Abstract
It is well known that BMD measured by DXA is a major determinant of bone strength and fracture risk, although most individuals with a fragility fracture will have a BMD in the osteopenic or even in the normal range. Skeletal and extraskeletal risk factors that contribute to overall fracture risk should be identified to better select patients for treatment. We report a case of a 51-year-old perimenopausal woman who sustained a typical osteoporotic fracture, despite not having osteoporosis by DXA. Her trabecular bone score (TBS), a gray-level texture measure derived from lumbar DXA images that provides an indirect index of bone architecture, was low. The patient’s 10-year probability of fracture by the Fracture Risk Assessment Tool (FRAX) was very high. The use of FRAX and TBS in clinical practice to identify patients that need pharmacological intervention and their role in monitoring antiosteoporotic therapy are reviewed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Case Presentation
A 51-year-old Brazilian perimenopausal woman presented to the emergency department with right wrist pain after a fall from standing height. Right wrist radiographs confirmed a distal radius fracture associated with ulnar styloid fracture, successfully treated by closed reduction and cast immobilization. Her stature was 172 cm, with a body weight of 71.4 kg, and a body mass index (BMI) of 24.1 kg/m2. Her physical examination was otherwise unremarkable. Her medical history included hypertension, dyslipidemia, impaired glucose tolerance, and vitiligo. She was compliant with atenolol, hydrochlorothiazide, and simvastatin. Her family history was positive for type 2 diabetes, but negative for fractures. She denied smoking or alcohol consumption, and her diet was low in calcium. The patient’s menopause occurred approximately 6 months after her first evaluation, at the age of 52, but she did not have significant hot flashes or other bothersome menopausal symptoms.
Initial workup revealed a bone mineral density by DXA in the osteopenic range, with a T-score of −2.3 at the lumbar spine, −2.3 at the femoral neck, and −2.1 at the total hip. Trabecular bone score (TBS) was low at 1.11. Secondary causes of osteoporosis were excluded based on extensive laboratory evaluation that included complete blood count, serum calcium, phosphate, total protein, albumin, liver enzymes, alkaline phosphatase, creatinine, 25-hydroxyvitamin D, intact parathyroid hormone, TSH, tissue transglutaminase antibodies, and urinary calcium. The bone resorption marker, serum C-terminal telopeptide (CTX), was 0.447 ng/mL (reference range for premenopausal women: 0.025–0.573 ng/mL). Spine radiographs did not show vertebral fractures.
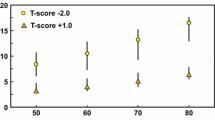
The patient’s 10-year fracture probability was calculated using the Fracture Risk Assessment Tool (FRAX) with BMD. The TBS-adjusted FRAX was also assessed. The risks of major osteoporotic fracture (MOF) and hip fracture were, respectively, 7.4% and 1.8%, and after adjusting for TBS, 12% and 3.7% (Fig. 3.1). The 10-year probability of MOF and hip fractures exceeded the country-specific intervention thresholds by 20%, using both FRAX and TBS-adjusted FRAX, which would identify this patient as having a very high risk of fracture. Thus, despite the BMD in the osteopenic range, pharmacologic therapy with alendronate was recommended based on her history of prior fragility fracture and the finding of a very high fracture risk by FRAX . She was also counseled to keep an active lifestyle, including weight-bearing exercises, to adopt fall prevention strategies, and to increase her intake of calcium to 1200 mg/day from diet and supplements. Vitamin D supplements were also indicated to maintain her serum level of 25-hydroxyvitamin D above 30 ng/mL.
Output of FRAX Brazil v4.2 (a), adjusted for TBS (b). Age-specific 10-year probability of major osteoporotic fracture (c, d) and hip fracture (e, f) for Brazilian women, as proposed by the UK National Osteoporosis Guideline Group (NOGG). The line denotes intervention thresholds for Brazilian women, which at the age of 51 years corresponds to 5% for major osteoporotic fractures and 0.7% for hip fractures. Dots represent, respectively, major osteoporotic and hip fracture probabilities of 7.4% (c) and 1.8% (e) using FRAX with BMD, and 12% (d) and 3.7% (f), using the TBS-adjusted FRAX. It is noted that the 10-year probabilities of fractures exceed the intervention thresholds by 20%, using both FRAX and TBS-adjusted FRAX. BMD, bone mineral density; BMI, body mass index; TBS, trabecular bone score; FRAX, Fracture Risk Assessment Tool
Assessment and Diagnosis
This patient experienced a typical osteoporotic fracture despite having a BMD by DXA in the osteopenic range. In fact, although low BMD is a strong predictor of fracture, most individuals with fragility fractures have BMD values that do not fall within the osteoporotic range [1, 2]. Thus, skeletal and extraskeletal risk factors that contribute to overall fracture risk should be identified to better select patients for treatment.
Among extraskeletal features, readily assessable clinical risk factors contribute to fracture risk, independently of BMD, and have been incorporated in risk assessment tools to calculate an individual’s probability of fracture. FRAX is the most widely used and comprehensively evaluated risk assessment tool currently available [3]. It integrates the following risk factors: age, BMI, sex, previous fragility fracture, parental history of hip fracture, smoking, prolonged glucocorticoid use, rheumatoid arthritis, excessive alcohol consumption, and other causes of secondary osteoporosis. FRAX was developed through a series of meta-analyses of prospective cohort studies from Europe, North America, Asia, and Australia including more than 40,000 individuals. Its ability to predict fractures has been validated in independent cohorts [4,5,6]. FRAX incorporates the risk of fracture with risk of death, and country-specific FRAX calculators have been developed to account for geographical variations in fracture incidence and mortality [7]. The tool estimates 10-year probabilities of major osteoporotic fractures (clinical spine, hip, distal forearm, and proximal humerus) and hip fractures, in individuals between the ages of 40 and 90 years [7].
FRAX does not directly yield an indication for treatment [8]. The estimated FRAX-probability of fracture needs to be interpreted, and thresholds set above which pharmaceutical intervention is justified. To this end, the cost-effectiveness of a therapy can be considered to set the intervention threshold. Alternatively, the threshold can be clinically derived and then validated using a cost-effectiveness analysis [8]. The approach to set the threshold varies across the world. The National Osteoporosis Foundation in the United States recommends treatment for those with BMD in the osteopenic range associated with a 10-year FRAX-probability of major osteoporotic fractures ≥20% or hip fracture ≥3% from a cost-effectiveness analysis [9]. In contrast, the UK National Osteoporosis Guideline Group (NOGG) developed its guidance on the basis of clinical appropriateness, setting the threshold at the age-specific 10-year FRAX-probability of fracture equivalent to women having already sustained a fracture. This approach has also been shown to be cost-effective, being adopted in many countries, particularly in Europe and Latin America [7, 8].
The probability of fracture can be estimated with or without femoral neck BMD [7]. When the assessment is made without BMD, fracture probability will be categorized as low, intermediate, or high. Patients with intermediate risk of fracture should undergo a DXA test, whereas patients with high fracture risk should be considered for treatment. When the assessment is made with BMD, two categories will be defined, namely, low and high fracture risk. Recently, the International Osteoporosis Foundation (IOF) and the European Society for Clinical and Economic Evaluation of Osteoporosis and Osteoarthritis (ESCEO) published an algorithm that further divide the high-risk category into high- and very-high-risk classifications [10]. To this end, a fracture probability that exceeds the intervention threshold by 20% would identify individuals with very high risk of fracture [7, 10]. The patient reported here had a history of previous fragility fracture, which according with the majority of guidelines worldwide can be considered for treatment without the need for further risk assessment. However, her 10-year probability of fracture was further assessed by FRAX. Interestingly, although the patient’s BMD was not in the osteoporotic range, her 10-year probability of fracture exceeded the country-specific intervention threshold by 20%, identifying this patient as having a very-high risk of fracture [10]. This approach could not only define the need for pharmacological intervention but also guide the choice of the initial anti-osteoporotic agent and the duration of therapy [9, 10].
Additional fracture risk tools such as Garvan Fracture Risk Calculator and QFracture risk calculator are also available, but they were developed based on data from single countries, limiting their use worldwide [11, 12].
In addition to clinical risk factors, skeletal features other than areal BMD contribute to overall fracture risk. Bone geometry, microarchitecture, microdamage, rate of bone turnover, and mineralization contribute to bone strength and risk of fracture [13,14,15]. However, methodologies that evaluate bone strength independent of BMD are not readily available, being currently used as research tools. A major challenge, therefore, has been to develop a clinically available tool that permits evaluation of skeletal structure beyond BMD by DXA. To this end, TBS was developed as another approach for assessing skeletal bone structure noninvasively from DXA projection images [16]. TBS (unitless) is a gray-level texture measure that provides an indirect index of bone architecture [17, 18]. It is measured at the lumbar spine with specialized software (TBS iNsight®, MedImaps) that uses the same region of interest as for conventional BMD measurement [19]. Of note, TBS can be artefactually reduced by excessive abdominal soft tissue [20, 21] and should not be measured in individuals with BMI outside of the range 15–37 kg/m2 [17].
TBS predicts the risk of fracture independent of BMD by DXA and clinical risk factors [22,23,24,25,26]. The International Society for Clinical Densitometry (ISCD) and the ESCEO support the use of TBS to assess fracture risk in postmenopausal women and older men [27, 28]. However, although a low TBS is associated with greater risk of fracture, a TBS threshold to initiate treatment has not been defined, and TBS should not be used as a single measurement to guide treatment decisions [27]. Alternatively, TBS can be entered in the FRAX calculator online, allowing for the calculation of TBS-adjusted 10-year probability of fracture, assisting in treatment decisions (Fig. 3.1). In general, the use of TBS to adjust the FRAX score has a lower impact with increasing age, and a greater clinical effect in those patients who are close to the intervention threshold by FRAX without TBS [19].
There is no consensus regarding what represents low versus normal TBS values, but a metanalyses involving 17,809 men and women from 14 prospective population-based cohorts from North America, Asia, Australia, and Europe described TBS thresholds of 1.23 and 1.31 using a tertile analysis. Patients whose TBS was lower than 1.23 presented a high fracture risk, while those with TBS between 1.23 and 1.31 had an intermediate risk, and individuals with TBS greater than 1.31 had the lowest fracture risk. The patient reported here had a TBS of 1.11, which would indicate a high fracture risk. The use of TBS to adjust the FRAX score in this case increased the 10-year probability of MOF by 62% and doubled the risk of hip fracture calculated by FRAX) without TBS (Fig. 3.1).
Management
According to the recently published Endocrine Society (ES) guidelines for the management of osteoporosis in postmenopausal women, the patient presented here would be considered at high risk for fracture and could be treated with a bisphosphonate [29, 30]. Women with vertebral fractures associated with a BMD T-score in the osteoporotic range are at very high risk of fracture and may be treated with bone-forming agents as the initial therapy [29, 30]. Accordingly, the IOF/ESCEO and the American Association of Clinical Endocrinologists (AACE) guidelines also support the use of osteoanabolic agents as the first line of treatment in such patients [9, 10]. There are differences between the guidelines with regard to classification of patients at very high risk. The AACE guideline suggests that patients at very-high risk of fracture include those with recent fracture (within the past 12 months), those who have fractures while on approved osteoporosis therapy, history of multiple fractures, fractures while on drugs that increase fracture risk (e.g., long-term glucocorticoids), those with a very low T-score (e.g., <−3.0), high risk of falls or history of injurious falls, and those with a very-high fracture probability by FRAX [9]. Similarly, the IOF/ESCEO guidelines use the FRAX score to categorize the risk of fracture, such that patients with a 10-year fracture probability that exceeds the intervention threshold by 20% would be classified at very high risk [10]. This is the case for the patient presented here, so that one could argue that she should have been treated with an osteoanabolic agent. However, the evidence supporting the superiority of anabolic agents over antiresorptive agents in reducing fracture risk was demonstrated in patients with very high fracture risk due to the presence of previous vertebral fractures and/or prolonged glucocorticoid use [31,32,33]. These risk factors were absent in the patient presented here, and initial treatment with alendronate was supported by the ES guidelines [29, 30].
The patient’s treatment was monitored using BMD by DXA and serum CTX, whereas TBS was not reassessed. The 2019 ISCD Position Development Conference concluded that the role of TBS in monitoring antiresorptive therapy is unclear [34]. Several studies have shown minimal changes in TBS in patients treated with bisphosphonates or denosumab for up to 3 years, with the majority of patients presenting a TBS improvement much lower than the least significant change [27, 34]. The use of TBS for monitoring patients on osteoanabolic therapy may be useful [27].
Some guidelines recommend FRAX reassessment in patients on bisphosphonates to define the duration of treatment and suggest a drug holiday in individuals whose FRAX risk falls below the intervention threshold [35]. This approach, however, has limitations, since the 10-year FRAX-probabilities of fracture can be overestimated in patients on osteoporosis treatment [36].
Outcome
The patient increased her calcium intake to 1200 mg/day, including 500 mg of calcium supplements, started on vitamin D3 supplementation to maintain 25-hydroxyvitamin D levels >30 ng/mL and was treated with alendronate 70 mg weekly. At approximately 3 months on treatment, her serum 25-hydroxyvitamin D was 31.8 ng/mL, and her serum CTX was at 0.165 ng/mL, representing a decline of 63% compared to the baseline measurement, indicating a satisfactory level of compliance and good response to treatment. The BMD by DXA 1 year following treatment remained stable, with a nonsignificant change of +1.2% at the lumbar spine, and +2.2% at the total hip. The patient is now on year 4 of treatment, without incident fractures.
Clinical Pearls and Pitfalls
-
Skeletal features other than BMD by DXA, as well as extraskeletal risk factors, contribute to overall fracture risk and should be identified to better select patients for anti-osteoporotic treatment.
-
FRAX uses readily assessable clinical risk factors, with or without BMD, to estimate 10-year probabilities of major osteoporotic fractures and hip fractures, with the adoption of country-specific thresholds to guide treatment decisions.
-
FRAX can be used without BMD, although the use of clinical risk factors in conjunction with BMD improves fracture prediction, particularly in the case of hip fractures.
-
Limitations of FRAX include the lack of validation in patients on anti-osteoporotic treatment; the use of T-score at the femoral neck only, disregarding other sites (e.g., lumbar spine); lack of dose response for several risk factors (e.g., number of prior vertebral fractures); and the absence of important clinical risk factors such as history of falls.
-
Trabecular bone score (TBS) is a gray-level textural measurement derived from lumbar spine DXA images that provides an indirect index of bone architecture and predicts the risk of fracture, independent of BMD by DXA and clinical risk factors.
-
TBS can be entered into the FRAX calculator online, allowing for the calculation of TBS-adjusted 10-year probability of fracture, assisting in treatment decisions.
-
The role of TBS in monitoring antiresorptive therapy is unclear.
References
Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA, et al. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab. 2005;90(5):2787–93.
Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286(22):2815–22.
Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18(8):1033–46.
Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA, et al. Independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J Bone Miner Res. 2010;25(11):2350–8.
Hillier TA, Cauley JA, Rizzo JH, Pedula KL, Ensrud KE, Bauer DC, et al. WHO absolute fracture risk models (FRAX): do clinical risk factors improve fracture prediction in older women without osteoporosis? J Bone Miner Res. 2011;26(8):1774–82.
Tamaki J, Iki M, Kadowaki E, Sato Y, Kajita E, Kagamimori S, et al. Fracture risk prediction using FRAX(R): a 10-year follow-up survey of the Japanese Population-Based Osteoporosis (JPOS) Cohort Study. Osteoporos Int. 2011;22(12):3037–45.
Kanis JA, Harvey NC, Johansson H, Liu E, Vandenput L, Lorentzon M, et al. A decade of FRAX: how has it changed the management of osteoporosis? Aging Clin Exp Res. 2020;32(2):187–96.
Liu J, Curtis EM, Cooper C, Harvey NC. State of the art in osteoporosis risk assessment and treatment. J Endocrinol Investig. 2019;42(10):1149–64.
Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26(Suppl 1):1–46.
Kanis JA, Harvey NC, McCloskey E, Bruyere O, Veronese N, Lorentzon M, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. 2020;31(1):1–12.
Hippisley-Cox J, Coupland C. Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort study. BMJ. 2012;344:e3427.
Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int. 2008;19(10):1431–44.
Nishiyama KK, Macdonald HM, Hanley DA, Boyd SK. Women with previous fragility fractures can be classified based on bone microarchitecture and finite element analysis measured with HR-pQCT. Osteoporos Int. 2013;24(5):1733–40.
Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11(10):1531–8.
Sroga GE, Vashishth D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep. 2012;10(2):141–50.
Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom. 2011;14(3):302–12.
Silva BC, Leslie WD. Trabecular bone score: a new DXA-derived measurement for fracture risk assessment. Endocrinol Metab Clin N Am. 2017;46(1):153–80.
Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29(3):518–30.
Silva BC, Leslie WD. Trabecular bone score. In: Primer on the metabolic bone diseases and disorders of mineral metabolism. Hoboken: Wiley; 2018. p. 277–86.
Kim JH, Choi HJ, Ku EJ, Hong AR, Kim KM, Kim SW, et al. Regional body fat depots differently affect bone microarchitecture in postmenopausal Korean women. Osteoporos Int. 2016;27(3):1161–8.
Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27(8):2467–75.
Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int. 2013;24(1):77–85.
Briot K, Paternotte S, Kolta S, Eastell R, Reid DM, Felsenberg D, et al. Added value of trabecular bone score to bone mineral density for prediction of osteoporotic fractures in postmenopausal women: the OPUS study. Bone. 2013;57(1):232–6.
Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26(11):2762–9.
McCloskey EV, Oden A, Harvey NC, Leslie WD, Hans D, Johansson H, et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res. 2016;31(5):940–8.
Schousboe JT, Vo T, Taylor BC, Cawthon PM, Schwartz AV, Bauer DC, et al. Prediction of incident major osteoporotic and hip fractures by trabecular bone score (TBS) and prevalent radiographic vertebral fracture in older men. J Bone Miner Res. 2016;31(3):690–7.
Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 2: trabecular bone score. J Clin Densitom. 2015;18(3):309–30.
Harvey NC, Gluer CC, Binkley N, McCloskey EV, Brandi ML, Cooper C, et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216–24.
Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595–622.
Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105(3):dgaa048.
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417–27.
Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230–40.
Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357(20):2028–39.
Krohn K, Schwartz EN, Chung YS, Lewiecki EM. Dual-energy X-ray absorptiometry monitoring with trabecular bone score: 2019 ISCD official position. J Clin Densitom. 2019;22(4):501–5.
Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31(1):16–35.
Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA, et al. Does osteoporosis therapy invalidate FRAX for fracture prediction? J Bone Miner Res. 2012;27(6):1243–51.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Silva, B.C., Soares, M.M.S. (2021). The Utility and Applicability of Risk Assessment Tools and Trabecular Bone Score. In: Cusano, N.E. (eds) Osteoporosis. Springer, Cham. https://doi.org/10.1007/978-3-030-83951-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-83951-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83950-5
Online ISBN: 978-3-030-83951-2
eBook Packages: MedicineMedicine (R0)