Abstract
This chapter describes several major projects undertaken by Boojum Research since 1982 in Canada, Brazil, and Germany. These projects ran between 5 and 16 years. The biological polishing tool reduced the concentrations of arsenic and nickel in a 5 million cubic meter pit lake and also the concentrations of zinc and iron from the effluent of a gloryhole using a series of polishing ponds. In Germany, a constructed wetland with rooted vascular plants was expected to reduce the concentrations of radionuclides, arsenic and iron from the effluent of a uranium mine. The removal was inefficient. The settling pond of the constructed wetland was used as a pilot system, by suspending curtains and introducing Chara, the multitasking alga, to sequester contaminants.
Using Carbonaceous Phosphate Mining Waste (CPMW), Acid Reduction Using Microbiology (ARUM) and biological polishing, a complete decommissioning concept was developed for an operating zinc mine in Quebec. Similarly, all tools were partially scaled up at a copper-zinc mine in northern Ontario. Finally, a coarse coal pile in Nova Scotia was treated with CPMW and compared to other piles treated with limestone, and at a different Nova Scotian site, the tools were used to treat effluents from waste coal tailings.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Decommissioning
- Acid mine drainage
- Alkaline drainage
- Mine waste management
- Sulfide oxidation
- Carbonaceous phosphate mining wastes
- Ecological engineering
9.1 Arsenic and Nickel Removal from a Pit Lake – Saskatchewan
A mined-out uranium pit in northern Saskatchewan was force-flooded from an adjacent fishing lake. Once filled, the lake would hold 5 million m3, with the only influxes coming from precipitation. With environmental approval, the barrier between the pit lake and an adjacent fishing lake would be removed. The arsenic and nickel concentrations of 0.22 mg.L−1 and 0.26 mg.L−1, respectively, were low, but exceeded regulatory limits. Before the lake and the pit water could be combined, the arsenic and nickel concentrations had to be lowered. Reductions in these low concentrations were not only difficult to achieve with chemical treatment, but also expensive. If biological polishing could be used to reduce the concentration further, chemical treatment costs could be avoided. The company decided to fund a detailed investigation using biological polishing to remove the nickel and arsenic (Fig. 9.1).
Sediment traps used to collect and analyze particulates. (a) The traps on the left were installed at depths between 3 and 45 m. The traps closest to the boat had a fine layer of clay which was not visible on any of the following traps. (b) Photograph of the 3 m depth traps one year after flooding, showing the high quantity of suspended solids (Boojum Research, 1994; Cao & Kalin, 1999; Kalin et al., 2001). (Photographs by Boojum Research)
Four sets of sedimentation traps were placed at various depths to collect suspended solids. The traps suspended at 3 m (close to the extinction of light) collected particulates and algae, which were subjected to SIMS microscopy (Secondary-Ion Mass Spectroscopy). This method examines the surface of materials to a depth of 1 to 2 nanometers. From the data generated by SIMS, a schematic was constructed (Fig. 9.2).
Schematic of particle aggregation based on SIMS microscopy, depicting algae as flocculating agents. The green circles and spirals are algal cells and EPS molecules, reddish brown shapes symbolize iron hydroxide, yellowish shapes are clay particles, pink shapes are nickel hydroxide, and violet crosses represent arsenic.
The force-flooding produced high concentrations of total suspended solids (TSS) in the first 2 years. The first summer after flooding, the particulates were mainly inorganic clay which gave way in the second year to an algal bloom of Dictyosphaerium sp. (Fig. 9.3a, b). These algae form colonies of cells embedded in a dense extracellular polysaccharide (EPS) sheath. The dense sheath around the algae was also a ‘magnet’ for inorganic particulates. The algae were acting like living flocculants, removing inorganic particulates, arsenic and nickel from the water column.
If the algae and associated particulates were relegating the contaminants to the sediments, the nickel and arsenic removal from the water column should balance with the arsenic and nickel found in the sediments. To produce this mass balance, bottom sediments were retrieved at about 35 m depth in several locations on the pit lake bottom with an Eckman grab sampler. The top 5 cm were retrieved from the sampler, dried and submitted for elemental analysis, pooling the samples. The concentrations of relevant elements in the sediment were compared to the sum of the elemental concentrations determined from the sedimentation traps over eight years. The results were close for the two contaminants, As and Ni (Table 9.1).
The elemental concentrations in the sediment traps compared well with the same elemental concentrations in the top 5 cm of the bottom sediment (Table 9.1). Exceptions were aluminum and iron, with higher concentrations in the sediment than found in the traps. Aluminum discrepancies can be explained by the presence of a layer of clay which was placed at the bottom of the pit to cover hazardous materials before filling. The higher iron concentrations could be explained by grabs that caught muskeg sediment that would have increased iron concentrations. Given these numbers, though, the mass balance survived the scrutiny of the company engineers.
The next step was to support and possibly increase the growth of the phytoplankton in the pit lake. In the first couple of years, the phytoplankton population density was relatively low, and a nutrient limitation was suspected. Using a simple Redfield ratio (Falkowski, 2000), it was estimated that an addition of 720 kg of calcium nitrate would probably serve to maintain or increase the biomass, thereby increasing the polishing capacity (Boojum Research Ltd., 1997; Kalin et al., 2002; Kalin & Wheeler, 2013). One to two fertilizer applications over time would suffice, since growth and decay of the biomass would recycle the nutrients within the pit lake. It is unknown if this recommendation was implemented, as the project ended in 2003.
9.2 Zinc Removal from Circum-Neutral Gloryhole Effluent – Newfoundland
Boojum Research was retained in 1988 to evaluate the applicability of using ecological engineering measures at a very complex mining system in Newfoundland. The old, polymetallic mine operated from the 1920s to 1984, and used the historic gloryhole method of mining. The ore was taken along the ore veins, dumped on rail cars into a gloryhole, and then transported through a haulage tunnel to the mill. Many orebodies were connected to the haulage tunnel, which, at the time of decommissioning, was referred to as the drainage tunnel. The major contaminant was zinc, which would precipitate with oxidized iron at neutral pH. At decommissioning, all workings were hydraulically connected and nearly all discharged to the Oriental East Pit (OEP), and thence to the Buchans River. Adjacent to the OEP (pH 5.8 to 7.0) is the Oriental West Pit (OWP; pH range between 2.7 and 3.4) which has no direct outflow. Both pit lakes did not stratify, but froze during the winter. The combined effluents exiting from the OEP contained between 20 and 25 mg.L−1 zinc throughout the year.
One of the goals of the project was to lower the concentrations of zinc that flowed into the Buchans River from the OEP. Since there was a large meadow downstream from the OEP, Boojum decided to use biological polishing to treat the effluent. A series of 6 small ponds were constructed and filled with cut brush as substrate for periphyton growth (Fig. 9.4).
(a) Martin Smith, a Boojum researcher, working on one of the pilot ponds. Note brush in the pond, used as a substrate for periphyton growth. (b) A schematic showing the pilot-scale ponds in yellow, and the scale-up in blue. The series of 6 yellow circles extending through pools 14 and 15 were the pilot ponds which served as design criteria for the scale up. First to be scaled up were pools 10–13 (in lighter blue), followed later by pools 14–17 (darker blue). Yellow arrows denote direction of flow. The three yellow circles in the lower left were ARUM test cells treating the drainage from the waste rock pile. Organics were added to a completely rust-covered sphagnum bog. (c) Aerial photograph of the OEP and polishing ponds in the meadow below the OEP. All photographs by Boojum Research.
These pilot, biological polishing ponds were shallow, and froze during the winter. Later, these 6 pilot ponds were expanded to cover the entire meadow. The scaled-up ponds worked only in the summer dropping the zinc concentration to 5 mg.L−1 or below. Winter removal rates were essentially absent. Winter results were attributed to ice which covered the OEP, preventing oxidation and particle formation. Since the iron could not oxidize, iron and zinc did not precipitate, hence no zinc removal. Periphyton on the cut brush collected the iron/zinc particles in the summer reducing zinc and iron concentrations (Fig. 9.5).
The two scaled-up ponds closest to the OEP, ponds 10 and 14, would have to cleaned out periodically, as they would fill up with precipitate quite rapidly (see Fig. 9.6). But, before the ponds could fill with precipitate, the project came to an abrupt halt, when the mine manager passed away suddenly in 1995. Shortly after, the responsibility for the site changed hands and a consultancy took over management. Boojum obtained system monitoring data from the Newfoundland government in 2007, when Boojum representatives visited the site. Upon reviewing the site, they were stunned, as the alder cuttings, serving as substrates for the periphyton had been removed. The locals, who had worked with Boojum in the past reported that the consultancy had requested that all brush be cleaned out of the pools. Shortly thereafter, a truckload of sugar arrived with instructions to the local caretaker to deposit it into the OEP. Sugar dissolves and leaves with the water! It was clear from the government monitoring data, that the ponds had ceased working sometime after the consultancy took over management. Ecological systems need to be monitored, and managed with knowledge of their function.
The effluents from the OEP were not the only challenge. Zinc ore concentrate (55% zinc) had spilled onto a water-saturated muskeg area below the mill. The acidity values ranged between 1,000 and 10,000 mg.L−1 CaCO3. Here, Boojum recommended pilot tests with CPMW from a shutdown phosphate plant available on the island. It was anticipated that an application might lead to a hardpan forming a drain which would precipitate some of the zinc along the way.
The mine manager had established several 5 L open bottom buckets with different addition ratios between (1:4 and 1:20) over the muskeg area. The buckets were sent to the Boojum laboratory after a short exposure of 10 days and after a 3 year exposure in the muskeg area. In the laboratory, the same routine was used as described in Chap. 8 with tailings. Supernatants were produced and the samples were kept under oxidizing conditions. The experiment was terminated after 1200 days (Kalin, 2004). The most important observations were that, regardless of the mixing ratios, the pH and acidity in the supernatants remained constant or improved compared to controls (Fig. 9.7a). An application of CPMW would produce lower acidity and zinc concentrations being discharged into the river. Shortly before his death, the manager had the local CPMW spread onto the muskeg area with the concentrate spill (Fig. 9.8). The project is described in detail in Kalin (2009).
Acidity and pH of bucket samples from the concentrate spill. The acidity (a) and pH (b) are shown for different ratios of concentrate soil mixtures (w:w, soil:CPMW) over time. Red lines are two different controls, i.e., no additions of CPMW. These data show a decline (control 2) in pH after a slight initial increase over the time, or almost no change in pH for control 1. Acidity of controls remained fairly constant after an initial increase. The best performance was noted in sample number one with ratio 1:4, indicating that, once reacted, no further acidity was produced
9.3 Limestone and CPMW Application to Coal Waste Piles – Nova Scotia
At a coal mining and processing facility in Nova Scotia, metallurgical and lower grade coal was produced. Four relatively large waste piles were set up as test piles, where management was addressing various methods of integrating a layer of limestone to reduce acid mine drainage. A fifth pile was added, to which CPMW was supplemented in the same configuration as the other 4 piles (Fig. 9.9a). All five piles were heavily instrumented with lysimeters to collect drainage. The work was carried out by an engineering consultancy.
Experiments using CPMW on coal piles in Nova Scotia. (a) Schematic for the application of both CPMW and of differently-compacted limestone, testing construction of coarse coal waste piles. (b) An autopsy of all piles showed the pile containing CPMW produced a hardpan, which reduced infiltration, preventing acidic effluents. Note hardpan layer at bottom of excavated pit with cracks which were described as self-healing, i.e., closed at bottom of crack. (c) Laboratory columns with hardpan formation seen in rust-colored layers set up in the Boojum laboratory. Column photograph by Boojum Research, coal pile photograph and schematic by Fred Baechler
Four piles produced drainage, but the fifth pile, with CPMW, produced no or very little drainage. Instead, the layer of CPMW produced large erosion channels along the sides of the pile. The same consultancy that had set up the test piles was hired by Boojum to perform an autopsy of all test piles (Fig. 9.9b). An extensive hard pan had formed within the CPMW pile, but was not found in any of the piles where limestone was added. The hardpan was extensively documented, and may have been self-sealing (Fig. 9.9b; Baechler, 1997).
Concurrently, Boojum set up laboratory columns which were monitored periodically by adding water and collecting effluent (Fig. 9.9c). The two columns with the yellow or brown stripes produced less and less drainage, eventually plugging. Boojum monitored the collected drainage from the columns for several years, but failed to obtain funding to analyze the drainage and complete the data interpretation. Both the field piles and laboratory columns demonstrated that CPMW could form hardpans, preventing the penetration of precipitation.
9.4 Tailings Hardpan Development Ontario
In general, a hardpan in tailings is desirable, as it prevents or slows penetration of precipitation, and thus, the oxidation of the sulfides. Ideally, this hardpan should be generated in the tailings just before the facility is shut down. Boojum experimented using CPMW in both fresh pyrrhotite tailings and aged uranium tailings.
At a mine site in Sudbury, Ontario. Boojum set up experiments with CPMW plowed into the fresh tailings (Fig. 9.10a). At this site, straw and grass seed were crimped into the tailings. At the aged tailings site (a uranium mine in Elliot Lake, Ontario; Fig. 9.10b), instead of straw, horse manure was crimped with the grass seed. CPMW was tilled into the tailings with a handheld plow. These measures (grass seed, horse manure, and straw) were used to prevent erosion. Samples from the plots were collected after a little more than 3 years in the field.
(a) Fresh pyrrhotite tailings in N. Ontario. CPMW was plowed into the tailings in the same fashion as lime would be integrated in tailings for revegetation. Straw was crimped into the surface and the tailings were seeded to control erosion. (b) The plots on abandoned uranium tailings. The control plots are shown in the foreground, while the plots with the highest CPMW and organic applications are in the dark area with a Boojum employee. All plots were seeded with manure and grass seed. (Photographs by Boojum Research)
The application of CPMW was expected to form a hardpan to reduce infiltration of rain and snowmelt. After 3 years, the markers delineating the plots had been lost. Since it was manually impossible to dig up the plots looking for the hardpan, an EM39 conductivity meter was used. This instrument performs a geophysical survey with fixed frequency electromagnetic (EM) profiling techniques employing a Geonics EM39 instrument. The EM survey was supposed to find differences in apparent conductivity in plots with and without CPMW additions. The instrument provided measurements of both the quad-phase (conductivity) and in-phase (magnetic susceptibility) components within two distinct depth ranges, simultaneously. However, the apparent conductivity, ECa, of the plots, which should have indicated the hardpan, failed because the surrounding conductivity was out of range for the instrument, i.e., the control plots and the surroundings were highly conductive. Perhaps if a backhoe had been available, the hardpan would have been found. Different EMs (EM 31- DL Em31 and Em34-3 instruments were very successful on the mine site locating boreholes, shafts and adits where acid drainage production occurred (Kalin & Pawlowski, 1994; Hutchinson & Barta, 2000).
While a hardpan could not be confirmed in the fresh and aged tailings, its presence in the Nova Scotian coal piles and in Newfoundland concentrate spill suggests that this approach was worth trying on a larger scale. The application of CPMW has to be simple, with easily-available equipment. The dosage of CPMW was estimated by the mine operator based on the same cost of hauling and distributing limestone. It is hoped that commercial applications of CPMW to actual, large-scale waste rock piles and tailings will reduce the production of weathering products leading to an improved quality of drainage, but these tests have yet to be performed.
9.5 Decommissioning Concepts Applying ARUM, CPMW and Biological Polishing – Quebec
Both tools, ARUM and biological polishing, were sufficiently understood at the time Boojum received a contract to develop a decommissioning plan for a zinc mine in Quebec. However, at that time, there was only limited evidence to suggest that microbes and phosphate wastes might be playing important roles slowing pyrite oxidation. The literature on phosphate and acid mine drainage was growing. Evangelou (1995), Georgopoulou et al. (1996), and Chen et al. (1997) carried out intensive studies on pyrite in coal, postulating that direct adsorption of phosphate molecules onto iron atoms on the pyrite surface leads to the subsequent elimination of electron transfer between pyrite and oxidizing agents. Boojum thought that applications of carbonaceous phosphate mine waste (CPMW) to mine waste rock and tailings might be beneficial in the reduction of sulfide oxidation. Thus, CPMW application became the cornerstone of the decommissioning scenario.
For Boojum, this mine waste management area in Quebec was the first opportunity to present an environmental management system for an operating mine (Boojum Research, 1992). All Boojum’s ecological tools were needed, but, most importantly, tools for reducing sulfide oxidation. Although the waste rock pile was just about starting the second lift, acid drainage was already destroying the muskeg (Fig. 9.11). The photograph shows the brown dead muskeg where drainage took its toll. The green shrubs mark the future path of the ARD drainage. In the distance, a light brown line can be seen on the horizon. This was a new ditch which would collect future drainage to the chemical treatment plant. A thorough hydrological reconnaissance prior to locating the waste rock might have prevented or lessened the impact.
Cost associated with conventional decommissioning options for a zinc mine in Quebec were estimated at the start of the 1990s. Operation estimated that decommissioning costs would be from $10 to $50 million for the tailings, and $5 to $15 million for the waste rock pile. The mine’s owner engaged the services of Boojum Research in 1990 to identify a less costly decommissioning scenario. Ideally, this scenario would not include the operation of a conventional treatment plant, which would be operated in perpetuity.
A plan by the mine’s owner to neutralize the acid mine drainage produced during operation would require approximately 5.7 million tonnes of lime over 285 years and generate 30 million m3 of sludge. The costs and sludge production estimates were provided by BP Selco. These outrageous numbers argued for a more sustainable and less costly alternative. An ecological engineering decommissioning concept was developed for the tailings and waste rock management areas, based on an assessment of an existing water quality monitoring program.
The decommissioning scenario Boojum suggested included adding a layer of granular CPMW to each waste rock lift. Ditches would be filled with haybales, initially to reduce the iron loading to the treatment plant. In time, these ditch treatment cells would be covered with floating, living islands, like those described in Chap. 6. In this proposal, Boojum would build ARUM cells to treat ARD in the perimeter ditch. Effluents would first encounter a cell containing CPMW to precipitate oxidized iron. The effluents would then enter several ARUM cells to remove acidity and metals. Finally, the ‘cleaned’ effluents would be directed to the finished pit. A schematic of the proposed system is shown in Fig. 9.12.
To test the CPMW application to waste rock, three tonnes of variously-sized rocks were shipped from the mine to the Boojum facility in Toronto. Rocks were distributed into 12, 55-gallon drums. CPMW was distributed in various ways to the drums. The drums sat outside for about 3 years, as natural precipitation drained through them. Details of this experiment are presented in Chap. 8.
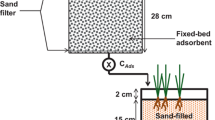
Another experiment was set up on site in Quebec. This system was designed to test applications of CPMW, ARUM and biological polishing to the mine’s effluents. The pilot treatment system is shown in Fig. 9.13. Here, effluents would enter a tank with CPMW, removing much of the oxidized iron. The water would then be processed first in a biological polishing tank, followed by an ARUM tank, and then finally a 2nd biological polishing tank. This system was built and treatment was initiated when the mine was sold, and the project terminated.
The other major source of contaminated effluents was the tailings. In the decommissioning scenario developed for the mine, fresh tailings would be plowed with CPMW and seeded with grass. Boojum was given a completed section of tailings pond, together with a spigot line for experimentation.
Here, the transport of water through the tailings can be reduced with the formation of a hardpan within 0.5 m depth of the tailings surface (Fig. 9.14). The tailings above the hardpan are tilled with organics and grass seeds, as shown in the schematic shown in Fig. 9.14. Heterotrophic microbes living in the organics would remove oxygen from the upper strata of tailings. The combination of low oxygen and hardpan would restrict water and air access to the tailings, reducing the production of AMD.
(a) Schematic showing the concept of unvegetated (barren) tailings as they dry. Water and air enter drying cracks oxidizing pyrite in the tailings on the left. (b) Concept of tailings in which a layer of CPMW is added to fresh tailings, but buried to react in the vadose zone, as exemplified in section 9.6. The CPMW (phosphate hardpan) would initiate iron precipitation and, in part, neutralize the acid. Most important, though, would be the presence of heterotrophic microbes which would consume oxygen in the vadose zone
The first experiment on fresh tailings involved adding vegetation seeds to the spigot line. Then, as the tailings were laid down, the seeds would sprout and cover the tailings with vegetation. However, this result did not occur. Tests carried out with this option failed, as growth only occurred in cracks of the drying tailings (Fig. 9.15a).
Field experiments to grow grass and vegetation on fresh tailings. (a) Vegetation seeds were added to the fresh tailings spigot line. Results were not satisfactory, as vegetation only grew in the cracks. (b) The more satisfying efforts with straw mats and CPMW. (c) Boojum researcher, Martin Smith, monitoring the grass plots. (Photographs by Boojum Research)
Field trials were carried out on fresh tailings using erosion control mats. A plot 70 m long by 8 m wide was established in June 1990 over a moisture gradient which existed along the tailings slope (wet, moist, and dry). Verdyol™ strips (4.0 × 3.75 m) were used to blanket the tailings. They were further divided into areas with different arrays of seeds and CPMW. Water penetrating the tailings should result in development of a low permeability stratum (iron precipitate hardpan) beneath the vegetation layer. This will provide suitable conditions for heterotrophic (oxygen-consuming) bacteria in the root zone of the grass layer, conventionally established (Kalin et al. 1993; Fig. 9.15b). Tailings, shortly after discharge, are not yet totally solid, requiring researchers like Martin Smith to constantly move (Fig. 9.15c).
A reduction in the rate at which tailings oxidize might be achieved if a population of oxygen-consuming bacteria (heterotrophs) could be maintained in the root region of a vegetation cover. Heterotrophs growing in the root zone would consume the oxygen in the water entering the tailings. Such bacteria have much higher potential growth rates than chemo-autotrophs and other pyrite-oxidizing bacteria. Boojum then asked the question: are there any heterotrophic, oxygen-consuming microbes in fresh tailings? Hence the entire mill circuit was tested for their presence and viability. Water samples at various stations throughout the mill were plated onto commercial agar dishes, and the viable colonies were counted (Table 9.2). The results were clear, tailings have viable heterotrophic microbe populations.
9.6 CPMW and ARUM in a Coal, Tailings Dump in Nova Scotia
A coal, tailings dump in Nova Scotia produced acidic effluent, which was impacting the local vegetation (Fig. 9.16). Boojum was tasked with decommissioning the effluent stream. The concept consisted of ditches which were to pretreat the un-oxidized iron in the effluent with CPMW. The oxidized iron and other contaminants would then be treated downstream to remove acidity and metals using ARUM (Kalin 1993). Finally, as a last step, biological polishing would remove remaining contaminants. Fig. 9.16a shows the system in the first year after installation. At this point, iron was being oxidized throughout the ditch system, and fouling hay bales installed for ARUM. Installation of curtains in the first ditch (Fig. 9.16b), enhanced the oxidation process, allowing only oxidized iron and contaminants to enter ditches downstream. A test area with ARUM (and floating cattails) was planned once the system was working. The effectiveness was evident in the successively clearer water entering the proposed ARUM area (Fig. 9.16b).
A CPMW, ARUM, and biological polishing treatment system established on a coal tailings dump in Nova Scotia. (a) System shortly after installation. Iron oxidation was occurring throughout the ditch system. (b) System after installation of curtains and CPMW dikes were constructed with the coarse gravel to foster iron oxidation in the first cells only. ARUM cells are shown as clear of iron. (Photographs taken by Fred Baechler)
9.7 Biological Polishing of 226Ra, Iron and Arsenic – Germany
With the fall of the Berlin Wall, Wismut GmbH became responsible for the remediation of the East German uranium mining district in the Erzgebirge. Wismut had experimented with the classical wetland treatment for the removal of iron, arsenic, uranium and radium. They used plants rooted in wetland sediment which overwintered and regrew above-ground biomass every year. These aquatic plants are referred to in German as helophytes. The wetland was set up in an old swimming pool. One side housed a regular wetland with plants rooted in sediment. Iron oxidation was accomplished using cascade ‘waterfall’ on the left side of Fig. 9.17 shown as a green bar. The open water side of the pool was used to collect the iron precipitate. The results were not very satisfactory, and Boojum was ask to implement some of its ecologically engineered tools.
Wismut-designed mine water treatment system with rooted plants receiving water from the right side for treatment. On the right side, in the open water, Boojum recommended installing curtains for biofilm formation and floating cattail islands to address fine iron particles. In the foreground some cattail islands grew well, but others were plagued leaf lice. The light blue buckets were installed to provide a flow-through system for Chara. Growth data were used to derive design criteria for scale-up
With iron precipitation in the cascade, arsenic and 226Ra were partially co-precipitated and removed from the circumneutral water. At the recommendation of Boojum, mats or curtains were suspended in the open water part of the pool to grow periphyton and biofilms which were to polish the arsenic, iron and 226Ra. Floating vegetation islands were tested as a means to supply dissolved organic carbon for biofilm development (Figs. 9.18 and 9.19).
Graph showing the specific activity of 226Ra removal by Chara at different radium concentrations. The Chara removal line (dotted) is compared to removal rates by other periphyton (solid) from the treatment system. The lines suggest that Chara can remove low levels of 226Ra, and it has a higher affinity for radium than other periphyton, even though there are only a few data points to support these conclusions. After 6 months in the containers, Chara thalli, starting from an initial concentration of < 1 Bq.kg−1, had a specific activity of 8700 Bq.kg−1, suggesting that Chara at high 226Ra effluent concentrations showed a concentration factor greater than 1000
Both the biofilm curtains and the above-ground plants were added to the pool at roughly the same time and grew for a maximum of 6 months. The biofilm scrapings were more effective at contaminant removal than the above-ground vegetation parts (Table 9.3). However, the root systems of the wetland vegetation were not compared to the biofilm scrapings. If they had been analysed, the differences might have been less. One potential drawback of the wetland approach was the potential for food chain contamination, which would not occur with biofilms and periphyton, as the latter would, ideally, become biomineralized. Given the radioactivity of the accumulating sediments, they were to be handled as low-level radioactive sludge and treated as any other sludge from a chemical treatment plant.
The results from the pilot project looked promising so the system was scaled up (Fig. 9.20a). Shortly after the scale-up, the management of the system was turned over to a consultancy, which discontinued Boojum’s participation. After Boojum left the project, Prof. Schubert from the University of Rostock, an expert in Chara growth, was asked to determine the health of the Chara growing in the ponds. He compared the photosynthesis of C. vulgaris from an unpolluted reference site (Fig. 9.20b) and from the bioremediation pond (Fig. 9.20c).
Bioremediation ponds in Pöhla (Boojum and Wismut, GmbH), Germany (a). Scale-up of the pilot test system at Pöhla (b). Non-encrusted C. vulgaris from the unpolluted reference site near the bioremediation ponds. (c) Heavily encrusted C. vulgaris from the bioremediation pond, scale bar = 1cm. (Photographs: H. Schubert)
Even though the charophytes from the bioremediation ponds were heavily encrusted, they maintained photosynthetic activity. However, respiration rates of non-encrusted C. vulgaris were higher than those of encrusted individuals. After removal of the crust, rinsed specimens revealed intact thalli which showed similar respiration rates to non-encrusted Chara (Marquardt & Schubert, 2009). Several years later, Boojum was informed that the system had been totally shut down. This was quite disappointing, as so much time and effort had been invested, with excellent support from Wismut. However, Herbst et al. (2019) started to look at utilizing algae for a mine effluent in the Mansfeld region in Germany.
9.8 Decommissioning with ARUM, CPMW, & Biological Polishing – Ontario
During the operation of a copper/zinc mine in northern Ontario, one million tonnes of tailings were deposited on a peninsula adjacent to a trophy fishing lake, which formed the headwaters of the English River. The tailings contained 45% pyrite and 5% pyrrhotite. A chemical treatment plant installation was not feasible since there was no secured space for the generated sludge. Based on calculations, given the pyrite and pyrrhotite content, the site tailings were expected to generate acid for thousands of years (Kalin et al., 1992). For these two reasons, Boojum was contracted to develop a decommissioning plan for the site. With an agreement between the mine’s operator and the Ontario regulatory agencies, the mine and surroundings were declared an R&D site in 1986 with the provision that no discharges reach the trophy fisheries lake. Ownership was returned to the crown, and funding for decommissioning was secured for 16 years. An overview of all of the tools and processes tested on the site is provided in Kalin (2003).
Within the mine waste and water management area, lay a 1 million cubic meter lake. The mine, when operated, generated drainage and underground seepages, which were diverted into the lake. Upon Boojum’s arrival, the lake was already at pH 4. Boojum decided to use this lake as a large treatment pond, and experimented with a number of tools. Boojum’s efforts were focused mainly on stabilizing and possibly improving the lake’s water quality, as the outflow joined the trophy fishing lake.
The sources of contaminants were the mill site with remnant concentrate, the drainage from the underground workings, and groundwater discharge from the tailings. These contaminant sources produced sediments heavily-laden with iron, zinc and copper. The contaminant loading had to remain in the lake sediments. This was compounded by the fact that the lake only had a 3-year retention period. Boojum’s efforts centered on using a combination of biological polishing to sequester the contaminants in the treatment lake, and ARUM to bio-mineralize the contaminants in the narrow channel of the lake forming the outflow. A floating cover was initiated with brush cuttings placed on the ice during the winter. The cuttings extended the shores of the outflow channel.
Table 9.3 shows a mass balance between contaminants entering the lake and its sediments. Boojum quantified the tonnage of the major contaminants, copper, iron, sulfur and zinc. Three time periods were differentiated in preparing the mass balance. The first period was before any ecological engineering measures were implemented. The second period started when brush cuttings for the attachment of periphyton were added to the lake. At generally the same time, moss transplants were initiated, along with a onetime -phosphate fertilization. The third period began with the experimental suspension of metallic magnesium on barges.
The tonnages of contaminants which were retained in the sediments was estimated by determining the load of contaminants entering the lake (In) minus the load leaving the lake (Out). The historic or background loadings reported in Table 9.4 have been estimated from sediment grab samples obtained in 1990. The stratum at 5 cm was analyzed and used as background. The contaminant mass balance demonstrated that ecological measures not only led to the retention of contaminants, but these measures counteracted any deterioration of the water quality over the three decades since work began. Extensive documentation of the site can be found at the Laurentian Library as all the reports are available there under the title, South Bay.
The work carried out employed nearly all processes and tools discussed in this contribution. They are summarized here.
-
Seasonal turnover of the shallow lake caused the iron-rich sediments to oxidize every year, driving the pH lower. A living moss cover over these sediments was initiated to prevent seasonal iron oxidation (See Chap. 5).
-
Metallic magnesium was used as a means to relatively quickly increase the lake pH. The method worked, but could not be scaled enough to alter the lake pH (see Chap. 7).
-
Approx. 140 tonnes of ground calcium phosphate mining wastes were added to the lake water and sediment to stimulate microbial, phytoplankton and moss cover growth (see Chap. 8).
-
Cut brush was placed along the perimeter of the lake to add surface area for the establishment and growth of biofilms and periphyton and to jump start the process of terrestrialization (see Chaps. 6 and 7).
-
Oxygen and water ingress into the tailings was slowed with 14 tonnes of calcium phosphate by forming a hardpan in the tailings, where annual ingress was suspected. The hardpan was expected to reduce seasonal water movement in the vadose zone and slow the groundwater movement (see Chap. 8).
Boojum terminated cooperation with Ministry of Mines and Northern Development in 2002 over disagreements about the ownership of the mine wastes and the associated responsibility. The site was returned to the crown decades ago with the full approval of the regulatory bodies of the time. To close the project, Boojum’s client requested that a summary of the contaminant sinks and sources within the mine waste management area be prepared. The largest sink in the waste management area was the contaminated lake, with sediments heavily-laden with iron, zinc and copper. By that time, the ecological measures used on the lake had matured. The underwater meadow which developed from the transplanted moss, was completely covering the sediments. The narrow outflow of the lake, where brush was placed, had begun the process of ‘landing in’ (Fig. 9.21a).
The experimental lake and tailings 4 years after Boojum’s departure. The lush green vegetation (both aquatic and terrestrial) is likely due to the application of CPMW to both lake and tailings. (a) Periphyton growth on cut brush in the biological polishing lake. (b) The growth of indigenous vegetation on the tailings after a CPMW addition. (Photographs taken in 2006 by the University of Windsor)
The Ministry of Northern Development and Mines requested a comparison between the lake water quality monitoring completed by Boojum Research in 2002 and those measured by the ministry in 2013 and 2015. The unexpectedly good lake water quality in 2015, given the loadings from the contaminant sources, suggests that the ecological engineering processes continue to work, not only in the treatment lake (Fig. 9.21a), but in the tailings, as well (Fig. 9.21b). The tailings have a dense vegetation cover which might reduce infiltration of atmospheric precipitation. A summary report of the program, along with detailed descriptions of work accomplished is detailed Kalin (2003).
Boojum’s ecological engineering tools will retain a large fraction of the metals within the mine waste management area and with that, a reasonable chance to solve many of mining’s environmental challenges. All that is needed is a paradigm shift from thinking about mine wastes as toxic wastes to natural, weathering of uncovered, extreme ecosystems.
References
Baechler, F. (1997). Investigation of PERD piles at CBDC VJCPP plant. pp 57. https://zone.biblio.laurentian.ca/handle/10219/2923
Boojum Research Ltd. (1992). La Mine Selbaie. Decommissioning with Ecological Engineering. Boojum Research Ltd 88 pages. Retrieved from: https://zone.biblio.laurentian.ca/handle/10219/3008
Boojum Research Ltd. (1994). The decommissioning of Buchans Unit and implementation of biological polishing. Final report / prepared for G. Neary, Buchans Unit, ASARCO. Retrieved from https://zone.biblio.laurentian.ca/handle/10219/2969
Boojum Research Ltd. (1997). B-Zone Pit: Limnology 1993–1996 and the Fate of Arsenic and Nickel: Final Report. Produced for Cameco Corporation as CA105. Retrieved from https://zone.biblio.laurentian.ca/handle/10219/3037.
Boojum Research Ltd. (2002) Development of a pit lake and fate of contaminants 1992–2001, pp156 https://zone.biblio.laurentian.ca/bitstream/10219/2915/1/CA112.pdf
Cao, Y., & Kalin, M. (1999). Phytoplankton in mine waste water community structure, control factors and biological monitoring. Natural Resources Canada, Biotechnology for Mining. Contract 23440–8. Retrieved from https://zone.biblio.laurentian.ca/handle/10219/3015
Chen, X., Wright, J. J. V., Conca, J. J. L. J., & Peurrung, L. L. M. (1997). Evaluation of heavy metal remediation using mineral apatite. Water, Air & Soil Pollution, 98, 57–78. http://www.springerlink.com/index/l7l2p67804476680.pdf
Evangelou, V. P. (1995). Pyrite oxidation and its control. New York: CRC Press. pp 293 ISBN 9780849347320 – CAT# 4732.
Falkowski, P. G. (2000). Rationalizing elemental ratios in unicellular algae. Journal of Phycology, 36(1), 3–6.
Georgopoulou, Z. J., Fytas, K., Soto, H., & Evangelou, B. (1996). Feasibility and cost of creating an iron-phosphate coating on pyrrhotite to prevent oxidation. Environmental Geology, 28(2), 61–69.
Herbst, A., Patzelt, L., Schoebe, S., Schubert, H., & von Tümpling, W. (2019). Bioremediation approach using charophytes-preliminary laboratory and field studies of mine drainage water from the Mansfeld Region, Germany. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-019-06552-6
Hutchinson, P. J., & Barta, L. S. (2000). Geophysical applications to solid waste analysis. In The 16th international conference on solid waste technology and management, Philadelphia, PA, USA (pp. 2–68).
Kalin, M. (2003). Closure with ecological engineering of a remote Cu/Zn concentrator: overview of 16 years R & D field program pp25. https://zone.biblio.laurentian.ca/handle/10219/2986
Kalin, M. (2004). Improving pore water quality in reactive tailings with phosphate mining wastes. Proceedings of the Fifth International Symposium on Waste Processing and Recycling in Mineral and Metallurgical Industries, and the 43rd Annual Conference of Metallurgists of CIM, Hamilton, Ontario, August 22–25, pp. 427–437.
Kalin, M. (1993). The application of ecological engineering to Selminco Summit. Final Report prepared for G. Landry, DEVCO. Retrieved from: https://zone.biblio.laurentian.ca/handle/10219/2996
Kalin, M. (2009). Buchans: Ecological Engineering Treatment Assessment: Long-term performance evaluation and Site Visit Report. https://zone.biblio.laurentian.ca/handle/10219/2892
Kalin, M., & Pawlowski, J. (1994). Electromagnetic surveys in acid-generating waste management areas. In Proceedings of the international symposium on extraction and processing for the treatment and minimization of wastes, San Francisco, California, February 27–March 3, pp. 727–736.
Kalin, M., & Wheeler, W. N. (2013). Biological polishing of arsenic, nickel and zinc in an acidic lake and two alkaline lakes. In W. Geller, M. Schulze, R. Kleinman, & C. Wolkersdorfer (Eds.), Acidic pit lakes: The legacy of coal and metal surface mines (pp. 387–407). Springer.
Kalin, M., van Everdingen, R. O., & McCready, R. G. L. (1992). Ecological engineering- interpretation of hydrogeochemical observations in a sulphide tailings deposit. CIM Bulletin, 85(965), 64–67. Retrieved from https://www.researchgate.net/profile/Margarete_Kalin/publication/285730714_Ecological_engineering_-_Interpretation_of_hydrogeochemical_observations_in_a_sulphide_tailings_deposit/links/568e438a08aef987e56760cc/Ecological-engineering-Interpretation-of-hydr
Kalin, M., Fyson, A., & Smith, M. P. (1993). Heterotrophic bacteria and grass covers on fresh, base metal tailings. In Proceedings of the Canadian land reclamation conference 18th annual meeting, Lindsay, Ontario, August 11–13, pp. 81–88.
Kalin, M., Cao, Y., Smith, M. P., & Olaveson, M. M. (2001). Development of the phytoplankton community in a pit lake in relation to water quality changes. Water Research, 35(13), 3215–3225.
Kalin, M., Kiessig, G., & Küchler, A. (2002). Ecological water treatment processes for underground uranium mine water: Progress after three years of operating a constructed wetland. In B. J. Merkel, B. Planer-Friedrich, & C. Wolkersdorfer (Eds.), Uranium in the aquatic environment (pp. 587–596). Springer.
Küchler, A., Kiessig, G., & Kunze, C. (2006). Passive biological treatment systems of mine waters at WISMUT sites. In Uranium in the environment (pp. 329–340). Springer.
Marquardt, M., & Schubert, H. (2009). Photosynthetic characterization of Chara vulgaris in bioremediation ponds. Charophytes, 2, 1–8. https://www.researchgate.net/publication/229066985
WISUTEC. (2002). Jahresbericht 2002: Ergebnisse des Pilotversuches zur passiv/biologischen Behandlung multitasking on Grubenwasser der Grube Pohla-Tellhauser. Page 2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kalin-Seidenfaden, M. (2022). R&D Field Applications. In: Kalin-Seidenfaden, M., Wheeler, W.N. (eds) Mine Wastes and Water, Ecological Engineering and Metals Extraction. Springer, Cham. https://doi.org/10.1007/978-3-030-84651-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-84651-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84650-3
Online ISBN: 978-3-030-84651-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)