Abstract
In this chapter, we discuss the effects of acute and chronic exercise in metabolic reprograming. The exercise causes considerable systemic disturbance in the homeostasis. However, exercise increases the blood flow and nutrient delivery to the skeletal muscle to supply the energy substrate and oxygen via sustained muscle contraction. The disruption in homeostasis is proportional to the skeletal muscle mass involved in exercise as well as the intensity and duration of exercise. Thus, different types of cells undergo metabolic reprograming for adaptation to exercise. This adaptation starts after one bout of exercise, with increased mRNA expression to supply the new challenges to cells; however, the effects of adaptive reprograming are slow, and it is achieved with continuous exercise training. We focused on the skeletal muscle, adipose tissue, and immune cells adaptations induced by acute and chronic exercises. Exercise training induces an anti-inflammatory milieu and improves cellular metabolism; thus, as we discuss in the following sections, training is an excellent non-pharmacological tool in the treatment of chronic inflammatory diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Metabolic Reprograming with Aerobic and Resistance Exercise
Chronic exercise improves cardiovascular health and increases skeletal muscle hypertrophy, promoting several adaptations. In the liver, exercise reduces inflammation, resulting in improved insulin sensitivity with superior, fine-tuned endogenous glucose production; in terms of brain health, the benefits include neuroprotection and neurogenesis. This improvement promoted by regular exercise is due to the adaptations of the whole body to exercise-induced stress. These adaptations can be defined as “reprograming.”
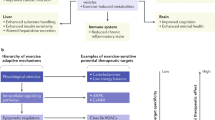
Metabolic reprogram is a cellular adaptation in the internal machinery via regulation of transcription factors, epigenetic alterations, mRNA expression, enzyme activity, and storage of energy substrates. This exercise-induced reprograming depends of the type (aerobic, strength, or concurrent exercise session), volume (short or long duration), intensity (high, moderate, or low), recovery during the exercise session (passive, active, short, or long), and duration of the exercise program (2 week, 6 week, 12 week, 24 week, 48 week, 1 year, 5 year, or 10 year).
Here, we will focus on metabolic reprograming in the skeletal muscles, adipose tissues, and immune cells after aerobic and strength training. By all of regular exercise training, strength and endurance are the most studied. Strength training improves s capacity of keletal muscle to contract, while aerobic training improves oxygen Uptake, from the blood, and delivery to the muscle. Thus, both strength and aerobic exercise training are prescribed to improve cardiovascular fitness, force production, and body composition. Independent of the exercise type, actually, exercise is considered an excellent non-pharmacological method of preventing and treating several chronic inflammatory diseases [1]. In contrast, a sedentary lifestyle is an independent risk factor in condition called the diseasome of physical inactivity [2].
Aerobic exercise, classically adopted for cardiorespiratory fitness and control of body fat, is usually performed as a moderate-intensity (50–70% VO2max) continuous training (MICT) (running, bike, indoor or outdoor). Recent studies have suggested that aerobic exercise can exert the same or similar adaptation when performed as high-intensity (90–120% VO2max) interval training (HIIT) in the same modalities and can be adopted in various forms. Some studies have shown that HIIT can positively impact the neuroendocrine axis, such as the leptin levels, and improve lipid metabolism in overweight/obese and lean subjects [3–6].
In order to obtain a deeper understanding about how exercise training can induce metabolic reprograming, the acute effects of each exercise session need to be examined in detail.
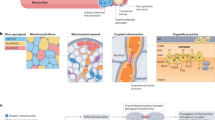
Figure 1 shows main acute and chronic effects of aerobic exercise performed at different intensities on the skeletal muscle and adipose tissue.
A substantial proportion of the alterations caused by a single bout of aerobic exercise is related to neuro-endocrine axis activation. Acute exercise enhances several hormones, especially those related to lipolysis in the adipose tissue and glycogenolysis in the skeletal muscle and liver (cortisol and adrenaline) that increase the availability of energy substrates (Non-esterified fatty acids (NEFA) and glucose) for muscle workload. With aerobic exercise both, carbohydrates and fat can be utilized for oxidation, and the metabolic changes are dependent on the exercise intensity and training status of the subjects.
The crossover point that leads to better oxidation of carbohydrate that the fatty acid in proportion, it is approximately in 65% of VO2max. However, the point of this bypass characterized by the predominance of carbohydrates as the major fuel for oxidation is dependent on the training levels. Sedentary subjects have a lower capacity of oxidation with exercise performed at intensities >45% VO2max, while well-trained athletes showed this threshold at intensities of approximately 75% VO2max. This is because fatty acid mobilization, transport, and oxidation are not easy, and athletes have superior adaptation to lipid metabolism [7, 8].
Thus, it is necessary to coordinate the mobilization of fatty acids from the adipose tissue, transport in the plasma, and fatty acid uptake by the skeletal muscle; once FA is inside the skeletal muscle, it has to be transported to the mitochondria and finally oxidized for ATP production [8]. The main adaptations involved in lipids metabolic reprograming are associated with increased delivery of fatty acids by the subcutaneous adipose tissue (SAT) and increased fatty acid uptake by the skeletal muscle [9]. The blood flow in the SAT increases 2–3 folds in endurance athletes [9]. This is an important step in the delivery of fatty acids from the adipose tissue to the skeletal muscle; however, albumin is the other limiting factor for fatty acid transport. Moreover, fatty acid transportation from circulation to inside the skeletal muscle is carried out by CD36 transporter in the plasma membrane of myocytes. Acute exercise and training induce increase in the CD36 in the sarcolemma [10–12].
Thus, metabolic reprograming can be observed when aerobic exercise is maintained for a long time all machinery is organizing for better efficiency for mobilization and oxidation of FA.
Well-trained individuals tend to store lipids in the skeletal muscle, similar to that found in diabetic patients; however, with exercise, fat accumulation does not harm cellular functioning, as is observed in metabolic diseases [13, 14].
Lipid storage in myofibers is mediated by perilipins, the perilipin-2, perilipin-3, and perilipin-5, the most common perilipins founded in lipid droplets (LD) in the skeletal muscle. An interesting study conducted by Daemmen et al. 2018 showed that the localization and size of the LD was different between diabetic subjects and athletes although the content of the LD was the same. In this sense, the diabetic subjects showed larger LD in type II fyber, and the athletes have smaller LD in type I fiber [15]. Moreover, athletes present lipid deposition near the mitochondria with higher expression of ATGL and CGI-58 [16]. Thus, these adaptations cause high LD turnover, optimize the energy production machinery to supply energy during exercise, and mitigate the lipotoxicity that could be generated by increased fatty acid accumulation [17].
AMP-activated protein kinase (AMPK), MAP kinase p38, calmodulin-dependent protein kinase (CAMKK), PGC-1alpha, and p53 form the axis that induces mitochondrial remodeling and increases electron chain transporters to maximize the oxidative phosphorylation. Increased Ca2+ concentration via muscle contraction directly activates CAMKK [18].
In general, both types of aerobic exercise training (MICT and HIIT) promote similar metabolic reprograming in the skeletal muscle and adipose tissue. However, short-term (<12 weeks) exercise training is insufficient to promote these cellular adaptations, while long-term (>12 weeks) training is able to promote them [4, 19, 20].
It is noteworthy that the same pathways responsible for energy-induced metabolic reprograming also lead to the production of proteins called myokines [21]. Myokines are a group of proteins formed by cytokines, chemokines, and growth factors produced by muscle cells that are released into the circulation and act in distant organs and different cell types [22, 23]. The most studied myokine is IL-6 [24].
IL-6 is a major moderator involved in the regulation of the acute-phase response to injury and infection. The skeletal musclecontraction induces the activation of the mitogen-activated protein kinase and Janus kinase (JAK)-signal transducers and activators of transcription cascade phosphorylation, culminating with IL-6 transcription in skeletal muscle [25]. The IL-6 produced and secreted by the skeletal muscle during contraction plays an important role in metabolism. Moreover, it induces the translocation of GLUT4, in the muscle, increasing glycogen synthesis and insulin sensitivity in the central and peripheral organs as well as augments lipid oxidation in the skeletal muscle to deliver fuel to source the skeletal muscle during exercise [22].
Prolonged and intense running on a treadmill at 70% VO2 max until exhaustion increased the muscle plasma levels for IL-6, and the post-run muscle glycogen concentrations were negatively correlated with modifications in muscle IL-6. Cabral-Santos et al. [26] found that during acute high-intensity interval exercise, there was a volume-dependent increase in the IL-6 levels.
IL-6 modifies the anti-inflammatory responses once it can induce anti-inflammatory cytokines production, such as IL-10 and IL-1ra [27]. IL-10 is essentiall in the anti-inflammatory response. It promotes the preservation of IkB, inhibiting the nuclear transcription factor kappa B (NF-kB) signaling, the main transcription factor of TNF-α [27]—a cytokine with a pro-inflammatory character that regulates insulin sensitivity and induces lipolysis. Cabral-Santos et al. [28] found significantly increased IL-6 and IL-10 levels after HIIE (1:1-min at vVO2 max, 5 km run), indicating a crucial role of HIIE in preventing a persistent inflammatory tendency.
Strength training also promotes metabolic reprograming; however, the cellular mechanism and the metabolic pathways are different because strength (resistance) training induces anabolic pathways that are contrary to the catabolic pathways induced by aerobic training. The main cellular response is the increase in the cross sectional area by the induction of the hypertrophy pathway controlled by the mTOR-p70S6 axis.
Different forms can activate the mTOR-P70S6. In physiological context, the insulin and growth factors (in special IGF-1) lead to activation of mTOR toward the activation of PI3K-Akt, this pathway is upstream to mTOR. It was previously believed that the activation of exercise-induced hypertrophy was caused by increase in the anabolic hormones and the PI3k pathway; however, simulation with IGF-1 was insufficient to mimic the hypertrophy induced by mechanical induction. Currently, the principal hypothesis is that the mechanical overload activates the dissociation of TSC2 (an inhibitor of mTOR-Rheb complex) in the lysosomes assembly and allows downstream signaling with the activation of p70. However, it is unclear how TSC2 phosphorylation and dissociation of mTOR-rheb complex occur, and more studies are necessary to identify the mechanism [29, 30].
The effects of resistance training are dependent on the size of the muscle skeletal recruited during exercise; for example, training of the biceps brachial induces local adaptation; however, this effect is not endocrine, and leg exercise training induces a huge endocrine response, with increase in the production and release of myokines. The fall in the serum myokine concentration is more rapid than that with endurance training.
Intensity and recovery intervals are the fundamental factors for changes in the cytokine response, main IL-6 alterations in resistance training [31, 32]. These factors influence the number of repetitions per- formed in subsequent set. In this sense, Izquierdo et al. [33] verified that the amount of metabolic demand or the fatigue experienced during the strength exercise session affects the hormonal and cytokine response patterns.
Myokines, specifically IL-6, play a role in skeletal muscle tissue regeneration after exercise as well as the recruitment of neutrophils, monocytes, and lymphocytes that phagocytize debris resources. The IL-6 aids the activation, differentiation and proliferation of satellite cells, which contribute to the formation of new myonuclei. IL-6 act on mTORC1 signaling and protein synthesis in the cultured myotubes [34]. It was observed that IL-6 activates gp130-Akt and mTORC1 pathways, inducing protein synthesis, suggesting that the IL-6 response to strength exercise potentiates muscle hypertrophy [34].
A combination of strength and aerobic exercises in the same session can be called concurrent exercise. Acute and chronic concurrent sessions promote alterations in the IL-6 and TNF-α responses that can be associated with an acute decline in the strength performance observed when high-intensity interval aerobic exercise is performed before strength exercise. This response was related a possible role of TNF-α and IL-6 as a trigger to restore the energy demand by providing substrates to help maintain contractile activity in skeletal muscle [35]. Long-term (12 weeks) practice for concurrent training leads to improved cardiorespiratory fitness and maximal strength accompanied with an increased cytokines response, leading to an anti-inflammatory response after an acute session of concurrent exercise. However, the effects of concurrent training in cellular reprograming remain unclear [36, 37].
The high-energy demand of exercise, especially in the case of long-duration and high-intensity exercise, such as that performed by long-distance runners, aquatic marathoners, and triathletes, is associated with stress hormonal response (increased catecholamines and cortisol) causing deep alterations in the immune cells.
2 Exercise and Immune System
The relationship of exercise and the immune system was first discussed during the late 70s and early 80s when some researchers observed that a single bolt of exercise (mainly aerobic) could cause an acute increase in the number of leucocytes in the circulation, with the levels returning to pre-exercise levels within some hours after activity cessation [38]. At this time, researches had already established the potential role of some hormones in this relationship and proposed that cortisol could mediate the re-sequestration of the immune cells. Several years thereafter, a research group from Denmark showed that this exercise-induced leukocytosis was, in part, catecholamine-dependent once catecholamines were released during exercise and the leucocytes expressed β2-adrenergic receptors [39].
Neutrophils are released in the highest amount after exercise, and their increased levels are maintained in the circulation for up to 6 h after exercise cessation. Complete recovery is generally achieved within 24 h [40]. Most of these cells are immature, less differentiated, and precursor neutrophils; thus, despite increased numbers, the function is decreased, especially after high-intensity and prolonged exercise (>2 h).
Monocytes also increase in response to high-intensity and long-duration exercise. In case of very prolonged exercise sessions, monocytosis is delayed by 1–2 h. In most cases, monocytes return to the rest levels 6 h after exercise cessation. Some studies also report a decrease in the function of these cells in response to exhaustive exercise [41].
Lymphocytes play a different role. Immediately after prolonged and/or high-intensity exercise, lymphocytes are also increased in the circulation; however, the number of lymphocytes decreases below the pre-exercise values within 30 min, returning to rest levels by up to 6 h after exercise cessation [41]. This lymphopenia is more prominent in the lymphocyte subtypes with potent effector functions (e.g., natural killer (NK) cells, γδT cells, and CD8+T cells). Moreover, T cells proliferation, NK cell cytotoxicity, and IgA salivary concentration are reportedly inhibited after a bout of very prolonged exercise [42].
The decrease in the function of immune cells in response to exhaustive exercise has been well documented in the literature. Two decades previously, the “open window” hypothesis was postulated proposing that up to 72 h after a high-intensity and prolonged exercise season, the host body would be more susceptible to viral infections because of immunodepression [43]. It could be due to a high concentration of cortisol in the blood in response to exercise stress and the decrease of substrates (glucose and glutamine) availability to the immune cells. This classic paradigm in the field of exercise immunology is still being understood with several contradictory results.
However, some studies, have reported a positive correlation between exhaustive exercise and upper respiratory tract infection (URTI). In 1994, Nieman [44] proposed a “J-shaped” curve relationship between the exercise workload and URTI risk. It indicates that sedentary individuals are at a higher risk of URTI than those who exercised moderately; however, those performing heavy exercise may have a higher risk than who led a sedentary lifestyle.
This “J-shaped” curve was challenged in many studies where they failed to find this relationship. Thus, an “S-shaped” curve was proposed [45], suggesting that very elite athletes are better adapted to the demands of their training and are less susceptible to URTI. It is noteworthy that this population was at a lower risk of URTI and had a better quality of recovery and nutrition, suggesting that exhaustive exercise by itself may not promote deep immunodepression and increase URTI risk; rather, these consequences were attributable to inappropriate recovery and poor nutrition.
The inability to maintain an exercise workload can be classified as acute fatigue. It may be caused by repeated bouts of intense exercise on the same day and/or during consecutive days without adequate recovery. Athletes who aim to improve their performance are often close to this fatigue and intend to reach an “overreaching” state when performance is enhanced. Continuous intense training without proper balance between training and recovery may surpass “overreaching” and result in an “overtraining syndrome.” [46]. At this point, fatigue is persistent, performance is considerably decreased, hormonal status is disturbed, and immune functions are declined. Over-trained individuals present with a decrease in the plasma glutamine concentration and salivary IgA and are at a higher risk of URTI.
High-intensity and prolonged training can cause transient immunodepression because overreaching athletes present reduction in resting neutrophil degranulation, lymphocyte proliferation, and antibody production. However, their immune functions are restored, preventing a rise in the URTI risk. In contrast, over-trained athletes cannot recover from the overreaching stage and frequently experience upper respiratory tract illnesses and chronic decrease in immune cells cytokines and antibody production [46].
Not only exercise workload and immune system, but also nutritional status influences the exercise recovery. During the previous 30 years, scientists have investigated nutritional strategies to prevent exercise-induced immunodepression and its complications.
The energy substrate is strongly related to immune cell function. Immune cells are dependent on the substrate not only for energy generation, but also for the creation of new molecules, via the action of biosynthetic pathways.
Long-distance athletes showed reduction in the glucose and glutamine disposal to the immune cells. The skeletal muscles utilize a high proportion of the available glucose to generate ATP via the glycolytic and oxidative pathways. Furthermore, the carbon skeletons provided by amino acids generated via induction on muscle proteolysis, which is induced by stress hormones (in special cortisol), are delivered to liver to formation of glucose by gluconeogenesis (Kou et al., 2013). Thus, the disposal of glucose and glutamine to the immune cells is reduced. This reduction causes the activation of AMPK that induces anti-inflammatory signature in the immune cells. It is very interesting that metabolic reprograming in immune cells is similar to that in the muscle cells, with an antagonist response between AMPK and mTOR activation.
AMPK has been demonstrated as a key metabolic regulator of T cells, essential for ATP homeostasis; further, it allows lymphocytes metabolic plasticity to adapt to the energy stress. However, AMPK does not play a role in cell effector function. Once activated, AMPK shuts off the anabolic pathways, including mTOR. mTOR complex 1 (mTORC1) is essential for immune cell activation and proliferation; however, its constant activation increases the reactive oxygen species and induces cell senescence. Therefore, the activation of AMPK or mTOR is essential for determining the fate of the immune cells and deciding the activation of the pro-inflammatory or anti-inflammatory profile [47].
It is unclear exactly how exercise induces the reprograming of immune cells; however, the myokines released after exercise, increased hormonal stress (particularly cortisol levels), and substrate available due to the reduction of glucose and specific amino acids in the circulation are responsible for inducing this reprograming [48].
Carbohydrate is the most researched nutrient, and several researches have investigated its supplementation before, during, and after a high-intensity and/or prolonged exercise. Carbohydrate ingestion during prolonged exercise can maintain the blood glucose levels and attenuate the release of stress hormones and exercise-induced cytokines. They also influence recovery by reducing the suppression of TCD4 and TCD8 lymphocytes for 24 h after exercise cessation [49].
Similar results were found when carbohydrate was consumed till up to 15 min before the exercise, preventing immunoendocrine disturbances. However, carbohydrate ingestion only during the recovery period has not shown such effects.
Carbohydrate supplementation is important for the maintenance of immune system functions during exercise and better recovery. Thus, it could be an effective strategy for decreasing the potential cumulative immunodepression over consecutive days of exercise to avoid overtraining. Researchers have found that carbohydrate ingestion during consecutive days of a heavy training regime was able to attenuate plasma cortisol levels and enhance lymphocytes proliferation and function, thus reducing exercise-induced adaptive immune depression [50].
Protein ingestion is also a concern in the immune nutrition area because it is essential for proper immune system function. A high-protein diet (3 g/kg/day) during a period of intense training decreased exercise-induced impairment in lymphocytes trafficking when compared to a carbohydrate-matched normal protein diet [51]. When supplemented for 6 days, during a heavy training period, protein ingestion decreased neutrophil degranulation during the post exercise recovery period [52]. It indicates that protein administration could be an approach to attenuate chronic immunodepression after a long period of intense training; however, it did not appear to have the same acute effect as carbohydrates.
In contrast to the immunodepression caused by high-intensity and prolonged exercise, moderate-intensity single or repeated bouts of exercise appear to exert a contradictory role. Several studies indicate that frequent participation in regular exercise can stimulate the immune system and decrease respiratory infections.
Moderate aerobic exercise appears as an effector-T cells activator, stimulating them to transmigrate to the peripheral tissues where immune surveillance is enhanced by physical stress. Recent data has shown that short-term intense exercise (30 min of cycling) increased virus-specific T cells mobilization, increasing the success of immunotherapy for viral infections. It also mobilized CD8 naïve T cells and high differentiated and EMRA CD8+ subsets [53]. Furthermore, moderate exercise may mobilize the senescent phenotype lymphocytes to undergo apoptosis in the tissues allowing “fresh” ones to take their place, thus possibly improving the immune response against infections.
A recent study showed that acute exercise mobilizes angiogenic T cells, facilitating vascular remodeling during recovery after exercise [54]. Hematopoietic stem cells are also mobilized after aerobic exercise, and this could aid skeletal muscle repair and regeneration for exercise recovery (Kruger et al. 2015). This exercise-induced stem cells mobilization has been studied as an adjuvant therapy for hematopoietic stem cell transplantation.
Neutrophil function is also enhanced by moderate exercise. One hour of cycling at 50% of VO2 max increased the neutrophil oxidative burst and during the early stage of recovery, the bacterial activity of these cells continues to increase up to 1 h after exercise [55]. Monocytes mobilized by exercise might infiltrate the skeletal muscle and differentiate into tissue-resident macrophages facilitating tissue repair and regeneration. Moreover, the expression of pathogen-recognition receptors, such as toll-like receptors, tends to decrease in response to moderate-intensity exercise that could contribute to the anti-inflammatory role of exercise training in metabolic diseases. In general, short bouts of moderate-intensity exercise might enhance cellular immune function [56].
Exercise training has the ability to decrease low‐grade chronic inflammation, restore antioxidant mechanisms, and stimulate immune cells response, which may reduce age‐related immunosenescence [57]. In a study conducted on Judo masters athletes, it was observed that they senescent naïve and effector memory CD8+ T cells and CD4+ T cells and VO2 max was positively correlated with naïve CD4+ T cells population, indicating that lifelong exercise could positively regulate immune function [58].
In studies on vaccination and exercise, physically active individuals appear to have better response than sedentary individuals. Older adults (62 years old) who performed at least 20 min of aerobic exercise 3–4 times a week showed higher anti-influenza IgG and IgM titers versus sedentary adults 2 weeks after vaccination. The exercise group also exhibited increased peripheral blood mononuclear cells in response to antigen-specific stimulation (Araujo et al. 2015). Moreover, in an animal model, mice who exercised before being exposed to the influenza virus showed lower severity of infection and inflammation in the lungs [59]. In another study, exercise before virus exposure was able to increase the survival rates in mice.
Thus, high-intensity and prolonged exercise without a proper recovery and nutrition can lead to an immunodepression and increase the risk of URTI. However, these finds indicate that exercise is a potent immunomodulator because continuous lower doses in moderate intensity can improve the immune response and decrease inflammation in metabolic diseases (Fig. 2).
3 Exercise for the Treatment and Prevention of Metabolic Disease
The increased prevalence of metabolic disorders and its various features, such as obesity and type 2 diabetes (T2D), represents an enormous challenge for health systems worldwide. It affects more than 20% of the general population in most Western countries. In addition to genetic factors and population aging, lifestyle plays a major role in the development and progression of the “‘metabolic syndrome’’ that represents a cluster of metabolic disorders, including abdominal obesity, dyslipidemia, increased blood pressure, and increased fasting glucose levels [60].
Improved accessibility to healthcare systems and general economic developments that support population aging are believed to increase medical costs to levels no longer sustainable in Western societies. Accordingly, lifestyle modification in the prevention and treatment of metabolic disorders represents one of the most promising approaches that is affordable, nontoxic, and highly efficient compared to medications [61].
A major feature of metabolic diseases is obesity. Several clinical and experimental studies have provided evidence for an association between visceral adipose tissue mass and a condition of chronic systemic and local inflammation. It is suggested that inflammatory processes represent an important cause of many obesity-associated risk factors and metabolic diseases.
There are differences in the risk of obesity based on the heterogeneity of adipose tissue within and among individuals. High visceral and ectopic fat accumulation is shown to be associated with higher cardiovascular risk compared with subcutaneous fat. Due to this distribution of body fat, there is a small subset of apparently metabolic healthy obese individuals [3]. These individuals have preserved insulin sensitivity, low fat storage in the liver and skeletal muscle, normal adipose tissue function characterized by lower adipose tissue infiltration of leukocytes and a physiological adipokine secretion pattern. However, in most cases, excessive body fat accumulation leads to adverse metabolic effects, like adipose tissue inflammation, insulin resistance, impaired glucose tolerance, dyslipidemia, and hypertension [62].
The reason for the pathological changes in the visceral and ectopic adipose tissue is massive tissue expansion resulting from chronic metabolic overload in adipocytes. Adipocytes represent a type of metabolic cells that not only minimally increase in number, but also increase in size. Subsequently, the chronic overload induces cellular stress pathways and inflammatory signals, including the activation of IκB kinase (IKK), c-jun N-terminal kinase (JNK), and protein kinase R (PKR). Downstream, the phosphorylation of IκB results in the dissociation of IκBα from NF-κB that is translocated into the nucleus inducing the expression of various inflammatory genes [63].
This signaling cascade is suggested to translate a primarily metabolic complication to immunological activation [64]. Simultaneously, the chronically enhanced calorie intake exceeds adipose tissue lipid storage capacity. The fatty acid metabolism products and advanced glycated end-products, accumulating due to hyperglycemia, activate various pattern-recognition such as the Toll-like receptors (TLRs) receptors and the NLRP3 inflammasome in hepatocytes and other tissues, thereby inducing endoplasmatic reticulum stress and aggravating cytokine production. Self-energizing inflammatory processes are initiated accompanied by increased expression and release of cytokines, such as TNF-α, IL-1β, IL-18, and several chemokines that induce the recruitment of leukocytes.
Accordingly, metabolic stress induces an inflammatory switch in the metabolic cells, such as adipocytes and hepatocytes, followed by invasion of leukocytes [63, 65]. The temporal and functional dynamics of adipose tissue residents or invading leukocytes has been partly defined. The increased concentration of pro-inflammatory cytokines and chemokines induces chemotactic recruitment of circulating monocytes that later culminates in the M1 polarization of macrophages that infiltrate the adipose tissue. The condition of chronic hyperlipidemia is also driven by excess fatty acids that bind to fatty acid-binding protein 4 (FABP4) on adipocytes or stromal macrophages in the adipose tissue microenvironment, followed by the induction of other chemokines that primarily recruit M1 macrophages [66, 67].
These cells seem to represent the most important producer of pro-inflammatory cytokines in the adipose tissue during obesity. M1 macrophages that express the surface molecule CD11c are able proliferate during obesity in a monocyte chemotactic protein 1 (MCP-1)-dependent mechanism. CD11c+ macrophages seem to play a major role in the development of insulin resistance and contribute to the formation of crown-like structures that represent a cluster of dead adipocytes surrounded by macrophages [68, 69].
In parallel, other immune cells, such as NK cells and lymphocytes, increase in the adipose tissue. NK cells are important producers of interferon-gamma (IFN-γ) that supports macrophage polarization and pro-inflammatory cytokine production [68, 70]. Regarding T cells, mainly cytotoxic CD8+ cells infiltrate the expanded adipose tissue and contribute to the recruitment, differentiation, and activation of macrophages. In contrast, the levels of regulatory T cells decrease, potentially favoring inflammatory and metabolic complications [71].
Hence, inflammation in the adipose tissue is affected by the imbalance of pro- and anti-inflammatory immune cell homeostasis toward a more pro-inflammatory status. The long-time metabolic surplus during the development of obesity leads to immunometabolic alterations in various other organs and tissues, such as the liver, brain, skeletal and cardiac muscle, blood vessels, lung, kidney, and gut. However, the adipose tissue is suggested to be an important source of inflammation that at least partially contributes to the induction of systemic inflammatory processes via a “spill over” into circulation [72].
Here, chronically increased levels of pro-inflammatory cytokines, such as TNF-α and IL-6, are involved in the development of other metabolic complications, such as progressive insulin resistance in the skeletal muscle [73]. In particular, TNF-α reduces the level of the inhibitor of IκB, followed by the upregulation of NF-κB and JNK pathways in the muscle. Subsequently, insulin resistance increased through the inhibition of insulin receptor substrate 1 (IRS-1). Non-esterified fatty acids (NEFA) have also been shown to cause insulin resistance in the muscle tissue through increased levels of intramuscular diacylglycerol (DAG) and ceramides.
Here, insulin resistance is also promoted via the upregulation of NF-κB. Moreover, NEFAs induce catabolic responses in the muscle via atrophy-related signaling and protein degradation through impaired activation of the PI3K-Akt pathway [74, 75].
Lifestyle modification appears to be the most appealing approach, and physical activity and exercise represent fundamental components. In general, weight loss is necessary for reducing the cardiovascular risk in obese subjects; however, physical activity is also effective in the treatment of many diseases, irrespective of weight loss. Exercise is well proven to reduce the risk of coronary heart disease in obese subjects. Exercise burns calories and may contribute to a calorie deficit in combination with specific dietary restrictions. Therefore, physical activity plays a pivotal role in long-term weight loss or maintenance to prevent obesity or as an important therapeutic option [76].
In parallel, most exercise regimes significantly improve the functional status and physical fitness. Regular exercise training induces a graded dose response in fitness in both sedentary and obese subjects that is associated with a lower risk for all-cause and cardiovascular disease mortality. It is well known that both moderate endurance as well as resistance exercise training reduces blood pressure in hypertensive individuals. The initiation of regular activity programs in previously inactive subjects also influences dyslipidemia by reducing small, dense LDL and triglyceride particles. In contrast, LDL particle size and high-density lipoprotein (HDL) cholesterol increase. Furthermore, regular physical activity is shown to reduce the ratio of total to HDL cholesterol [77].
Regarding T2D, a considerable association between insulin resistance and low cardiorespiratory fitness has been demonstrated [78]. Data from the Nurses’ Health Study suggests that the activity time spent per week reduces the relative risk of developing T2D. Similarly, exercise represents an effective tool for the therapeutic management of prediabetes and diabetes [79]. Based on the available data, it is recommended that at least 150 min/week of moderate to vigorous aerobic exercise should be performed in combination with one or two sessions of resistance exercise for prevention [80]. Various other studies, such as the Look AHEAD (Action for Health in Diabetes), has shown various beneficial effects of regular physical activity in subjects with T2D. Thereby, long-term activity programs in a diabetic population have shown to improve all the aspects of metabolic syndrome, including BMI and HbA1c [81].
Epidemiological studies demonstrate a negative association between the level of physical inactivity and systemic inflammatory status in patients with metabolic diseases. Similarly, physical fitness is negatively associated with the level of inflammatory biomarkers [82]. Accordingly, physical activity, particularly endurance training or combined endurance and strength training programs, seem to interact with the immune system by exerting anti-inflammatory effects. Patients who suffer from metabolic diseases can perform exercise training to lower both systemic and local levels of inflammation that have consistently shown to lower disease activity [83]. Some exercise-effects might address inflammatory processes indirectly, such as by regulating body composition. Maintaining low visceral fat mass prevents the development of adipose tissue dysfunction and inflammation. Regulation of dyslipidemia may improve T-cell receptor signaling and the translocation of MHC molecules for antigen presentation. Furthermore, regular exercise is shown to increase the body’s antioxidant defense system that prevents oxidative DNA damage to the immune cells and tissues [84].
In addition to these indirect effects, there are other direct mechanisms for exercise-immune crosstalk. During muscle contraction, various myokines are released into the circulation that exert immune-regulating effects in the circulation or organs and tissues. In response to muscular glycogen depletion, IL-6 is released from the skeletal muscle; this induces the increase of anti-inflammatory cytokines, such as IL-10, IL-1 receptor antagonist (IL-1ra), soluble TNF-α receptors, and inhibits the endotoxin-induced TNF-α production [85].
Similarly, each acute bout of exercise releases stress hormones with anti-inflammatory properties, such as cortisol and adrenaline, by activating both the SNS as well as the HPA axes [86]. While cortisol suppresses the activity of different leukocyte subpopulations, adrenaline attenuates the production of IL-1β and TNF-α. Regarding circulating leukocytes, regular exercise increases the percentage of Tregs in the blood and downregulates TLRs on monocytes and macrophages [87, 88]. Similarly, regular exercise increases the percentage of classical monocytes expressing CD14hiCD16–, while an inactive lifestyle promotes an increased percentage of non-classical monocytes characterized by the surface profile CD14l°wCD16+ or CD14hiCD16+ [89].
An important mechanism of the immune-regulating potential of exercise is energy expenditure that reduces metabolic stress. In particular, regular exercise training increases adipocyte-specific gene and protein expression of AMPK and PGC-1α followed by an increased β-oxidation and mitochondrial biogenesis [90]. Enhanced lipid turnover decreases metabolic overload and adipocyte dysfunction, thus limiting stress signaling and pro-inflammatory cytokine production in the adipose tissue. Similarly, exercise reduces signals for ER stress-induced inflammation. In parallel, the expression of CD163, a marker for M2 macrophages, increases, suggesting a switch from M1 to M2 macrophage polarization. In line with these observations, reduced expression of TNF-α and IL-6 in the adipose tissue of diabetic subjects was shown after exercise training [91].
Finally, exercise affects the type of adipose tissue that is generally composed of the following two types of adipose tissues: white adipose tissue (WAT) and brown adipose tissue (BAT). WAT plays a role storing energy and releasing hormones and cytokines that affect metabolism and insulin resistance. BAT, however, expends energy to produce heat through non-shivering thermogenesis, via mitochondrial uncoupling protein 1 (UCP-1) (Bargut et al. 2016). It is noteworthy that exercise can induce a browning process of WAT, leading to increased energy expenditure, thus aiding in the treatment of obesity. Mechanistically, the release of the myokine irisin and activity of the sympathetic nervous system seem to be involved in exercise-induced browning of the adipose tissue. However, the detailed contribution of BAT in the prevention and treatment of metabolic diseases remains to be clarified [92].
Moreover, exercise is an excellent tool for preventing and treating other metabolic and inflammatory diseases, such as neurodegenerative diseases, cardiovascular diseases, and cancer.
4 Exercise for the Prevention and Treatment of Cancer
Cancer is a multifactorial disease driven by chronic inflammation. Aerobic exercise training is considered a very effective tool for inducing an anti-inflammatory response. Thus, nowadays is well established that athletes of long-distance competitions showed reduced risk to many types of cancers. Moore et al. [93] showed that the duration of physical exercise is negatively correlated with the occurrence of 13 types of tumor. Exercise is able to reduce the most common tumors in Western countries, such as cancers of the breast, prostate, colon, pancreas, kidneys, and lungs [94]. It is noteworthy that exercise is less effective in murine models with deletion of p53 [95]. Thus, tumors that have a great genetic trigger and therefore, higher expressions of oncogenes, are less susceptible to exercise-induced prevention [96].
The mechanisms for reducing the risk of cancer are associated with the anti-inflammatory myokines released after exercise with a systemic effect. This anti-inflammatory milieu caused by exercise practice is enough to inhibit tumor generation and growth [97]. Hanahan and Weiberg [98] proposed that inflammation is a hallmark of cancer because the pro-inflammatory signaling can promote resistance to apoptosis and increase tumor cell proliferation.
Other mechanism related to cancer prevention in well-trained subjects is the reduction in the hormones and growth factors, such as sex hormones, insulin, and IGF-1, because exercise training increases the receptors in the target cells and improves the signaling cascade, such as that for insulin. Thus, the circulating levels of these factors can be reduced without affecting the cellular response [94] (Xi et al. 2007).
Most recently, the positive effects of exercise in the treatment of cancer have been shown. Thus, aerobic and resistance exercise can exert effects by reducing the growth of the tumor and metastasis, thus improving the life quality and expectancy [97].
The molecular mechanisms through which exercise induces this anti-tumor response are not completely clear; however, several hypotheses have been proposed. A large number of blood vessels are present in the tumor; however, it is nonfunctional, with reduction in blood flux and generation of hypoxia. Hypoxia is a characteristic of tumor cells, and these reductions in blood flux impair the delivery of immune cells and chemotherapy drugs. Aerobic exercise is able to increase VEGF and improve intra-tumoral perfusion in murine models of breast and prostate cancer [99, 100].
The cell metabolism in tumor is characterized by increased aerobic glycolysis owing to a higher proliferation ratio. Exercise can decrease the availability of energy substrate, particularly special glucose and glutamine, which reduces the supply of nutrients to the tumor. The tumors that have a high metabolic demand are most affected by exercise. In this sense, the metabolic program of tumor cells showed increased in AKT-mTOR axis [101]. Endurance exercise reduces mTOR activation in different types of solid tumors in murine models by reducing the growth factors. Moreover, exercise induces AMPK activation in tumor cells; this activation inhibits the mTOR pathway and inhibits the proliferative pathway. The role of AMPK and the activation of its enzyme is not fully understood. In many types of tumors, AMPK activation decreases tumor growth; however, others studies have shown that this enzyme effectively protects the tumor from oxidative stress and apoptosis [102, 103]. More studies are necessary to clarify the role of AMPK in cancer cells.
Catecholamine released in response to exercise and myokines are probably important for reducing tumor growth and activating the immune system. Pedersen et al. (2016) showed that in a murine model, exercise increased IL-6 and adrenaline that together mobilized NK cells, increasing its activity. It is noteworthy because the levels of IL-6 in intra-tumoral environment are associated with poor prognosis and high malignance. The results not published of our group showed that the aerobic training induces an increase in the IL-6 protein expression in the skeletal muscle and a reduction in the tumor.
The potential therapeutic role of exercise in the reduction of tumor growth (range 45%–67%) in a murine model is well documented. The incubation of several tumor cell lineages with the serum of trained animals reduces in 10%–15% tumor cells proliferation. Furthermore, well-trained athletes have a 40% lower risk of cancer mortality than the general population [94].
The strong evidence of exercise on treatment of cancer patients lead to the Exercise and Sport Science Australian published the position statement in 2019, with the guidelines about the practice of exercise in cancer patients. Several aspects must be considered, such as the type of cancer, pre-status of physical fitness, chemotherapy and radiotherapy, aging, and other co-morbidities; however, all intensities of exercise are preconized during the training. In this statement, the authors suggested at least 20 min for each session (Hayes et al. 2019).
Finally, exercise training is very important in the counter-regulation of the side effects of chemotherapy. This class of drugs causes several undesirable effects in different organs. Doxorubicin, an effective anti-tumor drug widely used in clinical practice, causes substantial toxicity in the heart, kidneys, adipose tissues, liver, and skeletal muscle. In skeletal muscle, patients suffer with proteolysis and fatigue, mimicking sarcopenia induced by cachexia. Resistance training is an efficient method of reducing weight and skeletal muscle mass loss. Moreover, endurance training mitigates the effects of doxorubicin in the reduction of running capability in mice and protects the decline in oxidative metabolism induced by this drug in the skeletal muscle. Moreover, aerobic training improves the delivery of drugs to the tumor. Some studies have shown that aerobic training is safe and potentiate the chemotherapy treatment reducing the dosage without losing efficacy.
Thus, exercise leads to metabolic reprograming in many tissues of the body and can prevent cancer initialization, thus reducing the cancer risk. Starting a fitness program after cancer diagnosis is safe and recommended. Several studies have shown that exercise reduces cancer growth and metastasis. Finally, exercise is extremely effective for reducing the side effects promoted by anti-tumoral drugs.
5 Conclusion
We discussed the metabolic pathways that induce cellular reprograming, especially with respect to the relationship between the AMPK and mTOR in the skeletal muscle, adipose tissue, and immune cells.
This is an important way to understand how exercise induces mobilization, recruitment, polarization, and production of inflammatory mediators in immune cells. Thus, we showed the state of art in the cellular adaptations induced by acute and training exercise. However, many steps of reprograming are unclear and need be investigated. In sum, exercise is an excellent alternative method for prevention and non-pharmacological treatment of inflammatory chronic diseases (obesity and cancer) that have reached epidemic proportions worldwide.
References
Neto JCR, Lira FS, De Mello MT, Santos RVT. Importance of exercise immunology in health promotion. Amino Acids 41(5):1165–1172
Pedersen BK (2009) The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J Physiol 587(Pt 23):5559–5568
Blüher S, Käpplinger J, Herget S, Reichardt S, Böttcher Y, Grimm A, Kratzsch J, Petroff D (2017) Cardiometabolic risk markers, adipocyte fatty acid binding protein (aFABP) and the impact of high-intensity interval training (HIIT) in obese adolescents. Metabolism 68:77–87
Caldeira RS, Panissa VLG, Inoue DS, Campos EZ, Monteiro PA, Giglio BM, Pimentel GD, Hofmann P, Lira FS (2018) Impact to short-term high intensity intermittent training on different storages of body fat, leptin and soluble leptin receptor levels in physically active non-obese men: a pilot investigation. Clin Nutr ESPEN. 28:186–192
Inoue DS, Panissa VL, Antunes BM, Oliveira FP, Malta RB, Caldeira RS, Campos EZ, Pimentel GD, Franchini E, Lira FS (2018) Reduced leptin level is independent of fat mass changes and hunger scores from high-intensity intermittent plus strength training. J Sports Med Phys Fitness 58(7–8):1045–1051
Lira FS, Dos Santos T, Caldeira RS, Inoue DS, Panissa VLG, Cabral-Santos C, Campos EZ, Rodrigues B, Monteiro PA (2017) Short-term high- and moderate-intensity training modifies inflammatory and metabolic factors in response to acute exercise. Front Physiol 31(8):856
Michallet AS, Tonini J, Regnier J, Guinot M, Favre-Juvin A, Bricout V, Halimi S, Wuyam B, Flore P (2008) Methodological aspects of crossover and maximum fat-oxidation rate point determination. Diabetes Metab 34(5):514–523
Purdom T, Kravitz L, Dokladny K, Mermier C (2018) Understanding the factors that effect maximal fat oxidation. J Int Soc Sports Nutr 12(15):3
Frayn KN (2010) Fat as a fuel: emerging understanding of the adipose tissue-skeletal muscle axis. Acta Physiol (Oxf) 199(4):509–518
Jeppesen J, Kiens B (2012) Regulation and limitations to fatty acid oxidation during exercise. J Physiol 590(5):1059–1068
Schenk S, Horowitz JF (2006) Coimmunoprecipitation of FAT/CD36 and CPT I in skeletal muscle increases proportionally with fat oxidation after endurance exercise training. Am J Physiol Endocrinol Metab 291(2):E254–E260
Yoshida Y, Jain SS, McFarlan JT, Snook LA, Chabowski A, Bonen A (2013) Exercise- and training-induced upregulation of skeletal muscle fatty acid oxidation are not solely dependent on mitochondrial machinery and biogenesis. J Physiol 591(18):4415–4426
Shaw CS, Clark J, Wagenmakers AJ (2010) The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu Rev Nutr 21(30):13–34
Watt MJ, Heigenhauser GJ, Dyck DJ, Spriet LL (2002) Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J Physiol 541(Pt 3):969–978
Daemen S, Gemmink A, Brouwers B, Meex RCR, Huntjens PR, Schaart G, Moonen-Kornips E, Jörgensen J, Hoeks J, Schrauwen P, Hesselink MKC (2018) Distinct lipid droplet characteristics and distribution unmask the apparent contradiction of the athlete’s paradox. Mol Metab. 17:71–81
Gemmink A, Daemen S, Brouwers B, Huntjens PR, Schaart G, Moonen-Kornips E, Jörgensen J, Hoeks J, Schrauwen P, Hesselink MKC (2018) Dissociation of intramyocellular lipid storage and insulin resistance in trained athletes and type 2 diabetes patients; involvement of perilipin 5? J Physiol 596(5):857–868
Gemmink A, Daemen S, Kuijpers HJH, Schaart G, Duimel H, López-Iglesias C, van Zandvoort MAMJ, Knoops K, Hesselink MKC (2018) Super-resolution microscopy localizes perilipin 5 at lipid droplet-mitochondria interaction sites and at lipid droplets juxtaposing to perilipin 2. Biochim Biophys Acta Mol Cell Biol Lipids 1863(11):1423–1432
Hawley JA, Krook A (2016) Metabolism: one step forward for exercise. Nat Ver Endocrinol. 12(1):7–8
Gerosa-Neto J, Panissa VLG, Monteiro PA, Inoue DS, Ribeiro JPJ, Figueiredo C, Zagatto AM, Little JP, Lira FS (2019) High- or moderate-intensity training promotes change in cardiorespiratory fitness, but not visceral fat, in obese men: a randomised trial of equal energy expenditure exercise. Respir Physiol Neurobiol 266:150–155
Keating SE, Johnson NA, Mielke GI, Coombes JS (2017) A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev 18(8):943–964
Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B (2003) Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil 24(2–3):113–119
Pedersen BK, Akerström TC, Nielsen AR, Fischer CP (2007) Role of myokines in exercise and metabolism. J Appl Physiol (1985) 103(3):1093–8.
Pedersen BK, Fischer CP (2007) Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr Opin Clin Nutr Metab Care 10(3):265–271
Pedersen BK, Febbraio MA (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88(4):1379–1406
Pedersen BK, Febbraio M (2005) Muscle-derived interleukin-6–a possible link between skeletal muscle, adipose tissue, liver, and brain. Brain Behav Immun 19(5):371–376
Cabral-Santos C, Castrillón CI, Miranda RA, Monteiro PA, Inoue DS, Campos EZ, Hofmann P, Lira FS (2016) Inflammatory cytokines and bdnf response to high-intensity intermittent exercise: effect the exercise volume. Front Physiol 7:509
Petersen AM, Pedersen BK (2005) The anti-inflammatory effect of exercise. J Appl Physiol (1985) 98(4):1154–62
Cabral-Santos C, Gerosa-Neto J, Inoue DS, Panissa VL, Gobbo LA, Zagatto AM, Campos EZ, Lira FS (2015) Similar anti-inflammatory acute responses from moderate-intensity continuous and high-intensity intermittent exercise. J Sports Sci Med 14(4):849–56
Camera DM, Smiles WJ, Hawley JA (2016) Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic Biol Med 98:131–143
Marcotte GR, West DW, Baar K (2015) The molecular basis for load-induced skeletal muscle hypertrophy. Calcif Tissue Int 96(3):196–210
Gerosa-Neto J, Rossi FE, Campos EZ, Antunes BM, Cholewa JM, Lira FS (2016) Impact of short and moderate rest intervals on the acute immunometabolic response to exhaustive strength exercise: part II. J Strength Cond Res 30(6):1570–1576
Rossi FE, Gerosa-Neto J, Zanchi NE, Cholewa JM, Lira FS (2016) Impact of short and moderate rest intervals on the acute immunometabolic response to exhaustive strength exercise: part I. J Strength Cond Res 30(6):1563–1569
Izquierdo M, Ibañez J, Calbet JA, Navarro-Amezqueta I, González-Izal M, Idoate F, Häkkinen K, Kraemer WJ, Palacios-Sarrasqueta M, Almar M, Gorostiaga EM (2009) Cytokine and hormone responses to resistance training. Eur J Appl Physiol 107(4):397–409
Rossi FE, de Freitas MC, Zanchi NE, Lira FS, Cholewa JM (2018) The role of inflammation and immune cells in blood flow restriction training adaptation: a review. Front Physiol 9(9):1376
Inoue DS, Panissa VL, Monteiro PA, Gerosa-Neto J, Rossi FE, Antunes BM, Franchini E, Cholewa JM, Gobbo LA, Lira FS (2016) Immunometabolic responses to concurrent training: the effects of exercise order in recreational weightlifters. J Strength Cond Res 30(7):1960–1967
Monteiro PA, Campos EZ, de Oliveira FP, Peres FP, Rosa-Neto JC, Pimentel GD, Lira FS (2017) Modulation of inflammatory response arising from high-intensity intermittent and concurrent strength training in physically active males. Cytokine 91:104–109
Panissa VLG, Fukuda DH, de Oliveira FP, Parmezzani SS, Campos EZ, Rossi FE, Franchini E, Lira FS (2018) Maximum strength development and volume-load during concurrent high intensity intermittent training plus strength or strength-only training. J Sports Sci Med 17(4):623–632
Robertson AJ, Ramesar KC, Potts RC, Gibbs JH, Browning MC, Brown RA, Hayes PC, Beck JS (1981) The effect of strenuous physical exercise on circulating blood lymphocytes and serum cortisol levels. J Clin Lab Immunol 5(1):53–57
Steensberg A, Toft AD, Schjerling P, Halkjaer-Kristensen J, Pedersen BK (2001) Plasma interleukin-6 during strenuous exercise: role of epinephrine. Am J Physiol Cell Physiol 281(3):C1001–C1004
Suzuki K, Nakaji S, Yamada M, Liu Q, Kurakake S, Okamura N, Kumae T, Umeda T, Sugawara K (2003) Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med Sci Sports Exerc 35:348–355
Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P (2011) Position statement. Part one: immune function and exercise. Exerc Immunol Rev 17:6–63
Simpson RJ, Bigley AB, Spielmann G, LaVoy EC, Kunz H, Bollard CM (2016) Human cytomegalovirus infection and the immune response to exercise. Exerc Immunol Rev 22:8–27
Nieman DC (1994) Exercise, infection, and immunity. Int J Sports Med 15(Suppl 3):S131–S141
Nieman DC (1994) Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exer 6(2):128–39
Malm C (2006) Susceptibility to infections in elite athletes: the S-curve. Scand J Med Sci Sports 16(1):4–6
Peake JM, Neubauer O, Walsh NP, Simpson RJ (1985) Recovery of the immune system after exercise. J Appl Physiol 122(5):1077–1087
Batatinha HAP, Biondo L, Lira FS, Castell LM, Rosa-Neto JC (2018) Nutrients, immune system and exercise: where it will take us? Nutrition. https://doi.org/10.1016/j.nut.2018.09.019
Saskia Heijnen, Bernhard Hommel, Armin Kibele,and Lorenza S. Colzato (2015) Neuromodulation of aerobic exercise. A Rev Front Psychol 6:1890
Lancaster GI, Khan Q, Drysdale PT, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M (2005) Effect of prolonged exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J Appl Physiol (1985) 98(2):565–71
Bishop NC, Walker GJ, Bowley LA, Evans KF, Molyneux K, Wallace FA, Smith AC (1985) Lymphocyte responses to influenza and tetanus toxoid in vitro following intensive exercise and carbohydrate ingestion on consecutive days. J Appl Physiol 99(1327–1335):2005
Witard OC, Turner JE, Jackman SR, Kies AK, Jeukendrup AE, Bosch JA, Tipton KD (2014) High dietary protein restores overreaching induced impairments in leukocyte trafficking and reduces the incidence of upper respiratory tract infection in elite cyclists. Brain Behav Immun 39:211–219
Costa RJ, Oliver SJ, Laing SJ, Waiters R, Bilzon JL, Walsh NP (2009) Influence of timing of postexercise carbohydrate-protein ingestion on selected immune indices. Int J Sport Nutr Exerc Metab 19:366–384
Kunz HE, Spielmann G, Agha NH, O’Connor DP, Bollard CM, Simpson RJ (2018) A single exercise bout augments adenovirus-specific T-cell mobilization and function. Physiol Behav 1(194):56–65
Lansford KA, Shill DD, Dicks AB, Marshburn MP, Southern WM, Jenkins NT (2016) Effect of acute exercise on circulating angiogenic cell and microparticle populations. Exp Physiol 101:155–167. https://doi.org/10.1113/EP085505
Robson PJ, Blannin AK, Walsh NP, Castell LM, Gleeson M (1999) Effects of exercise intensity, duration and recovery on in vitro neutrophil function in male athletes. Int J Sports Med 20:128–135
Simpson RJ, McFarlin BK, McSporran C, Spielmann G, ó Hartaigh B, Guy K (2009) Toll-like receptor expression on classic and pro-inflammatory blood monocytes after acute exercise in humans. Brain Behav Immun 23:232–239. https://doi.org/10.1016/j.bbi.2008.09.013
Batatinha HAP, Diniz TA, de Souza Teixeira AA, Krüger K, Rosa-Neto JC (2019) Regulation of autophagy as a therapy for immunosenescence-driven cancer and neurodegenerative diseases: the role of exercise. J Cell Physiol. https://doi.org/10.1002/jcp.28318
Minuzzi LG, Rama L, Chupel MU, Rosado F, Dos Santos JV, Simpson R, Martinho A, Paiva A, Teixeira AM (2018) Effects of lifelong training on senescence and mobilization of T lymphocytes in response to acute exercise. Exerc Immunol Rev 24:72–84
Lowder T, Padgett DA, Woods JA (2006) Moderate exercise early after influenza virus infection reduces the Th1 inflammatory response in lungs of mice. Exerc Immunol Rev 12:97–111
Bhupathiraju SN, Hu FB (2016) Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res 118:1723–1735
Ortega FB, Lavie CJ, Blair SN (2016) Obesity and cardiovascular disease. Circ Res 118:1752–1770
Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Haring HU (2008) Identification and characterization of metabolically benign obesity in humans. Arch Int Med 168:1609–1616
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445
Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS (2002) A central role for JNK in obesity and insulin resistance. Nature 420(6913):333–336
Krüger K, Mooren FC, Pilat C (2016) The immunomodulatory effects of physical activity. Curr Pharm Des 22(24):3730–3748
Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M (2014) Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab 19(1):162–171
Orr JS, Puglisi MJ, Ellacott KLJ, Lumeng CN, Wasserman DH, Hasty AH (2012) Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes 61(11):2718–2727
Batatinha HAP, Rosa Neto JC, Krüger K (2019) Inflammatory features of obesity and smoke exposure and the immunologic effects of exercise. Exerc Immunol Rev 25:96–111
Wouters K, Gaens K, Bijnen M, Verboven K, Jocken J, Wetzels S, Wijnands E, Hansen D, van Greevenbroek M, Duijvestijn A, Biessen EA, Blaak EE, Stehouwer CD, Schalkwijk CG (2017) Circulating classical monocytes are associated with CD11c+ macrophages in human visceral adipose tissue. Sci Rep 15(7):42665
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15(8):914–920
Chatzigeorgiou A, Karalis KP, Bornstein SR, Chavakis T (2012) Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia 55(10):2583–2592
Krüger K (2017) Inflammation during obesity—pathophysiological concepts and effects of physical activity. Dtsch Z Sportmed 68:163–169
Perry BD, Caldow MK, Brennan-Speranza TC, Sbaraglia M, Jerums G, Garnham A, Wong C, Levinger P, Asrar Ul Haq M, Hare DL, Price SR, Levinger I (2016) Muscle atrophy in patients with Type 2 Diabetes Mellitus: roles of inflammatory pathways, physical activity and exercise. Exerc Immunol Rev 22:94–109
Aguirre V, Uchida T, Yenush L, Davis R, White MF (2000) The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J Biol Chem 275:9047–9054
Liang H, Tantiwong P, Sriwijitkamol A, Shanmugasundaram K, Mohan S, Espinoza S, Defronzo RA, Dube JJ, Musi N (2013) Effect of a sustained reduction in plasma free fatty acid concentration on insulin signalling and inflammation in skeletal muscle from human subjects. J Physiol 591:2897–2909
Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K (2011) Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 364(13):1218–1229
Kelly AS, Wetzsteon RJ, Kaiser DR, Steinberger J, Bank AJ, Dengel DR (2004) Inflammation, insulin, and endothelial function in overweight children and adolescents: the role of exercise. J Pediatr 145(6):731–6. https://doi.org/10.1016/j.jpeds.2004.08.004. PMID: 15580192
Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M (2001) Finnish diabetes prevention study group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344(18):1343–50
Chae JS, Kang R, Kwak JH, Paik JK, Kim OY, Kim M, Park JW, Jeon JY, Lee JH (2012) Supervised exercise program, BMI, and risk of type 2 diabetes in subjects with normal or impaired fasting glucose. Diabetes Care 35(8):1680–1685
Hallal PC, Bauman AE, Heath GW, Kohl HW 3rd, Lee IM, Pratt M (2012) Physical activity: more of the same is not enough. Lancet 380(9838):190–91
Di Loreto C, Fanelli C, Lucidi P, Murdolo G, De Cicco A, Parlanti N, Santeusanio F, Brunetti P, De Feo P (2003) Validation of a counseling strategy to promote the adoption and the maintenance of physical activity by type 2 diabetic subjects. Diabetes Care 26(2):404–8
Parsons TJ, Sartini C, Welsh P, Sattar N, Ash S, Lennon LT, Wannamethee SG, Lee IM, Whincup PH, Jefferis BJ (2017) Physical activity, sedentary behavior, and inflammatory and hemostatic markers in men. Med Sci Sports Exerc 49(3):459–465
Krüger K, Pilat C, Schild M, Lindner N, Frech T, Muders K, Mooren FC (2015) Progenitor cell mobilization after exercise is related to systemic levels of G-CSF and muscle damage. Scand J Med Sci Sports 25:e283–e291. https://doi.org/10.1111/sms.12320
Kruk J, Aboul-Enein HY, Kładna A, Bowser JE (2019) Oxidative stress in biological systems and its relation with pathophysiological functions: the effect of physical activity on cellular redox homeostasis. Free Radic Res 53(5):497–521
Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK (2003) IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285(2):E433–E437
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA (2011) The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11(9):607–615
Rada I, Deldicque L, Francaux M, Zbinden-Foncea H (2018) Toll like receptor expression induced by exercise in obesity and metabolic syndrome: a systematic review. Exerc Immunol Rev 24:60–71
Weinhold M, Shimabukuro-Vornhagen A, Franke A, Theurich S, Wahl P, Hallek M, Schmidt A, Schinköthe T, Mester J, von Bergwelt-Baildon M, Bloch W (2016) Physical exercise modulates the homeostasis of human regulatory T cells. J Allergy Clin Immunol 137(5):1607–1610
Aw NH, Canetti E, Suzuki K, Goh J (2018) Monocyte subsets in atherosclerosis and modification with exercise in humans. Antioxidants (Basel) 19;7(12)
DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J (1985) Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76:149–155
Kawanishi N, Yano H, Yokogawa Y, Suzuki K (2010) Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev 16:105–118
Rodríguez A, Becerril S, Ezquerro S, Méndez-Giménez L, Frühbeck G (2017) Crosstalk between adipokines and myokines in fat browning. Acta Physiol (Oxf) 219(2):362–381. https://doi.org/10.1111/apha.12686. Epub 2016 Apr 20. PMID: 27040995
Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, Adami HO, Blair CK, Borch KB, Boyd E, Check DP, Fournier A, Freedman ND, Gunter M, Johannson M, Khaw KT, Linet MS, Orsini N, Park Y, Riboli E, Robien K, Schairer C, Sesso H, Spriggs M, Van Dusen R, Wolk A, Matthews CE, Patel AV (2016) Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Me 176(6):816–25
Ruiz-Casado A, Martín-Ruiz A, Pérez LM, Provencio M, Fiuza-Luces C, Lucia A (2017) Exercise and the hallmarks of cancer. Trends Cancer 3(6):423–441
Colbert LH, Westerlind KC, Perkins SN, Haines DC, Berrigan D, Donehower LA, Fuchs-Young R, Hursting SD (2009) Exercise effects on tumorigenesis in a p53-deficient mouse model of breast cancer. Med Sci Sports Exerc 41(8):1597–1605
Jones LW, Kwan ML, Weltzien E, Chandarlapaty S, Sternfeld B, Sweeney C, Bernard PS, Castillo A, Habel LA, Kroenke CH, Langholz BM, Queensberry CP Jr, Dang C, Weigelt B, Kushi LH, Caan BJ (2016) Exercise and prognosis on the basis of clinicopathologic and molecular features in early-stage breast cancer: the LACE and pathways studies. Cancer Res 76(18):5415–5422. https://doi.org/10.1158/0008-5472.CAN-15-3307
Hojman P, Gehl J, Christensen JF, Pedersen BK (2018) Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab 27(1):10–21. https://doi.org/10.1016/j.cmet.2017.09.015
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. https://doi.org/10.1016/j.cell.2011.02.013
Jones LW et al (1985) (2010) Effect of aerobic exercise on tumor physiology in ananimal model of human breast cancer. J Appl Physiol 108(343–348):48
Jones LW et al (1985) (2012) Exercise modulation of the host–tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol 113(263–272):49
Zhu Z et al (2008) Effectofnonmotorizedwheelrunningon mammary carcinogenesis:circulatingbiomarkers, cellularpro- cesses, andmolecularmechanismsinrats. Cancer Epidemiol Biomarkers Prev 17:1920–1929
Kishton RJ, Barnes CE, Nichols AG, Cohen S, Gerriets VA, Siska PJ, Macintyre AN, Goraksha-Hicks P, de Cubas AA, Liu T, Warmoes MO, Abel ED, Yeoh AE, Gershon TR, Rathmell WK, Richards KL, Locasale JW, Rathmell JC1 (2016) AMPK is essential to balance glycolysis and mitochondrial metabolism to control T-ALL cell stress and survival. Cell Metab 23(4):649–62. https://doi.org/10.1016/j.cmet.2016.03.008
Vara-Ciruelos D, Russell FM, Hardie DG (2019) The strange case of AMPK and cancer: Dr Jekyll or Mr Hyde? (†). Open Biol 9(7):190099
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Batatinha, H.P., Lira, F.S., Kruger, K., Rosa Neto, J.C. (2022). Physical Exercise and Metabolic Reprogramming. In: Camara, N.O.S., Alves-Filho, J.C., Moraes-Vieira, P.M.M.d., Andrade-Oliveira, V. (eds) Essential Aspects of Immunometabolism in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-86684-6_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-86684-6_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86683-9
Online ISBN: 978-3-030-86684-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)