61/EC of the European Parliament and of the Council of 19th July 2002. Off J Eur Commun L243:15
Reisch MS (1996) Chem Eng News 10
Correia VM, Stephenson T, Judd SJ (1994) Environ Technol 15:917–929
Wampler TP (ed) (1995) Handbook of pyrolysis. Marcel Dekker, New York, Ch 1
Saferstein R, Manura JJ (1977) J Forensic Sci 22:748
DeFelippi J, Kenion G, Ludicky R (1995) In: Proc 43rd ASMS Conf on Mass Spectrometry and Allied Topics, 21–26 May 2005, Atlanta, GA
Sonoda N (1999) Stud Conserv 44:195
Fabbri D, Chiavari G, Ling H (2000) J Anal Appl Pyrolysis 56:167
Plum A, Engewald W, Rehorek A (2003) Chromatographia 57:243
Van de Meent D, De Leeuw JW, Schenck PA (1980) J Anal Appl Pyrolysis 2:249
McEvoy J, Giger W (1986) Environ Sci Technol 20:376
Trehy ML, Gledhill WE, Orth RG (1990) Anal Chem 62:2581
Field JA, Miller DJ, Field TM, Hawthone SB, Giger W (1992) Anal Chem 64:3161
Van Loon WMGM, Boon JJ, De Groot B (1991) J Anal Appl Pyrolysis 20:275
Van Loon WMGM, Boon JJ, De Groot B (1993) Anal Chem 65:1728
Blazsó M (1993) J Anal Appl Pyrolysis 25:25
Ericsson I (1985) J Anal Appl Pyrolysis 8:73
Wagner B, Xu M (2004) Reactive dye printing process. US Patent 20040219296
Acknowledgements
We thank Dr. Konrad Bootz from DyStar GmbH, Leverkusen, Germany for kindly providing dyes and dye educts. We owe much gratitude to Ruth Meurer for supporting the performance of Py-GC MS analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Werner Engewald on the occasion of his 70th birthday.
Rights and permissions
About this article
Cite this article
Rehorek, A., Plum, A. Characterization of sulfonated azo dyes and aromatic amines by pyrolysis gas chromatography/mass spectrometry. Anal Bioanal Chem 388, 1653–1662 (2007). https://doi.org/10.1007/s00216-007-1390-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1390-0