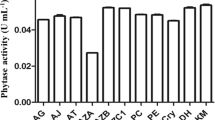
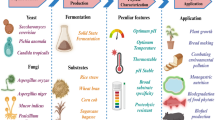
Abstract
Phytases have so far only found use in diets for monogastric animals as an animal feed additive, although there is significant potential for the use of this class of enzymes in the production and manufacture of food for human consumption. The near-complete elimination of phytate in the human stomach and upper small intestine during food processing and/or food digestion results in an increase in the bioavailability of essential minerals such as iron and zinc. It is seen as a way to reduce the likelihood that disadvantaged groups such as child-bearing mothers, strictly vegetarians, and inhabitants of developing countries may run into mineral deficiencies. Additionally, phytate removal may affect the production process economy as well as the yield and purity of the final products as stated for bread making, plant protein isolate processing, maize wet milling, and cereal bran fractionation. Not complete but controlled phytate degradation is the goal of using phytase to produce food with regulated content and composition of myo-inositol phosphate esters with health benefits or to generate these compounds as supplements for the food. Various fungal species have been reported for the production of enzyme phytase that has several industrial applications in the food sector. In this chapter, phytase-producing fungi and their further applications have been discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Phytases have been one of the most important enzymes in nutrition, environmental protection, and human health over the last two decades. These enzymes sequentially isolate orthophosphate groups from the core of phytate inositol, the principal chemical source of phosphorus in plants (Kumar et al. 2017). Various phytases have been isolated from plants or microbes and can be categorized according to their optimal pH and catalytic methods (histidine acid phosphatases, ß-propellate phytases, cysteine, or purple acid phosphatases). The urgent need to boost phytate phosphorus in diets for animals with simple stomachs to reduce the excretion of phosphorus fume into the environment has been motivated by recent phytase activities. However, future phytase applications may be extended to release dietary phytate-bound minerals for human nutrition and to produce specific inositol phosphates for human health (Konietzny and Greiner 2003; Kour et al. 2020).
There is also an excellent opportunity to use phytases in the production and manufacture of food for human consumption, where research focuses on the enhancement of the nutritional value of plant-based food as well as the technical development of food processing. A high diet of phytate contributes to a considerably reduced absorption of dietary mineral products (Sandberg et al. 1999). During food processing, phytate dephosphorylation results in the formation of only partly phosphorylated myo-inositol phosphate esters with a lower capacity to interfere with the dietary intake (Sandström and Sandberg 1992; Han et al. 1999; Shears 1998). Specific myo-inositol phosphate esters have proved to have many essential physiological functions in humans (Greiner et al. 2002). Phytases, therefore, that can be used for food processing to produce functional foods (Haros et al. 2001) should generate these biochemically active phosphate esters of myo-inositol and absorb them into the food tract. This chapter contains technical improvements to the implementation of phytases for bread making (Wang et al. 1999), plant protein isolation production (Fredrikson et al. 2001; Caransa et al. 1988), corn waters (Antrim et al. 1997; Kvist et al. 2005), and cereal bran fractionation (Andlid et al. 2004).
In the gastrointestinal monogastric tract, phytate (Inositol hexaphosphate, IP6), the dominant source of phosphorous and mineral food complexes (calcium, ferrous, magnesium, and zinc), is indigestible (Hellström et al. 2010; Brinch-Pedersen et al. 2007). Phytate is a key antinutritional factor for the bioavailability of dietary minerals that rely exclusively on cereals to dietary consumers (Cao et al. 2007). It involves the global burden of iron deficiency and the resulting complications, particularly among women and children, in low-income countries. Diät mineral bioavailability can be increased with the use of phytase, a catalytic enzyme for phytate sequence hydrolysis (Raghavendra and Halami 2009; Ogunremi et al. 2020; Mullaney and Ullah 2003).
As members of previously recognized phosphatases classes, the basic structural characteristics of several phytate-degrading enzymes were determined (Mullaney and Ullah 2003; Chu et al. 2004). X-ray crystallographic studies, among others, have confirmed their belonging to a class with a new catalytic mechanism (Ha et al. 2000). The description of the molecular 3D structure of the various phytate degrading enzymes strengthened our understanding of the relationship between the molecular structure and the catalytic functioning of the molecular structure. It is now clear that specific phytases have been established to satisfy the unique nutritional requirements of various forms of life in plants, animals, and microbials (Rastegari et al. 2020). A strong connection between the catalytic domain of an enzyme and unique molecular architectural elements also seems to exist. Although certain structural components are important, certain nonessential parts of the molecule may therefore be altered to adapt the catalytic mechanism to the different substrates. In future research, the exact number of catalytic mechanisms formed to hydrolyze the phytate can be determined. It is now recognized that four phosphatase enzyme groups are representative of phytic acid that can be degraded as described in Table 12.1.
12.2 Phytases of Fungal Origin
The majority of phytases isolated from fungi and yeast, typically known as 3-phytases, are histidine acid phosphatases, glycosylated, and active for a wide variety of substrates (Wyss et al. 1999b). Aspergillus niger PhyA was the first phytase to be well characterized and marketable. This enzyme, encoded by a 1.4 kb DNA fragment, is a monomer with an estimated molecular weight of 80 kDa, a bi-hump pH profile with two optimal pH at 2.5 and 5.0–5.5, an optimal temperature at 55–60 °C, and a high phytic acid affinity (Han et al. 1999). Aspergillus fumigatus phytase has a sequence similarity of 66% to A. PhyA Phytase niger, however, exhibits greater thermo-tolerance (Pasamontes et al. 1997a; Wyss et al. 1998).
The thermo-tolerance was associated with high refolding efficiency after heat denaturation, and the specificity of the buffers used in heat treatment can be modulated (Rodriguez et al. 2000a). The enzyme has a wide range of pH and is highly active against low phosphorylation inositol phosphates (Wyss et al. 1999b; Rodriguez et al. 2000a). Yet its unique phytate activity is small (Tomschy et al. 2000). PhyA phytase Peniophora lycii was also sold out. It is a 6-phytase with optimum pH at 4.0–4.5 and optimum temperature at 50–55 °C and has dimeric conformation (Lassen et al. 2001). It seems vulnerable to thermal and protease treatments (Simon and Igbasan 2002) or low pH (Quan et al. 2004) isolated a low-molecular-weight (32.6 kDa) phytase from Cladosporium sp., an airborne fungus. PS-1.
The enzyme is not glycosylated and has an average 3.5 pH and an average 40 °C temperature. It produces tri-phosphate inositol as the product. Phytases isolated from thermophilic fungi Myceliophtora thermophila and Talaromyces thermophilus (Mitchell et al. 1997; Pasamontes et al. 1997b) show a high degree of homology of sequence to other fungal phytases A. niger, A. Terreus, and A. Fumigate. Berka et al. (1998) isolated a phytase from the Thermomyces lanuginosus thermophilic fungus which showed better thermostability and catalytic performance and a higher transition temperature than the A. Phytase to the niger. Chadha et al. (2004) reported that phytase produced by the Mucor pusillus thermophilic fungus was active in a wide pH range of 3–7.8. Nakamura et al. (2000) found substantial levels of phytase activity in 35 species from a survey on 738 strains of yeast, with a wide range of optimal pH and temperature. Arxula adeninivorans developed well in media containing phytate as the sole source of phosphate and secreted phytase with an optimum pH of 4.5–5.0, and an optimum temperature of around 75 °C (Sano et al. 1999; Quan et al. 2002) also reported substantial phytase development from soil-isolated yeast Candida Krusei WZ-001. The isolated phytase produced two different subunits with 116- and 31-kDa molecular masses, had a glycosylation rate of about 35%, and had optimal pH and temperature at 4.6 and 40 °C, respectively. Pichia anomala (Vohra and Satyanarayana 2001), Saccharomyces cerevisiae (Türk et al. 2000), and Schwanniomyces castellii (Segueilha et al. 1992) also showed phytase activity (Loewus 2002). These enzymes were active in the range of acid pH, with the optimum temperature at 60–74 °C.
Phytats Dietary Effects Salts of phytic acid, designated as phytates, are regarded as the primary storage form of both phosphate and inositol in plant seeds and grains. Phytate is formed during the maturation of the plant seed, and in dormant seeds, it represents 60–90% of the total phosphate (Reddy 2002). Phytate is therefore a common constituent of plant-derived foods. Depending on the amount of plant-derived foods in the diet and the grade of food processing, the daily intake of phytate can be as high as 4500 mg (Grases et al. 2000). On average, daily intake of phytate was estimated to be 2000–2600 mg for vegetarian diets as well as diets of inhabitants of rural areas in developing countries and 150–1400 mg for mixed diets. Potential health benefits of phytate-rich diets. Consumption of phytate, however, does not seem to have negative aspects on human health. Dietary phytate was reported to prevent kidney stone formation (Jariwalla et al. 1990) and to protect against atherosclerosis and coronary heart disease (Vucenik and Shamsuddin 2003) as well as against a variety of cancers (Grases et al. 2001).
The levels of phytate and its dephosphorylation products in the urine, plasma, and other biological fluids are fluctuating with ingestion or deprivation of phytate in the human diet. Therefore, the reduction in phytate intake in developed compared to developing countries might be a factor responsible for the increase in diseases typical for Western societies, such as diabetes mellitus, renal lithiasis, cancer, atherosclerosis, and coronary heart diseases. It was suggested that phytate exerts beneficial effects in the gastrointestinal tract and other target tissues through its chelating ability, but other mechanisms have also been discussed. Because several myo-inositol phosphates, including phytate, are present as intracellular molecules, and because the second messenger D-myo-inositol(1,4,5)trisphosphate is bringing about a range of cellular functions including cell proliferation via mobilizing intracellular Ca2+ (16), phytate was proposed to exert its anticancer effect by affecting cell signaling mechanisms in mammalian cells (Grases et al. 2001). An effect of extracellular phytate on the concentration of several intracellular myo-inositol phosphate esters has already been demonstrated in human erythroleukemia cells (Ferry et al. 2002).
Furthermore, it has recently been reported that highly negatively charged myo-inositol polyphosphates can cross the plasma membrane and be internalized by cells. Myo-inositol hexakisphosphate was shown to enter HeLa cells followed by intracellular dephosphorylation to partially phosphorylated myo-inositol phosphates (Maffucci et al. 2005), whereas turnover of myo-inositol(1,3,4,5,6)pentakisphosphate was quite slow after internalization by SKOV-3 cells (Carrington et al. 1993). In addition, individual myo-inositol phosphate esters have been proposed to be metabolically active. D-myo-inositol (1,2,6), for example, has been studied with respect to prevention of diabetes complications and treatment of chronic inflammations as well as cardiovascular diseases (Claxson et al. 1990; Konietzny and Greiner 2003), and due to its antiangiogenic and antitumor effects, myo-inositol(1,3,4,5,6)pentakisphosphate was suggested as a promising compound for anticancer therapeutic strategies (Carrington et al. 1993).
12.3 Phytase Adverse Effects
Phytate is an antinutrient that functions as a strongly negatively charged ion in a wide pH range and thus has a considerable affinity to positive-charge food components, such as minerals, trace elements, and proteins (Lopez et al. 2002; Sandberg et al. 1999). This relationship not only has nutritional effects but also affects the yield and consistency of food ingredients such as starch, steep corn liquor, or isolates of plant protein (Sandström and Sandberg 1992; Han et al. 1999; Wang et al. 1999; Fredrikson et al. 2001; Caransa et al. 1988). The main issue about phytate’s role in the human diet is its detrimental impact on mineral absorption. In this sense, minerals of concern include zinc, iron, calcium, magnesium, manganese, and copper (Antrim et al. 1997). Since such complexes are basically nonabsorbable from the human gastrointestinal tract, the development of insoluble mineral–phytate complexes at physiological pH values is known to be the main explanation for the poor bioavailability. In addition, owing to the lack of endogenous phytate-degrading enzymes and the restricted microbial community in the upper part of the digestive tract, the human small intestine has only a very limited capacity to hydrolyze phytate (Kvist et al. 2005). Myo-inositol phosphate–mineral complexes have been found to decrease in solubility and stability as the amount of phosphate residues on the myo-inositol ring decreases. The removal of phytate phosphate residues, therefore, results in a decreased impairment of the intestinal absorption of essential dietary minerals (Cheryan 1980; Iqbal et al. 1994; Sandberg 1991).
Only myo-inositol pentakisphosphate in isolated form inhibited human absorption of iron, zinc, and calcium, while myo-inositol tetrakis and trisphosphates had no effect in the concentrations under investigation. Nonetheless, in the presence of higher phosphorylated myo-inositol phosphates, myo-inositol tetrakis- and trisphosphates have been shown to lead to the phytate’s negative impact on iron absorption (Cheryan 1980). Since there has been a clear negative association between zinc absorption and the amount of myo-inositol tris- by hexakisphosphate from cereal and legume meals (Sebastian et al. 1998), such a contribution is also possibly true for zinc absorption. Phytate is well known to form protein complexes at both acidic and alkaline pH (Knuckles and Betschart 1987). This interaction can affect changes in protein structure that may reduce enzymatic activity, solubility of proteins, and digestibility of proteolytics. The importance of protein–phytate complexes in feeding is, however, still under scrutiny. There is clear evidence that phytate–protein interactions have an adverse effect on in vitro protein digestibility, and the degree of this effect depends on the source of the protein. Nonetheless, a negative effect of phytate on the protein’s nutritional value was not explicitly established in monogastric animal studies (Desphande and Cheryan 1984).
Although some have indicated phytate does not influence the digestibility of proteins, others have found an increase in the supply of amino acids with declining phytate levels. This disparity may be attributed at least in part to the use of various sources of protein. The inhibition by phytate of digestive enzymes such as a-amylase (Knuckles 1988), lipase (Singh and Krikorian 1982), or proteinases (Desphande and Damodaran 1989; Inagawa et al. 1987; Jenab and Thompson 2002), such as pepsin, trypsin, and chymotrypsin, may also be of nutritional significance as shown in in vitro studies. With the amount of phosphate residues per myo-inositol molecule, and the concentration of myo-inositol phosphate, the inhibitory effect increases. This inhibition may be due to the nonspecific nature of phytate–protein interactions, the chelation of calcium ions necessary for the trypsin and a-amylase function, or the interaction with these enzyme substrates. Protease inhibition may be partially responsible for the decreased digestibility of the proteins. Phytate was also considered an in vivo a-amylase inhibitor as shown by the negative relationship between phytate intake and blood glucose response (D’Souza et al. 1987).
Food rich in phytate has therefore been regarded as having significant nutritional significance in the prevention and management of diabetes mellitus, one of Western society’s most common nutrition-dependent diseases. The most serious phytate-attributable effects have occurred as a major dietary variable in populations with unrefined cereals and/or pulses. Deficiencies in zinc and iron have been reported due to high intakes of phytate (Lönnerdal 2000; Maberly et al. 1994). Various approaches have been developed to reduce the risk of mineral deficiency in vulnerable groups, such as child-bearing mothers, strictly vegetarians, inhabitants of developing countries, and fast-growing children. Supplementation with nutritional formulations, food fortification, dietary diversification, and disease prevention (Raboy 2002) is the most known methods for reducing micronutrient malnutrition.
None has been very good for different reasons. An alternative solution would be to increase the overall level of micronutrients in the edible parts of staple crops while at the same time increasing the concentration of compounds that encourage their uptake and/or decrease the amount of compounds that inhibit their uptake, either by plant breeding or by genetic engineering. Low phytate mutants have recently been isolated in maize, barley, rice, and soybeans (Mendoza 2002), and their capacity for enhancing the absorption of iron, zinc, and calcium has been shown (Lucca and Hurrell 2001). To boost rice as an iron source, three proteins were expressed in the rice seed’s central endosperm: a Phaseolus phytoferritin , an endogenous metallothionine-like protein rich in cysteine, and an Aspergillus fumigatus phytase (Coello et al. 2001).
If properly managed, phytase overexpression during seed production may result in reduced levels of phytate in the mature seed (Greiner and Konietzny 2006). Increased levels of seed phytase can also lead to an increase in mineral absorption by decreasing phytate levels in plant-based food during human stomach processing and digestion after a meal is eaten. Furthermore, phytate degradation may be improved during food processing by adding exogenous phytases or modifying optimal conditions for the native plant or microbial phytases. In addition to enzymatic degradation, nonenzymatic phytate hydrolysis during food processing or physical separation of phytate-rich sections of plant seed may lead to reduced phytate levels in the final foods. In general, a loss of valuable nutrients that are either extracted along with the phytate-rich sections of the plant or destroyed by the strong acids or high temperatures needed for nonenzymatic phytate dephosphorylation must compensate for the lower phytate rates. However, enzymatic phytate degradation also occurs under moderate conditions and does not affect other components of food (Egli et al. 2002).
12.4 Enzymatic Phytate Dephosphorylation to Increase Mineral Bioavailability During Food Processing
Different methods of food processing and preparation contribute to a reduction of the phytate content of the raw products. As regards enzymatic phytate dephosphorylation during food processing and preparation, it is important to distinguish the modification of optimal conditions during food processing for the native plant or microbial phytases from the introduction of exogenous ones. For example, phytate hydrolysis during germination, soaking, cooking, and fermentation is a consequence of the naturally occurring phytate degrading behavior in plants and microorganisms. Due to variations in their intrinsic phytate-degrading activities (Viveros et al. 2000; Eeckhout and de Paepe 1994; Konietzny and Greiner 2002) and the properties of enzymes such as protein stability and pH, as well as optimum temperature for phytate degradation (Hurrell 2003), the ability to dephosphorylate phytate significantly differs between different plant and microbial organisms. To understand phytate hydrolysis, it is important to recognize and account not only for phytase activity but also for other phosphatase activities present in the plant material.
All enzymes capable of dephosphorylated phytate are known as phytases per description. However, the products of phytase activity on phytate, myo-inositol pentakis-, tetrakis-, tris-, bis-, and monophosphates can be further dephosphorylated during food processing by phytases as well as phosphatases that do not accept phytate as a substratum. Phytate is generally not completely hydrolyzed during food processing or preparation by the phytases which occur naturally in plants and microorganisms. However, it has been found that phytate must be reduced to very low levels to greatly increase the bioavailability of minerals, particularly iron (Greiner and Konietzny 1999). Knowing the properties of the natural phytases is important to improve food processing and preparation for phytate degradation. Several cereal phytases (Greiner 2002; Greiner and Konietzny 1998; Hayakawa et al. 1989; Konietzny et al. 1995; Laboure et al. 1993; Nakano et al. 1999; Greiner et al. 2001; Greiner 2002;), legumes (Houde et al. 1990; Mandel et al. 1972; Gibson and Ullah 1988; Nayini and Markakis 1984; de Angelis et al. 2003), and microorganisms used for food fermentation (Greiner and Konietzny 1998, 1999) have been isolated in recent years, and their enzymatic properties have been established. However, the properties of a purified enzyme in a food matrix are not necessarily identical to the properties of the same enzyme. For example, the optimum temperature for phytate dephosphorylation was determined by a phytase of black beans (Phaseolus vulgaris var. Preto) as 50 °C for the isolated enzyme and 65 °C for the enzyme in the bean matrix (Fredlund et al. 1997) (Table 12.1).
12.4.1 Soaking
Soaking is also used as a pretreatment to promote the production of grains from the legumes and cereals. Soaking can last for a short time, about 15–20 min, or a very long time, about 12–16 h. Cereals and legumes are usually immersed in water overnight at room temperatures in household circumstances. Since phytate is water-soluble, by discarding the soak water, a significant reduction in phytate can be achieved. Additionally, endogenous phytase activity contributes to the reduction of phytate. Temperature and pH values during soaking (Fredlund et al. 1997; Greiner et al. 1997; Vidal-Valverde et al. 1998) have been shown to have a significant effect on enzymatic hydrolysis of the phytate. If the soaking stage is performed at temperatures between 45 and 65 °C and pH values between pH = 5.0 and 6.0, which are similar to the optimum conditions for phytate dephosphorylation by intrinsic plant phytases, a large percentage of phytate (26–100%) has been enzymatically hydrolyzed (Greiner et al. 1997; Vidal-Valverde et al. 1998; Egli et al. 2002).
12.4.2 Cooking
Since phytate is heat stable, phytate is not expected to cause significant heat loss during cooking. Thus, substantial phytate dephosphorylation during cooking occurs only by either discarding the cooking water or by enzymatic phytate hydrolysis due to the action of intrinsic plant phytases during the early part of the cooking process (Fredlund et al. 1997). Prolonged periods at high temperatures lead to a gradual inactivation of the endogenous enzymes. The supply of heat-stable phytases to plants or the introduction of exogenous heat-stable phytases is therefore possible.
12.4.3 Germination
Germination is a process widely used in legumes and cereals, especially through the breakdown of certain anti-nutrients, such as phytate and protease inhibitors, to increase their palatability and nutrient value. A little intrinsic phytate-degrading activity is found in nongerminated legume grains and cereal seeds, with the exception of rye and to some degree wheat, triticale, and barley (85–88), but a marked increase in phytate-degrading activity was observed during germination, with a concomitant decrease in phytate content (Mandel et al. 1972; Türk et al. 1996; Greiner et al. 2003). During germination, phytate is gradually hydrolyzed by phytases or concerted action of phytases and phosphatases that do not accept phytate as a substratum to supply the plant’s nutritional needs without the accumulation of less phosphorylated intermediate myo-inositol. After 6–10 days of germination, phytate levels that resulted in a strong increase in mineral uptake could be achieved. Since there is a need for long periods of time to increase mineral bioavailability by germination, this approach is intended to be useful for household applications, but it does not appear to be an economical industrial food processing method.
12.4.4 Fermentation
Food fermentation covers a wide range of microbial and enzymatic processing of food and ingredients to achieve desirable features such as extended shelf-life, enhanced safety, attractive taste, nutritional enrichment, removal of anti-nutrients, and health promotion. Many cereals, legumes, and vegetables are used extensively to make a variety of fermented foods. Microorganisms used for the fermentation of food may be part of the normal microflora present in the fermented raw material or specially cultivated crops engineered to bring about changes in the fermented content. Established starter cultures and controlled conditions are widely used today in the fermentation of food. During the fermentation process, the form of microorganism, the fermentation conditions used, and the starting amount of phytate present in the raw material greatly affect the extent of phytate removal. Major microorganisms for fermentation include lactic acid bacteria, molds, and yeast. For starters, in many countries, yeast and/or lactic acid bacteria are used to produce bread, a staple food. Phytate reduction takes place in the different stages of bread making and naturally depends on the type of bread that is being made. Phytase present in the cereal flour is primarily responsible for phytate dephosphorylation during bread fermentation, whereas the contribution of microbial phytate degrading activity from baker’s yeast and lactic acid bacteria is very small or even nonexistent (Leenhardt et al. 2005; Lopez et al. 2000; Sutardi and Buckle 1988).
There is still some debate about the ability of the lactic acid bacteria to produce a phytate-degrading enzyme. Some studies appear to demonstrate the ability of lactic acid bacteria to hydrolyze phytate (Greiner and Konietzny 1999; Fujita et al. 2003), while others have failed to identify a phytate-degrading enzyme (Lopez et al. 2000). Lowering the pH value of the dough to a more suitable one for the operation of the endogenous cereal phytases is therefore very likely the contribution of the microorganisms during fermentation to phytate hydrolysis. However, there is convincing evidence in Oriental food fermentation that phytases of the micro-organisms used for fermentation contribute significantly to phytate degradation (Fujita et al. 2003; Konietzny et al. 1995). Food products such as tempeh, miso, koji, and soy sauce are produced by fermenting soybeans with, respectively, Rhizopus oligosporus and Aspergillus oryzae . Both molds have been shown to develop phytate-degrading activity intra- as well as extracellular (Kerovuo et al. 1998).
12.5 Isolated Phytases and Food Processing in Recent Times
It has been shown that adding a phytase preparation during food processing is an alternative to maximizing phytate dephosphorylation by enzymes already present in the raw material used in food processing. The effectiveness of supplementary phytase in reducing phytate content during food processing was demonstrated for cereals as well as for food products derived from legumes (Greiner et al. 1997; Shimizu 1992), and even full phytate degradation was demonstrated to be feasible. During food processing, the level of phytate hydrolysis is influenced by the raw material used, the manufacturing process, the source of phytase, and the amount of added enzyme activity. There is no suitable phytase for all diet applications. The added phytase must be highly active when processing or preparing food. Since temperature and pH value are the major factors deciding enzyme activity, high phytate degrading capability even at room temperature, appropriate heat resistance, and high activity over a broad pH range are beneficial properties for phytase that should be used in food processing (Kim et al. 1998; Jog et al. 2005; Yang et al. 1991; Wyss et al. 1999a; Greiner et al. 1993; Vohra and Satyanarayana 2002). The activity of the enzymes increases with temperature to the limit. A further rise in temperature results in enzyme denaturation which is heat-induced. The optimum temperature for phytate hydrolysis ranges from 35 to 80 °C, depending on the source of the enzyme.
Plant phytases generally exhibit maximum activity at lower temperatures as opposed to their microbial equivalent. The higher pH and thermal stability as well as the higher specific microbial activity relative to plant phytases make the former more suitable for food processing applications. Specific activity is a crucial factor in the commercial use of an enzyme, as it affects the expected use on the economy. Thus microbial phytases appear to exhibit higher specific activities compared to plant phytases. The stability of most plant phytases decreased significantly at pH values below pH = 4 and above pH = 7.5, while most of the corresponding microbial enzymes even at pH values above pH = 8.0 and below pH = 3.0 are very stable. For example, when exposed to 4 °C for 2 h (Segueilha et al. 1992), a phytase from Escherichia coli did not lose any activity at pH = 2.0 and pH = 10.0. Most plant phytases are irreversibly inactivated within minutes at temperatures above 70 °C while most of the corresponding microbial enzymes maintain significant activity even after extended incubation periods. Pichia anomala (Doekes et al. 1999), Schwanniomyces castellii (O’Conner et al. 2001), and Lactobacillus sanfranciscensis (Greiner and Konietzny 1999) have isolated the phytases most resistant to high temperatures reported so far. Incubation of these enzymes at 70 °C for 10 min did not result in significant loss of activity, and it was even confirmed that the phytase of Pichia anomala tolerated a 30-h treatment at 70 °C without any loss of activity (Doekes et al. 1999).
It is of practical interest for the technical application of phytases in food processing that a crude enzyme preparation as well as an enzyme present in a food matrix are more pH- and heat-resistant than the corresponding highly purified enzyme. Although microbial phytases are best suited for a food processing application, cereal and legume phytases are thought to be an alternative due to their higher consumer acceptance and their presumed low allergenic potential. Phytases that are found in cereals and legumes are already part of the human diet and none of them have been reported to be an allergen. Conversely, Aspergillus niger phytase was thought to be a high-risk factor for occupational asthma and rhinitis. The enzyme has been demonstrated to trigger unique immune responses to IgE among workers exposed to powered phytase preparation (Baur et al. 2002; Rodriguez et al. 2000; Garrett et al. 2004). The phytase preparations of Aspergillus niger are commercially available, making their use technically feasible in the food processing industry (Abdel-Azeem et al. 2021; Yadav et al. 2019a, b). Since phytases with the properties needed for food processing applications have so far not been found in nature, phytase engineering is seen as a promising strategy to optimize their catalytic features.
Improving thermal tolerance and rising specific activity are two important issues not only for animal feed but also for phytase applications for food processing. To obtain an enzyme capable of withstanding higher temperatures, various techniques were employed. After the introduction of three glycosylation sites into the amino acid sequence of the Escherichia coli phytase by site-directed mutagenesis (Lehmann et al. 2002), a shift in the optimum temperature of the Escherichia coli phytase from 55 to 65 °C and a significant increase in its thermal stability at 80 and 90 °C was achieved by expression of the enzyme in the yeast Pichia pastoris. A further technique used to improve the efficiency of the Escherichia coli phytase (Tomschy et al. 2000) was the saturation mutagenesis technology of the gene site. A library of clones incorporating all 19 possible changes in amino acids in the 431 residues of the Escherichia coli phytase sequence was generated and screened for mutants showing increased thermal tolerance.
When exposed to 62 °C for 1 h and 27% of its initial activity after 10 min at 85 °C, the best mutant displayed no loss of activity, which is a major improvement over parental phytasis. Additionally, there has been a 3.5-fold enhancement in gastric stability. By using a consensus approach based on the comparison of amino acid sequences of homologous proteins and the subsequent calculation of a consensus amino acid sequence using one of the standard programs available, a fully synthetic phytase was generated, showing an increase in intrinsic thermal stability of 21–42 °C compared to the 19 parental fungal phytases used in its design (Tomschy et al. 2000) In addition, a threefold increase in specific activity was achieved by replacing one single amino acid in a fungal phytase sequence with site-directed mutagenesis (Miksch et al. 2002; Mayer et al. 1999). Finally, a phytase will not be efficient if an inexpensive device cannot produce it in high yield and purity. Due to the small amount of phytase obtained from wild-type organisms and their tedious and cost-intensive purification, wild-type organisms are not an appropriate source of enzymes for industrial applications. This has led to the development of highly efficient and cost-effective processes to produce phytase by recombinant microorganisms. The use of economically efficient expression/secretion systems for Escherichia coli (Yao et al. 1998) as well as for the yeasts has identified high rates of phytate-degrading activity accumulating in the fermentation media Hansenula (Kerovuo and Tynkkynen 2000) and Pichia pastoris (Haraldsson et al. 2005). There is no need for protein purification if phytate-degrading capability is introduced or increased in microorganisms used for food fermentation, such as Saccharomyces cerevisiae, Lactobacillus sanfranciscensis, or Lactobacillus plantarum. Improved use of microorganisms in the fermentation of raw material extracted from plants is expected to result in food products with substantially lower levels of phytate. A genetically modified phytase-secreting strain of Lactobacillus plantarum has recently been reported (Haros et al. 2001), but the levels of secretion were far too low for an industrial application. In addition, a Saccharomyces cerevisiae strain was constructed which produces high levels of extracellular phytase activity (Sandberg et al. 1996), but its ability to contribute significantly to phytate hydrolysis during fermentation needs to be studied first.
12.6 Isolated Phytase Applications in Food Production
In addition to improving the bioavailability of the mineral and trace elements, phytase addition during food processing has been documented to affect the economy of the production process as well as the yield and quality of the end products. Technical advances have been recorded by the introduction of phytase during food processing for bread making (Pasamontes et al. 1997), plant protein isolate production (Sandström and Sandberg 1992; Han et al. 1999), maize wet milling (Wang et al. 1999; Fredrikson et al. 2001), and cereal bran fractionation (Caransa et al. 1988).
12.6.1 Bread Making
Phytase proved to be an outstanding improver in bread making (Pasamontes et al. 1997). In addition to reducing phytate content in doughs and fresh breads, phytase addition reduced fermentation time without affecting the pH of the dough. An increase in bread volume and an improvement in crumb texture were also observed. For both formulations, the breadcrumbs’ hardness or firmness was popular so that softer crumbs were obtained with the addition of phytase. Certain criteria of texture such as gumminess and chewiness were also reduced. It has been proposed that these changes in bread consistency are consistent with an indirect effect of phytase on a-amylase activities. In the final breads, the introduction of phytase during bread making results in lower levels of phytate. Even so, a complete phytate removal was not attainable. This, in effect, releases calcium ions from calcium–phytate complexes that are important for a-amylase action. No phytase activity could be found in final breads. Therefore, both intrinsic cereal and augmented microbial phytases were inactivated during baking.
12.6.2 Generation of Isolated Plant Proteins
The application of plant protein isolates and concentrates has been finding increasingly important in food production because of their strong nutritional and functional properties. Nonetheless, the relatively high phytate content present in plant seeds and grains and their association with proteins under alkaline conditions, which are typically applied for protein extraction, adversely affect the yield and quality of the protein isolates obtained using standard production processes. The solubility of the proteins decreased by interacting with the phytate resulting in decreased protein content in the final concentrate. Therefore, a considerable amount of phytate ends up in the protein isolate which affects both its nutritional and its functional properties.
However, it was confirmed that the introduction of exogenous phytase into the production process resulted in significantly higher protein yields and almost complete removal of myo-inositolhexakis-, pentakis-, tetrakis-, and trisphosphates from the final plant protein isolate (Sandström and Sandberg 1992; Han et al. 1999). Such phytate-reduced plant protein isolates have been proposed as ideal protein sources for infant formulae due to an increase in mineral bioavailability, their amino acid composition, and their in vitro protein digestibility. In addition, certain phytate-reduced plant protein isolates are addressed in food products as functional additives, due to their strong properties of foaming, emulsifying, and gelling.
12.6.3 Wet Corn Milling
Steeping is a process needed to obtain the valuable corn steep liquor and soften the maize kernel as well as break the maize cell wall in wet maize milling. Starch yield, corn steep liquor consistency, and steeping time are the main issues of corn wet milling. Maize consists of phytate, which in large measure ends up in the steep liquor of corn and constitutes an undesirable portion. Phytate-free corn steep liquor is easier to absorb and is used in the fermentation industry to manufacture compounds such as enzymes, yeast, polysaccharides, antibiotics, and amino acids as well as a high-energy liquid feed product for animals. Through adding phytases to the steep liquor along with plant cell wall degrading enzymes, corn steep liquor was obtained which was completely free of phytate (Wang et al. 1999; Fredrikson et al. 2001). However, the steeping time was substantially decreased which was accomplished by promoting the separation of starch from fiber which gluten, higher starch and gluten yields, and lower energy consumption.
12.6.4 Cereal Branch Split
It is commonly recognized and accepted that the cereal bran, the by-product of flour production, is the most nutritious component of a grain of cereals. Recently, an industrial method was created to isolate economically the main branch fractions to produce high-value protein, soluble non-starch carbohydrates, oil fractions, and insoluble fibers (Antrim et al. 1997). The bran is first subjected to a combination of enzymatic treatment using starch- and phytate-hydrolyzing enzyme group proteins and wet milling, accompanied by concurrent centrifugation and ultrafiltration. The second step is to fractionate the insoluble phase of the above-mentioned first step by enzymatic treatment with xylanase and/or b-glucanase and wet milling, followed again by sequential centrifugation and ultrafiltration. All fractions obtained have much broader business applications and higher values than the branch initial.
12.7 Phytase Degradation in Human Body
Phytate hydrolysis in the human gastrointestinal tract can be achieved by the action of phytate-degrading enzymes from three sources: dietary phytases, small intestine mucosal phytases, and bacterial flora phytases in the colon. Except for calcium, phytate degradation in the colon is not expected to greatly affect mineral absorption, as minerals are mainly absorbed in the upper small intestine. In addition, it has been shown that only very low phytate-degrading activity exists in the small intestine of humans.
The human small intestine, therefore, has a very limited ability to hydrolyze phytate. In comparison, dietary phytases are an essential factor for phytate degradation during digestion because these enzymes are involved in the stomach of humans (Berka et al. 1998).
Generally speaking, the intrinsic phytate-degrading activity in plant-derived foods is not strong enough to hydrolyze the dietary phytate during the passage through the human stomach to such a degree that there is a substantial increase in iron absorption. The production of plants with higher phytase-degrading activities in the edible parts or the application of phytase preparations to the raw materials or the final foods may lead to more extensive phytate degradation in the human stomach. Such phytases should be effective in releasing phytate phosphate into the human stomach, stable to resist inactivation by storage, and may also be suitable to withstand food processing and preparation. Thermal stability is a concern of special significance as food processing and preparation typically require exposure to high temperatures. Until now, phytases with the necessary degree of thermal stability to withstand thermal treatments such as isolated cooking or within a certain food matrix have not been found in nature. It is therefore no surprise that isolation and characterization of thermostable enzymes, as well as engineering phytases, are hot spots of current phytase work (Phillippy 1999; Kim et al. 2003; Lehmann et al. 2002; Tomschy et al. 2000) to boost stability at elevated temperatures and the quest for determinants of thermal stability. Likewise, it is undisputedly desirable to have a phytase that can withstand long-term storage or transport at ambient temperature.
The enzymatic properties of a phytase are determined by its ability to hydrolyze phytate in the digestive tract. Since the stomach is the key functional site of dietary and/or supplementary phytase, it is beneficial to provide an enzyme with optimum acid pH, good stability under acidic pH conditions, and good pepsin resistance. Microbial phytases are thought to have advantages over their plant counterparts regarding their phytate-degrading ability in the human stomach. Microbial phytases demonstrate substantial enzymatic activity over a wide range of pH and are also active below pH = 3.5. Additionally, the stability of certain microbial phytases below pH = 3.0 is noteworthy. Additionally, plant phytases are more susceptible to gastrointestinal enzyme inactivation. It was confirmed that wheat phytase was less resistant to pepsin and pancreatin than Aspergillus niger phytase (Simon and Igbasan 2002) and that the phytases of Escherichia coli and Citrobacter braakii were even more resistant to pepsin and pancreatin than the Aspergillus niger phytase (Rodriguez et al. 1999).
Furthermore, Citrobacter braakii phytase was stable to trypsin (Rodriguez et al. 1999). The corresponding enzyme from Bacillus subtilis demonstrated a comparable vulnerability to pancreatin compared with the Escherichia coli phytase but a much higher resistance to pepsin (Haros et al. 2005). As recently recorded for the Escherichia coli and Aspergillus niger phytase produced in Pichia pastoris (Blanquet et al. 2004), it must also be noted that recombinant enzymes can differ in proteolytic resistance compared to their wild-type counterparts.
Quite recently (Sandberg et al. 1996), a radically different approach to enhancing phytate degradation in humans’ stomach and upper small intestines was brought up. Healthy for human consumption microorganisms such as baker’s yeast (Saccharomyces cerevisiae), lactobacilli, or bifidobacteria have been proposed as carriers of phytate reducing activity throughout the gastrointestinal tract. Tolerance to the conditions in the stomach and small intestine and the ability to produce extracellular phytate-degrading activity under gastrointestinal conditions are therefore characteristics that microorganisms require for such an application. Saccharomyces cerevisiae (Shears 1998), as well as some strains of Lactobacillus and Bifidobacterium (Vucenik and Shamsuddin 2003), has also demonstrated the ability to survive the passage across the human gastrointestinal tract.
Neither for Saccharomyces cerevisiae nor for any Lactobacillus or Bifidobacterium strains, however, was a sufficiently high extracellular phytate degrading activity demonstrated. Genetic engineering may be used to solve this restriction for the development of high-recombinant, phytase-producing strains. To be able to hydrolyze the dietary phytate, the microorganisms must secrete this phytase or attach it to its outer cell wall. Two separate strategies for improving the production and secretion of phytase in the target microorganisms were successfully applied. A Bacillus subtilis phytase-encoding gene was inserted into a Lactobacillus plantarum strain, but the secreted phytate degrading activity was far too small for any application (Haros et al. 2001).
Nevertheless, in yeast, the regulation of phytase synthesis has been changed by removing a gene encoding a negative regulator for the phytase-encoding genes to express themselves. Compared with the corresponding wild-type yeast, the recombinant yeast exhibited multiple-fold higher phytate hydrolysis capacity, both in the presence and in the absence of orthophosphate (Sandberg et al. 1996). However, recombinant yeast was shown to degrade up to 40% of the phytate present in wheat gruel under simulated gastric conditions, while with wild-type yeast no phytate hydrolysis was observed. Studies on in vivo are still lacking, however. Since yeast phytase at pH values above pH = 7 is practically inactive, this phytase will only be active in the human stomach, and no further phytate degradation is expected to occur in the small intestine. Thus, using one or a mixture of food-grade microorganisms with both characteristics, secretion of an optimally active phytase at acidic and another optimally active at alkaline conditions may increase phytate breakdown in the human stomach and upper small intestine during digestion with a concomitant improvement in the bioavailability of mineral products.
12.8 Production of Metabolically Active Phytate Breakdown Products
In recent years, a great deal of scientific evidence has been published connecting diet, foods, or individual food components with preserving human health and preventing chronic diseases such as coronary heart disease, cancer, or osteoporosis. Specific phosphate esters with myo-inositol have been shown to have essential physiological functions in humans (Brinch-Pedersen et al. 2007).
Several of these compounds, in particular D-myo-inositol(1,4,5)trisphosphate and D-myo-inositol(1,3,4,5)tetrakisphosphate, have been shown to play an important role as secondary intracellular messengers (Greiner and Konietzny 1996), and some isomers of myo-inositol phosphates have demonstrated major pharmacological effects, such as complications of diabetes and anti-inflammatory effects as well as an anti-inflammatory effect (Claxson et al. 1990; Konietzny and Greiner 2003). In addition, it has been proposed that dietary myo-inositol phosphates offer benefits for human health, such as improving heart disease conditions by regulating hypercholesterolemia and atherosclerosis (Vucenik and Shamsuddin 2003), preventing the development of renal stone (Jariwalla et al. 1990), and protecting against a variety of cancers, especially colon cancer (Grases et al. 2001).
Phytate can be partially dephosphorylated during food processing and digestion to produce many positional isomers of pentakis, tetrakis, tris, bis, and monophosphates in myo-inositol (Singh et al. 2020). The number and distribution of the residues of phosphates on the myo-inositol ring determine the metabolic effects caused by the individual phosphate isomer of myo-inositol. Different phytases exhibit varied pathways of phytate degradation, resulting in the generation and accumulation of various intermediate myo-inositol phosphate. Nonenzymatically, attempts to produce specified isomers of the various partially phosphorylated myo-inositol phosphates have resulted in mixtures of pentakis, tetrakis, tris, bis, and monophosphate isomers. The purification from the mixture of these isomers is arduous and uneconomical. An alternative approach to making phytate pure breakdown products available in appropriate amounts for physiological studies is the use of a bioreactor based on immobilized enzymes followed by the hydrolysis mixture anion-exchange chromatography. The quantity of the desired product for phytate degradation may be regulated by the number of phytate-containing solution passing through the bioreactor (Sandberg et al. 1987).
When individual phytate degradation products are known to be metabolically active, phytases can be used in food processing to produce foods with enhanced nutritional value, health benefits, and sensory (functional foods) properties. Through attaching phytase to the raw material, phytate is converted during food processing into metabolically active myo-inositol phosphates. To end up with foods with a reduced phytate content and a regulated content and composition of partially phosphorylated myo-inositol phosphate esters with health benefits, phytate dephosphorylation must be closely managed during food processing. An alternative may be using pure phytate as the source material to produce metabolically active myo-inositol phosphates as food supplements.
Since partially phosphorylated myo-inositol phosphate esters are subject to degradation in the human gastrointestinal tract even if all dietary phosphatases like phytases are inactivated, the desired physiological effects may need to be stimulated by enriching foods with a precursor of the true active myo-inositol phosphate ester. It has already been demonstrated in ileostomy patients (Sandberg and Andersson 1988; Sandberg et al. 1987) that the human intestinal alkaline phosphatase exhibits activity against lower myo-inositol phosphate esters and that the microflora in the human colon is also considered capable of degrading phytate and phytate breakdown items. Myo-inositol phosphates must be consumed in the gastrointestinal tract to achieve their metabolic effects in tissues far away from the food tract. There is some evidence that the human digestive tract consumes myo-inositol phosphates since the levels of phytate and its dephosphorylation products in biological fluids fluctuate with ingestion or phytate deficiency in the human diet (Shamsuddin et al. 1992).
12.9 Conclusion
Phytases, the most significant enzyme in nutrition, human health, and environmental protection, are helpful in the elimination of phytate in the human stomach and upper small intestine during food processing. Surprisingly, in mankind phytase enzyme is reduced. In conclusion, research is required to classify metabolically active isomers and phytases of myo-inositol phosphate or a mixture of phytases and/or phosphatases without phytate-degrading activity able to produce such isomers. There isn’t one phytase suitable for all food applications. Thus, scanning nature for phytases with more desirable properties for food applications and engineering phytases to improve their catalytic and stability characteristics are appropriate approaches to make a suitable phytase available for a particular food processing application. The phytases can be used in isolated form or generated at high levels in recombinant microorganisms used for the fermentation of food and/or in recombinant plant edible pieces. If the intrinsic phytases present in the material to be processed are to be used during food processing for phytate dephosphorylation, their catalytic properties must be elucidated to maximize phytate degradation with respect to the intended use of the product.
References
Abdel-Azeem AM, Yadav AN, Yadav N, Usmani Z (2021) Industrially important fungi for sustainable development. In: Biodiversity and ecological perspective, vol 1. Springer, Cham
Andlid TA, Veide J, Sandberg A-S (2004) Metabolism of extracellular inositol hexaphosphate (phytate) by Saccharomyces cerevisiae. Int J Food Microbiol 97:157–169. https://doi.org/10.1016/j.ijfoodmicro.2004.04.016
Antrim RL, Mitchinson C, Solheim LP (1997) Method for liquefying starch. US patent 5652127
Baur S, Melching-Kollmuss F, Koops K, Straßburger A (2002) Zober, IgE-mediated allergy to phytase—a new animal feed additive. Allergy 57:943–945
Berka RM, Rey MW, Brown KM, Byun T, Klotz AV (1998) Molecular characterization and expression of a phytase gene from the thermophilic fungus Thermomyces lanuginosus. Appl Environ Microbiol 64:4423–4427
Blanquet R, Antonelli L, Laforet S, Denis S, Marol-Bonin M (2004) Alric, living recombinant Saccharomyces cerevisiae secreting proteins or peptides as a new drug delivery system in the gut. J Biotechnol 110:37–49
Brinch-Pedersen H, Borg S, Tauris B, Holm PB (2007) Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J Cereal Sci 46:308–326. https://doi.org/10.1016/j.jcs.2007.02.004
Cao L, Wang W, Yang C, Yang Y, Diana J, Yakupitiyage A, Luo Z, li D (2007) Application of microbial phytase in fish feed. Enzyme Microbial Technol 40: 497–507. doi: https://doi.org/10.1016/j.enzmictec.2007.01.007
Caransa A, Simell M, Lehmussaari A, Vaara M, Vaara T (1988) A novel enzyme application for corn wet milling. Starch 40:409–411
Carrington AL, Calcutt NA, Ettlinger CB, Gustafsson T, Tomlinson DR (1993) Effects of treatment with myo-inositol or its 1,2,6-trisphosphate (PP56) on nerve conduction in streptozotocin-diabetes. Eur J Pharmacol 237:257–263
Chadha BS, Harmeet G, Mandeep M, Saini HS, Singh N (2004) Phytase production by the thermophilic fungus Rhizomucos pusillus. World J Microbiol Biotechnol 20:105–109
Cheryan (1980) Phytic acid interactions in food systems. Crit Rev Food Sci Nutr 13:297–355
Chu HM, Guo RT, Lin TW, Chou CC, Shr HL, Lai HL, Tang TY, Cheng KJ, Selinger BL, Wang AHJ (2004) Structures of Selenomonas ruminantium phytase in complex with persulfated phytate: DSP phytase fold and mechanism for sequential substrate hydrolysis. Structure 12:2015–2024
Claxson A, Morris C, Blake D, Siren M, Halliwell B, Gustafsson T et al (1990) The anti-inflammatory effects of D-myo-inositol-1.2. 6-trisphosphate (PP56) on animal models of inflammation. Agents Actions 29:68–70
Coello JP, Maughan A, Mendoza R, Philip DW, Bollinger TL, Veum LO, Vodkin JC (2001) Polacco, Generation of low phytic acid Arabidopsis seeds expressing an E. coli phytase during embryo development. Seed Sci Res 11:285–291
D’Souza SW, Lakhani P, Waters HM, Boardman KM, Cinkotai KI (1987) Iron deficiency in ethnic minorities: associations with dietary fibre and phytate. Early Hum Dev 15:103–111
de Angelis M, Gallo G, Corbo MR, McSweeney PLH, Faccia M, Giovine M, Gobbetti M (2003) Phytase activity in sourdough lactic acid bacteria: purification and characterization of a phytase from Lactobacillus sanfranciscensis CB1. Int J Food Microbiol 87:259–270
Desphande SS, Cheryan M (1984) Effects of phytic acid, divalent cations, and their interactions on alpha-amylase activity. J Food Sci 49:516–519
Desphande SS, Damodaran S (1989) Effect of phytate on solubility, activity and conformation of trypsin and chymotrypsin. J Food Sci 54:695–699
Doekes G, Kamminga N, Helwegen L, Heederik D (1999) Occupational IgE sensitisation to phytase, a phosphatase derived from Aspergillus niger. Occup Environ Med 56:454–459
Eeckhout W, de Paepe M (1994) Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim Feed Sci Technol 47:19–29
Egli L, Davidsson L, Juillerat MA, Barclay D, Hurrell RF (2002) The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feeding. J Food Sci 67:3484–3488
Ferry S, Matsuda M, Yoshida H, Hirata M (2002) Inositol hexakisphosphate blocks tumor cell growth by activating apoptotic machinery as well as by inhibiting the Akt/NFcappaB-mediated cell survival pathway. Carcinogenesis 23:2031–2041
Fredrikson M, Biot P, Alminger ML, Carlsson N, Sandberg A-S (2001) Production process for high-quality pea-protein isolate with low content of oligosaccharides and phytate. J Agric Food Chem 49(3):1208–1212. https://doi.org/10.1021/jf000708x
Fredlund K, Asp NG, Larsson M, Marklinder I, Sandberg AS (1997) Phytate reduction in whole grains of wheat, rye, barley and oats after hydrothermal treatment. J Cereal Sci 25:83–91
Fujita S, Shigeta Y, Yamane H, Fukuda Y, Kizaki S, Wakabayashi K (2003) Ono, Production of two types of phytase from Aspergillus oryzae during industrial koji making. J Biosci Bioeng 95:460–465
Garrett JB, Kretz KA, O’Donoghue E, Kerovuo J, Kim W, Barton NR, Hazlewood GP, Short JM, Robertson DE, Gray KA (2004) Enhancing the thermal tolerance and gastric performance of a microbial phytase for use as a phosphate-mobilizing monogastric-feed supplement. Appl Environ Microbiol 70:3041–3046
Gibson DM, Ullah AHJ (1988) Purification and characterization of phytase from cotyledons of germinating soybean seeds. Arch Biochem Biophys 260:503–513
Grases F, March JG, Prieto RM, Simonet BM, Costa-Bauza A, García-Raja A, Conte A (2000) Urinary phytate in calcium oxalate stones formers and healthy people. Scand J Urol Nephrol 34:162–164
Grases F, Simonet BM, Prieto RM, March JG (2001) Variation of InsP4, InsP5 and InsP6 levels in tissues and biological fluids depending on dietary phytate. J Nutr Biochem 12:595–601
Greiner R (2002) Purification and characterization of three phytate-degrading enzymes from germinated lupin seeds (Lupinus albus var. Amiga). J Agric Food Chem 50:6858–6864
Greiner R, Konietzny U (1996) Construction of a bioreactor to produce special breakdown products of phytate. J Biotechnol 48:153–159
Greiner R, Konietzny U (1998) Endogenous phytate-degrading enzymes are responsible for phytate reduction while preparing beans (Phaseolus vulgaris). J Food Process Preserv 29:321–331
Greiner R, Konietzny U (1999) Improving enzymatic reduction of myo-inositol phosphates with inhibitory effects on mineral absorption in black beans (vulgaris var. Preto). J Food Process Preserv 23:249–261
Greiner R, Konietzny U (2006) Phytase for food application. Food Technol Biotechnol 44(2):125–140. (paper from which reference was taken)
Greiner R, Konietzny U, Jany KD (1993) Purification and characterization of two phytases from Escherichia coli. Arch Biochem Biophys 303:107–113
Greiner R, Konietzny U, Jany KD (1997) Properties of phytate-degrading enzymes and their biotechnological application in phytate reduction. In: Sandberg AS, Andersson H, Amadó R, Schlemmer U, Serra F (eds) Cost 916: bioactive inositol phosphates and phytosterols in foods. Office for the Official Publications of the European Communities, Luxembourg, pp 61–67
Greiner R, Muzquiz M, Burbano C, Cuadrado C, Pedrosa MM, Goyoaga C (2001) Purification and characterization of a phytate-degrading enzyme from germinated faba beans (Vicia faba var. Alameda). J Agric Food Chem 49:2234–2240
Greiner R, Larsson Alminger M, Carlsson NG, Muzquiz M, Burbano C, Cuadrado C, Pedrosa MM, Goyoaga C (2002) Enzymatic phytate degradation—a possibility to design functional foods? Pol J Food Nutr Sci 11:50–54
Greiner R, Konietzny U, Villavicencio ALCH (2003) Reduction in phytate content during lactic acid fermentation. In: Proceedings of the EURO Food Chem XII Congress: strategies for safe foods, Brugge, Belgium, vol 2, pp 670–673
Ha NC, Oh BC, Shin S, Kim HJ, Oh TK, Kim YO, Choi KY, Oh BH (2000) Crystal structures of a novel, thermostable phytase in partially and fully calcium-loaded states. Nat Struct Biol 7:147–153
Han YM, Wilson DB, Lei XG (1999) Expression of an Aspergillus niger phytase gene (phyA) in Saccharomyces cerevisiae. Appl Environ Microbiol 65:1915–1918
Haraldsson AK, Veide J, Andlid T, Larsson Alminger M, Sandberg AS (2005) Degradation of phytate by high-phytase Saccharomyces cerevisiae strains during simulated gastrointestinal digestion. J Agric Food Chem 54:5438–5444
Haros M, Rosell CM, Benedito C (2001) Fungal phytase as a potential breadmaking additive. Eur Food Res Technol 213:317–322
Haros M, Bielecka M, Sanz Y (2005) Phytase activity as a novel metabolic feature in bifidobacterium. FEMS Microbiol Lett 247:231–239
Hayakawa T, Toma Y, Igaue I (1989) Purification and characterization of acid phosphatases with or without phytase activity from rice bran. Agric Biol Chem 53:1475–1483
Hellström AM, Vázques-juárez R, Svanberg U, Andlid TA (2010) Biodiversity and phytase capacity of yeasts isolated from Tanzanian togwa. Int J Food Microbiol 136:352–358. https://doi.org/10.1016/j.ijfoodmicro.2009.10.011
Houde RL, Alli I, Kermasha S (1990) Purification and characterization of canola seed (Brassica sp.) phytase. J Food Biochem 114:331–351
Hurrell RF (2003) Influence of vegetable protein sources on trace element and mineral bioavailability. J Nutr 133(Suppl):2973–2977
Inagawa J, Kiyosawa I, Nagasawa T (1987) Effect of phytic acid on the digestion of casein and soyabean protein with trypsin, pancreatin and pepsin. Nippon Eiyo Shokuryo Gakkaishi 40:367–373
Iqbal TH, Lewis KO, Cooper BT (1994) Phytase activity in the human and rat small intestine. Gut 35:1233–1236
Jariwalla RJ, Sabin R, Lawson S, Herman ZS (1990) Lowering of serum cholesterol and triglycerides and modulation of divalent cations by dietary phytate. J Appl Nutr 42:18–28
Jenab M, Thompson LU (2002) Role of phytic acid in cancer and other diseases. In: Reddy NR, Sathe SK (eds) Food phytates. CRC Press, Boca Raton, FL, pp 225–248
Jog SP, Garchow BG, Mehta BD, Murthy PPN (2005) Alkaline phytase from lily pollen: investigation of biochemical properties. Arch Biochem Biophys 440:133–140
Kerovuo J, Tynkkynen S (2000) Expression of Bacillus subtilis phytase in Lactobacillus plantarum 755. Lett Appl Microbiol 30:325–329
Kerovuo J, Lauraeus M, Nurminen P, Kalkinnen N, Apajalahti J (1998) Isolation, characterization, molecular gene cloning and sequencing of a novel phytase from Bacillus subtilis. Appl Environ Microbiol 64:2079–2085
Kim YO, Kim HK, Bae KS, Yu JH, Oh TK (1998) Purification and properties of a thermostable phytase from Bacillus sp. DS11. Enzym Microb Technol 22:2–7
Kim HW, Kim YO, Lee JH, Kim KK, Kim YJ (2003) Isolation and characterization of a phytase with improved properties from Citrobacter braakii. Biotechnol Lett 25:1231–1234
Knuckles BE (1988) Effect of phytate and other myo-inositol phosphate esters on lipase activity. J Food Sci 53:250–252
Knuckles BE, Betschart AA (1987) Effect of phytate and other myo-inositol phosphate esters on alpha-amylase digestion of starch. J Food Sci 52:719–721
Konietzny U, Greiner R (2002) Molecular and catalytic properties of phytate-degrading enzymes (phytases). Int J Food Sci Technol 37:791–812
Konietzny U, Greiner R (2003) Phytic acid: nutritional impact. In: Caballero B, Trugo L, Finglas P (eds) Encyclopedia of food science and nutrition. Elsevier, London, UK, pp 4555–4563
Konietzny U, Greiner R, Jany KD (1995) Purification and characterization of a phytase from spelt. J Food Biochem 18:165–183
Kour D, Kaur T, Yadav N, Rastegari AA, Singh B, Kumar V et al (2020) Phytases from microbes in phosphorus acquisition for plant growth promotion and soil health. In: Rastegari AA, Yadav AN, Yadav N (eds) Trends of microbial biotechnology for sustainable agriculture and biomedicine systems: diversity and functional perspectives. Elsevier, Amsterdam, pp 157–176. https://doi.org/10.1016/B978-0-12-820526-6.00011-7
Kumar V, Yadav AN, Verma P, Sangwan P, Saxena A, Kumar K et al (2017) β-Propeller phytases: diversity, catalytic attributes, current developments and potential biotechnological applications. Int J Biol Macromolec 98:595–609. https://doi.org/10.1016/j.ijbiomac.2017.01.134
Kvist S, Carlsson T, Lawther JM, DeCastro FB (2005) Process for the fractionation of cereal brans. US patent application US 20050089602
Laboure AM, Gagnon J, Lescure AM (1993) Purification and characterization of a phytase (myo–inositolhexakisphosphate phosphohydrolase) accumulated in maize (Zea mays) seedlings during germination. Biochem J 295:413–419
Lassen SF, Breinholt J, Østergaard PR, Brugger R, Bischoff A, Wyss M, Fuglsang CC (2001) Expression, gene cloning, and characterization of five novel phytases from four basidiomycete fungi: Peniophora lycii, Agrocybe pediades, a Ceriporia sp. and Trametes pubescens. Appl Environ Microbiol 67:4701–4707
Leenhardt F, Levrat-Verny MA, Chanliaud E, Rémésy C (2005) Moderate decrease of pH by sourdough fermentation is sufficient to reduce phytate content of whole wheat flour through endogenous phytase activity. J Agric Food Chem 53:98–102
Lehmann M, Loch C, Middendorf A, Studer D, Lassen SF, Pasamontes L, van Loon APGM, Wyss M (2002) The consensus concept for thermostability engineering of proteins: further proof of concept. Protein Eng 15:403–411
Loewus F (2002) Biosynthesis of phytate in food grains and seeds. In: Reddy NR, Sathe SK (eds) Food phytates. CRC Press, Boca Raton, FL, pp 53–61
Lönnerdal B (2000) Dietary factors influencing zinc absorption. J Nutr 130(Suppl):1378–1383
Lopez HW, Ouvry A, Bervas E, Guy C, Messager A, Demigne C, Rémésy C (2000) Strains of lactic acid bacteria isolated from sour doughs degrade phytic acid and improve calcium and magnesium solubility from whole wheat flour. J Agric Food Chem 48:2281–2285
Lopez HW, Leenhardt F, Coudray C, Rémésy C (2002) Minerals and phytic acid interactions: is it a real problem for human nutrition? Int J Food Sci Technol 37:727–739
Lucca R, Hurrell I (2001) Potrykus, approaches to improve the bioavailability and level of iron in rice seeds. J Sci Food Agric 81:828–834
Maberly GF, Trowbridge FL, Yip R, Sullivan KM, West CE (1994) Programs against micronutrient malnutrition: Ending hidden hunger. Ann Rev Public Health 15:277–301
Maffucci T, Piccolo E, Cumashi A, Iezzi M, Riley AM, Saiardi A, Godage HY, Rossi C, Broggini M, Iacobelli S, Potter BVL, Innocenti P, Falasca M (2005) Inhibition of the phosphatidylinositol-3-kinase/Akt pathway by inositol pentakisphosphate results in antiangiogenic and antitumor effects. Cancer Res 65:8339–8349
Mandel NC, Burman S, Biswas BB (1972) Isolation, purification and characterization of phytase from germinating mung beans. Phytochemistry 11:495–502
Mayer AF, Hellmuth K, Schlieker H, Lopez-Ulibarri R, Oertel S, Dahlems U, Strasser AWM, van Loon APGM (1999) An expression system matures: a highly efficient and cost-effective process for phytase production by recombinant strains of Hansenula polymorpha. Biotechnol Bioeng 63:373–381
Mendoza C (2002) Effect of genetically modified low phytic acid plants on mineral absorption. Int J Food Sci Technol 37:759–767
Miksch G, Kleist S, Friehs K, Flaschel E (2002) Overexpression of the phytase from Escherichia coli and its extracellular production in bioreactors. Appl Microbiol Biotechnol 59:685–694
Mitchell DB, Vogel K, Weimann BJ, Pasamontes L, van Loon APGM (1997) The phytase subfamily of histidine acid phosphatases: isolation of genes for two novel phytases from the fungi Aspergillus terreus and Myceliophthora thermophila. Microbiol 143:245–252
Mullaney EJ, Ullah AHJ (2003) The term phytase comprises several different classes of enzymes. Biochem Biophys Res Commun 312:179–184
Nakamura Y, Fukuhara H, Sano K (2000) Secreted phytase activities of yeasts. Biosci Biotechnol Biochem 64:841–844
Nakano T, Joh T, Tokumoto E, Hayakawa T (1999) Purification and characterization of phytase from bran of Triticum aestivum L. cv. Nourin #61. Food Sci Technol Res 5:18–23
Nayini NR, Markakis P (1984) The phytase of yeast. Lebensm Wiss Technol 17:24–26
O’Conner TM, Bourke JF, Jones M, Brennan N (2001) Report of occupational asthma due to phytase and b-glucanase. Occup Environ Med 58:417–419
Ogunremi OR, Agrawal R, Sanni A (2020) Production and characterization of volatile compounds and phytase from potentially probiotic yeasts isolated from traditional fermented cereal foods in Nigeria. J Genet Eng Biotechnol 18:16. https://doi.org/10.1186/s43141-020-00031-z
Oh BC, Choi WC, Park S, Kim YO, Oh TK (2004) Biochemical properties and substrate specificities of alkaline and histidine acid phosphatase. Appl Microb Biotechnol 63:362–372
Pasamontes M, Haiker M, Wyss M, van Tessier APGM (1997) Loon, Gene cloning, purification, and characterization of a heat-stable phytase from the fungus Aspergillus fumigatus. Appl Environ Microbiol 63:1696–1700
Pasamontes L, Haiker M, Wyss M, Tessier M, van Loon APGM (1997a) Gene cloning and characterization of a heat-stable phytase from the fungus Aspergillus fumigatus. Appl Environ Microbiol 63:1696–1700
Pasamontes L, Haiker M, Henriquez-Huecas M, Mitchell DB, van Loon APGM (1997b) Cloning of the phytase from Emericella nidulans and the thermophilic fungus Talaromyces thermophilus. Biochim Biophys Acta 1353:217–223
Phillippy BQ (1999) Susceptibility of wheat and Aspergillus niger phytases to inactivation by gastrointestinal enzymes. J Agric Food Chem 47:1385–1388
Quan CS, Fan SD, Zhang LH, Wang YJ, Ohta Y (2002) Purification and properties of a phytase from Candida krusei WZ-001. J Biosci Bioeng 94:419–425
Quan CS, Tian WJ, Fan SD, Kikuchi Y (2004) Purification and properties of a low-molecularweight phytase from Cladosporium sp. FP-1. J Biosci Bioeng 94:260–266
Raboy (2002) Progress in breeding low phytate crops. J Nutr 132(Suppl):503–505
Raghavendra P, Halami PM (2009) Screening, selection and characterization of phytic acid degrading lactic acid bacteria from chicken intestine. Int J Food Microbiol 133:129–134. https://doi.org/10.1016/j.ijfoodmicro.2009.05.006
Rastegari AA, Yadav AN, Yadav N (2020) New and future developments in microbial biotechnology and bioengineering: trends of microbial biotechnology for sustainable agriculture and biomedicine systems: perspectives for human health. Elsevier, Amsterdam
Reddy NR (2002) Occurrence, distribution, content, and dietary intake of phytate. In: Reddy NR, Sathe SK (eds) Food phytates. CRC Press, Boca Raton, FL, pp 25–51
Rodriguez E, Porres JM, Han Y, Lei XG (1999) Different sensitivity of recombinant Aspergillus niger phytase (r-PhyA) and Escherichia coli pH 2.5 acid phosphatase (r-AppA) to trypsin and pepsin in vivo. Arch Biochem Biophys 365:262–267
Rodriguez E, Wood ZA, Karplus PA, Lei XG (2000) Site-directed mutagenesis improves catalytic efficiency and thermostability of Escherichia coli pH 2.5 acid phosphatase/ phytase expressed in Pichia pastoris. Arch Biochem Biophys 382:105–112
Rodriguez E, Mullaney EJ, Lei XG (2000a) Expression of the Aspergillus fumigatus phytase gene in Pichia pastoris and characterization of the recombinant enzyme. Biochem Biophys Res Commun 268:373–378
Sandberg AS (1991) The effect of food processing on phytate hydrolysis and availability of iron and zinc. Adv Exp Med Biol 289:499–508
Sandberg AS, Andersson H (1988) Effect of dietary phytase on the digestion of phytate in the stomach and small intestine of humans. J Nutr 118:469–473
Sandberg AS, Andersson H, Carlsson NG, Sandström B (1987) Degradation products of bran phytase formed during digestion in the human small intestine: effect of extrusion cooking on digestibility. J Nutr 117:2061–2065
Sandberg AS, Rossander Hulthén L, Türk M (1996) Dietary Aspergillus niger phytase increases iron absorption in humans. J Nutr 126:476–480
Sandberg AS, Brune M, Carlsson NG, Hallberg L, Skoglund E, Rossander-Hulthén L (1999) Inositol phosphates with different numbers of phosphate groups influence iron absorption in humans. Am J Clin Nutr 70:240–246
Sandström B, Sandberg AS (1992) Inhibitory effects of isolated inositol phosphates on zinc absorption in humans. J Trace Elem Electrol Health Dis 6:99–103
Sano K, Fukuhara H, Nakamura Y (1999) Phytase of the yeast Arxula adenivorans. Biotechnol Lett 21:33–38
Sebastian S, Touchburn SP, Chavez ER (1998) Implications of phytic acid and supplemental microbial phytase in poultry nutrition: a review. World Poult Sci J 54:27–47
Segueilha L, Lambrechts C, Boze H, Moulin G, Galzy P (1992) Purification and properties of the phytase from Schwanniomyces castellii. J Ferment Bioeng 74:7–11
Shamsuddin AM, Baten A, Lalwani ND (1992) Effects of inositol hexaphosphate on growth and differentiation in K-562 erythroleukemia cell line. Cancer Lett 64:195–202
Shears SB (1998) The versatility of inositol phosphates as cellular signals. Biochim Biophys Acta 1436:49–67
Shimizu M (1992) Purification and characterization of a phytase from Bacillus subtilis (natto) N-77. Biosci Biotechnol Biochem 56:1266–1269
Simon O, Igbasan F (2002) In vitro properties of phytases from various microbial origins. Int J Food Sci Technol 37:813–822
Singh M, Krikorian AD (1982) Inhibition of trypsin activity in vitro by phytate. J Agric Food Chem 30:799–800
Singh B, Boukhris I, Pragya KV, Yadav AN, Farhat-Khemakhem A et al (2020) Contribution of microbial phytases to the improvement of plant growth and nutrition: a review. Pedosphere 30:295–313. https://doi.org/10.1016/S1002-0160(20)60010-8
Sutardi M, Buckle KA (1988) Characterization of extra- and intracellular phytases from Rhizopus oligosporus used in tempeh production. Int J Food Microbiol 6:67–79
Tomschy A, Tessier M, Wyss M, Brugger R, Broger C, Schnoebelen L et al (2000) Optimization of the catalytic properties of Aspergillus fumigatus phytase based on the three-dimensional structure. Protein Sci 9:1304–1311
Türk M, Carlsson NG, Sandberg AS (1996) Reduction in the levels of phytate during wholemeal bread making; Effect of yeast and wheat phytases. J Cereal Sci 23:257–264
Türk M, Sandberg AS, Carlsson N, Andlid T (2000) Inositol hexaphosphate hydrolysis from baker’s yeast. Capacity, kinetics, and degradation products. J Agric Food Chem 48:100–104
Vidal-Valverde C, Frias J, Sotomayor C, Diaz-Pollan C, Fernandez M, Urbano G (1998) Nutrients and antinutritional factors in faba beans as affected by processing. Z Lebensm Unters Forsch A 207:140–145
Viveros C, Centeno A, Brenes R, Canales A, Lozano A (2000) Phytase and acid phosphatase activities in plant feedstuffs. J Agric Food Chem 48:4009–4013
Vohra A, Satyanarayana T (2001) Phytase production by the yeast, Pichia anomala. Biotechnol Lett 23:551–554
Vohra A, Satyanarayana T (2002) Purification and characterization of a thermostable and acid-stable phytase from Pichia anomala. World J Microbiol Biotechnol 18:687–691
Vucenik I, Shamsuddin AM (2003) Cancer inhibition by inositol hexaphosphate (IP6) and inositol: from laboratory to clinic. J Nutr 133(Suppl):3778–3784
Wang M, Hettiarachchy NS, Qi M, Burks W, Siebenmorgen T (1999) Preparation and functional properties of rice bran protein isolate. J Agric Food Chem 47:411–416
Wodzinski RJ, Ullah AHJ (1996) Phytase Adv Appl Microbiol 42:263–302
Wyss M, Pasamontes L, Rémy R, Kohler J, Kusznir E, Gadient M, Müller F, van Loon APGM (1998) Comparison of the thermostability properties of three acid phosphatases from molds: Aspergillus fumigatus phytase, A. niger phytase, and A. niger pH 2.5 acid phosphatase. Appl Environ Microbiol 64:4446–4451
Wyss M, Brugger R, Kronenberger A, Remy R, Fimbel R, Oesterhelt G, Lehmann M, van Loon APGM (1999a) Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolase): catalytic properties. Appl Environ Microbiol 65:367–373
Wyss M, Brugger R, Kronenberger A, Rémy R, Fimbel R, Oesterhelt G, Lehmann M, van Loon APGM (1999b) Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): catalytic properties. Appl Environ Microbiol 65:367–373
Yadav AN, Mishra S, Singh S, Gupta A (2019a) Recent advancement in white biotechnology through fungi. Volume 1: Diversity and enzymes perspectives. Springer International Publishing, Cham
Yadav AN, Singh S, Mishra S, Gupta A (2019b) Recent advancement in white biotechnology through fungi. Volume 2: Perspective for value-added products and environments. Springer International Publishing, Cham
Yang WJ, Matsuda Y, Sano S, Masutani H, Nakagawa H (1991) Purification and characterization of phytase from rat intestinal mucosa. Biochim Biophys Acta 1075:75–82
Yao B, Thang C, Wang J, Fan Y (1998) Recombinant Pichia pastoris overexpressing bioactive phytase. Sci China 41:330–336
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dar, P.M. (2021). Fungal Phytases: Current Research and Applications in Food Industry. In: Abdel-Azeem, A.M., Yadav, A.N., Yadav, N., Sharma, M. (eds) Industrially Important Fungi for Sustainable Development. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-030-85603-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-85603-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-85602-1
Online ISBN: 978-3-030-85603-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)