Abstract
As the incidence of anterior cruciate ligament (ACL) reconstructions continues to increase, the rate of revision surgery continues to climb. Despite advances in the technical aspects and rehabilitation protocols following primary ACL surgery, the rate of revision and even re-revision remains higher than desired.
Since the outcomes of revision ACL reconstruction remain poor compared to those of primary procedures it is crucial to identify and address the factors that may have contributed to graft failure.
Many biomechanical studies have highlighted the importance of the posterior tibial slope (PTS) in increasing the forces on native and graft ACL. This issue was confirmed by clinical studies that confirmed the PTS as an important and independent risk factor for ACL (re)rupture and instability. It becomes crucial, then, to address this feature in cases of ACL-deficiency. For this purpose, a deflexion osteotomy performed with an anterior closing-wedge osteotomy has been described. Preliminary results of this technique combined with ACL reconstruction in patients with ACL re-ruptures and high PTS have been promising with regard to restoring knee stability, obtaining satisfactory functional levels and reducing recurrent failures. Larger series and longer follow-up are needed, however, to confirm the efficacy of this technique.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anterior cruciate ligament
- ACL re-rupture
- ACL reconstruction
- ACL revision
- Knee biomechanics
- Anterior tibial translation
- Posterior tibial slope
- Anterior closing wedge osteotomy
- Tibial deflexion osteotomy
12.1 Introduction
As the incidence of anterior cruciate ligament (ACL) reconstructions continues to increase, the rate of revision surgery continues to climb. Despite technical and rehabilitation advances in primary ACL surgery, the rate of revision and even re-revision remains higher than desired.
Primary anterior cruciate ligament reconstruction (ACLR) generally leads to good outcomes and has a revision rate of between 1.6% and 2.1% [1]. With follow-up longer than 10 years, the ACL graft rupture rate increases to 6% and clinical failure occurs in approximately 10% of ACLR cases (range, 2–26%) [2]. Revisions of previously reconstructed ACLs show even higher re-rupture rates of approximately 13.7% [3]. Furthermore, outcomes with subsequent revisions can be dismal, with one study showing less than one-third of patients returning to their prior levels of activity [4]. Additionally, based on long-term follow-up, osteoarthritis develops in 21–48% of patients after combined ACL, meniscus, and cartilage injuries but in only 0–13% of patients with isolated ACL rupture [5].
Despite a relatively high failure rate of revision ACL reconstructions, additional reconstructive procedures are not commonly performed. This is for multiple reasons, including decreased patient activity and expectations with increasing age and concerns of undergoing a further surgical procedure [6].
Since the outcomes of revision ACL procedures remain poor compared to the results of primary reconstructions [7,8,9,10] it is crucial to identify and address the factors that may have contributed to graft failure. These factors can be divided in “extrinsic” or “intrinsic”. Extrinsic factors, related to surgical technique and rehabilitation, are often determined according to the patients’ intended sports activities, [11, 12] while intrinsic factors include specific anatomic features such as an increased posterior tibial slope (PTS), a narrow intercondylar notch, hyperlaxity or gender.
12.2 Biomechanics
The factors that contribute to the abnormal knee kinematics after ACL injury and reconstruction remain unclear. Bone shape has been implicated in the development of hip and knee osteoarthritis, although there is little knowledge about the effects of bone shape on knee kinematics.
Many risk factors have already been identified for ACL injuries. These include a decreased notch width, generalized joint laxity, subtalar pronation, hormonal factors, body mass index, knee recurvatum, and increased Q angle. Previously, it has been demonstrated that anterior tibial translation (ATT) increased 0.6 mm per degree of posterior tibial slope (PTS) in ACL-deficient knees [13] and, more recently, studies have shown that increased PTS is an independent risk factor for primary ACL injuries [14].
The normal PTS is within the range of 5–7°, depending on the measurement technique, and it is considered pathologic if it exceeds 12° [15].
An increase in PTS leads to an anterior shift of the tibia’s resting position, which not only intensifies the stress on the ACL, but can also lead to abnormal loading of the knee, resulting in damage to menisci and articular cartilage [16].
Andrew et al. [17], in their cadaveric study, quantified the effect of changes in sagittal plane tibial slope on ACLR graft force at varying knee flexion angles. They proved with axial loading that the PTS had an independently significant, linearly increasing effect on graft force regardless of flexion angle (coefficient = 0.92, SE = 0.08, P < 0.001). Meanwhile, significantly higher graft force was observed at 0° of flexion as compared with all other flexion angles for the loaded (all P < 0.001) and unloaded (all P < 0.001) conditions.
In a more recent clinical study, Dejour et al. [18] have proved that static ATT increased significantly in knees with tibial slopes >7°, by approximately 0.3 mm per degree, and that dynamic ATT increased significantly in knees with tibial slope ≥12°, by approximately 0.2 mm per degree. These findings confirm the relationship between tibial slope and ATT first described by Dejour [19, 20] and confirmed more recently by Schatka et al. [21].
It is important to highlight that these studies did not find an association between the pivot shift test and tibial slope, possibly because the latter is generally measured at the medial compartment, while rotational stability may depend more on lateral tibial slope [14, 22].
Of course, meniscal status is another significant contributor to knee stability and anterior tibial translation control that has been well established in the literature.
The role of the menisci in limiting ATT can be explained by considering them as part of a ‘soft tissue slope’ which increases the coverage and reduces the bony slope [23].
The association of medial meniscal tears with dynamic ATT, as well as the pivot shift has been corroborated by cadaveric studies [24, 25] gait analyses and simulations, which detected a significant impact of medial meniscal tears on ATT during gait [24, 26, 27].
Samuelsen et al. [28] found a significant interaction between PTS and posterior medial meniscal root tear (PMMR), where a PMMR tear was observed to potentiate the effect that increased PTS has on ACLR graft forces at 30° of knee flexion. A PMMR tear also led to a significant increase in ACLR graft force when compared with the intact state, whereas the meniscal repair state was not significantly different from the intact state. This finding corroborates and highlighted the importance of the medial meniscus as a secondary stabilizer of the knee.
Meanwhile, it is important to notice that Dejour et al. [18] recently found that the lateral meniscus was not a major component of either static anteroposterior or rotational knee stability in ACL-deficient knees.
Taking all these biomechanical considerations into account, a slope-reducing osteotomy procedure can significantly reduce ACL force and anterior tibial translation with tibio-femoral compression (TFC) alone as well as combined with anterior force or valgus moment [29].
12.3 Measurement
Radiographic examination includes an antero-posterior view (in monopodal stance when it is possible at 20° of flexion), a Rosenberg view (postero-anterior at 35–40° of knee flexion), a true lateral view (in monopodal stance at 25–30° of knee flexion), and an axial view at 30° of knee flexion. Assessments are made using true lateral views of the knee under fluoroscopic control to ensure that the femoral condyles are superimposed.
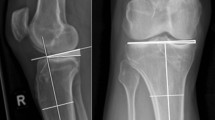
As for the PTS measurement, there is little consensus on the ideal references to be used. Many anatomical references have been described but there is little information about the values measured with the different methods, and, for this reason, it is difficult to compare the measurements of different studies. The authors utilize the method described by Dejour and Bonnin [19], using a goniometer with a precision of ±1° to measure the angle between the line perpendicular to the proximal tibial diaphyseal axis and the line tangent to the most superior points at the anterior and posterior edges of the medial tibial plateau (Fig. 12.1). According to this method, the physiological PTS measures ~7°. Other anatomical references, however, have been described and applied as the longitudinal axis.
Jae Ho Yoo et al. [30] have utilized the mechanical axis (MA, a line connecting the midpoints of the tibia plateau and the tibia plafond), the anterior tibial cortex (ATC, a line connecting 2 points on the anterior tibial cortex at 5 and 15 cm distal to the knee joint line), the proximal tibial anatomical axis (PTAA, a line connecting midpoints of outer cortical diameter at 5 and 15 cm distal to the knee joint line), the central tibial anatomical axis (CTAA, a line connecting midpoints of outer cortical diameter at 10 cm distal to the knee joint line and at 10 cm proximal to the ankle joint line), the posterior tibial cortex (PTC, a line connecting 2 points on the posterior tibial cortex at 5 and 15 cm distal to the knee joint line), and the fibular shaft axis (FSA, a line connecting midpoints of outer cortical diameter of proximal and distal ends of the fibular diaphysis) (Fig. 12.2). They evaluated 90 knees in 60 consecutive female patients and found that the mean PTS varied up to 5° based on the axis chosen. It was 10.6° with the mechanical axis, 13.8° with the anterior tibial cortex, 10.8° with the proximal anatomical axis, 12.9° with the central anatomical axis, 7.8° with the posterior tibial cortex, and 9.5° with the fibular shaft diaphysis.
The ATT is defined as the distance between two lines parallel to the posterior tibial cortex: the first, tangent to the posterior aspect of the medial tibial plateau, and the second, tangent to the posterior femoral condyles. Static ATT is measured on monopodal weight-bearing radiographs with the knee flexed by 20° (Fig. 12.1). The dynamic tibial translation (DTT) measure is realized by using the Telos™ stress device (Telos GmbH, Marburg, Germany) with 150 N at 20° of knee flexion and the side-to-side difference (SSD) between the injured and healthy knee is then calculated (Fig. 12.3).
The patellar height is expressed by the Caton–Deschamps index [31, 32].
The presence of radiographic signs of osteoarthritis is graded following the classification of Ahlback [15].
It is also important to consider the presence of meniscal lesions or previous menisectomy that could exacerbate the effects of a high PTS. The recent study of Lustig et al. [23] demonstrated how “soft tissue tibial slope”, measured using magnetic resonance imaging (MRI), is influenced by menisci which shift the tibial slope toward the horizontal.
In this regard, it has to be mentioned that much of what is known about the geometry of the asymmetric, three-dimensional, osseous portion of the tibial plateau is based on two-dimensional measurements obtained from lateral radiographs. With that approach, it is difficult to differentiate between the medial and lateral aspects of the plateau because they are superimposed.
As such, Hashemi et al. [33] performed the slope measurements with the accepted radiographic methods using MRI (Fig. 12.4a–c), which allowed them to characterize the slope of the tibial plateau at the center of the articular surfaces of both the medial and lateral compartments.
Magnetic resonance images illustrating the method used to determine the medial and lateral tibial slopes. (a) The sagittal plane (represented by the green lines in the axial view at the center of the tibial spines) was used to determine the orientation of the diaphyseal axis in the sagittal plane. The axis L is the line connecting the midpoints of the lines 1 and 2 drawn at 4–5 cm from the joint line. (b) The axis L is copied and pasted in the sagittal plane (represented by the green lines in the axial view) that can clearly show the orientation of the tibia. The peak anterior and posterior points on the tibial plateau are identified (A and B) and a line perpendicular to the axis is drawn (P). The slope of the line extending between A and B represents the medial tibial slope, and it is measured by the angle between the line itself and the line P. (c) The same procedure is utilized in order to measure the lateral tibial plateau slope
Furthermore, they advocate for measuring the depth of the concavity of the medial compartment to better characterize the complex three-dimensional geometry of the tibial plateau. A deep medial plateau will constrain the femoral condyle to a greater extent and result in increased resistance to displacement of the tibia relatively to the femur. Conversely, the combination of a high medial tibial slope and low depth of concavity may be associated with a decreased resistance to displacement of the tibia relative to the femur, placing the knee at increased risk for ligament injury.
These recent findings indicate that a surgeon will need to combine the assessment of radiographic images and MRI in order to have a more complete idea of the three-dimensional geometry of the “soft tissue tibial slope” and the knee kinematics for each patient.
12.4 Clinical Application
The importance of the adverse effect of a high PTS on the ACL biomechanics has been confirmed by many clinical studies, evaluating the correlation between PTS and the rate of native and grafted ACLs (re-)rupture.
Regarding primary ACL injuries, Sonnery-Cottet et al. [34] performed a case-control study comparing a study group of 50 patients who had sustained an isolated, complete rupture of the ACL and a control group of 50 patients who had consulted for other reasons to determine whether there was a difference in the PTS and notch width index (NWI) between them. They found a statistically significantly steeper PTS and narrower NWI in the patients with a ruptured ACL than in the uninjured group. Waiwaiole et al. [35] reviewed medical records of 221 patients who underwent MRI of the knee between January 2003 and December 2009 and separated them into two subgroups: a study group of 107 subjects who had undergone surgery for ACL injury and a control group of 114 patients diagnosed with patellofemoral syndrome. They found significantly greater values for lateral PTS (6° ± 4°; P < 0.001) and medial PTS (7° ± 4°; P = 0.002) in the study group compared with controls (5° ± 3° and 5° ± 4°, respectively). They found also a statistically significant relationships between young age and lateral PTS with ACL injury. Zeng et al. [36] performed a case-control study containing 146 patients in total (73 noncontact ACL injuries and 73 meniscus injuries) and found that the mean PTS of the ACL-injured group was significantly higher than that of the control group (P < 0.001).
Historically, anatomic features such as an increased PTS or narrow intercondylar notch have not been routinely addressed during ACL reconstructions, despite the biomechanical studies documenting their negative effect on the ACL graft. As such, there are many studies that investigated the correlation between them and the risk of ACL graft failures.
Webb et al. [37] enrolled 200 consecutive patients who underwent primary ACLR with hamstring autografts in a prospective longitudinal study over 15 years and found that the mean tibial slope was significantly greater in patients with ACL graft tear (50 of them) compared with patients with no further injury (9.9° vs 8.5°). The mean PTS for those with both an ACL graft and contralateral ACL rupture was 12.9°. Patients with a tibial slope of 12° or higher had an odds ratio of further ACL injuries increased by a factor of 5, to an incidence of 59%. Christensen et al. [14] compared, in a case-control study, 35 patients with early (within 2 years) failure of primary ACLR with 35 control patients who underwent ACLR with a minimum of 4 years of clinical follow-up and no evidence of graft failure. They compared their lateral tibial posterior slope (LTPS) and they found it to be significantly higher in the early ACL failure group (8.4° vs 6.5°; p = 0.012), with a 1.6, 2.4, and 3.8 odds ratio for graft failure considering respectively a 2°, 4°, and 6° increase in the LTPS value. The most striking correlation between an increasing LTPS and graft failure was observed in women. In this population, a 4° change in the slope increased the risk of graft failure nearly five times, and a 6° change resulted in over ten times the increased risk. Lately, Salmon et al. [38] further highlighted the negative effect that a steep PTS has on ACL grafts. They reviewed 179 patients who underwent isolated primary ACLR with hamstring autograft and they found out that ACL graft survival was significantly affected by age <18 years at the time of reconstruction (hazard ratio, 3.3; 95% CI, 1.7–6.4; P = 0.001) and a PTS of 12° or more (hazard ratio, 3.1; 95% CI, 1.5–5.9; P = 0.001), while contralateral ACL injury was significantly affected only by a PTS of 12° or more (hazard ratio, 7.3; 95% CI, 3–18; P = 0.001). In particular, adolescents with a tibial slope of 12° or more were 11 times more likely to rupture their ACL graft and seven times more likely to rupture their contralateral ACL than were adults with tibial slopes of 12° or less. At 20 years, the ACL survival for adolescents with a PTS of 12° or more was 22%, showing the catastrophic effect of tibial slope on further ACL injuries.
The negative correlation between PTS and ACL graft survival has also been shown in cases of combined ACL reconstruction and alignment correction osteotomies addressing chronic cases of early osteoarthritis and instability. It is well known that bony procedures treating coronal deformity can incidentally change the PTS and affect the sagittal balance. Schuster et al. [39] retrospectively evaluated 50 cases of combined high tibial osteotomy (HTO), ACL reconstruction, and chondral resurfacing (CR, abrasion plus microfracture), and analyzed the graft failure rates in relation to the tibial slope. They found that graft insufficiency was strongly dependent on tibial slope, with a failure rate of 7% in cases of postoperative tibial slope <7.5°, 24% in cases of slope between 7.5° and 12.5°, and 36% in cases of slope >12.5°.
Finally, some studies have shown a high PTS in rare cases of congenital absence of the ACL. Frikha et al. [40] presented a descriptive analysis of 8 knees with congenital agenesia of the ACL in five patients of the same family and they found that the tibial slope was increased in all knees (mean 20.6°).
All of this evidence draws attention on PTS as an important and independent risk factor for ACL (re)rupture and instability. It becomes crucial, then, to address this feature in cases of ACL-deficiency.
A high PTS can be associated with deformities in the coronal plane (frequently a double varus), and in these cases it is recommended to combine the ACL reconstruction with a HTO.
In general, the tibial slope tends to increase after opening-wedge HTO while it has an inverse tendency after closing-wedge HTO [41, 42]. Arun et al. [43] retrospectively analyzed data from 30 patients who underwent arthroscopic ACLR along with medial opening-wedge osteotomy, measuring preoperative and postoperative tibial slopes. They found that patients who had a PTS decrease of 5° or more postoperatively had the best results compared with the others in terms of ACL graft survival and functional outcomes. Thus, they suggested that placing the tricortical graft posterior to midline in the opening wedge could reduce the PTS and, consequently, the stress on the graft, leading to better functional outcomes.
However, not all patients who present with an increased PTS has a coronal plane deformity or arthritis issues. In order to properly treat these patients, it has been suggested that decreasing the PTS could potentially protect ACL grafts and reduce the risk of failed revisions. As such, several authors have described a deflexion osteotomy performed with an anterior closing-wedge osteotomy.
That is a complex and technically demanding procedure, and limited studies can be found in literature about it.
In the first article, Dejour et al. [13] reported a series of 22 knees with chronic anterior laxity and excessive PTS (average 16.5°). Four of them were isolated tibial deflexion osteotomies while the other 18 were combined with ACLR. Better clinical results were observed in the latter group. PTS was corrected to an average of 7° postoperatively. ATT in monopodal stance decreased from 12.5 mm preoperatively to 3 mm at last follow-up.
Sonnery-Cottet et al. [6] retrospectively evaluated five patients after a slope-reducing anterior closing-wedge osteotomy in combination with an ACL re-revision after a mean 32 months follow-up. All patients presented an excessive PTS as an intrinsic risk factor for graft failure. The mean PTS decreased from 13.6° preoperatively to 9.2° postoperatively and the anterior laxity measured with the KT-1000 arthrometer decreased from 10.4 mm to 2.8 mm. As regards clinical outcomes, Lysholm score and IKDC score both improved from preoperatively to the last follow-up and the mean Tegner activity score reached the same level as before the last ACL injury (7.4 and 7.2).
Finally, Dejour et al. [15] reported the outcomes, at a minimum of 2-year follow-up, of nine patients that underwent second revision ACLR combined with tibial deflexion osteotomy for a high PTS value (all patients had a PTS > 12°). The mean PTS decreased from 13.2° ± 2.6° (median 13°; range 12–18°) preoperatively to 4.4° ± 2.3° (median 4°; range 2–8°) postoperatively. With regard to the clinical outcomes, the mean Lysholm score was 73.8 ± 5.8 (median 74; range 65–82), and the IKDC-SKF was 71.6 ± 6.1 (median 72.8; range 62.2–78.5) showing satisfactory results and suggesting that tibia slope correction protects reconstructed ACLs from fatigue failure.
12.5 Indications and Contraindications
In patients with multiple ligament tears and surgeries, it is of paramount importance to carefully analyze the causes of the previous failures and to address the risk factors in order to avoid another rerupture. The authors, therefore, recommend a correction of the PTS with an anterior closing-wedge osteotomy in patients with a failed ACLR and a PTS > 12°. Contraindications include hyperextension (>10°), a significant deformity in the coronal plane, and end-stage osteoarthritis. In patients with more than 10° of hyperextension, it is not possible to perform the osteotomy because it would generate an excessive genu recurvatum while patients with lower degree of hyperextension (0–10°) are treated like all the other. Since there are very few studies reporting outcomes of slope reduction techniques, there is still no consensus regarding their indications and contraindications.
12.6 Preoperative Planning
All the patients must undergo a standard radiographic evaluation as explained before. The PTS must be carefully measured and the amount of slope correction is estimated in order to obtain a PTS between 3° and 5° considering 1 mm of resection equal to 1° of correction.
12.7 Surgical Technique
In the literature, there are two surgical techniques described to perform a tibial deflexion osteotomy. Originally, Dejour et al. [13] proposed an approach to the osteotomy site above the patellar tendon insertion. On the contrary, Sonnery-Cottet et al. [6] proposed to detach the tibial tubercle to reach the osteotomy site 4 to 5 cm distal to the joint line. The main advantages of the original procedure are that it maintains the patellar height while keeping both the patellar tendon and tibial tuberosity intact, though it is technically challenging to position the osteotomy at the appropriate level above the anterior tibial tuberosity. The alternative procedure allows more comfortable exposure of the osteotomy site, but it requires detachment of the patellar tendon and the tibial tuberosity, which may be associated with increased morbidity and tougher rehabilitation.
In the technique proposed by the authors, the patient is positioned in the supine position and a tourniquet is placed high on the thigh. A lateral post at the level of the tourniquet maintains the leg position in the frontal plane, and a distal support holds the knee at 90° of flexion, allowing full range of motion when desired (Fig. 12.5).
The first step is the harvesting of the autograft, depending on where the previous grafts were taken (hamstrings, patellar tendon, or quadriceps tendon).
Second, the knee is arthroscopically assessed through the anterolateral and anteromedial portals, in order to evaluate the status of the cartilage and the menisci, as well as the shape of the intercondylar notch (a notchplasty is performed if needed) and previous tunnel positions. Third, the femoral and tibial tunnels are drilled to match the graft diameter, but the graft is not yet inserted. Fourth, all meniscal interventions (either meniscal suture or removal) are performed if required. Fifth, the tibial deflexion osteotomy is performed through an anterior longitudinal incision medial to the tibial tuberosity. The deep medial collateral ligament and the iliotibial band on Gerdy’s tubercle are detached up to the posterior part of the tibia, in order to expose the place of the osteotomy site, and the patellar tendon insertion on the tibia. Detachment of the tibial tubercle is not necessary because the osteotomy is performed at the level of the insertion of the patellar tendon and a biplanar osteotomy will be done. Two monocortical parallel K-wires are positioned under fluoroscopy on both sides of the patellar tendon aimed towards the posterior tibial cortex, 1 cm below the joint line. The level of the osteotomy always starts at the superior margin of the patellar tendon insertion and continues distally. Other two monocortical parallel K-wires are positioned distally in accordance with the amount of correction decided at the preoperative planning. They are orientated distally to proximally aiming to the end of the first two K-wires and their position is controlled under fluoroscopy (Fig. 12.6). The K-wires are placed until their tips are 10 mm away from the tibial plateau surface just below the tibial insertion of the posterior cruciate ligament. These 4 K-wires will serve as the guide for the bone cuts for the closing wedge osteotomy (Fig. 12.7). The first bone cut will be exactly behind the tibial tubercle in the coronal plane in order to perform a biplanar osteotomy. The tibia is then osteotomized cutting under the proximal pins (to be sure not to violate the joint cartilage), keeping the posterior cortex intact as a hinge. The patellar tendon is protected from the oscillating saw using spreaders. Before the distal osteotomy is executed, the measurement of the correction is confirmed. The distal osteotomy is performed with a convergent orientation toward the posterior part of the proximal osteotomy. The proper orientation of the saw is assessed under fluoroscopic guidance. In patients with a slightly varus-valgus deformity, the osteotomy can also be 2-dimensional with an anterior and lateral-medial based wedge respectively.
After making the distal cut, the anterior wedge of bone is removed (Fig. 12.8a, b). With the intact posterior cortex acting like a hinge, the osteotomy gap can be decreased by manipulation of the limb. To decrease the gap, it is possible to push down on the proximal tibial plateau, or simply extend the leg. Extending the leg will exert pressure through the femoral condyles onto the tibial plateau. The amount of slope correction is confirmed and measured under fluoroscopy. Then the osteotomy fixation is achieved using two staples on both sides of patellar tendon (Figs. 12.9 and 12.10). After osteotomy fixation (checked at fluoroscopy), the final step is completing the ACL graft. The tibial tunnel is gently hand-redrilled to debride tibial tunnel from bone fragments. The graft is then pulled and fixed using interference screws or suspensory fixation. The fixation is done at 90° of flexion in order to give a little bit of stiffness to the knee in order to compensate for the negative effect of the “genu recurvatum” caused by the osteotomy. As a final step, a double fixation of the graft is obtained by passing the sutures under the staples and tying them together firmly.
12.8 Postoperative Rehabilitation
After surgery, patients are immobilized in an extension brace during transfers, in order to address the “genu recurvatum”, and weight bearing is not allowed for the first 3 weeks. According to the patient tolerance, nonaggressive rehabilitation is immediately begun with passive and active motion exercises and full range of motion. The main objectives in the first phase are reduction in knee swelling, quadriceps control, and recovery of range of motion, while always avoiding hyperextension. After 3 weeks, weight bearing is gradually progressed in the extension brace with the goal of full weight bearing at day 45. Afterward, patients are placed on a standard phase 2 ACL protocol from days 45 to 90, with swimming and cycling activities. They are finally moved to phase 3 for 3 months (3–6 months post- operation), during which the schedule comprises a progressive return-to-sport program. After 6 months, isokinetic and functional tests are performed: patients can return to full sports activities if they have a good quadriceps/hamstrings ratio and muscle recovery comparable with the contralateral side.
12.9 Conclusions
Preliminary outcomes of ACLR combined with anterior closing-wedge osteotomy in patients with ACL re-ruptures and high PTS show promising results in restoring good knee stability, satisfactory functional levels, and reduced recurrent failures. The authors emphasize the importance of this procedure since it is the only way to properly address the increased forces produced by high values of PTS to which ACL grafts are subjected, even while standing and during normal activities like walking [44].
Given the mounting evidence from recent studies of the strong correlation between high PTS and ACL re-rupture rate as well as the promising results obtained by the deflexion osteotomy procedure, it is worth discussing whether this intervention may also be indicated in the high-risk patient with primary ACL ruptures (PTS > 15° and/or ATT > 10 mm in monopodal weight-bearing).
Larger series and longer follow-up are needed, however, to confirm the efficacy of this technique, considering that still few studies are published on this topic.
References
Björnsson H, Andernord D, Desai N, et al. No difference in revision rates between single- and double-bundle anterior cruciate ligament reconstruction: a comparative study of 16,791 patients from the Swedish National Knee Ligament Register. Arthroscopy. 2015;31:659–64.
Crawford SN, Waterman BR, Lubowitz JH. Long-term failure of anterior cruciate ligament reconstruction. Arthroscopy. 2013;29:1566–71.
Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94:531–6.
Griffith TB, Allen BJ, Levy BA, Stuart MJ, Dahm DL. Outcomes of repeat revision anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41:1296–301.
Øiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37:1434–43.
Sonnery-Cottet B, Mogos S, Thaunat M, Archbold P, Fayard J-M, Freychet B, Chambat P. Proximal tibial anterior closing wedge osteotomy in repeat revision of anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(8):1873–80.
Colombet P. Knee laxity control in revision anterior cruciate ligament reconstruction versus anterior cruciate ligament reconstruction and lateral tenodesis: clinical assessment using computer-assisted navigation. Am J Sports Med. 2011;39(6):1248–54.
Gifstad T, Drogset JO, Viset A, Grøntvedt T, Hortemo GS. Inferior results after revision ACL reconstructions: a comparison with primary ACL reconstructions. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2011–8.
Lind M, Lund B, Fauno P, Said S, Miller LL, Christiansen SE. Medium to long-term follow-up after ACL revision. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):166–72.
Wright RW, Gill CS, Chen L, Brophy RH, Matava MJ, Smith MV, Mall NA. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531–6.
Bien DP. Rationale and implementation of anterior cruciate ligament injury prevention warm-up programs in female athletes. J Strength Cond Res. 2011;25(1):271–85.
Ebben WP, Fauth ML, Petushek EJ, Garceau LR, Hsu BE, Lutsch BN, Feldmann CR. Gender-based analysis of hamstring and quadriceps muscle activation during jump landings and cutting. J Strength Cond Res. 2010;24(2):408–15.
Dejour D, Kuhn A, Dejour H. Tibial deflexion osteotomy and chronic anterior laxity: a series of 22 cases. Rev Chir Orthop. 1998;84:28–9.
Christensen JJ, Krych AJ, Engasser WM, Vanhees MK, Collins MS, Dahm DL. Lateral tibial posterior slope is increased in patients with early graft failure after anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43(10):2510–4.
Dejour D, Saffarini M, Demey G, Baverel L. Tibial slope correction combined with second revision ACL produces good knee stability and prevents graft rupture. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2846–52.
van de Pol GJ, Arnold MP, Verdonschot N, van Kampen A. Varus alignment leads to increased forces in the anterior cruciate ligament. Am J Sports Med. 2009;37:481–7.
Bernhardson AS, et al. Tibial slope and its effect on force in anterior cruciate ligament grafts: anterior cruciate ligament force increases linearly as posterior tibial slope increases. Am J Sports Med. 2019;47(2):296–302.
Dejour D, Pungitore M, Valluy J, Nover L, Saffarini M, Demey G. Preoperative laxity in ACL-deficient knees increases with posterior tibial slope and medial meniscal tears. Knee Surg Sports Traumatol Arthrosc. 2019;27(2):564–72.
Dejour H, Bonnin M. Tibial translation after anterior cruciate ligament rupture. Two radiological tests compared. J Bone Joint Surg Br. 1994;76(5):745–9.
Dejour H, Walch G, Neyret P, Adeleine P. Results of surgically treated chronic anterior laxities. Apropos of 251 cases reviewed with a minimum follow-up of 3 years. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(7):622–36.
Schatka I, Weiler A, Jung TM, Walter TC, Gwinner C. High tibial slope correlates with increased posterior tibial translation in healthy knees. Knee Surg Sports Traumatol Arthrosc. 2018;26(9):2697–703.
Rahnemai-Azar AA, Abebe ES, Johnson P, Labrum J, Fu FH, Irrgang JJ, Samuelsson K, Musahl V. Increased lateral tibial slope predicts high-grade rotatory knee laxity preoperatively in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1170–6.
Lustig S, Scholes CJ, Leo SP, Coolican M, Parker DA. Influence of soft tissues on the proximal bony tibial slope measured with two-dimensional MRI. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):372–9.
Ahn JH, Bae TS, Kang KS, Kang SY, Lee SH. Longitudinal tear of the medial meniscus posterior horn in the anterior cruciate ligament-deficient knee significantly influences anterior stability. Am J Sports Med. 2011;39(10):2187–93.
Stephen JM, Halewood C, Kittl C, Bollen SR, Williams A, Amis AA. Posteromedial meniscocapsular lesions increase tibiofemoral joint laxity with anterior cruciate ligament deficiency, and their repair reduces laxity. Am J Sports Med. 2016;44(2):400–8.
Ali AA, Harris MD, Shalhoub S, Maletsky LP, Rullkoetter PJ, Shelburne KB. Combined measurement and modeling of specimen-specific knee mechanics for healthy and ACL-deficient conditions. J Biomech. 2017;57:117–24.
Lorbach O, Kieb M, Herbort M, Weyers I, Raschke M, Engelhardt M. The influence of the medial meniscus in different conditions on anterior tibial translation in the anterior cruciate deficient knee. Int Orthop. 2015;39(4):681–7.
Samuelsen BT, Aman ZS, Kennedy MI, Dornan GJ, Storaci HW, Brady AW, LaPrade RF. Posterior medial meniscus root tears potentiate the effect of increased tibial slope on anterior cruciate ligament graft forces. Am J Sports Med. 2019;48(2):334–40.
Yamaguchi KT, Cheung EC, Markolf KL, Boguszewski DV, Mathew J, Lama CJ, Petrigliano FA. Effects of anterior closing wedge tibial osteotomy on anterior cruciate ligament force and knee kinematics. Am J Sports Med. 2018;46(2):370–7.
Yoo JH, Chang CB, Shin KS, Seong SC, Kim TK. Anatomical references to assess the posterior tibial slope in total knee arthroplasty: a comparison of 5 anatomical axes. J Arthroplast. 2008;23(04):586–92.
Caton J. Method of measuring the height of the patella. Acta Orthop Belg. 1989;55:385–6.
Caton J, Deschamps G, Chambat P, et al. Patella infera. Apropos of 128 cases. Rev Chir Orthopédique Réparatrice Appar Mot. 1982;68:317–25.
Hashemi J, Chandrashekar N, Gill B, et al. The geometry of the tibial plateau and its influence on the biomechanics of the tibiofemoral joint. J Bone Joint Surg Am. 2008;90(12):2724–34. https://doi.org/10.2106/JBJS.G.01358.
Sonnery-Cottet B, Archbold P, Cucurulo T, et al. The influence of the tibial slope and the size of the intercondylar notch on rupture of the anterior cruciate ligament. J Bone Joint Surg Br. 2011;93(11):1475–8.
Waiwaiole A, Gurbani A, Motamedi K, Seeger L, Sim MS, Nwajuaku P, Hame SL. Relationship of ACL injury and posterior tibial slope with patient age, sex, and race. Orthop J Sports Med. 2016;4(11):2325967116672852.
Zeng C, Yang T, Wu S, Gao SG, Li H, Deng ZH, Zhang Y, Lei GH. Is posterior tibial slope associated with noncontact anterior cruciate ligament injury? Knee Surg Sports Traumatol Arthrosc. 2016;24(3):830–7.
Webb JM, Salmon LJ, Leclerc E, Pinczewski LA, Roe JP. Posterior tibial slope and further anterior cruciate ligament injuries in the anterior cruciate ligament–reconstructed patient. Am J Sports Med. 2013;41:2800–4.
Salmon LJ, Heath E, Akrawi H, Roe JP, Linklater J, Pinczewski LA. 20-year outcomes of anterior cruciate ligament reconstruction with hamstring tendon autograft: the catastrophic effect of age and posterior tibial slope. Am J Sports Med. 2018;46(3):531–43.
Schuster P, Geßlein M, Schlumberger M, Mayer P, Richter J. The influence of tibial slope on the graft in combined high tibial osteotomy and anterior cruciate ligament reconstruction. Knee. 2018;25(4):682–91.
Frikha R, Dahmene J, Ben Hamida R, Chaieb Z, Janhaoui N, Laziz Ben Ayeche M. Congenital absence of the anterior cruciate ligament: eight cases in the same family. Rev Chir Orthop Reparatrice Appar Mot. 2005;91(7):642–8.
El-Azab H, Halawa A, Anetzberger H, Imhoff AB, Hinterwimmer S. The effect of closed- and open-wedge high tibial osteotomy on tibial slope: a retrospective radiological review of 120 cases. J Bone Joint Surg Br. 2008;90:1193–7.
Hohmann E, Bryant A, Imhoff AB. The effect of closed wedge high tibial osteotomy on tibial slope: a radiographic study. Knee Surg Sports Traumatol Arthrosc. 2006;14:454–9.
Arun GR, Kumaraswamy V, Rajan D, et al. Long-term follow up of single-stage anterior cruciate ligament reconstruction and high tibial osteotomy and its relation with posterior tibial slope. Arch Orthop Trauma Surg. 2016;136:505–11.
Shelburne KB, Kim HJ, Sterett WI, Pandy MG. Effect of posterior tibial slope on knee biomechanics during functional activity. J Orthop Res. 2011;29(02):223–31.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 ISAKOS
About this chapter
Cite this chapter
Muzzi, S., Muniagurria, C., Gruskay, J., Dejour, D. (2022). Osteotomy: Slope Change Tibial Osteotomy to Address ACL Deficiency. In: Nakamura, N., Marx, R.G., Musahl, V., Getgood, A., Sherman, S.L., Verdonk, P. (eds) Advances in Knee Ligament and Knee Preservation Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-84748-7_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-84748-7_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84747-0
Online ISBN: 978-3-030-84748-7
eBook Packages: MedicineMedicine (R0)