Abstract
Obesity, a disorder of body weight regulatory systems that is characterized by the accumulation of excess body fat is increasingly becoming a global pandemic. Despite several approaches that have been applied to mitigate obesity, they have not been able to totally reverse the obesity and its mediated complication . Advanced glycation end products (AGEs) refer to a group of prooxidant heterogenous compounds whose formation results from nonenzymatic reactions between reactive sugars and proteins, lipids and nucleic acids. While supporting evidence has suggested that AGEs may have contributory roles in the pathogenesis of obesity, there are indications that increased consumption of dietary AGEs increases the circulating AGEs levels and their deposition in the tissues including the adipose tissue, increasing the risk of development of obesity and its comorbidities. Identification of the underlying mechanism may provide an important strategy for novel therapeutic approaches against obesity. This chapter therefore provided novel insights into the role of dietary AGEs in the pathogenesis of obesity and the purported mechanisms of action.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Obesity is a disorder of body weight regulatory systems that is characterized by the accumulation of excess body fat [1]. According to the World Health Organization [2], obesity is an abnormal or excessive fat accumulation that presents a risk to human health. The development of obesity involves both adipocyte hypertrophy and hyperplasia. Obesity in childhood has been reported to involve adipocyte hyperplasia and hypertrophy while adipocyte hypertrophy arising from imbalanced energy intake was generally suggested to be responsible for most adult-onset obesity. However, adipocyte hyperplasia has also been suggested to contribute to the development of adult-onset obesity, especially morbidly obese patients [3].
Advanced glycation end products (AGEs) refer to a group of prooxidant heterogeneous compounds whose formation results from nonenzymatic reactions between reactive sugars and proteins, lipids and nucleic acids in a reaction that is otherwise known as ‘the Maillard reaction’. Recent studies have revealed that consumption of foods that are rich in these AGEs could play fundamental roles in the pathogenesis of chronic diseases including obesity [4]. Given the inability of the current therapeutic approaches for obesity to totally reverse it [5], identification of the underlying mechanism involved in the contribution of AGEs to obesity may provide an important strategy for novel therapeutic approaches against obesity. In line with the above, this chapter discussed the role of dietary AGEs in the pathogenesis of obesity. The mechanisms of action deriving from preclinical and clinical studies that were carried out were also reported.
Global Prevalence of Overweight and Obesity
In current report, the global prevalence of overweight and obesity doubled since 1980 to such an extent that about one third of the world's population is now classified as being overweight or obese [6]. Additionally, studies that were carried out revealed that a total of 1.9 billion and 609 million adults were estimated to be overweight and obese in 2015, respectively, representing approximately 39% of the world’s population [6]. The prevalence of obesity was generally higher in women than in men in all age groups, with sex differences being maximal between 50 and 65 years old [6]. Obesity adversely affects nearly all the physiological functions of the body and has become a significant public health threat [6].
Measurement of Obesity
The body mass index (BMI) usually expressed as weight (kg) divided by the square of height (meters)2 is the most acceptable index of measurement of obesity. This index measures the relative weight, adjusted for height which makes for comparisons both within and between populations [1]. Based on BMI cut off point, individuals with BMI values of 18.5 and 24.9 are regarded to have normal weight; individuals with a BMI value between 25 and 29.9 are considered overweight, those with a BMI value equal to or greater than 30 are classified as obese, while BMI values above 40 are regarded as extremely/morbidly obese. Measurement of the waist circumference using a tape has also been found to be diagnostic of obesity as it reveals the amount of fat (visceral fat) in the central abdominal region of the body [1]. This approach has also been reported to reduce the risk of developing cardiovascular diseases, independent of BMI [1, 5, 7].
Advanced Glycation End Products—Overview
AGEs are a group of heterogeneous compounds whose formation results from nonenzymatic reactions between reactive sugars and proteins, lipids and nucleic acids in a reaction that is otherwise known as Maillard reaction as earlier stated [4, 8]. In this reaction, the carbonyl group of a reducing sugar reacts with the amino group of a protein, lipid or nucleic acid, generating Schiff bases which rearrange to Amadori products. Since the Amadori products are relatively unstable, further reactions occur, which eventually lead to the formation of irreversible AGEs [9]. Examples of AGEs include: glycated hemoglobin (HbA1c), Nε-carboxymethyllysine (CML), Nε-carboxyethyl-lysine (CΕL), ALI, pentosidine, methylglyoxal, pyrraline and imidazolone, N-fructosyl-lysine, Alkyl formyl glycosyl pyrroles, and others [10, 11].
With respect to their chemical structure, AGEs can be classified as: non-fluorescent crosslinked (e.g. glucosepane, imidazolium dilysine), fluorescent crosslinked (e.g. crossline, pentosidine) and non-crosslinked molecules (e.g. pyralline, CML) [11]. These AGEs exert their effects through their receptors (RAGE) (described in detail in our review, [5] and book chapter [7]).
Sources of AGEs
Based on the differences in their origins, AGEs are categorised into two major classes namely: biologically derived AGEs (biological-AGEs) and environmentally derived AGEs. Biologically derived AGEs are produced endogenously (in vivo) during normal body metabolism , aging, prolonged oxidative stress or due to hyperglycemia [11]. On the other hand, environmentally derived AGEs are obtained exogenously (in vitro) while cigarette smoke and diet (food) are two major sources of environmentally derived AGEs.
Cigarette smoke contains glycation products that are highly reactive (AGEs) which may contribute to the increased AGEs accumulation in serum and tissue as observed in cigarette smokers. Cigarette smoke derived AGEs have been shown to increase the risk of developing cancers and cardiovascular diseases in former smokers [10]. Dietary AGEs constitute a class of pro-oxidant foods and their formation depends directly on the protein, lipid and carbohydrate content of the food as well as on the temperature and conditions of cooking, especially time used for cooking and moisture [12].
Heat treatment of food results in the generation of AGEs and other Maillard reaction products that improve the aroma and flavour of food products. Therefore, dietary AGEs are commonly found in processed foods with their levels being increased by food processing at high temperatures [13, 14]. In fact, foods may contain up to 200 times the initial AGEs content after cooking depending on the cooking method [10]. Food processing and cooking techniques that utilise dry heat (frying, roasting, baking, grilling, barbecuing) result in greater AGEs formation compared with techniques that use lower temperatures for longer periods of time with higher water content, such as boiling or steaming [4, 15, 16].
Higher pH levels have also been reported to increase the formation of AGEs, as the alkaline conditions promote the amino groups to be in their basic deprotonated form, increasing their reactivity. Pre-treating foods with acidic solutions before cooking (to lower their pH) such as marinating with vinegar or lemon has been reported to decrease the formation of dietary AGEs in food products [15]. Animal-derived foods cooked at high temperature, for a prolonged time and under dry conditions have the highest content of AGEs [12]. Other examples of such foods that contribute to high amounts of dietary AGEs include processed cereal products such as biscuits, bakery products and extruded breakfast cereals [15].
In contrast, dairy products, fruits, and vegetables have lower AGEs content. Higher humidity, lower temperatures, and low pH also make minor contributions to the formation of AGEs [17]. Table 9.1 shows the differences between food groups and cooking methods that contribute to low or high levels of AGEs [4, 18,19,20,21].
Digestion, Absorption, Distribution and Excretion of Dietary AGEs
Digestion
Protein and peptide bound AGEs are the major AGEs in the diet [8]. It has been suggested that the structural features of AGEs may have a significant effect in the digestion of glycated proteins and peptides. For instance, reports have shown that AGEs with non-crosslinked structures result in a considerable decrease in glycated protein digestibility. In addition, the digestibility of CML-casein (60% modification of target lysine) was reported to be significantly lower than that of native casein [8].
Absorption
AGEs can exist in either free form as a single amino acid or a free low-molecular-weight peptide, or bound to proteins, forming high-molecular-weight compounds. Dietary AGEs are reportedly absorbed in circulation mostly in the form of free AGEs eg. CML (by simple diffusion) and peptide bound AGEs following gastrointestinal digestion [8] and thereafter, they can get deposited in various tissues [17].
Approximately 10–30% of dietary AGEs are absorbed into the systemic circulation [8], one third of which is excreted by the kidneys while two-thirds linger in the body, contributing to the body’s AGEs pool and which binds to several tissues [17, 22]. Therefore, decreased intake of dietary AGEs has been associated with approximately 40% decreases in the levels of AGEs in the body [8]. On the other hand, intestinal absorption of pyrraline is considered to happen mostly as a dipeptide rather than a free amino acid, with the dipeptide form of pyrraline absorbed across the intestinal epithelium using peptide transporter 1 [15].
Distribution of Dietary AGEs
After crossing the epithelial cells of the digestive tract, dietary AGEs enter into circulation and merge with the biological AGEs [8]. Dietary AGEs are distributed in most tissues following their absorption and supporting evidence has shown that intake of dietary AGEs can cause AGEs accumulation throughout the body, including the tissues (gastrointestinal tract, liver, kidneys, lungs, adipose tissue, heart and spleen), serum, urine and faeces [8]. The distribution of dietary AGEs in the body is driven by their higher affinity for some tissues on the basis of covalent or noncovalent binding interactions [8].
Metabolism
It has been reported that AGEs may not act as substrates for detoxification by phase I and phase II enzymes of xenobiotic metabolism [8, 23]. According to the hydrophile-lipophile balance, hydrophilic AGEs do not act as substrates for phase I enzymes in the fatty membranes of the endoplasmic reticulum and due to the glycation of side groups, most AGEs have insufficient typical side groups for phase II coupling reactions, except acidic groups for esterification [8].
Excretion
The renal excretion of dietary AGEs has been estimated to be approximately 30% of the absorbed amount in healthy adults, but less than 5% in patients with renal disease [8]. Between 20 and 50% of ingested CML are reportedly excreted in the faeces, suggesting that there is a proportion of ingested AGEs that are not absorbed and not defecated, and may be metabolised intraluminally by the microbiome [15].
Dietary AGEs and Human Health
Consumption of dietary AGEs have been reported to contribute to oxidative stress and inflammation in animal models, although results were less consistent in human studies [15, 24]. Excessive dietary AGEs consumption in mice was implicated in the development of hepatic inflammation in the absence of steatosis. In a rat model of non-alcoholic fatty liver disease, high dietary AGEs were reported to exacerbate liver injury, inflammation and liver fibrosis while chronic dietary AGEs intake was suggested to cause cognitive decline and Alzheimer’s disease [15]. Furthermore, regular consumption of dietary AGEs in healthy individuals was reported to promote the accumulation of CML in some organs such as kidneys, heart, liver, tendons and lungs [8]. On the contrary, consumption of low dietary AGEs was reported to improve markers of inflammation and oxidative stress in haemodialysis patients and those with stage 3 chronic kidney disease [15].
Dietary AGEs and Obesity
Supporting evidence from studies that were carried out have implicated dietary AGEs in the pathogenesis of obesity [17, 25, 26]. In fact, it has been suggested that increased consumption of processed foods in the last 50 years may have favoured increased consumption of dietary AGEs, leading to increased development of obesity, and its mediated complications in the world’s population [4, 27].
In a study that was conducted in rats, high-AGEs diet reportedly increased serum levels of AGEs and upregulated ovarian RAGE. These effects were reversed following consumption of a low-AGE diet, administration of an AGE blocker and the use of orlistat (obesity suppressing drug) [17, 28, 29]. To examine the relationship between methylglyoxal accumulation and the development of obesity, Jia et al. [3] compared methylglyoxal accumulation in the white fat tissues from Zucker lean and obese rats. The authors found significantly increased methylglyoxal accumulation in the kidney, fat tissue and serum of the obese rats at age of 16 weeks relative to the lean rats.
Earlier studies by Koyama et al. [30] showed that plasma levels of sRAGE were inversely correlated with BMI. Additionally, sRAGE levels were shown to be significantly reduced in obese women compared to their normal weight counterparts. Furthermore, short-term weight loss programs in the obese women were reported to significantly increase their plasma sRAGE levels [31]. Since sRAGE (acts as a decoy receptor for AGEs that decreases their circulating levels) was inversely associated with obesity [26], these studies are further pointers to the role of AGEs in the pathogenesis of obesity.
Later experimental studies that were conducted by Sayej et al. [10] showed that mice that were fed high-AGEs high-fat diet accumulated more fat than those fed low-AGEs high-fat diet and low-AGEs low-fat diet, respectively. This was evidenced by their larger weight gain and larger epididymal fat pad, indicating the role of dietary AGEs in the pathogenesis of obesity [10]. The authors further reported that the mice that were fed high-AGEs low-fat diet had similar outcomes to those that were fed high-AGEs high-fat diet in terms of weight gain and epididymal fat pad but milder development of hepatosteatosis compared to the group fed high-AGEs high-fat diet [10], further affirming the role of dietary AGEs in the pathogenesis of obesity.
Studies conducted by Uribarri et al. [12], showed a positive relationship between visceral fat and elevated serum concentration of AGEs, suggesting a contributory role of exogenous AGEs in the development of obesity and metabolic syndrome. This statement was further buttressed by the decreased circulating and urinary AGE markers and the improved anthropometric indices that were reported in overweight and obese individuals placed on low-AGEs diets [4, 32]. Additionally, a low-AGEs diet was reported to decrease circulating and urinary AGEs markers, and was further associated with improved anthropometric, glycemic, and cardiometabolic indices, as well as overall decrease in body weight compared with a high-AGEs diet [4].
Since these studies revealed that consumption of foods rich in AGEs play a fundamental role in the pathogenesis of obesity, reduced intake of AGEs is suggested to be beneficial in the management of obesity, independent of consumption of standard energy-restricted diets [4].
Evidence from Pre-clinical and Clinical Studies on the Mechanism of AGEs Mediated Pathogenesis of Obesity
AGEs act as appetite stimulating agents that simultaneously stimulate excessive food intake and inflammation, increasing the risk of obesity [4, 27]. The report by these authors was affirmed by the study that was carried out by Sayej et al. [10] in mice which found increased leptin levels in high-AGEs high-fat diet fed mice compared to other groups, suggesting leptin desensitization, leading to excessive food intake as a potential mechanism of obesity development following feeding of diets high in AGEs.
Leptin is an adipose tissue derived protein hormone that plays important roles in regulating food intake and energy expenditure, including appetite and metabolism [10, 33]. Leptin levels control food intake and energy expenditure by acting on receptors in the mediobasal hypothalamus [34]. Therefore, desensitization of this leptin hormone will lead to its increased plasma levels due to decreased negative feedback control mechanism that regulates adipocyte leptin production [35] leading to increased food intake, decreased energy expenditure and obesity.
The PI3K/Akt signaling pathway is another pathway that has been implicated in dietary AGEs mediated pathogenesis of obesity. The pathway is required for normal body metabolism, regulation of cell proliferation, promotion of lipid biosynthesis, inhibition of lipolysis, etc. [36]. PI3Ks refer to a family of lipid kinases composed of regulatory and catalytic subunits that are activated by phosphorylating the 3-hydroxyl group of the inositol ring of phosphatidylinositol lipids in the plasma membrane [37, 38]. PI3K is activated by various stimuli, including growth factors (epidermal growth factor, platelet-derived growth factor and insulin-like growth factor), cytokines and hormones [38].
A number of polymerization targets receive signals generated by the PI3K downstream cascade. However, the most important mediator is Akt [38]. Akt refers to a class of serine/threonine protein kinase B which controls several cellular functions such as modulation of growth, survival, proliferation and metabolism. Akt has 3 isoforms namely Akt 1 (PKBα), Akt2 (PKBβ) and Akt3 (PKBγ). Akt is phosphorylated at two sites: the catalytic domain by phosphatide-dependent kinase 1 and the carboxy domain by the mammalian target of rapamycin complex2 [39]. Akt 1 is the isoform that contributes to cell proliferation and cell growth, Akt2 is involved in the regulation of glucose transport and uptake by fat and muscle cells while Akt3 is critical in neuronal development [3, 39]. The different functions of these Akt isoforms have been suggested to contribute to Akt signaling diversity [39].
A mutation in the catalytic domain of Akt2 causes severe insulin resistance and T2DM in humans. Additionally, targeted deletion of Akt2 in mice but not Akt1 or Akt3, reportedly led to insulin insensitivity, hyperglycemia, hyperinsulinemia, glucose intolerance, and impaired glucose uptake by the muscle and adipose tissue [39], further affirming the role of Akt2 in glucose homeostasis and glucose uptake by the muscle and adipose tissue.
Activation of insulin receptor causes phosphorylation of tyrosine residues in insulin receptor substrate (IRS)-1 and IRS-2, leading to the activation of the PI3K complex. Activation of PI3K further activates Akt and phosphoinositide-dependent kinase-1, which then convey most of the intracellular effects induced by insulin [36, 37], one of which is the regulation of food intake at the hypothalamus through leptin regulation. Therefore, the PI3K/Akt signaling pathway integrates the effect of insulin and leptin in the regulation of food intake and studies have shown that this pathway is required for the acute effects of leptin, such as leptin mediated reduction in food intake [37].
There are indications that interaction between AGEs and their receptors in the adipose tissue, trigger insulin insensitivity in the adipocytes. These could be evidenced in these two studies:
-
(a)
In an in vitro study that was conducted on 3T3-L1 adipocytes, the presence of AGEs inhibited glucose uptake in the presence or absence of insulin. Additionally, it increased the generation of ROS and the expression of monocyte-1-chemoattractive protein [40].
-
(b)
The study by Monden et al. [41] showed that increased RAGE expression increases adipocyte hypertrophy, suppression of glucose transporter type 4 (GLUT-4), attenuation of insulin-stimulated glucose uptake, and reduction of IRS-1 phosphorylation.
These studies and more, reveal that AGEs/RAGE interactions in the adipocytes, inhibit glucose uptake through increased generation of ROS, cytokines, and other inflammatory mediators, decreased phosphorylation of IRS-1, thereby inhibiting the PI3K-Akt signaling pathway [4]. These lead to increased food intake, adipocyte hypertrophy and obesity [26]. Since Akt2 is the Akt isoform that is involved in glucose uptake in the muscle and adipose tissue, the implication is that AGEs/RAGE interactions in the adipocytes inhibit PI3K/Akt2 signaling, thereby leading to adipocyte hypertrophy. Although PI3K is required in the activation of Akt, Akt activation has also been reported to be mediated by a P13K independent mechanism [36].
In the study by Jia et al. [3] that reported the role of methylglyoxal in adipocyte proliferation using Zucker rat models, the authors found that methylglyoxal accumulation in the white fat tissue of obese Zucker rats aged 16 weeks old (relative to lean Zucker rats), stimulated the phosphorylation of Akt1 and its targets including P21 and P27, leading to adipocyte proliferation and adipogenesis, an action that was reversed by the administration of the AGEs breaker, alagebrium and Akt inhibitor.
Since Akt1 is the Akt isoform that contributes to cell proliferation and cell growth, the authors studied the phosphorylation of Akt1 isoforms from the Zucker lean and obese rats at the age of 16 weeks and found significantly elevated levels of phosphorylated Akt1 compared to Zucker lean rats. The authors further found that methylglyoxal promoted faster cell cycle progression in 3T3-LI cells by increasing the cell number in the S and G1 phases of the cell cycle. Their study therefore suggested that phosphorylation of Akt1 by methylglyoxal promotes phosphorylation of the downstream targets (P21 and P27), enhancing cell cycle, leading to adipogenesis. Since the increased Akt1 phosphorylation associated with methylglyoxal accumulation in obese rats was found in the obese rats aged 16 weeks old, these authors suggested that methylglyoxal stimulated adipogenesis by the up-regulation of Akt signaling pathway could contribute to the development of adult-onset morbid obesity [3].
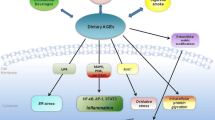
The findings from these studies as reported above, therefore reveal that consumption of dietary AGEs, increases the circulating AGEs levels and the deposition of AGEs in the tissues (including the adipose tissue). AGEs/RAGE interaction in the adipocytes, inhibits glucose uptake through increased generation of ROS, cytokines and inflammatory mediators, decreases phosphorylation of IRS-1, inhibits PI3K-Akt2 signaling, leading to increased food intake, adipocyte hypertrophy and obesity. Additionally, increased deposition of AGEs in the adipocytes can stimulate phosphorylation of Akt1 and its targets including p21 and p27, leading to adipocyte proliferation and adipogenesis. The summary of the mechanisms that explain the role of dietary AGEs in the pathogenesis of obesity is shown in Fig. 9.1.
The summary of the mechanisms that explain the role of dietary AGEs in the pathogenesis of obesity. Increased consumption of dietary AGEs leads to increased deposition of AGEs in the tissues including the adipose tissue. This ultimately leads to desensitization of leptin in the hypothalamus, leading to increased food intake and obesity. Increased AGEs in the adipose tissue also increases AGEs/RAGE interaction in the adipocytes leading to increased generation of ROS, cytokines and other inflammatory mediators which causes decreased glucose uptake. Then, this can cause decreased phosphorylation of IRS-1 and inhibition of PI3K-Akt2 signaling, leading to increased food intake and obesity. Additionally, the increased deposition of AGEs in the adipocytes can stimulate phosphorylation of Akt1 and its targets including p21 and p27, leading to adipocyte proliferation and adipogenesis, resulting to obesity
Conclusions
Preclinical and clinical studies that were conducted have supported the contributory role of dietary AGEs in the pathogenesis of obesity. Three key mechanisms such as: AGEs mediated leptin dysregulation, AGEs/RAGE mediated inhibition of PI3K/Akt2 signaling and AGEs mediated upregulation of Akt1 have been implicated in this pathogenesis. Therefore, decreased consumption of foods that are high in dietary AGEs could be helpful in the prevention of obesity.
Abbreviations
- Akt:
-
Protein kinase B
- ALI:
-
Arginine-lysine imidazole
- BMI:
-
Body mass index
- IRS-1:
-
Insulin receptor substrate 1
- IRS-2:
-
Insulin receptor substrate 2
- PI3K:
-
Phosphatidylinositol 3-kinase
- RAGE:
-
Receptor for Advanced Glycation End products
- ROS:
-
Reactive Oxygen Species
- sRAGE:
-
Soluble receptors for advanced glycation end products
References
Ferrier DR (2014) Lippincotts biochemistry, 6th edn, pp 634–635
World Health Organization (2015) Obesity and overweight. Fact sheet N°311 January 2015 [cited 2016 20 April 2016]. http://www.who.int/mediacentre/factsheets/fs311/en/
Jia X, Chang T, Wilson TW, Wu L (2012) Methylglyoxal mediates adipocyte proliferation by increasing phosphorylation of Akt1. PLoS One 7:e36610
Ribeiro PVM, Tavares JF, Costa MAC et al (2019) Effect of reducing dietary advanced glycation end products on obesity-associated complications: a systematic review. Nutr Rev 77:725–734
Eleazu C, Omar N, Lim OZ et al (2019) Obesity and comorbidity: could simultaneous targeting of esRAGE and sRAGE be the Panacea? Front Physiol 10:787
Chooi YC, Ding C, Magkos F (2019) The epidemiology of obesity. Metab 92:6–10
Eleazu C, Mohamed M (2020) Targeting advanced glycation end products (esRAGE and sRAGE) for obesity, diabetes, and its associated complications. In: Faintuch J Faintuch S (eds) Obesity and diabetes. Springer Nature Switzerland AG. In Press. https://doi.org/10.1007/978-3-030-53370-0_14
Liang Z, Chen X, Li L, Li B, Yang Z et al (2019) The fate of dietary advanced glycation end products in the body: from oral intake to excretion. Critic Rev Food Sci Nutr 2019. https://doi.org/10.1080/10408398.2019.1693958
Nowotny K, Jung T, Höhn A et al (2015) Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 5:194–222
Sayej WN, Lii KPR Guo WA et al (2016) Advanced glycation end products induce obesity and hepatosteatosis in CD-1 wild-type mice. Biomed Res Int 2016:12 pages
Kosmopoulos M, Drekolias D, Zavras PD et al (2019) Impact of advanced glycation end products (AGEs) signaling in coronary artery disease. Biochimica et Biophysica Acta (BBA)—Molec Basis Dis 1865:611–619
Uribarri J, Cai W, Woodward M et al (2015) Elevated serum advanced glycation end products in obese indicate risk for the metabolic syndrome: a link between healthy and unhealthy obesity? J Clin Endocrinol Metab 100:1957–1966
Ames JM (2008) Determination of N epsilon-(carboxymethyl) lysine in foods and related systems. Ann N Y Acad Sci 1126:20–24
Pouillart P, Mauprivez H, Ait-Ameur L et al (2008) Strategy for the study of the health impact of dietary Maillard products in clinical studies: the example of the ICARE clinical study on healthy adults. Ann N Y Acad Sci 1126:173–176
Snelson M, Coughlan MT (2019) Dietary advanced glycation end products: digestion, metabolism and modulation of gut microbial ecology. Nutrients 11:215
Delgado-Andrade C, Seiquer I, Haro A et al (2010) Development of the Maillard reaction in foods cooked by different techniques. Food Chem 122:145–153
Zhang JJ, Merhi Z (2016) Could advance glycation end products explain the poor response to controlled ovarian hyperstimulation in obese women? J Endocrinol Diab 3:1–9
Uribarri J, Woodruff S, Goodman S et al (2010) Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc 110:911–916.e12
Tessier FJ, Birlouez-Aragon I (2012) Health effects of dietary Maillard reaction products: the results of ICARE and other studies. Amino Acids 42:1119–1131
Barbosa JHP, Souza IT, Santana AEG, Goulart MOF (2016) Determination of advanced glycation (AGEs) and lipoxidation (ALEs) end products in foods and biological systems: advances, challenges and perspectives. Quım Nova 39. https://doi.org/10.5935/0100-4042.20160048
Kehm R, Rückriemen J, Weber D et al (2019) Endogenous advanced glycation end products in pancreatic islets after short-term carbohydrate intervention in obese, diabetes-prone mice. Nutr and Diab 9:9
Uribarri J, Cai W, Sandu O et al (2005) Diet-derived advanced glycation end products are major contributors to the body’s age pool and induce inflammation in healthy subjects. Ann New York Acad Sci 1043:461–466
Ott C, Jacobs K, Haucke E et al (2014) Role of advanced glycation end products in cellular signaling. Redox Biol 2:411–429
Kellow NJ, Coughlan MT (2015) Effect of diet-derived advanced glycation end products on inflammation. Nutr Rev 73:737–759
Leuner B, Max M, Thamm K et al (2012) RAGE influences obesity in mice. Effects of the presence of RAGE on weight gain, AGE accumulation, and insulin levels in mice on a high fat diet. Z Gerontol Geriatr 45:102–108
Tavares JF, Ribeiro PVM, Coelho OGL et al (2020) Can advanced glycation end-products and their receptors be affected by weight loss? A systematic review. Obes Rev 21:e13000
Vlassara H, Striker GE (2011) AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Endocrinol 7:526–539
Diamanti-Kandarakis E, Katsikis I, Piperi C et al (2007) Effect of long-term orlistat treatment on serum levels of advanced glycation end-products in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 66:103–109
Boor P, Celec P, Behuliak M et al (2009) Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metab 58:1669–1677
Koyama H, Shoji T, Yokoyama H et al (2005) Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 25:2587–2593
Vazzana N, Guagnano MT, Cuccurullo C et al (2012) Endogenous secretory RAGE in obese women: association with platelet activation and oxidative stress. J Clin Endocrinol Metab 97:E1726–E1730
Poulsen MW, Bak MJ, Andersen JM et al (2014) Effect of dietary advanced glycation end products on postprandial appetite, inflammation, and endothelial activation in healthy overweight individuals. Eur J Nutr 53:661–672
Frago LM (2015) Chowen JAHypothalamic leptin and ghrelin signaling as targets for improvement in metabolic control. Current Pharm Design 21:3596–3605
Williams KW, Scott MM, Elmquist JK (2009) From observation to experimentation: leptin action in the mediobasal hypothalamus. Am J Clin Nutr 89:985S–990S
Balland E, Cowley MA (2015) New insights in leptin resistance mechanisms in mice. Front Neuroendocrinol 39:59–65
Huang X, Liu G, JGuo J, Zhengquan SZ (2018) The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci 14:1483–1496
Donato J, Frazão R, Elias CF (2010) The PI3K signaling pathway mediates the biological effects of leptin. Arquivos Brasileiros de Endocrinologia & Metabologia 54:591–602
Shi X, Wang J, Lei Y et al (2019) Research progress on the PI3K/AKT signaling pathway in gynecological cancer. Mol Med Rep 19:4529–4535
Gonzalez E, McGraw TE (2009) The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle 8:2502–2508
Unoki H, Bujo H, Yamagishi S et al (2007) Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes Res Clin Pract 76:236–244
Monden M, Koyama H, Otsuka Y et al (2013) Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of toll-like receptor 2. Diabetes 62:478–489
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Eleazu, C.O., Nna, V.U., Suleiman, J.B., Mohamed, M. (2021). Dietary Advanced Glycation End Products as Mediators of Obesity: Cellular and Molecular Mechanisms of Action. In: Tappia, P.S., Ramjiawan, B., Dhalla, N.S. (eds) Cellular and Biochemical Mechanisms of Obesity. Advances in Biochemistry in Health and Disease, vol 23. Springer, Cham. https://doi.org/10.1007/978-3-030-84763-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-84763-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84762-3
Online ISBN: 978-3-030-84763-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)