Abstract
Among several soil pollutants, the heavy metal effluents discharged from different industries directly or indirectly influence the global environmental balance and eventually decrease agricultural productivity. As a result of these harmful activities, soil pollution due to heavy metal toxicity is a potentially crucial environmental issue globally. The conventional methods of removing the huge metals from the environment are not eco-friendly, and these processes produce huge toxic residues. So, in this situation, bioremediation is the most preferred way to minimise the effects of heavy metals on the environment. Under such circumstances, the impact of plant growth-promoting rhizobacteria (PGPR) in remediation of metal toxicated areas has gained importance in sustainable agriculture systems. PGPRs increase plant growth by solubilising phosphate, synthesising IAA, producing enzymes, fixing the nitrogen, etc. So, the inoculation of suitable and specific heavy metal-tolerant PGPR strains associated with plants can maximise the phytoremediation. In this work, the impact of PGPR on remediation of the heavy metal contaminated zone is adequately described.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
The continued expansion of industrial activities and, more particularly, the dense industrial effluents are the main reasons contributing to soil pollution. Among various soil pollutants, heavy metals are highly phytotoxic, and their toxicity has a significant effect not only on plant growth but also on mass crop yield and health. It is a well-known fact that to enhance crop production, the deliberate application of chemical fertilisers, especially nitrogen and phosphorus, has led to extreme deleterious effects on soil structure and total plant health. In this situation, rhizosphere researchers have been throwing up surprises regarding the rhizospheric microorganisms. Plant growth-promoting rhizobacteria (PGPR) are bacteria that live in plant roots. In recent years, substantial attention has been paid to the potential of PGPR to substitute agrochemicals (fertilisers and pesticides) for plant growth promotion through a variety of mechanisms including organic matter decomposition, soil structure formation, organic pollutant degradation, mineral solubilisation and biocontrol of seed-borne pathogens. Heavy metal stress has become a big issue in whole terrestrial ecosystems worldwide. Damage to soil texture means disturbing the pH of soil. Heavy metal accumulation is the chief factors responsible for the reduction of plant growth and development. The huge industrial discharge, particularly wastewater discharge, contains a heavy load of metal effluents. When these materials get accumulated in agricultural land through irrigation, they produce severe problems in human bodies and the entire living systems. Under such circumstances, PGPR can be the safest option to decrease the notorious impacts of heavy metals on these environments.
11.2 An Introduction to PGPR
The rhizosphere is a layer of soil that is tightly regulated by the root system of the plant. This area is nutrient-dense as a result of the accumulation of a variety of nutritious plant exudates, such as sugars and amino acids. It is home to a diverse array of bacteria that colonise this region. Rhizobacteria are the microorganisms and bacteria that inhabit this area. Numerous rhizobacteria genera have been classified as PGPR, but Pseudomonas and Bacillus are the most prevalent.
Due to the ever-increasing hunger of the excessively increasing human population, the use of PGPR for reducing the application of agrochemicals is a critical issue. PGPR, the beneficial root-inhabiting bacteria, stimulates plant growth and protects them from various seed-borne pathogens by establishing a symbiotic relationship.
11.3 Mechanisms of PGPR’s Action
PGPRs promote plant development in a number of ways, both overt and indirect with phosphate solubilisation, nitrogen fixation, IAA synthesis and siderophore synthesis as the examples of direct pathways. Indirect pathways, on the other hand, involve the suppression of fungal, bacterial, fungal and nematode infections by the synthesis of various enzymes such as cellulases proteases and chitinases. Additional indirect pathways include quorum sensing, signal interference, mineral nutrient solubilisation, biofilm inhibition and systemic acquired tolerance (Fig. 11.1).
The root-colonising bacteria can improve plant growth by N-fixation (Djordjevic et al., 1987; Strzelczyk et al., 1994), phosphate solubilisation (Kloepper et al., 1988), phytohormone (auxin, gibberellins, cytokinin) production and decreasing the ethylene level in plants (Glick et al., 2007; Glick et al., 1999). Promoting water absorption and nutrient translocation, promoting rhizo anatomical development (Okon & Kapulnik, 1986), improving the whole enzyme system and cooperating with other groups of beneficiary soil microbes to perform better are the other mechanisms by which they improve the plant growth.
11.3.1 Direct Mechanism
PGPRs enhance the growth of plants through the following direct (Arora et al., 2012; Bhardwaj et al., 2014).
11.3.1.1 Nitrogen Fixation
PGPRs are widely applied to fix nitrogen , the most significant nutrient for plant growth and development (Fig. 11.2). Irrespective of the presence of nitrogen in the highest concentration in air, the plants are incapable of converting it into ammonia, thus remaining unavailable to plants. PGPRs convert dinitrogen into ammonia, utilising nitrogenase enzyme (Gaby & Buckley, 2012). They fix nitrogen either by building symbiotic association or by non-symbiotic pathway. Among different nutrients, nitrogen is one of the most essential, specifically in rice production. Every year 50–70% loss in rice yield occurs due to the failure of fulfilment of nitrogen demand of rice plants by chemical fertilisers (Ladha et al., 2005). As new varieties of rice demand a higher amount of nitrogen, it is getting impossible to provide it only by chemical fertilisers.
Among the root-colonising bacteria population that fix atmospheric nitrogen and benefit plant growth are plant growth-promoting rhizobacteria (PGPR). Alternatively, they are known as bio-enhancers or biofertilisers (Kloepper et al., 1980; Shamsuddin et al., 2014).
It is stated that PGPRs fix nitrogen in cereals, banana and grasses (Döbereiner, 1997). They also increase the nutrient absorption rate and resistance to droughts (Arzanesh et al., 2011). Among several naturally occurring host-microbe interactions, the symbiotic relationship between Rhizobium and leguminous plants is well established. This symbiosis is best understood and is a well-applied nitrogen-providing system to leguminous plants. Nowadays, research is going on to develop Rhizobium-non-legume interactions as well. This approach involves the integration of nitrogen-fixing gene into the rice-Rhizobium system. The rhizobial gene manipulation and modulation have lots of benefits. It develops a high level of root architecture, increases root hairs and enhances the rate of nutrient absorption (Yanni et al., 1997).
The co-inoculation of Rhizobium with two PGPR strains, Pseudomonas fluorescens P-93 and Azospirillum lipoferum S-21, effectively controlled the total nitrogen uptake, nutrient uptake and translocation of nutrients in Phaseolus vulgaris L. The PGPRs first make entry into the plants following the nodule formation where nitrogen fixation starts. The rhizo-microbial population showing symbiotic association includes several symbionts, like Bradyrhizobium, Rhizobium, Mesorhizobium and Sinorhizobium with legume-forming plants (Zahran, 2001).
Non-symbiotic nitrogen fixation is carried out by free-living diazotrophs, which concurrently promote the growth and yield of non-leguminous plants. Azotobacter, Azoarcus, Acetobacter, Burkholderia, Cyanobacteria and Pseudomonas are all examples of non-symbiotic nitrogen fixers, 2012; Vessey, 2003).
11.3.1.2 Phosphate Solubilisation
Phosphorus is another vital mineral element for a plant’s nutrition . It plays a pivotal role in photosynthesis, signal transduction, respiration and energy transfer. In soil, phosphate is present in inorganic and organic form with inadequate amounts, but plants can’t absorb phosphate because 98% of phosphate is present in insoluble and precipitated form (Pandey & Maheshwari, 2007). Plants can utilise phosphate only in two forms, the monobasic form (H2PO4) and the dibasic (HPO42−) form (Bhattacharyya & Jha, 2012).
It remains in the soil either in mineral salt form or in organic form. Hence, irrespective of the abundance of phosphorus in the soil, the plants can’t absorb it because of its insolubility which becomes a major limiting factor for the proper development of plants. That’s why it gradually becomes necessary to apply phosphorus in soluble form through fertilisers in the agricultural field.
Recent research indicates that inoculating crops with phosphate-solubilising microbes (PSM) will result in a 50% reduction in phosphate fertiliser application without affecting crop output (Yazdani et al., 2009). PSB (phosphate-solubilising bacteria) can also be beneficial in the phytoremediation of soils contaminated with heavy metals or in the bioleaching of rare earth elements from mined ores.
PGPRs solubilise inorganic phosphates by releasing phosphatase enzyme during substrate degradation (Sharma et al., 2013). Phosphate-solubilising PGPRs belong to the genera Arthrobacter, Beijerinckia, Microbacterium, Erwinia, Rhodococcus, Burkholderia, Flavobacterium, Enterobacter and Serratia (Bhattacharyya & Jha, 2012). It is also reported that the phosphate solubilisation rate gradually increases by the application of other beneficial soil microbes along with PGPRs (Zaidi et al., 2009).
11.3.1.3 Siderophore Production
Iron is a critical micronutrient for all living species and is found in abundance in soil. Irrespective of its high presence in soil, plants can’t utilise it because of its low solubility rate. Iron is the fourth in position according to its abundance on earth. It is readily assimilated neither by the plants nor by any bacteria because of its presence in the aerobic soils in ferric ion (Fe3+) form that is not readily soluble in water (Ma, 2005). But some microorganisms have developed some unique mechanisms for iron assimilation, including the formation of low-molecular-weight iron-chelating products called siderophores (Arora et al., 2012; Schwyn & Neilands, 1987). Through siderophores, it enters within the plant body. The siderophores perform an important function like iron’s extracellular solubilisation from minerals.
According to distinct functional groups, siderophores can be divided into three major categories: carboxylates, hydroxamates and catecholates (Cornelis, 2010), and bacteria can produce all four types of siderophores. Examples of some active siderophore-producing bacteria are Salmonella, Enterobacter, Vibrio cholerae, Escherichia coli, Aeromonas and Yersinia.
Some fungi, for examples, A. versicolor (Holinsworth & Martin, 2009), Ustilago sphaerogena (Shanmugaiah et al., 2015), Rhizopus (Shenker et al., 1992), etc., are also reported to produce siderophore . A large number of PGPRs, e.g. Streptomyces (Dimkpa et al., 2008), Azotobacter (Fekete et al., 1983), Rhizobium (Datta & Chakrabartty, 2014), Burkholderia (Ong et al., 2016), Aeromonas (Hirst et al., 1991), Pseudomonas, etc. (Sujatha & Ammani, 2013), also produce siderophores and improve plant growth.
11.3.1.4 Production of Phytohormone
Phytohormones are usually organic, and their impact on plant occurs in a meagre amount. They are synthesised in different tissues and are then transported to their target sites. Hormones are categorised into five groups by plant biologists: auxin, cytokinins, ethylene, gibberellins and abscisic acid (ABA). Recently, two novel hormones, brassinosteroid and strigolactones, are also reported to be produced by plants (Zwanenburg et al., 2016). Microorganisms in the rhizosphere generate growth-stimulating hormones such as indole acetic acid (IAA), cytokinins and gibberellins, among others (Arora et al., 2013), which significantly enhance plant growth.
11.3.1.4.1 Indole Acetic Acid (IAA)
The fungi Rhizopus suinus and Absidia ramosa were identified to produce auxin. About 80% of root-colonising microbial populations isolated from different crops and vegetables are proven to produce auxin due to secondary metabolism (Vessey, 2003). IAA is the natural auxin, and it has positive effects on the root and shoot elongation. The primary precursor of IAA is tryptophan which is found to occur in root exudates (Miransari & Smith, 2014). Different PGPRs like Pseudomonas, Agrobacterium, Klebsiella and Enterobacter produce IAA either via the formation of indole-3-pyruvic acid or via indole-3-acetic-aldehyde (Shilev, 2013).
Several free-living PGPRs like Alckaligenes faecalis, Acetobacter diazotrophicous and Enterobacter cloacae are also related to the low level of IAA production. IAA increases the rate of cell division, differentiation, lateral and adventitious root development and pigment production and provides resistance to stress conditions (Spaepen & Vanderleyden, 2011).
IAA is a secondary metabolite produced through either tryptophan-dependent pathway or an independent tryptophan pathway in plants and bacteria. In Azospirillum brasilense IAA is produced through tryptophan-independent pathway. It is reported that IAA produced in wheat plants by Azospirillum brasilense stimulates a high number and length of lateral roots.
Irrespective of plant growth promotion and root nodulation, IAA also helps in root proliferation and root branching. The function of IAA, produced by the PGPB Pseudomonas putida GR12-2, is well established by the experiment done on canola roots. The IAA-deficient mutant bacterial strain was applied on other canola plant sets and eventually showed no such root and shoot growth compared to PGPB-treated set. Inoculation in a seed with Pseudomonas putida GR12-2 showed root formations that were 35–50% longer than the roots grown from seeds inoculated with IAA-deficient mutant PGPB strain. In another set where the mung bean plant was used in experimentation, the same PGPB showed overproduction of IAA in plants compared to the uninoculated control set. IAA is proven in taking a role in root and shoot growth and necessary for the formation of nodules.
The production of IAA in plants is a stimulator of the cell wall loosening. It is secreted in higher amounts as a root exudate, which provides excess nutrients to root-colonising bacteria.
11.3.1.4.2 Gibberellins and Cytokinins
Among different rhizobacteria, Azotobacter sp., Bacillus subtilis, Pseudomonas fluorescens, Paenibacillus polymyxa and Rhodospirillum rubrum are known to produce either gibberellins or cytokinins or both, which play an essential role in plant growth promotion (Kang et al., 2010). PGPRs produce a lower level of cytokinins as compared to different phytopathogens. According to Barea et al. (2005), 90% of root-colonising bacteria isolated from other crops exhibit the ability of cytokinin like compound production, e.g. a free-living soil bacterium, Pseudomonas polymyxa, was reported to produce cytokinins (Timmusk et al., 2014).
Gibberellins are involved in seed germination, floral induction, stem and floral growth and crop and fruit development. Simultaneously, GA processing by PGPRs encourages the growth and yield of a wide variety of grain plants and vegetables.
It is also reported that Azospirillum brasilense and Arthrobacter giacomelli produce a dense concentration of cytokinins grown in mixed culture condition (Lippi et al., 1991). In 1972, Philips and Torrey experimentally proved the presence of zeatin like compounds around the Rhizobium nodules.
11.3.2 Indirect Mechanisms
The application of PGPR is a promising approach for sustainable agriculture, as it upsurges plant growth and soil fertility in an indirect way. Based on their indirect mechanism, researchers are trying to reduce the application of different agrochemicals. PGPR can improve soil fertility via antibiosis, lytic enzyme production, the implication of induced systemic resistance, etc. (Lugtenberg & Kamilova, 2009).
11.3.2.1 Antibiotic Production
According to researchers, PGPRs develop some antibiotics that are highly effective against a variety of soil-borne phytopathogens (Shilev, 2013). Numerous antibiotics are developed by various Pseudomonas bacteria, including phenazine, oomycin A, pyrrolnitrin, tensin and cyclic lipopeptides (Loper & Gross, 2007). Streptomyces and Bacillus contain antibiotics such as kanamycin, oligomycin A and zwittermicin A (Compant et al., 2005).
Pseudomonas sp. produces 2,4-DAPG in soil, which can be used to monitor Gaeumannomyces graminis var. tritici in wheat (de Souza et al., 2003). Bacillus amyloliquefaciens produces lipopeptides and polyketides that are effective against soil-borne pathogens (Ongena & Jacques, 2008). Certain PGPRs can also synthesise a volatile compound called HCN, which is used as a biocontrol agent against Thielaviopsis basicola (Sacherer et al., 1994).
11.3.2.2 Lytic Enzyme Production
PGPRs can produce different types of lytic enzymes like chitinases, lipases, proteases, dehydrogenase, phosphatase, etc. (Hayat et al., 2010; Joshi et al., 2012). They show hyperparasitic function in attacking phytopathogens by their cell wall hydrolysis. PGPRs can also tolerate different living and non-living stresses by suppressing several pathogenic fungi, including Fusarium oxysporum, Pythium ultimum, Rhizoctonia solani, etc. (Nadeem et al., 2013; Upadyay et al., 2012). Azotobacter chroococcum has been reported to give good performance on Sesamum indicum at field trial (Maheshwari et al., 2012). Similarly, Trichoderma sp. inoculation in peanut acts as a biocontrol against Aspergillus niger which causes collar rot disease in the plant (Rabeendran et al., 2000).
11.3.2.3 Development of Induced Systemic Resistance (ISR)
When the environmental stimuli activate, the innate defence mechanism of the plant gets power against different challenges, called ISR (Avis et al., 2008). As PGPRs provide systemic resistance against several pathogens, like bacteria, fungi, insects and nematodes, they can be applied to host plants (Naznin et al., 2013). ISR stimulates jasmonates and ethylene, which help plants against several types of pathogens (Glick, 2012).
Induced resistance is a physiological condition that occurs when plant growth-promoting rhizobacteria induce an increase in defensive capacity (PGPR). The enzymes which have so far been reported to be involved with ISR include chitinase, peroxidase, superoxide dismutase, phenylalanine lyase, catalase, polyphenol oxidase and ascorbate peroxidase (Annapurna et al., 2013).
11.4 Impact of PGPR on Plants
PGPR is a distinct group of root-colonising bacteria that facilitate rooting and promote overall plant growth (Glick et al., 1995). According to Piao et al. (1992), plant growth-improving bacteria are collectively called YIB (Yield Increasing Bacteria). Some workers referred to them as plant beneficial bacteria (PBB). Gaind and Gaur (1991) referred to them as ‘direct PGPR’, and Grayston and Germida (1991) supported the term ‘direct PGPR’, whereas described them as non-biocontrol PGPRs.
The PGPR can enhance plant growth by nitrogen fixation (Roy Chowdhury et al., 2017), phosphate solubilisation (; Yazdani et al., 2009), phytohormone production (Vejan et al., 2016), potassium solubilisation (Han & Lee, 2005; Parmar & Sindhu, 2013) and siderophore production (Beneduzi et al., 2012; Pahari & Mishra, 2017).
11.4.1 As Biofertilisers
The host plant-PGPR relationship is critical for optimising plant growth and production on a broad scale. As the PGPRs are efficient for producing different phytohormones, specifically IAA, the phytohormonal network is still understudied. Among several PGPRs, C138 is proven to supply iron in iron-starved tomato plants. Similarly, Bacillus amyloliquefaciens is reported as growth and yield improver in soybean in India.
Burkholderia kururiensis (Estrada-de los Santos et al., 2001) and Burkholderia vietnamensis (Gillis et al., 1995) are examples of nitrogen fixers. Besides nitrogen fixation, phosphate solubilisation and siderophore productions are also fulfilled by the PGPRs. For these reasons, PGPRs are used as biofertilisers.
11.4.2 As Biocontrol Agent
Few root-colonising bacteria, especially Pseudomonads, inhibit the growth of some soil-inhabited pathogens. These bacteria not only impart resistance but also help in the improvement of plant growth and yield. In such circumstances, the term ‘biocontrol plant growth-promoting rhizobacteria’ was proposed by soil microbiologists (Tilak et al., 1999).
In paddy, P. fluorescens strains exhibited inhibitory action on hyphal growth of Rhizoctonia solani (Radjacommare et al., 2004). Stenotrophomonas marcescens strain, another biocontrol PGPR, inhibits different soil-borne pathogens, including Fusarium oxysporum. Different strains of Bacillus spp. constitute the ability to indulge ISR in a wide variety of crops. The biocontrol power of Bacillus spp. is one of the critical agents that can combat rhizo- and soil-borne pathogens in the case of chickpea (Landa et al., 1997).
They offer biological control to plants either by antibiotic production or by siderophore/phytohormone production. Genetic modification has opened a new way for developing PGPRs as biocontrol agents.
11.4.3 As Environmental Stress Controller
Due to climate change, rainfall patterns become more erratic, resulting in a tremendous reduction in crop production. As a result, the plants either become exposed to severe drought condition or floods. In addition to these, the continuous rising of the pollutant level in the environment, specifically the toxic gases in the atmosphere and heavy metals in the soil, leads to a drastic reduction of crop yield. The chemical effluents from several industries are also getting mixed in the river water, which subsequently passes to the agricultural fields. All these situations are giving birth to different environmental stresses.
Under stress conditions, plants produce a high concentration of ethylene, utilising ACC as a precursor. The ethylene retards the root and shoot elongation and suppresses leaf expansion. So, it is clear that if the PGPRs can produce ACC deaminase, they can tolerate the stress to a certain level (Akhgar et al., 2014). The ACC deaminase synthesising PGPRs reduces several environmental stresses by producing different exopolysaccharides, which immediately binds with cations (Na++) and eventually form a sheath on the plant roots. Few rhizobacteria also develop heavy metal tolerance mechanisms (Maxton et al., 2018) in plants.
ACC deaminase producers can relieve different stresses, such as heavy metals, drought, polyaromatic hydrocarbons and salts (Glick et al., 2007). Jacobson et al. (1994) showed that Pseudomonas putida contains ACC deaminase that helps reduce the adverse stress level in plants. Under water stress conditions, Pseudomonas sp. can improve CAT enzyme activity in basil plants. In GPX and APX function and total chlorophyll content, combinations of three PGPRs (Pseudomonas sp., Bacillus lentus, Azospirillum brasilense) showed tremendously good result under water stress condition (Heidari & Golpayegani, 2012).
11.5 Reports on the Effect of PGPRs in the Role of Biofertilisers
Biofertiliser is becoming a fundamental pillar of eco-friendly organic farming and a significant component of sustainable agriculture globally. They contain products that can be inoculated to seeds, soil or epidermal plant portion. They subsequently colonise the rhizospheric area and ultimately enhance plant growth by providing nutrients to the host plant. As bio-formulation, biofertilisers contain many microorganisms responsible for enriching the plants’ nutrient uptake status.
Azotobacter is a cytokinin synthesiser, which showed increased yield in cucumber (Alori et al., 2017). It fixes nitrogen in wheat, barley, rice, maize, lime, coconut, tobacco, etc. (Wani et al., 2013). Azorhizobium is highly efficient for nitrogen fixation in wheat, and it is applied as biofertiliser in wheat cultivation (Sabry et al., 1997). Bacillus bacterisation develops more lateral roots in cucumber (Sokolova et al., 2011) and synthesises gibberellins in pepper (Joo et al., 2005). It can also solubilise potassium in these crops (Han & Lee, 2005). Some other PGPRs are reported to act as biofertilisers on different crops and vegetables enlisted in Table 11.1.
From the above-mentioned examples, it is clear that PGPRs have outstandingly worked on different plant species as biofertilisers. They provide the safest and the most eco-friendly approach to sustainable agriculture. The importance of PGPRs in yield development and their capacity to elicit ISR against several abiotic stresses has been reported (Avis et al., 2008). The symbiotic association between PGPR and host plants is the most promising way for developing a new approach for sustainable agriculture.
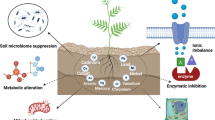
11.6 Heavy Metal Stress in the Environment
Heavy metals are a significant cause of soil pollution. Numerous metals contribute to soil pollution, including Ni, Cd, Zn, Cr, Cu and Pb. Heavy metals exert toxic impacts on the soil microflora; hence their population size, diversity and total activities get drastically affected. Different physiological activities of plants like photosynthesis, water absorption and cell division get affected tremendously. The toxic symptoms include the appearance of dark green leaves, permanent wilting of plants, stunted growth, brown short leaves and roots. The plants’ uptake metals from soil and these metals eventually enter the food chain and result in high health risk for living animals, including humans. The agricultural runoff containing heavy metal discharge enters the aquatic environment and leads to toxic effects on aquatic animals and plants.
Heavy metals reduce bacterial species richness in the contaminated soils. Among different heavy metals, cadmium (Cd) is considered the most toxic one to the microbial enzymes, whereas lead (Pb) decreases catalase, urease, alkaline phosphatase and acid phosphatase. The nature of sensitivity of soil enzymes to different heavy metals is quite different from each other.
11.6.1 Effects of PGPRs on Plants in Heavy Metal-Contaminated Soil
Hyperaccumulator plants can accumulate a high level of heavy metals and tolerate heavy metal stress to an extent. The plants growing in the heavy metal-polluted soils harbour a wide group of microbes that can tolerate heavy metal concentrations to a higher limit and provide several nutrients to host plants. Among the rhizospheric microbes, the plant growth-promoting rhizobacteria (PGPR) attract special attention because they can enhance the phytoremediation method by releasing chelators, synthesising different phytohormones, etc. The following table (Table 11.2) summarises the existing reports regarding the effects of PGPR on phytoremediation in metal-polluted soil.
PGPRs are known to affect the metal mobility and availability to the host plant, and it may occur through redox changing, acidification, siderophore production, mobilisation of inorganic phosphate, etc. The sensitivity and sequestration power of rhizospheric microbes towards heavy metal stress broaden the way of bioremediation. The PGPRs can also alter the plant metabolism to better withstand the heavy metal stress in the soil.
11.7 Conclusions
Phytoremediation is a new cost-effective way for sustainable agriculture. The recent trends of research on remediation of heavy metals in soil by applying PGPRs show a brilliant prospect for modern agriculture. The application of PGPRs in enhancing crop growth and development helps in heavy metal mobilisation, which is quite advantageous to applying chemical fertilisers. The microbial metabolites are less toxic, biodegradable and eco-friendly. So, to remove the harmful impact of heavy metals from the agricultural soil, it is the safest option to use the PGPR, which will open a new gateway to sustainable agriculture.
References
Aboushanab, R. A. I., Angle, J. S., & Chaney, R. L. (2006). Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high Ni soils. Soil Biology and Biochemistry, 38(9), 2882–2889. https://doi.org/10.1016/j.soilbio.2006.04.045.
Afzal, A., & Bano, A. (2008). Rhizobium and phosphate solubilizing bacteria improve the yield and phosphorus uptake in wheat (Triticum aestivum). International Journal of Agriculture and Biology, 10(8530), 1560.
Akhgar, A., Arzanlou, M., Bakker, A. H. M., & Hamidpour, M. (2014). Characterisation of 1-aminocyclopropane-1-carboxylate (ACC) deaminase-containing Pseudomonas spp. in the rhizosphere of salt-stressed canola. Pedosphere, 24(4), 461–468.
Alori, E. T., Glick, B. R., & Babalola, O. O. (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol 8, 971.
Annapurna, K., Kumar, A., Kumar, L. V., Govindasamy, V., Bose, P., & Ramadoss, D. (2013). PGPR-induced systemic resistance (ISR) in plant disease management. In D. Maheshwari (Ed.), Bacteria in agrobiology: Disease management. Berlin, Heidelberg: Springer.
Arora, N. K., Tewari, S., & Singh, R. (2013). Multifaceted plant-associated microbes and their mechanisms diminish the concept of direct and indirect PGPRs. In N. K. Arora (Ed.), Plant microbe symbiosis: Fundamentals and advances (pp. 411–449). Berlin: Springer.
Arora, N. K., Tewari, S., Singh, S., Lal, N., & Maheshwari, D. K. (2012). PGPR for protection of plant health under saline conditions. In D. K. Maheshwari (Ed.), Bacteria in agrobiology: Stress management (pp. 239–258). Springer-Verlag.
Arzanesh, M. H., Alikhani, H. A., Khavazi, K., Rahimian, H. A., & Miransari, M. (2011). Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World Journal of Microbiology and Biotechnology, 27(2), 197–205. https://doi.org/10.1007/s11274-010-0444-1.
Avis, T. J., Gravel, V., Antoun, H., & Tweddell, R. J. (2008). Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biology and Biochemistry, 40(7), 1733–1740. https://doi.org/10.1016/j.soilbio.2008.02.013.
Beneduzi, A., Ambrosini, A., & Passaglia, L. M. (2012). Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genetics and Molecular Biology, 35(4(Suppl.)), 1044–1051. https://doi.org/10.1590/s1415-47572012000600020.
Bhardwaj, D., Ansari, M. W., Sahoo, R. K., & Tuteja, N. (2014). Biofertilisers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microbial Cell Factories, 13, 66. https://doi.org/10.1186/1475-2859-13-66.
Bhattacharyya, P. N., & Jha, D. K. (2012). Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World Journal of Microbiology and Biotechnology, 28(4), 1327–1350. https://doi.org/10.1007/s11274-011-0979-9.
Barea, J-M., Pozo, M., Azcón, R., & Azcón-Aguilar, C., (2005). Microbial co-operation in the rhizosphere. Journal of Experimental Botany, 56(417):1761–1778. https://doi.org/10.1093/jxb/eri197.
Burd, G. I., Dixon, D. G., & Glick, B. R. (2000). Plant growth- promoting bacteria that decrease heavy metal toxicity in plants. Canadian Journal of Microbiology, 46(3), 237–245. https://doi.org/10.1139/w99-143.
Cavaglieri, L., Orlando, J., Rodríguez, M. I., Chulze, S., & Etcheverry, M. (2005). Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level. Research in Microbiology, 156(5–6), 748–754. https://doi.org/10.1016/j.resmic.2005.03.001.
Chakraborty, U., Chakraborty, B., & Basnet, M. (2006). Plant growth promotion and induction of resistance in Camellia sinensis by Bacillus megaterium. Journal of Basic Microbiology, 46(3), 186–195. https://doi.org/10.1002/jobm.200510050.
Chen, L., Luo, S., Li, X., Wan, Y., Chen, J., & Liu, C. (2014). Interaction of Cd hyperaccumulator Solanum nigrum L. and functional endophyte Pseudomonas sp. Lk9 on soil heavy metals uptake. Soil Biology and Biochemistry, 68, 300–308. https://doi.org/10.1016/j.soilbio.2013.10.021.
Compant, S., Reiter, B., Sessitsch, A., Nowak, J., Clément, C., & Ait Barka, E. (2005). Endophytic colonisation of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain 45. Applied and Environmental Microbiology, 71(4), 1685–1693. https://doi.org/10.1128/AEM.71.4.1685-1693.2005.
Cornelis, P. (2010). Iron uptake and metabolism in pseudomonads. Applied Microbiology and Biotechnology, 86(6), 1637–1645. https://doi.org/10.1007/s00253-010-2550-2.
Datta, B., & Chakrabartty, P. K. (2014). Siderophore biosynthesis genes of Rhizobium sp. isolated from Cicer arietinum L. 3. Biotech, 4(4), 391–401. https://doi.org/10.1007/s13205-013-0164-y.
de Souza, J. T., Weller, D. M., & Raaijmakers, J. M. (2003). Frequency, diversity and activity of 2, 4-diacetylphloroglucinol producing fluorescent Pseudomonas spp. in Dutch take-all decline soils. Phytopathology, 93(1), 54–63. https://doi.org/10.1094/PHYTO.2003.93.1.54.
Djordjevic, M. A., Gabriel, D. W., & Rolfe, B. G. (1987). Rhizobium-the refined parasite of legumes. Annual Review of Phytopathology, 25(1), 145–168. https://doi.org/10.1146/annurev.py.25.090187.001045.
Dimkpa, C., Svatos, A., Merten, D., Büchel, G., & Kothe, E. (2008). Hydroxamate siderophores produced by Streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Canadian Journal of Microbiology, 54(3), 163–172. https://doi.org/10.1139/w07-130.
Döbereiner, J. (1997). Biological nitrogen fixation in the tropics: Social and economic contributions. Soil Biology and Biochemistry, 29(5–6), 771–774. https://doi.org/10.1016/S0038-0717(96)00226-X.
Egamberdiyeva, D. (2007). The effect of plant growth promoting bacteria on growth and nutrient uptake of maise in two different soils. Applied Soil Ecology, 36(2–3), 184–189. https://doi.org/10.1016/j.apsoil.2007.02.005.
El-Akhal, M. R., Rincón, A., Coba de la Peña, T., Lucas, M. M., El Mourabit, N., Barrijal, S., & Pueyo, J. J. (2013). Effects of salt stress and rhizobial inoculation on growth and nitrogen fixation of three peanut cultivars. Plant Biology, 15(2), 415–421. https://doi.org/10.1111/j.1438-8677.2012.00634.x.
Elbeltagy, A., Nishioka, K., Sato, T., Suzuki, H., Ye, B., Hamada, T., … Minamisawa, K. (2001). Endophytic colonisation and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Applied and Environmental Microbiology, 67(11), 5285–5293. https://doi.org/10.1128/AEM.67.11.5285-5293.2001.
Estrada-de los Santos, P., Bustillos-Cristales, R., & Caballero-Mellado, J. (2001). Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Applied and Environmental Microbiology, 67(6), 2790–2798. https://doi.org/10.1128/AEM.67.6.2790-2798.2001, PubMed: 27902798.
Fekete, F. A., Spence, J. T., & Emery, T. (1983). Siderophores produced by nitrogen-fixing Azotobacter vinelandii OP in iron-limited continuous culture. Applied and Environmental Microbiology, 46(6), 1297–1300. https://doi.org/10.1128/aem.46.6.1297-1300.1983.
Flores-Félix, J. D., Silva, L. R., Rivera, L. P., Marcos-García, M., García-Fraile, P., Martínez-Molina, E., … Rivas, R. (2015). Plants probiotics as a tool to produce highly functional fruits: The case of Phyllobacterium and vitamin C in strawberries. PLoS One, 10(4), e0122281. https://doi.org/10.1371/journal.pone.0122281.
Gaby, J. C., & Buckley, D. H. (2012). A comprehensive evaluation of PCR primers to amplify the nifH gene of nitrogenase. PLoS One, 7(7), e42149. https://doi.org/10.1371/journal.pone.0042149.
Gaind, S., & Gaur, A. C. (1991). Thermo tolerant phosphate solubilising microorganisms and their interaction with mung bean. Plant and Soil, 133(1), 141–149. https://doi.org/10.1007/BF00011908.
Gholami, A., Shahsavani, S., & Nezarat, S. (2009). The effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. International Journal of Agricultural and Biosystems Engineering, 3, 1.
Gillis, M., Kesters, K., Hoste, B., Janssens, D., Kropenstedt, R. M., Stephen, M. P., … de Ley, J. (1995). Acetobacter diazotrophicussp. Nov. a nitrogen fixing acid bacterium associated with sugarcane. International Journal of Systematic and Evolutionary Microbiology, 39, 361–364.
Glick, B. R. (2012). Plant growth-promoting bacteria: Mechanisms and applications. Scientifica, 2012, 963401. https://doi.org/10.6064/2012/963401.
Glick, B. R., Cheng, Z., Czarny, J., & Duan, J. (2007). Promotion of plant growth by ACC deaminase-producing soil bacteria. European Journal of Plant Pathology, 119(3), 329–339. https://doi.org/10.1007/s10658-007-9162-4.
Glick, B. R., Karaturovíc, D. M., & Newell, P. C. (1995). A novel procedure for rapid isolation of plant growth promoting pseudomonads. Canadian Journal of Microbiology, 41(6), 533–536. https://doi.org/10.1139/m95-070.
Glick, B. R., Penrose, D. M., & Li, J. (1999). A model for the lowering of plant ethylene concentrations by plant growth promoting rhizobacteria. Journal of Theoretical Biology, 190, 63–68.
Govindarajan, M., Balandreau, J., Kwon, S. W., Weon, H. Y., & Lakshminarasimhan, C. (2008). Effects of the inoculation of Burkholderia vietnamensis and related endophytic diazotrophic bacteria on grain yield of rice. Microbial Ecology, 55(1), 21–37. https://doi.org/10.1007/s00248-007-9247-9.
Grayston, S. J., & Germida, J. J. (1991). Sulphur-oxidising bacteria as plant growth promoting rhizobacteria for canola. Canadian Journal of Microbiology, 37(7), 521–529. https://doi.org/10.1139/m91-088.
Han, H. S., & Lee, K. D. (2005). Phosphate and potassium solubilizing bacteria effect on mineral uptake, soil availability and growth of eggplant. Research Journal of Agriculture and Biological Sciences, 1(2), 176–180.
Hayat, R., Ali, S., Amara, U., Khalid, R., & Ahmed, I. (2010). Soil beneficial bacteria and their role in plant growth promotion: A review. Annals of Microbiology, 60(4), 579–598. https://doi.org/10.1007/s13213-010-0117-1.
He, L. Y., Zhang, Y. F., Ma, H. Y., Su, L. N., Chen, Z. J., & Wang, Q. Y. (2010). Characterisation of copper resistant bacteria and assessment of bacterial communities in rhizosphere soils of copper-tolerant plants. Applied Soil Ecology, 44(1), 49–55. https://doi.org/10.1016/j.apsoil.2009.09.004.
Heidari, M., & Golpayegani, A. (2012). Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). Journal of the Saudi Society of Agricultural Sciences, 11(1), 57–61. https://doi.org/10.1016/j.jssas.2011.09.001.
Hirst, I. D., Hastings, T. S., & Ellis, A. E. (1991). Siderophore production by Aeromonas salmonicida. Journal of General Microbiology, 137(5), 1185–1192. https://doi.org/10.1099/00221287-137-5-1185.
Holinsworth, B., & Martin, J. D. (2009). Siderophore production by marine-derived fungi. Biometals: an International Journal on the Role of Metal Ions in Biology, Biochemistry, and Medicine, 22(4), 625–632. https://doi.org/10.1007/s10534-009-9239-y.
Retrieved from. https://www.google.com/search?hl=en&biw=1366&bih=577&tbm=isch&sa=1&ei=pBMZXPXgKdHbrQHRs5KoDw&q=mechanism+of+pgpr+action+in+cycle+form&oq=mechanism+of+pgpr+action+in+cycle+form&gs_l=img.3. 1.0.0.240.2902.0j11j4.. . . . 0. . . .1..gws-wiz-img.iFqJeLp4_80#imgrc=8hiQbTaie4kuXM, 42372(48609), 49154.
Jiang, C. Y., Sheng, X. F., Qian, M., & Wang, Q. Y. (2008). Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere, 72(2), 157–164. https://doi.org/10.1016/j.chemosphere.2008.02.006.
Joo, G. J., Kim, Y. M., Kim, J. T., Rhee, I. K., Kim, J. H., & Lee, I. J. (2005). Gibberellins-producing rhizobacteria increase endogenous gibberellins content and promote growth of red peppers. Journal of Microbiology, 43(6), 510–515.
Joshi, M., Shrivastava, R., Sharma, A. K., & Prakash, A. (2012). Screening of resistant verities and antagonistic Fusarium oxysporum for biocontrol of Fusarium Wilt of Chilli. Plant Pathologia et Microbiologia, 3, 134.
Kang, B. G., Kim, W. T., Yun, H. S., & Chang, S. C. (2010). Use of plant growth-promoting rhizobacteria to control stress responses of plant roots. Plant Biotechnology Reports, 4(3), 179–183. https://doi.org/10.1007/s11816-010-0136-1.
Khan, A. L., Waqas, M., Kang, S. M., Al-Harrasi, A., Hussain, J., Al-Rawahi, A., … Lee, I. J. (2014). Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. Journal of Microbiology, 52(8), 689–695. https://doi.org/10.1007/s12275-014-4002-7.
Kloepper, J. W., Leong, J., Teintze, M., & Schroth, M. N. (1980). Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature, 286(5776), 885–886. https://doi.org/10.1038/286885a0.
Kloepper, J. W., Lifshitz, R., & Schroth, M. N. (1988). Pseudomonas inoculants to benefit plant production. ISI Atlas of Science – Animal and Plant Sciences, 1, 60–64.
Kumar, H., Bajpai, V. K., Dubey, R. C., Maheshwari, D. K., & Kang, S. C. (2010). Wilt disease management and enhancement of growth and yield of Cajanus cajan (L.) var. Manak by bacterial combinations amended with chemical fertiliser. Crop Protection, 29(6), 591–598. https://doi.org/10.1016/j.cropro.2010.01.002.
Ladha, J. K., Pathak, H., Krupnik, J., Six, T., & van Kessel, C. (2005). Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. Advances in Agronomy, 87, 85–156. https://doi.org/10.1016/S0065-2113(05)87003-8.
Landa, B. B., Hervás, A., Bettiol, W., & Jiménez-Díaz, R. M. (1997). Antagonistic activity of bacteria from the chickpea rhizosphere against Fusarium oxysporum f. sp. ciceris. Phytoparasitica, 25(4), 305–318. https://doi.org/10.1007/BF02981094.
Liang, X., He, C. Q., Ni, G., Tang, G. I., Chen, X. P., & Lei, Y. R. (2014). Growth and Cd accumulation of Orychophragmus violaceus as affected by inoculation of Cd-tolerant bacterial strains. Pedosphere, 24(3), 322–329. https://doi.org/10.1016/S1002-0160(14)60018-7.
Lippi, D., Cacciari, I., Paola, Q., & Pietrosanti, T. (1991). Interactions between Azospirillum and sorghum rhizosphere isolates under different cultural conditions.
Loper, J. E., & Gross, H. (2007). Genomic analysis of antifungal metabolite production by Pseudomonas fluorescens Pf-5. European Journal of Plant Pathology, 119(3), 265–278. https://doi.org/10.1007/s10658-007-9179-8.
Lugtenberg, B., & Kamilova, F. (2009). Plant-growth-promoting rhizobacteria. Annual Review of Microbiology, 63, 541–556. https://doi.org/10.1146/annurev.micro.62.081307.162918.
Ma, Y., Rajkumar, M., & Freitas, H. (2009). Isolation and characterisation of Ni mobilising PGPB from serpentine soils and their potential in promoting plant growth and Ni accumulation by Brassica spp. Chemosphere, 75(6), 719–725. https://doi.org/10.1016/j.chemosphere.2009.01.056, PubMed: 19232424.
Ma, J. F. (2005). Plant root responses to three abundant soil minerals: Silicon, aluminum and iron. Critical Reviews in Plant Sciences, 24(4), 267–281. https://doi.org/10.1080/07352680500196017.
Maheshwari, D. K., Dubey, R. C., Aeron, A., Kumar, B., Kumar, S., Tewari, S., & Arora, N. K. (2012). Integrated approach for disease management and growth enhancement of Sesamum indicum L. utilising Azotobacter chroococcum TRA2 and chemical fertiliser. World Journal of Microbiology and Biotechnology, 28(10), 3015–3024. https://doi.org/10.1007/s11274-012-1112-4.
Maxton, A., Singh, P., Andy, A., Prasad, S. M., & Masih, S. A. (2018). PGPR: A boon in stress tolerance and bio control. Research Journal of Biotechnology, 13, 105–111.
Miransari, M., & Smith, D. L. (2014). Plant hormones and seed germination. Environmental and Experimental Botany, 99, 110–121. https://doi.org/10.1016/j.envexpbot.2013.11.005.
Nadeem, S. M., Naveed, M., Zahir, Z. A., & Asghar, H. N. (2013). Plant-microbe interactions for sustainable agriculture: Fundamentals and recent advances. In N. K. Arora (Ed.), Plant microbe symbiosis: Fundamentals and advances (pp. 51–103). India: Springer.
Naznin, H. A., Kimura, M., Miyazawa, M., & Hyakumachi, M. (2013). Analysis of volatile organic compounds emitted by plant growth promoting fungus Phoma sp. GS83 for growth promotion effects on tobacco. Microbes and Environments, 28(1), 42–49. https://doi.org/10.1264/jsme2.me12085.
Okon, Y., & Kapulnik, Y. (1986). Development and function of Azospirillum-inoculated roots. Plant and Soil, 90(1–3), 3–16. https://doi.org/10.1007/BF02277383.
Ong, K. S., Aw, Y. K., Lee, L. H., Yule, C. M., Cheow, Y. L., & Lee, S. M. (2016). Burkholderia paludis sp. nov., an antibiotic-siderophore producing novel Burkholderia cepacia complex species, isolated from Malaysian tropical peat swamp soil. Frontiers in Microbiology, 7, 2046. https://doi.org/10.3389/fmicb.2016.02046.
Ongena, M., & Jacques, P. (2008). Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends in Microbiology, 16(3), 115–125. https://doi.org/10.1016/j.tim.2007.12.009.
Pahari, A., & Mishra, B. B. (2017). Characterisation of siderophore producing rhizobacteria and its effect on growth performance of different vegetables. International Journal of Current Microbiology and Applied Sciences, 6(5), 1398–1405. https://doi.org/10.20546/ijcmas.2017.605.152.
Pandey, P., & Maheshwari, D. K. (2007). Two sp. microbial consortium for growth promotion of Cajanus cajan. Current Science, 92, 1137–1142.
Parmar, P., & Sindhu, S. S. (2013). Potassium solubilisation by rhizosphere bacteria: Influence of nutritional and environmental conditions. Journal of Microbiology Research, 3(1), 25–31.
Piao, C. G., Tang, W. H., & Chen, Y. X. (1992). Study on the biological activity of yield-increasing bacteria. Chinese Journal of Microecology, 4, 55–62.
Rabeendran, N., Moot, D. J., Jones, E. E., & Stewart, A. (2000). Inconsistent growth promotion of cabbage and lettuce from Trichoderma isolates. New Zealand Journal of Plant Protection, 53, 143–146.
Radjacommare, R., Kandan, A., Nandakumar, R., & Samiyappan, R. (2004). Association of the hydrolytic enzyme chitinase against Rhizoctonia solani in rhizobacteria treated rice plants. Journal of Phytopathology, 152(6), 365–370. https://doi.org/10.1111/j.1439-0434.2004.00857.x.
Rivas, R., Martens, M., de Lajudie, P., & Willems, A. (2009). Multilocus sequence analysis of the genus Bradyrhizobium. Systematic and Applied Microbiology, 32(2), 101–110. https://doi.org/10.1016/j.syapm.2008.12.005.
Roy Chowdhury, A., Kundu, S., & SenGupta, C. (2017). Plant growth promoting rhizobacteria (PGPR): One step ahead to sustainable agriculture. International Journal of Innovative Science Engineering and Technology, 4(7), 41–48.
Ryder, M. H., Yan, Z., Terrace, T. E., Rovira, A. D., & Tang, W. (1999). Uses of Bacillus isolated in China to suppress take all and Rhizoctonia root rot, and promote seedling growth of glasshouse grown wheat in Australian soils. Soil Biology and Biochemistry, 31, 19–29.
Sabry, S. R. S., Saleh, S. A., Batchelor, C. A., Jones, J., Jotham, J., Webster, G., … Cocking, E. C. (1997). Endophytic establishment of Azorhizobium caulinodans in wheat. Proceedings of the Royal Society of London. Series B, 264(1380), 341–346. https://doi.org/10.1098/rspb.1997.0049.
Sacherer, P., Défago, G., & Haas, D. (1994). Extracellular protease and phospholipase C are controlled by the global regulatory gene gacA in the biocontrol strain Pseudomonas fluorescens CHA0. FEMS Microbiology Letters, 116(2), 155–160. https://doi.org/10.1111/j.1574-6968.1994.tb06694.x.
Schwyn, B., & Neilands, J. B. (1987). Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry, 160(1), 47–56. https://doi.org/10.1016/0003-2697(87)90612-9.
Shamsuddin, H. Z., TanZuan, K., Radziah, O., Halimi, M. S., Khairuddin, R. A., & Sheikh, H. (2014). Isolation and characterisation of rhizobia and plant growth-promoting rhizobacteria and their effects on growth of rice seedlings. American Journal of Agricultural and Biological Sciences, 9(3), 342–360. https://doi.org/10.3844/ajabssp.2014.342.360.
Shanmugaiah, V., Karmegham, N., Harikrishnan, H., Jayaprakashvel, M., & Natesan, B. (2015). Biocontrol mechanisms of siderophores against bacterial plant pathogens. Sustainable approaches to controlling plant pathogenic bacteria, Edition: First [Chapter]. In V. R. Kannan & K. K. Bastas (Eds.), Biocontrol mechanisms of siderophores against bacterial plant pathogens (pp. 167–186). CRC Press.
Shenker, M., Oliver, I., Helmann, M., Hadar, Y., & Chen, Y. (1992). Utilisation by tomatoes of iron mediated by a siderophore produced by Rhizopus arrhizus. Journal of Plant Nutrition, 15(10), 2173–2182. https://doi.org/10.1080/01904169209364466.
Shilev, S. (2013). Soil rhizobacteria regulating the uptake of nutrients and undesirable elements by plants. In N. K. Arora (Ed.), Plant microbe symbiosis: Fundamentals and advances (pp. 147–150). India: Springer.
Singh, S., & Kapoor, K. K. (1999). Inoculation with phosphate-solubilising microorganisms and a vesicular arbuscular mycorrhizal fungus improves dry matter yield and nutrient uptake by wheat grown in a sandy soil. Biology and Fertility of Soils, 28, 139–144.
Sokolova, M. G., Akimova, G. P., & Vaishlia, O. B. (2011). Effect of phytohormones synthesised by rhizosphere bacteria on plants. Prikladnaia Biokhimiia i Mikrobiologiia, 47, 302–307.
Spaepen, S., & Vanderleyden, J. (2011). Auxin and plant-microbe interactions. Cold Spring Harbor Perspectives in Biology, 3(4), a001438. https://doi.org/10.1101/cshperspect.a001438.
Strzelczyk, E., Kampert, M., & Li, C. Y. (1994). Cytokinin-like substances and ethylene production by Azospirillum in media with different carbon sources. Microbiological Research, 149(1), 55–60. https://doi.org/10.1016/S0944-5013(11)80136-9.
Sujatha, N., & Ammani, K. (2013). Siderophore production by the isolates of fluorescent pseudomonads. International Journal of Current Research and Review, 5, 1–7.
Sharma, S. K., Ramesh, A., & Johri, B. N. (2013). Isolation and characterisation of plant growth promoting Bacillus amyloliquefaciens strain Sks_bnj_1and its influence on rhizosphere soil properties and nutrition of soybean (Glycine max L. Merrill). Journal of Virology & Microbiology, 2013, 1–19.
Tilak, K. V. B. R., Singh, G., & Mukerji, K. G. (1999). Biocontrol—Plant growth promoting rhizobacteria: Mechanism of action. In K. G. Mukerji, B. P. Chamola, & R. K. Upadhyay (Eds.), Biotechnological approaches in biocontrol of plant pathogens (Vol. 10, pp. 114–115). Kluwer Academic/Plenum Publishers.
Timmusk, S., Abd El-Daim, I. A., Copolovici, L., Tanilas, T., Kännaste, A., Behers, L., … Niinemets, Ü. (2014). Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS One, 9(5), e96086. https://doi.org/10.1371/journal.pone.0096086.
Upadyay, S. K., Maurya, S. K., & Singh, D. P. (2012). Salinity tolerance in free living plant growth promoting rhizobacteria. Indian Journal of Scientific Research, 3, 73–78.
Vejan, P., Abdullah, R., Khadiran, T., Ismail, S., & Nasrulhaq Boyce, A. N. (2016). Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules, 21(5), 573. https://doi.org/10.3390/molecules21050573.
Verma, V. C., Singh, S. K., & Prakash, S. (2011). Bio-control and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A. Juss. Journal of Basic Microbiology, 51(5), 550–556. https://doi.org/10.1002/jobm.201000155.
Vessey, J. K. (2003). Plant growth promoting rhizobacteria as biofertilisers. Plant and Soil, 255(2), 571–586. https://doi.org/10.1023/A:1026037216893.
Wani, S. A., Chand, S., & Ali, T. (2013). Potential use of Azotobacter chroococcum in crop production: An overview. Current Agriculture Research Journal, 1(1), 35–38. https://doi.org/10.12944/CARJ.1.1.04.
Wu, S. C., Cheung, K. C., Luo, Y. M., & Wong, M. H. (2006). Effects of inoculation of plant growth promoting rhizobacteria on metal uptake by Brassica juncea. Environmental Pollution, 140(1), 124–135. https://doi.org/10.1016/j.envpol.2005.06.023.
Yanni, Y. G., Rizk, R. Y., Corich, V., Squartini, A., Ninke, K., … Dazzo, F. B. (1997). Natural endophytic association between Rhizobium leguminoserum bv. trifolii and rice roots and assessment of its potential to promote rice growth. Plant and Soil, 194(1/2), 99–114. https://doi.org/10.1023/A:1004269902246.
Yazdani, M., Bahmanyar, M. A., Pirdashti, H., & Esmaili, M. A. (2009). Effect of phosphate solubilization microorganisms (PSM) and plant growth promoting rhizobacteria (PGPR) on yield and yield components of corn (Zea mays L.). International Journal of Agricultural and Biosystems Engineering, 3, 1.
Zahran, H. H. (2001). Rhizobia from wild legumes: Diversity, taxonomy, ecology, nitrogen fixation and biotechnology. Journal of Biotechnology, 91(2–3), 143–153. https://doi.org/10.1016/s0168-1656(01)00342-x.
Zaidi, A., Khan, M. S., Ahemad, M., & Oves, M. (2009). Plant growth promotion by phosphate solubilising bacteria. Acta Microbiologica et Immunologica Hungarica, 56, 263–284.
Zhang, H., Sekiguchi, Y., Hanada, S., Hugenholtz, P., & Kim, H. (2003). Gemmatimona saurantiacagen. nov, sp. nov., a Gram-negative, aerobic, polyphosphate accumulating microorganism, the firrst cultured representative of the new bacterial phylum Gemmatimonadetesphyl. International Journal of Systematic and Evolutionary Microbiology, 53, 1155–1163.
Zwanenburg, B., Pospíšil, T., & Ćavar Zeljković, S. (2016). Strigolactones: New plant hormones in action. Planta, 243(6), 1311–1326. https://doi.org/10.1007/s00425-015-2455-5.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Roy Chowdhury, A. (2022). Plant Growth-Promoting Rhizobacteria (PGPR): Strategies to Improve Heavy Metal Stress Under Sustainable Agriculture. In: Bandh, S.A. (eds) Sustainable Agriculture. Springer, Cham. https://doi.org/10.1007/978-3-030-83066-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-83066-3_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83065-6
Online ISBN: 978-3-030-83066-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)