Abstract
Pulmonary hypertension is a life-threatening condition that affects people of all ages that can occur as an idiopathic disorder at birth or as part of a variety of cardiovascular and infectious disorders. It is commonly treated with inhaled pulmonary vasodilators such as nitric oxide and less frequently using formulations and analogs of prostacyclin. To minimize systemic effects and preserve pulmonary vasodilation, vasodilators are often administered directly into the airway. Nitric oxide is the only USA Food and Drug Administration-approved inhaled pulmonary vasodilator that can be used during mechanical ventilation. Over the past two decades, interest has grown in the use of aerosolized prostacyclin and prostacyclin analogs for the treatment of pulmonary hypertension during mechanical ventilation. Clinicians who administer inhaled prostacyclin may not have a clear understanding of its risks because of the lack of data from large clinical trials examining safety and efficacy; moreover, its safe use remains poorly documented. The off-label use of drugs is legitimate, but prescribers must recognize the potential complications and liability in doing so. This manuscript aims to address potential problems related to the aerosol administration of pulmonary vasodilators in the mechanically ventilated neonatal patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Limited safety data exist pertaining to inhaled prostacyclin during mechanical ventilation. |
Several concerns should be taken into consideration when delivering inhaled pulmonary vasodilators. |
The alkaline excipients aerosolized with prostacyclin may have unwanted effects on the airways. |
1 Introduction
Pulmonary hypertension (PHTN) is a life-threatening condition that affects people of all ages, and is commonly treated with inhaled pulmonary vasodilators (IPVs) such as nitric oxide (NO) and less frequently prostacyclin (PGI2) [1]. PHTN can occur as an idiopathic disorder at birth, diagnosed as persistent PHTN of the newborn (PPHN), or it can occur as part of a variety of cardiovascular and infectious disorders. PPHN occurs in two per 1000 live births, and is particularly difficult to treat in newborns with anatomic connections between the pulmonary and systemic circulations, with a mortality of 4–33% [2]. To minimize systemic effects and preserve pulmonary vasodilation, vasodilators are often administered directly into the airway. Aerosol administration of IPVs has disadvantages along with advantages. One disadvantage to the inhaled route of administration is that abrupt discontinuation of treatment or reduction in dosage can result in vasoconstriction and rebound PHTN that exacerbates hypoxemia. Topical effects within the lung must also be considered when administering a chemical by inhalation, particularly during mechanical ventilation, since the upper airways are bypassed and the topical effects with the lung may increase. NO is the only Food and Drug Administration (FDA)-approved IPV that can be used during mechanical ventilation. Inhaled nitric oxide (iNO, INOmax®) is indicated for treatment of term and late preterm neonates with PPHN complicated by hypoxemic respiratory failure (HRF) [3]. iNO also has an FDA-cleared delivery system that has been validated for more than 60 commercially available ventilators. Over the past 2 decades, interest has grown in the use of aerosolized prostacyclins for the treatment of PHTN during mechanical ventilation. Although two analogs of prostacyclin have been approved by the FDA for inhalation, no formulation of prostacyclin has an FDA-cleared delivery device for use in conjunction with mechanical ventilation. Clinicians who administer inhaled prostacyclin may not have a clear understanding of its risks because of the lack of data from large clinical trials examining safety and efficacy; moreover, its safe use remains poorly documented. The off-label use of drugs is legitimate, but prescribers must recognize the potential complications and liability in doing so [4], [5]. This manuscript aims to address potential problems related to the aerosol administration of pulmonary vasodilators, especially prostacyclin, in the mechanically ventilated neonatal patient.
2 The Pathophysiology of Pulmonary Hypertension (PHTN)
The neonate is particularly susceptible to hypoxemia from pulmonary hypertension (PHTN) because of intracardiac and extracardiac shunts between the pulmonary and systemic circulations through the foramen ovale and ductus arteriosus, respectively (Fig. 1). The distribution of blood flow is inversely proportional to the resistance on either side of the shunt. As an example, using the ductus arteriosus, the higher the vascular resistance in either of these circulations (pulmonary or systemic), the lower the fraction of flow to that circulation, where PVR is pulmonary vascular resistance and SVR is systemic vascular resistance:
Models depicting the flow of blood through the hearts of a normal neonate (a) and a neonate with right-to-left intracardiac shunting (b). The blue arrows represent the flow of deoxygenated blood. The red arrows represent the flow of oxygenated blood. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
This equation demonstrates the relationship between pulmonary vascular resistance and pulmonary blood flow. The pathogenesis of PHTN occurs because increased pulmonary vascular resistance limits pulmonary blood flow and thus gas exchange. The therapeutic strategy involves agents to dilate the pulmonary vascular bed to reduce pulmonary vascular resistance and improve pulmonary blood flow.
Another important physiologic principle governing vascular resistance is Poiseuille’s law:
Three pathophysiologic processes in the newborn can raise pulmonary vascular resistance: (1) increased viscosity of the blood, which occurs with polycythemia (hematocrit >65%) [6]; (2) reduced vessel number, associated with pulmonary hypoplasia, such as that occurring with congenital diaphragmatic hernia [7–9]; or (3) reduction of the radius of the pulmonary vessel lumen, which occurs with vasoconstriction, hypertrophy of the pulmonary vascular media muscularis [10], and diffuse pulmonary vascular thrombosis [11]. Vessel narrowing causes an increase in pulmonary vascular resistance proportional to the fourth power of the vessel radius.
Pulmonary vasoconstriction in the newborn is often associated with perinatal infections, especially group B streptococcus (GBS) [12, 13]. The vasoconstrictor effects of GBS appear to be mediated by the release of thromboxane-A2, which can be blocked by indomethacin, a nonspecific cyclooxygenase inhibitor, and by dazmegrel, a selective thromboxane antagonist [12]. Endotoxin associated with gram-negative infections is also a potent pulmonary vasoconstrictor.
Hypoxemic respiratory failure (HRF) frequently leads to PHTN because oxygen is one of the most potent pulmonary vasodilators and, conversely, hypoxemia is a potent pulmonary vasoconstrictor. Pulmonary artery flow of relatively desaturated blood may continue to alveoli that are not participating in gas exchange because of inflammatory cells occluding the airway, or from severe alveolar collapse, as occurs in respiratory distress syndrome in premature newborns. On the other hand, pulmonary vasoconstriction from a variety of stimuli (such as endotoxin or acidic blood) may reduce or eliminate blood flow to areas that are participating in gas exchange. Both situations cause ventilation/perfusion mismatch in which blood perfusing the lungs through the pulmonary artery leaves the lung through the pulmonary veins without adequate oxygenation, leading to systemic hypoxemia, which leads to further acidosis and pulmonary vasoconstriction, exacerbating this vicious cycle.
2.1 Treatment of PHTN
Understanding our therapeutic options has furthered our understanding of the etiology of PHTN. Following the report by Gersony et al. [14], who described the syndrome of persistent fetal circulation in two infants with profound hypoxemia and clear lung fields, the search began for an effective, therapeutic, selective pulmonary vasodilator. Gersony et al. initiated treatment with tolazoline, a non-specific vasodilator used to lower pulmonary vascular resistance in adults and children with ventricular septal defects [15]. While many of the pharmacologic properties of tolazoline relate to its agonistic and antagonistic effects on different mediators and receptors, its most important vascular effects are mediated through release of histamine [16]. In the newborn, histamine dilates the pulmonary arteries as well as the systemic vessels, and decreases both pulmonary and systemic vasculature resistances. Histamine also stimulates gastric acid release, so much that it can cause gastric ulceration and perforation in newborns with persistent PHTN of the newborn (PPHN) [16–19]. Tolazoline required administration through a vein draining through the superior vena cava to minimize shunting through the foramen ovale, but its long half-life and histamine release aggravated systemic hypotension and led to further right-to-left shunting [15, 16, 20]. It was clearly not a selective pulmonary vasodilator and was removed from the US market.
The development of extracorporeal membrane oxygenation (ECMO) in the 1980s allowed rescue of many newborns with PPHN until their pulmonary vasculature reverted from a thick-walled, high-resistance circulation to the thinned pulmonary arteries typical of older children and adults [21, 22]. Later, a new substance isolated from the endothelium was found to dilate the pulmonary vasculature. Initially, it was referred to as endothelium-derived relaxant factor (EDRF); subsequently, researchers identified EDRF as nitric oxide (NO) [23, 24]. Inhaled NO (iNO) was shown to dilate the pulmonary vasculature through increased synthesis of cyclic guanosine monophosphate (cGMP) [25]. Much of its selectivity comes from administration through the airway. iNO diffuses into the pulmonary interstitium, where it dilates the pulmonary capillaries before entering the bloodstream. Once in the blood, NO immediately binds to hemoglobin to form methemoglobin. Adverse effects of methemoglobinemia produce a dose limitation to iNO treatment; however, this rarely occurs at doses used clinically (≤20 ppm).
Besides NO, several other chemicals and conditions have been identified that can either constrict or relax the pulmonary vasculature (Table 1). Kinsella and Abman [26] outlined many of these factors and directions for further treatments.
2.2 Prostacyclin
Attempts to selectively lower pulmonary vascular resistance by local drug administration have led to inhalation of various vasoactive compounds, such as prostacyclin and its structural analogs, prostaglandin E compounds, milrinone [a phosphodiesterase (PDE)-3 inhibitor], and even the now discontinued tolazoline. The prostanoids derive their selectivity for dilating the pulmonary vasculature by inactivation before they reach the systemic circulation. Should the dosage exceed the clearance capacity within the lung and its vasculature, dilation of the systemic vasculature occurs; this may induce systemic hypotension and result in or exacerbate right-to-left vascular shunts, especially in the newborn.
Clearance of traditional prostacyclin occurs rapidly within the lung (half-life of 6.5–10 min), and has been studied extensively since its discovery as PG-X by Moncada and Gryglewski [27–30]. Of note, newer-generation analogs of prostacyclin have longer half-lives; treprostinil, for example, has a half-life of 4.4–4.6 h [31, 32].
After release of arachidonic acid (eicosa-all cis-5,8,11,14-tetraenoic acid) from its phospholipid base, cyclooxygenase-1 (COX1), the constitutive form, and cyclooxygenase-2 (COX2), the inducible form, generate the endoperoxides PGG2 and PGH2. In the endothelium, COX2 provides the mitochondrial prostacyclin synthase (PGI-S) with PGH2, from which it forms prostacyclin, a potent vasodilator. The pulmonary endothelium is the major source of prostacyclin in the body [27]. Prostacyclin is then inactivated by PDE-3 and PDE-4 to 6-keto prostaglandin F1α. Elucidation of this pathway has led to treatment with inhaled prostacyclin along with intravenous milrinone, an inhibitor of PDE-3, an inotrope and a vasodilator. This combination of vasodilators is effective, but excessive dosages can lead to systemic hypotension.
The pivotal pharmacologic feature that supports efficacy is dilation of the pulmonary vasculature more than the systemic vasculature. Both prostaglandin E1 (PGE1) and prostaglandin I2 are rapidly inactivated within the pulmonary vasculature by PDE-3 and PDE-4. They have the potential to provide selective pulmonary vasodilation as long as the enzymes are not saturated.
The desire for an alternative treatment to iNO non-responders and a cheaper PHTN treatment has generated significant interest in the use of aerosolized prostacyclin. Studies evaluating the use of aerosolized prostacyclin date as far back as the 1980s; however, interest in this as an alternative to iNO greatly increased in the mid-1990s. In fact, more than 90% of reports on aerosolized prostacyclin have been published after 1995. It is important to note that no formulation of prostacyclin is available that is Food and Drug Administration (FDA) approved for inhalation during mechanical ventilation. Initially, researchers and clinicians aerosolized intravenous formulations of prostacyclins. Recently, two formulations of prostacyclin have been developed and approved for aerosolization in spontaneously breathing (not intubated) subjects [33, 34].
2.3 Concerns Related to the Aerosolized Administration of Prostacyclin
2.3.1 Off-Label Use
It is permissible to use drugs off-label under various clinical circumstances. These require a reasonable scientific basis, reasonable use under the circumstances, and disclosure when used for investigational purposes [5]. Whether a pharmacologic agent is approved or off-label, what constitutes negligent use would be the lack of a valid scientific basis and whether reasonable physicians would refrain from doing the same under similar circumstances. Does the fact that there is another approved drug make the off-label use of the drug unreasonable? This is not necessarily the case, but it places the user at a higher risk should an adverse event occur. This would rest on a factual determination, specific to the safety factors, relative risk-to-benefit ratio, alternatives, and costs (frequently, third-party payers will not reimburse the costs of drugs used off-label). Patients and parents of neonates need to be informed of these facts and the circumstances under which the off-label drug is being prescribed [35].
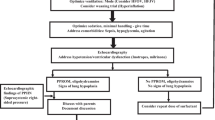
Prostacyclin poses some specific challenges for aerosolized therapy. Most of these challenges stem from limited study of this route of administration in neonates and adults [28–30]. There have been no prospective, multicenter, randomized, controlled clinical trials of inhaled prostacyclin during mechanical ventilation in any patient population, nor is there a consensus as to the safest and most effective way to administer it. Furthermore, there are no FDA-approved devices for aerosolizing prostacyclin in conjunction with mechanical ventilation (Fig. 2, bullet 1). For pediatric patients, the Flolan package insert indicates that safety and effectiveness have not been established [36]. However, a fair number of case reports and retrospective reviews have been conducted in recent years that indicate a growing use of inhaled prostacyclin during mechanical ventilation; although none of these publications were powered or designed to evaluate safety of inhaled prostacyclin, several do report similar efficacy with regard to pulmonary vasodilation and oxygenation as iNO [37–40].
2.3.2 Pharmacy Logistics
Serious or potentially serious errors have been reported with the use of prostacyclin in the critical care setting. Medication errors associated with prostacyclin treatment often stem from its preparation or administration (Fig. 2, bullet 2). The Institute of Safe Medication Practices lists intravenous prostacyclin as a high alert medication [41]. Classic formulations of prostacyclin must be dissolved shortly prior to their use because they have a limited activity in solution and cannot be stored for more than 48 h [36]. A recent survey of specialists who treat PHTN revealed that 65 of 95 respondents (68%) reported serious or potentially serious errors with the administration of intravenous prostacyclin [42]. The use of the wrong dose because of a calculation or concentration error (n = 29) and the use of a prostacyclin cassette or bag that was intended for another patient (n = 25) were two notable sources of medication errors [42]. Medication errors were examined in an observational medical record review of 16 patients receiving prostacyclin via inhalation in the Florida Hospital Health System. Medication errors were reported in three patients who incorrectly received prostacyclin intravenously, and one patient who was administered the incorrect dose [43]. Most of these errors are less likely to occur with newer-generation analogs of prostacyclin that are intended to be delivered via the inhaled route.
2.3.3 Drug Concentration Monitoring
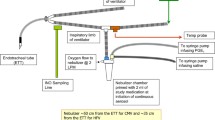
Technology to continuously monitor the administered dose of prostacyclin does not exist, which is potentially dangerous when the dosage is being adjusted to effect and when the dosage is being weaned. This concern is compounded by the fact that different nebulizers deliver different amounts of drug to the airways and that different formulations of prostacyclins have different recommended doses. In early reports of use in adults, prostacyclin caused initial improvement, but weaning from 50 to 20 ng/kg/min reversed the initial beneficial effects, with resumption of shunting and an increase in the alveolar-to-arterial oxygen pressure difference (AaDO2) [29]. During a dose-response study by Siobal et al. [44], the drug chamber both overflowed and dried. The investigators also pointed out, “The effects of evaporation during jet nebulization are often unrecognized and usually ignored, especially during continuous aerosolization”. At this time, there is no administration apparatus able to measure the concentration of drug in the nebulization container or the concentration of drug being delivered to the ventilator circuit or endotracheal tube (ETT). Consequently, the delivered dosage of continuously aerosolized drug cannot be accurately monitored. This is most relevant for drugs with very short half-lives, which require constant replenishment to continue producing pharmacologic effects (Fig. 2, bullet 4). The initial dose of prostacyclin was approximately 20% less than the desired dose because of evaporative volume loss when the reservoir is refilled [44].
Siobal et al. [44] developed a complicated dual infusion device for aerosol administration of prostacyclin. This system used a MiniHEART nebulizer (Westmed, Inc., Tuscon, AZ, USA) with a 30-mL capacity, intended to provide varying dosages of prostacyclin by aerosol, using two infusion pumps to deliver drug and normal saline into the nebulizer in different ratios. Several factors in this study prevented knowing what dosage was actually delivered. The initial dosage was always lower than the intended dosage and, as evaporation progressed, the dosage varied, but at an uncontrolled rate. The formulation prostacyclin used is unstable at pH < 10.2, and the lowest dosages delivered had a pH of 10.13 ± 0.03, so inactive drug may have been delivered [45]. The concentration of prostacyclin in the drug chamber was not measured, nor was the presence of inactive drug.
2.3.4 Drug Delivery
The factors that contribute to these inconsistencies include the specific nebulizer used, its placement within the ventilator circuit, and ventilator settings used during drug delivery. These variables ultimately determine the concentration of the drug maintained inside the nebulizer and the concentration delivered to the airways.
2.3.5 Delivery Devices
Van Heerden et al. reported the first two patients with severe acute respiratory distress syndrome (ARDS) treated with multiple vasopressors to which inhaled prostacyclin was added, with improvement in oxygenation [29]. The nebulizer for administration was locally engineered and did not account for drug evaporation or loss of drug in passage through the ventilator tubing and ETT. In the second patient, the dosage was varied from 8.3 to 50 ng/kg/min, but it is not indicated how this variation in dosage was achieved. The authors acknowledged that the delivered dose was unknown, as was how much drug was lost through the expiratory limb of the circuit. This is particularly relevant during critical care ventilation as opposed to during anesthesia, as critical care ventilators use a high and variable amount of bias flow in the ventilator circuit; this bias flow circulates aerosolized medication that bypasses the patient between breaths.
Administering aerosolized drug is affected by several uncontrolled, yet important, variables [44]. These include the carrier gas flow rate, ambient humidity, temperature of the nebulizer, and the specific device used [44]. All of these variables determine how much drug can be carried in the aerosolized mist and how much will reach the patient. If the concentration varies in the chamber, such as during dose escalation or weaning, the actual dose delivered to the ETT is not known. Based on in vitro studies with working lung models, the dosage reaching the end of the ETT can vary widely (Fig. 2, bullet 3). The concentration of drug has been shown to change as the drug and diluent in the device chamber evaporates. Finally, if aerosolization slows because of a slow carrier gas flow [44], the container may overflow as the drug continues to be infused [44].
2.3.6 Delivery Device Configuration
There is currently no consensus as to the optimal set-up for the aerosolization of prostacyclin. In the case of albuterol (the most commonly aerosolized medication), the location of the nebulizer within the ventilator circuit has varied from study to study and influences the delivered dose [46, 47]. The position of the nebulizer within the circuit, the type of nebulizer used, the amount of drug being dispensed, the ventilation modality, and the ventilator settings can affect drug delivery [46, 48]. No comprehensive studies have been carried out to determine the most effective nebulized PGI2 set-up; however, it is safe to assume that the factors that affect albuterol delivery also affect PGI2 delivery. Therefore, set-up of nebulized PGI2 delivery systems continues to be left to the discretion of respiratory therapists, who may or may not be using the most ideal configuration. Additional research is needed to validate the safest and most consistent delivery strategy (Fig. 2, bullet 5).
2.3.7 Drug Delivery Monitoring
The nebulization apparatus used to administer prostacyclin has no instrumentation to continuously monitor the concentration in the container, the delivered concentration and, thus, the delivered dosage. Experts working in this field predict that the mechanical properties of nebulizers will vary during use [49]. This is a concern when a medical device that is intended for intermittent use is utilized continuously. Nebulizers lack an alarm to warn if the concentration/dosage is too high or too low (Fig. 2, bullet 7). None of the nebulizers has received FDA clearance for continuous administration for several days, the time that is often required to treat severe PPHN.
2.3.8 Airway Deposition
Although much has been characterized about medication aerosolization and delivery, the distribution of aerosolized drug within the airway is unpredictable because of the variation in particle sizes produced by the nebulizer. In one of the first reports of inhaled prostacyclin treatment of adults, 49% of the particles were larger than 5.17 μM [50]. The particle size delivered varies with the specific brand of nebulizer and, potentially, even among nebulizers from the same manufacturer [51]. Larger particles will collect in the more proximal airway, while particles of 1–3 μM are likely to reach the alveolus. The site of deposition is important for variations in the amount of the dose delivered to the pulmonary interstitial space around the alveolus and to the route of absorption. Proximal airway absorption will return to the right atrium through systemic veins, from which it is likely to enter the systemic circulation through right-to-left shunts. This raises the risk of systemic vasodilation before the drug can be metabolized (Fig. 2, bullet 8).
2.3.9 Local Effects of the Delivered Drug
During mechanical ventilation, the upper airways are bypassed by an artificial airway. This can be of benefit when delivering inhaled medications since less medication will deposit in the upper airways and more will deposit in the lower airways. This increased targeted delivery also increases unwanted local effects; when delivering aerosolized prostacyclin, the pH and topical irritant effects of the medications should be taken into consideration.
While the pH of the lung airway lining fluid is difficult to measure accurately, measurements of exhaled breath condensate (EBC) suggest that the surface of the lung is slightly alkaline, with a pH between 7.4 and 8.2 [30]. Disturbances in the pH of the lung lining, either acidification or alkalinization, can adversely affect lung tissue. Airway acidification is associated with neutrophilic and eosinophilic inflammation, bronchospasm, bronchial hyper-reactivity, ciliary dysfunction, epithelial dysfunction, augmented oxidative damage, abnormal fluid transport, inhibition of transport of cationic drugs (such as albuterol), and inhibition of programmed cell death [46–48, 52–54, 56]. Airway alkalinization is associated with decreased mucociliary clearance, increased epithelial damage and sloughing, and the inhibition of transepithelial transport of albuterol [46–48, 53, 54, 56, 57].
To remain stable in solution, prostacyclin must be dissolved in a buffered solution. The two most common excipients of aerosolized prostacyclin are a glycine buffer (pH 10.3–12) and an arginine buffer (pH 11–13) [36, 45]. Of note, these formulations are designed for intravenous administration, not inhalation. It is not known whether inhalation of prostacyclin solution causes airway alkalinization and lung injury in humans; however, a single, 3-mL nebulized dose of glycine buffer mixed with albuterol sulfate has been shown to cause an increase in human EBC pH of 0.235 pH units [58]. The control group of this study received standard albuterol sulfate, which has a pH of 3–5 [45]. No pH change was seen in this group even though the aerosolized solution was acidic, indicating the relevance of the buffers used in the alkaline diluents as opposed to the relatively unbuffered acidic solutions in which most other inhaled medications are dissolved (Table 2). Without a potent buffer, inhaled medications are likely to assume the pH of the airway lining fluid upon which they deposit as opposed to what was observed with the glycine buffer.
Potentially irritating effects of one formulation of prostacyclin and its glycine buffer on the airway itself have been studied in animals, although the effects of the more alkaline arginine buffer have yet to be evaluated. A 5- to 7-h study in five piglets (intubated but not mechanically ventilated) showed mild tracheitis in all animals exposed to the glycine or prostacyclin diluents. Potential airway toxicity was also evaluated in mechanically ventilated lambs treated with epoprostenol by jet nebulizer for 8 h compared to animals receiving nebulized saline [29]. The lung and airways were evaluated in detail after a standardized dissection. No definite abnormalities could be demonstrated from the inhaled drug as opposed to the effects of mechanical ventilation, which were seen predominately in the trachea. The animals were large and mature, weighing 28.5–48.5 kg, and were ventilated at a rate of 12 bpm, with a positive end-expiratory pressure (PEEP) of 10 cmH2O and inspiratory time of 1.66 s. These settings are quite different from those used to support newborns and may have minimized airway trauma, whereas higher pressures and more rapid rates may predispose the neonatal trachea to trauma from mechanical ventilation that further exposes damaged tissue to prostaglandins. Very few newborns with PPHN are treated for only 8 h. Long-term effects of airway exposure to this pH must be determined, but have not been reported to date. Airway trauma from prolonged mechanical ventilation, when combined with exposure to alkaline conditions, may produce damage not seen in a short experiment and requires further study (Fig. 2, bullet 9).
Of note, subjects with inflammatory airway diseases have been shown to have a low airway pH, in which case the alkalinity of inhaled prostacyclin may have less of a caustic effect [30, 58]. Also, the concerns related to the pH of aerosolized prostacyclins are not relevant when using one of the two formulations currently approved for inhalation, both of which are buffered at a pH closer to normal physiologic levels (Table 2) [33, 34]. However, in the FDA Drug Approval Packages of treprostinil and iloprost noted cough/respiratory irritation as a common side effect of inhalation of these medications, suggesting local irritant effects of these compounds [31, 32]. These effects were observed in spontaneously breathing (not intubated) patients and may be increased when the upper airways are bypassed during mechanical ventilation.
2.3.10 Systemic Hypotension
Evaporation of drug or diluent within the nebulizer varies the concentration and the delivered dose (Fig. 2, bullet 10). This is important for a drug in which the dosage can exceed the pulmonary clearance, so that it reaches the systemic circulation where it may cause vasodilation, reduce systemic resistance, decrease systemic blood pressure, and increase shunting. Vasodilation by prostacyclin was suspected to be the cause for increased splanchnic blood flow during a study of adults comparing aerosolized prostacyclin to iNO [57].
2.3.11 Evidence of Efficacy
Few studies have compared the efficacy of iNO and aerosolized prostacyclin in patients [57, 59]. In one such study [59], the efficacy was compared in adults by measuring changes in mean pulmonary artery pressure (PAP) and pulmonary vascular resistance. The investigators failed to adjust for differences in potency and stated that prostacyclin (dosed in ng/kg/min) produced greater reductions in mean PAP and pulmonary vascular resistance than did iNO (dosed in parts per million). Pharmacologically, this is not a meaningful comparison, because differences in potency will produce different slopes for the dose-response curves. This comparison is further complicated by the lack of verification of the aerosolized dosage of prostacyclin by direct measurement of the concentration of active drug that was aerosolized. The author stated that there is no assay for prostacyclin, but a gas chromatography mass spectrometric assay was used to measure the metabolism of prostacyclin in rats in 1978 [60] and in humans in 1981 [61]. Large, randomized, controlled studies are needed to accurately determine the comparative efficacies (Fig. 2, bullet 11).
3 Summary
Airway administration of drugs to treat PHTN can improve oxygenation, lower pulmonary vascular resistance, increase pulmonary blood flow, and reduce right-to-left shunting. Although several drugs have been shown to work acutely, their pulmonary vascular “selectivity” is dependent upon metabolic clearance before they reach the systemic circulation. Complete clearance of prostacyclin and PGE1 (alprostadil) within the lung will be dose related. Minimizing the systemic effects of prostaglandins will be a challenge because of the variation in particle size and wide variation in the delivered dose. An apparatus to continuously deliver aerosolized prostacyclin during mechanical ventilation has not been developed. The prescriber must take responsibility for both the drug selection and its administration, which have been demonstrated to be variable.
There are several limitations to this review. Most of the published data regarding aerosolized prostacyclin reported the use of older-generation nebulizers and traditional formulations of prostacyclin that were not designed to be delivered via inhalation. As standard-of-care for aerosolized drug delivery during mechanical ventilation evolves towards newer-generation vibrating-mesh micropump nebulizers, concerns related to drug device selection, configuration, and delivery monitoring that are listed above will be significantly lessened. Moreover, safety concerns related to drug half-life and airway pH are greatly reduced with newer-generation prostacyclin analogs. Many institutions are already using these technologies; unfortunately, peer-reviewed safety data are still lacking [40].
As discussed in statements by the American Academy of Pediatrics Committee on Drugs, off-label treatment is permissible, but the prescriber must exercise appropriate medical judgment and should inform the parents of the status of the treatment [4, 5]. When considering aerosolized pulmonary vasodilators, use of a compound designed for pulmonary administration is recommended.
References
Hill NS, Farber HW. Pulmonary hypertension. Totowa: Springer; 2008.
Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105:14–20.
INOmax®. Nitric oxide gas. 2015. http://inomax.com/wp-content/uploads/2016/02/INOmax-PI-web-2015-10.pdf. Accessed 30 Mar 2016.
American Academy of Pediatrics Committee on Drugs. Unapproved uses of approved drugs: the physician, the package insert, and the Food and Drug Administration: subject review. Pediatrics 1996;98:143–5.
Off-label use of drugs in children. Pediatrics. 2014;133(3):563–7.
Ozek E, Soll R, Schimmel MS. Partial exchange transfusion to prevent neurodevelopmental disability in infants with polycythemia. Cochrane Database Syst Rev. 2010:CD005089.
Rabinovitch M. Morphology of the developing pulmonary bed: pharmacologic implications. Pediatr Pharmacol (New York). 1985;5:31–48.
Levin DL. Morphologic analysis of the pulmonary vascular bed in congenital left-sided diaphragmatic hernia. J Pediatr. 1978;92:805–9.
Kitagawa M, Hislop A, Boyden EA, Reid L. Lung hypoplasia in congenital diaphragmatic hernia. A quantitative study of airway, artery, and alveolar development. Br J Surg. 1971;58:342–6.
Naeye RL, Shochat SJ, Whitman V, Maisels MJ. Unsuspected pulmonary vascular abnormalities associated with diaphragmatic hernia. Pediatrics. 1976;58:902–6.
Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S.
Philips JB 3rd, Lyrene RK, Godoy G, Graybar G, Barefield E, Sams JE, et al. Hemodynamic responses of chronically instrumented piglets to bolus injections of group B streptococci. Pediatr Res. 1988;23:81–5.
Shankaran S, Farooki ZQ, Desai R. beta-hemolytic streptococcal infection appearing as persistent fetal circulation. Am J Dis Child. 1982;136:725–7.
Gersony WM, Duc GV, Sinclair JC. “PFC” syndrome (persistence of the fetal circulation). Circulation 1969;40:Suppl III-87.
Grover RF, Reeves JT, Blount SG Jr. Tolazoline hydrochloride (Priscoline): an effective pulmonary vasodilator. Am Heart J. 1961;61:5–15.
Ward RM. Pharmacology of tolazoline. Clin Perinatol. 1984;11:703–13.
Goetzman BW, Sunshine P, Johnson JD, Wennberg RP, Hackel A, Merten DF, et al. Neonatal hypoxia and pulmonary vasospasm: response to tolazoline. J Pediatr. 1976;89:617–21.
Stevens DC, Schreiner RL, Bull MJ, Bryson CQ, Lemons JA, Gresham EL, et al. An analysis of tolazoline therapy in the critically-ill neonate. J Pediatric Surg. 1980;15:964–70.
Stevenson DK, Kasting DS, Darnall RA Jr, Ariagno RL, Johnson JD, Malachowski N, et al. Refractory hypoxemia associated with neonatal pulmonary disease: the use and limitations of tolazoline. J Pediatr. 1979;95:595–9.
Ward RM, Daniel CH, Kendig JW. Oliguria and tolazoline pharmacokinetics in the newborn. Pediatrics. 1986;77:307–15.
Andrews AF, Roloff DW, Bartlett RH. Use of extracorporeal membrane oxygenators in persistent pulmonary hypertension of the newborn. Clin Perinatol. 1984;11:729–35.
Beck R, Anderson KD, Pearson GD, Cronin J, Miller MK, Short BL. Criteria for extracorporeal membrane oxygenation in a population of infants with persistent pulmonary hypertension of the newborn. J Pediatric Surg. 1986;21:297–302.
Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9.
Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–79.
Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6.
Kinsella JP, Abman SH. Recent developments in the pathophysiology and treatment of persistent pulmonary hypertension of the newborn. J Pediatr. 1995;126:853–64.
Gryglewski RJ. Prostacyclin among prostanoids. Pharmacol Rep. 2008;60:3–11.
Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–5.
Van Heerden PV, Webb SA, Hee G, Corkeron M, Thompson WR. Inhaled aerosolized prostacyclin as a selective pulmonary vasodilator for the treatment of severe hypoxaemia. Anaesth Intensive Care. 1996;24:87–90.
Davis MD, Hunt J. Exhaled breath condensate pH assays. Immunol Allergy Clin N Am. 2012;32:377–86.
United States Food and Drug Administration Center for Drug Evaluation and Research Approval Package for Iloprost. Submission Number 21779. 2004.
United States Food and Drug Administration Center for Drug Evaluation and Research Approval Package for Treprostinil. Submission Number 22387. 2009.
Actelion. Ventavis prescribing information. South San Francisco: Actelion; 2013
United Therapeutics Corp. Tyvaso prescribing information. Research Triangle Park: United Therapeutics Corp; 2016.
Horen B, Montastruc JL, Lapeye-Mestre M. Adverse drug reactions and off-label drug use in paediatric outpatients. Br J Clin Pharmacol. 2002;54(6):665–70.
GSK. Flolan prescribing information. Research Triangle Park: GSK; 2008.
Yilmiz O, Kahyeci Zeybek C, et al. Inhaled iloprost in preterm infants with severe respiratory distress syndrome and pulmonary hypertension. Am J Perinatol. 2014;31:321–6.
Tissot C, Beghetti M. Review of inhaled iloprost for the control of pulmonary artery hypertension in children. Vasc Health Risk Manag. 2009;5:325–31.
Brown A, Gillespie J, Miquel-Vergas F, et al. Inhaled epoprostenol therapy for pulmonary hypertension: Improves oxygenation index more consistently in neonates than in older children. Pulmon Circ. 2012;2:61–6.
Cosa N, Costa E. Inhaled pulmonary vasodilators for persistent pulmonary hypertension of the newborn: safety issues relating to drug administration and delivery devices. Med Dev Evid Res. 2016;9:45–51.
Institute for Safe Medication Practices (IMSP). ISMP list of high-alert medications in acute care settings. 2014. https://www.ismp.org/tools/institutionalhighAlert.asp. Accessed 30 Mar 2016.
Kingman MS, Tankersley MA, Lombardi S, Spence S, Torres F, Chin KS, et al. Prostacyclin administration errors in pulmonary arterial hypertension patients admitted to hospitals in the United States: a national survey. J Heart Lung Transplant. 2010;29:841–6.
Dunkley KA, Louzon PR, Lee J, Vu S. Efficacy, safety, and medication errors associated with the use of inhaled epoprostenol for adults with acute respiratory distress syndrome: a pilot study. Ann Pharmacother. 2013;47:790–6.
Siobal MS, Kallet RH, Pittet JF, Warnecke EL, Kraemer RW, Venkayya RV, et al. Description and evaluation of a delivery system for aerosolized prostacyclin. Respir Care. 2003;48:742–53.
Actelion. Veletri prescribing information. South San Francisco: Actelion; 1995.
Luk CK, Dulfano MJ. Effect of pH, viscosity and ionic-strength changes on ciliary beating frequency of human bronchial explants. Clin Sci (Lond). 1983;64:449–51.
Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol. 2004;113:610–9.
Holma B, Lindegren M, Andersen JM. pH effects on ciliomotility and morphology of respiratory mucosa. Arch Environ Health. 1977;32:216–26.
Le Brun PP, de Boer AH, Heijerman HG, Frijlink HW. A review of the technical aspects of drug nebulization. Pharm World Sci. 2000;22:75–81.
Van Heerden PV, Blythe D, Webb SA. Inhaled aerosolized prostacyclin and nitric oxide as selective pulmonary vasodilators in ARDS—a pilot study. Anaesth Intensive Care. 1996;24:564–8.
Berlinski A, Willis JR. Albuterol delivery by 4 different nebulizers placed in 4 different positions in a pediatric ventilator in vitro model. Respir Care. 2013;58:1124–33.
Cancado JE, Mendes ES, Arana J, Horvath G, Monzon ME, Salathe M, et al. Effect of airway acidosis and alkalosis on airway vascular smooth muscle responsiveness to albuterol. BMC Pharmacol Toxicol. 2015;16:9.
Clary-Meinesz C, Mouroux J, Cosson J, Huitorel P, Blaive B. Influence of external pH on ciliary beat frequency in human bronchi and bronchioles. Eur Respir J. 1998;11:330–3.
Horvath G, Schmid N, Fragoso MA, Schmid A, Conner GE, Salathe M, et al. Epithelial organic cation transporters ensure pH-dependent drug absorption in the airway. Am J Respir Cell Mol Biol. 2007;36:53–60.
Shin HW, Shelley DA, Henderson EM, Fitzpatrick A, Gaston B, George SC. Airway nitric oxide release is reduced after PBS inhalation in asthma. J Appl Physiol. 2007;102:1028–33.
Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, et al. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161:694–9.
Eichelbronner O, Reinelt H, Wiedeck H, Mezody M, Rossaint R, Georgieff M, et al. Aerosolized prostacyclin and inhaled nitric oxide in septic shock—different effects on splanchnic oxygenation? Intensive Care Med. 1996;22:880–7.
Davis MD, Walsh BK, Dwyer ST, et al. Safety of an alkalinizing buffer designed for inhaled medications in humans. Respir Care. 2013;58(7):1226–32.
Siobal M. Aerosolized prostacyclins. Respir Care. 2004;49:640–52.
Sun FF, Taylor BM. Metabolism of prostacyclin in rat. Biochemistry. 1978;17:4096–101.
Rosenkranz B, Fischer C, Frolich JC. Prostacyclin metabolites in human plasma. Clin Pharmacol Ther. 1981;29:420–4.
Nephron Pharmaceuticals. Albuterol sulfate prescribing information. Orlando: Nephron Pharmaceuticals.
Nephron Pharmaceuticals. Ipratropium bromide prescribing information. Orlando: Nephron Pharmaceuticals.
AstraZeneca. Pulmicort Respule prescribing information. Sodertalji: AstraZeneca; 2010.
Hospira, Inc. Acetylcysteine prescribing information. Lake Forest: Hospira, Inc; 2004.
Genentech, Inc. Pulmozyme prescribing information. South San Francisco: Genentech, Inc; 2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Robert Ward, MD, Steven Donn, MD, and Michael D. Davis have served as paid consultants to Mallinckrodt Pharmaceuticals (formally Ikaria, Inc.). Mallinckrodt Pharmaceuticals had no input into the content of this review and had no editorial involvement with the content of this manuscript. None of the authors own stock or serve as employees of companies with an interest in this paper.
Rights and permissions
About this article
Cite this article
Davis, M.D., Donn, S.M. & Ward, R.M. Administration of Inhaled Pulmonary Vasodilators to the Mechanically Ventilated Neonatal Patient. Pediatr Drugs 19, 183–192 (2017). https://doi.org/10.1007/s40272-017-0221-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-017-0221-9