Abstract
Hyperthyroidism is a set of disorders that involve excess synthesis and secretion of thyroid hormones by the thyroid gland, which leads to thyrotoxicosis. The most common forms of hyperthyroidism include diffuse toxic goiter (Graves’ disease), toxic multinodular goiter (Plummer’s disease), and a solitary toxic adenoma. The most reliable screening measure of thyroid function is the thyroid-stimulating hormone (TSH) level. Options for treatment of hyperthyroidism include antithyroid drugs, radioactive iodine therapy (the preferred treatment of hyperthyroidism among US thyroid specialists), or thyroidectomy. Massive thyroid enlargement with compressive symptoms, a suspicious nodule, Graves’ orbitopathy, and patient preference are indications for surgical treatment of thyrotoxicosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hyperthyroidism
- Graves’ disease
- Thyroid-stimulating hormone
- Anti-TSH receptor Antibodies
- Toxic nodular goiter
- Plummer’s disease
- Antithyroid drugs
- Radioactive iodine
- Surgery
Case Presentation
A 48-year-old female, with a past medical history of Graves’ disease and 2 years status post–radioiodine ablation, presented to a clinic, after a 2-year lapse in healthcare. She had increasing anxiety over the past several months regarding her perceived worsening state of health, particularly the recent weight gain and facial edema. Upon further questioning, the patient also reported hair loss, dry skin, hoarse voice, constipation, difficulty sleeping, fatigue, depression, anxiety, arthralgias, and myalgias. Her past medical history was significant only for Graves’ disease treated with radioiodine ablation. She denied ever taking thyroid replacement medication after her thyroid ablation due to a lack of such a recommendation.
On initial presentation, her body mass index (BMI) was 28.7 kg/m2, temperature 36.0 °C, blood pressure 100/70 mmHg, and pulse 54 beats per minute. Physical exam was remarkable for hoarse voice, bradycardia, dry skin on her hands and legs bilaterally, tender scalp, and facial edema. Electrocardiogram (ECG) revealed marked sinus bradycardia (heart rate (HR) 44), low voltage, and nonspecific T wave changes.
Although the patient’s longstanding untreated hypothyroidism was quickly identified and confirmed with TSH serum level of 102.4 mIU/L (reference range: 0.27–4.5 mIU/L), a comprehensive evaluation was completed to rule out other contributing factors. Lipid panel, comprehensive metabolic panel, and complete blood count revealed hypercholesterolemia, elevated liver enzymes, and mild normocytic anemia, respectively.
The patient was started on levothyroxine 50 mcg/day, and the dose was slowly adjusted according to serial TSH levels. On week 8, from the onset of levothyroxine replacement therapy, the dose was 112 mcg/day and serum TSH level was 2.4 mIU/L. Once she became euthyroid, her cholesterol, low-density lipoprotein (LDL), and triglycerides improved, while her hemoglobin, creatinine, and aspartate aminotransferase (AST) levels normalized. The patient reported improved balance and stability, facial edema disappeared, increased body hair growth, softer skin, more regular bowel movements, uninterrupted sleep, increased energy, and improved mood. She continued to have myalgias and arthralgias, which were treated conservatively with nonsteroidal anti-inflammatory medication with clinical improvement. On exam, her voice was less hoarse, heart rate increased, skin appeared normal, face was normal, and she was more relaxed. Follow-up ECG showed improved sinus bradycardia and nonspecific T wave changes. After treatment, the patient stated that she felt better than she had in many months. She continues to come to the clinic every 6 months for TSH monitoring.
Questions
-
1.
The symptoms of hyperthyroidism include
-
1.
Nervousness, anxiety, irritability, emotional liability
-
2.
Weight gain and facial edema
-
3.
Hair loss, thinning of the hair
-
4.
More frequent bowel movements/diarrhea
-
5.
Increased sweating
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (2) and (4) are correct.
-
(c)
Only (1) and (3) and (4) are correct.
-
(d)
Only (1) and (3) and (4) and (5) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
2.
Signs of hyperthyroidism include
-
1.
Menstrual disorders
-
2.
Tachycardia
-
3.
Systolic hypertension
-
4.
Proximal muscle weakness
-
5.
Diarrhea
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (2) and (4) are correct.
-
(c)
Only (1) and (3) and (4) are correct.
-
(d)
Only (2) and (3) and (4) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
3.
Low serum TSH and normal serum FT4 and FT3 correspond to
-
1.
Overt hyperthyroidism
-
2.
Subclinical hyperthyroidism
-
3.
Overt hypothyroidism;
-
4.
Subclinical hypothyroidism
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (2) and (4) are correct.
-
(c)
Only (2) is correct.
-
(d)
Only (1) and (2) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
4.
The diagnosis of Graves’ disease is very likely in the following situations:
-
1.
Overt or subclinical hyperthyroidism and an increased anti-TSHR serum level
-
2.
Hyperthyroidism accompanied by thyroid orbitopathy with clear involvement of the soft tissues of the orbits or thyroid dermopathy
-
3.
Hyperthyroidism with goiter confirmed by ultrasound (diffuse parenchymal hypoechogenicity) – if anti-TSHR antibodies cannot be determined
-
4.
Isolated thyroid orbitopathy and increased anti-TSHR serum level
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (2) and (4) are correct.
-
(c)
Only (2) and (4) are correct
-
(d)
Only (2) is correct.
-
(e)
All are correct.
-
(a)
-
1.
-
5.
The diagnosis of Graves’ disease is likely if
-
1.
There are relapses of hyperthyroidism, separated by periods of euthyroidism lasting >6 months without the use of antithyroid drugs.
-
2.
There are cases of Graves’ disease or Hashimoto’s disease in the family of a patient with hyperthyroidism, or another autoimmune disease.
-
3.
There is limited but diffuse 123I thyroid uptake on scintigraphy.
-
4.
There is limited but diffuse 99mTc thyroid uptake on scintigraphy.
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (2) and (4) are correct.
-
(c)
Only (3) and (4) are correct.
-
(d)
Only (1) and (2) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
6.
What statements regarding differential diagnosis of hyperthyroidism are correct?
-
1.
The diagnosis of Graves’ disease should be based on signs of thyrotoxicosis, elevation in serum fT4 and fT3 levels, suppression of TSH, and elevated level of anti-TSHR antibody.
-
2.
Thyroid scintigraphy, which demonstrates diffuse and remarkably elevated uptake of 123I, suggests a diagnosis of Graves’ disease.
-
3.
Patients with Plummer’s disease have thyrotoxicosis but are negative for anti-TSHR antibody and show scintigraphic patterns of multifocal uptake of 123I.
-
4.
Increased thyroid blood flow in whole thyroids on ultrasound in patients with hyperthyroidism suggests the existence of Graves’ disease, whereas nodules showing increased thyroid blood flow in anti-TSHR-negative patients with hyperthyroidism are suggestive for Plummer’s disease.
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (2) and (4) are correct.
-
(c)
Only (3) and (4) are correct.
-
(d)
Only (1) and (2) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
7.
What statements regarding Graves’ disease are correct?
-
1.
There is no effective causative treatment of Graves’ disease, only the symptoms are treated like hyperthyroidism and orbitopathy.
-
2.
The first goal of treatment is to achieve euthyroidism as soon as possible and to make a decision together with the patient on a further treatment strategy.
-
3.
There are three therapeutic alternatives for GD: antithyroid drugs, radioactive iodine, and thyroidectomy.
-
4.
Each modality has its own advantages and disadvantages.
-
(a)
Only (1) and (4) are correct.
-
(b)
Only (1) and (3) are correct.
-
(c)
Only (2) and (4) are correct.
-
(d)
Only (2) and (3) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
8.
Which statements regarding toxic multinodular goiter are correct?
-
1.
The etiologic factors involved in the formation of a toxic multinodular goiter include an inherent functional heterogeneity of thyroid follicles.
-
2.
The etiologic factors involved in the formation of a toxic multinodular goiter include the effect of growth factors and goitrogens.
-
3.
The etiologic factors involved in the formation of a toxic multinodular goiter include iodine deficiency.
-
4.
The etiologic factors involved in the formation of a toxic multinodular goiter include genetic abnormalities that include somatic activating mutations of genes that regulate thyroid growth and hormone synthesis.
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (1) and (2) and (4) are correct.
-
(c)
Only (1) and (3) and (4) are correct.
-
(d)
Only (2) and (3) and (4) are correct.
-
(e)
All are correct.
-
(a)
-
1.
-
9.
Which statement regarding toxic multinodular goiter are correct?
-
1.
There are two main goals for treatment in patients with TMNG: to eliminate an autonomously functioning thyroid tissue and to alleviate compressive symptom.
-
2.
Pharmacological treatment with antithyroid drugs (methimazole) allows for controlling the symptoms of hyperthyroidism, but its withdrawal always leads to the relapse of the hyperthyroidism (after a few days to several weeks).
-
3.
The beta-blocker is used similarly to other types of hyperthyroidism, but in TMNG, it is needed more often and in higher doses than in Graves’ disease due to the greater severity of cardiac symptoms.
-
4.
Definitive treatment options for TMNG are either surgical (subtotal or total thyroidectomy) or radioiodine ablation, and the choice of method is not individualized for each patient.
-
(a)
Only (1) and (2) and (3) are correct.
-
(b)
Only (2) and (3) and (4) are correct.
-
(c)
Only (3) and (4) are correct.
-
(d)
Only (4) is correct.
-
(e)
All are correct.
-
(a)
-
1.
-
10.
Which statements regarding toxic multinodular goiter are correct?
-
1.
Surgical resection is the preferred treatment option in patients with a nodule with cytological or clinical features of malignancy and also in patients with a large goiter giving compression symptoms, especially in the presence of inactive thyroid nodules, unless there are contraindications for surgery.
-
2.
The operation is possible only after euthyroid state is achieved.
-
3.
Treatment with methimazole should be stopped on the day of surgery, and the dose of beta-blocker should be gradually reduced to discontinue it within a few days after surgery.
-
4.
Potassium iodide should be given 5–10 days before surgery.
-
(a)
Only (1) and (2) are correct.
-
(b)
Only (1) and (2) and (3) are correct.
-
(c)
Only (2) and (3) are correct.
-
(d)
Only (4) is correct.
-
(e)
All are correct.
-
(a)
-
1.
-
11.
Which statements regarding toxic multinodular goiter are correct?
-
1.
The presence of substernal thyroid extension or airway obstruction is a relative contraindication for RAI because of the potential for transient increase in size of the goiter and worsening of airway compromise through RAI-induced transient radiation thyroiditis.
-
2.
The extent of surgery should be total or near-total thyroidectomy.
-
3.
Subtotal thyroidectomy (Dunhill operation) may be considered in patient who experienced intraoperative loss of signal of neuromonitoring after removal of the first-attempted lobe, and usually dominant thyroid lobe (a staged thyroidectomy).
-
4.
The risk of permanent surgical morbidity is low (<1–2%) if surgery is performed by a high-volume thyroid surgeon.
-
5.
In untreated TMNG, the risk of arrhythmias and other cardiovascular complications as well as thyroid crisis is not increased.
-
6.
The risk of cancer is far lower than in other forms of nodular goiter.
-
(a)
Only (1) and (2) and (3) and (4) are correct.
-
(b)
Only (2) and (3) and (5) and (6) are correct.
-
(c)
Only (1) and (3) and (4) and (6) are correct.
-
(d)
Only (2) and (3) and (4) and (6) are correct.
-
(e)
All are correct.
-
(a)
-
1.
1 Introduction
Primary hyperthyroidism is a set of disorders in which the thyroid gland synthesizes and secretes too much thyroid hormones for the body’s needs, which leads to the hypermetabolic condition of thyrotoxicosis. The thyroid gland is a small organ located at the base of the neck. It is responsible for the production and release of two hormones: triiodothyronine (T3) and thyroxine (T4), which regulate the function of most tissues of the body, influence the metabolism of our body and thermogenesis (heat production). The thyroid gland is controlled by the pituitary gland, which releases thyroid-stimulating hormone (TSH), which stimulates the thyroid gland to produce the hormones T3 and T4.
The activities of the thyroid gland and pituitary gland are closely related (known as negative feedback), in which the increased concentration of thyroid hormones reduces the release of TSH by the pituitary gland, and the deficiency of these hormones increases the production of TSH, which in turn stimulates the thyroid to produce more T3 and T4.
The term hyperthyroidism is distinct from thyrotoxicosis, a clinical state in which there is an inappropriately high thyroid hormone action in tissues. Thyrotoxicosis can result from hyperthyroidism as well as from other etiologies like subacute, painless, or radiation-induced thyroiditis, excessive intake of thyroid hormone, struma ovarii, and functional metastatic thyroid cancer.
The most common causes of hyperthyroidism include: Graves’ disease (an autoimmune disease in which your own antibodies stimulate the thyroid gland to produce hormones), thyroid nodules (hyperactive [toxic] multinodular goiter [Plummer’s disease], or a solitary thyroid autonomic tumor). The less common causes of hyperthyroidism include subacute thyroiditis (a disease associated with a previous viral infection), postpartum thyroiditis, and amiodarone-induced thyrotoxicosis.
The overall prevalence of hyperthyroidism, which is approximately 1.3% (it occurs in 2% of women and 0.5% in men), increases to 4–5% in older women. Hyperthyroidism is also more common in smokers. Graves’ disease is seen most often in younger women, while toxic nodular goiter is more common in older women [1].
1.1 Clinical Presentation of Hyperthyroidism
The main symptoms and manifestations that suggest thyrotoxicosis are presented in ◘ Box 3.1, while signs of hyperthyroidism are listed in ◘ Box 3.2.
The extent of symptoms may differ from patient to patient. In the elderly, the symptoms of hyperthyroidism may be less pronounced and subtle. Weakness, asthenia, fatigue, and problems with the circulatory system may predominate – heart rhythm disturbances (atrial fibrillation), ischemic heart disease, and symptoms of congestive heart failure.
1.2 Natural History of Hyperthyroidism
Hyperthyroidism should not be taken lightly, and if left untreated, it can lead to dangerous complications, for example, arrhythmias, heart failure, osteoporosis, or thyroid crisis (with a life-threatening increase in T3 and T4 levels). In pregnant women, hyperthyroidism is detrimental to both the mother and the fetus. Treatment of hyperthyroidism requires compliance with medical recommendations, regular medication, and regular medical check-ups.
1.3 Diagnosis of Hyperthyroidism
A person who finds symptoms suggesting the presence of hyperthyroidism should see a general practitioner who, after a medical examination (after taking an anamnesis and after the physical examination), will decide on the need to measure the serum TSH level. The test is performed with a blood sample that does not have to be taken on an empty stomach. In the case of very severe hyperthyroidism, it is necessary to urgently refer the patient to a hospital.
To confirm hyperthyroidism, hormonal tests are performed. The best initial screening test to assess thyroid function is a serum TSH level, and it may be ordered by the general practitioner. If the result is incorrect, it is necessary to measure thyroid hormone tests: free T4 (FT4) and/or free T3 (FT3). Hyperthyroidism is diagnosed if a decreased TSH concentration is accompanied by an increased FT4 and/or FT3 concentration in the serum (the higher FT4 and FT3 serum levels, the more severe is hyperthyroidism).
Patients with a low serum TSH level and normal FT4 and FT3 levels are defined as having subclinical hyperthyroidism. A FT3 serum level is important to make a diagnosis of “T3 thyrotoxicosis” in a patient with a suppressed serum TSH level and a normal FT4 level. In most cases, T3 thyrotoxicosis is an early manifestation of Graves’ disease.
If hyperthyroidism is diagnosed, its cause and etiology should be determined, which is important when deciding on the treatment method. The following studies are helpful for this: thyroid ultrasound, serum antithyroid antibodies (especially anti-TSH receptor [anti-TSHR] antibodies, and antithyroid peroxidase [anti-TPO] antibodies), fine-needle aspiration biopsy (if there are focal lesions of the thyroid), and thyroid scintigraphy performed in selected cases. Sometimes clinical examination may readily reveal the etiology of the disease like in case of orbitopathy associated with Graves’ disease (◘ Fig. 3.1).
Thyroid uptake scintigraphy with iodine-123 (123I) or technetium-99m (99mTc) can be helpful in determining the cause of hyperthyroidism. Thyroid uptake is elevated in patients with Graves’ disease and may be elevated or normal in patients with toxic multinodular goiter or a solitary toxic nodule [2]. It is low or undetectable in patients with thyrotoxicosis from thyroiditis. Thyroid scintigraphy typically demonstrates diffuse symmetrical uptake in patients with Graves’ disease, heterogeneous uptake in patients with toxic multinodular goiter, and a single area of hyperfunctioning with a variable degree of suppression of the remaining thyroid gland in patients with a solitary toxic nodule (◘ Fig. 3.2).
The diagnosis of Graves’ disease should be based on signs of thyrotoxicosis, elevation in serum fT4 and fT3 levels, suppression of TSH and elevated level of anti-TSHR antibody. In addition, thyroid scintigraphy demonstrates diffuse and remarkably elevated uptake of 123I, suggesting a diagnosis of Graves’ disease. Contrary to these findings, patients with Plummer’s disease have thyrotoxicosis but are negative for anti-TSHR antibody and show scintigraphic patterns of multifocal uptake of 123I. When we consider hyperthyroidism based on thyroid ultrasound, increased thyroid blood flow in whole thyroids in patients with hyperthyroidism suggests the existence of Graves’ disease. When we find nodules showing increased thyroid blood flow in anti-TSHR-negative patients with hyperthyroidism, we consider such patients likely having Plummer’s disease.
1.4 Treatment of Hyperthyroidism
There are many ways to treat hyperthyroidism. There is no single best treatment as each method has advantages and disadvantages that can be discussed with a healthcare professional. In addition, the treatment of hyperthyroidism depends on its cause, severity, age of the patient, and coexisting diseases. Hence, the best method of treatment is tailored individually for each patient. Most often, it begins with the use of drugs that reduce the production of thyroid hormones, usually also when treatment is planned by another method (surgery or radioiodine 131I).
In the treatment of hyperthyroidism, the following are used: drug treatment with antithyroid drugs (thyreostatic drugs), radioiodine treatment (131I), and surgical treatment (strumectomy, thyroidectomy).
1.4.1 Antithyroid Drugs
Antithyroid drugs include methimazole (thiamazole) and propylthiouracil. Thyreostatics inhibit the production of hormones in the thyroid gland, and their effect becomes apparent after about 2–4 weeks of use. Each time the physician determines the starting dose of the drug individually. Adjuvant therapy with ß-blockers, for example, propranolol, is also often used, as they do not lower thyroid hormone levels by themselves, but help to control some symptoms, such as trembling hands and the feeling of a rapid heartbeat. During the treatment, the physician controls the effectiveness of the therapy through clinical evaluation (interview and examination of the patient) and determination of the concentration of thyroid hormones, and adjusts the dose accordingly. Undesirable effects may occur during treatment with thyreostatic drugs.
In the event of any side effects that may be related to the commencement of treatment, the patient should report it to the physician as soon as possible. In minor complications (e.g., itching of the skin, joint pain), it is enough to change the drug or its dose. In some, very rare cases, it may be necessary to discontinue thyreostatic treatment.
A particularly dangerous, but fortunately extremely rare complication can be agranulocytosis, a significant decrease in the number of neutrophils (a type of white blood cell) in the serum due to reversible toxic bone marrow damage that resolves after drug discontinuation, but requires close medical supervision. This is a very dangerous condition, because the body’s resistance to infection is greatly impaired. Therefore, in the event of fever, weakness, or sore throat, the patient should immediately stop taking the drug and urgently visit the clinic or hospital for complete blood count control with a smear. If the neutrophil count is not decreased, the earlier treatment should be continued immediately. If agranulocytosis is confirmed, this group of drugs must never be used again in the future.
1.4.2 Radioactive Iodine (RAI)
A single oral administration of radioiodine is intended to slow irreversible damage to the thyroid cells that actively take up iodine from the blood. The radioiodine effect develops within a few months after therapy. The development of permanent hypothyroidism (requiring treatment with thyroxine tablets) cannot be treated as a complication, but as an effect of effective treatment. This form of therapy must not be used in pregnant women and during breastfeeding. Additionally, the treated person should not contact young children and pregnant women for a period of about a week. Finally, women should not plan a pregnancy for at least 6–12 months after treatment.
1.5 Indications for Surgery in Hyperthyroidism
This form of therapy is absolutely indicated in the case of suspected or diagnosed thyroid cancer, including coexisting hyperthyroidism. Moreover, surgical treatment is considered in patients with high volume goiter compressing the trachea (◘ Fig. 3.3). After surgical removal of the thyroid gland, hypothyroidism occurs, which requires constant treatment with levothyroxine. Unfortunately, during the operation, one must take into account the possibility of complications that should be discussed with the consulting surgeon. Serious postoperative complications include paresis/paralysis of one or both vocal cords as a result of intraoperative injury to the recurrent laryngeal nerve(s), damage to the external branch of the superior laryngeal nerve(s), and transient or permanent hypoparathyroidism.
1.6 Prognosis in Hyperthyroidism
The possibility of a complete recovery (the patient does not require any thyroid medication) depends mainly on the cause of the hyperthyroidism. Hypothyroidism is common after radioiodine therapy or thyroidectomy, requiring continued treatment with thyroxine preparations for life.
After the treatment of hyperthyroidism is finished, the patient requires further constant medical care. Periodic hormonal control (serum TSH level) and ultrasound (ultrasound of the thyroid) are recommended. In some cases, there is a possibility of relapse of hyperthyroidism, recurrence of nodular goiter, or development of hypothyroidism after the treatment, even many months after its completion. If the patient, after completing the therapy, requires treatment with oral thyroid hormones due to hypothyroidism, it is necessary to take them regularly and periodically check the effectiveness of this treatment.
This chapter focuses on the clinical presentation, diagnosis, and surgical management of hyperthyroidism caused by Graves’ disease and multinodular toxic goiter (Plummer’s disease).
2 Graves’ Disease
2.1 Pathogenesis
Graves’ disease (GD) is an autoimmune disease in which the autoantigen is the TSH receptor (TSHR). Its activation by anti-TSHR antibodies causes increased secretion of thyroid hormones and leads to symptoms of hyperthyroidism, stimulates the growth of the thyroid gland, and the development of its vascularization [3]. Activation of the cellular response mechanisms against the same antigen, which is present in orbital and skin fibroblasts, leads to increased secretion of pro-inflammatory cytokines and the development of nonthyroid symptoms of the disease. Thyroid orbitopathy is a set of eye symptoms caused by immunological inflammation of the soft tissues of the orbit in the course of GD, leading to temporary or permanent damage to the organ of vision.
2.2 Epidemiology
Graves’ disease is the most common cause of hyperthyroidism in developed countries. An annual incidence of Graves’ disease is estimated to be 30–38 per 100,000 population [4, 5]. It is more common between 30 and 60 years; 5–10 times more frequent in women [6]. The genetic predisposition accounts for 79% of the risk for GD, while environmental factors for 21%. About 70% of genes associated with autoimmune thyroid disorders are implicated in T-cell function. Among GD environmental risk factors, smoking, iodine excess, selenium or vitamin D deficiency, and interferon- α (IFN-α) treatment in history for chronic HCV hepatitis are well recognized.
It can be also associated with other autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, chronic lymphocytic thyroiditis, Sjögren’s syndrome, vitiligo, pernicious anemia, type 1 diabetes mellitus, Addison’s disease, myasthenia gravis, and idiopathic thrombocytopenic purpura [7].
2.3 Clinical Presentation
The clinical manifestations of GD include typical presentation of symptoms and signs of overt hyperthyroidism (◘ Boxes 3.1 and 3.2) in a patient with diffuse goiter (◘ Fig. 3.4). However, in the elderly, only cardiac symptoms may be present. Typically, GD is characterized by periods of exacerbation and remission, there is a diffuse symmetrical and well-vascularized goiter with a characteristic vascular murmur, there may be exophthalmos (overt orbitopathy is not a necessary condition for the diagnosis of GD), less often symptoms of autoimmune dermatitis – pretibial edema (thyroid dermopathy – a pathognomonic symptom, but rare), and thyroid acropachy (thickened and rounded end phalanges of the hands; symptom very rare).
Orbitopathy manifests itself simultaneously with hyperthyroidism or later within 18 months, may precede other symptoms of hyperthyroidism, and is rarely the only symptom of GD; exceptionally, it may accompany hypothyroidism. Patients complain of pain in the eyeballs, burning sensation, lacrimation, decreased visual acuity, a feeling of sand under the eyelids, photophobia and double vision; physical examination reveals exophthalmos, swelling of the eyelids and periorbital tissues, conjunctival redness, and limited eye movement. The risk of blindness occurs when there is ulceration of the cornea due to regurgitation of the eyelids and when there is pressure on the optic nerve (initially impaired color vision).
Clinically relevant orbitopathy occurs in 20–30% of patients with GD and is vision-threatening in 3–5% of patients [8]. Malignant exophthalmos is a rare but severe form of progressive edematous infiltrative orbitopathy with a particularly high risk of permanent complications [9].
2.4 Diagnosis
Graves’ disease may present as overt or subclinical primary hyperthyroidism, accompanied by features of autoimmune inflammation, clinically evident or detected in additional tests.
In laboratory tests, decreased serum TSH level and increased (less frequently normal) FT4 and FT3 levels (usually FT4 determination is sufficient; if correct – FT3 determination is needed) is found. In overt hyperthyroidism, the significant advantage of the FT3 increase over the FT4 increase is a prognostic signal – the response to antithyroid therapy is worse. In the remission phase, the results of hormonal tests are normal.
Increased serum level of anti-TSHR antibodies confirms the diagnosis (determine before or during the first 3 months of antithyroid treatment), whereas normalization indicates immune remission of the disease.
Characteristic for GD on ultrasound of the thyroid gland is the hypoechoic parenchyma (◘ Fig. 3.5), enlarged thyroid with increased vascular flow; the presence of thyroid nodules does not exclude GD. Thyroid scintigraphy is especially helpful in the presence of nodules and qualification for radioiodine treatment.
Computed tomography (CT) of the eye sockets without contrast allows for assessment of the soft tissues of the orbit, its bones (important in planning decompression surgery), and external thickening of the eyeball muscles.
Magnetic resonance imaging (MRI) of the eye sockets allows for assessment of the swelling or fibrosis of the eyeball muscles.
The diagnosis of GD may be certain or probable depending on the set of available data (◘ Table 3.1). An isolated increase in the serum level of anti-TSHR antibodies is not sufficient for the diagnosis of GD (it may occur in relatives of patients with GD who do not develop symptoms of the disease).
It is also important not only to diagnose inflammation of the orbital tissues and to diagnose thyroid orbitopathy (GO), but above all to assess whether the severity of symptoms warrants treatment (requires full ophthalmological examination and often CT of the orbits). Classification of thyroid orbitopathy taking into account the activity of immune inflammation (according to EUGOGO, 2016) distinguishes the following: mild GO with a limited influence on daily functioning, moderate to severe GO without risk of vision loss, and very severe GO with a risk of blindness [10]. In addition, Clinical Activity Score (CAS) serves to assess of the activity of the thyroid orbitopathy based on the presence of inflammatory features including (sign present 1, sign absent 0): spontaneous retro-orbital pain, pain when looking up or down, redness of the eyelids, conjunctival redness, inflammation of the lacrimal muscle and/or conjunctival crescent fold, swelling of the eyelids, swelling of the conjunctiva (chemosis). An active GO is indicated by a CAS value of 3 out of 7 or more. Inactive GO is diagnosed at CAS below 3.
2.5 Treatment
An ideal treatment of GD would include the following: prompt control of the disease manifestations, return to and maintenance of the euthyroid state, minimal morbidity, no mortality risk, and a reasonable cost.
Unfortunately, there is no effective causative treatment of GD, only the symptoms are treated like hyperthyroidism and orbitopathy.
The first goal of treatment is to achieve euthyroidism as soon as possible and to make a decision together with the patient on a further treatment strategy.
There are three therapeutic alternatives for GD: antithyroid drugs, radioactive iodine, and thyroidectomy. Each modality has its own advantages and disadvantages. Patient, physician, institutional, and geographical preferences often influence the choice of therapy. In the United States, radioactive ablation has traditionally been the primary treatment used. However, a 2011 survey indicates that there has been a trend toward increasing the long-term use of antithyroid drugs in lieu of radioactive iodine [11]. In Europe, Japan, and South America, prolonged antithyroid drugs therapy still remain a preferred option [12,13,14].
2.5.1 Antithyroid Drugs
If the main method of treatment is antithyroid drugs, efforts should be made to achieve and maintain immune remission. The prognosis of anti-TSHR normalization is favorable, as well as a decrease in the volume of the goiter and the disappearance of the vascular features (the stimulating effect of anti-TSHR antibodies decreases and lymphocytic infiltration subsides), which represent indirect features of immune remission. In the event of recurrence of hyperthyroidism, radical treatment – radioiodine treatment or surgery – is preferable, but chronic treatment with a low dose of antithyroid drug is acceptable, if radical treatment cannot be performed or if it is contraindicated or if this is the patient’s preferences.
The optimal duration of pharmacological treatment is 18 months, or at least 12 months, if the goal is to achieve permanent immune remission [15]. The risk of relapse increases with the disease severity, and it was reported to be as high as 48% [16].
The classic antithyroid treatment regimen is methimazole (thiamazole) to achieve euthyroidism (which takes 3–6 weeks), usually at a dose of 20–30 mg per day, then gradually reduce the dose to the maintenance dose (continued for 18 months); only if allergic to methimazole, if treatment is necessary, propylthiouracil can be used (as time to achieve euthyroidism is usually longer).
Manifestation of the ineffectiveness of pharmacotherapy in GD is summarized in ◘ Box 3.3.
Propylthiouracil is preferred for the management of GD during pregnancy, because methimazole has been associated with birth defects [17, 18].
Initial pharmacological preparation before radical treatment should last 4–6 weeks (at least 2 weeks) before surgery, and methimazole is preferred due to the shorter time to achieve euthyroid state. On the other hand, methimazole is recommended to be used 1–3 months prior to treatment with radioactive iodine because of the lesser inhibition of thyroid sensitivity to ionizing radiation [2, 13].
2.5.2 Radioactive Iodine (RAI)
Radioactive iodine was introduced for treatment of hyperthyroidism in the 1940s (subtotal thyroidectomy was in use before that era) [19]. Radioactive iodine is nowadays the method of choice in the radical treatment of hyperthyroidism in the course of GD (at a dose of 10–15 mCi). 131I emits beta particles, which destroy the follicular cells of the thyroid. In approximately 3/4 of cases, a single administration of 131I is sufficient; in the remaining cases, it is necessary to administer the second dose, usually after 6 months [20]. Treatment with 131I is contraindicated in active severe or moderate orbitopathy [2, 13]. In mild active orbitopathy, treatment with 131I should be performed in a corticosteroid shield (due to the risk of temporary exacerbation) – prednisone 0.3–0.5 mg/kg/d from 1–3 days after 131I administration for 1 month, then gradually reduce the dose to stop treatment within 3 months. The majority of patients with GD gradually enter remission of TSH-receptor autoimmunity during medical or after surgical therapy, with no difference between the types of therapy. Remission of TSH-receptor autoimmunity after radioiodine therapy is less common [21, 22]. Side effects of RAI ablation include neck pain and tenderness from radiation-induced thyroiditis, transient increase in thyroid hormone levels, and worsening of Graves’ orbitopathy [2, 13].
2.6 Indications for Surgery and Surgical Details
A clear indication for surgery in GD is a coexisting nodule with cytological proven or clinical risk of malignancy (the risk of thyroid cancer in GD is similar to that in other forms of nodular goiter and accounts for 2–7%). Surgical approach is also preferred in the case of coexisting severe orbitopathy and in the case of large goiter (>80 ml) with compressive symptoms, especially in the case of large radioiodine non-avid foci (area of the nodule refractory to RAI).
It can be also considered based on patient preference, and in pregnant women requiring high doses of antithyroid drugs, or who are intolerant to the drugs. Patient preference is often related to desire for the most rapid amelioration of symptoms (RAI take several months to render a patient hypothyroid), a reluctance to receive RAI because of having young children , a planned pregnancy, and a fear of exposure to radiation [2, 13].
The advantages of surgical therapy are the following: euthyroid state is achieved rapidly and consistently, long-term risks of RAI and antithyroid medications are avoided, tissues for histology are provided (concomitant thyroid nodules and carcinoma are removed), childbearing renders immediately possible, with total thyroidectomy risk of recurrent hyperthyroidism is practically abolished, and absolute titration of thyroid hormone is allowed. In addition, total thyroidectomy has been associated with improvement in eye manifestation attributable to excess adrenergic activity [23].
The disadvantage of surgical treatment of Graves’ disease is the risk of surgical morbidity, which includes laryngeal nerve(s) injury, hypoparathyroidism, bleeding, hematoma, and thyroid storm. When total thyroidectomy is performed by a high-volume endocrine surgeon, the risk of permanent complication is approximately 1–2%, and neck hematoma is 1% or less. Advancements such as preoperative preparation, intraoperative neural monitoring of the laryngeal nerves, and intraoperative parathyroid hormone monitoring together with novel technologies based on autofluorescence phenomenon and currently available for intraoperative parathyroid glands identification and viability assessment have decreased risks greatly and improved outcomes at hands of high-volume endocrine surgeons [24].
Patients should be rendered euthyroid before operation to decrease thyroid vascularity, to improve surgical planes, and to prevent life-threatening thyroid storm. The latter complication is characterized by severe manifestation of hyperthyroidism along with fever, nausea, vomiting, diarrhea, tachyarrhythmias, congestive heart failure, agitation, and delirium. The risk of thyroid storm can be eliminated by adequate preoperative preparation [25,26,27]. Antithyroid drugs are used to normalize FT4 and FT3 serum levels before the planned operation. A beta-blocker is used for symptomatic treatment of adrenergic symptoms and tachycardia. Once the patient’s FT4 and FT3 are normalized, a saturated solution of potassium iodide (SSKI) or Lugol’s solution is administered 5–10 days before surgery. This treatment has also been shown to reduce the thyroid blood flow and vascularity, which allows for surgical dissection in a dry operative field and improves identification and preservation of vital perithyroidal structures like laryngeal nerves and parathyroid glands. Despite convincing data in the literature on utility of preoperative preparation with iodide among patient with GD and the American Thyroid Association (ATA) and European Thyroid Association (ETA) recommendations to use iodide in the presurgical management [2, 13], the need for this treatment approach has recently been challenged [28].
In addition, the vitamin D serum level should be checked and corrected preoperatively to reduce the risk of symptomatic hypocalcemia.
The extent of thyroidectomy for the treatment of GD has been controversial in the past. This controversy regarding the extent of surgical resection in Graves’ disease presumably arose because of the previously reported higher prevalence of permanent complications following total thyroidectomy. More recent data support the safety of total thyroidectomy for benign thyroid disease including GD if surgery is performed in a high-volume unit [24]. Subtotal thyroidectomy (leaving unilateral or bilateral thyroid remnants) is associated with a high rate of hypothyroidism (40–60% within 20 years of operation), and large remnants (more than 4 g; volume of the residual thyroid fragments strongly correlates with the risk of GD recurrence) have a potential for recurrence [29, 30]. Total thyroidectomy or near-total thyroidectomy obviates these disadvantages and can be performed without increased complication rates, and hence, it is the preferred surgical option for treatment of GD when thyroid hormone replacement therapy is readily available (However, bilateral subtotal thyroidectomy still remains an option in the treatment of GD, particularly in the low-income countries with limited access to thyroid hormones medication or in case of no patient compliance.). In addition, total thyroidectomy eliminates the antigen that is the source for the TSH receptor antibodies and other antibodies that cross-react with antigens in the extraocular muscles, the retro-orbital connective tissue, and the optic nerve [31,32,33,34].
Postoperatively, patients must be monitored carefully for hypocalcemia, a potentially serious complication. Patients will require lifelong thyroid hormone replacement with levothyroxine. Radioactive iodine ablation should be considered for disease recurrence after surgery.
2.7 Prognosis
If hyperthyroidism is untreated, sometimes, it comes to spontaneous remission, but sooner life-threatening complications may occur. Pharmacological treatment eliminates the symptoms of thyroid hormones excess and accelerates remission, but relapse occurs in approximately 50% of patients [16]. The concentration of anti-TSHR antibodies is normalized in most patients after approximately 6 months of treatment, but this does not ensure that the achieved remission will be long-lasting. The risk of relapse is increased in men and in those below 20 years of age, as well as in patients with high volume goiter and a high initial FT3/FT4 ratio. Hypothyroidism always occurs after radical surgery (total thyroidectomy) and very often after successful radioiodine treatment; it can also develop in GD treated with pharmacological treatment for a long time.
Untreated Graves’ orbitopathy may resolve without permanent sequelae, especially if it is mild, but in the active severe form, the risk of permanent damage to the orbital tissues (impaired eye mobility and visual acuity, and even blindness) is high, especially in malignant exophthalmia. Early treatment (in the active phase of the disease) often avoids serious consequences. If the advancement of exophthalmos and the involvement of the soft tissues and muscles of the eyeball are significant, or if the cornea is affected or the optic nerve is compressed, the risk of permanent damage to the organ of vision and permanent changes in the patient’s appearance is high. Strabismus and exophthalmia are surgically corrected after disappearance of the active phase of the disease [10].
3 Toxic Multinodular Goiter (Plummer’s Disease)
Toxic nodular (hyperactive) goiter (TMNG), also known as Plummer’s disease, is a disease in which hyperthyroidism develops on the basis of nodular hyperplasia of the thyroid gland and there is no autoimmune background. A characteristic feature is the presence of a nodule or nodules that secrete thyroid hormones in an autonomous manner independent of TSH regulation.
3.1 Pathogenesis
A toxic multinodular goiter contains multiple autonomously functioning nodules, resulting in hyperthyroidism. These nodules function independently of TSH regulation and are almost always benign. However, nonfunctioning thyroid nodules in the same goiter may be malignant.
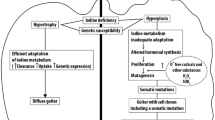
The etiologic factors involved in the formation of a multinodular goiter include an inherent functional heterogeneity of thyroid follicles, the effect of growth factors and goitrogens, iodine deficiency, and genetic abnormalities that include somatic activating mutations of genes that regulate thyroid growth and hormone synthesis [35,36,37]. It is postulated that in iodine-deficient areas, chronic TSH stimulation increases the replication of the follicular cells and results in the appearance of expression of mutations in the TSH receptor gene [38, 39]. Nodular growth results from hyperplasia of clusters of follicular cells with abnormal growth potential at scattered sites in the thyroid gland. The final phase in the evolution of goiter is when nodules become autonomous, progress form a nontoxic to a toxic state with loss of TSH regulation and nonsuppressability with thyroxine administration. A nontoxic multinodular goiter is present for 10–25 years before becoming toxic. During this evolution, patients with nontoxic multinodular goiter have a high prevalence of subclinical hyperthyroidism [40,41,42].
3.2 Epidemiology
Autonomous nodules are the second most common overall cause of thyrotoxicosis, accounting for 5–15% of all causes of hyperthyroidism [43]. The prevalence of TMNG increases with age, making it the most common cause of thyrotoxicosis in the elderly, whereas solitary toxic adenomas are more often found in young individuals [35, 36, 44, 45].
3.3 Clinical Presentation
Hyperthyroidism usually develops very slowly (the onset of an overt disease is preceded by subclinical hyperthyroidism in nodular goiter), but it can also occur suddenly, under the influence of a large dose of iodine (iodine-induced thyrotoxicosis, or the Jod-Basedow phenomenon), administered, for example, in the form of a radiological contrast agent or contained in drugs (amiodarone or some disinfectants) [46]. The growth of a goiter or the appearance of a lump(s) often goes unnoticed by the patient. If the goiter is large, there may be a feeling of pressure in the neck (compressive symptoms attributable to mass effect, particularly in case of substernal extension), difficulty in breathing (due to trachea deviation or narrowing), or, more rarely, dysphagia and coughing. The hyperthyroidism is usually less severe than in GD. Over manifestations of thyrotoxicosis are often masked in older patients, whereas cardiac manifestations, such as atrial fibrillation, tachycardia, congestive heart failure, and accelerated angina pectoris, are predominant. Unexplained weight loss, anxiety, insomnia, and muscle wasting are also more likely to occur in the elderly hyperthyroid patients [47].
3.4 Diagnosis
Visible or palpable nodular goiter of various sizes (the primary importance is the finding of 2 or more nodules or ultrasound), with laboratory findings of hyperthyroidism: marked suppression of TSH secretion and markedly increased concentrations of FT4 and FT3 or only FT3 in the serum, are sufficient findings to make a diagnosis of TMNG.
As TMNG is often asymmetrical, and substernal extension may make it less apparent on physical examination (◘ Fig. 3.6), thyroid imaging tests are helpful. Ultrasound of the thyroid gland allows to measure the size of the goiter and accurately assess the nodules. Thyroid scintigraphy enables an accurate assessment of tracer uptake and distribution and the diagnosis of autonomic nodules, which is the basis for the decision to treat with 131I [47].
An autonomic nodule (hot in scintigraphy), in diameter <3 cm on ultrasound, usually does not require fine-needle aspiration biopsy (FNAB) due to a very low risk of cancer; in other focal lesions, indications for FNAB are the same as in nontoxic nodular goiter.
A CT scan may be necessary to assess for substernal extension, and trachea narrowing, but iodine contrast should only be administered with caution (◘ Fig. 3.7).
3.5 Treatment
There are two main goals for treatment in patients with TMNG: to eliminate an autonomously functioning thyroid tissue and to alleviate compressive symptoms.
Pharmacological treatment with antithyroid drugs (methimazole) allows for controlling the symptoms of hyperthyroidism, but its withdrawal always leads to the relapse of the hyperthyroidism (after a few days to several weeks) [48]. The beta-blocker is used similarly to other types of hyperthyroidism, but in TMNG, it is needed more often and in higher doses than in GD due to the greater severity of cardiac symptoms.
Hence, radical treatment is necessary and definitive treatment options are surgical (subtotal or total thyroidectomy) or radioiodine ablation. The choice of method is individual for each patient [2, 37, 43, 47]. Nevertheless, most recent data indicate on safety of long-term, low-dose methimazole treatment for TMNG for 60–100 months, which is not inferior to RAI treatment [49].
3.5.1 Radioactive Iodine (RAI)
The radiosensitivity of autonomic nodules is lower than in Graves’ disease (this is taken into account when planning RAI treatment). Inactive nodules do not respond to treatment, and most active nodules do not disappear, but only shrink, but the hyperthyroidism subsides (although sometimes 131I must be readministered after 6 months). Small goiter with no signs of malignancy risk is more inclined to treatment with 131I, as are contraindications for surgery (most elderly patients with comorbidities that increase their risk for surgery). Because TMNG contains nonfunctioning nodules and areas of fibrosis and calcifications, RAI treatment is only variably effective in reducing goiter size and relieving compressive symptoms. However, a reduction in thyroid volume and an increase in the cross-sectional area of the tracheal lumen have been demonstrated by MRI imaging after RAI treatment [50,51,52].
3.6 Indications for Surgery and Surgical Details
Surgical resection is the preferred treatment option in patients with a nodule with cytological or clinical features of malignancy and also in patients with a large goiter giving compression symptoms, especially in the presence of inactive thyroid nodules, unless there are contraindications for surgery [2, 48, 53]. The operation is possible only after euthyroid state is achieved. Treatment with methimazole should be stopped on the day of surgery, and the dose of beta-blocker should be gradually reduced to discontinue it within a few days after surgery. Potassium iodide should not be given before surgery.
The presence of substernal thyroid extension or airway obstruction is a relative contraindication for RAI because of the potential for transient increase in size of the goiter and worsening of airway compromise through RAI-induced transient radiation thyroiditis. The extent of surgery should be total (◘ Fig. 3.8) or near-total thyroidectomy. Subtotal thyroidectomy (Dunhill operation) may be considered in patient who experienced intraoperative loss of signal of neuromonitoring after removal of the first-attempted, and usually, dominant thyroid lobe (a staged thyroidectomy) [54]. The risk of permanent surgical morbidity is low (<1–2%) if surgery is performed by a high-volume thyroid surgeon [55,56,57,58,59,60]. Surgery was also demonstrated to be more cost-effective than treatment with RAI for TMNG in patients aged 62 years or less [59]. Surgery also allows for curing incidental concomitant thyroid cancer, which was reported in 3–9% of patients with TMNG [61, 62]. In general, age older than 50 years, cold nodule(s), and ultrasound characteristics of nodule(s) may preselect patients with higher risk of malignancy for surgical treatment of TMNG [63, 64].
3.7 Prognosis
In untreated TMNG, the risk of arrhythmias and other cardiovascular complications as well as thyroid crisis is increased. The risk of cancer is the same as with other forms of nodular goiter. Recurrence of thyrotoxicosis after incomplete surgery for TMNG was reported to be as high as 50–78%. Hence, total or near-total thyroidectomy is the preferred surgical approach in patients not eligible for RAI treatment [55,56,57,58,59,60].
Answers to the Questions
1. (d); 2. (e); 3. (c); 4. (e); 5. (d); 6. (e); 7. (e); 8. (e); 9. (a); 10. (e); 11. (a)
References
Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T-4 and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–99.
Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–421.
Morshed SA, Latif R, Davies TF. Delineating the autoimmune mechanisms in Graves’ disease. Immunol Res. 2012;54(1–3):191–203.
Smith TJ, Hegedüs L. Graves’ disease. N Engl J Med. 2016;375(16):1552–65.
McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42(2):252–65.
McLeod DS, Caturegli P, Cooper DS, Matos PG, Hutfless S. Variation in rates of autoimmune thyroid disease by race/ethnicity in US military personnel. JAMA. 2014;311(15):1563–5.
Antonelli A, Ferrari SM, Ragusa F, et al. Graves’ disease: epidemiology, genetic and environmental risk factors and viruses. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101387.
Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21(2):168–99.
Kubota S, Gunji K, Stolarski C, Kennerdell JS, Wall J. Reevaluation of the prevalences of serum autoantibodies reactive with “64-kd eye muscle proteins” in patients with thyroid-associated ophthalmopathy. Thyroid. 1998;8(2):175–9.
Bartalena L, Baldeschi L, Boboridis K, et al. The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J. 2016;5(1):9–26.
Burch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves’ disease. J Clin Endocrinol Metab. 2012;97(12):4549–58.
Wartofsky L, Glinoer D, Solomon B, et al. Differences and similarities in the diagnosis and treatment of Graves’ disease in Europe, Japan, and the United States. Thyroid. 1991;1(2):129–35.
Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH. 2018 European thyroid association guideline for the management of Graves’ hyperthyroidism. Eur Thyroid J. 2018;7(4):167–86.
Moon JH, Yi KH. The diagnosis and management of hyperthyroidism in Korea: consensus report of the Korean Thyroid Association. Endocrinol Metab (Seoul). 2013;28(4):275–9.
In H, Pearce EN, Wong AK, Burgess JF, McAneny DB, Rosen JE. Treatment options for Graves’ disease: a cost-effectiveness analysis. J Am Coll Surg. 2009;209(2):170–9.
Masiello E, Veronesi G, Gallo D, et al. Antithyroid drug treatment for Graves’ disease: baseline predictive models of relapse after treatment for a patient-tailored management. J Endocrinol Investig. 2018;41(12):1425–32.
Yoshihara A, Noh J, Yamaguchi T, et al. Treatment of Graves’ disease with antithyroid drugs in the first trimester of pregnancy and the prevalence of congenital malformation. J Clin Endocrinol Metab. 2012;97(7):2396–403.
Andersen SL, Olsen J, Wu CS, Laurberg P. Birth defects after early pregnancy use of antithyroid drugs: a Danish nationwide study. J Clin Endocrinol Metab. 2013;98(11):4373–81.
Hertz S, Roberts A. Radioactive iodine in the study of thyroid physiology. The use of radioactive iodine therapy in hyperthyroidism. J Am Med Assoc. 1946;131:81–6.
Bogazzi F, Giovannetti C, Fessehatsion R, et al. Impact of lithium on efficacy of radioactive iodine therapy for Graves’ disease: a cohort study on cure rate, time to cure, and frequency of increased serum thyroxine after antithyroid drug withdrawal. J Clin Endocrinol Metab. 2012;95(1):201–8.
Laurberg P, Wallin G, Tallstedt L, et al. TSH-receptor autoimmunity in Graves’ disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur J Endocrinol. 2008;158(1):69–75.
Ponto KA, Zang S, Kahaly GJ. The tale of radioiodine and Graves’ orbitopathy. Thyroid. 2010;20(7):785–93.
Bhargav PRK, Sabaretnam M, Kumar SC, Zwalitha S, Devi NV. Regression of ophthalmopathic exophthalmos in Graves’ disease after total thyroidectomy: a prospective study of a surgical series. Indian J Surg. 2017;79(6):521–6.
Lorenz K, Raffaeli M, Barczyński M, Lorente-Poch L, Sancho J. Volume, outcomes, and quality standards in thyroid surgery: an evidence-based analysis-European Society of Endocrine Surgeons (ESES) positional statement. Langenbeck’s Arch Surg. 2020;405(4):401–25.
Yilmaz Y, Kamer KE, Ureyen O, Sari E, Acar T, Karahalli O. The effect of preoperative Lugol’s iodine on intraoperative bleeding in patients with hyperthyroidism. Ann Med Surg (Lond). 2016;9:53–7.
Whalen G, Sullivan M, Maranda L, Quinlan R, Larkin A. Randomized trial of a short course of preoperative potassium iodide in patients undergoing thyroidectomy for Graves’ disease. Am J Surg. 2017;213(4):805–9.
Erbil Y, Ozluk Y, Giriş M, et al. Effect of Lugol’s solution on thyroid gland blood flow and microvessel density in the patients with Graves’ disease. J Clin Endocrinol Metab. 2007;92(6):2182–9.
Mercier F, Bonal M, Fanget F, et al. Does surgery without Lugol’s solution pretreatment for graves’ disease increase surgical morbidity? World J Surg. 2018;42(7):2123–6.
Jörtsö E, Lennquist S, Lundström B, Norrby K, Smeds S. The influence of remnant size, antithyroid antibodies, thyroid morphology, and lymphocytic infiltration on thyroid function after subtotal resection for hyperthyroidism. World J Surg. 1987;11(3):365–71.
Alsnea O, Clark OH. Treatment of Graves’ disease: the advantages of surgery. Endocrinol Metab Clin N Am. 2000;29(2):321–37.
Barczyński M, Konturek A, Hubalewska-Dydejczyk A, Gołkowski F, Nowak W. Randomized clinical trial of bilateral subtotal thyroidectomy versus total thyroidectomy for Graves’ disease with a 5-year follow-up. Br J Surg. 2012;99(4):515–22.
Maurer E, Maschuw K, Reuss A, et al. Total versus near-total thyroidectomy in Graves’ disease: results of the randomized controlled multicenter TONIG-trial. Ann Surg. 2019;270(5):755–61.
Liu ZW, Masterson L, Fish B, Jani P, Chatterjee K. Thyroid surgery for Graves’ disease and Graves’ ophthalmopathy. Cochrane Database Syst Rev. 2015;(11):CD010576.
Bobanga ID, McHenry CR. Treatment of patients with Graves’ disease and the appropriate extent of thyroidectomy. Best Pract Res Clin Endocrinol Metab. 2019;33(4):101319.
Berghout A, Wiersinga WM, Smits NJ, Touber JL. Interrelationships between age, thyroid volume, thyroid nodularity, and thyroid function in patients with sporadic nontoxic goiter. Am J Med. 1990;89(5):602–8.
Gozu HI, Lublinghoff J, Bircan R, Paschke R. Genetics and phenomics of inherited and sporadic non-autoimmune hyperthyroidism. Mol Cell Endocrinol. 2010;322(1–2):125–34.
Siegel RD, Lee SL. Toxic nodular goiter: toxic adenoma and toxic multinodular goiter. Endocrinol Metab Clin N Am. 1998;27(1):151–68.
Krohn K, Führer D, Bayer Y, et al. Molecular pathogenesis of euthyroid and toxic multinodular goiter. Endocr Rev. 2005;26(4):504–24.
Vitti P, Rago T, Tonacchera M, Pinchera A. Toxic multinodular goiter in the elderly. J Endocrinol Invest. 2002;25(10 suppl):16–18.
Vadiveloo T, Donnan PT, Cochrane L, Leese GP. The Thyroid Epidemiology, Audit, and Research Study (TEARS): the natural history of endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab. 2011;96(1):E1–8.
Rosario PW. Natural history of subclinical hyperthyroidism in elderly patients with TSH between 0.1 and 0.4 mIU/l: a prospective study. Clin Endocrinol (Oxf). 2010;72(5):685–8.
Das G, Ojewuyi TA, Baglioni P, Geen J, Premawardhana LD, Okosieme OE. Serum thyrotrophin at baseline predicts the natural course of subclinical hyperthyroidism. Clin Endocrinol. 2011;77(1):146–51.
Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ. 2006;332(7554):1369–73.
Laurberg P, Pedersen KM, Vestergaard H, Sigurdsson G. High incidence of multinodular toxic goitre in the elderly population in a low iodine intake area vs. high incidence of Graves’ disease in the young in a high iodine intake area: comparative surveys of thyrotoxicosis epidemiology in East-Jutland Denmark and Iceland. J Intern Med. 1991;229(5):415–20.
Abraham-Nordling M, Byström K, Törring O, et al. Incidence of hyperthyroidism in Sweden. Eur J Endocrinol. 2011;165(6):899–905.
Fradkin JE, Wolff J. Iodide-induced thyrotoxicosis. Medicine (Baltimore). 1983;62(1):1–20.
Nayak B, Hodak SP. Hyperthyroidism. Endocrinol Metab Clin N Am. 2007;36(3):617–56.
van Soestbergen MJ, van der Vijver JC, Graafland AD. Recurrence of hyperthyroidism in multinodular goiter after long-term drug therapy: a comparison with Graves’ disease. J Endocrinol Investig. 1992;15(11):797–800.
Azizi F, Takyar M, Madreseh E, Amouzegar A. Treatment of toxic multinodular goiter: comparison of radioiodine and long-term methimazole treatment. Thyroid. 2019;29(5):625–30. https://doi.org/10.1089/thy.2018.0397.
Huysmans DA, Hermus AR, Corstens FH, Barentsz JO, Kloppenborg PW. Large, compressive goiters treated with radioiodine. Ann Intern Med. 1994;121(10):757–62.
Nygaard B, Hegedüs L, Ulriksen P, Nielsen KG, Hansen JM. Radioiodine therapy for multinodular toxic goiter. Arch Intern Med. 1999;159(12):1364–8.
Schiavo M, Bagnara MC, Camerieri L, et al. Clinical efficacy of radioiodine therapy in multinodular toxic goiter, applying an implemented dose calculation algorithm. Endocrine. 2015;48(3):902–8.
Ríos A, Rodríguez JM, Balsalobre MD, Torregrosa NM, Tebar FJ, Parrilla P. Results of surgery for toxic multinodular goiter. Surg Today. 2005;35(11):901–6.
Schneider R, Randolph GW, Dionigi G, et al. International neural monitoring study group guideline 2018 part I: staging bilateral thyroid surgery with monitoring loss of signal. Laryngoscope. 2018;128(Suppl 3):S1–S17.
Miccoli P, Antonelli A, Iacconi P, Alberti B, Gambuzza C, Baschieri L. Prospective, randomized, double-blind study about effectiveness of levothyroxine suppressive therapy in prevention of recurrence after operation: result at the third year of follow up. Surgery. 1993;114(6):1097–101.
Agarwal G, Aggarwal V. Is total thyroidectomy the surgical procedure of choice for benign multinodular goiter? An evidence-based review. World J Surg. 2008;32(7):1313–24.
Ríos A, Rodríguez JM, Galindo PJ, Montoya MJ, Canteras M, Parrilla P. Surgical treatment of multinodular goiter in young patients. Endocrine. 2005;27(3):245–52.
Moalem J, Suh I, Duh QY. Treatment and prevention of recurrence of multinodular goiter: an evidence-based review of the literature. World J Surg. 2008;32(7):1301–12.
Vidal-Trecan GM, Stahl JE, Eckman MH. Radioiodine or surgery for toxic thyroid adenoma: dissecting in important decision. A cost-effectiveness analysis. Thyroid. 2004;14(11):933–45.
Barczyński M, Konturek A, Hubalewska-Dydejczyk A, Gołkowski F, Nowak W. Ten-year follow-up of a randomized clinical trial of total thyroidectomy versus Dunhill operation versus bilateral subtotal thyroidectomy for multinodular non-toxic goiter. World J Surg. 2018;42(2):384–92.
Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157–60.
Alexopoulou O, Beguin C, Buysschaert M, et al. Predictive factors of thyroid carcinoma in non-toxic multinodular goiter. Acta Clin Belg. 2004;59(2):84–9.
Senyurek Giles Y, Tunca F, Boztepe H, Kapran Y, Terzioglu T, Tezelman S. The risk factors for malignancy in surgically treated patients for Graves’s disease, toxic multinodular goiter, and toxic adenoma. Surgery. 2008;144(6):1028–36.
Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Barczyński, M. (2021). Graves’ Disease and Toxic Nodular Goiter (Plummer’s Disease). In: Shifrin, A.L., Raffaelli, M., Randolph, G.W., Gimm, O. (eds) Endocrine Surgery Comprehensive Board Exam Guide. Springer, Cham. https://doi.org/10.1007/978-3-030-84737-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-84737-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84736-4
Online ISBN: 978-3-030-84737-1
eBook Packages: MedicineMedicine (R0)