Abstract
Assessment of the circulatory system in trauma is vital to give clues about the presence and degree of ongoing bleeding, adequacy of resuscitation and guide ongoing management in terms of necessary interventions and their immediacy. This chapter provides an overview of basic cardiovascular physiology and the causes of shock in trauma, clinical and biochemical assessment and current concepts on volume replacement and ongoing cardiovascular management in the context of ongoing and controlled bleeding. Consideration is given to various fluid replacement strategies and future developments that may occur, with practical advice on how to optimise circulation preservation in clinical care.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Circulation
- Bleeding
- Transfusion
- Permissive hypotension
- Hybrid resuscitation
- Blood products
- Haemostasis
- Synthetic haemoglobin
-
Physiology of normal blood pressure and its determinants
-
Definition, classification and causes of shock
-
Signs and assessment of shock
-
Hypotension in trauma
-
Historic and current treatment of hypovolaemia
-
Practical aspects of massive blood transfusion
Introduction
The cardiovascular system has evolved to deliver oxygenated blood and nutrients to the tissues and vital organs of the body while removing waste products to the liver and kidney for excretion. A simplified overview of this system is that there is a pump, a series of connecting pipes and a fluid which is necessary for the correct functioning of all other body systems. In trauma, any one of these components can fail in isolation or conjunction with each other and lead to morbidity or mortality.
Gross Physiology of Circulation and Blood Pressure
Blood pressure is regulated by the balance between the sympathetic and parasympathetic nervous system on both the heart and vessels. The degree of vasoconstriction or vasodilatation (otherwise known as the systemic vascular resistance or SVR) and the cardiac output (the amount of blood ejected from the left ventricle per minute) can both be increased or decreased by an intact nervous system or by certain hormones such as adrenaline.
Figure 10.1 gives an overview of the common factors that combine or can be manipulated to influence blood pressure and circulation. The cardiac output is the product of the heart rate and the stroke volume. In order to increase cardiac output, there either needs to be more beats per minute or each beat has to eject more blood. This leads to the first compensatory mechanism that can be observed in patients with shock—mounting a tachycardic response to increase blood flow around the body. If there is not enough blood returning to the heart, then the heart speeds up to try and offset decreased stroke volume by increasing its rate. This brings us onto stroke volume, which is itself controlled by three factors—preload (volume of blood in the ventricle just before it contracts), contractility (the amount of strength the heart muscle can contract with) and afterload (the amount of resistance to blood being ejected from the heart, for example by a tight aortic valve or high SVR). By increasing the amount of blood returning to the heart, the fibres in cardiac muscle are increasingly stretched and will contract with more force up to a point when they become overstretched and less efficient. Beyond this point, although the amount of filling of the heart increases, the contractility and volume of blood ejected per beat decreases. This ultimately leads to a lower cardiac output and ventricular failure with pulmonary oedema and is known as the Frank-Starling law. This rarely happens in trauma, but occasionally, patients who are given large volumes of blood products or fluid and have an underlying cardiac condition may develop acute heart failure via this mechanism.
Decreases in contractility can be seen in patients who have developed cardiac contusions as a result of blunt chest trauma, or alternately patients who have suffered a cardiac event which has subsequently led to trauma. An example of this may be the patient who has a myocardial infarction or arrhythmia and subsequently blacks out and crashes their car. Depending on the underlying nature of the injury, decreases in contractility can be mitigated against by judicious use of inotropes to increase myocardial work. However, this is a complicated area of management as the failing heart may subsequently fail altogether if it is pushed too hard pharmacologically. It may be possible, or even preferable in some cases, to tolerate a slightly lower than normal blood pressure or to use small fluid boluses to see if the heart will cope with additional preload if there are no other considerations such as ongoing bleeding.
Shock
Shock is simultaneously very simple to define but very difficult to quantify objectively on occasion. On a systemic level, any state where there is an imbalance between the body’s demands for delivery of blood and oxygen to the vital organs and its supply is termed shock. This usually is because the circulatory system’s output has fallen below demand, but occasionally the imbalance is due to increasing demand rather than dropping supply, and this is termed high output cardiac failure. This occurs in conditions such as beriberi, severe hyperthyroidism and morbid obesity. The types of shock are defined by their underlying mechanism (distributive, obstructive, cardiogenic or hypovolaemic), but ultimately the result is the same—inadequate blood and oxygen delivery. When considering shock, it should be conceptualised in the basic terms explained above if the problem is with the pump, the pipes or the fluid. All treatments of shock are targeted against treating the underlying cause or mitigating the effects that it causes.
Distributive Shock
Distributive shock is where the heart is functioning well, and there is sufficient blood or fluid in the body, but it is not in the correct location, i.e. a problem with the pipes. The classic examples of this are anaphylaxis, septic shock and neurogenic shock. It should be noted that neurogenic shock and spinal shock are two different things—neurogenic shock can be a cardiovascular consequence of spinal shock, but spinal shock is a specific injury to the spinal cord. Spinal shock is a sensorimotor phenomenon, usually caused by hyperextension or hyperflexion of the neck—this is covered more in Chap. 32 (Spinal and neurological trauma). In distributive shock, the vasomotor tone (amount of constriction in the arterial and venous systems) is decreased, and the effective volume that the normal circulating volume has had to fill is vastly increased as a consequence. Imagine a bucket that has a capacity of 5 litres and contains 4 litres of water initially. If the pressure of the water at the bottom of the bucket is measured, it is directly related to the vertical height of water above it. If the same 4 litres of water remain but the diameter of the bucket is doubled, the pressure of the water at the bottom of the bucket will be much lower as the volume it has to fill is larger, and hence the vertical height reduced. The volume of water in the bucket is the same both times and is an analogy for the circulating volume of blood. By changing the capacitance of the bucket (increasing vasodilation—increasing the capacity of the pipes in the circulatory system), the pressure is lowered.
Anaphylaxis
In anaphylaxis, vasomotor tone is lost due to histamine release from mast cells, causing sudden and profound vasodilation. There is also increasing porosity of the circulatory system so that fluid can leak out of the capillary system and not re-enter into the venous side of the circulation as it would under normal circumstances. This is known as capillary leak and adds an element of hypovolaemia to the picture (i.e. the fluid component of the circulatory system is also decreased). This leads to a hyperdynamic circulation with the widespread vasodilation leading to profound hypotension, warm peripheries, a sudden onset erythematous rash and a compensatory tachycardia. In severe cases, this can also cause a large amount of tissue swelling, which if it affects the airway, can be rapidly fatal. The vasodilation itself can cause cardiac collapse and arrest without airway involvement. Anaphylaxis in trauma is rare, but potentially any drug that a patient receives can cause an allergic reaction and anaphylaxis is the worst-case scenario. The immediate treatment of anaphylaxis is with adrenaline/epinephrine, usually given by an intramuscular injection (e.g. with an EpiPen as seen in Fig. 10.2), but in patients with IV access, small aliquots of intravenous adrenaline can be given by those with appropriate expertise.
Sepsis
Similarly to anaphylaxis, sepsis is a combination of both high output failure and vasodilation, but also may have an element of increased oxygen demand. The body’s basal metabolic rate increases as it attempts to mount an immune response to invading pathogens and so it requires enhanced oxygen delivery. At the same time, bacterial toxins and cytokines are released, which cause peripheral vasodilation and a drop the systemic vascular resistance. There may also be capillary leak as described in anaphylaxis. In both these cases, the correct treatment is to address the underlying cause, and provide organ support, usually by giving additional vasoactive medications (such as noradrenaline, vasopressin or adrenaline) to counteract the vasodilation and increase SVR. In anaphylaxis, this may only require a short treatment for a few minutes while the histamine release has been counteracted, but in sepsis, the administration of vasoactive drugs may be required for days. Successful treatment involves controlling the source of infection by either surgery, antibiotics or both while counteracting direct effects of the pathogen and the body’s immune response. The increased oxygen requirement may also induce an element of high output failure as described above.
Neurogenic Shock
Neurogenic shock is where there is a high thoracic or cervical cord lesion, which decreases the vasomotor tone of the cardiovascular system. The degree of vasoconstriction or vasodilation usually is held in balance by the opposing forces of the sympathetic and parasympathetic nervous system. The main outflow of the sympathetic nervous system is in the thoracolumbar spinal cord, whereas the parasympathetic system originates in the cranial, cervical and sacral nerves (see Fig. 10.3). If an injury to the cord occurs above the level of T3-4 (the upper limits of sympathetic innervation), then the balance is lost, and the parasympathetic nervous system dominates. This leads to vasodilation and increased capacitance of all the circulatory vessels (but without capillary leak as described in anaphylaxis). However, there is also a compounding cardiac response.
In the same way that vasomotor tone is balanced by sympathetic and parasympathetic innervation, the heart is equally dual innervated. The sympathetic cardio-acceleratory fibres originate from T2-T4, and parasympathetic cardioinhibitory fibres arise from a branch of the vagus nerve, the 10th cranial nerve. If the level of the injury is higher than the cardio-acceleratory fibres, then in addition to the distributive hypotension caused by vasodilation the body is unable to mount a compensatory tachycardia. Indeed, unopposed vagal stimuli will cause worsening bradycardia and subsequent decrease in cardiac output and compound the hypotension further. The correct treatment of neurogenic shock involves infusions of vasopressors such as noradrenaline, vasopressin, phenylephrine or metaraminol to maintain blood pressure and cord perfusion. If the injury is above T2-T4 then directly acting cardiac inotropes/chronotropes may be needed in addition to vasopressors. Isoprenaline may be sufficient if added to a vasopressor or alternatively adrenaline may be used initially to act as a combined inotrope/chronotrope and vasopressor. By maintaining this perfusion pressure to the damaged cord (and the rest of the vital organs), the risk of secondary injury is minimised.
Obstructive Shock
Stressing the heart by increasing the afterload significantly can cause the heart to fail. Due to the way that the heart has evolved, the right ventricle does not need to be as strong as the left as it only pushes blood through the low-pressure circulatory system of the lungs. The left ventricle, in contrast, has to supply the whole body so needs to be able to generate more forceful contractions. Consequently, it is easier to cause the right side of the heart to fail than the left. The common trauma scenarios in which this is seen are pulmonary embolus and tension pneumothorax.
Pulmonary Embolus
In pulmonary embolus, a large blood clot or fat embolus obstructs the pulmonary artery and increases the right ventricular afterload, causing impairment of blood flow to the lungs and increased dead space. This causes both hypoxia and hypotension, as obstruction of blood flow to the left ventricle causes decreased preload and subsequent decrease in cardiac output. Depending on the nature and size of the embolus and other factors associated with trauma such as bleeding risk, the options for treatment may vary [1, 2]. These can range from observation only, anticoagulation to reduce the risk of the clot increasing in size, thrombolytic drugs to break down the clot with enzymes, removal of the embolus with interventional radiology guidance or open surgery with cardiac bypass to remove large, life-threatening clots. Each step grows particularly more aggressive and increases the risk of side effects, so each case requires an individualised approach.
Tension Pneumothorax
While pulmonary embolus causes increased afterload on the right ventricle from inside the circulation, external compression of the lung and pulmonary artery by a tension pneumothorax causes the same issues. This is compounded by hypoxia and decreased venous return caused by twisting of the mediastinum—please refer to Chap. 9 (Breathing and chest trauma) for more details.
Cardiogenic Shock
Cardiogenic shock is when the heart itself cannot generate sufficient force to pump blood effectively, despite having adequate blood volume returned to it and no problems with afterload. This may be due in broad terms as a result of intrinsic heart diseases such as cardiomyopathies, an arrhythmia such as supraventricular tachycardia or a myocardial infarction; these causes of failure are outside the scope of this text. The two leading causes in trauma are as a result of cardiac contusions as described in Chap. 9 (Breathing and chest trauma), or rarely as a result of cardiac tamponade.
Cardiac Tamponade
The heart is surrounded by a tight, fibrous sack called the pericardium. This structure has evolved to keep the heart in place in the mediastinum, is filled with fluid to decrease friction on surrounding structures when the heart contracts and is a physical barrier against infection. As it is a fibrous structure, it does not stretch when the pressure inside it increases. Because the pericardium is so inflexible and closely opposed to the heart, if fluid accumulates between the heart and the pericardium, then it will compress the heart from the outside and decrease the ventricular volume available to fill with blood (see Fig. 10.4). This decreases the stroke volume in both ventricles, though it affects the right more than the left due to the previously mentioned weakness of the right ventricle compared to the left. Any fluid that accumulates in the pericardium can compress the ventricle, but in trauma, it is most commonly blood. In cases of patients presenting with penetrating chest or upper abdominal trauma, the heart may have been pierced, and blood may leak from the injured chamber or vessel into the pericardium. This is an emergency and requires surgical intervention. If the patient arrests, this may need to be by a resuscitative thoracotomy (see Chap. 12 - Traumatic cardiac arrest). There is no role for needle aspiration of pericardial blood (needle pericardiocentesis) in trauma as blood tends to clot quickly and become too viscous to aspirate through a needle. There is also the risk of missing the collection altogether and causing further cardiac injury if using a blind technique, and even ultrasound-guided techniques are not guaranteed to succeed [3].
Cardiac tamponade—blood fills the pericardial space and prevents diastolic filling (Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436)
Tamponade itself is not an intrinsic cardiac muscular problem, but the mechanism by which it produces shock is due to pump failure as opposed to fluid or pipe failure; hence its inclusion in this section. There is an element of obstruction to filling, but the mechanism of failure is different from those pathologies listed in the Obstructive Shock section. With tamponade, the issue is with the heart filling whereas, with pure obstructive shock, the pathological mechanism is impairment of the heart emptying.
Hypovolaemic Shock
The final mechanism of shock is hypovolaemia. This is by far the most common cause of shock in trauma as the principal cause is bleeding. A cardinal maxim is that all shock in trauma should be considered to be hypovolaemic until conclusively proven otherwise. More than one pathology can coexist, so clinicians must aggressively search for haemorrhage before concluding that shock is not due at least in part to hypovolaemia.
Failure to Identify Active Bleeding
Typically this is occult bleeding into a major body cavity which, if not appreciated from the mechanism of injury or developing signs, will result in rapid deterioration or death. These patients typically need rapid transport to a hospital which can manage such bleeds (which usually means surgery or angiography, e.g. in the case of splenic bleeds as in Fig. 10.5) but could also include REBOA or resuscitative thoracotomy with cross-clamping of the descending aorta. The latter course of action is not recommended except in extremis due to increased morbidity and extremely high mortality when performed for extra-thoracic causes of hypovolaemia. Management before this point should concentrate on active volume replacement with blood and rapid access to facilities to obtain definitive haemostatic control.
A ruptured spleen such as this example can cause significant hypovolaemia. Current practice is to try and preserve the spleen and treat with interventional radiology measures, but a severely shattered spleen with ongoing bleeding needs surgical intervention (Thomas Zimmerman via Wikimedia under the Creative Commons Attribution-Share Alike 3.0 Germany license)
Failure to Control Active Bleeding
There is no excuse for not trying to manage external bleeding. Every drop of blood is precious circulating volume for that patient, and transfusion or intravenous fluids are not equivalent replacements. Every possible effort should be made to preserve circulating volume where possible. This concept of circulation preservation is now a fundamental part of bleeding and shock management and is far more effective than relying on replacement strategies. The bottom line is that at present, the patient’s own blood is the best substance to be circulating in their vessels. Circulation preservation may be as simple as a pressure dressing, tourniquet or may be as involved as urgent laparotomy or angiography for internal bleeding.
In the MABCD assessment, if there is no visible massive external haemorrhage, move on but be aware of the signs of more occult bleeding during the assessment of other systems such as A-airway and B-breathing and inevitably C-circulation and D-disability.
Key Points
Typical features that may be identified include:
-
Rapid, shallow breathing. Increased respiratory rate is the best single indicator or sign of an unwell patient. This could be from many causes and not just shock, but should never be ignored. In shock, the reduced tissue perfusion and metabolic acidosis which occur result in signs of ‘air hunger’. Casualties may appear to be distressed or gasping to get their breath as they hyperventilate to compensate in respiratory terms for the metabolic acidosis and rising lactate. Whilst it is a sensitive sign, it is unfortunately not highly specific for any one pathology.
-
The oxygen saturation may be difficult to obtain, due to lack of perfusion causing a low signal and generalised peripheral shut down. It may also be genuinely low as a result of increasing ventilation/perfusion mismatching in the lungs.
-
Confusion, agitation or anxiety may occur as cerebral perfusion drops and blood supply to the brain is reduced. This may lead to feeling faint and ultimately to a falling conscious level and responsiveness
Remember these are also features which may suggest a pneumothorax. Hopefully, this has been excluded as part of the respiratory assessment, but the possibility of a significant pneumothorax should be considered in trauma patients presenting with cardiovascular compromise.
The management of hypovolaemia has changed over many years as the understanding of traumatic haemorrhage has evolved. This is driven primarily from military experience in war, and some lessons are re-learned at the start of each new conflict. The three principles that should be adhered to are 1) preserving circulation whenever possible (e.g. with the use of tourniquets and other devices and techniques described in Chap. 6 (Massive haemorrhage control), 2) achieving haemostatic control as soon as possible and 3) employing a damage control resuscitation strategy. Definitive haemostasis can be either via surgical or radiological means or both [4], and resuscitative radiology is becoming more advanced as time passes. It has several advantages including specific targeting of individual vessels without the morbidity of some surgical approaches, for example some pelvic arterial bleeding can be controlled by minimally invasive arteriography and coiling rather than a laparotomy to access the vessels and may decrease the time to definitive haemostasis [5].
“Classical” Clinical Signs of Shock
As previously stated, shock is easy to define but can be hard to quantify. As the body attempts to compensate for blood loss, adaptive physiological changes include increased heart rate to compensate for decreased stroke volume, increasing diastolic blood pressure to maintain perfusion pressure and diversion of blood from the periphery to vital organs. This means that patients may feel peripherally cool to the touch, have a prolonged capillary refill time and look pale on clinical examination. As blood pressure decreases further, the end organs become less perfused, and other clinical signs may become apparent. These include decreased urine output as the kidneys attempt to retain circulating volume or become hypoperfused, and confusion or unconsciousness if cerebral perfusion is not maintained.
The ATLS classification of hypovolaemic shock has been graded from level 1 to 4 depending on ranges of heart rate, blood pressure and Glasgow Coma Scale (GCS) with approximate blood loss inferred from these three signs. This has been part of the ATLS course since 1980, but the validity of this concept (while attractive based on first principles) has not been established. A paper by Little et al. [6] from 1995 first explained why these measurements are imperfect in practice, and a more recent paper by Mutschler et al. [7] retrospectively reviewed the German Trauma Register and the UK TARN database with these measurements. They concluded that only 9.3% of all trauma patients could be allocated to one of the ATLS classifications based on their vital signs. Further, this classification may overestimate the degree of tachycardia and underestimate the degree of GCS impairment associated with hypovolaemic shock; i.e. patients may have lost more than the 40% of their circulating volume that classifies them as category 4 shock, but their vital signs are not as deranged as the ATLS classification suggests they should be. Young, fit patients may be more hypovolaemic than their vital signs suggest. This is before any other confounding factors are taken into consideration such as medications, age and other comorbidities, which may mean that patients have “abnormal” vital signs before they have any trauma inflicted on them. Classically ATLS and other courses teach that tachycardia is shock until proved otherwise and this is true. However, the corollary is not true—the absence of tachycardia does not mean the absence of shock. Consider an elderly, bleeding patient on regular beta-blockers who may be unable to mount a tachycardia or a triathlete with a very low resting heart rate. Both may be bleeding and in shock but have a heart rate of less than an arbitrarily defined 100 BPM. Thankfully there are other useful clinical signs of developing shock and failure of compensation mechanisms such as skin colour, capillary refill time and level of consciousness. For more information on this concept and specific examples, please refer to Chap. 37 (Silver Trauma).
Conscious level is particularly useful as a practical indicator of the level of brain perfusion. Tachycardia may be present and signify blood loss, but if the patient is coherent and not confused, then they are adequately perfusing their brain at present and may not warrant fluid resuscitation immediately, especially with crystalloids. This was the basis for US forces pre-hospital fluid resuscitation in recent conflicts in Iraq and Afghanistan. If the patient could recite their name, date of birth and serial number when asked, they were deemed to be adequately perfusing their brain and not given any fluids.
While previous editions of the ATLS course manual have given specific values for specific classes of shock, the 10th edition [8] has done away with these but retained a table illustrating the features associated with progressive shock. Other courses such as the European Trauma Course have done away with the classification altogether, and advocate multi-factorial assessment of shock on clinical, physiological and biochemical grounds such as lactate, pH and base deficit on arterial or venous blood gas analysis [9]. This is more in keeping with current practice, as the arbitrary division of patients into four groups which are not supported by data adds little if anything to their management. What is very clear is that while tachycardia is one indicator of shock, it should no longer be considered a ‘target for resuscitation’. In other words, for a bleeding patient with a high heart rate, the traditional approach was to continue fluid resuscitation and avoid anaesthesia until the tachycardia has resolved, indicating that the patient was then “resuscitated”. This approach is fundamentally flawed when a patient is still actively bleeding, and the high volumes of fluid required to “treat the tachycardia” will create even more severe problems and could even be fatal.
Another proposal which appeals to first principles is that as patients become progressively more hypotensive, peripheral pulses become impalpable at specific blood pressures. This is often quoted as a radial pulse requires a pressure of ≥80 mmHg, femoral ≥70 mmHg and carotid ≥60 mmHg. The presence of a palpable peripheral or central pulse in itself is a reasonable generalised indicator of adequate perfusion [10, 11] and the radial > femoral > carotid pressures appear to hold clinically. However there is no reproducible evidence that the presence of a specific pulse equates to a specific blood pressure, and this approach may overestimate blood pressure [12, 13].
Metabolic Assessment of Shock
While clinical signs may give a trend of decline or response to therapy and extreme values may be useful in defining severe forms of shock, as discussed, vital signs are not entirely reliable in assessing some patients. The metabolic consequences of shock may be apparent before clinical signs develop, or before young and fit patients (who make up a large proportion of trauma patients) exhaust their reserve and start to decompensate. No one test will accurately, consistently and infallibly predict major haemorrhage, shock, the need for mass transfusion or adequate resuscitation early [14]. While the below tests, their basis, merits and current evidence are discussed, having multiple data points to correlate and interpret in the context of both mechanism of injury and physiological signs is far more useful.
pH and Base Excess/Deficit
Base excess is defined as the relative amount of acid or alkali that must be added to each litre of fully oxygenated blood to return the pH to 7.40 at a temperature of 37 °C once the effect of carbon dioxide dissolved in the blood has been accounted for and normalised to 5.3 kPa [15]. Carbon dioxide is an acidic gas which dissociates into H+ and HCO3 in water in a reaction catalysed by carbonic anhydrase, so changes in the amount of CO2 in the blood will subsequently cause a change in pH. The more CO2 present, the more dissociation occurs and the lower the pH, purely from the effect of CO2. Of course, CO2 is not the only contributor to pH, and metabolic acids and alkalis may have a more significant role depending on how much is present. By calculating the amount of H+ present due to CO2 dissociation and subtracting it from the total acid burden, whatever remains must be the degree of acidity or alkalinity which is due to metabolic rather than respiratory causes. The usual range of base excess or deficit is +2.0 to −2.0 MEq/l, and values below −2 indicate an excess of metabolic acids, and values above +2 indicate an excess of alkali (base).
To be explicit, base deficit and base excess are corollary measurements of the same thing—a negative base excess means that there is more metabolic acid present and is the same as a positive base deficit. This occasionally is not clear from reading some physiology textbooks and has been a cause of some confusion when teaching this subject clinically. For the avoidance of confusion, this text will refer to base excess only when discussing this area.
In trauma, base excess is used as a surrogate marker of perfusion, as hypoperfusion (assumed to be from haemorrhage and/or tissue ischaemia) causes the production of acidic metabolites. The use of base excess to quantify hypoperfusion and infer haemorrhage has been studied in several papers and found to have better discriminatory value than the physiological parameters described in ATLS [16]. Whilst purists will state that base excess must be drawn from an arterial sample (which may be more challenging in hypovolaemic trauma patients), there are data that base excess calculated from venous samples is at least as sensitive (if not better) than arterial samples as a marker of shock and predictor of survival [17]. It has been hypothesised that this is due to post-capillary acid-base balance being a better marker of tissue perfusion than arterial measurements [18], and also that changes occur earlier (at least in animal models) in the venous circulation in haemorrhage than in the arterial [19]. The more severe the hypoperfusion, the larger the base deficit. The Mutschler paper referenced above stratified patients into four groups based on base excess—no shock (BE ≤ 2.0), mild shock (BE > 2–6.0), moderate shock (BE > 6–10.0) and severe shock (BE > 10). These groups showed a linear progression in terms of transfusion requirements, lactate levels, morbidity and mortality when their outcomes and length of ICU and hospital stay were followed up. This is an important paper as it was a retrospective analysis of over 16,000 patients in the German Trauma Register, which correlated with similar findings from the UK TARN database.
While there is a role for arterial blood gasses in the assessment of the adequacy of oxygenation and ventilation in trauma [20], a venous blood gas taken at the time of initial venipuncture can give reliable and actionable prognostic results in minutes. Clinical pitfalls in interpretation are similar to those around lactate measurement as described below, but also other causes of an increased base deficit can interfere with clinical interpretation.
A comparatively recent change in the understanding of pH is the Stewart model of acid-base balance [21] which looks at the concentration of strong and weak ions in a solution and their effect on pH. A full explanation of this model is impractical in this textbook, suffice to say that the contribution of ions other than H+ and OH− are essential in determining pH and subsequently base excess. Amongst these strong ions are Cl-, Na+, lactate, Ca2+, proteins, Mg2+, K+ and albumin—all of which may be deranged or administered at some point in resuscitation and consequently have a knock-on effect on pH and base deficit. For example, giving “normal” 0.9% or hypertonic saline will increase the concentration of Cl− ions, which under the Stewart model will lower the strong ion difference and subsequently cause a hyperchloraemic metabolic acidosis [22, 23]. This has been verified in clinical publications [24] and a corresponding decrease in this effect when balanced salt solutions such as Plasmalyte, Hartmann’s solution or Ringer’s Lactate are used [25]. It has been suggested that this translates to an increased rate of adverse effects such as blood transfusion requirement and ventilator days in at least one meta-analysis [26]. While base deficit may be an accurate reflection of perfusion on admission, if large volumes of saline are given, then this may lead to clinical decisions being made on iatrogenic complications rather than evolving pathology. This is yet another argument against crystalloid resuscitation generally, but thoughtless administration of saline solutions specifically. While there is a role for hypertonic saline in the role of brain-injured patients (see Chap. 11 - Disability and head injury), in a well-resourced centre there is no role for 0.9% saline as a resuscitation fluid for acute trauma. In more austere environments where blood and blood products are not readily available, 0.9% saline could be considered in the minimum amount necessary in accordance with permissive hypotension and damage control strategies if it is all that is available [27]. One redeeming feature is that while Hartmann’s and Ringer’s solutions are mildly hypotonic and could theoretically worsen cerebral oedema in brain-injured patients, 0.9% saline is theoretically isotonic so should not cause this issue.
Lactate
As tissue perfusion becomes impaired, oxygen-starved tissues may revert to anaerobic respiration and produce lactate as a result. The initial lactate on presentation has been found to correlate with mortality and the need for operative intervention, and the subsequent clearance of lactate at 6 h is an independent predictor of mortality [28, 29]. Lactate in itself is a misunderstood entity at times; high serum lactate levels are occasionally the source of concern for many healthcare staff, but lactate is an intrinsically biologically useful molecule. It can be used as an energy substrate by the brain, heart and kidneys and may act to mitigate against acidosis by acting as a “shuttle” to mop up hydrogen ions in anaerobic tissue and transfer them to aerobic tissue, where ATP can be produced more efficiently than by glycolysis [30]. Lactate has been found to improve cardiac output [31] in addition to being the primary energy substrate of injured brain and may be neuroprotective [32, 33]. Lactate has also been shown to be superior to the base deficit when assessing the initial severity of trauma [34]. Caution is advised though as high lactate levels automatically equating to hypoperfusion is not a universal truth; specific pathology, normal physiology and some therapeutic agents can raise lactate levels. For example, a runner who falls and twists his ankle may have a high lactate level from exercise if it is checked acutely—he is a trauma patient who is not bleeding but will have a high lactate. Similarly, patients who have brain parenchymal injury or high brain glucose usage may have slightly raised lactate as a consequence of acute lactate release from an outward brain: blood lactate gradient, though this effect is more likely to be localised and small [35]. Finally, patients who have been given beta-agonists such as salbutamol or adrenaline will also have a raised lactate due to the pharmacological action of these agents—it does not mean they are hypoperfused.
Haemoglobin
Traditional teaching is that haemoglobin does not correlate with acute blood loss as the blood that is lost has the same haemoglobin concentration as the blood that stays in the body, i.e. the total volume of blood decreases but the measured haemoglobin concentration remains the same because whole blood is lost. This is undoubtedly true in hyperacute haemorrhage at the initial point of injury and the immediate post-injury minutes, but physiological compensation may have started by the time the pre-hospital team reach the patient. A study by Bruns et al. [36] looked at the initial haemoglobin concentration taken within 30 min of arrival in the emergency department and correlated this with physical and biochemical signs of blood loss and ultimately the need for urgent interventions to stop haemorrhage. There was a small but statistically significant difference in average presenting haemoglobin concentrations in patients who required urgent intervention compared to those who did not (mean of 12 g/dl ± 2 g/dl in those requiring intervention versus 13 g/dl ± 2 g/dl in those who did not). What was noted was that a presenting haemoglobin concentration of less than 10 g/dl was associated with over a threefold increase in the need for haemostatic intervention. A similar (but more extensive and multi-centre) study was undertaken in France in 2018 by Figureiredo et al. [37]. Enrolling over 6400 patients, this study went further and compared sequential pre-hospital and in-hospital blood results on the point of care testing machines with against a formal laboratory sample on arrival in hospital. The volume of crystalloid given (which may have contributed to an iatrogenic dilutional anaemia) was taken into account in the analysis, and again similar results were found. A pre-hospital haemoglobin concentration of less than 12 g/dl in women or 13 g/dl in men was modestly predictive for significant haemorrhage (defined as requiring ≥ four units of blood or death within 24 h attributable to exsanguination). In hospital, the same point of care test could discriminate between those who had a significant haemorrhage, though the cut off points dropped to 10 g/dl for women and 12 g/dl for men. This is presumably as a result of a combination of ongoing haemorrhage, physiological compensation by drawing fluid from the periphery into the circulating volume and as a result of crystalloid administration. Of note, the point of care device was as sensitive as a formal laboratory result but without the wait for processing. A drop in haemoglobin concentration between pre- and in-hospital point of care testing of 2 g/dl or more was also predictive of significant haemorrhage. Patients who required IV fluids for hypotension with significant haemorrhage were also compared to those who were hypotensive and also required IV fluids but did not meet the definition of significant haemorrhage. It was found that for the same amount of fluid administration, there was a significantly higher drop in the haemoglobin concentration of those who had significant haemorrhage than those who were not bleeding as severely.
Other Biomarkers
There has been much interest in the use of biomarkers in conditions such as sepsis to identify and treat patients early, and trauma is no different. Most research has been done in animal models and identified specific proteins or other biomarkers which may be up- or down-regulated in response to trauma, and potential treatments such as valproic acid [38, 39]. What is not known is whether these markers are specific for trauma, whether treatment is effective for mitigating the underlying cause of the rise, whether they will have cross-species validity or whether they would be part of a practical and timely laboratory test that could be used to guide management in these patients. It has not stopped early development and patent applications for the use of these biomarkers [40]. There are currently no other specific predictive biomarkers in routine clinical use other than the ones explored above, though this may be an area for development in the future.
Hypotension in Trauma
While hypotension is a manifestation of shock, the modality of treatment of hypotension can have significant impacts on mortality. While the idea of having a specific number to target seems appealing, several factors interact to make this a complex treatment decision. Should hypotension be treated at all? If so, with fluids or vasopressors? Which ones and when? Is there a role for antifibrinolytic agents such as tranexamic acid?
History of Permissive Hypotension
Permissive hypotension is a concept which appears to have been rediscovered at the start of every major conflict in the twentieth century, but thankfully is now being accepted as the standard of care in trauma. W B Cannon first described it in World War One [41], amongst many other fascinating discoveries that seem to have been largely forgotten for the better part of a century. Cannon also described acidosis in hypoperfused states, observations on raised blood pressure in severe head injuries that are consistent with current interpretations of cerebral perfusion pressure and the deleterious effects of hypothermia on trauma patients [42]. A professor of physiology at Harvard University, Cannon volunteered to be a field investigator at a casualty clearing station in Bethune, France and was appointed the president of the Red Cross medical research society in November 1917. He was a shrewd observer and documenter of observations and encouraged the publication of case reports as a learning tool. In his 1918 paper The Preventive Treatment of Wound Shock [42], he advised caution in administering IV fluids to shocked patients without the facility to achieve surgical haemostasis:
Injection of a fluid that will increase blood pressure carries danger in itself. Haemorrhage in the case of shock may not have occurred to a large degree because the blood pressure is too low, and the flow too scant to overcome the obstacle offered by a clot. If the pressure is raised before the surgeon is ready to check any bleeding that may take place, blood that is sorely needed may be lost.
This lesson was forgotten after the war but re-emerged with the advent of World War 2 and further combat casualties. Lt Col Henry Beecher in his 1945 publication Preparation of Battle Casualties for Surgery [43] argued that a systolic blood pressure of 85 mmHg was adequate and that the minimum amount of plasma should be given to achieve this aim. He also advanced the idea that resuscitation should continue during surgery rather than operate late (as was the preferred French and Italian model at the time)—a concept integral to what we now consider Damage Control Resuscitation. Again, this lesson appeared to be lost after the conflict ended. Patients who presented to medical treatment facilities during the Vietnam and Falklands conflicts often survived for prolonged periods with low blood pressure, before being “resuscitated” with large volumes of crystalloid and dying of their injuries. It was not until 1994 when Bickell et al. published a prospective trial showing improved survival, fewer complications and decreased length of hospital stay in patients who had delayed fluid resuscitation while hypotensive from penetrating abdominal trauma [44]. From then, the direction of research and current practise has been to understand what blood pressure to tolerate before intervention, what intervention to perform and when.
Permissive Hypotension as a Concept
A baseline understanding of the first principles and pathophysiology of bleeding is essential when discussing strategies for treating haemorrhage-induced hypotension. If bleeding occurs and a significant amount of circulating volume is lost, the ventricular pre-load drops. In turn, this drops the stroke volume, and the cardiac output is initially maintained or slightly increased by a compensatory tachycardia. Eventually, if there is enough blood loss and decompensation occurs, the cardiac output will drop and the blood pressure will also decrease (even if circulating catecholamines cause an increase in the SVR). This low blood pressure reduces the flow of blood to peripheral tissues, and the hypoperfusion causes the raised lactate referred to earlier (with the already expressed caveats).
At this point, the hydrostatic pressure of the blood in the vessels drops, and circulating factors may be able to form a clot over the bleeding vessel temporarily sealing it. Two important points need highlighting—firstly, this clot has formed under the best possible circumstances in terms of the amount of available circulating clotting factors, temperature, pH and presence of cofactors such as calcium. Secondly, at this point the clot is immature and vulnerable to breakdown by either rough handling of the patient or further increases in the hydrostatic pressure in the injured vessel, e.g. by administering fluids. The ethos behind permissive hypotension is to protect this clot while it matures and prevents further bleeding while maintaining an acceptable degree of end-organ perfusion. To reiterate—while the patients’ blood pressure is not normal, if there is adequate perfusion to vital organs then this should be tolerated until definitive haemostasis can be assured. If the pressure continues to fall, then the resuscitative target should be to restore an adequate blood pressure only, not a normal one. This is usually defined as a systolic pressure of approximately 80–90 mmHg and is mostly pragmatic and based on entrance criteria of trials of liberal vs restrictive fluid resuscitation in trauma. An editorial on the concept of permissive hypotension from Nevin and Brohi appeared in Anaesthesia in December 2017 and is well worth reading for a more comprehensive overview [45].
More recent work has looked at the effect of permissive hypotension over a prolonged period and concluded that (in animal models at least) prolonged permissive hypotension can lead to increased morbidity and mortality. The approach trialled in animal models by Doran et al. is ‘Hybrid’ or ‘targeted’ resuscitation [46]. This aims to offer the best compromise between prolonged hypotension and overly aggressive resuscitation. It is based on the principle that it is not blood pressure that matters, but rather blood ‘flow’ and perfusion of tissues as described above.
The following recommendations were made:
-
Maintain a Systolic BP of 80 mmHg for the first hour of care
-
Increase the systolic BP to 110 mmHg after this period even if definitive haemostasis has not been achieved
-
Then fully restore normotension with blood and blood products once definitive control has been secured.
By doing this the overall extent of the shock is reduced, base deficit improved, the degree of coagulopathy and systemic inflammation reduced and this may well be the kind of best compromise approach that will be adopted in future years.
When this approach was applied to a blast model in rats [47], survival was significantly improved in the group that had a short period of permissive hypotension followed by slow resuscitation to normotension (Blast NH), rather than prolonged hypotensive resuscitation (Blast Hypot), until bleeding was adequately controlled (see Fig. 10.6).
Whether or not this can be applied to human cases is still not clear, but the animal work does suggest promising results. However, this further confirms that the shorter the period of hypotensive ‘hit’ the better, but this has to be balanced against how it is corrected. Merely filling the actively bleeding casualty with any available resuscitation fluid is potentially a greater risk.
This is a complicated picture requiring some compromises and caution, and a single definitive solution is unlikely ever to exist. When considering the other effects of shock, the picture becomes even more complicated. However, some interventions have been demonstrated to worsen outcome and should be avoided:
-
Excessive volumes of resuscitation fluid while still bleeding
-
Delays in achieving control of bleeding
-
Prolonged or extreme shock
Cyclic Hyper-resuscitation and Crystalloids
Before permissive hypotension was accepted, traditional teaching was to aggressively treat hypotension by the administration of a fluid challenge—usually 2000 ml of a crystalloid solution such as Hartmann’s or Ringer’s lactate. The thinking behind this approach was that by restoring a normal blood pressure, the flow of blood to the vital organs would be restored and the patient stabilised so they were in the best possible condition for an operative prcedure. In some cases, the timing of operations would depend on whether the patient was a responder or non-responder to the initial administration of fluids. From the perspective of having an endpoint to target and assess against, it is easy to see why this approach was undertaken. It does, however, have several flaws which have been discovered as research has progressed. Consider the above emphasis on clot protection and preservation—by increasing the blood pressure with crystalloids, hydrostatic pressure increases and stress on any clot which has formed increases. This may cause the clot to rupture and cause further bleeding and hypotension (sometimes referred to colloquially as “popping the clot”). Once the blood pressure decreases to its previous level, a clot may form again to prevent further haemorrhage. However, the problem with this is that the second clot that forms may not be as strong as the first for several reasons. Firstly, the initial clot was formed under the best conditions with the highest number of clotting factors available, and many of these factors and cofactors may have been consumed in the production of the first clot. As a result, the formation of a subsequent clot (if possible) may start with 50% or less of the available clotting factors used in the first purely from a consumptive perspective. Secondly, the action of giving a 2000 ml crystalloid bolus will dilute the remaining clotting factors and cofactors that are present further and impair their effectiveness. Thirdly, as a result of further bleeding from the clot being disrupted (and more often than not the 2000 ml bolus not being as warm as body temperature), the patients’ temperature falls, decreasing the efficiency of the enzymatic systems involved in forming a clot. Finally, as crystalloid has no oxygen-carrying capacity it cannot intrinsically improve the delivery of oxygen to tissues (other than by a transient rise in stroke volume/cardiac output which is subsequently offset by deleterious effects), and so hypoperfusion and acidosis worsen, causing a decrease in plasma pH which adds a second hit to the function of enzymatic-dependent clotting.
If the patient is fortunate enough to be able to form a second clot despite being replete of coagulation factors, cold, acidotic and having suffered further bleeding, they will be hypotensive. This may trigger further crystalloid resuscitation and the downward spiral continues in the same manner until no clot can be formed, with the patient bleeding to death if surgical control cannot be achieved. This repeated pattern of hypotension, crystalloid administration and further bleeding has been termed cyclic hyper-resuscitation and should be avoided [48]. Even if the patient survives to the intensive care unit, administration of large volumes of crystalloid has been associated with the development of Adult Respiratory Distress Syndrome (ARDS), abdominal compartment syndrome and the worsening of pulmonary contusions if present [49,50,51,52,53,54]. Another paper by Sharpe et al. [55] compared high and low ratios of crystalloid to blood products in resuscitation acutely and concluded that there was no appreciable difference between the strategies in terms of mortality. This paper raises a couple of methodological questions, as the range of crystalloid doses in the high ratio group (1000–11,000 ml, mean of 4000 ml) compared to the low group (500–8200 ml mean of 1300 ml) does not appear significantly different and the low ratio group had a higher amount of HES administered in their initial resuscitation. The volumes of crystalloids given in both groups appear at first glance to be excessive, but even in this paper there is a statistically significant difference in the rate of development of ARDS!
Fluid Therapy in Trauma
While some patients may develop hypotension, form a clot and maintain an acceptable blood pressure, many may not and will require some degree of fluid resuscitation in order to get to the point of definitive haemorrhage control. The question then becomes “which fluid should be given”? Crystalloid solutions do not transport oxygen and excess administration causes further bleeding, hypothermia, dilution of clotting factors and ARDS as previously described, so the alternatives become colloids or blood products.
Colloids
Colloids are large molecules that exert an osmotic pressure and theoretically stay in the circulation and exert their effects for longer than crystalloids. The most common colloids in recent clinical use are dextrans, modified gelatine or starches. Blood is technically a colloid, but in this textbook, blood is considered a separate entity and the use of the word “colloid” refers exclusively to non-blood, non-crystalloid resuscitative solutions.
An often-quoted rule from historic ATLS teaching is “3:1”—students are often told that giving a bolus of colloid is the equivalent of giving three times the amount of crystalloid for the same haemodynamic effect. In terms of mitigating the effects of excessive volume, this would seem a good solution between the deleterious effects of crystalloid and the risks and expense of blood transfusion if it is not necessary. The problem lies in the fact that this is an urban myth—the actual ratio is only somewhere between 1:1.3 and 1:1.6 [27, 56,57,58,59,60]. Moreover, a Cochrane review [61] showed no evidence of benefit in using colloids over crystalloids in the resuscitation of patients following burns, trauma or surgery and that the use of hydroxyethyl starch (HES) may increase mortality in these patients. A further review which excluded papers which had been retracted because of research fraud further condemned HES as it showed an increase in mortality and acute kidney injury in comparison to other resuscitation solutions [62]. As some colloids are modified gelatine proteins they also carry a risk of provoking anaphylaxis in susceptible patients [63]. They are also more expensive to produce than crystalloids, and with no overall benefit demonstrated, it is difficult to justify their continued use in clinical practice.
Synthetic Oxygen Carriers
An off-the-shelf, oxygen-carrying solution which does not need to be cross-matched, stored under certain constrictive conditions, is cheap to produce and with few serious side effects is considered the Holy Grail of trauma resuscitation fluids. The morbidity and mortality from blood transfusions like acute transfusion reactions, mismatched blood groups, the passage of blood-borne diseases before the advent of effective screening and immunologically mediated problems such as Transfusion Related Acute Lung Injury is not insignificant. Because of the lack of an alternative solution, these complications remain a risk of this life-saving therapy. Efforts have rightly been made to reduce the risk by focussing on systems and reporting to minimise it as much as possible. If a synthetic drug were produced that could perform the same function as even just haemoglobin as opposed to the clotting aspects of blood, that would be game-changing. The current mortality in the USA from a blood transfusion in isolation is currently estimated as 2.3 deaths for every 1 million component units transfused [64]. Some improvements have been made due to the evolution of reporting networks such as the annual SHOT (Serious Hazards of Transfusion) report in the UK [65] and subsequent changes in practice. One example is the use of male-only FFP in transfusions in the UK due to a recognised increase in immune-mediated complications when female plasma is used. However, sidestepping these issues altogether with a synthetic product would be much preferred.
There has been much research done on synthetic haemoglobins and perfluorocarbons over the last 25–30 years, but the results have not been promising. Only one perfluorocarbon has been approved for use in humans by the FDA since research began (Fluosol-DA-20 in 1989), but it was withdrawn 5 years later due to side effects ranging from transient hypertension to myocardial infarction, arrhythmias and death. Notably, one of the main drivers for the development of haemoglobin substitutes was for patients with haemoglobinopathies such as sickle cell disease rather than trauma, and there has yet to be any agents approved for use internationally for any indication.
Hemopure has been approved in South Africa for treatment of acutely anaemic adult surgical patients, but despite trials in other countries, it has not been accepted into mainstream practice. There have been some phase three clinical trials of using synthetic haemoglobins in trauma or emergency surgery that were small scale but initially positive [66, 67], though these were conducted by the product manufacturers (PolyHeme from Northfield Laboratories). A recent review by Ferenz and Steinbicker [68] has highlighted many of the outstanding issues with synthetic haemoglobins from clinical and pre-clinical trials from 2013 to 2018. It is an interesting article, highlighting issues around the short lifespan of products, side effects, inflammatory reactions, difficulties in either loading or unloading oxygen appropriately and different approaches to solving these problems. The takeaway message, however, is that while research in this field is ongoing and making some progress, it will likely be many years before these products make it to market, or the medical literature advances much beyond case studies and small trial series. An alternate source of blood may be from mass production or manufacture from stem cells. There is work in progress at DARPA, though the cost is currently prohibitively high to scale up. Currently, each unit of blood costs £30,000 to produce [69] in comparison to £120–150 per unit of cross-matched blood in the UK. Twenty-five years in the future this may be an option as production costs decrease and scale increases, but it is not yet a viable option.
Blood Transfusions in Trauma
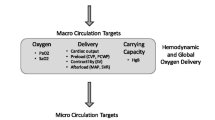
Blood has a distinct advantage over all other transfusion fluids in that it delivers oxygen to tissues in addition to expanding volume. While red blood cells will increase oxygen-carrying capacity, if given in isolation, then they will also dilute clotting factors that are present. Interestingly, more and more research has shown that red blood cells themselves are essential components in haemostasis due to their adhesion to endothelial cells and platelets and their ability to generate thrombin [70, 71], with anaemia increasing bleeding times [72]. A full discussion of the role of blood transfusion in trauma and the management of trauma-associated coagulopathy is found in Chap. 15 (Haemostasis and coagulopathy) later in this textbook. Suffice it to say the best strategy is to preserve as much of the patient’s blood as possible (including the use of cell salvage where possible), and replace blood with blood—either by units of whole blood or balanced component therapy. Ultimately, blood transfusion aims to promote the delivery of oxygen to tissues (otherwise known as DO2). This is dependent on two factors—the cardiac output and the oxygen content of arterial blood that reaches the tissues.
Oxygen Content of Blood
This content is principally due to the amount of haemoglobin present in the blood, but there is also a small amount of oxygen dissolved in plasma itself. The overall formula for calculating DO2 is, therefore:
Where CO is cardiac output and CaO2 is the oxygen content of arterial blood that is being delivered to the tissues. This can be expanded into individual components further by examining what constitutes the oxygen content of the blood.
The fully expanded equation is seen if Fig. 10.7, where:
-
[Hb] = Concentration of haemoglobin in grams per deciliter (g/dl)
-
1.34 = Hüfner’s constant—the amount of oxygen carried by fully saturated haemoglobin (1.39 ml O2/g Hb is the theoretical maximum, but in direct measurement, the maximum is 1.34)
-
SpO2 = Percentage arterial O2 saturation
-
PaO2 = arterial oxygen tension in kilopascals (kPa)
-
0.023 = amount of oxygen in ml dissolved per 100 ml of plasma per kPa
It can be seen that the most significant determinant of oxygen content in the blood is haemoglobin concentration. The amount of oxygen dissolved in plasma is itself a minor contributor, and assuming a patient is on 100% dry oxygen, with maximum efficiency in metabolism with a normal PaCO2 of 5 kPa, the theoretical maximum amount of oxygen that could be dissolved is only 2.19 ml O2/100 ml plasma In contrast, by increasing the haemoglobin concentration by only 1.6 g by the administration of just over 1 unit of blood to an average adult, the same raise in CaO2 can be achieved while still breathing room air. The dissolved concentration of oxygen rarely makes a significant contribution to DO2 overall, as physiologically a PaO2 of 11–13 is a normal value, giving a dissolved oxygen concentration of 0.25 ml O2/100 ml blood, in comparison to 1 g of 100% oxygenated blood which transports 1.34 ml O2/100 ml blood, i.e. 5.36 times more. This dissolved fraction only tends to be physiologically significant when there is severe lung damage or exceptionally high oxygen demand, and a high FiO2 is required.
Cardiac Output, Blood Pressure and Flow
As previously discussed, cardiac output is the product of the stroke volume and heart rate. When this is combined with systemic vascular resistance, it determines the blood pressure. While physiological first principles are sound and give a good mental model of how the circulatory system works, there are a few other factors to consider when discussing oxygen delivery to tissues outside of gross systemic models. The flow of oxygenated blood to tissues is the important measure, and there are a couple of factors which may affect flow rather than blood pressure. Examining the mathematical model described at the start of the chapter, by increasing SVR (causing vasoconstriction) blood pressure is increased. While blood pressure is a driving force for perfusion, downstream vasoconstriction may decrease the flow of blood in hypovolaemic states and subsequently decrease DO2. The reason for this is that the Hagen-Poiseuille equation governs laminar flow:
Where:
-
Q = Flow
-
P = Driving pressure
-
r = radius of the tube
-
η = Viscosity
-
l = length of tubing
As the factor with the greatest influence in terms of flow is the radius of the tube (as it is raised to the fourth power), it is therefore obvious why peripheral vasoconstriction decreases flow. Mathematically, doubling the radius of the tube (or in this case, blood vessel) and keeping everything else the same will increase flow by a factor of 16 times. This relationship is also why having short, large-bore IV access in patients who may require large amounts of blood products is preferable to smaller access. The rest of the equation is also important to consider. There needs to be a driving pressure (P) in order to have flow into a vessel, so in most cases adequate filling is necessary to achieve this by increasing preload and stroke volume. Flow rate is inversely proportional to viscosity, so thicker solutions such as FFP and red blood cells may not be as quickly transfused as crystalloids through the same IV access due to this. There is also a slight evolutionary advantage, however. When a patient bleeds and their haemoglobin levels fall, the blood becomes less viscous and flow improves as a consequence. As flow improves, DO2 must also become more efficient, and this may possibly be why management in ICU of patients with a haemoglobin level of 7 g/dl is sufficient. Current practice is to avoid transfusion above this threshold unless there is a history of ischaemic heart disease [73, 74]. It must be pointed out that this strategy in acute blood loss does not work—if a patient presents with a haemoglobin of 7 g/dl before intervention, then they are bleeding and require resuscitation with blood and blood products, not crystalloid. After achieving definitive haemostasis and restoring an adequate circulating volume with blood products, there may be a role for crystalloids on ICU. One pitfall to point out is that when a patient arrives in ICU, they may not be adequately resuscitated and still have an ongoing requirement for blood products to catch up. This is not the time to employ a restrictive transfusion strategy or to use vasopressors/inotropes to correct a low blood pressure caused by undertreated hypovolaemia. Inappropriate use of vasopressors in this group of patients may increase mortality by at least 200% [75,76,77,78].
Blood Components or Warm Fresh Whole Blood?
As blood is usually split into its component fractions (red blood cells, fresh frozen plasma and platelets) once it has been donated and cold-stored, there has been much debate over the effectiveness of how best to use blood. Questions posed include the optimum ratio of blood products to administer to patients who are acutely bleeding [79], whether there is a difference in outcomes in patients administered blood stored for longer or shorter periods [80,81,82] and whether using warm fresh whole blood (WFWB) is superior to individual component therapy. The military experience of using warm fresh whole blood (WFWB) to resuscitate patients (i.e. taking a unit of blood from a walking donor panel and administering it without fractionation to a bleeding casualty [83]) has generally been positive. Small series reports and trial data have shown increased survival and decreased transfusion volume requirements over 24 h compared to component therapy [84,85,86]. The exact reasons for this are not entirely understood and this approach also throws up many questions on the effectiveness of troops who have donated blood whilst on deployment (though marksmanship [87] and cognitive performance [88] have been reported as being preserved or improved). Is WFWB intrinsically superior to fractionated components? Is the process of buddy-buddy donation between non-medically trained personnel safe? There are many aspects which need further consideration and research, not only in medical but also military spheres.
Practical Aspects of Blood Transfusion
A full exploration of the current state of evidence behind blood transfusion ratios is available in Chap. 15 (Haemostasis and coagulopathy), but there are some practical points which are worth following in managing blood transfusions in major trauma. Firstly, the time to review a mass transfusion policy is not the first time it is needed—a thorough stress testing of the policy should be undertaken well in advance of it being used in vivo. Simulation (and specifically in situ simulation) is a readily available tool to assess the weak points of policies. If a blinded exercise is undertaken by the people who may be expected to use a mass transfusion policy in real-time, there may be some surprising results. Unanticipated chokepoints can be elucidated [89], explored and improved on, and there are several ready-made scenarios which can be implemented to test departmental responses [90]. Investing a couple of hours in simulation and debriefing will pay dividends in the event of the mass transfusion policy being enacted. It is also vital to let blood bank know where to send products; if a mass transfusion has been declared and the patient moves from the emergency department to interventional radiology or the operating theatre then blood bank and portering staff should be made aware. If issues are identified in simulation, then these should be fed into the live organisational reporting system—no patients have been harmed but risks/problems may be identified which could lead to future harm. If these problems are not captured, they may well lead to actual morbidity or mortality in a live patient in future.
Secondly, make sure IV access is adequate and appropriate for what is needed. Per the Hagen-Poiseuille equation already mentioned, flow is optimum in wide and short tubes so IV access should be established with this in mind. When comparing flow rates through different devices in vitro in a paper by Khoyratty et al. [91] the Hagen-Poiseuille equation predicts the salient points. Peripheral access outperforms central lines of the same width, the use of a needle-free access device decreases flow rates in cannulae of 16G or larger (with or without pressured infusion), Rapid Infusion Catheters (RIC) are superior to 14G cannulae, and flow is improved when a pressure system is used to increase the rate in all devices. These findings are similar to papers by Reddick et al. from 2011 [92] and Wrenn et al. from 2017 [93], reinforcing the basic principle of IV access being short, wide and peripheral in the first instance.
Third, several mnemonics can be used to prepare a patient and team for a mass transfusion and trauma anaesthetic (see TRAUMATIC and POLYTRAUMAS examples, above in Figs. 10.8 and 10.9 respectively). Use of cognitive aids should be encouraged as any tool which can increase the use of evidence-based practice and the available cognitive bandwidth to deal with complex trauma are undoubtedly a good thing [94, 95]. It is crucial, however, to consider how these aids are designed as a poorly thought out tool may decrease performance [96]. NASA have produced guidelines on how to produce checklists which are based on experience and sound research methodology [97, 98], and work best when internally designed by the people who will use them rather than being externally imposed [99].
Fourth, having a dedicated clinician managing the mass transfusion is exceptionally useful [100, 101]. Due to the time needed for checking of blood, ensuring correct ratios of plasma, platelets, PRBCs and calcium, a dedicated anaesthetist or emergency medicine physician should be tasked to keep track of blood products administered as well as the practicalities of running a mass transfusion device such as a Belmont or Level 1. Practically, a count is best maintained by either a tally chart or keeping bags of transfused products in a set space and separating them by type for later documentation. Some centres have included a check box system in the blood boxes that are issued from the lab for units to be ticked off when administered to keep track. This allows the lead anaesthetist to concentrate on other aspects of resuscitation and request volume boluses as needed without having the additional cognitive burden of ensuring compliance with transfusion protocols.
Fifth, ensure that adequate amounts of calcium are given. A general rule of thumb is to try and keep the ionised calcium above 1.0 mmol/l on ABG testing. In cases where product administration is rapid due to ongoing loss and ABG’s cannot be checked, give 10 ml of 10% calcium chloride for every four units of PRBCs or FFP that are administered. This should counteract the leaching effect of the sodium citrate that is used to preserve red blood cells and FFP, and an adequate calcium level is essential to ensure clotting. Calcium is also a positive inotrope and may contribute to increased blood pressure by increasing stroke volume. Calcium gluconate is not an ideal substitute in comparison to calcium chloride as it has approximately one-third of the amount of elemental calcium of calcium chloride, so three times the volume would be required for the same clinical effect [102]. Higher levels of calcium are also cardioprotective when dealing with hyperkalaemia which may be associated with muscle damage from trauma or massive transfusion itself [103, 104].
Sixth, give a 1 g tranexamic acid (TXA) bolus early, or 15 mg/kg in children—the same dose as paracetamol/acetaminophen. The CRASH-2 [105] and MATTERS [106] trials have been widely accepted as proof of decreased morbidity and mortality from bleeding if TXA is given within 3 h of injury. There have been a few unconvincing post-hoc analyses which have attempted to show an increase in thrombotic complications, but these mainly refer to administration outside the 3-h window or are methodologically flawed. The source data from CRASH-2 is open for public scrutiny and analysis. Professor Brohi has offered to collaborate and publish an analysis of the data with groups sceptical of the effect of TXA as long as he had a right of reply in the paper but has not yet been taken up on this offer.
Seventh, make use of near-patient testing of coagulation such as ROTEM or TEG devices where available. Both will give real-time indications of coagulation and may guide further blood product administration, and have been associated with lower overall transfusion requirements and may lead to improved mortality [107,108,109]. This is covered in more detail in the Chap. 15 (Haemostasis and coagulopathy).
Active Management of Circulatory Failure
In light of the complex state of the evidence base, some contradictory research and uncertainty on how to proceed, Revell, Porter and Greaves proposed a new UK consensus statement in 2002 [110] regarding the pre-hospital management of hypotension in trauma.
They stated that:
-
Trauma victims with a radial pulse do not require fluid until haemostasis is achieved
-
If a radial pulse is absent, then a 250 ml bolus of normal saline is given
-
In penetrating torso trauma, the presence of a major pulse, e.g. femoral, is considered adequate
They went on to explain that this approach must be combined with rapid transfer to theatre or control of bleeding, but they did also identify some exceptional situations:
-
Head injuries—they were unsure of what best evidence to recommend.
-
Children—titration to a brachial pulse was recommended.
Shock is a pathological, abnormal physiological state, and every patient has varying degrees of tolerance of it. Even advocates of hypotensive resuscitation/delayed fluid resuscitation will accept that the limits of this strategy are not only defined by the level of the blood pressure, but also the duration of the shock.
In-Hospital On-Going Care
In-hospital, many of the fundamental principles remain the same such as the importance of rapid control of bleeding and preservation of circulating volume. The Task Force for Advanced Bleeding in Trauma was established in 2004 and publishes regular updates to the latest evidence, with the fifth edition published in 2019 [111].
The first iteration of the guidelines made some key recommendations, with the main beneficial factor that they identified in cases of severe haemorrhage was to achieve the shortest time to theatre.
They also highlighted the importance of circulation preservation with measures such as:
-
Use of a damage control approach
-
Early recognition of bleeding
-
Pelvic stabilisation
-
Consideration of early angiography and embolisation for pelvic bleeding
-
A multi-disciplinary approach.
The latest critical messages from the 2019 guidelines are:
-
Traumatically injured patients should be transported quickly and treated by a specialised trauma centre whenever possible.
-
Measures to monitor and support coagulation should be initiated as early as possible and used to guide a goal-directed treatment strategy.
-
A damage-control approach to surgical intervention should guide patient management.
-
Coagulation support and thromboprophylaxis strategies should consider trauma patients who have been pre-treated with anticoagulants or platelet inhibitors.
-
Local adherence to a multidisciplinary, evidence-based treatment protocol should serve as the basis of patient management and undergo regular quality assessment
Continuing the same key principles started in the pre-hospital arena, external bleeding should be controlled, excessive crystalloids should be avoided, and rapid assessments should be made before making decisions about ongoing care and moving forward to CT, theatre or interventional radiology.
Circulation preservation remains a key priority by whatever means necessary, and at this point, it should be noted that immobilisation devices such as pelvic binders or traction splints are considered part of ‘C’ for circulation management. Their application can significantly reduce ongoing blood loss and also reduce fracture movement and pain.
Finally, do not forget that simple measures such as ‘gentle patient handling’ can also have profound effects in terms of minimising further blood loss. The increased use of the scoop stretcher to reduce bleeding from log-rolling has little firm supporting evidence, but anecdotally it may reduce sudden internal blood loss and pain by avoiding the need for a full log-roll if appropriately used. If used badly with poor technique, however, it can result in two log-rolls in opposite directions to introduce the two halves of the scoop.
Others would argue that a ‘log-roll’ will have to be performed at some point to check the back of the patient thoroughly, and it can be performed without compromise once the pelvis is strapped. This is also controversial, as those that actively promote limited movement would suggest that the traditional rolling and checking of the back offers very little unless searching for wounds. Major trauma casualties almost invariably going through the CT scanner regardless, which should pick up back injuries far more effectively than simple examination.
As it stands at present, the scoop is favoured over the long-board by UK ambulance services, but there are concerns that this is being proposed as better in terms of its ‘pressure area’ risk when in reality, it is just another hard plastic board. Ultimately, a vacuum mattress or “vac-mat” is probably a far better device than both of these for the casualty, but this does have both cost and practical limitations. Movement of patients onto a vac-mat from a scoop when they arrive in hospital is possible and may minimise other additional movements before and during CT scan. It can also be used to minimise heat loss if the vac-mat is wrapped securely and has a heating or warmed blanket enclosed next to the patient’s skin where possible.
Throughout the early time in the hospital, it is essential to look for any signs of ongoing uncontrolled bleeding. If identified, then this should be managed quickly and effectively to preserve the patient’s circulation. Practitioners should not be over-reliant on the lab and transfused blood, as transfused blood will never be as good as the patient’s own.
Brain Injury and Permissive Hypotension
Neurosurgeons would like normotension (or perhaps slight hypertension) and rapid delivery to their care within 4 h to optimise their outcome (see disability and head injury chapter for more information). The application of permissive hypotension in patients with concomitant brain injury is a compromise at best, and there is still no precise data to guide this approach despite several reviews and meta-analyses [112]. The best approach would undoubtedly be to deal with both severe haemorrhage and surgically amenable brain injury at the same operative sitting in a damage control approach, but outside a few centres of excellence in the world, this is rarely possible. Conventional wisdom is that active haemorrhage takes precedence over brain injury, but once bleeding is controlled, then blood pressure should be increased to maintain a cerebral perfusion pressure of 50–60 mmHg. The Brain Trauma Foundation Guidelines [113] are discussed in the disability and head injury chapter and form the mainstay of current treatment.
Damage Control Resuscitation
A damage control resuscitation and damage control surgical approach should be adopted, and the bleeding arrested in the shortest time possible. Once control has been achieved, circulating volume can be restored and the shock and hypoperfusion addressed (typically correcting the base deficit and lactate).
During damage control surgery, other factors will help to minimise further blood loss, including the use of a cell saver system, anti-fibrinolytic agents and other blood products such as FFP, platelets and cryoprecipitate.
In theatre during surgery, the temptation exists to reach for many of the numerous clinical devices traditionally associated with assessing resuscitation, cardiac output and hydration. In reality, few if any of these offer much value until bleeding is controlled and coagulopathy corrected.
Even relatively simple monitors such as arterial lines (often erroneously considered immediately essential) can delay transfer and definitive control of bleeding. Therefore, much as in in the pre-hospital domain, every action must be considered; is it appropriate and necessary to perform a procedure right now or will it introduce unnecessary delay?
Failure to Respond to Resuscitation
For some patients, attempts to maintain an ideal target MAP may fail, there may be continued deterioration and absence of a normal response to filling with blood or other fluids. Similarly, even after haemorrhage is controlled, it may remain difficult to improve the shocked state of the patient. This may be as a result of such rapid and massive bleeding that the volume loss has not been adequately replaced, there may be myocardial damage that is starting to manifest or the patient may be still falling further behind as a result of concealed or occult ongoing bleeding. There may be no response, inadequate response or even deterioration to any filling and then the prospect of a missed injury or intervention should be considered. These may include
Conditions which may mimic hypovolaemic shock
-
Tension pneumothorax—ultrasound is quick and sensitive
-
Cardiac tamponade—ultrasound could quickly reveal a tamponade
-
Adrenal insufficiency or crisis—consider a dose of hydrocortisone
-
Hypocalcaemia—especially after massive transfusion of stored blood and FFP/cryoprecipitate
-
Hypoglycaemia—always check a blood sugar
-
Spinal injury—mechanism, history, examination, CT
Summary
Shock and fluid resuscitation seem so simple if the circulation is merely considered as an empty bucket that needs re-filling, or a simple system of a pump, a fluid and connecting pipes. While it is an excellent theoretical analogy to understand the basic concepts of the circulatory system, it is a gross oversimplification in practice.
Filling patients to a normal blood pressure while still bleeding risks not only further bleeding, but also producing a wide range of severe side effects from the large volumes of resuscitation fluids, no matter what type is chosen. Every casualty will cope with blood loss and shock differently, and this is not merely down to cardiovascular fitness but a far broader concept of ‘shock tolerance’.
Blood pressure is a poor marker in this respect as patients may have low blood pressure, but with adequate blood flow maintained. They may display few signs of under-perfusion while others may demonstrate worsening acidosis and increasing base deficit with minimal blood loss and no drop in blood pressure. The focus must still be circulation preservation and rapid haemostasis, usually with surgery or interventional radiology. Until this is achieved, permissive hypotension, minimal crystalloid use, blood and possibly hybrid resuscitation will all help to minimise complications and achieve the best chances of survival.
As yet, no synthetic alternative to blood has proved itself to a satisfactory degree, but they may be a panacea in future. Stem cell-produced blood cells or fresh whole blood are currently likely to offer better alternatives but are prohibitively expensive or unavailable in everyday practice.
Damage control resuscitation is a hugely complex challenge and requires excellent diagnostic and decision skills and continuous ongoing evaluation of patient physiology. It is not just filling a bucket, and there is a very challenging balance to achieve despite the large amount of research that has been completed.
Questions
-
1.
Patients who present with a normal blood pressure by definition cannot have suffered significant hypovolaemia
-
(a)
True
-
(b)
False
-
(a)
-
2.
Synthetic haemoglobins are commonly used in current practice
-
(a)
True
-
(b)
False
-
(a)
-
3.
Permissive hypotension means actively lowering a patient’s blood pressure to stop bleeding
-
(a)
True
-
(b)
False
-
(a)
-
4.
The most important determinant of flow through resuscitative IV access is its diameter
-
(a)
True
-
(b)
False
-
(a)
-
5.
All trauma patients should be given a 2000 ml fluid challenge as part of standard resuscitation
-
(a)
True
-
(b)
False
-
(a)
Answers
-
1.
b
-
2.
b
-
3.
b
-
4.
a
-
5.
b
References
Lapner ST, Kearon C. Diagnosis and management of pulmonary embolism. BMJ. 2013;346:f757.
Yamamoto T. Management of patients with high-risk pulmonary embolism: a narrative review. J Intensive Care. 2018;6(1):16.
Kumar R, Sinha A, Lin MJ, Uchino R, Butryn T, O’Mara MS, et al. Complications of pericardiocentesis: a clinical synopsis. Int J Crit Illn Inj Sci. 2015;5(3):206–12.
Otsuka H, Sato T, Sakurai K, Aoki H, Yamagiwa T, Iizuka S, et al. Importance of the capability for complete resuscitative treatment combining surgery and interventional radiology for potentially lethal multiple injuries: a case report. Trauma Case Rep. 2017;11:13–7.
Chakraverty S, Zealley I, Kessel D. Damage control radiology in the severely injured patient: what the anaesthetist needs to know. Br J Anaesth. 2014;113(2):250–7.
Little RA, Kirkman E, Driscoll P, Hanson J, Mackway-Jones K. Preventable deaths after injury: why are the traditional ‘vital’ signs poor indicators of blood loss? Emerg Med J. 1995;12(1):1–14.
Mutschler M, Paffrath T, Wölfl C, Probst C, Nienaber U, Schipper IB, et al. The ATLS® classification of hypovolaemic shock: a well established teaching tool on the edge? Injury. 2014;45:S35–8.
American College of Surgeons, Committee on Trauma. Advanced trauma life support: student course manual. 10th ed. Chicago, IL: American College of Surgeons; 2018.
Goode P, Mountain A, Thies K, editors. The European Trauma Course Manual. 4.0. European Trauma Course Organisation; 2019. 164 p.
Duchesne JC, McSwain NE, Cotton BA, Hunt JP, Dellavolpe J, Lafaro K, et al. Damage control resuscitation: the new face of damage control. J Trauma Inj Infect Crit Care. 2010;69(4):976–90.
McManus J, Yershov AL, Ludwig D, Holcomb JB, Salinas J, Dubick MA, et al. Radial pulse character relationships to systolic blood pressure and trauma outcomes. Prehospital Emerg Care Off J Natl Assoc EMS Physicians Natl Assoc State EMS Dir. 2005;9(4):423–8.
Deakin CD. Accuracy of the advanced trauma life support guidelines for predicting systolic blood pressure using carotid, femoral, and radial pulses: observational study. BMJ. 2000;321(7262):673–4.
Poulton TJ. ATLS paradigm fails. Ann Emerg Med. 1988;17(1):107.
Porter JM, Ivatury RR. In search of the optimal end points of resuscitation in trauma patients: a review. J Trauma. 1998;44(5):908–14.
Kibble JD, Halsey CR. The big picture medical physiology [Internet]. New York: McGraw-Hill Medical; 2009. http://site.ebrary.com/id/10346923. Accessed 22 May 2019
Mutschler M, Nienaber U, Brockamp T, Wafaisade A, Fabian T, Paffrath T, et al. Renaissance of base deficit for the initial assessment of trauma patients: a base deficit-based classification for hypovolemic shock developed on data from 16,305 patients derived from the TraumaRegister DGU®. Crit Care. 2013;17(2):R42.
Wijaya R, Ng JH, Ong L, Wong ASY. Can venous base excess replace arterial base excess as a marker of early shock and a predictor of survival in trauma? Singapore Med J. 2016;57(2):73–6.
Rudkin SE, Kahn CA, Oman JA, Dolich MO, Lotfipour S, Lush S, et al. Prospective correlation of arterial vs venous blood gas measurements in trauma patients. Am J Emerg Med. 2012;30(8):1371–7.
Oropello JM, Manasia A, Hannon E, Leibowitz A, Benjamin E. Continuous fiberoptic arterial and venous blood gas monitoring in hemorrhagic shock. Chest. 1996;109(4):1049–55.
Schmelzer TM, Perron AD, Thomason MH, Sing RF. A comparison of central venous and arterial base deficit as a predictor of survival in acute trauma. Am J Emerg Med. 2008;26(2):119–23.
Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61(12):1444–61.
Thomas R, Power KJ. Interpreting the base deficit. Anaesthesia. 2000;55(7):696–7.
Handy JM, Soni N. Physiological effects of hyperchloraemia and acidosis. Br J Anaesth. 2008;101(2):141–50.
Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90(5):1265–70.
McFarlane C, Lee A. A comparison of Plasmalyte 148 and 0.9% saline for intra-operative fluid replacement. Anaesthesia. 1994;49(9):779–81.
Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg. 2015;102(1):24–36.
Wise R, Faurie M, Malbrain MLNG, Hodgson E. Strategies for intravenous fluid resuscitation in trauma patients. World J Surg. 2017;41(5):1170–83.
Odom SR, Howell MD, Silva GS, Nielsen VM, Gupta A, Shapiro NI, et al. Lactate clearance as a predictor of mortality in trauma patients. J Trauma Acute Care Surg. 2013;74(4):999–1004.
Parsikia A, Bones K, Kaplan M, Strain J, Leung PS, Ortiz J, et al. The predictive value of initial serum lactate in trauma patients. Shock. 2014;42(3):199.
Handy J. Lactate—The bad boy of metabolism, or simply misunderstood? Curr Anaesth Crit Care. 2006;17(1–2):71–6.
Nalos M, Leverve XM, Huang SJ, Weisbrodt L, Parkin R, Seppelt IM, et al. Half-molar sodium lactate infusion improves cardiac performance in acute heart failure: a pilot randomised controlled clinical trial. Crit Care. 2014;18(2):R48.
Glenn TC, Martin NA, Horning MA, McArthur DL, Hovda DA, Vespa P, et al. Lactate: Brain fuel in human traumatic brain injury: a comparison with normal healthy control subjects. J Neurotrauma. 2015;32(11):820–32.
Patet C, Suys T, Carteron L, Oddo M. Cerebral lactate metabolism after traumatic brain injury. Curr Neurol Neurosci Rep. 2016;16(4):31.
Raux M, Manach YL, Gauss T, Baumgarten R, Hamada S, Harrois A, et al. Comparison of the prognostic significance of initial blood lactate and base deficit in trauma patients. Anesthesiol J Am Soc Anesthesiol. 2017;126(3):522–33.
Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32(7):1107–38.
Bruns B, Lindsey M, Rowe K, Brown S, Minei JP, Gentilello LM, et al. Hemoglobin drops within minutes of injuries and predicts need for an intervention to stop hemorrhage. J Trauma. 2007;63(2):312–5.
Figueiredo S, Taconet C, Harrois A, Hamada S, Gauss T, Raux M, et al. How useful are hemoglobin concentration and its variations to predict significant hemorrhage in the early phase of trauma? A multicentric cohort study. Ann Intensive Care. 2018;8(1):76.
Li Y, Liu B, Dillon ST, Fukudome EY, Kheirbek T, Sailhamer EA, et al. Identification of a novel potential biomarker in a model of hemorrhagic shock and valproic acid treatment. J Surg Res. 2010;159(1):474–81.
Abassi ZA, Okun-Gurevich M, Salah NA, Awad H, Mandel Y, Campino G, et al. Potential early predictors for outcomes of experimental hemorrhagic shock induced by uncontrolled internal bleeding in rats. PLoS One. 2013;8(11):e80862.
Alam HB, Li Y. Biomarkers of hemorrhagic shock [Internet]. US9435813B2, 2016. https://patents.google.com/patent/US9435813B2/en. Accessed 22 May 2019.
Horne S. WB Cannon: a trauma pioneer. Trauma. 2004;6(1):79–81.
Cannon WB, Fraser J, Cowell EM. NATURE AND TREATMENT OF WOUND SHOCK AND ALLIED CONDITIONS. J Am Med Assoc. 1918;70(8):520–35.
Beecher HK. Preparation of battle casualties for surgery. Ann Surg. 1945;121(6):769–92.
Bickell WH, Wall MJ, Pepe PE, Martin RR, Ginger VF, Allen MK, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105–9.
Nevin DG, Brohi K. Permissive hypotension for active haemorrhage in trauma. Anaesthesia. 2017;72(12):1443–8.
Doran CM, Doran CA, Woolley T, Carter A, Male K, Midwinter MJ, et al. Targeted resuscitation improves coagulation and outcome. J Trauma Acute Care Surg. 2012;72(4):835–43.
Kirkman E, Watts S, Cooper G. Blast injury research models. Philos Trans R Soc Lond B Biol Sci. 2011;366(1562):144–59.
Mattox KL. Timing of transfusion in trauma. Vox Sang. 2004;87(s2):222–6.
Kasotakis G, Sideris A, Yang Y, de Moya M, Alam H, King DR, et al. Aggressive early crystalloid resuscitation adversely affects outcomes in adult blunt trauma patients: an analysis of the Glue Grant Database. J Trauma Acute Care Surg. 2013;74(5):1215–22.
Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care. 2014;4:21.
Malbrain MLNG, Roberts DJ, Sugrue M, De Keulenaer BL, Ivatury R, Pelosi P, et al. The polycompartment syndrome: a concise state-of-the-art review. Anaesthesiol Intensive Ther. 2014;46(5):433–50.
Marik PE, Monnet X, Teboul J-L. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1(1):1.
Hahn RG, Bergek C, Gebäck T, Zdolsek J. Interactions between the volume effects of hydroxyethyl starch 130/0.4 and Ringer’s acetate. Crit Care. 2013;17(3):R104.
Strunden MS, Heckel K, Goetz AE, Reuter DA. Perioperative fluid and volume management: physiological basis, tools and strategies. Ann Intensive Care. 2011;1:2.
Sharpe JP, Magnotti LJ, Croce MA, Paulus EM, Schroeppel TJ, Fabian TC, et al. Crystalloid administration during trauma resuscitation: does less really equal more? J Trauma Acute Care Surg. 2014;77(6):828–32. discussion 832
Lira A, Pinsky MR. Choices in fluid type and volume during resuscitation: impact on patient outcomes. Ann Intensive Care. 2014;4:38.
Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901–11.
Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124–34.
Annane D. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310(17):1809.
Guidet B, Martinet O, Boulain T, Philippart F, Poussel JF, Maizel J, et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS study. Crit Care. 2012;16(3):R94.
Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev [Internet]. 2013;(2). https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000567.pub6/full. Accessed 20 May 2019.
Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309(7):678–88.
Apostolou E, Deckert K, Puy R, Sandrini A, Leon MPD, Douglass JA, et al. Anaphylaxis to Gelofusine® confirmed by in vitro basophil activation test: a case series*. Anaesthesia. 2006;61(3):264–8.
Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406–17.
NHS Blood and Transplant. Serious hazards of transfusion home page [Internet]. Serious Hazards of Transfusion. https://www.shotuk.org/. Accessed 22 May 2019.
Gould SA, Moore EE, Hoyt DB, Ness PM, Norris EJ, Carson JL, et al. The life-sustaining capacity of human polymerized hemoglobin when red cells might be unavailable. J Am Coll Surg. 2002;195(4):445–52.
Gould SA, Moore EE, Hoyt DB, Burch JM, Haenel JB, Garcia J, et al. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J Am Coll Surg. 1998;187(2):113–20.
Ferenz KB, Steinbicker AU. Artificial oxygen carriers—past, present, and future—a review of the most innovative and clinically relevant concepts. J Pharmacol Exp Ther. 2019;369(2):300–10.
Lee N. The Lancet technology: pharming blood. The Lancet. 2016;387(10037):2496.
Litvinov RI, Weisel JW. Role of red blood cells in haemostasis and thrombosis. ISBT Sci Ser. 2017;12(1):176–83.
Tombak A. Red blood cells and relation to thrombosis. Transfus Med Sci Dev [Internet]. 2017. https://www.intechopen.com/books/transfusion-medicine-and-scientific-developments/red-blood-cells-and-relation-to-thrombosis. Accessed 20 May 2019.
Valeri CR, Cassidy G, Pivacek LE, Ragno G, Lieberthal W, Crowley JP, et al. Anemia-induced increase in the bleeding time: implications for treatment of nonsurgical blood loss. Transfusion (Paris). 2001;41(8):977–83.
Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–17.
Hébert PC. The TRICC Trial: a focus on the sub-group analysis. Vox Sang. 2002;83(s1):387–96.
Aoki M, Abe T, Saitoh D, Hagiwara S, Oshima K. Use of vasopressor increases the risk of mortality in traumatic hemorrhagic shock: a nationwide cohort study in Japan. Crit Care Med. 2018;46(12):e1145–51.
Tsuneyoshi I, Onomoto M, Yonetani A, Kanmura Y. Low-dose vasopressin infusion in patients with severe vasodilatory hypotension after prolonged hemorrhage during general anesthesia. J Anesth. 2005;19(2):170–3.
Gupta B, Garg N, Ramachandran R. Vasopressors: Do they have any role in hemorrhagic shock? J Anaesthesiol Clin Pharmacol. 2017;33(1):3–8.
Sperry JL, Minei JP, Frankel HL, West MA, Harbrecht BG, Moore EE, et al. Early use of vasopressors after injury: caution before constriction. J Trauma Inj Infect Crit Care. 2008;64(1):9–14.
McQuilten ZK, Crighton G, Brunskill S, Morison JK, Richter TH, Waters N, et al. Optimal dose, timing and ratio of blood products in massive transfusion: results from a systematic review. Transfus Med Rev. 2018;32(1):6–15.
Anand T, Ramnanan R, Skinner R, Martin M. Impact of massive transfusion and aging blood in acute trauma. Am Surg. 2016;82(10):957–9.
Sowers N, Froese P, Erdogan M, Green R. Impact of the age of stored blood on trauma patient mortality: a systematic review. Can J Surg. 2015;58(5):335–42.
Heddle NM, Cook RJ, Arnold DM, Liu Y, Barty R, Crowther MA, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med. 2016;375(20):1937–45.
Olszewski A, Korzeniewski K, Lass A. Selected epidemiological aspects of fresh whole blood application in the Polish Field Hospital in Afghanistan. Int Marit Health. 2014;65(1):23–7.
Cotton BA, Podbielski J, Camp E, Welch T, del Junco D, Bai Y, et al. A randomized controlled pilot trial of modified whole blood versus component therapy in severely injured patients requiring large volume transfusions. Ann Surg. 2013;1.
Jones AR, Frazier SK. Increased mortality in adult patients with trauma transfused with blood components compared with whole blood. J Trauma Nurs. 2014;21(1):22–9.
Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66(4 Suppl):S69–76.
Strandenes G, Skogrand H, Spinella PC, Hervig T, Rein EB. Donor performance of combat readiness skills of special forces soldiers are maintained immediately after whole blood donation: a study to support the development of a prehospital fresh whole blood transfusion program. Transfusion (Paris). 2013;53(3):526–30.
Eliassen HS, Hervig T, Backlund S, Sivertsen J, Iversen VV, Kristoffersen M, et al. Immediate effects of blood donation on physical and cognitive performance–A randomized controlled double-blinded trial. J Trauma Acute Care Surg. 2018;84(6S Suppl 1):S125–31.
Langston A, Downing D, Packard J, Kopulos M, Burcie S, Martin K, et al. Massive transfusion protocol simulation: an innovative approach to team training. Crit Care Nurs Clin North Am. 2017;29(2):259–69.
Kamerer JL. Massive transfusion protocol simulation: compound femur fracture and hypovolemic shock. Simul Healthc. 2012;7(3):196.
Khoyratty SI, Gajendragadkar PR, Polisetty K, Ward S, Skinner T, Gajendragadkar PR. Flow rates through intravenous access devices: an in vitro study. J Clin Anesth. 2016;31:101–5.
Reddick AD, Ronald J, Morrison WG. Intravenous fluid resuscitation: was Poiseuille right? Emerg Med J. 2011;28(3):201–2.
Wrenn EA, Wohlers R, Montgomery MH, Cobb H, Condra J, Tharpe S. Comparison of flow dynamics of peripherally and centrally inserted intravenous catheters using a rapid infusion system (ThermaCor 1200). Am Assoc Nurse Anaesth. 2017;85(4):256–60.
Marshall SD, Sanderson P, McIntosh CA, Kolawole H. The effect of two cognitive aid designs on team functioning during intra-operative anaphylaxis emergencies: a multi-centre simulation study. Anaesthesia. 2016;71(4):389–404.
Long E, Fitzpatrick P, Cincotta DR, Grindlay J, Barrett MJ. A randomised controlled trial of cognitive aids for emergency airway equipment preparation in a Paediatric Emergency Department. Scand J Trauma Resusc Emerg Med. 2016;24(1):8.
Marshall S. The use of cognitive aids during emergencies in anesthesia: a review of the literature. Anesth Analg. 2013;117(5):1162–71.
Degani A, Wiener EL. Cockpit checklists: concepts, design, and use. Hum Factors J Hum Factors Ergon Soc. 1993;35(2):345–59.
Schultz C. Here are NASA’s top 19 typography tips [Internet]. Smithsonian. https://www.smithsonianmag.com/smart-news/here-are-nasas-top-19-typography-tips-180947788/. Accessed 26 May 2019
Gawande A. The Checklist Manifesto: how to get things right. London: Profile Books Ltd: [distributor] TBS The Book Service Ltd; 2011.
Mercer SJ, Whittle CL, Mahoney PF. Lessons from the battlefield: human factors in defence anaesthesia. BJA Br J Anaesth. 2010;105(1):9–20.
Mercer SJ, Tarmey NT, Woolley T, Wood P, Mahoney PF. Haemorrhage and coagulopathy in the Defence Medical Services. Anaesthesia. 2013;68(s1):49–60.
Cote’ CJ, Drop LJ, Daniels AL, Hoaglin DC. Calcium chloride versus calcium gluconate: comparison of ionization and cardiovascular effects in children and dogs. Anesthesiology. 1987;66(4):465–70.
Raza S, Ali Baig M, Chang C, Dabas R, Akhtar M, Khan A, et al. A prospective study on red blood cell transfusion related hyperkalemia in critically ill patients. J Clin Med Res. 2015;7(6):417–21.
Vraets A, Lin Y, Callum JL. Transfusion-associated hyperkalemia. Transfus Med Rev. 2011;25(3):184–96.
CRASH-2 Trial Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. The Lancet. 2010;376(9734):23–32.
Morrison JJ. Military application of tranexamic acid in trauma emergency resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113.
Gonzalez E, Moore EE, Moore HB. Management of trauma-induced coagulopathy with thrombelastography. Crit Care Clin. 2017;33(1):119–34.
Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17(2):R76.
Schöchl H, Maegele M, Solomon C, Görlinger K, Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med. 2012;20(1):15.
Revell M. Fluid resuscitation in prehospital trauma care: a consensus view. Emerg Med J. 2002;19(6):494–8.
Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019;23(1):98.
Kudo D, Yoshida Y, Kushimoto S. Permissive hypotension/hypotensive resuscitation and restricted/controlled resuscitation in patients with severe trauma. J Intensive Care. 2017;5(1):11.
Carney N, Totten AM, OʼReilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, Fourth EditionNeurosurgery. 2016;1.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lax, P. (2022). Circulation. In: Lax, P. (eds) Textbook of Acute Trauma Care . Springer, Cham. https://doi.org/10.1007/978-3-030-83628-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-83628-3_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83627-6
Online ISBN: 978-3-030-83628-3
eBook Packages: MedicineMedicine (R0)