Abstract
Respiratory care equipment includes devices that: (1) provide supplemental oxygen, (2) provide humidification to the inspired gas, (3) are used to deliver therapeutic aerosols, and (4) are used for airway secretion clearance. Devices used for delivery of supplemental oxygen differ in their ability to increase the inspired oxygen concentration. High flow nasal cannula (HFNC) is a special form of supplemental oxygen where the gas is heated and humidified. HFNC use has increased in infants and children with concomitant reduction in the use of NIV. HFNC has also resulted in improved outcomes. Optimal use of therapeutic aerosols requires an understanding the performance characteristics of the different aerosol-generating equipment so that it can be adequately applied with specific disease conditions and patient population. Respiratory therapeutics, medications administered through the respiratory system, includes bronchodilators, antimicrobials and specialty gases such as helium–oxygen mixture, nitrogen, and inhaled nitric oxide.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Respiratory care
- Humidification
- Inspired O2 content
- High flow nasal cannula
- Therapeutic aerosols
- Airway secretion clearance
11.1 Oxygen Delivery Devices
Supplemental oxygen administration is the most common therapeutic intervention provided to infants and children presenting with acute or chronic respiratory disease. In order to match the patient’s needs with the appropriate device requires an understanding of the patient’s pathophysiology and the capabilities of a particular device. The acronym AIM has been suggested as a helpful means to select oxygen delivery device. The AIM mnemonic stands for (A) assessment of patient need, (I) identification of device capability, and (M) matching device/technology with need. Clinical evaluation for the need for supplemental oxygen includes general appearance, responsiveness, pulse-oximetry, and heart rate. Knowing each oxygen delivery device’s capabilities and limitations is critical in selecting the right device for the patient’s needs. Matching the device capacities with the patient’s oxygen needs begins with an evaluation of severity of hypoxemia, patient inspiratory rate, and tolerance of the applied device. Intolerance of the applied device can the stress the patient and increase the work of breathing which may lead to further compromise. Common reasons for ineffective supplemental oxygen therapy include improper fit, inadequate flow rates and educational gaps in understanding technical capabilities and published consensus guidelines.
Blenders and Low Flow Meters
Air-oxygen blenders allow for mixing medical grade air and oxygen to any concentration from 21 to 100% oxygen. The output from the blender may be delivered to a variety of respiratory care devices. Oxygen blenders allow the clinician to set a specific concentration of oxygen supplied to the oxygen device, most frequently a nasal cannula in neonates and small infants. Both the flow rate and the blender concentration can be adjusted to deliver the required FiO2. It is important to understand that air-oxygen blenders cannot be used reliably to deliver other gases such as helium–oxygen mixture since there is considerable difference in gas density which will not only affect the flow through the blender but also the FiO2 of the gas output from the blender. Table 11.1 gives a suggested guideline for managing and weaning FiO2 when air-oxygen blenders are used with flow meters.
Blow-by Oxygen Delivery
Blow-by oxygen method of supplemental oxygen delivery is the simplest and easiest to tolerate. This method is provided in several different ways, which include a high-flow oxygen source connected to large bore or small caliber oxygen tubing with or without a face tent/simple face mask placed in close proximity and directed towards the patient’s face. It is most commonly used in the delivery-room for oxygen supplementation during infant stabilization, during the initial evaluation, and during the initial patient presentation with respiratory distress. Blow-by oxygen is the least consistent means to provide a known FiO2 and for this reason it is only recommended for brief oxygen support until a more definitive device can be applied. But, in patients who don’t tolerate more bulky devices or who may have undergone facial surgery or suffered trauma to the face, head, or neck, blow-by oxygen may be the only reliable method to provide supplemental oxygen. Blow-by oxygen therapy delivers relatively low concentrations of oxygen.
Oxygen Hood or Tent
An oxygen hood or tent is a plastic enclosure surrounding the patient’s head or the whole body that provides continuous flow of humidified air-oxygen mixture. The source gas can be delivered by either an air entrainment device or more commonly from an air-oxygen blender. Oxygen hoods are used for neonates and small infants and surround the head and upper torso. One benefit of this device is direct access to the patient’s chest and body for ongoing assessment. With adequate enclosure seal, an oxygen hood can provide a delivered FiO2 from 0.22 to 0.8 with a range of 7–10 L/min. Oxygen tent covers the child’s entire body and the range of flow rates used is between 15 and 30 L/min. Due to its size, it is difficult to maintain FiO2 higher than 0.5 and may not be appropriate for patients who need a higher FiO2. With these devices, exhaled CO2 is removed by providing an adequate amount of fresh gas into the device. If the gas flow into the device is inadequate, there is a risk of CO2 rebreathing. There is a risk of hypothermia if the gas is not adequately heated. Widespread use of nasal cannulas, both for low flow and high flow oxygen delivery, has resulted in these devices being less preferable, especially in infants and small children.
Low-flow Nasal Cannula (Low-flow)
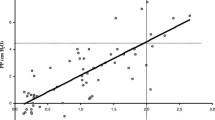
Low-flow nasal cannula is one of the most frequently used oxygen devices in infants and children. Low flow oxygen is delivered via 2 prongs situated in the patient’s nares. The proximal end of the cannula is connected to 100% oxygen gas source flow meter or an air-oxygen blender allowing for adjustment of source gas FiO2. Use of a blender provides more control over the delivered oxygen concentration in smaller patients where device flow rates equal or exceed the patient’s inspiratory flow demand. It has been reported that delivered FiO2 to the neonate via a nasal cannula ranges from 0.22 to 0.95 at a set maximum flow of 2 L/min (Fig. 11.1).
Actual FiO2 delivered via a nasal cannula is associated with several important factors such as the set flow rate and its proportion to the patient’s inspiratory flow demand. Other determinants of FiO2 include room-air entrainment and the device’s inability to meet an acceptable proportion of the patient’s inspiratory demand in times of increase minute ventilation needs. This will result in a decreased FiO2 that may require switching to another means to deliver adequate oxygen delivery. Conversely, patients with decreased minute ventilation as a result of sedation, or being post-ictal following a seizure will have increased FiO2, as flow will fill the anatomic dead space increasing the FiO2 delivered. Because anatomic dead space is less in the neonate and infant, low flow nasal cannula will provide greater FiO2 than in adult population. Oxygen delivered with this method may also be passively humidified to increase patient comfort. Prolonged use or high flow rates without adequate humidification may lead to tissue irritation by drying the mucosa and contribute to patient discomfort. Heating and humidifying the gas source can decrease irritation by adequate humidification and increase patient tolerance.
Heated Humidified High-Flow Nasal Cannula
Definition and Equipment
Heated Humidified High-Flow Nasal Cannula (HHHFNC or shortened to HFNC), is defined as the delivery of heated and humidified oxygen at a flow rate of 0.5–2 L/kg/min through a specially designed nasal delivery system. During normal breathing, the normal peak inspiratory flow rate is about 0.5–1 L/kg/min. The usual starting flow for HFNC can range between 0.5 and 2 L/kg/min. Flow greater than 2 L/kg/min may increase expiratory resistance and decrease the efficacy of HFNC therapy. The FiO2 of HFNC can range from 0.21 to 1.0. The gas is heated and humidified to increase patient comfort, decrease drying of airway mucosa and facilitate airway clearance (Table 11.2).
HFNC system consist of several primary components, which include:
-
1.
Medical-grade gas source—depending on the configuration, system will require access to air and oxygen to power gas blenders for delivery of varying FiO2. Commercially available systems have integrated gas-blending systems, removing the need for multiple gas sources.
-
2.
Heated humidifier—conditions gas to a relative humidity of approximately 100% at temperature of 34 and 37 °C, improving patient tolerance, decreasing insensible losses, and improving mucociliary clearance. Additionally, heated and humidified gas decreases resistance in the nasal cavity, which is an important consideration as it accounts for 50% of the total resistance of the respiratory system.
-
3.
Heated circuit—systems should have an integrated heated circuit to increase temperature control, decrease circuit rain-out, and increase humidity of the inspired gas. These circuit monitor temperature at the chamber and at the distal end of the circuit, which helps the clinician ensure adequate humidity and temperature management. Heated circuit increase patient comfort at high liter flows, increasing device tolerance.
-
4.
High-flow nasal cannula—by definition, high-flow nasal cannula interfaces are non-occlusive, with proper sizing occluding 50% of the circumference of the nares. The leak limits the risk of auto-PEEP/gas-trapping and facilitates CO2 elimination through an open system during nose breathing with a closed mouth.
-
5.
Oxygen analyzer—commercially available systems have integrated oxygen analyzers to ensure accurate FiO2 delivery, which adds a margin of safety, facilitates oxygen weaning, and documentation. If an institutionally developed system has been created using a blender, heated humidification device, and heated circuit, an oxygen analyzer should be placed in-line for accurate FiO2 measurement.
-
6.
Pressure relief valve—provides a mechanism to relieve pressure at a set level within the circuit to decrease the risk of over-pressurization in the event too large a cannula is used or an obstruction occurs within the system. These are often supplied with the manufacturer supplied circuit systems. These valves should be used as indicated to limit risk to the patient.
The mechanisms by which HFNC may improve the work of breathing and oxygenation are:
-
1.
Decreased inspiratory work of breathing by providing a flow of gas that matches or exceeds the peak inspiratory flow of the patient
-
2.
Decreased dead space
-
a.
Approximately a third of the exhaled tidal volume is rebreathed during normal respiration
-
b.
This terminal portion of the exhaled tidal volume contains carbon dioxide which constitutes about 5–6% of this space
-
c.
HFNC washes out this gas with fresh oxygen-rich gas
-
a.
-
3.
Maintenance of a higher FiO2 in the pharyngeal space due to decreased entrainment of atmospheric air
-
4.
With a tight fit in the nares and with the mouth closed, HFNC can generate positive pressure in the airways and produce CPAP. However, the level of positive airway pressure generated in the alveoli is variable and would depend on the ability of the patient to keep the mouth closed, and the tightness of the fit of the nasal cannulas.
Indications and Contraindications
Administration of supplemental oxygen via HFNC is most commonly indicated in children with mild to moderate hypoxemia unresponsive to low-flow oxygen delivery devices. High-flow nasal cannula has also been found to be effective in treating infants and children with underlying lung disorders that require enhanced oxygenation with possibly reducing the work of breathing.
Bronchiolitis Most of the evidence supporting the use of HFNC in the pediatric population is in bronchiolitis. In a large multi-center randomized, controlled trial in infants less than 12 months of age with bronchiolitis, those treated with HFNC received less escalation of care. Additionally, those infants who failed standard therapy, 61% had a response to high-flow rescue therapy. There was no difference in hospital length of stay or duration of oxygen support. High-flow nasal cannula therapy has also been associated with decreased rates of intubation in infants admitted to the PICU with bronchiolitis. A single center retrospective review demonstrated a 68% decrease in intubation in infants less than 24 months of age admitted to the PICU with bronchiolitis after introduction of HFNC into practice. The authors also reported a reduction in PICU length of stay from 6 to 4 days post introduction of HFNC. These results need to be validated in prospective studies.
Successful use of HFNC in infants with bronchiolitis begins with an early assessment and identification of severity of respiratory distress and therapeutic need. Respiratory Distress Score and oxygen requirement are useful in determining the level of support needed. Infants with mild to moderate respiratory distress and an FiO2 requirement greater than 0.60 may benefit from HFNC. Severe respiratory distress and higher FiO2 requirements should be considered for either non-invasive or invasive positive pressure ventilation. Initiation of HFNC should begin at a rate of 0.5–2 L/kg/min and an FiO2 of 1.0, with FiO2 decreased to maintain acceptable SpO2.
Asthma Use of HFNC for asthmatic patients has potential physiologic benefits, which include reduced work of breathing due to auto-PEEP, and potential amelioration of bronchoconstriction by delivering heated and humidified gas. Recently it has been reported that early initiation of HFNC is superior when compared to conventional oxygen therapy in moderate-to-severe asthma exacerbations. It is recommended that use of HFNC in this patient population is based on clinical indications which incorporate clinical respiratory distress scoring. Low flow delivery systems may be adequate to support less severe exacerbations and the application of HFNC may result in an increased resource utilization, length of stay, and costs. Furthermore, use of HFNC may mask the need for more intensive support such as a need for non-invasive positive pressure ventilation. Delivery of nebulized bronchodilator therapy has been suggested via HFNC, however much controversy remains as the delivered dose varies from only 0.5% to 25% of the administered dose. Consideration should be given in the method of aerosol delivery in children supported via HFNC, as an exceedingly low dose is delivered to the lung, with increased nasal disposition, which may result in local toxicity.
Interfacility Transport Interfacility transport is a dynamic low-resource environment, often complicated by patient severity of illness and lack of confirmed diagnosis. Evidence demonstrating the association of the use of HFNC with decreased rate of intubation and escalation of care in both the emergency department and intensive care units, has led to its use in the interfacility transport setting. Benefits associated with HFNC in this environment include the administration of variable level of CPAP, reduced work of breathing through flow-related reduction in anatomic dead space, and patient comfort. A few operational concerns need be addressed in using high-flow therapy in the transport setting. The primary issue is related to a continuous power source for the heater-humidifier. Loss of power will result in a rapid loss of heat delivered humidity and temperature.
Other Potential Uses The success of HFNC with bronchiolitis has increased its potential applications in pneumonia, after cardiothoracic surgery, as well as after extubation.
Contraindications, Risks and Complications
Contraindications for HFNC include suspected or confirmed pneumothorax, severe upper airway obstruction and decreased respiratory drive. Use of HFNC comes with several risks. Most significant concern is the inability to measure the exact level of positive pressure produced. Level of potential PEEP generated by the therapy varies from patient to patient and is affected by patient size, air flow, open-mouth, and the percent occlusion of the nares. These variables allow for inconsistent end-expiratory pressure and may result in gastric distention and/or lung over expansion. Most pediatric lung disease is heterogeneous—producing areas of increased compliance and other areas with decreased compliance. Inconsistent positive end expiratory pressure from a non-occlusive interface results in non-uniform pressure distribution with areas of atelectasis and over-expansion. This may lead to escalation in care such as increased FiO2 requirement due to V/Q mismatching. A complication rate of 0.9/100 HFNC treatment days has been reported. Of these complications, 4% of the total cohort had either development of new pneumothoraces or chest tube-related air leaks following the initiation of HFNC therapy. A secondary concern is the concentration of oxygen that can be delivered. At high flow rates, it is quite easy to deliver a FiO2 > 0.60, which may mask progressing hypoxic respiratory failure and introduce lung tissue to toxic levels of oxygen. Supplemental oxygen concentration should be weaned to maintain adequate SpO2. If high levels of oxygen are required to maintain a clinically acceptable arterial saturation, occlusive non-invasive positive pressure ventilatory support should be considered as a more stable mean airway pressure will recruit collapsed alveolar units and result in increased FRC. Paradoxical respiration is a sign of upper airway collapse in small children that is better managed by an occlusive airway device such as nasal prongs, nasal masks, or full-face masks.
Simple Face Mask (Low-flow)
Simple Facemask is a lightweight reservoir mask that fits over the patient’s nose and mouth with an elastic strap that is secure around the child’s head just above the ears (Fig. 11.2). The mask has open ports on each side—allowing for exhalation and for the patient to draw in room-air during inspiration. Rubber flaps maybe placed on one side of the mask, decreasing room air entrainment, increasing FiO2 delivered. Set flow rates between 6 and 10 L/min deliver a variable FiO2 between 0.35 and 0.50. The conical shape and reservoir design may accumulate exhaled carbon dioxide (CO2) if minimal flows are not ensured. A minimum flow of 6 L/min is recommended for older children and adults to ensure flushing of exhaled CO2. Sound practice includes that all patients receive no less than 6 L/min when receiving supplemental oxygen via a simple face mask.
No data pertaining to newborns or infants have been reported regarding effective FiO2 delivery via simple facemasks. Several hazards and complications are associated with the use of simple facemasks. Because the mask is strapped to the infant or child’s face, phonation, eating including breast- and bottle-feeding increases the risk of aspiration of vomitus. Particular caution should be taken in patients with altered consciousness and potentially a full-stomach. They should be evaluated for the presence of cough and gag. The elastic strap is also uncomfortable and may cause skin redness and irritation with prolonged use, most notably on the top of the ears.
Air-Entrainment (Venturi) Masks (High-flow)
Air-entrainment or Venturi masks are high-flow masks that provide a fixed concentration of oxygen at a flow rate that meets or exceeds the patient total inspiratory demand. The set flow rate, indicated by the specific device combined with the fixed air-entrainment, exceed the patient respiratory flow demand—ensures that only a fixed amount of room air is entrained. For this reason, these devices are ideal in clinical situations where a reliable concentration of oxygen is indicated. For example, patients with known CO2 retention whose breathing is dependent on hypoxic drive such as cystic fibrosis, a fixed FiO2 delivery will decrease the risk of hyperoxia related hypoventilation. Such patients will benefit from an air-entrainment mask. Another clinical situation would be those hypoxic patients with high respiratory rate and tidal volumes, where an air-entrainment mask is capable of meeting the patient’s inspiratory flow demand.
The mask fits over the patient’s nose and mouth with large ports on each side. The port allows for removal of exhaled gas while providing a means to entrain room air during times of high inspiratory flow demand. The mask commonly has a 50 ml portion of corrugated tubing which acts as a small reservoir. At the end of the corrugated tubing there is a jet-orifice, which is connected to small-bore tubing. Some air-entrainment masks come with multiple jet-orifices specifically designed to deliver the desired FiO2 (Fig. 11.3), while others have manufacturer provided adjustable jet orifices. Venturi masks deliver concentrations ranging from 24 to 50%. Each system has oxygen flow requirements indicated to deliver the precise FiO2. Increase or decrease in this flow rate will affect delivered oxygen concentration. It must also be noted that backpressure in the system will increase the delivered oxygen. Back-pressure is commonly caused by blockage of the entrainment ports. Particular attention should be paid to this, as it will limit patient inspiratory flow and increase FiO2.
Supplied medical gas is dry and devoid of any humidity. When using an air-entrainment mask, the high flows needed to meet the patient needs may result in mucosal drying and airway irritation. The high flows will also result in excessive backpressure build up if a bubble humidifier is used, resulting in backpressure pop-off alarm. To address this, some manufacturers supply a humidification hood. The 22-mm plastic collar (Fig. 11.3) can be attached to a bland-aerosol nebulizer to provide humidity. Bland Aerosols can either be cool or heated as indicated by the clinical situation.
As previously mentioned, performance of an air-entrainment mask may be altered by resistance to gas flow occurring distal to the jet–orifice, resulting in less room-air entrainment and lower total delivered flow. If the flow is decreased significantly enough, the patient may only receive room-air. This is the first step in troubleshooting the system when periods of hypoxemia are noted. In addition, at the 50% oxygen setting, total flow delivered is far less than at lower oxygen concentrations. This creates the potential for the patient to receive a lower FiO2 in times of increased inspiratory flow requirements.
Reservoir Masks
Reservoir masks consist of a mask and plastic reservoir bag with or without a one-way valve to hold oxygen while barring exhaled CO2 form being rebreathed. Fresh oxygen is supplied to the system via the neck of the mask, directed into the bag reservoir where it can be easily withdrawn during inspiration. The bag increases the total volume of fresh gas supplied for each breath, functionally delivering a higher oxygen concentration. The mask is designed with exhalation ports for exhaled gas elimination. These ports can have plastic valves added to either one or both sides to limit room air entrainment and increase the delivered FiO2.
Reservoir masks have the capability to provide moderate to high oxygen concentrations. Ensuring these concentrations requires an appropriately sized mask with a tight-fit, which makes these devices less then optimal for long-term use. Reservoir mask are not well-tolerated by infants and small children. They are not recommended for neonates.
Partial Non-rebreather
Partial non–rebreather mask is similar to a simple facemask, but it includes a plastic reservoir bag at the end of the bottom of the mask. It differs from a non-rebreather mask (discussed in detail later) as they do not have a one-way valve to prevent rebreathing of the exhaled breath. The device is designed to conserve oxygen by delivery of 100% oxygen and allowing for partial rebreathing of the exhaled gas, which increases FiO2 at lower flow-rates. A majority of the exhaled gas is vented through 2 ports, one on each side of the mask. As in all masks, the mask should fit securely on the patient’s face with little to no leak. A leak will allow for room-air entrainment decreasing delivered oxygen concentration—a common mistake when utilizing high concentration masks. Oxygen flow should be set at a rate to ensure that the bag remains partially inflated during inspiration. Usually, 6–15 L/min is adequate. In the event the bag deflates totally, more flow is need and the oxygen flow rate should be increased. If inadequate flow is not addressed it may result in CO2 retention. With a good seal and sufficient flow, a partial-rebreather mask can deliver FiO2 of up to 0.6. It must be noted as in other oxygen delivery devices, the delivered oxygen concentration will be influenced by the patient’s respiratory pattern. Caution should be taken in patients considered to have a full stomach with altered mental status, as the closed design of the mask may increase the risk of aspiration (Fig. 11.4).
Non-rebreather Mask
Non-rebreathing masks are very similar to partial rebreather mask, except they have a one-way valve located between the mask and the reservoir bag to prevent rebreathing of exhaled gas which is directed through the exhalation ports located on either side of the mask. Exhalation ports have one-way rubber/plastic leaflets to prevent room air entrainment. These design characteristics allow for the delivery of higher oxygen concentrations when compared to either a simple facemask or a partial non-rebreathing mask. Like a partial non-rebreathing mask, flow rates should be set at a level high enough to ensure the bag does not completely deflate. If the bag deflates completely, additional flow should be added to meet the patient’s inspiratory flow demand. With proper and snug fit, non-rebreather mask can provide oxygen concentrations greater than 90%, and in the best situations close to 100%. Because of its design, these masks can be used to deliver specialty gas mixtures such as Helium–oxygen or sub-atmospheric FiO2 via a blender set-up. It must be noted that these masks are not intended for long-term use and must be evaluated frequently for pressure breakdown form the strap or non-compliance. Also aspiration risk and CO2 retention should also be considered when using these mask devices (Fig. 11.5).
Oxymask™ (High and Low-flow)
The Oxymask™ is a high-flow system that incorporates a “Pin and Diffuser” technology, which is designed to concentrate and redirect oxygen flow towards the patient’s nose and mouth. The device has an open facemask allowing for room air entrainment, thus not limiting the patient’s inspiratory flow demand while removing the need for valves and reservoirs used in partial- and non-rebreather masks. Oxygen delivery is a function of oxygen flow rate ratio to the patient’s inspiratory flow and tidal volume. The device delivers FiO2 rate form 24 to 90% at flow rates between 1 and >15 L/min. The open mask design allows for carbon dioxide to disperse into the environment during exhalation, removing the risk of CO2 retention. Additionally, the open mask design decrease the risk of aspiration of vomitus. Other benefits include reduction of setup errors, simplified flow adjustments, decreased oxygen use compared with the traditional oxygen interfaces, and the ability to use one device to manage oxygen delivery across all supplemental oxygen needs (low and high flow delivery systems). Oxymasks come in four sizes, from standard adult to infant appropriate.
An additional option available using the Oxymask™ technology is the OxyMask™ ETCO2, which allows for non-invasive end-tidal carbon dioxide (ETCO2) monitoring. The device allows for low and high flow oxygen delivery with uninterrupted side-stream ETCO2 monitoring. This device delivers FiO2 ranging from 24 to 65% at 1 to >15 L/min during end-tidal CO2 monitoring. Oxygen administration and monitoring of ETCO2 are useful during conscious sedation, bronchoscopy, endoscopy, and interventional radiology. The design, oxygen delivery range, and ETCO2 monitoring allow this device the ability to have one oxygen delivery device that supports a wide range of supplemental oxygen needs and flow rates. The OxyMaskTMETCO2 version comes in 3 sizes that include standard adult, large adult and pediatric (Table 11.3).
11.2 Humidification Systems
The upper respiratory tract warms, humidifies, and filters the inspired gas. The primary location where this occurs is the nasopharynx, where the highly vascularized moist mucus membrane efficiently conditions inhaled gas because of its large surface area and turbulent flow created by the nasal turbinates. The system is so efficient that in the coolest and dry conditions, the inspired gas reaching the alveolus is fully saturated at body temperature.
Insensible water losses are also a consideration when evaluating airway humidification in children. It is estimated that 30% of insensible losses are from the respiratory tract in children, one and a half times greater than in adults. The remaining 70% loss is from the skin, which is also greater than in adults because of greater body surface to weight ratio. In the clinical setting, adding humidification during artificial respiratory support will decrease insensible water losses from the respiratory tract.
Fundamentals of Humidification
The physical properties of humidification and their role in temperature regulation and mucociliary clearance are essential in supporting respiratory tract homeostasis during both non-invasive and invasive ventilatory support.
Absolute humidity (AH) is the total amount of water vapor that can be contained in a volume of gas, often expressed as mg/L or as partial pressure of water (mmHg). AH increases commensurately with temperature. Clinically, heating the inspired gas in the humidifier provides more humidity to the respiratory tract enhancing mucociliary clearance.
Relative humidity (RH) is the percentage (%) of water vapor contained in a volume of gas relative to its total maximum water carrying capacity. RH may be 100% at low temperatures, as cool or cold gas has a decreased capacity to hold water vapor. For this reason, the relationship of AH with temperature is more important to consider in clinical practice. For example, at a RH of 50%, the column of gas is holding ½ of its maximum possible water vapor it can hold. Clinically, providing only 50% RH will result in water being pulled from the respiratory tract resulting in tissue drying and thickening of secretions. Inadequate humidity may result in mucus plugging and sometimes and airway bleeding.
The temperature at which a gas is 100% saturated with water is the dew point. Clinically, the amount of water vapor in the inspired gas is important as it is responsible for condensation of water in the circuit, often referred to as “rainout”. The greater the temperature drop from the heating chamber to the airway, greater the potential for circuit rainout. Heated wire circuits have helped decrease the rainout as the inspiratory gas temperature drop is minimized.
Types and Function of Humidifiers
All patients undergoing mechanical ventilator support via an artificial airway require gas conditioning by either active or passive humidification systems. Passive devices such as heat moisture exchangers (HMEs) are better suited for short-term (≤96 h) or during transport. Chronically ventilated patients benefit from HMEs during trips out of the home, as the device provides some filtration while providing acceptable humidification. They are useful in clinical situations where short-term ventilation is needed such as post-operatively. Active humidification is well suited for clinical situations where prolonged ventilation is needed.
Active humidifiers use an external energy source to heat and condition inspiratory gas within a reservoir. Once the water vapor is added to it, the gas travels through the inspiratory limb or oxygen supply line to the patient’s airway. Current active devices include a heated-wire in the inspiratory limb of the circuit limiting temperature loss as the column of gas moves away from the heat source toward the airway.
Passover Humidifiers Passover humidifiers add water vapor as inspiratory gas “passes” over a reservoir. This type of humidifier is the simplest and least efficient type of high-flow humidifier. These systems may or may not be heated, and are infrequently used for invasive mechanical ventilator support. These are used for short-term and temporary indications such as use in the emergency department.
Bubble Humidifiers Bubble humidifiers are most commonly used on low-flow oxygen delivery systems such as nasal cannula. Source gas is directed into a tube submerged in a column of water held in a reservoir. The gas exits through a grid at the tube creating bubbles that increases the surface area and adds humidity to the gas. Bubble humidifiers are cost effective for short-term use in non-invasive low-flow systems. However, they do not add enough water vapor for use in invasively supported patients. This is used most commonly in neonates and young infants.
Cascade Humidifiers Cascade humidifiers provide humidity as gas from the ventilator is directed below the water surface contained in the reservoir. The gas bubbles pass through a grid, essentially making the device an efficient bubble humidifier. The incorporated grid creates a foam or froth of small bubbles that absorb water. Cascade humidifiers have a thermostat built into the device to ensure an adequate temperature which is usually set at approximately body temperature (34–37 °C). It is important to note, that cascade humidifiers deliver water vapor, however they may also deliver micro-aerosols to the patient, increasing the risk of bacteria transmitted to the patient if the reservoir is contaminated.
Wick Humidifiers Wick humidifiers are passover humidifiers modified with a “wick” constructed of blotter paper, which is surrounded by a heating element. The base of the wick is submerged in water, which is absorbed. Gas surrounds the heated moist wick, increasing the relative humidity. The large gas/liquid interface adds water vapor without increasing the volume of the reservoir. These types of humidifiers are efficient.
Passive Humidifiers Passive humidifiers use the patient’s own temperature and hydration to add humidity, functioning without the electricity or additional water source. They are often referred to as artificial noses since they mimic the action of the nasal cavity by conditioning the inspired gas.
Heat and Moisture Exchangers (HMEs) HMEs contain a condenser element retaining moisture from the exhaled breath returning it back to the less humid inspired gas. Unlike active humidifiers, which are placed in the proximal portion of the inspiratory limb, HMEs are placed at the hub of the endotracheal tube. Limitations of HMEs include the risk of increased airway resistance, increased work of breathing, inadvertent PEEP generation, the need to be removed for aerosol administration, adding dead space and the need to be changed every 48 h.
Several types of HMEs exist, with nomenclature based on device design. Some of the HMEs incorporate layered aluminum with no fibrous component. Aluminum transfers temperature efficiently during exhalation, which allows for condensation to form between the layers. The heat and humidity is then transferred back to the airway during inspiration. Some designs add a fibrous element that aids in moisture retention, and decreases pooling of condensation. These types of HMEs are the least efficient design, though they are most cost effective for short-term use—ideal for operating room setting. Newer HME designs incorporate media that are more efficient in providing both heat and humidity. These include hydrophobic, hygroscopic, and combined designs.
Contraindications: Contraindications to the use of HMEs include thick copious secretions, large leak such that exhaled tidal volume is <70% of delivered tidal volume, use of low tidal volume lung protective strategies, hypothermia (body temperature <32 °C), high minute ventilation, and the need for in-line drug aerosols.
11.3 Respiratory Care Therapeutics
Aerosolized drug administration is perhaps the most widely used therapy in the treatment of infants and children with respiratory diseases. Drug aerosols are used for a variety of diseases from reactive airway disease to lung infections. The unique challenge in drug aerosol therapy in patients with respiratory illnesses is to identify the most effective and practical method of delivery to ensure optimal therapeutic effect without compromising the safety of the patient. The therapeutic index of the drug when administered as an aerosol is enhanced since it is delivered directly to the site of action. Optimal dosing will depend upon the size of the patient, delivery devices used, the procedure used to deliver the medication, and the type of drug used.
Compared to adults, delivery of aerosolized particles to the distal airways in infants and children is poor. Small airway caliber with greater airway resistance, high respiratory rate with a short inspiratory time, increased chest wall compliance, ineffective coordination of effort and inconsistent breath-holding maneuvers all contribute to the poor delivery of aerosols in the airways of infants and children. Despite poor aerosol delivery, a clinical response to inhaled medications is often observed. Knowing that a physiologic response to inhaled medications is determined by the amount of drug that reaches the site of action in the respiratory tract, the goal would be to control as many variables responsible that affect its delivery.
Delivery Devices
The generic term nebulizer includes a number of aerosol-generating devices. Each device has both benefits and limitations. The ideal particle size should be at least 1–5 micron for deposition in the distal airways. Currently, four types of delivery systems are available for clinical use that generate medication aerosols. These are the jet nebulizers (small volume and large volume), ultrasonic nebulizers, metered-dose inhalers, and dry-powder inhalers. Nebulizer performance and the efficacy of therapy depend on the type of nebulizer, gas flow rate, nebulizer volume fill, breathing pattern of the patient, and airway geometry.
Small-Volume Nebulizers Small-volume nebulizers generate aerosols by converting a liquid medication into small particles using a compressed gas source. The primary benefit in the pediatric patient population is the desired dose is given over a longer period of time rather than in one to four breaths, as young children often have irregular breathing pattern. There are many technical and patient-related factors that must be considered when using small volume nebulizers for intermittent therapy. The aerosol deposition in the lungs using these nebulizers with a mouthpiece or a face mask is about 8–12% with almost 30% remaining in the nebulizer.
Large volume nebulizers Large volume nebulizers utilize a similar jet nebulization principle found in the small volume nebulizer but with a larger medication basin, and therefore can be used for longer periods. Duration of therapy is dependent upon the output performance and the amount of medication made available in the basin. This type of nebulizer has been developed primarily for continuous aerosol delivery.
Jet Nebulizers (Pneumatically-Driven) Also known as handheld nebulizers, updraft nebulizers, and unit-dose nebulizers, jet nebulizers are small reservoir gas-powered devices that are the most cost-effective means to deliver an aerosolized medication. These nebulizers utilize the “jet-shearing” principle, created by an external gas source forced through a small lumen contained in a medication cup. As the gas expands—localized negative pressure develops pulling the medication up a feeder tube. The liquid enters the gas stream resulting in the formation of droplets, which then enter a baffle. The smaller particles exit the reservoir following the baffling process. Larger particles drop back into the reservoir for re-nebulization. Two limitations of these devices include large amount of drug wastage, and evaporation with recirculation resulting in increased amount of drug necessary for the therapeutic effect.
Mesh Nebulizers Vibrating mesh nebulizers create a fine aerosol by moving a drug solution through a plate or mesh with small holes. The diameter of these holes or apertures determines the particle size. These devices do not use an external gas source, instead the nebulizer is powered by an electrical power source, which can be battery powered for travel. Lack of additional flow provides the benefit of more normal breath delivery and triggering capacity. In addition to these benefits, vibrating mesh nebulizers also have small dead space volume, ranging between 0.1 and 0.5 mL.
Ultrasonic Nebulizers Ultrasonic nebulizer uses a piezoelectric crystal that produces a highly concentrated output of aerosol particles and has historically been used for cough and sputum production. Ultrasonic nebulizer with its highly concentrated output, may perform better than small volume nebulizer in accomplishing greater deposition of medications in children.
Metered-Dose Inhaler The metered-dose inhaler uses a pressurized canister that dispenses a single bolus of aerosolized medication. They are convenient, cost-effective, versatile, and generally have an effective deposition rate of 10–15%. To optimize the delivery of the drug, the patient must be able to coordinate a series of inspiratory maneuvers while activating the canister. Low inspiratory flow rates, inspiratory pause or sustained maximal inflation maneuver facilitates better deposition. Lower flow rates reduce aerosol impaction in the oropharynx and inner walls of the airways while breath-holding improves deposition by gravity. A spacer device can be a valuable attachment if the metered-dose inhaler is used by children. By adding a spacer device to the inhaler, the synchronized effort is less of a concern and drug delivery is maximized. The canister is activated into the spacer and the medication remains suspended in the chamber until the patient inhales. For younger children or infants, a mask is added to the spacer for more efficient delivery (Table 11.4).
Aerosols Delivered Through Ventilators
Aerosol delivery is not very efficient when delivered through a ventilator. The endotracheal tube is the most significant barrier to effective delivery. Smaller the inner diameter of the tube, the less efficient is aerosol delivery. In addition to the endotracheal tube, several other factors impact the delivery of aerosols by mechanical ventilators. The nebulizer is most effective when placed near the inspiratory portion of the ventilator circuit which serves as a spacer chamber, similar to the spacer used for metered dose inhalers. The aerosolized particles remain suspended in the inspiratory limb awaiting to be delivered with the ensuing ventilator breath. Aerosols must be generated during the expiratory phase of the ventilator cycle to fill the inspiratory limb. Therefore, a synchronized nebulizer mode is essential where a portion of the preset inspiratory gas is diverted to power the nebulizer. One of the concerns of using aerosols with ventilators is the potential for collection of the particles in the expiratory filter increasing the expiratory resistance or complete blockage of the expiratory port. Filters must be monitored and changed frequently to avoid obstruction or added resistance. In such a circumstance, airway pressures and tidal volumes delivered to the patient should be measured at the hub of the endotracheal tube. An alternative to nebulizers is a metered-dose inhaler, especially with the use of a spacer. Variables affecting aerosol delivery during mechanical ventilation include the nebulizer power source, nebulizer characteristics, ventilator settings, temperature and humidity, location of the aerosol device, and the size of the artificial airway. It is important to consider whether a particular device would be tolerated well by the patient. The aerosol output characteristics are also equally important to consider when selecting an aerosol device.
11.4 Specialty Gases
Altering Inspired Oxygen and Carbon Dioxide Concentration
With certain types of congenital heart disease with single ventricle physiology such as hypoplastic left heart syndrome, it is critical to control pulmonary blood flow and prevent pulmonary over-circulation and systemic hypoperfusion. This can be accomplished by increasing pulmonary vascular resistance and reducing pulmonary blood flow while increasing blood flow to the systemic circulation. One approach is to decrease the FiO2 to less than 0.21 with a blending of room air with nitrogen which causes hypoxic pulmonary vasoconstriction. The exact FiO2 delivered must be monitored to avoid administering excessively low inspired oxygen and causing severe hypoxemia. The other approach, especially in patients undergoing mechanical ventilation, both preoperatively and postoperatively, is to increase the inspired carbon dioxide concentration (FiCO2). Increased FiCO2 also increases pulmonary vascular resistance. During mechanical ventilation, increased FiCO2 allows one to hyperventilate, recruit the lungs, and prevent atelectasis without producing hypocarbia. One of the difficulties with a boost in FiCO2 is increased spontaneous ventilatory drive due to an increased PaCO2. This increases the work of breathing and with marginal cardiac reserve, may impose an undue strain on the heart. Therefore, neuromuscular blockade and total ventilatory support may be necessary with increased FiCO2 to avoid an increased workload on the heart.
Helium–Oxygen Mixture
Based on the physics of airflow and the properties of helium oxygen mixture (Heliox), certain outcomes can be predicted with its use: (1) Heliox will results in a higher flow when transairway pressures are held constant, (2) Heliox will result in a lower airway pressure when the airflow is constant, (3) Density-dependent flow meters will underestimate flow, (4) Heliox can decrease the degree of air-trapping and hyperinflation associated with lower airway obstruction, (5) Heliox can decrease the work of breathing, and (6) Heliox can result in better deposition of aerosols administered with it. Helium is usually administered in at least 30% to 40% oxygen. However, for helium to be effective, it should constitute at least 50–60% of the inspired gas. Heliox therapy is therefore not helpful in patients requiring greater than 0.5 FiO2. Oxygenation should be monitored during administration of helium–oxygen mixture to avoid hypoxemia, especially in neonates.
Inhaled Nitric Oxide
Inhaled nitric oxide produces selective pulmonary vasodilation. Indications for inhaled nitric oxide include diaphragmatic hernia, pulmonary hypertension after repair of congenital heart disease, primary pulmonary hypertension, and isolated right heart failure. Not all patients respond to inhaled nitric oxide. It is prudent to test whether a patient will respond to inhaled nitric oxide. A 2-h trial of inhaled nitric oxide with 20–40 ppm is administered to infants and children with hypoxemic respiratory failure. A good response is defined as improvement in PaO2/FiO2 ratio of greater than 100%. A partial response is defined as an improvement in PaO2/FiO2 ratio between 50 and 100%. If the response is less than 50%, the patient is considered a non-responder. Inhaled nitric oxide is then continued in only those patients who show a partial or good response. Nitric oxide binds to hemoglobin to produce methemoglobin. Therefore methemoglobin levels should be monitored during the administration of nitric oxide. In addition, nitric oxide combines with oxygen to form nitrogen dioxide. Nitrogen dioxide is known to cause lung injury. Therefore the concentration of nitrogen dioxide should be monitored in the inspired gas to keep it below 1–2 ppm. To minimize complications, the inhaled nitric oxide therapy should be continued at the lowest possible concentrations that are still effective in producing the desired therapeutic effects.
Suggested Readings
McKienan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannula therapy in infants with bronchiolitis. J Pediatr. 2010;156(4):634–8.
Milesi C, Pierre AF, Deho A, et al. A multicenter randomized controlled trial of a 3-l/kg/min versus 2-l/kg/min high-flow nasal canula flow rate in young infants with severe viral bronchiolitis (TRAMONTANE 2). Intensive Care Med. 2018;44(11):1870–1870.
Wing R, James C, Maranda LS, Armsby CC. Use of high-flow nasal cannula in the emergency department reduces the need for intubation in pediatric acute respiratory insufficiency. Pediatr Emerg Care. 2012;28(11):1117–23.
Franklin D, Babl FE, Schlapbsch LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378:1121–31.
Bhashyam AR, Wolf MT, Marcinkowski AL, Saville A, Thomas K, Carcillo JA, Corcoran TE. Aerosol delivery through nasal cannulas: an in vitro study. J Aerosol Med Pulm Drug Deliv. 2008;21:181–8.
Corcoran TE, Sallille A, Adams PS, et al. Deposition studies of aersol delivery by nasal cannula to infants. Pediatr Pulmonol. 2019;54:1319–25.
Fontanari P, Burnet H, Zattara-Hartmann MC, Jammes Y. Changes in airway resistance induce by nasal inhalation of cold dry, dry, or moist air in normal individuals. J Appl Physiol. 1996;81:1739–43.
Byerly FL, Haithcock JA, Buchnana IB, et al. Use of high flow nasal cannula on a pediatric burn patient with inhalation injury and post-extubation stridor. Burns. 2006;32:121.
AL Ashry HS, Modrykamien AM. Humidification during mechanical ventilation in the adult patient. BioMed Res Int. 2014;Article ID 715434:12 pages.
Baudin F, Gagnon S, Crulli, et al. Modalities and complications associated with the use of high-flow nasal cannula: experience in a pediatric ICU. Respir Care. 2016;61(10):1305–10.
Sarnaik SM, Saladino RA, Manole M, Pitetti R, Arora G, Kuch BA, Orr RA, Felmet KA. Diastolic hypotension is an unrecognized risk factor for ß-agonist-associated myocardial injury in children with asthma. Pediatr Crit Care Med. 2013;14(6):e273–9.
Kalister H. Treating children with asthma, a review of drug therapies. West J Med. 2001;174:415–20.
Petersen W, Karup-Pedersen F, Friis B, Howitz P, Nielsen F, Stromquist LH. Sodium cromoglycate as a replacement of inhaled corticosteroids in mild-to-moderate childhood asthma. Allergy. 1996;51:870–87.
de Benedictis FM, Tuteri G, Pazzelli P, Berotto A, Bruni L, Vaccaro R. Cromolyn versus nedocromil: duration of action in exercise-induced asthma in children. J Allergy Clin Immunol. 1995;96:510–4.
Wisecup S, Eades S, Hashmi SS, Samuels C, Mosquera RA. Diastolic hypotension in pediatric patients with asthma receiving continuous albuterol. J Asthma. 2015;52(7):693–8.
Davis MD, Donn SM, Ward RM. Administration of inhaled pulmonary vasodilators to the mechanically ventilated neonatal patient. Pediatr Drugs. 2017;19(3):183–92.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kuch, B.A., Venkataraman, S.T. (2022). Respiratory Care Equipment. In: Sarnaik, A.P., Venkataraman, S.T., Kuch, B.A. (eds) Mechanical Ventilation in Neonates and Children. Springer, Cham. https://doi.org/10.1007/978-3-030-83738-9_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-83738-9_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83737-2
Online ISBN: 978-3-030-83738-9
eBook Packages: MedicineMedicine (R0)