Abstract
The study of semiconductor photocatalysts is essential to achieve sustainable solutions for application in energy generation and treatment of environmental pollution. Titanium dioxide (TiO2) is one of the most researched photocatalysts because of its abundance and non-toxicity, but its low efficiency, when deployed as a photocatalyst, can sometimes hinder its potential for these applications. Combining nanostructured TiO2 with carbon nanotubes (CNTs) to form hybrid materials can help overcome some of the metal oxide limitations, such as a large band-gap and a high electron–hole recombination rate. This chapter summarizes some of the CNT/TiO2 nanostructured materials that have been reported in the last years and outlines their morphologies, methods of synthesis, and applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Titanium dioxide is the naturally occurring oxide of titanium and can be found in nature in its three primary crystalline forms: anatase, rutile, and brookite [1]. Due to its low cost, non-toxicity, and strong oxidizing power [2, 3], TiO2 is a promising material to be used in applications such as photocatalysis [4], dye-sensitized solar cells (DSSC) [5], and H2 production via water splitting [6]. However, its relatively high band-gap (3.2 eV for the anatase phase) and high charge carrier recombination rate limit its solar photoconversion [7]. The three main ways this can be overcome is by band-gap tuning, which can be achieved using dopants [8] or by synthesizing oxygen-rich titania [9], suppressing the electron–hole recombination [10], and by improving surface reactions [11]. Carbon nanotubes (CNT) present a high surface area, high-quality active sites, and high electronic conductivity [12, 13], therefore allowing it to enhance the photocatalytic activity of TiO2 through all of these routes.
Since their discovery by Iijima in 1991 [14], CNTs have attracted attention because of their unique properties. They can be either single-walled (SWCNTs), which consists of a single graphite sheet wrapped into a cylindrical tube, or multi-walled (MWCNTs), which are an arrangement of multiple concentrical SWCNTs [7]. The SWCNTs have a surface area of 400–900 m2 g−1, while MWCNTs have a surface area of 200–400 m2 g−1, which means that they can provide a large reactive surface area that is comparable to activated carbon. According to Woan et al. [15], TiO2 and CNTs may form a Schottky barrier similar to metal–semiconductor junctions, which results in the CNTs having an excess negative charge and the TiO2 an extra positive charge. In addition, they may accept photo-induced electrons in CNT/TiO2 hybrid materials, thus increasing the recombination time.
The formation of CNT/TiO2 heterostructures was reported by Banerjee and Wong in the early 2000s [16]. Thenceforth, a variety of TiO2/CNTs hybrid nanomaterials have been researched [7], including TiO2 nanoparticles on the CNTs surface [17], TiO2 coated with CNTs [18], and CNTs grown on TiO2 nanotubes [19].
2 Synthesis and Morphology
As mentioned above, both the CNTs and the TiO2 used in the hybrid nanostructures can present different morphologies. The titanium dioxide can be synthesized in the form of nanoparticles [17], nanotubes [19, 20], nanorods [21, 22], nanofibers [23], or thin films [24]. Regarding the methods employed for the synthesis of these hybrid nanostructures, some widely performed techniques can be mentioned: mixture in solution with pre-functionalized surfaces [4, 25], sol–gel [26, 27], hydrothermal [28, 29], chemical vapor deposition (CVD) [19], and atomic layer deposition (ALD) [17]. This section summarizes some of the main synthesis routes and promising nanostructures that have been studied recently.
2.1 CNT/TiO2 Nanoparticles
Hybrid nanostructures of CNTs and TiO2 nanoparticles can be obtained through multiple routes, such as hydrothermal synthesis [28], modified microwave method [30], and ALD [17]. However, sol–gel is the most employed technique [7]. Sol–gel is a relatively low-cost method that presents good controllability of the synthesis conditions [31]. In addition, it has the advantage of using precursors in a liquid state, which allows good dispersion of the filler phase when producing composites [32]. However, because CNTs present strong van der Waals interactions, they tend to form agglomerates, which requires a functionalization step to be dispersed in an aqueous solution [33]. Thus, the synthesis is divided into two significant steps, one organic and one inorganic. The CNTs are functionalized in the organic step, and surfactants are used to disperse them in the inorganic step. The composite materials are formed during the gelification process of the condensed sol, and the porous sol–gel can encapsulate nanoparticles and carbon nanotubes. In addition, sol–gel synthesis provides the prospect of adding CNTs alongside the precursors, allowing for a better connection between the carbon and the nanotubes and providing a uniform mesoporosity throughout the material [31].
Da Dalt et al. [4] produced CNT@TiO2 composites by a modified sol–gel method using commercial MWCNT (Baytubes®), titanium propoxide, nitric acid, isopropyl alcohol, and deionized water. Different heat treatment temperatures for the obtained sol were evaluated. The authors synthesized spherical-shaped TiO2 nanoparticles (NPs) aggregated on the CNT surface, as shown in Fig. 1. The TiO2 NPs presented an average diameter smaller than 30 nm. The photocatalytic activity of the composites was evaluated and compared to commercial TiO2 (AEROXIDE®—P25) and its combination with MWCNT. The CNT@TiO2 composites performed better than pure TiO2 nanoparticles when the catalyst concentration in the dye solution increased. The authors concluded that the CNT/TiO2 heterojunctions formed reduced the recombination rate of photo-induced electron–hole pairs.

Edited and reprinted with permission of Da Dalt et al. [4]
TEM images of the CNT@TiO2 composites using. a P25. b Sol–gel obtained nanoparticles.
Yao et al. [18] synthesized anatase/CNTs composite materials. Anatase nanoparticles with diameters of 5 and 100 nm were added to different proportions of commercially SWCNTs and MWCNTs. First, the CNTs were functionalized with nitric acid and dispersed in water. Then, they were combined with the composite structures by simple evaporation and drying process. The photocatalytic activity for the degradation of a solution containing phenol was tested. The TiO2/SWCNTs composites presented a lower electron–hole recombination rate than TiO2/MWCNTs composites, probably because of the attachment area of the nanotubes on the surface of the nanoparticles.
Banerjee and Wong [16] synthesized heterostructures by oxidizing raw SWCNTs bundles using acidic potassium permanganate solution and hydrochloric acid and then etched the nanotubes using HNO3. This procedure removed most of the amorphous carbon and the metal catalysts from the nanotubes. Next, the TiO2 nanoparticles were synthesized by slow hydrolysis of titanium tetraisopropoxide in the presence of an alcoholic solution of 11-aminoundecanoic acid. Finally, in order to achieve TiO2/CNTs heterostructures, solutions containing the nanoparticles and the oxidized SWCNTs were sonicated for 24 h in a dimethylformamide (DMF) solution. After filtering, washing, and drying, the authors confirmed the formation of the heterostructures by Transmission Electronic Microscopy (TEM), Energy Dispersive X-ray Spectroscopy (EDS), and Fourier Transform Infrared Spectroscopy (FT-IR). In addition, the FT-IR analysis indicated that charge transfer from nanotube to nanoparticles had been achieved.
Zhao et al. [34] deposited TiO2 on the surface of CNTs by solvothermal reaction. To achieve that, the authors functionalized commercial-grade CNTs with HNO3, which was later dispersed in an ethanol and glycerol solution. Titanium butoxide (TBT) was added dropwise to this solution, and the formed liquid was heated at 110 ºC for 24 h. Finally, the products were dried and then calcinated at 350 ºC for 2 h. Figure 2a shows a schematic illustration of the experimental procedure adopted and Fig. 2b presents the scanning electron microscopy (SEM) image of the CNT@TiO2 morphology obtained, in which can be noticed the TiO2 grains on the surface of CNTs. The SEM analysis showed that composites with a CNT content of 42 wt% maintained the original morphology of tangled CNTs, and EDS analysis showed that the TiO2 nanoparticles were connected and coated on the surface of the CNTs.

Edited and reprinted with permission of Zhao et al. [34]
a Schematic illustration of the experimental procedure for the synthesis of CNTs@TiO2 composites. b SEM image of the CNTs@TiO2 composite with 42 wt%. of CNT.
MWCNT forests coated with TiO2 films via Atomic Layer Deposition (ALD) were obtained by Kaushik et al. [35]. Two types of layer configuration were obtained: (1) MWCNT with TiO2 films and (2) MWCNT with a coating of carboxyl plasma polymer (PP) and TiO2 films. Figure 3a presents the SEM image of the lateral view of the MWCNTs forest after 400 cycles of ALD. It can be seen that the tips of the forests are brighter, which means that TiO2 film was not deposited uniformly on CNTs, only on the surface of the forests. The region selected at TEM analysis (Fig. 3b) shows a uniform TiO2 film covering a MWCNT. The carboxyl coating created more nucleation sites for the deposition of TiO2, thereby producing a more uniform layer.

Reprinted with permission of Kaushik et al. [35]
a Backscattered electrons (BSE) SEM image of the lateral view of the MWCNTs forest coated with TiO2. b TEM image of the TiO2-coated MWCNT.
2.2 CNT/TiO2 Fibers
One-dimensional (1D) nanoarchitectures attract attention because of their length‑to‑diameter ratio, which can enhance their chemical and optic properties when compared to nanoparticles [36]. That may occur because TiO2 nanoparticles have reduced electron mobility caused by the contact between the particles, which enhances the scattering of free electrons [5]. Nevertheless, when employed as a photoanode, structures such as nanofibers present some limitations caused by their reduced surface area, which can be surpassed by using nanoparticles decorated on their surface [37]. Another innovative way to overcome these limitations is by using CNTs combined with 1D nanostructures.
One versatile and simple method to produce ultrathin TiO2 fibers is the electrospinning technique [38]. It requires a relatively simple basic setup that involves electrifying a liquid droplet to generate a jet, which is stretched into a fiber. The droplet is deformed into a Taylor cone due to the electrostatic repulsion among the surface. Initially, the formed jet is stretched through a straight line before whipping due to electrostatic instability, and finally, the thinner jet solidifies and is deposited into an electrified collector [39].
Macdonald et al. [40] fabricated a TiO2 nanofiber-CNTs composite to be applied as a photoanode in a DSSC. To achieve that, the authors synthesized SWCNTs by arc discharge and TiO2 nanofibers by electrospinning. First, they electrospun a sol–gel containing titanium n-butoxide, poly(vinyl pyrrolidone) (PVP), and glacial acetic acid in absolute ethanol. Next, the fiber mats were subject to pyrolysis at 500 ºC to remove the PVP. The remaining TiO2 nanofibers were then sonicated in an SWCNT/ethanol solution until a uniform TiO2 nanofibers/CNTs mixture was formed. Finally, the mixture underwent rotary evaporation to remove the ethanol. The inclusion of CNTs in the mixture was confirmed by Raman spectroscopy, which showed the disorder-induced D-band at 1345 cm−1 and the G-band at 1575 cm−1 for the samples containing SWCNTs. Following, the authors fabricated DSSC using TiO2 nanofibers and TiO2 nanofibers/SWCNT photoanodes and applied J-V curves to characterize the photovoltaic activity. The solar cells composed of TiO2/CNTs photoanodes presented JSC of 12.65 mA cm−2, VOC of 0.72 V, FF of 0.53, and PCE of 4.83%. The addition of SWCNTs improved the efficiency of the solar cells by 67%, probably due to the CNTs contributing with addition charge-transfer channels which ultimately increased the diffusion coefficient.
Jung et al. [41] synthesized hollow TiO2 nanofibers with embedded CNTs through electrospinning and impregnation. To achieve that, they electrospun a solution containing polyacrylonitrile (PAN), DMF, and MWCNTs. The as‑spun fibers were then impregnated using a solution containing 0, 1, 5, and 10 wt% titanium isopropoxide (TTIP)/ isopropyl alcohol for 5 h, dried, and later calcinated at 550 ºC for 1 h. The characterization was made by field emission scanning electron microscopy (FE‑SEM), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and the surface area was calculated via Brunauer‑Emmett‑Teller (BET) method. Finally, the adsorption and photocatalytic capability of the obtained materials were investigated by adding the photocatalyst in a solution containing methylene blue under magnetic stirring in a dark room for 30 min before exposing the solution to UV light at 365 nm for 70 min. For comparison, the authors also prepared hollow TiO2 nanofibers without the presence of CNTs. A schematic representation of the experimental procedure is shown in Fig. 4. The obtained CNT-embedded hollow TiO2 fibers (Fig. 5) had a diameter that ranged from 430 to 550 nm, and the CNTs were aligned with the fibers. The authors attributed this variation in diameter to the irregular distribution of CNTs within the nanofiber. They suggested that the alignment of the CNTs could be due to the electric field lines during the injection.

Reprinted with permission of Jung et al. [41]
Schematic representation of the experimental procedure to obtain hollow TiO2 nanofiber embedded with CNTs.

Reprinted with permission of Jung et al. [41]
SEM images of the a PAN-CNT fibers, b impregnated PAN-CNT fibers, c CNT embedded hollow TiO2 fibers, and d hollow TiO2 fibers.
Furthermore, the addition of CNTs improved the adsorption capability of the TiO2 nanofibers. The photocatalytic activity was highest for the sample impregnated in the solution containing 5 wt% TTIP concentration, and the CNT-TiO2 photocatalysts more efficiently degraded the methylene blue compared to the pure TiO2 nanofibers. The authors proposed that the CNTs might have introduced photogenerated electrons into the conduction band of the TiO2 fibers, contributing to the enhancement of the photocatalytic capability of the TiO2 fibers.
2.3 TiO2 as Catalyst for CNT Synthesis
Carbon nanotubes can be synthesized by three main methods: the laser deposition method, electric arc method, and CVD [42]. The latter is the most employed method to synthesize nanomaterials and to mass-produce CNTs. When employed to synthesize CNTs, CVD has the advantage of requiring a lower temperature (550–1000 ºC). Moreover, the method allows the control of the morphology and structure of the nanotubes and the growth of CNTs with the desired alignment [43].
In order to produce CNTs, the CVD method involves employing a carbon-bearing precursor over a substrate in the presence of a catalyst [43]. This catalyst has the function of decomposing and nucleate the carbon source in the form of nanotubes. The most common catalysts are transition metals such as Fe, Ni, and Co. Hydrocarbons such as methane or acetylene are commonly used as carbon sources. As for the substrates, the most common are Ni, SiO2, Cu/Ti/Si, glass, and stainless steel [44]. Lately, a particular type of substrate (doped TiO2) has been researched, where the doping atoms act as a catalyst for the CNTs [19].
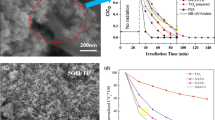
Recently, Guaglianoni et al. [45] investigated the influence of CVD parameters on synthesizing CNTs using cobalt doped TiO2 nanotubes (Co-TiO2) as catalyst. For that, the authors produced Co-TiO2 nanotubes via a one-step anodization process using titanium foil as substrate. Instead of performing the traditional heat treatment to transform the amorphous titania to anatase, they used the as-synthesized metal oxide as catalyst to produce MWCNTs. They employed hexane as the carbon source and studied the synthesis temperatures of 700 and 800 ºC, as well as different synthesis times (10, 20, and 30 min). The Co-TiO2/CNT structures were characterized by X-ray diffraction (XRD), Raman spectroscopy, SEM, and TEM. The Raman spectra showed the characteristic modes for CNTs in all synthesized samples. The presence of CNTs on the surface of the TiO2 nanotubes was also confirmed by SEM (Fig. 6). By comparing the intensity between the D and the G bands (ID/IG), the authors found out that the CNTs obtained in longer synthesis times are more defective. Linear voltammetry essays showed that the combination of CNTs and Co-TiO2 developed a photocurrent density 65 times greater than the Co-TiO2 nanotubes.
3 Applications
3.1 Lithium-Ion Batteries
TiO2 is a promising material to be employed as anode for lithium-ion batteries (LIBs) because of its stability, non-toxicity, low cost, high theoretical capacity and low volume expansion during the charging/discharging processes (<4%) [34, 46]. However, the electrochemical performance of this semiconductor is hindered by its poor electronic conductivity and low lithium-ion diffusivity [25, 34, 47]. A strategy to surpass these limitations is the formation of composites with carbonaceous materials, such as carbon nanotubes (CNTs).
Lo et al. [48] reported the application of TiO2-CNT layers deposited on titanium thin plate as anodes for LIBs. The composites (Fig. 7a, b) were obtained using a micro-arc oxidation (MAO) process with different CNT quantities in the electrolyte. The charge–discharge behavior (Fig. 7c) was evaluated between 0.1 and 3.0 V (vs. Li/Li+) at a current rate of 0.2 C. The sample without CNTs (Ti/nTiO2) presented charge–discharge capacities of 176 and 170 mAh g−1 in the initial cycle. The formation of the CNT composite greatly increased the charge–discharge capacities. For instance, the capacities were 292/248 mAh g−1 for the sample with higher CNT content (Ti/nTiO2-CNT3). Thus, the presence of CNTs provided electrons to the TiO2 nanostructure and promoted the Li-ion incorporation. Additionally, the discharge capacity of the Ti/nTiO2 decreased to 69 mAh g−1 at the 20th cycle (Fig. 7d), probably due to changes in the anode surface generated by the Li-ion insertion/extraction. On the other hand, the TiO2-CNT composites showed high cycle stability and discharge capacities. The discharge capacities of the Ti/nTiO2-CNT3 sample at discharge rates of 0.2 C, 1 C, and 2 C were 201, 53 and 38 mAh g−1, respectively. The authors attributed the enhancement of electron transport and discharge capacity observed for the composites to the conductive CNT network that supported the TiO2 nano-flaky structures.

Edited and reprinted with permission of Lo et al. [48]
FE−SEM cross-sectional images of a Ti/nTiO2-CNT1 and b Ti/nTiO2-CNT3 composites. c Charge–discharge curves for Ti/nTiO2, Ti/nTiO2-CNT1, and Ti/nTiO2-CNT3 anodes. d Cycling performance of Ti/nTiO2 and Ti/nTiO2-CNT composites at discharge rates of 0.2 C, 1 C, and 2 C.
Another interesting TiO2/CNT configuration that produced outstanding results for application as anode material for LIBs was obtained by Zhao et al. [34]. TiO2 nanoparticles connected to the surface of the CNTs were obtained. The samples had their specific capacities tested at different current densities (Fig. 8a) and their cyclic performances were evaluated at 10C (Fig. 8b). The composites exhibited superior performance than the TiO2 alone for all current densities tested. The sample with higher CNT content (i.e., 42 wt%) showed an increased specific capacity (approximately 200 mAh g−1), with a discharge capacity almost 2.5 times that of pure TiO2 after 1000 cycles (Fig. 8b). These results indicate that the CNTs provided a prompt pathway for the electrons and ions movement throughout the composites.

Reprinted with permission of Zhao et al. [34]
a Specific capacities at different current densities and b cyclic performances of the CNTs@TiO2 composites.
3.2 Supercapacitors
Originated from the patent applied by Becker in 1954 [49], supercapacitors or ultracapacitors are electrochemical energy storage devices that present high power-density in discharge and recharge, basically combining the capabilities of batteries and capacitors [50, 51]. The supercapacitors can be divided into three categories based on their charge storage mechanism: (1) electric double-layer capacitors (EDL); (2) pseudocapacitors; and (3) hybrid capacitors [52].
Due to their high surface area, high chemical stability, and low electric resistance, CNTs are promising materials to be used as an electrode of supercapacitors [53]. To further enhance the CNTs performance for this application, methods such as modifying the nanotubes with transition metal oxides or conductive polymers are often used [54, 55].
Zhang et al. [56] produced a CNT/TiO2 composite via hydrothermal method and enhanced its capacitance by pretreating the material with UV light irradiation. To achieve this, the authors placed commercial-grade CNTs, titanium sulfate, and urea in an autoclave and baked it at 150 ºC for two hours. The remaining product was centrifuged and later dried. Electrodes were manufactured with the composite to test the electrochemical properties by cyclic voltammetry, chronopotentiometry, and AC impedance method. Finally, the UV light pretreatment was made by placing the CNT/TiO2 electrode in a black-box-type analyzer and irradiated it for 1 h under 254 and 365 nm UV light. In addition, pristine CNT electrodes were used for comparison. The SEM analysis (Fig. 9a–b) revealed that the TiO2 nanoparticles and dispersed evenly and well-mixed with the CNTs. The AC impedance analysis (Fig. 9c) showed that the CNT/TiO2 electrodes pretreated with UV light presented the largest polarization resistance. The authors attributed the results to the oxygen-containing groups on the TiO2 surface and its larger Faradic pseudo capacitance from the oxidation–reduction reaction of TiO2. In addition, the calculated impedance derived from the AC impedance method was 10.7 F g−1 for the UV-irradiated CNT/TiO2 electrode and 4.1 F g−1 for the pristine CNT electrode.

Reprinted and edited with permission of Zhang et al. [56]
a–b SEM images of the CNT/TiO2 electrodes. c AC impedance analysis performed on the CNT, CNT/TiO2 and UV-irradiated CNT/TiO2 electrodes.
3.3 Gas Sensors
TiO2/CNT composites have been studied as gas sensors for detection of ammonia, toluene, hydrogen, oxygen, NO, CO, among other gases [35, 57,58,59,60]. The TiO2/CNT hybrid materials present enhanced sensing performance, when compared to sensors made by the single constituents, due to the formation of p-n junctions, facilitated electron transfer and increased surface area [61, 62].
MWCNT forests coated with TiO2 films via ALD were employed as ammonia gas sensors [35]. The samples synthesized were exposed to different concentrations (100, 250, and 500 ppm) of ammonia gas, as shown on Fig. 10. The sensors with the thinnest TiO2 layers, nominal thickness of 5 nm, presented the best response for ammonia detection. TiO2 layers with greater thicknesses may have reduced the diffusion of charge carriers, which decreased the sensitivity of the sensor. Regarding the type of layer configuration, the sensors formed by TiO2-coated MWCNTs with carboxyl PP film had the higher response. According to Kaushik et al. [35], this result can be attributed to the formation of a conduction path for charge carriers that facilitated the charge transfer between the MWCNTs and the TiO2. The authors proposed that the improved sensing behavior of the TiO2/CNT composites is due to the formation of two p-n junctions: (1) between the MWCNTs (p-type semiconductor) and the TiO2 (n-type semiconductor); (2) the TiO2 surface.

Reprinted with permission of Kaushik et al. [35]
Sensor response for ammonia detection of the MWCNTs-TiO2 sensors (a) with and (b) without carboxyl PP layer.
3.4 H2 Production via Water Splitting
Hydrogen (H2) is a promising compound used as an energy source in the replacement of petroleum because of its low molecular weight and high-power density. It can be produced by steam reforming of natural gas; however, it can be harnessed more environmentally friendly way. A greener alternative to using fossil fuels is to reduce water to hydrogen using semiconductor and solar energy, but the high charge carrier recombination of these materials is a limiting factor [63]. Fujishima and Honda [6] were the first to publish a report on splitting water using solar radiation. For that, they used an n-type TiO2 coupled with a Pt counter electrode. The relatively high band-gap of the TiO2 (3.2 eV) makes only ultraviolet light excite the semiconductor, hindering the TiO2 application. Thus, solutions to this limitation have been widely researched.
The photocatalytic activity of TiO2 can be enhanced in numerous ways. One that is commonly researched is the use of dopants, such as metal ions, to decrease the band-gap and better the optical response to sunlight [64, 65]. The most popular dopants are transition metals since their incorporation in the TiO2 lattice can form a new energy level near the conduction band [66]. The use of CNTs combined with TiO2 can also increase the photocatalytic activity of the semiconductor. Carbon Nanotubes can promote charge transfer because of their high electrical conductivity and high surface area [67,68,69].
Recently, Ahmed et al. [70] managed to combine hydrothermal synthesis and CVD to produce a nanoribbon-shaped TiO2 and CNT hybrid material with a lower band-gap than commercially available TiO2. They evaluated the hybrid nanomaterial using different electrolytes (KOH, Na2S2O3, and HCl) and prepared two configurations of electrodes, one using the titania nanoribbons (TNRs) and other using CNT@TNRs. They found a substantial increase in current density voltage from the CNT@TNRs electrode compared with the TNRs electrode under the same configuration. The authors concluded that this was probably because of the decrease of electron–hole recombination on the CNT@TNRs electrode. Moreover, its large surface area, nanoporous structure, and high optical absorption in the visible region made the electrode suitable for H2 production. However, the downside of their method was that a long time was required to synthesize the TNRs.
Another interesting configuration is the usage of TiO2 in the form of nanotubes in combination with CNTs. Because of their 1D structure, nanotubes can present enhanced optical and chemical properties when compared to nanoparticles [36]. For example, Guaglianoni et al. [19] produced cobalt doped titania nanotubes via one-step anodization followed by CVD treatment to grow CNTs on its surface. The method allowed the production of connected arrays of titania nanotubes and CNT. When tested, the Co-TiO2/CNT nanotubes presented an improved photocurrent performance compared with the cobalt doped titania nanotubes and CNT and TiO2 nanoparticles composites reported in literature [71].
4 Conclusion
TiO2 is a widely employed semiconductor, but some of its applications are hindered due to its relatively high band‑gap and recombination rate. The addition of CNTs to form hybrid nanomaterials with TiO2 has been extensively studied for various applications, from photocatalysts to supercapacitors. The unique properties and high specific surface area of the carbon nanotubes allow them to overcome these limitations. CNTs can enhance the photocatalytic activity of the TiO2 by decreasing the recombination rate of electron‑hole pairs and contributing to adsorptive active sites. In addition, TiO2 may improve the capacitance of carbon nanotubes, thus making it a more suitable material to be used as an anode in supercapacitors. Novel CNT/TiO2 nanostructures have been reported in recent years, and a better understanding of the mechanisms that cause this synergic effect and the development of reliable, low‑cost synthesis would make these materials more suitable for energy and eco‑friendly applications.
Abbreviations
- 1D :
-
One-dimensional
- AC:
-
Alternating current
- ALD:
-
Atomic layer deposition
- BET:
-
Brunauer‑Emmett‑Teller
- CNT:
-
Carbon nanotube
- CO:
-
Carbon monoxide
- Co-TiO2 :
-
Cobalt doped titanium dioxide
- CVD:
-
Chemical vapor deposition
- DMF:
-
Dimethylformamide
- DSC:
-
Differential scanning calorimetry
- DSSC:
-
Dye-sensitized solar cell
- EDL:
-
Electric double-layer capacitor
- EDS :
-
Energy dispersive X-ray spectroscopy
- FE-SEM:
-
Field emission scanning electron microscopy
- FF:
-
Fill factor
- FT-IR:
-
Fourier transform infrared spectroscopy
- H2 :
-
Hydrogen gas
- HCl:
-
Hydrochloric acid
- HNO3 :
-
Nitric acid
- JSC :
-
Short circuit current density
- KOH :
-
Potassium hydroxide
- LIB :
-
Lithium-ion battery
- MAO :
-
Micro-arc oxidation
- MWCNT :
-
Multi-walled carbon nanotube
- Na2S2O3:
-
Sodium thiosulfate
- NO :
-
Nitric oxide
- NP :
-
Nanoparticle
- PAN :
-
Polyacrylonitrile
- PCE :
-
Power conversion efficiency
- PP :
-
Plasma polymer
- PVP :
-
Poly(vinyl pyrrolidone)
- SEM:
-
Scanning electron microscopy
- SiO2 :
-
Silicon dioxide
- SWCNT :
-
Single-walled carbon nanotube
- TBT :
-
Titanium butoxide
- TEM :
-
Transmission electronic microscopy
- TGA :
-
Thermogravimetric ananalysis
- TiO2 :
-
Titanium dioxide
- TNR :
-
Titania nanoribbon
- TTIP :
-
Titanium isopropoxide
- UV :
-
Ultraviolet
- VOC :
-
Open circuit voltage
- XRD :
-
X-ray diffraction
References
Haider, A.J., Jameel, Z.N., Al-Hussaini, I.H.M.: Review on: Titanium dioxide applications. In: Energy Procedia, pp. 17–29. Elsevier Ltd. (2019). https://doi.org/10.1016/j.egypro.2018.11.159
Abdel Messih, M.F., Ahmed, M.A., Soltan, A., Anis, S.S.: Facile approach for homogeneous dispersion of metallic silver nanoparticles on the surface of mesoporous titania for photocatalytic degradation of methylene blue and indigo carmine dyes. J. Photochem. Photobiol. A Chem. 335, 40–51 (2017). https://doi.org/10.1016/j.jphotochem.2016.11.001
Hamaloğlu, K.Ö., Sağ, E., Tuncel, A.: Bare, gold and silver nanoparticle decorated, monodisperse-porous titania microbeads for photocatalytic dye degradation in a newly constructed microfluidic, photocatalytic packed-bed reactor. J. Photochem. Photobiol. A Chem. 332, 60–65 (2017). https://doi.org/10.1016/j.jphotochem.2016.08.015
Da Dalt, S., Alves, A.K., Bergmann, C.P.: Photocatalytic degradation of methyl orange dye in water solutions in the presence of MWCNT/TiO2 composites. Mater. Res. Bull. 48, 1845–1850 (2013). https://doi.org/10.1016/j.materresbull.2013.01.022
Mor, G.K., Shankar, K., Paulose, M., Varghese, O.K., Grimes, C.A.: Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 6, 215–218 (2006). https://doi.org/10.1021/nl052099j
Fujishima, A., Honda, K.: Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972). https://doi.org/10.1038/238037a0
Leary, R., Westwood, A.: Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon N. Y. 49, 741–772 (2011). https://doi.org/10.1016/j.carbon.2010.10.010
Ullah, I., Haider, A., Khalid, N., Ali, S., Ahmed, S., Khan, Y., Ahmed, N., Zubair, M.: Tuning the band gap of TiO2 by tungsten doping for efficient UV and visible photodegradation of Congo red dye. Spectrochim. Acta—Part A Mol Biomol. Spectrosc. 204, 150–157 (2018). https://doi.org/10.1016/j.saa.2018.06.046
N. G., D.R. A., A.I. A., J. R.L.: Tuning the optical band Gap of pure TiO2 via photon induced method. Optik (Stuttg). 179, 889–894 (2019). https://doi.org/10.1016/j.ijleo.2018.11.009.
Pesci, F.M., Wang, G., Klug, D.R., Li, Y., Cowan, A.J.: Efficient suppression of electron-hole recombination in oxygen-deficient hydrogen-treated TiO2 nanowires for photoelectrochemical water splitting. J. Phys. Chem. C. 117, 25837–25844 (2013). https://doi.org/10.1021/jp4099914
Al Jitan, S., Palmisano, G., Garlisi, C.: Synthesis and surface modification of TiO2-based photocatalysts for the conversion of CO2. Catalysts (2020)
Baughman, R.H., Zakhidov, A.A., De Heer, W.A.: Carbon nanotubes—The route toward applications. Science 297, 787–792 (2002). https://doi.org/10.1126/science.1060928
Serp, P., Figueiredo, J.L.: Carbon Materials for Catalysis. John Wiley & Sons, Inc., Hoboken, NJ, USA (2008). https://doi.org/10.1002/9780470403709
Iijima, S.: Helical microtubules of graphitic carbon. Nature 354, 56–58 (1991). https://doi.org/10.1038/354056a0
Woan, K., Pyrgiotakis, G., Sigmund, W.: Photocatalytic Carbon-Nanotube-TiO2 Composites. Adv. Mater. 21, 2233–2239 (2009). https://doi.org/10.1002/adma.200802738
Banerjee, S., Wong, S.S.: Synthesis and characterization of carbon nanotube-nanocrystal heterostructures. Nano Lett. 2, 195–200 (2002). https://doi.org/10.1021/nl015651n
Acauan, L., Dias, A.C., Pereira, M.B., Horowitz, F., Bergmann, C.P.: Influence of different defects in vertically aligned carbon nanotubes on TiO2 nanoparticle formation through atomic layer deposition. ACS Appl. Mater. Interfaces. 8, 16444–16450 (2016). https://doi.org/10.1021/acsami.6b04001
Yao, Y., Li, G., Ciston, S., Lueptow, R.M., Gray, K.A.: Photoreactive TiO2/carbon nanotube composites: synthesis and reactivity. Environ. Sci. Technol. 42, 4952–4957 (2008). https://doi.org/10.1021/es800191n
Guaglianoni, W.C., Florence, C.L., Bonatto, F., Venturini, J., Arcaro, S., Alves, A.K., Bergmann, C.P.: Novel nanoarchitectured cobalt-doped TiO2 and carbon nanotube arrays: synthesis and photocurrent performance. Ceram. Int. 45, 2439–2445 (2019). https://doi.org/10.1016/j.ceramint.2018.10.169
Zhu, Z., Zhou, Y., Yu, H., Nomura, T., Fugetsu, B.: Photodegradation of humic substances on MWCNT/nanotubular-TiO2 composites. Chem. Lett. 35, 890–891 (2006). https://doi.org/10.1246/cl.2006.890
Yang, L., Leung, W.W.F.: Electrospun TiO2 nanorods with carbon nanotubes for efficient electron collection in dye-sensitized solar cells. Adv. Mater. 25, 1792–1795 (2013). https://doi.org/10.1002/adma.201204256
Zhu, Y.E., Yang, L., Sheng, J., Chen, Y., Gu, H., Wei, J., Zhou, Z.: Fast sodium storage in TiO2@CNT@C Nanorods for High-Performance Na-Ion Capacitors. Adv. Energy Mater. 7, 1701222 (2017). https://doi.org/10.1002/aenm.201701222
Hieu, N.T., Baik, S.J., Chung, O.H., Park, J.S.: Fabrication and characterization of electrospun carbon nanotubes/titanium dioxide nanofibers used in anodes of dye-sensitized solar cells. Synth. Met. 193, 125–131 (2014). https://doi.org/10.1016/j.synthmet.2014.04.010
Wang, G.J., Lee, M.W., Chen, Y.H.: A TiO2/CNT coaxial structure and standing CNT array laminated photocatalyst to enhance the photolysis efficiency of TiO2. Photochem. Photobiol. 84, 1493–1499 (2008). https://doi.org/10.1111/j.1751-1097.2008.00374.x
Liu, J., Feng, H., Jiang, J., Qian, D., Li, J., Peng, S., Liu, Y.: Anatase-TiO2/CNTs nanocomposite as a superior high-rate anode material for lithium-ion batteries. J. Alloys Compd. 603, 144–148 (2014). https://doi.org/10.1016/j.jallcom.2014.03.089
Koli, V.B., Dhodamani, A.G., Delekar, S.D., Pawar, S.H.: In situ sol-gel synthesis of anatase TiO2-MWCNTs nanocomposites and their photocatalytic applications. J. Photochem. Photobiol. A Chem. 333, 40–48 (2017). https://doi.org/10.1016/j.jphotochem.2016.10.008
Nguyen, K.C., Ngoc, M.P., Van Nguyen, M.: Enhanced photocatalytic activity of nanohybrids TiO2/CNTs materials. Mater. Lett. 165, 247–251 (2016). https://doi.org/10.1016/j.matlet.2015.12.004
Naffati, N., Sampaio, M.J., Da Silva, E.S., Nsib, M.F., Arfaoui, Y., Houas, A., Faria, J.L., Silva, C.G.: Carbon-nanotube/TiO2 materials synthesized by a one-pot oxidation/hydrothermal route for the photocatalytic production of hydrogen from biomass derivatives. Mater. Sci. Semicond. Process. 115, 105098 (2020). https://doi.org/10.1016/j.mssp.2020.105098
Dai, K., Zhang, X., Fan, K., Peng, T., Wei, B.: Hydrothermal synthesis of single-walled carbon nanotube-TiO2 hybrid and its photocatalytic activity. Appl. Surf. Sci. 270, 238–244 (2013). https://doi.org/10.1016/j.apsusc.2013.01.010
Alosfur, F.K.M., Jumali, M.H.H., Radiman, S., Ridha, N.J., Yarmo, M.A., Umar, A.A.: Modified microwave method for the synthesis of visible light-responsive TiO2/MWCNTs nanocatalysts. Nanoscale Res. Lett. 8, 1–6 (2013). https://doi.org/10.1186/1556-276X-8-346
Bazli, L., Siavashi, M., Shiravi, A.: A Review of Carbon nanotube/TiO2 Composite prepared via Sol-Gel method. J Compos. Compd. 1, 1–12 (2019). https://doi.org/10.29252/jcc.1.1.1
De Andrade, M.J., Lima, M.D., Stein, L., Bergmann, C.P., Roth, S.: Single-walled carbon nanotube silica composites obtained by an inorganic sol-gel route. Phys. Status Solidi Basic Res. 244, 4218–4222 (2007). https://doi.org/10.1002/pssb.200776114
de Andrade, M.J., Lima, M.D., Bergmann, C.P., de Ramminger, G.O., Balzaretti, N.M., Costa, T.M.H., Gallas, M.R.: Carbon nanotube/silica composites obtained by sol–gel and high-pressure techniques. Nanotechnology 19, 265607 (2008). https://doi.org/10.1088/0957-4484/19/26/265607
Zhao, S., Ding, H., Chen, J., Yang, C., Xian, X.: Facile synthesis of CNTs@TiO2 composites by solvothermal reaction for high-rate and long-life lithium-ion batteries. J. Phys. Chem. Solids. 152, 109950 (2021). https://doi.org/10.1016/j.jpcs.2021.109950
Kaushik, P., Eliáš, M., Michalička, J., Hegemann, D., Pytlíček, Z., Nečas, D., Zajíčková, L.: Atomic layer deposition of titanium dioxide on multi-walled carbon nanotubes for ammonia gas sensing. Surf. Coatings Technol. 370, 235–243 (2019). https://doi.org/10.1016/j.surfcoat.2019.04.031
Chen, C., Fan, Y., Gu, J., Wu, L., Passerini, S., Mai, L.: One-dimensional nanomaterials for energy storage. J. Phys. D. Appl. Phys. 51, 113002 (2018). https://doi.org/10.1088/1361-6463/aaa98d
Anjusree, G.S., Deepak, T.G., Pai, K.R.N., Joseph, J., Arun, T.A., Nair, S.V., Nair, A.S.: TiO2 nanoparticles @ TiO2 nanofibers-an innovative one-dimensional material for dye-sensitized solar cells. RSC Adv. 4, 22941–22945 (2014). https://doi.org/10.1039/c4ra03701d
Marinho, B.A., de Souza, S.M.A.G.U., de Souza, A.A.U., Hotza, D.: Electrospun TiO2 nanofibers for water and wastewater treatment: a review. J. Mater. Sci. 56, 5428–5448 (2021). https://doi.org/10.1007/s10853-020-05610-6
Xue, J., Wu, T., Dai, Y., Xia, Y.: Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 119, 5298–5415 (2019). https://doi.org/10.1021/acs.chemrev.8b00593
MacDonald, T.J., Tune, D.D., Dewi, M.R., Gibson, C.T., Shapter, J.G., Nann, T.: A TiO2 nanofiber-carbon nanotube-composite photoanode for improved efficiency in dye-sensitized solar cells. Chemsuschem 8, 3396–3400 (2015). https://doi.org/10.1002/cssc.201500945
Jung, J.Y., Lee, D., Lee, Y.S.: CNT-embedded hollow TiO2 nanofibers with high adsorption and photocatalytic activity under UV irradiation. J. Alloys Compd. 622, 651–656 (2015). https://doi.org/10.1016/j.jallcom.2014.09.068
Anzar, N., Hasan, R., Tyagi, M., Yadav, N., Narang, J.: Carbon nanotube—a review on synthesis properties and plethora of applications in the field of biomedical science. Sensors Int. 1, 100003 (2020). https://doi.org/10.1016/j.sintl.2020.100003
Manawi, Y.M., Ihsanullah, Samara, A., Al-Ansari, T., Atieh, A.: A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials (Basel) 11, 822 (2018). https://doi.org/10.3390/ma11050822
Prasek, J., Drbohlavova, J., Chomoucka, J., Hubalek, J., Jasek, O., Adam, V., Kizek, R.: Methods for carbon nanotubes synthesis—review. J. Mater. Chem. 21, 15872–15884 (2011). https://doi.org/10.1039/c1jm12254a
Campos Guaglianoni, W., Garcia, A.P., Basegio, T.M., Arcari Bassani, M.A., Arcaro, S., Pérez Bergmann, C.: Influence of CVD parameters on Co-TiO2 /CNT properties: a route to enhance energy harvesting from sunlight. Int. J. Appl. Ceram. Technol. 18, 1297–1306 (2021). https://doi.org/10.1111/ijac.13773
Roy, P., Srivastava, S.K.: Nanostructured anode materials for lithium ion batteries. J. Mater. Chem. A. 3, 2454–2484 (2015). https://doi.org/10.1039/c4ta04980b
Ventosa, E., Chen, P., Schuhmann, W., Xia, W.: CNTs grown on oxygen-deficient anatase TiO2-δ as high-rate composite electrode material for lithium ion batteries. Electrochem. Commun. 25, 132–135 (2012). https://doi.org/10.1016/j.elecom.2012.09.031
Lo, W.C., Su, S.H., Chu, H.J., He, J.L.: TiO2-CNTs grown on titanium as an anode layer for lithium-ion batteries. Surf. Coatings Technol. 337, 544–551 (2018). https://doi.org/10.1016/j.surfcoat.2018.01.029
Becker, H.J.: United States Patent Office Low Voltage Electolytic Capacitor. https://patentimages.storage.googleapis.com/a2/f8/a9/b7d5c04a415c8b/US2800616.pdf (1957). Accessed 31 May 2021
Conway, B.E.: Electrochemical Supercapacitors. Springer US, Boston, MA (1999). https://doi.org/10.1007/978-1-4757-3058-6
Burke, A.: Ultracapacitors: Why, how, and where is the technology. J. Power Sources. 91, 37–50 (2000). https://doi.org/10.1016/S0378-7753(00)00485-7
Mendoza, R., Al-Sardar, M., Oliva, A.I., Robledo-Trujillo, G., Rodriguez-Gonzalez, V., Zakhidov, A., Oliva, J.: Improving the electrochemical performance of flexible carbon nanotubes based supercapacitors by depositing Ni@TiO2: W nanoparticles on their anodes. J. Phys. Chem. Solids. 155, 110128 (2021). https://doi.org/10.1016/j.jpcs.2021.110128
Liu, C.G., Liu, M., Li, F., Cheng, H.M.: Frequency response characteristic of single-walled carbon nanotubes as supercapacitor electrode material. Appl. Phys. Lett. 92, 143108 (2008). https://doi.org/10.1063/1.2907501
Xiao, Q., Zhou, X.: The study of multiwalled carbon nanotube deposited with conducting polymer for supercapacitor. Electrochim. Acta. 48, 575–580 (2003). https://doi.org/10.1016/S0013-4686(02)00727-2
Zhou, Y.K., He, B.L., Zhang, F.B., Li, H.L.: Hydrous manganese oxide/carbon nanotube composite electrodes for electrochemical capacitors. J. Solid State Electrochem. 8, 482–487 (2004). https://doi.org/10.1007/s10008-003-0468-7
Zhang, B., Shi, R., Zhang, Y., Pan, C.: CNTs/TiO2 composites and its electrochemical properties after UV light irradiation. Prog. Nat. Sci. Mater. Int. 23, 164–169 (2013). https://doi.org/10.1016/j.pnsc.2013.03.002
Seekaew, Y., Wisitsoraat, A., Phokharatkul, D., Wongchoosuk, C.: Room temperature toluene gas sensor based on TiO2 nanoparticles decorated 3D graphene-carbon nanotube nanostructures. Sensors Actuators, B Chem. 279, 69–78 (2019). https://doi.org/10.1016/j.snb.2018.09.095
Khalilian, M., Abdi, Y., Arzi, E.: Formation of well-packed TiO2 nanoparticles on multiwall carbon nanotubes using CVD method to fabricate high sensitive gas sensors. J. Nanoparticle Res. 13, 5257–5264 (2011). https://doi.org/10.1007/s11051-011-0511-z
Ueda, T., Takahashi, K., Mitsugi, F., Ikegami, T.: Preparation of single-walled carbon nanotube/TiO2 hybrid atmospheric gas sensor operated at ambient temperature. Diam. Relat. Mater. 18, 493–496 (2009). https://doi.org/10.1016/j.diamond.2008.08.017
Frontera, P., Malara, A., Stelitano, S., Leonardi, S.G., Bonavita, A., Fazio, E., Antonucci, P., Neri, G., Neri, F., Santangelo, S.: Characterisation and H2O2 sensing properties of TiO2-CNTs/Pt electro-catalysts. Mater. Chem. Phys. 170, 129–137 (2016). https://doi.org/10.1016/j.matchemphys.2015.12.030
Nunes Simonetti, E.A., Cardoso de Oliveira, T., Enrico do Carmo Machado, A., Coutinho Silva, A.A., Silva dos Santos, A., de Simone Cividanes L.: TiO2 as a gas sensor: The novel carbon structures and noble metals as new elements for enhancing sensitivity—a review. Ceram. Int. 47, 17844–17876 (2021). https://doi.org/10.1016/j.ceramint.2021.03.189
Li, Z., Yao, Z.J., Haidry, A.A., Plecenik, T., Xie, L.J., Sun, L.C., Fatima, Q.: Resistive-type hydrogen gas sensor based on TiO2: a review. Int. J. Hydrogen Energy. 43, 21114–21132 (2018). https://doi.org/10.1016/j.ijhydene.2018.09.051
Moniz, S.J.A., Shevlin, S.A., Martin, D.J., Guo, Z.X., Tang, J.: Visible-light driven heterojunction photocatalysts for water splitting-a critical review. Energy Environ. Sci. 8, 731–759 (2015). https://doi.org/10.1039/c4ee03271c
Venturini, J., Bonatto, F., Guaglianoni, W.C., Lemes, T., Arcaro, S., Alves, A.K., Bergmann, C.P.: Cobalt-doped titanium oxide nanotubes grown via one-step anodization for water splitting applications. Appl. Surf. Sci. 464, 351–359 (2019). https://doi.org/10.1016/j.apsusc.2018.09.093
Dholam, R., Patel, N., Adami, M., Miotello, A.: Hydrogen production by photocatalytic water-splitting using Cr- or Fe-doped TiO2 composite thin films photocatalyst. Int. J. Hydrogen Energy 34, 5337–5346 (2009). https://doi.org/10.1016/j.ijhydene.2009.05.011
Khlyustova, A., Sirotkin, N., Kusova, T., Kraev, A., Titov, V., Agafonov, A.: Doped TiO2: the effect of doping elements on photocatalytic activity. Mater. Adv. 1, 1193–1201 (2020). https://doi.org/10.1039/d0ma00171f
Yang, H., Wu, S., Duan, Y., Fu, X., Wu, J.: Surface modification of CNTs and enhanced photocatalytic activity of TiO2 coated on hydrophilically modified CNTs. Appl. Surf. Sci. 258, 3012–3018 (2012). https://doi.org/10.1016/j.apsusc.2011.11.029
Wang, S., Ji, L., Wu, B., Gong, Q., Zhu, Y., Liang, J.: Influence of surface treatment on preparing nanosized TiO2 supported on carbon nanotubes. Appl. Surf. Sci. 255, 3263–3266 (2008). https://doi.org/10.1016/j.apsusc.2008.09.031
Wang, H., Wang, H.L., Jiang, W.F., Li, Z.Q.: Photocatalytic degradation of 2,4-dinitrophenol (DNP) by multi-walled carbon nanotubes (MWCNTs)/TiO2 composite in aqueous solution under solar irradiation. Water Res. 43, 204–210 (2009). https://doi.org/10.1016/j.watres.2008.10.003
Ahmed, A.M., Mohamed, F., Ashraf, A.M., Shaban, M., Aslam Parwaz Khan, A., Asiri, A.M.: Enhanced photoelectrochemical water splitting activity of carbon nanotubes@TiO2 nanoribbons in different electrolytes. Chemosphere 238, 124554 (2020). https://doi.org/10.1016/j.chemosphere.2019.124554
Chaudhary, D., Singh, S., Vankar, V.D., Khare, N.: A ternary Ag/TiO2/CNT photoanode for efficient photoelectrochemical water splitting under visible light irradiation. Int. J. Hydrogen Energy. 42, 7826–7835 (2017). https://doi.org/10.1016/j.ijhydene.2016.12.036
Acknowledgements
The authors would like to thank the Human Resources Program of the National Agency of Petroleum, Natural Gas and Biofuels (PRH-ANP 13.1; grant number 042319) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 for the financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
da Rosa Cunha, G., Guaglianoni, W.C., Bergmann, C.P. (2022). CNT/TiO2 Hybrid Nanostructured Materials: Synthesis, Properties and Applications. In: Kopp Alves, A. (eds) Environmental Applications of Nanomaterials. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-86822-2_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-86822-2_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86821-5
Online ISBN: 978-3-030-86822-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)