Abstract
Aging is a process which leads to gradual redox status deterioration at the subcellular level. Proteostasis is a dynamic event that regulates protein’s redox status within the aging process to maintain redox stability of proteome. Proteostasis also includes the highly complex redox regulatory signaling pathways that affect various functions in the aging cell. At the subcellular level, other cellular organelles besides mitochondria, such as lysosomes, peroxisomes, and endoplasmic reticulum (ER), also produce reactive oxygen species (ROS) that contribute to proteomic aging. The optimum stability and function of proteome may be deteriorated by many aging-related factors such as impaired cellular redoxtasis, nonenzymatic post-translational modifications, and ER stress. Misfolded protein accumulation in the ER lumen interferes signal transduction-related events. Proteasome-autophagy systems possess the removal activity for oxidatively modified proteins and aging organelles. The ubiquitin–proteasome system is major intracellular protein degradation system that controls the garbage recycle process in the aging proteome. Aging-related impaired redoxtasis may cause nonenzymatic post-translational modification- related proteinopathies. The gradual accumulation of oxidatively modified and misfolded protein aggregates is the main characteristics of proteinopathies. Aging-induced interorganellar redox imbalance, impaired oxidative garbage removal, and deposition of modified proteins like amyloid β, tau proteins, α-synuclein, and amyloid polypeptides are all related to age-related protein misfolding diseases. Thus, in the long term, novel antiaging and senolytic strategies to restore proteostasis in aging proteome may provide an effective way to establish promising therapies for Alzheimer’s disease and other aging-induced protein misfolding diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Aging mainly depends on the optimum efficiency of several interconnected subcellular networks in long-lived postmitotic cells which are also referred to as non-dividing cells (e.g., cardiac myocytes, neurons, and retinal pigment epithelial cells). Interorganellar communication needs to be precisely tuned to prevent subcellular oxidative damage during the aging process. Recent experimental evidence indicates that variations in the subcellular redox status of aging organelles have a pivotal role in regulating their physiological functions with the aging process (Gil-Hernández and Silva-Palacios 2020). Postmitotic cells possess inadequate regenerative activity because of the division and differentiation of stem cells. Therefore, biological waste materials which cannot be removed from the aging cell gradually accumulate and replace normal cellular structures. All these changes lead to interorganellar communication disorders, and eventually cellular death (Çakatay 2010). Cellular functions of the aging organelle mainly depend on communication and proximity with subcellular systems to sustain specific functions (Silva-Palacios et al. 2020).
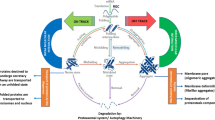
Available data from previous studies support the presence of subcellular “redox triangle” failure in senescence postmitotic cells related to mitochondria, peroxisomes, and endoplasmic reticulum (ER) (Fig. 10.1). Redox triangle detects ROS-mediated oxidative damage signals and redoxtasis imbalances. Redox signaling process and related enzymes take place in the “redox triangle” created by ER, peroxisomes, and mitochondria (Yoboue et al. 2018). Signaling free radicals can be released by redox triangle via aquaporins. Redox triangle-induced ROS overproduction affects the functioning of ER-mitochondria Ca2+ ion transport, ATP synthesis, and oxidative folding activity of proteins within the cisternal lumen of ER. It has been known that redox triangle-controlled relationship occurs during peroxisomal beta-oxidation process. Peroxisomal β-oxidation of very long chain fatty acids (VLCFA) is partially completed and forms NADH that should be moved to mitochondria via outer membrane located porins for its complete oxidation. The optimum redox triangle activity may become inadequate with the advance of the aging process, as determined by decreased activity of peroxisomal catalase in senescent cells (Yoboue et al. 2018) (Fig. 10.1). A list of specific protein oxidation biomarkers in subcellular organelles correlating with aging is given in Table 10.1.
Interorganellar redox regulation and oxidative garbage removal mechanisms of aging cell. Reactive oxygen species (ROS), immunoglobulin binding protein (BiP), ubiquitin–proteasome system, inositol requiring element 1 (IRE-1), serine–threonine ER kinase (PERK), activating transcription factor 6 (ATF6), X-box-binding protein 1 (XBP1), activating transcription factor 4 (ATF4), and homologous protein (CHOP)
The progressive redox failure plays the leading role in the occurrence of degenerative processes with the advancing age. Proteostasis is a highly complicated, subcellularly controlled process that regulates redox stability of proteins within the aging metabolome. Impaired redoxtasis is an inevitable part of subcellular aging which leads to gradual failure of the proteostasis-dependent vital systems, removal of oxidatively damaged proteins, and quality control of related metabolic events in aging (Janikiewicz et al. 2018). At the subcellular level, besides mitochondria, other cellular organelles such as lysosomes, peroxisomes, and ER also produce reactive oxygen species (ROS) that contribute to proteomic aging. Physiologically essential or detrimental properties of ROS depend on their subcellular concentrations. Interorganellar diffusion rate of ROS is likely to be regulated. The place of each organelle on total ROS formation and proteomic aging exhibits a significant variation between cell types and ages (Cecarini et al. 2007).
Giacomello and Pellegrini's terminology (2016) “MAM (mitochondria-associated membrane) fraction” has been used to describe isolated or purified membranes of mitochondria–ER interactions; however, when the subcellular architecture of such contacts has (had) considered, authors refer them as mitochondria–ER contacts “MERCs”. Redox status-sensitive proteins are localized to contact sites between the mitochondria and ER, known as MERCs. ER cisternae create membrane contact surfaces with peroxisomes and mitochondria (MAM). Among the advantages of MAM in the aging cell is that it allows the lower rate of ROS formation, the reduced oxidation state of mitochondrial proteins as well as better uptake of Ca2+ ions. The close interactions of these contact surfaces increase the ROS and Ca2+ ion reuptake which leads to cell death (Gil-Hernández and Silva-Palacios 2020). Unusual organization of MAM can lead to the disruption of ER–mitochondria contact sites with the advance of aging (Cherubini et al. 2020). These contact sites are also implicated in neuronal longevity (De Mario et al. 2017). Hence, MAM dysfunction leads to the development of various neurodegenerative diseases (Xu et al. 2020). Interorganellar matrix and physical interactions permit the transport of various metabolites and ROS that affects the cellular redoxtasis systems.
MAM-localized modulators of ER-mitochondria signal transduction-related crosstalk are known as Ero1-α, calnexin, and selenon/selenoprotein N1 gene (SEPN1). Some of the ROS-generated chaperones and oxidoreductases bind to ER Ca2+ ion handling proteins which regulate ER–mitochondria Ca2+ ion flux via redox-dependent interactions. ER is an interconnected network of cisternae that fulfills many of the functions related to cellular proteome such as protein biosynthesis, peptide translocation, protein folding, and various enzymatic post-translational modifications such as disulfide formation, glycosylation, and chaperone-related folding. Growing polypeptide chain should remain in unfolded state during its translocation into cisternal lumen. Maturing newly synthesized polypeptides are prone to misfolding as a result of exceeding the critical concentration in cisternal lumen. The accomplishment of protein folding is even more problematic in a massive cisternal network of the ER for secreted proteins, where luminal proteins need to keep their native conformation while being constantly exposed by high-energy collisions with neighboring cisternal proteins (Valastyan and Lindquist 2014). Because of these unfavorable luminal conditions, many of the ER proteins cannot gain their native conformations, or stably assume the incorrect ones. However, ER blocks improperly folded or incompletely assembled proteins from exiting the ER and destinating to the cytosol or other subcellular components. Unfavorable folding conditions may result in protein folding diseases. Majority of the resident ER proteins such as molecular chaperons and folding enzymes (foldases) collaborate in order to achieve native folding of newly synthesized polypeptide chain and its subsequent release from ER. Chaperones are expressed continuously at the constant level and their expression induces in response to the gradual deposition of unfolded and/or misfolded proteins. ATP-binding and carbohydrate-binding chaperon systems which interact directly to the growing polypeptide chain and indirectly hydrophilic glycosyl groups accomplish together to ensure proper protein passage through the ER and the secretory pathway. Folding enzymes catalyze proline cis–trans isomerization and/or disulfide bond formation, both are necessary for folding to the native conformation (Braakman and Hebert 2013). Incorrectly folded proteins are destined for ER-associated degradation (ERAD) with the help of ubiquitin–proteasome system. The proteasome system is located in the cytoplasm, nucleus, and ER to ensure proper protein folding and prevent aggregation, aforementioned chaperone groups and folding enzymes reduce excessive workload of ERAD (Zeeshan et al. 2016).
The close physical contact of the rough ER to the nucleus ensures the quality control process of protein folding. The rough ER is also able to activate its own signal transduction mechanisms to manage its workload by decreasing the overall rate of translation under ER stress and halting improper folding of proteins. Misfolded protein accumulation in the cisternal lumen triggers a sequence of reactions named unfolded protein response (UPR). Under prolonged and severe ER stress, UPR reduces translation rate, induces luminal protein folding capacity, and ER-related protein degradation rate. It is called the heat shock response in the cytosolic and nuclear compartments. Aging leads to decline in gene expression and folding function of ER-located chaperones. Folding enzymes ensure the fidelity of the protein folding process and UPR (Brown and Naidoo 2012). If the homeostatic response fails, aging cells are directed to undergo apoptosis. UPR also induces an autophagic pathway to eliminate misfolded proteins which cannot be degraded by ERAD. Functions of the UPR components decline with age. During the aging process, the balance between the protective function of UPR and pro-apoptotic signaling was reported; the protective function is significantly diminished and the apoptotic function is getting more robust.
Autophagy displays its protective role against subcellular aging through the removal of intracellular protein aggregates and damaged organelles. It has been suggested that autophagy can ensure neuroprotection by enhancing the removal of these protein aggregates. The growing experimental finding shows that autophagy also decreases with the advance in age; the rate of autophagosome biogenesis and the efficiency of autophagosome/lysosome fusion are getting reduced. Cross-linking is most commonly seen ROS-mediated post-translational modification in long-lived proteins which becomes undegradable by autophagocytosis (Terman and Brunk 2004).
Insoluble protein aggregates known as aggresomes are most commonly seen in senescent neurons. As has been reported in previous papers, prolonged ER stress, interorganellar redox imbalance, protein misfolding-initiated dysregulated ROS cascades, impaired oxidative garbage removal activity, and accumulation of aggresomes have important roles in proteomic aging and the physiopathological mechanism of various age-related proteinopathies such as neurodegenerative diseases, inflammaging, and diabetes mellitus (Terman 2006).
2 Mitochondrial Aging and Redox Proteostasis
Mitochondria carry out important physiological tasks such as the production of energy, Ca2+ homeostasis, regulation of cell cycle, differentiation, apoptosis, and aging. Insufficient mitochondrial function is generally accepted as one of the important indicators of aging (López-Otín et al. 2013). The role of mitochondria in age-related impaired redox status has been reviewed by the mitochondrial free radical theory of aging (Barja 2014). It is generally assumed that mitochondria contribute approximately 90% of the cellular ROS (Wang et al. 2020). During the cellular aging process, oxidatively damaged mitochondria produces lesser amount of ATP and a higher amount of ROS. Electron transfer chain is embedded in the inner membrane of mitochondria and includes five types of protein complexes: NADH dehydrogenase (Complex I), succinate dehydrogenase (SDH) (Complex II), cytochrome bc1 complex (Complex III), cytochrome c (Cytc) oxidase (Complex IV), and ATP synthase (Complex V). Complexes I, III, and IV pump generated protons across the inner membrane into the intermembrane space, producing electrochemical gradient, which is then utilized by ATP synthase (Figueiredo et al. 2008). Complexes I and III are known as the primary source of superoxide radical anion (O2•−) production. ROS is passed into the intermembrane space across the complex III. In addition to Complexes I and III-related ROS production, other complexes are also known as producers of ROS, including pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, cytochrome b5 reductase, flavoprotein– ubiquinone oxidoreductase, and the monoamine oxidase (Balaban et al. 2005).
The flavin mononucleotide and coenzyme Q sites of Complex I, and the quinol oxidase site of Complex III determine the course whether ROS is released to the inside or outside of the mitochondria. Variations in the activity of mitochondrial electron transfer chain with the aging process are likely due to the destabilization of Complexes I and III. If ROS accumulates inside the mitochondria, the mitochondrial DNA coding subunits of electron transfer chain complexes may be oxidatively damaged, resulting in impaired electron flow and insufficient ATP synthesis. If ROS-induced macromolecular damage accumulates outside of the mitochondria with age, downstream cytoplasmic organelles might be oxidatively deteriorated over the cellular life course (Genova and Lenaz 2015). Another characteristic of mitochondrial aging is related to the opening activity of mitochondrial permeability transition (MPT) pore. MPT pore complex is located between mitochondrial membranes which consist of different macromolecular complexes such as cyclophilin D, the adenine nucleotide translocase, and voltage-dependent ion channel (VDAC) (Bonora and Pinton 2019). MPT increases inner mitochondrial membrane permeability to lower weighted substances (<1.5 kDa) which is triggered by gradual deposition of Ca2+ ions in the matrix. The movement of H2O into the mitochondrial matrix causes mitochondrial swelling, the loss of the inner membrane potential (∆ψm), and the uncoupling of oxidative phosphorylation which leads to cellular death (Bonora et al. 2015). Functionally, mitochondria subject to age-related deteriorations include increased ROS formation rate, culminating in oxidative damage to cellular macromolecules as well as impaired bioenergetics. Dysregulated mitochondrial antioxidant enzyme activity and increased ROS formation can influence many of the metabolic pathways such as oxidative phosphorylation, tricarboxylic acid (TCA) cycle, glycolysis, and ATP synthesis. All these seem to be related to diminish mitochondrial dynamics like mitophagy and fusion/fission in the senescent organism that are linked to a higher probability of the occurrence of age-related diseases (Forrester et al. 2018; Gil-Hernández and Silva-Palacios 2020).
Brain mainly includes peroxidizable membrane lipids while featuring a high O2 consumption rate. Mitochondrial dysfunction has also been known as one of the earliest events in Alzheimer’s disease (AD) due to the gradual deposition of β-amyloid peptide (Aβ) in mitochondria, prior to plaque formation process. Even though the exact molecular mechanisms of how Aβ affects mitochondrial redox status are still obscure. The presence of inefficient mitochondrial redox status causes: (i) reduced activities of TCA cycle enzymes, oxidative phosphorylation and ATP production; (ii) a decrease in glucose metabolism and an increase in oxidative damage due to the increased formation rate of ROS (Wang et al. 2020).
Aging mitochondrion seems to be not the only source of ROS formation in the senescent cells in the brain. Microglia cell is considered to be a substantial source of increased ROS production and has been attributed to its own ROS-induced inflammatory activity. In a related context, superoxide forming NADPH oxidase is known as the leading producer of microglial ROS. It has been demonstrated that NADPH oxidase enzyme complex to be activated in the AD brain which can be toxic to neighboring neurons (Desler et al. 2018).
In the cytoplasm of microglia, highly reactive O2•- is removed by Cu,Zn superoxide dismutase catalyzing the generation of H2O2, which in turn is inactivated by reaction with reduced glutathione (GSH) catalyzed by glutathione peroxidase. If the ROS production rate exceeds the antioxidant capacity, O2•- and H2O2 levels will rise. In the presence of redox-active transition metal ions such as Fe2+ and/or Cu2+, highly reactive OH· radicals can be formed by Haber–Weiss or Fenton reactions. OH· radical has the highly reactive potential to induce oxidative damage to proteins, lipids, and DNA. Studies related to proteomic approach made the quantitative identification of carbonylated subcellular proteins feasible which are formed by protein oxidation in relation to aging. Considering to these assessments, during organism lifespan, mitochondrial proteins result in the overrepresented ones and also exhibited an enormous increase in carbonylation rate (Cabiscol et al. 2014).
Glial cells are prone to generate an enormous amount of nitric oxide (NO) through the inducible nitric oxide synthase. Activated microglial cells accelerate the formation of more reactive free radicals such as peroxynitrite (ONOO−). ONOO− initiates lipid peroxidation, protein oxidation, and DNA damage, which lead to neuronal death. There is also extensive evidence support the conclusion that impaired redox homeostasis affects the oxidation status of almost all types of macromolecules in the brain of AD patients (Swomley and Butterfield 2015; Erdoğan et al. 2017; Wang et al. 2020).
3 ER Proteome
ER is considered to be a highly active organelle like mitochondria. ER regulates its structural process (e.g., degradation, elongation, fission, and fusion of cisternal membranes) according to its metabolic tasks. The age-related variation in the levels of ER-resident chaperons has clarified to us that aged cell tries to maintain its redox homeostasis in this cellular compartment (Gil-Hernández and Silva-Palacios 2020). ER-resident chaperones such as immunoglobulin binding protein (BiP), thiol-disulfide oxidoreductases, protein disulfide isomerase (PDI), calnexin, glucose-regulated protein 94 (GRP94), and calreticulin are gradually oxidized with the aging process and this detrimental process may also be related to their functional impairment. All these oxidative modifications exhibit a significant correlation with impaired functions of several chaperones and foldases (Brown and Naidoo 2012).
BiP is a member of heat shock 70 protein family and also named glucose-regulated protein 78 (GRP78). It binds transiently to newly synthesized underglycosylated, misfolded, or unfolded proteins transported to the ER lumen. It is also known as heat shock protein 5A(HSP5A) or GRP78. Cisternal unfolded protein accumulation releases BiP. BiP induces transmembrane sensors of ER, serine–threonine ER kinase (PERK), the inositol requiring element 1 (IRE-1), and the activating transcription factor 6 (ATF6), whose signaling recruits several transcription factors such as activating transcription factor 4 (ATF4), X-box-binding protein 1 (XBP1), and homologous protein (CHOP) leading to the activation UPR genetic program. Removal of the BiP from PERK and IRE1 leads to the initiation of UPR through their oligomerization and trans-autophosphorylation. Activation of PERK leads to phosphorylation of the eukaryotic initiation factor (eIF2α) and stimulation of ATF4. Stimulated ATF4 induces its target genes and related redox reactions. ATF4 activation also leads to the induction of CHOP. The activated domain of ATF6 is moved to the nucleus to upregulate ER-related chaperons and protein degradation factors, as well as XBP1 and CHOP expression (Estébanez et al. 2018) (Fig. 10.1).
UPR causes the following processes: (i) upregulation of ER chaperones such as BiP/GRP78 to assist the refolding process of proteins; (ii) inhibition of mRNA translation which is accomplished by PERK which phosphorylates eIF2α thereby reducing the rate of translation; and preventing further synthesis and thus protein folding (iii) removal of misfolded proteins by the proteasome complex by a process known as ERAD (Brown and Naidoo 2012).
ER-resident transmembrane proteins such as IRE-1, PERK, and ATF6 regulate proteostasis in the cisternal lumen. Transmembrane ER stress transducers (e.g., IRE-1, PERK, and ATF6) have a crucial role to play in mitigating stress and ensuring proteostasis. However, persistent subcellular stress and aging may lead the cell toward apoptosis (Minakshi et al. 2017).
4 ER-Related Aging and Redox Proteostasis
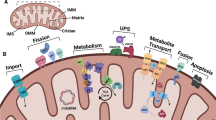
The ER is highly sensitive to impaired redox homeostasis and altered redox signaling. All these alterations can influence protein folding, Ca2+ ion release, and mitochondrial respiration (Forrester et al. 2018). ER stress and UPR initiate ROS-related cascades and have important roles in the pathogenesis of aging-induced protein misfolding diseases. Redox signaling mediators such as calcium, endoplasmic reticulum oxidoreductin (ERO)-1, GSH/glutathione disulfide (GSSG), glutathione peroxidase 8 (GPX8), NADPH oxidase 4 (NOX4), NADPH-P450 reductase (NPR), and PDI have a strong relationship with ER stress-induced ROS formation (Zeeshan et al. 2016) (Fig. 10.2). GSH/GSSG is considered as the principal thiol redox couple in the ER. Glutathione status of the ER changes during aging process and lower GSH level causes less antioxidant production upon oxidative attack (Rudzińska et al. 2020). GSH is involved in redoxtasis reactions of ER proteins such as the maintenance of protein thiol groups in the reduced form and elimination of H2O2. Reducing cytosolic redox environment is known to be unfavorable for the formation of protein disulfide bonds. The luminal molar ratio of [GSH]/[GSSG] in the ER lumen is 1:1 to 3:1 as compared to 30:1 to 100:1 for the ratio of outside of cisternal lumen. In the cisternal lumen, the relative concentration of the GSSG compared to the GSH may contribute to the function of GSSG as the oxidizing peptide during protein folding. Active site of the PDI needs its own oxidized state thiol groups to catalyze the formation of disulfide bonds. A slight shift in the reductive potential of the cisternal lumen transforms PDI to its reduced thiol state (Dixon et al. 2008; Rudzińska et al. 2020). GSH can transform into GSSG by GPX8. GSSG is transformed back to GSH by glutathione reductase with the consumption of NADPH (Fig. 10.2). A list of redoxtasis-related processes in the ER, mitochondria, and peroxisomes is given in Table 10.2.
Proteostasis mechanisms during ER stress-related aging. Prolonged ER stress and UPR that induce reactive oxygen species (ROS)-related cascades and are known to play important roles in the pathogenesis of aging-induced protein misfolding diseases. Protein disulfide isomerase (PDI)-endoplasmic reticulum oxidoreductin (ERO)-1, glutathione (GSH)/glutathione disulfide (GSSG), glutathione peroxidase 8 (GPX8), NADPH oxidase 4 (NOX4)
Knowledge in age-related modulation of ROS formation in the ER is still in the infancy period. However, ER-located proteins like the molecular chaperones BiP/Grp78 and PDI undergo oxidative modification and progressive impairment during aging by some of the studies related to senescent hepatocytes (Rabek et al. 2003; Nuss et al. 2008).
5 Mitochondria–ER Signaling-Related Communication
Variations in MAM ultrastructure and aberrant function of ER–mitochondria cooperation cause various age-related diseases such as cardiovascular diseases, cancer, neurodegenerative diseases, metabolic diseases, and inflammation (Gil-Hernández and Silva-Palacios 2020). Cellular functions (e.g., regulation of lipid transfer and Ca2+ ion interchange) were the initially clarified functions attributed to MERCs, but additional important physiological roles (e.g., ATP synthesis, the regulation of mitochondrial division, innate immunity, inflammasome assembly, autophagosome formation, processing of the amyloid precursor protein, apoptosis, and redox signaling control) have also been attributed to MERCs, recently (Ray et al. 2014; Moltedo et al. 2019; Garrido-Maraver et al. 2020).
Mitochondria accomplish many vital processes. Increased mitochondrial Ca2+ concentration activates matrix enzymes rolled in the TCA cycle, including pyruvate, isocitrate, and α-ketoglutarate dehydrogenases complexes, and stimulates oxidative phosphorylation, which leads to increased production of ATP and MPT pore opening (Janikiewicz et al. 2018; Garrido-Maraver et al. 2020). The gradual decrease in mitochondrial Ca2+ ion uptake and the reduced number of MERCs are commonly seen in senescent cells (Janikiewicz et al. 2018). Mitochondria include both crucial regulators of cell death and potent inductors of apoptosis such as second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein (Smac/DIABLO), apoptosis-inducing factor (AIF), and Cytc. Some of the MAM-resident proteins play a role in ER–mitochondria communication. The participation of glucose-regulated protein 75 (GRP75) is forming a link between Ca2+ channels inositol triphosphate receptor (IP3R) and VDAC, while Calnexin (CNX) modulates the activity of sarcoplasmic/ER calcium ATPase 2b (SERCA2b), directly. The ER releases its Ca2+ contents through ion channel IP3R and pumps it back through SERCA. Mitochondria imports these Ca2+ ions through a series of channels (mCU and VDAC) driven by ∆ψm which is also considered to be a significant signaling mechanism, particularly during mitophagy and cell survival decisions. Higher rate Ca2+ ions transport to matrix could serve as a homeostatic mechanism to counterbalance the loss of Δψm in aging cells (Janikiewicz et al. 2018).
Impairment in redox homeostasis needs a synchronic response from ER and mitochondria. ER signaling ensures mitochondrial integrity and mitochondria have crucial components for regulated UPR signaling (Bravo-Sagua et al. 2013).
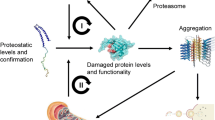
Mitochondria and ER are both known as ROS generation centers, and therefore the communication at MERCs participates to the detrimental effects of intracellular ROS formation. ER–MT redox crosstalk occurs at MERCSs where it is responsible for ROS formation: oxidative folding activity of the Ero1-α, Ca2+ ion movement from ER to MT with the help of IP3R/VADC Ca2+ channels, and the electron transport promoted at mitochondrial electron transfer chain. Ero1-α is known as FAD-dependent oxidase and plays an essential role in protein folding with PDI. Ero1-α activates IP3Rs to facilitate MPTs as a member of MAM proteome. Ero1-α reforms the oxidized PDI and transfers the electrons from PDI to O2, leading to H2O2 synthesis (Fig. 10.2). During ER stress, Ero1-α oxidizes IP3R1, which potentiates the release of Ca2+ ions from the ER. Ca2+ homeostatic ion flux from the ER to mitochondria maintains the TCA cycle. High production rate of ROS at MERCs leads to generate redox nanodomains at ER–MT contact sites that modulate ER–MT apposition (Fig. 10.3) (Fan and Simmen 2019; Moltedo et al. 2019).
Endoplasmic reticulum–Mitochondria (ER–MT) redox crosstalk occurs at mitochondria–ER contacts (MERCs) where different mechanisms are responsible for reactive oxygen species (ROS) formation: Ca2+ ion flux from the ER to MT through the subtype 3 of the 1,4,5- triphosphate receptor/ voltage-dependent anion-selective channel IP3R/VADC Ca2+ channels, the oxidative folding activity of the ER chaperone, ER oxidoreductase 1 alpha (Ero1-α), and the electron transport promoted by p66Shc at mitochondrial electron transfer chain. Ero1-α activates IP3Rs to facilitate mitochondrial permeability transitions as a member of MAM proteome. Ca2+ homeostatic ion flux from the ER to mitochondria maintains the TCA cycle. Unfolded protein response (UPR)-unrelated activities of ER transmembrane kinase/ribonuclease 1 (IRE1) and serine–threonine ER kinase (PERK) control mitochondrial ETS activity. Accelerated ROS at MERCs forms redox nanodomains at ER–MT interface that modulates ER–MT apposition
Nowadays, increasing experimental evidence supports the idea that MERCs-related molecular interactions are closely related to the development of age-related diseases (Moltedo et al. 2019). MERCs are known as the initial occurrence site of Aβ formation (Schreiner et al. 2015) and play a crucial role in the development of AD. The release of Aβ peptide occurs at MERCs throughout the processing of the amyloid precursor protein by the γ-secretase complex, composed of Presenilin 1 and Presenilin 2. Mutated Presenilin 2 proteins affect ER–MT connections and their related biofunctions in genetic types of AD (Zampese et al. 2011).
6 Mitochondria–Lysosome Signaling-Related Communication
The proteasome is not merely a subcellular structure able to degrade oxidatively modified proteins. The lysosomal system includes different types of proteases that contribute to protein turnover. Lysosome-related proteolysis targets long-lived proteins and is considered to be non-selective (Cecarini et al. 2007). Correlations between mitochondria and lysosomes in the execution of the apoptosis process are emphasized in the lysosomal-mitochondrial axis theory (Zhao et al. 2003). Lysosomes are the iron-dependent formation sites of OH· radicals and most likely sites for the formation of indigestible substances. Lipofuscin possesses a brown-yellow auto-fluorescent, electron-dense pigment which includes oxidatively modified protein and lipid residues. Autophagic capacity is insufficient in lipofuscin-loaded cells such as neurons, retinal pigment epithelial cells, and cardiac myocytes. Lysosomal membrane disintegration can be induced in several different ways resulting in apoptosis. Released enzymes can attack various cellular proteins and mitochondria. H2O2 diffuses from the mitochondria into lipofuscin-filled lysosomes which are rich in redox-active iron catalyzing transformation H2O2 to OH· with Fenton reaction. OH· causes oxidative protein damage to lysosomal membranes that induces leak of lysosomal enzymes and iron into cytosol (Terman and Brunk 2004; Cecarini et al. 2007). Lysosomal enzymes and cytosolic hydrolytic enzymes such as phospholipase A2 permeabilize the outer membranes of mitochondria and lead to releasing of Cytc, AIF, and Smac/DIABLO triggering cell death (Terman and Brunk 2004).
7 Peroxisomal Aging and Redox Proteostasis
Peroxisomes are known as multifunctional organelles involved in α-oxidation of branched chain fatty acids such as phytanic acid, β-oxidation of VLCFA, detoxification of glyoxylate, bile acid conjugation, ether lipid synthesis, bile acid conjugation, ROS, and reactive nitrogen species (RNS) formation (Islinger et al. 2018; Walker et al. 2018). Peroxisomal homeostasis needs to be adapted to the metabolic requirements such as peroxisomal proliferation and removal of extensively damaged organelles by autophagy (Walker et al. 2018). Over the last decade, the biological role of peroxisomes has widened well and cellular signaling pathways have also been included. More recent experimental evidences indicate the possible links between peroxisomal aging and impaired cellular redox status.
Peroxisome biogenesis is accomplished by de novo synthesis or division and growth of pre-existing peroxisomes. Peroxisomal biogenesis requires the fusion of two pre-peroxisomal vesicles formed by ER and mitochondria. The growth and division of peroxisomes are accomplished by elongation factors and fission regulators. These processes are strictly regulated by peroxisome biogenesis factors, named peroxins and peroxisomal membrane proteins (Jo et al. 2020). In recent years, the interest increased in nonphysiological roles of peroxisomes (e.g., in cellular stress responses, the combat of pathogens, and antiviral defense as cellular signaling platforms and health aging) (Islinger et al. 2018; Cook et al. 2019). Cytotoxic properties of VLCFA metabolism for inflammatory demyelination and axonopathy are reported. Death of oligodendrocytes and astrocytes, regulation of Ca2+ homeostasis, and a marked decrease of the membrane potential of mitochondria in oligodendrocytes are also related to peroxisomal VLCFA metabolism (Islinger et al. 2018). VLCFA triggers oxidative stress characterized by an overproduction of ROS.
It is generally considered that mitochondria are the main ROS formation sites in the aging cell. Expanding knowledge in the last decade showed that the peroxisomes and the ER produce as much or even more ROS than mitochondria. Peroxisome-originated ROS may not only induce aging-related effects, but also function as antiaging signaling effects. Since the peroxisomal matrix contains a high amount of H2O2 producing flavoenzymes/oxidoreductases, H2O2 is the main product of ROS metabolism in peroxisomes (Table 10.2). Peroxisomes do not only generate H2O2, but similar to mitochondria have the ability to form O2•− and NO radicals (Cipolla and Lodhi 2017). The superoxide radical anion mainly derives from the enzyme xanthine oxidase. Xanthine oxidase is located in the cytosol as well as in the peroxisomes and is the final enzyme and therefore plays a primary role in purine degradation. H2O2 has a comparatively long intracellular half-life with its relatively mild oxidant reactivity and a high diffusion rate. All these properties make H2O2 an efficient signaling molecule which has a significant role in cellular differentiation, migration, proliferation, and gene expression. Excess accumulation of H2O2 causes impaired proteostasis, which if not balanced will induce cellular dysfunction with aging. The reduction of H2O2 produces highly reactive hydroxyl radicals (OH) that can readily react with proteins, lipids, and nucleic acids. OHs may also alter their macromolecular structures and functions. Oxidation by ROS (like H2O2) leads to redox post-translational modifications of cysteine residues. Many of the reactions lead to disulfide bond formation: (i) intramolecular disulfide bonds are often inserted into a reduced protein by disulfide exchange (via formation of mixed disulfides) with GSSG or another oxidized protein molecule (e.g., PDI) and (ii) intermolecular disulfide bonds can be formed with another protein or low molecular weight thiols. Although the peroxisome is generally attributed to be a leading producer of O2·− and H2O2, it also significantly contributes to RNS. NO is formed with the catalytic activity of nitric oxide synthase activity with the transformation of l-arginine to NO· and citrulline. ONOO− is formed as a consequence of the reaction between NO· and O2·− (Fransen et al. 2012). Peroxisomal GSH reacts with ONOO- to form S-nitrosoglutathione, known as a signaling molecule. The cellular localization and activities of several peroxisomal matrix proteins are known to be regulated by the cellular redoxtasis system (Wang et al. 2015). ONOO− is also a powerful oxidizing agent and nitrated agent that may inactivate peroxisomal enzymes. Some of the nitrogenous species have structural ability and may trigger direct oxidative and nitrosative modification, often manifested as protein oxidation (Yanar et al. 2020). 3-nitrotyrosine as an important product of tyrosine side chain oxidation reactions is generated due to the reaction with ONOO− (Yanar et al. 2020). H2O2 inside peroxisomes may give rise to reactive OH formation through the Fenton reaction. Carbonylation is the most widely studied nonenzymatic protein modification that takes place as a consequence of aging-related oxidative stress. Metal ion-catalyzed protein carbonylation process is likely to involve the overproduction of OHs (Çakatay et al. 2001). It is very likely that peroxisomes also lead to formation of protein carbonyl group during aging process.
Peroxisomes are sources of ROS and also they protect cells from the oxidative damaging effects of ROS. Peroxisomes include some of the scavenger systems such as catalase (CAT: hemoprotein catalyzing the reduction of H2O2 in H2O), superoxide dismutase (superoxide dismutation into H2O2), and peroxiredoxin 5 (reduction of H2O2, ROOH, ONOO using catalytic cysteine residue). Epoxide hydrolase 2 (EPHX2) is a homodimeric enzyme which can bind and transforms epoxides into the corresponding dihydrothiols. It has been thought that the primary physiological role of EPHX2 is to detoxify fatty-acid-derived epoxides. GSH is synthesized in the cytosol, from where it is transferred into peroxisomes (Table 10.2). GSSG is thought to be transported to the cytosol with peroxisomal glutathione transporter, wherein it is reduced to GSH by NADPH-dependent cytosolic glutathione reductase (Wang et al. 2015). The diminished CAT targeting to the peroxisome is commonly seen with aging. Senescent cells exhibit a reduced amount of peroxisomal biogenesis factor 5 (PEX5) which leads to diminished recognition affinity. This, in turn, lowers the ability of CAT to be targeted to the peroxisome. It was reported that reduced levels of H2O2 cannot be inefficiently degraded by CAT due to its active site which needs the interaction of two molecules of H2O2, despite its higher catalytic efficiency. Catalase activity may not actually play a significant role in removing low levels of H2O2 from the peroxisomal matrix. CAT is highly prone to its protein oxidation and can be inactivated itself related to higher amounts of peroxisomal H2O2. H2O2 leakage into the cytosol induces peroxisomalredox signaling pathway at physiological amounts or causes oxidative damage at excessive amounts (Bonekamp et al. 2009). Gradual accumulation of H2O2 causes impaired cellular redox status which if not balanced with redoxtasis systems will promote impaired redoxtasis and cellular dysfunction with aging. As a result, increased H2O2 formation, coupled with decreased removal of oxidative damage, promotes impaired redox homeostasis as evidenced by increased lipid peroxidation rate and deposition of lipofuscin granules in hepatocytes. Age-related decline in the peroxisomal import of de novo synthesized CAT, coupled with its already diminished activity, limits its ability to the elimination of H2O2 (Walker et al. 2018).
Peroxisomes form a very close physical contact with the ER and with mitochondria (Fig. 10.1). Peroxisomes and mitochondria possess signaling network systems and have crucial roles in regulating redox signaling pathways. Peroxisome-related proteins are also prone to ROS and RNS-mediated oxidative protein damage. Peroxisomal Lon protease homolog-2 possesses the ability to eliminate such oxidatively modified proteins, thus prolonging the useful lifespan of the organelle. Senescent peroxisomes are also removed by autophagy. Autophagic degradation of dysfunctional peroxisomes is named pexophagy. ROS-activated ataxia-telangiectasia mutated kinase targets the peroxisome for degradation in two ways: (i) signaling mammalian target of rapamycin complex 1 to inhibit its suppression of pexophagy and (ii) phosphorylating PEX5 to promote its ubiquitination and its subsequent binding of p62. It was reported that PEX5 may serve as a redox/stress sensor to keep peroxisomal CAT in the cytosol to combat oxidative stress of non-peroxisomal origin (Wanders 2014).
The impaired peroxisomal function can also lead to mitochondrial dysfunction. It is widely assumed that peroxisomal activity is diminished with aging. Decreased expression of peroxisomal matrix proteins involved in ROS and lipid metabolism is commonly seen in aging and age-related disorders (Fransen et al. 2012; Cipolla and Lodhi 2017).
8 Concluding Remarks
The regulation of redox status of subcellular proteins has long-term effects on healthy aging and longevity. Recently, the redox-dependent signaling process has been integrated with intracellular ROS production which is no longer considered as just detrimental products of subcellular metabolism but are now highly appreciated for their role in regulating signaling networks in aging. Illumination of the processes related to the regulation of both redox triangle and proteostasis in the subcellular aging would help to identify clinically relevant senolytic and geroprotective therapeutic targets for combating age-related proteinopathies and even increase life expectancy in humans.
References
Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120:483–495
Barja G (2014) The mitochondrial free radical theory of aging. Prog Mol Biol Transl Sci 127:1–27
Beltran Valls MR, Wilkinson DJ, Narici MV, Smith K, Phillips BE, Caporossi D, Atherton PJ (2015) Protein carbonylation and heat shock proteins in human skeletal muscle: relationships to age and sarcopenia. J Gerontol A Biol Sci Med Sci 70:174–181
Bonekamp NA, Völkl A, Fahimi HD, Schrader M (2009) Reactive oxygen species and peroxisomes: struggling for balance. BioFactors 35:346–355
Bonora M, Wieckowsk MR, Chinopoulos C, Kepp O, Kroemer G, Galluzzi L, Pinton P (2015) Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene 34:1608
Bonora M, Pinton P (2019) A new current for the mitochondrial permeability transition. Trends Biochem Sci 44:559–561
Braakman I, Hebert DN (2013) Protein folding in the endoplasmic reticulum. Cold Spring Harb Perspect Biol 5:a013201
Bravo-Sagua R, Rodriguez AE, Kuzmicic J, Gutierrez T, Lopez-Crisosto C, Quiroga C, Díaz-Elizondo J, Chiong M, Gillette TG, Rothermel BA, Lavandero S (2013) Cell death and survival through the endoplasmic reticulum-mitochondrial axis. Curr Mol Med 13:317–329
Brown MK, Naidoo N (2012) The endoplasmic reticulum stress response in aging and age-related diseases. Front Physiol 3:263
Cabiscol E, Tamarit J, Ros J (2014) Protein carbonylation: proteomics, specificity and relevance to aging. Mass Spectrom Rev 33:21–48
Çakatay U, Telci A, Kayali R, Tekeli F, Akcay T, Sivas A (2001) Relation of oxidative protein damage and nitrotyrosine levels in the aging rat brain. Exp Gerontol 36:221–229
Çakatay U (2010) Protein redox-regulation mechanisms in aging. In: Bondy S, Maiese K (eds) Aging and age-related disorders, 1st edn. Humana Press, pp 3–25
Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN (2007) Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim Biophys Acta 1773:93–104
Chepelev NL, Bennitz JD, Wright JS, Smith JC, Willmore WG (2009) Oxidative modification of citrate synthase by peroxyl radicals and protection with novel antioxidants. J Enzyme Inhib Med Chem 24:1319–1331
Cherubini M, Lopez-Molina L, Gines S (2020) Mitochondrial fission in Huntington’s disease mouse striatum disrupts ER-mitochondria contacts leading to disturbances in Ca(2+) efflux and Reactive Oxygen Species (ROS) homeostasis. Neurobiol Dis 136:104741
Cipolla CM, Lodhi IJ (2017) Peroxisomal dysfunction in age-related diseases. Trends Endocrinol Metab 28:297–308
Cook KC, Moreno JA, Jean Beltran PM, Cristea IM (2019) Peroxisome plasticity at the virus-host interface. Trends Microbiol 27:906–914
De Mario A, Quintana-Cabrera R, Martinvalet D, Giacomello M (2017) (Neuro)degenerated mitochondria-ER contacts. Biochem Biophys Res Commun 483:1096–1109
Desler C, Lillenes MS, Tønjum T, Rasmussen LJ (2018) The role of mitochondrial dysfunction in the progression of Alzheimer’s disease. Curr Med Chem 25:5578–5587
Dixon BM, Heath S-HD, Kim R, Suh JH, Hagen TM (2008) Assessment of endoplasmic reticulum glutathione redox status is confounded by extensive ex vivo oxidation. Antioxid Redox Signal 10:963–972
Erdoğan ME, Aydın S, Yanar K, Mengi M, Kansu AD, Cebe T, Belce A, Çelikten M, Çakatay U (2017) The effects of lipoic acid on redox status in brain regions and systemic circulation in streptozotocin-induced sporadic Alzheimer’s disease model. Metab Brain Dis 32:1017–1031
Estébanez B, de Paz JA, Cuevas MJ, González-Gallego J (2018) Endoplasmic reticulum unfolded protein response, aging and exercise: an update. Front Physiol 9:1744
Fan Y, Simmen T (2019) Mechanistic connections between Endoplasmic Reticulum (ER) redox control and mitochondrial metabolism. Cells 8:9
Figueiredo PA, Mota MP, Appell HJ, Duarte JA (2008) The role of mitochondria in aging of skeletal muscle. Biogerontology 9:67–84
Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK (2018) Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122:877–902
Fransen M, Nordgren M, Wang B, Apanasets O (2012) Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim Biophys Acta 1822:1363–1373
Garrido-Maraver J, Loh SHY, Martins LM (2020) Forcing contacts between mitochondria and the endoplasmic reticulum extends lifespan in a Drosophila model of Alzheimer’s disease. Biol Open 9:1
Genova ML, Lenaz G (2015) The interplay between respiratory supercomplexes and ROS in aging. Antioxid Redox Signal 23:208–238
Giacomello M, Pellegrini L (2016) The coming of age of the mitochondria-ER contact: a matter of thickness. Cell Death Differ 23:1417–1427
Gil-Hernández A, Silva-Palacios A (2020) Relevance of endoplasmic reticulum and mitochondria interactions in age-associated diseases. Ageing Res Rev 64:101193
Islinger M, Voelkl A, Fahimi HD, Schrader M (2018) The peroxisome: an update on mysteries 2.0. Histochem Cell Biol 150:443–471
Janikiewicz J, Szymański J, Malinska D, Patalas-Krawczyk P, Michalska B, Duszyński J, Giorgi C, Bonora M, Dobrzyn A, Wieckowski MR (2018) Mitochondria-associated membranes in aging and senescence: structure, function, and dynamics. Cell Death Dis 9:332
Jo DS, Park NY, Cho D-H (2020) Peroxisome quality control and dysregulated lipid metabolism in neurodegenerative diseases. Exp Mol Med 52:1486–1495
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217
Marshall A, Lutfeali R, Raval A, Chakravarti DN, Chakravarti B (2013) Differential hepatic protein tyrosine nitration of mouse due to aging—effect on mitochondrial energy metabolism, quality control machinery of the endoplasmic reticulum and metabolism of drugs. Biochem Biophys Res Commun 430:231–235
Minakshi R, Rahman S, Jan AT, Archana A, Kim J (2017) Implications of aging and the endoplasmic reticulum unfolded protein response on the molecular modality of breast cancer. Exp Mol Med 49:e389
Moltedo O, Remondelli P, Amodio G (2019) The mitochondria-endoplasmic reticulum contacts and their critical role in aging and age-associated diseases. Front Cell Dev Biol 7:172
Murakami H, Guillet C, Tardif N, Salles J, Migné C, Boirie Y, Walrand S (2012) Cumulative 3-nitrotyrosine in specific muscle proteins is associated with muscle loss during aging. Exp Gerontol 47:129–135
Nakamura T, Lipton SA (2008) Emerging roles of S-nitrosylation in protein misfolding and neurodegenerative diseases. Antioxid Redox Signal 10:87–101
Naoi M, Maruyama W, Shamoto-Nagai M, Yi H, Akao Y, Tanaka M (2005) Oxidative stress in mitochondria: decision to survival and death of neurons in neurodegenerative disorders. Mol Neurobiol 31:81–93
Nuss JE, Choksi KB, DeFord JH, Papaconstantinou J (2008) Decreased enzyme activities of chaperones PDI and BiP in aged mouse livers. Biochem Biophys Res Commun 365:355–361
Rabek JP, Boylston WH 3rd, Papaconstantinou J (2003) Carbonylation of ER chaperone proteins in aged mouse liver. Biochem Biophys Res Commun 305:566–572
Ray S, Bonafede MM, Mohile NA (2014) Treatment patterns, survival, and healthcare costs of patients with malignant gliomas in a large US commercially insured population. Am Heal Drug Benefits 7:140–149
Rudzińska M, Parodi A, Balakireva AV, Chepikova OE, Venanzi FM, Zamyatnin AAJ (2020) Cellular aging characteristics and their association with age-related disorders. Antioxidants (basel, Switzerland) 9:2
Schreiner B, Hedskog L, Wiehager B, Ankarcrona M (2015) Amyloid-β peptides are generated in mitochondria-associated endoplasmic reticulum membranes. J Alzheimers Dis 43:369–374
Silva-Palacios A, Zazueta C, Pedraza-Chaverri J (2020) ER membranes associated with mitochondria: Possible therapeutic targets in heart-associated diseases. Pharmacol Res 156:104758
Sudheesh NP, Ajith TA, Ramnath V, Janardhanan KK (2010) Therapeutic potential of Ganoderma lucidum (Fr.) P. Karst. against the declined antioxidant status in the mitochondria of post-mitotic tissues of aged mice. Clin Nutr 29:406–412
Swomley AM, Butterfield DA (2015) Oxidative stress in Alzheimer disease and mild cognitive impairment: evidence from human data provided by redox proteomics. Arch Toxicol 89:1669–1680
Terman A (2006) Catabolic insufficiency and aging. Ann N Y Acad Sci 1067:27–36
Terman A, Brunk UT (2004) Aging as a catabolic malfunction. Int J Biochem Cell Biol 36:2365–2375
Uehara T, Nakamura T, Yao D, Shi Z-Q, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA (2006) S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441:513–517
Valastyan JS, Lindquist S (2014) Mechanisms of protein-folding diseases at a glance. Dis Model Mech 7:9–14
Walker CL, Pomatto LCD, Tripathi DN, Davies KJA (2018) Redox regulation of homeostasis and proteostasis in peroxisomes. Physiol Rev 98:89–115
Walton PA, Pizzitelli M (2012) Effects of peroxisomal catalase inhibition on mitochondrial function. Front Physiol 3:108
Wanders RJA (2014) Metabolic functions of peroxisomes in health and disease. Biochimie 98:36–44
Wang W, Zhao F, Ma X, Perry G, Zhu X (2020) Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Mol Neurodegener 15:30
Wang X, Li S, Liu Y, Ma C (2015) Redox regulated peroxisome homeostasis. Redox Biol 4:104–108
Xu L, Wang X, Tong C (2020) Endoplasmic reticulum-mitochondria contact sites and neurodegeneration. Front Cell Dev Biol 8:428
Yanar K, Atayik MC, Simsek B, Çakatay U (2020) Novel biomarkers for the evaluation of aging-induced proteinopathies. Biogerontology 21:531–548
Yoboue ED, Sitia R, Simmen T (2018) Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis 9:331
Zampese E, Fasolato C, Kipanyula MJ, Bortolozzi M, Pozzan T, Pizzo P (2011) Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc Natl Acad Sci U S A 108:2777–2782
Zeeshan HMA, Lee GH, Kim H-R, Chae H-J (2016) Endoplasmic reticulum stress and associated ROS. Int J Mol Sci 17:327
Zhao M, Antunes F, Eaton JW, Brunk UT (2003) Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur J Biochem 270:3778–3786
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest:
There is no conflict of interest.
Ethical Approval:
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Atayik, M.C., Yanar, K., Çakatay, U. (2022). Redox Proteostasis in Subcellular Aging. In: Çakatay, U. (eds) Redox Signaling and Biomarkers in Ageing. Healthy Ageing and Longevity, vol 15. Springer, Cham. https://doi.org/10.1007/978-3-030-84965-8_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-84965-8_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84964-1
Online ISBN: 978-3-030-84965-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)