Abstract
Addiction is a chronic relapsing disorder. Despite pharmacological and psychological interventions during rehabilitation, a majority of patients still relapse. In this seventh chapter, we present neuromodulation techniques as a complementary intervention for addiction. Firstly, while deep brain stimulation (DBS) has shown promising results, its cost–benefit–risk ratio is nonetheless too high to be implemented in routine clinical care. Secondly, repeated transcranial magnetic stimulation (rTMS) and transcranial direct courant stimulation (tDCS) over the dorsolateral prefrontal cortex (DLPFC) have shown reduced craving and relapses, but the results are mixed. To improve efficacy, new perspectives envisioned that the insula could be a promising target for rTMS and DBS in combination with cognitive remediation and while participants are exposed to key conditioned stimuli. Additionally, neurofeedback could be a useful tool in teaching patients to actively regulate their neural activity, although better controlled experimental designs and rigorous measures of brain changes are needed. Despite the heterogeneity of studies, neuromodulation techniques as complementary tools to conventional care seem to constitute a turning point in the management of addictions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Firmly grounded in a vision that addiction is a brain disease (Leshner, 1997), neurostimulation emerged as an encouraging set of techniques aimed at restoring brain functions and improving clinical trajectories (Ekhtiari et al., 2019). It capitalised on influential theories that considered abnormal neurocognitive functioning as a key dimension of addiction (Goldstein & Volkow, 2002, 2011; Koob & Volkow, 2016; Noël et al., 2013; Robbins & Everitt, 1996; Robinson & Berridge, 2008, 2016).
Indeed, the progress made in brain imaging over the last decades represents a marked advancement in our understanding of substance use disorder (SUD) (American Psychiatric Association, 2013), as it offers concrete and effective modelling of addictive states’ neurobiological underpinnings (Parvaz et al., 2011). While the initial investigations mainly focused on limbic-dysregulated activity and the reward system, the research emphasises a wider disrupted neuronal circuitry of addiction (Goldstein & Volkow, 2011; Noël et al., 2013; Koob & Volkow, 2016). Indeed, SUD could arise from an imbalance between three separate but interacting neural systems: a reflective, principally prefrontal cortex (PFC)-dependent system involving inhibitory control and decision-making, predicting the future consequences of behaviour; an impulsive system, mostly on the amygdala-striatum, promoting automatic, salient and habitual behaviours; and the insula that integrates interoception, which further integrates conscious feelings and decision-making processes involved in short risky profit (Noël et al., 2013).
In addition to pharmacological intervention (Mann et al., 2014), a growing amount of data has promoted the efficacy of non-pharmacological neurocognitive interventions for SUD (Coles et al., 2018; Noël et al., 2019). To date, the dorsolateral prefrontal cortex (DLPFC) has been the most targeted brain area for improving cognitive control, decision-making and reducing craving intensity (Bechara, 2005; Lüscher et al., 2020; Zilverstand et al., 2018). Insula has recently become a highly relevant subject for reducing SUD symptoms (Ibrahim et al., 2019).
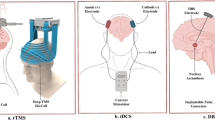
In this chapter, we briefly summarise key findings on the following: (1) deep brain stimulation (DBS), an invasive and focal electrical therapy using electrodes implanted deep into the brain; (2) repetitive transcranial magnetic stimulation (rTMS), a non-invasive technique using a stimulation coil over the scalp delivering a magnetic pulse through the skull over a period; (3) transcranial direct current stimulation (tDCS), a painless non-invasive device delivering low direct current across the scalp with a positive (anodal) and a negative (cathodal) electrode; and (4) neurofeedback (NF), a brain–computer interface using a real-time display of brain activity fed back to the patient so they can learn to implement strategies to regulate their brain activity.
2 Invasive Brain Stimulation
DBS is an invasive technique that continuously stimulates brain areas in the long term (Herrington et al., 2016; Montgomery & Gale, 2008). It consists of a pulse generator implanted with brain surgery, with four electrodes placed in deep brain areas. It is possible to turn the system off/on or modify its frequency and intensity. In the 1980s, DBS was applied as an intervention for movement disorders and treating tremors in patients with Parkinson’s disease (Benabid et al., 1987). During the 2000s, it was used in psychiatric disorders for patients with treatment-refractory disorders, first in obsessive-compulsive disorder (Nuttin et al., 1999), followed by major depression (Mayberg et al., 2005). Case studies observing the effect of DBS on the nucleus accumbens (NAc) in patients with Parkinson’s disease and psychiatric patients with concomitant alcohol and nicotine use disorders show an unexpected reduction in consumption (Ardouin et al., 2006; Kuhn et al., 2007, 2009). Following these observations, Luigjes et al. (2012) suggested stimulating the NAc, involved in motivation and inhibitory control.
Eight case studies have explored the effect of DBS on addiction among a total of 11 patients (meta-analysis Luigjes et al., 2019). Four focused on alcohol use disorder (Voges et al., 2006; Müller et al., 2009, 2016; Kuhn et al., 2011), three on opioid use disorder (Zhou et al., 2011; Valencia-Alfonso et al., 2012; Kuhn et al., 2014) targeting the bilateral NAc and one on cocaine use disorder focusing on the bilateral anterior cingulate cortex (Gonçalves-Ferreira et al., 2016). Most patients were still abstainers after at least 12 months. All opioid use disorder patients and half alcohol disorder patients were abstainers, while half alcohol use disorder patients and all cocaine use disorder patients were non-abstainers with reduced consumption. A study comparing methadone maintenance and DBS as a treatment for opioid use disorder showed that over 6 months, 47% of patients under methadone had opiate-free urine against 49% on DBS (Stephen et al., 2012).
The results were positive for addiction disorders, but the problem is that DBS is extremely invasive, with 0.4% surgeries leading to death and 2% leading to adverse events (e.g., haemorrhage problems) (Voges et al., 2006). Although DBS appears to be well tolerated after recovery from brain surgery, the risk seems too high and knowledge too unclear to suggest it as common clinical practice for addiction treatment (Carter & Hall, 2011). Indeed, the mechanisms underlying the beneficial effects of DBS have been investigated (Luigjes et al., 2012, 2019; Pierce & Vassoler, 2013; Herrington et al., 2016). The identified mechanisms include neuroplasticity and possibly neuroprotection and neurogenesis (Jakobs et al., 2019) for exhaustive cellular mechanisms.
In conclusion, studies on DBS have yielded positive clinical outcomes, but the cost–benefit ratio is questionable and ethically disputable. DBS should be reserved for patients refractory to any other less invasive treatment as a last resort.
3 Non-invasive Brain Stimulation
Strengthening the brain area for clinical improvement without brain surgery and fatal risk was the main aim of non-invasive brain stimulation (NIBS). Although NIBS are less powerful, they allow interventions with fewer risks and adverse events (Rossi et al., 2009, 2020). The first recommendations for using NIBS for clinical purposes date back to 1994 (Rossini et al., 1994). Since then, numerous clinical trials have been conducted in psychiatry (Tortella, 2015; Kekic et al., 2016; Lefaucheur et al., 2017; Fregni et al., 2020), including SUD (Jansen et al., 2013; Grall-Bronnec et al., 2014; Hone-Blanchet et al., 2015; Schluter et al., 2018; Ekhtiari et al., 2019; Luigjes et al., 2019; Stein et al., 2019; Song et al., 2019; Bollen et al., 2021). The most studied NIBS are repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS). rTMS is less invasive than DBS but more invasive than tDCS. rTMS can induce action potential by magnetic stimulation. tDCS, using electric stimulation, is easier and cheaper than rTMS. Multiple sessions of NIBS are safe even for children and adolescents with a similar rate of adverse events as adults, which are mainly headache (11.5%) for rTMS and redness (4.7%), itching (5.8%) and tingling (11.5%) for tDCS (Krishnan et al., 2015).
Both techniques are recommended to be repeated for at least five 5- to 30-min sessions over the DLPFC to be effective in SUD (Luigjes et al., 2019; Song et al., 2019). In SUD patients, DLPFC activation is disrupted, reflecting poor memory, attention and inhibitory capacity in the context of substance-related stimuli (Goldstein & Volkow, 2011). Indeed, the DLPFC is involved in inhibitory control and decision-making, resisting the urge (Bechara, 2005; Koechlin & Hyafil, 2007; Badre & Nee, 2018; Zilverstand et al., 2018). Induced craving is linked to DLPFC in eight functional magnetic resonance imaging (fMRI) studies (Wilson et al., 2004). It correlates with glutamatergic dysfunction in the NAc and the anterior cingulate cortex, which are two important areas of the reward system (Bauer et al., 2013). An imbalance between the hyperactive emotional system and the hypoactive executive function system is hypothesised to reflect the chronicity of addiction disorders (McClure & Bickel, 2014; Zilverstand et al., 2018; Lindgren et al., 2019). The DLPFC is the most relevant area to be strengthened via the NIBS (Lapenta et al., 2014; Sauvaget et al., 2015; Baeken et al., 2016; Lefaucheur et al., 2017; Luigjes et al., 2019; Song et al., 2019; Fregni et al., 2020; Bollen et al., 2021).
A meta-analysis of 48 NIBS studies concluded that DLPFC neuromodulation has a small effect on craving and a moderate effect on consumption, with no significant difference between the type of substance (alcohol, illicit drugs, nicotine or eating disorders), neuromodulation (rTMS or tDCS) or DLPFC stimulation laterality (left or right DLPFC). Several repeated sessions were more effective than a single session. Moreover, craving and the total sessions showed a positive linear association (Song et al., 2019).
3.1 Repetitive Transcranial Magnetic Stimulation
The rTMS uses a coil placed on the scalp stimulating magnetic pulses through the skull in intervals. The magnetic field involves a focal electrical current that depolarises underlying cortical neurons. The frequency, intensity and duration of current, with the properties and area, influenced the effect. The rTMS can be either low frequency (<2 Hz) to inhibit the target area and decrease its activity or high frequency (>5 Hz), also called deep TMS, to increase the regulating activity and excite the target area (Chen et al., 1997; Siebner et al., 2000; Luigjes et al., 2019).
Typically, for addictive disorders’ studies, rTMS targets the right DLPFC with a high frequency to excite the area (Amiaz et al., 2009) or the left DLPFC at a low frequency to inhibit it (Trojak et al., 2015). A recent meta-analysis based on 26 studies found that rTMS over the left DLPFC reduces craving and the bilateral DLPFC reduces consumption, compared to sham stimulation (medium and robust effect) (Zhang et al., 2019a). The DLPFC seems to be the most accurate area to target, but laterality remains a controversial issue. Song et al. (2019) did not find a difference between the right and left hemisphere, while Maiti et al. (2017) found an effect on nicotine craving with rTMS on the bilateral DLPFC. Enokibara et al. (2016) also found a craving reduction with rTMS on the right DLPFC. This controversy could be due to the small number of clinical trials, small sample sizes and high heterogeneity (e.g., type of SUD, baseline characteristics of the sample, rTMS method, number of sessions and context such as psychiatric and pharmacologic interventions). Although the rTMS effect did not significantly differ by the SUD type, the craving effect size was small for alcohol use disorder, medium for nicotine use disorder and large for illicit drugs. Additionally, more pulses during stimulation were associated with a greater craving effect size (Zhang et al., 2019a).
Five 8-min sessions of 10 Hz rTMS over the left DLPFC reduced the craving of methamphetamine use disorder patients compared to sham (Su et al., 2017). The blinding TMS procedure commonly uses a sham coil without an electromagnetic pulse or a considerably low current to imitate the cutaneous sensation on the scalp muscles (Ekhtiari et al., 2019). Many clinical trials do not include sham rTMS conditions for comparison but use controlled conditions such as waiting lists or habitual rehabilitation (e.g., Liu et al., 2020). A recent clinical trial showed that 20 sessions of 10 Hz rTMS with rehabilitating female methamphetamine use disorder patients reduced their craving for at least 30 days after discharge compared with the control group (Liu et al., 2019). Young female patients showed a greater craving reduction, possibly due to greater cortical plasticity. Additionally, rTMS was more effective in the high-craving subgroup. Indeed, induced craving to activate its neuronal network could make the intervention more effective. A recent randomised, double-blinded, sham-controlled trial showed that rTMS (10 sessions over 2 weeks at 10 Hz, 300 pulses per session) over the left DLPFC along with a smoking video allowed smokers to reduce not only craving cues but also cigarette consumption during the 2-week treatment and 1 month after the treatment (Li et al., 2020).
In addition, other potential target areas could be the insula and medial PFC, which are involved in the maladaptive response in a stressful or rewarding context, influencing decision-making (Euston et al., 2012). After brain damage in the insula, patients are likely to stop smoking easily and not feel a craving after quitting (Naqvi et al., 2007). It corroborates the involvement of the insula in craving (Bonson, 2002) and decision-making (Naqvi & Bechara, 2010). These findings suggest that the insula is a potential target for neuromodulation (Ibrahim et al., 2019). To date, the first and only SUD clinical trial targeting this area was a double-blinded, randomised study using bilateral stimulation over the DLPFC and insula (Dinur-Klein et al., 2014). In total, 115 smokers intending to stop smoking received 13 sessions of either high-frequency rTMS (10 Hz), low-frequency rTMS (1 Hz) or sham rTMS, with or without presentation of smoking cues (six subgroups in total; condition 3 × 2). High-frequency rTMS significantly reduced the number of cigarettes consumed, the Fargerström Test for Nicotine Dependence (evaluating the dependence), and increased the abstinence rate compared to low-frequency rTMS and sham rTMS, especially when applied with smoking cue exposure. High-frequency rTMS showed a reduction in cigarette consumption at 6-month follow-up compared to sham rTMS but not compared to low-frequency rTMS or the condition without exposure. Insula stimulation with deep rTMS is promising for reducing cigarette consumption in the long term.
The newer forms of rTMS have also yielded encouraging results, such as intermittent theta-burst stimulation (iTBS), which is a shorter intervention than typical rTMS (approximately 5 min against 8–10 min) (Chen et al., 2020b; Su et al., 2020). A clinical trial on methamphetamine use disorder patients showed that 20 daily sessions of iTBS over the DLPFC (900 pulses per day) reduced craving and improved cognition and sleep quality compared to sham conditions (Su et al., 2020).
3.2 Transcranial Direct Current Stimulation
The tDCS is the most investigated neuromodulation among transcranial electrical stimulation (tES) classification. The tES is a classification of non-invasive stimulation intended to change brain activity by passing an electrical current. Depending on the technique, there are several tESs (Bikson et al., 2019): transcranial random noise stimulation (tRNS) uses a random stimulus to desynchronise pathological rhythms (Terney et al., 2008); transcranial pulsed current stimulation (tPCS) uses either monophasic or biphasic pulsed waveforms (Jaberzadeh et al., 2014) and transcranial alternating current stimulation (tACS) uses a sinusoidal current waveform (Antal et al., 2008). For blinding, studies used sham tES, a short duration stimulation (10–30 s) to give the cutaneous sensation of being stimulated (Ekhtiari et al., 2019).
tDCS is electric neuromodulation that acts with a low intensity of constant current applied by two electrodes on the scalp. The current may vary between 0.5 and 2 mA and last between 10 and 40 min with fade in and fade out (10–30 s) of the current (Higgins & George, 2019). tDCS can be used in three montages (Zhao et al., 2017). The bi-cephalic montage indicates that the anode and cathode are placed on the scalp, the anode delivers current to the brain and increases cortical excitability, and the cathode inhibits brain excitability and current escapes from it (Nitsche & Paulus, 2000, 2001). The distance between the two electrodes on the scalp can influence the strength of neurostimulation (Bikson et al., 2010). In the mono-cephalic montage, the anode is placed on the scalp and the cathode on the body as the reference electrode (e.g., arm or neck). It makes possible to focus on the observation of the anode effect and limit confusion. The non-cephalic montage suggests non-cortical stimulation, such as the cerebellum (Zhao et al., 2017).
Similar to other NIBS, the mechanisms of tDCS are still unclear (Nitsche et al., 2003; Arul-Anandam & Loo, 2009; Stagg & Nitsche, 2011; Brunoni et al., 2012; Philip et al., 2017; Zhao et al., 2017). The literature suggests that tDCS mechanisms act like long-term potentiation and long-term depression (Nitsche et al., 2003). The action potentials are modulated by tDCS even after the stimulation period (Nitsche & Paulus, 2000, 2001), and several neuromodulation sessions could increase the duration of the effects (Boggio et al., 2007). The mechanisms of tDCS during and after stimulation are different. During stimulation, anodal and cathodal tDCS modulates neuronal excitability by altering the resting membrane potential of neurons. The modulation persists after stimulation and produces glutamatergic and GABAergic synaptic plasticity as an aftereffect (Stagg & Nitsche, 2011). Contrary to TMS, tDCS itself is not sufficiently high to directly cause neuronal firing. If the intrinsic fluctuation of the neuron voltage is close to the threshold, the tDCS excitation can make it a potential action (Philip et al., 2017). Therefore, tDCS responsivity depends on cortical excitability, influenced by age, gender, anxiety level, lack of sleep, hormonal status and medication (Sauvaget et al., 2015). There are significant inter-individual differences in response to tDCS (Strube et al., 2016), contextual or inherent (Fertonani & Miniussi, 2017; Li et al., 2015).
Although this neuromodulation has been investigated as an intervention in SUDs (Hone-Blanchet et al., 2015; Schluter et al., 2018; Luigjes et al., 2019; Chen et al., 2020a), not so much for behavioural addiction, the data have been encouraging (Sauvaget et al., 2015). The new guidelines of Fregni et al. (2020) categorised tDCS as an effective treatment for reducing craving and relapse in addictive disorders, especially in alcohol use disorder. The recommendation is bilateral stimulation with the right DLFC anodal and the left DLPFC cathodal tDCS (F4 and F3 positions, respectively, according to the 10–20 international system for electroencephalography [EEG] electrode placement). More tDCS studies are required to conclude regarding crack-cocaine or methamphetamine use disorders. A longer duration of stimulation is related to a larger effect size in reducing craving (Chen et al., 2020a). Multiple sessions improve craving reduction compared to a single tDCS session (Song et al., 2019; Chen et al., 2020a).
A recent clinical trial showed that 10 sessions of DLPFC tDCS over 5 weeks on methamphetamine use disorder patients reduced craving and improved executive functions immediately after and 1 month after the intervention. Interestingly, there is a significant correlation between the reduction in craving and cognitive control improvement (Alizadehgoradel et al., 2020). Most clinical trials have targeted craving. Numerous meta-analyses have reported a reduction in craving (Jansen et al., 2013; Sauvaget et al., 2015; Lupi et al., 2017; Spagnolo & Goldman, 2017; Chen et al., 2020a, b; Kang et al., 2019; Luigjes et al., 2019; Song et al., 2019; Bollen et al., 2021). A recent meta-analysis of 32 tDCS studies found a medium effect in reducing craving, indicating more effect with longer stimulation sessions and a higher number of sessions (Chen et al., 2020a).
Few clinical trials have investigated the effect of tDCS on the relapse rate. At the 6-month follow-up after 10 sessions of tDCS, eight patients suffering from alcohol use disorder were still abstainers in the active condition against two in the sham condition (50% vs. 11.8%) (Klauss et al., 2014). The DLPFC stimulation can increase the quality of life and can decrease the relapse rate in patients suffering from alcohol use disorder, sometimes without reduced craving, depressive and anxiety symptoms, or improved cognitive function (13 min × 10 sessions twice a day of 2 mA stimulation, anode-right and cathode-left DLPFC, and 6-month follow-up) (Klauss et al., 2014) and sometimes with reduced craving (20 min × 10 daily sessions of 2 mA stimulation, anode-right and cathode-left DLPFC, and 3-month follow-up) (Klauss et al., 2018b). In the same design as in Klauss et al. (2018b) but on patients with crack-cocaine use disorder (Klauss et al., 2018a), the relapse rate was not affected. Another clinical trial with guided tDCS (10 sessions of 2 mA, anodal-right DLPFC and cathodal-left DLPFC over 5 consecutive days) did not reduce the craving or relapse rate or improve cognitive function in patients with cocaine use disorder (Verveer et al., 2020). Nevertheless, active tDCS reduced the relapse rate in the crack-cocaine subgroup users compared to sham tDCS.
Despite the positive impact of tDCS on craving and relapse rate, results remain inconsistent across clinical trials. This is possibly due to the large heterogeneity of the experimental setting (e.g., type of substances, targeted area, localisation of anode/cathode, ampere of tDCS, duration of stimulation, the time between sessions, number of sessions) (Luigjes et al., 2019; Chen et al., 2020a) and variations between the studied samples (e.g., characteristics of the sample at baseline, sample size, study design) (Bollen et al., 2021). Although most studies use a between-subjects design comparing multiple conditions (e.g., active tDCS vs. sham), a within-subjects design (e.g., a sample performing both conditions in a counterbalanced order) could limit baseline bias. Further, cognitive remediation during tDCS could improve cognitive functions and reduce clinical symptoms (Elmasry et al., 2015; Dedoncker et al., 2016; Noël et al., 2019; Zhang et al., 2019b; Bollen et al., 2021).
3.3 Combined Non-invasive Brain Stimulation
NIBS techniques have the advantage of being combined with other interventions. In most cases, the participant is passively stimulated (i.e., in the absence of any effort), while in other cases, another intervention may be offered simultaneously (e.g., cognitive remediation, rehabilitation of cognitive biases, mindfulness or psychotherapy, simultaneously with tDCS). Combining complementary interventions could (1) combine the effects of the two interventions and (2) potentiate the effects through synergy (Dedoncker et al., 2021).
tDCS can be especially suitable for combined intervention because the sensation is minor and should not distract the patient during a task. According to the activity-selectivity assumption, tDCS preferentially induces modulation in already activated neuronal networks compared to inactive neuronal networks (Bikson et al., 2013). Therefore, it seems possible to target specific neuronal networks by inducing neuronal pre-activation with cognitive training or substance-related stimuli. For the synergistic effect on neuronal plasticity change, tDCS and cognitive training or psychotherapy should activate the same neuronal pathway (Dedoncker et al., 2021).
The combination of these therapeutic interventions, such as exogenous neuromodulation (e.g., tDCS) and endogenous activation (e.g., cognitive training or psychotherapy), has been investigated in a few studies with non-clinical participants and participants with cognitive impairments (Elmasry et al., 2015; Zhang et al., 2019b). To date, no clinical trial in SUD has combined tDCS with psychotherapy. However, eight have combined tDCS with cognitive training related to SUD. Two randomised controlled clinical trials in alcohol use disorder combined tDCS with cognitive bias modification (CBM) (den Uyl et al., 2017, 2018).
CBM is a broad classification of cognitive training focused on cognitive bias retraining. It uses images related to a specific SUD substance that needs treatment. The alcohol attentional bias modification (ABM) uses a dot-probe task to exercise visual disengagement from alcohol images by associating alcohol-related images on the opposite side of the dot to be viewed. It may also use the alcohol approach-avoidance task (AAT) to train the subject to push away the alcohol-related images with a joystick to create an avoidance tendency towards alcohol (developed by Wiers et al., 2009). Four alcohol AAT sessions during 1 week of hospitalisation for alcohol use disorder reduced the abstinence rate by 17% 2 weeks after discharge (Manning et al., 2021). Meta-analyses of CBM show a reduced relapse rate (Allom et al., 2016; Jones et al., 2016), cognitive biases and cue reactivity (Boffo et al., 2019; Loijen et al., 2020). However, their effects are still limited. Cognitive training could be an interesting add-on treatment for addictive disorders, at least in the short term (Manning et al., 2021).
Two studies combined tDCS with CBM during hospitalisation for inpatients with alcohol use disorder inpatients (den Uyl et al., 2017, 2018). They did not follow the recent guidelines (Fregni et al., 2020) but proposed 20-min neuromodulation at 2 mA with anodal tDCS over the left DLPFC (35 cm2) and cathodal tDCS over the right DLPFC (100 cm2) (den Uyl et al., 2017). The two studies differed in the type of CMB. The first retrained the AAT with a joystick (push 90% of alcohol images and 10% of soft drink images; pull 90% of soft drink images and 10% of alcohol images in the active training task; 50% of pull and push in the inactive training task) (den Uyl et al., 2017). The second was the alcohol ABM via a dot-probe task with two stimuli: either alcohol or soft drink images or, in some cases, two soft drink images (absent target or two objects as a surprise trial). In the active training, the contingency probe after alcohol was 90% and 10% after alcohol stimuli (50/50 in the inactive version) (den Uyl et al., 2018). The first study investigated the potential effects of four stimulation sessions on the DLPFC simultaneously with alcohol approach tendency retraining (4× tDCS + AAT, three groups in parallel design) on alcohol bias, craving and relapse after 3 months and 1 year post-discharge (den Uyl et al., 2017). The second study investigated the effects of four sessions of simultaneous simulation on DLPFC combined with ABM (4× tDCS + ABM, 2 × 2 factor parallel design) on craving, alcohol bias and relapse after 1 year (den Uyl et al., 2018). One year after hospitalisation with the combined intervention, den Uyl et al. (2018) showed non-significant results for alcohol relapse rate. Nonetheless, the relapse rate trend was in the expected direction: the combined condition (21%), followed by active tDCS with inactive ABM condition (31%), sham tDCS with active ABM condition (38%) and a worse relapse rate in sham tDCS with inactive ABM condition (45%). A trend-level effect appeared for the first study showing that tDCS concurrent with active training reduces the relapse rate at 1 year only compared to sham tDCS (no difference compared to tDCS separated to the CBM) (den Uyl et al., 2017). Notably, the trend effect appears only when they consider other predictors (gender, duration of alcohol problem, number of detoxifications, alcohol problems, duration of treatment, depression symptoms and scored craving) in the logistic regression. Moreover, there was no effect at the 3-month follow-up.
There are no significant results showing the interest in combining tDCS with CBM. Nonetheless, the two studies measured only long-term relapse (3-month and 1-year follow-up), and results went in the expected direction, showing an average lower relapse rate in the active tDCS condition simultaneous with CBM at 1-year follow-up (den Uyl et al., 2017, 2018). The combined intervention did not influence alcohol-scored craving. However, it was extremely low in patients. Perhaps induced craving is a more sensible measure, as den Uyl et al. (2016) found that active combined tDCS reduces the induced craving (by alcohol images) in EEG tasks in heavy drinkers. More combined clinical trials following the tDCS guidelines (anode-right DLPFC and cathode-left DLPFC and >5 sessions) (Noël et al., 2019) and measuring early relapse should be investigated (Manning et al., 2021). In addition, reducing the electrode surface could increase the effect (Bollen et al., 2021).
The learning effect of tDCS can be observed ‘online’ (i.e., during the training with the tDCS) or ‘offline’ (i.e., the same task after the intervention). With online learning data, it is possible to see if the stimulation increases the learning effect of the cognitive task by comparing the improvement in training in the active tDCS condition compared to the sham tDCS condition. The two studies on AUD patients by den Uyl et al. (2017, 2018) revealed in exploratory analyses that the learning process has been improved by active tDCS on the DLPFC. The first study showed a learning effect on the approach alcohol bias enhanced by tDCS between sessions 1 and 2; however, it disappeared in the last two sessions (mini-assessment before each of the four interventions) (den Uyl et al., 2017). The second study also found an enhancer effect of tDCS on the ABM. The combined intervention with active tDCS and active ABM had a stronger avoidance bias (only with the analysis of the mean of the four sessions) (den Uyl et al., 2018). In conclusion, although the results are fragile, they suggest that tDCS could accelerate the learning process of CBM with ABM and AAT. However, they failed to maintain the effect on offline measures with a similar task after treatment.
To our knowledge, only one clinical trial combining rTMS with another intervention has been reported (Trojak et al., 2015). It combined 10 sessions of low-frequency rTMS with nicotine replacement therapy (nicotine in the form of gum). It showed an improvement in cigarette abstinence directly after the 2-week intervention, but the effect did not last. As there are only two groups comparing sham and verum rTMS, it is impossible to determine if the effect is from this combination. To date, concurrently combining rTMS with CBM or cognitive training has not yet been studied in SUD. Muscular and cutaneous sensations induced by rTMS could not allow the patient to focus correctly on the other intervention. Thus, sequential complementary interventions would be more appropriate.
In conclusion, few studies have combined NIBS concurrently with a complementary intervention, and they did not follow the new recommendations. Further studies combining more than four sessions of bilateral tDCS with anode-right DLPFC and cathode-left DLPFC simultaneously with CBM, cognitive revalidation or psychotherapy are encouraged. Studies combining insula high-frequency rTMS or DLPFC high/low-frequency rTMS during sequential CBM, cognitive revalidation or psychotherapy will advance clinical research.
4 Neurofeedback
From Richard Caton’s first description of brain electrical activity (1875) to our actual knowledge of EEG, a history of neurophysiology has led to breakthrough advances in technology, allowing an in-depth assessment of brain functioning and neuromodulatory interventions. In 1935, after Berger (1929) discovered the now vastly recognised synchronised ‘alpha EEG rhythm’, Alfred Lee Loomis demonstrated that conditioning could be applied to EEG activity by bringing alpha rhythm under voluntary control. This period marked the first demonstration of EEG biofeedback. Subsequently, during the 1960s, Maurice (Barry) Sterman became a pioneer of EEG-biofeedback clinical application through his work on internal inhibition and basal forebrain modulation together with Carmine Clemente at UCLA (USA) (Arns & Sterman, 2019). Currently, there is a definite and growing interest, in both clinical and research domains, towards this neuromodulation technique evidenced by its application to a large sample of psychiatric ailments such as addiction (Cox et al., 2016; Dousset et al., 2020; Horrell et al., 2010), posttraumatic stress disorder (Reiter et al., 2016) and schizophrenia (Balconi & Vanutelli, 2019; Rieger et al., 2018). It has even extended to enhancing healthy subjects’ abilities, such as improving performance (Arns et al., 2008; Crivelli et al., 2019).
Essentially, biofeedback is the use of instrumentation to mirror psychophysiological processes of which the individual is not normally aware, and which may be brought under voluntary control (Thompson & Thompson, 2015). In that respect, neurofeedback (NF) stands for a specific kind of biofeedback, with reflected information being cerebral activity measurements. Interestingly, biofeedback is a natural and universally shared regulatory mechanism as our biological system evolves by constantly adapting itself according to the information sent by the peripheral nervous system, just as NF displays are sent by the optic or auditory pathways (Thompson & Thompson, 2015). In this subsection, we will expose the theoretical and methodological aspects of NF, its application through fMRI and EEG interfaces, and the future perspectives towards a better practice.
4.1 Theoretical and Methodological Aspects
From a methodological perspective, brain activity measures are converted into visual or auditory signals fed back in real-time to the patient. The patient is asked to work on this fed-back display via mental strategies such as imagining particular events happening (e.g., moving a limb of the body, thinking about negative consequences of drug consumption, etc.), and expected changes are positively reinforced (Cox et al., 2016). Consequently, patients control their responses, see their progress in real-time and achieve optimum performance to control their symptoms or an unwished behaviour (Cox et al., 2016). Thus, NF requires patients to take on an active role in their care—finding personal mental strategies impacting brain activity by themselves and actively implementing them repetitively. Therefore, contrary to medication or compared to the aforementioned neuromodulation techniques (TMS and tDCS) that imply passive involvement, as learning is an active process requiring repetition of training sessions, NF entails implication, motivation and dynamic engagement from the patient.
Theoretically, by rewarding successive approximations, we can shape a behaviour: The patient learns to switch on or switch off a specific network in the brain, and if it is often enough, neuroplastic changes will occur, based on learning (Thompson & Thompson, 2015). Indeed, ‘… brain plasticity can be induced by demands associated with training, practice or learning and is defined as the brain capacity to continuously remodeling the neuronal synaptic organization in order to optimize the brain’s networks functioning …’ (Kubben, 2012). To a large degree, this learning process relies on operant conditioning and the fundamental principle of Thorndike’s law of effect, whereby rewarding behaviour increases the likelihood of its recurrence (Sterman, 1996; Thompson & Thompson, 2015). In operant conditioning of brain waves, the patient receives a reward (e.g., a smiley or a sun, indicating the level of the current performance) when they successfully put themselves in the targeted mental state—a process that will become almost automatic after several practice sessions. Subsequently, as patients face salient stimuli leading to an intense and irrepressible desire of the substance and ultimately result in consumption, the last step is to apply the learned skill in ecological situations, a transfer process hypothesised to involve classical conditioning (Thompson & Thompson, 2015).
Thus, NF offers the possibility to modify cortical activity, a phenomenon that cannot be achieved without objective brain measurements (Micoulaud-Franchi et al., 2013; Thibault et al., 2016). There are several interfaces for the application of NF, including EEG, fMRI, functional near-infrared spectroscopy (fNIRS) and magnetoencephalography (MEG), many of which involve different technologies and, thus, different procedures (Alkoby et al., 2018; Orndorff-Plunkett et al., 2017; Thibault et al., 2016). Currently, the widespread forms mostly involve fMRI-NF and EEG-NF (Dickerson, 2018). Applied to NF, each method presents its advantages and drawbacks (Thibault et al., 2016; Orndorff-Plunkett et al., 2017). However, a common interesting feature is that it allows targeting neural networks and is not limited to the intervention on just one brain region. Addictive disorders are characterised by abnormal behaviours generated by dysfunctional neurocognitive networks (Kalivas & Volkow, 2005; Noël et al., 2013). By modulating these networks, NF investigation seems to be attractive and promising for reducing symptoms and promoting resilience in favour of an optimal intercession (Sitaram et al., 2017).
4.2 Functional Magnetic Resonance Imaging Neurofeedback
As the fMRI-NF offers a good spatial resolution, it has the advantage of localising brain signals to specific areas (Cox et al., 2016). Once the regions of interest (ROIs) have been identified through the peak of the BOLD signal, an NF-training protocol can be implemented to modulate (increase or reduce) neural activity in these particular ROIs (Bracht et al., 2021; Hanlon et al., 2013). Many studies have demonstrated the effectiveness of fMRI-NF training protocols in patients suffering from addiction by manipulating relevant brain regions related to the abnormal bottom-up system that generates a ‘wanting’ (craving) behaviour (Luigjes et al., 2013). In fact, it appears that NF training impacts abstinence by reducing the activity of craving-related regions—the anterior cingulate cortex (ACC) (Hartwell et al., 2016; Karch et al., 2019), PFC (Hartwell et al., 2016; Karch et al., 2019), insula and ventral striatum (Kirsch et al., 2016)—and the feedback on the connectivity between the anterior (frontal) and posterior regions (temporal and parietal) (Karch et al., 2019; Luigjes et al., 2019). Conjointly, ACC activity correlates with craving ratings, and patients might be more able to exert voluntary control over the ACC than the PFC (Fovet et al., 2015; Hanlon et al., 2013; Hartwell et al., 2016; Li et al., 2013). Notably, these areas are mostly included in the reward system, which plays a major role in adaptive behaviour, control of behaviours and learning processes (Bari et al., 2018; Karch et al., 2019). Some studies have already attempted to identify the network involved in the brain self-regulation process during real-time fMRI-NF training. This network mostly recruits the anterior insular cortex, basal ganglia and ACC. These regions are recurring targets of fMRI-NF protocols. Therefore, NF studies face a new challenge by considering the potential overlap between the activated regions in response to the NF-induced regulatory phenomenon and the regions whose activity constitutes the target of the experimental protocol for symptom reduction (Emmert et al., 2016).
4.3 Electroencephalography Neurofeedback
As NF relies on real-time processes, EEG-NF presents a clear advantage for optimal learning owing to its high temporal resolution (Dousset et al., 2020). EEG-NF is used as a neuromodulation technique to identify target brain frequencies, to increase or reduce specific forms of EEG activity (Gunkelman & Johnstone, 2005). Thus, EEG-NF protocols rely on electrical activity recorded from the scalp and mainly focus on alpha (8–12 Hz), beta (13–30 Hz), delta (0–4 Hz), theta (4–8 Hz) and gamma (30–50 Hz) frequencies or their combination such as alpha/theta ratio and beta/theta ratio (Marzbani et al., 2016; Orndorff-Plunkett et al., 2017; Pandey et al., 2012).
As mentioned, addictive behaviours result partly from an altered top-down process on craving, setting up a reduced cognitive control with impaired inhibition of the dominant response. This impairment has been attributed to abnormal neuroelectrical characteristics: a discrepancy in the N2/P3 complex component with either increased or decreased amplitudes and prolonged latencies (Campanella et al., 2014; Luijten et al., 2014; Petit et al., 2014). Given that identifying the relevant frequency patterns underlying this impairment remains difficult, researchers set out to pinpoint the most suitable NF protocol for treating SUD (Dousset et al., 2020). Since the 1980s, the most popular protocol has been Peniston and Kulkosky’s protocol (modulation of alpha/theta frequencies) (Peniston & Kulkosky, 1989), which targets a state of relaxation. By increasing alpha/theta activity while reducing β-endorphin levels, this protocol counterbalances anxiety-eliciting situations. Thus, patients are relieved from the tension linked with withdrawal in the early stages of abstinence (Peniston & Kulkosky, 1989; Saxby & Peniston, 1995). Scott and Kaiser’s protocol, connecting alpha/theta regulation with SMR-beta modulation, extends Peniston’s protocol to a larger panel of substances of abuse (Luigjes et al., 2019). On the one hand, alpha/theta modulation intends to soothe conditions of stress and anxiety. On the other hand, SMR-beta modulation aims to alleviate impulsivity by remediating cognitive deficits. Together, these protocols seem to have a pronounced impact on maintaining abstinence (Scott et al., 2005; Sokhadze et al., 2008; Dalkner et al., 2017). Although they have ample merit, the evolution of our knowledge regarding the underlying mechanisms of SUD provides us with the opportunity to investigate the modulation of other frequency bands (Dousset et al., 2020). For instance, as the N2/P3 complex may be viewed as an overlay of brain oscillatory components with the theta band shaping the N200 and the early part of the P300 wave, and the delta band shaping the main part of the P300 (Jones et al., 2006), a delta/theta protocol could be a promising perspective for the care of addicted patients (Kamarajan et al., 2004).
4.4 Neurofeedback: Future Perspectives and New Insights
Despite the conventional treatments devised for SUD, the relapse rate remains astonishingly high and outlines the limitations of the conventional systematic approach that offers medication and psychotherapy (Andersson et al., 2019). In fact, SUD induces long-lasting changes in brain functioning resulting from the interaction between chronic substance use, genetic disposition and environment. Hence, a psychiatric diagnosis must consider heterogeneous entities characterised by extremely complex changes in the brain (Perna et al., 2018). Assuming the idea of neurobiological heterogeneity within SUD, identifying biomarkers should allow us to move towards stratified psychiatry, meaning stratifying subgroups of patients’ profiles paving the way to personalised medicine to provide reliable and customised assistance (Arns, 2020; Perna et al., 2018). To quote Arns et al. (2011), ‘… in this area the goal is to prescribe the right treatment, for the right person at the right time as opposed to the current one-size-fits-all treatments’ (Arns et al., 2011). The underlying idea behind personalised medicine is that brain imaging data illustrate stable phenotypes incorporating both the effects of nature and nurture. It allows the identification of neurological biomarkers and leads to predictions regarding treatment outcomes (Perna et al., 2018). Ultimately, such insights could allow the implementation of tailor-made NF protocols related to precise alterations and should lead to a more targeted intervention, thereby fostering specific needs to be breached. For example, according to theoretical concepts, both the incentive-sensitization theory of Robinson and Berridge (1993) and the dual-process model introduced by Wiers et al. (2007) are linked to the I-RISA (impaired response inhibition and salience attribution) syndrome conceptualised by Goldstein and Volkow (2002), putting forward an increased salience of drug-related cues paired with disabled inhibition of the dominant response. In that frame, and as already discussed in our review published in 2020, ‘… the challenge of maintaining abstinence more directly, i.e., through tailor-made experimental NF protocols targeting inhibitory control and/or attentional bias, warrants increased attention to patient particularities: some benefit more from decreasing/suppressing attentional bias, others more from increasing inhibitory control, and others instead make the most of both’ (Dousset et al., 2020).
In the same vein, from a perspective relying upon this dualistic vision of inhibitory control, implementing NF protocols exclusively related to either proactive or reactive processes would lead to more targeted care, meeting a more specific need. In fact, a reactive course is involved in conflict resolution and interference resistance, while a proactive course operates as an anticipatory mechanism and avoids interference by actively maintaining the goal (Braver, 2012). Recent evidence suggests that these distinct modes of control call for both common and specific network activation patterns. More precisely, addictive-inhibited behaviours involve activating an anterior–posterior theta oscillatory network (Cooper et al., 2015). Nevertheless, imaging data put forward different modulations of this network depending on whether the task recruits a proactive or reactive state. On the one hand, a proactive neurobehavioural state is associated with a centro-parietal network involving delta–theta–beta oscillations and mostly recruiting the left putamen, bilateral parietal lobe and premotor cortex. On the other hand, reactive control seems to be strongly involved in right-lateralised frontal, parietal and temporal networks, along with alpha–theta band activity (Cooper et al., 2015; Garcia et al., 2017; van Belle et al., 2014).
Overall, from what we know, and we are still learning about NF, this non-invasive method seems attractive and promising for modulating dysfunctional brain networks associated with SUD to reduce symptoms and promote resilience. As NF is a new approach in managing psychiatric ailments, the challenge remains for establishing standardised procedures for mapping brain networks targeted with NF (Bracht et al., 2021). In this respect, all the investigations will refine our knowledge of how NF works through identifying variables and characteristics that make the NF training effective in favour of an optimal intercession.
5 General Conclusion
The primary aim of this chapter was to summarise the main neurocognitive interventions aimed at addressing the clinical aspects of SUDs. To the best of our knowledge, the neurobiology of addiction, that is, an overwhelming motivational drug-seeking and a low capacity to control the desire to consume, is indexed by long-lasting changes in brain function. To remodel these dysfunctional neural circuits, the development of neuromodulation techniques has evolved in response to the enduring vulnerability to relapse even after years of abstinence. We have explicitly focused on the therapeutic potential of DBS, rTMS, tES and NF approaches in this chapter. Experimental evidence for these neuromodulation techniques demonstrated encouraging results in consolidating abstinence, highlighting the critical role of cognitive functioning in regaining control over problematic behaviours when facing stimuli predicting the availability of the substance and its use. Importantly, despite compelling arguments favouring the previously stated neuromodulation procedures, more standardised and rigorous experimental designs and objective reports are needed to consolidate efficacy (Ekhtiari et al., 2019; Fried et al., 2021; Ros et al., 2020).
In line with the recommendation of several recent reviews (Bollen et al., 2021; Dedoncker et al., 2021), to increase their efficacy, tDCS and rTMS should be combined with psychological interventions (e.g., mindfulness, cognitive training), ideally, tailored to fit distinct endophenotypes (e.g., impaired inhibitory control, low working memory) and learning. Indeed, the state of the brain at the time of stimulation can be critical for optimal clinical outcomes (Dinur-Klein et al., 2014). The ‘activity-selectivity’ hypothesis stresses that tDCS preferentially modulates populations of active and inactive neurons (Bikson et al., 2013). Finally, the recent recognition that the insula, a region of the cerebral cortex, is involved in various critical aspects underlying SUDs (interoception, decision-making, etc.) led us to recommend targeting this region with brain stimulation in the future (Ibrahim et al., 2019), particularly with deep TMS (Dinur-Klein et al., 2014).
Regarding neurofeedback intervention, considering the progress on our fundamental understanding of the neurobiological underpinnings of SUD, the currently enforced protocols should be kept up to date to specifically target the needs of patients presenting distinguished profiles and move towards a stratified medicine. Further, according to the current fostering discussions in this area, an important aspect of neurofeedback practice is that future studies are required to provide (1) well-controlled experimental designs, (2) objective measures of brain changes and (3) links between neurological biomarkers, cognition and clinical improvements to reliably authenticate the specific impact of neurofeedback and demonstrate robust evidence of its efficiency (Thibault et al., 2017; Micoulaud-Franchi et al., 2018; Thibault & Raz, 2018).
References
Alizadehgoradel, J., Nejati, V., Sadeghi Movahed, F., Imani, S., Taherifard, M., Mosayebi-Samani, M., Vicario, C. M., Nitsche, M. A., & Salehinejad, M. A. (2020). Repeated stimulation of the dorsolateral-prefrontal cortex improves executive dysfunctions and craving in drug addiction: A randomized, double-blind, parallel-group study. Brain Stimulation, 13(3), 582–593. https://doi.org/10.1016/j.brs.2019.12.028
Alkoby, O., Abu-Rmileh, A., Shriki, O., & Todder, D. (2018). Can we predict who will respond to neurofeedback? A review of the inefficacy problem and existing predictors for successful EEG neurofeedback learning. Neuroscience, 378, 155–164. https://doi.org/10.1016/j.neuroscience.2016.12.050
Allom, V., Mullan, B., & Hagger, M. (2016). Does inhibitory control training improve health behaviour? A meta-analysis. Health Psychology Review, 10(2), 168–186. https://doi.org/10.1080/17437199.2015.1051078
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Author. https://doi.org/10.1176/appi.books.9780890425596
Amiaz, R., Levy, D., Vainiger, D., Grunhaus, L., & Zangen, A. (2009). Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction (Abingdon, England), 104(4), 653–660. https://doi.org/10.1111/j.1360-0443.2008.02448.x
Andersson, H. W., Wenaas, M., & Nordfjærn, T. (2019). Relapse after inpatient substance use treatment: A prospective cohort study among users of illicit substances. Addictive Behaviors, 90, 222–228. https://doi.org/10.1016/j.addbeh.2018.11.008
Antal, A., Boros, K., Poreisz, C., Chaieb, L., Terney, D., & Paulus, W. (2008). Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimulation, 1(2), 97–105. https://doi.org/10.1016/j.brs.2007.10.001
Ardouin, C., Voon, V., Worbe, Y., Abouazar, N., Czernecki, V., Hosseini, H., Pelissolo, A., Moro, E., Lhommée, E., Lang, A. E., Agid, Y., Benabid, A. L., Pollak, P., Mallet, L., & Krack, P. (2006). Pathological gambling in Parkinson’s disease improves on chronic subthalamic nucleus stimulation. Movement Disorders: Official Journal of the Movement Disorder Society, 21(11), 1941–1946. https://doi.org/10.1002/mds.21098
Arns, M. (2020). My personal neurofeedback journey (pp. 7–10). Academic Press.
Arns, M., & Sterman, M. B. (2019). Neurofeedback: How it all started. GVO.
Arns, M., Kleinnijenhuis, M., Fallahpour, K., & Breteler, R. (2008). Golf performance enhancement and real-life neurofeedback training using personalized event-locked EEG profiles. Journal of Neurotherapy, 11(4), 11–18. https://doi.org/10.1080/10874200802149656
Arns, M., Gunkelman, J., Olbrich, S., Sander, C., & Hegerl, U. (2011). EEG vigilance and phenotypes in neuropsychiatry: Implications for intervention. In Neurofeedback and neuromodulation techniques and applications (pp. 79–435). Academic Press.
Arul-Anandam, A. P., & Loo, C. (2009). Transcranial direct current stimulation: A new tool for the treatment of depression? Journal of Affective Disorders, 117(3), 137–145. https://doi.org/10.1016/j.jad.2009.01.016
Badre, D., & Nee, D. E. (2018). Frontal cortex and the hierarchical control of behavior. Trends in Cognitive Sciences, 22(2), 170–188. https://doi.org/10.1016/j.tics.2017.11.005
Baeken, C., Brunelin, J., Duprat, R., & Vanderhasselt, M. A. (2016). The application of tDCS in psychiatric disorders: A brain imaging view. Socioaffective Neuroscience and Psychology, 6, 29588. https://doi.org/10.3402/snp.v6.29588
Balconi, M., & Vanutelli, M. (2019). Neurofeedback intervention for emotional behavior regulation in schizophrenia: New experimental evidences from optical imaging. NeuroRegulation, 6(2), 71–80. https://doi.org/10.15540/nr.6.2.71
Bari, A., DiCesare, J., Babayan, D., Runcie, M., Sparks, H., & Wilson, B. (2018). Neuromodulation for substance addiction in human subjects: A review. Neuroscience and Biobehavioral Reviews, 95, 33–43. https://doi.org/10.1016/j.neubiorev.2018.09.013
Bauer, J., Pedersen, A., Scherbaum, N., Bening, J., Patschke, J., Kugel, H., Heindel, W., Arolt, V., & Ohrmann, P. (2013). Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 38(8), 1401–1408. https://doi.org/10.1038/npp.2013.45
Bechara, A. (2005). Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience, 8(11), 1458–1463. https://doi.org/10.1038/nn1584
van Belle, J., Vink, M., Durston, S., & Zandbelt, B. B. (2014). Common and unique neural networks for proactive and reactive response inhibition revealed by independent component analysis of functional MRI data. NeuroImage, 103, 65–74. https://doi.org/10.1016/j.neuroimage.2014.09.014
Benabid, A. L., Pollak, P., Louveau, A., Henry, S., & de Rougemont, J. (1987). Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Applied Neurophysiology, 50(1–6), 344–346.
Berger, H. (1929). Über das elektroenkephalogramm des menschen. Archiv für psychiatrie und nervenkrankheiten, 87(1), 527–570.
Berridge, K. C., & Robinson, T. E. (2016). Liking, Wanting and the Incentive-Sensitization Theory of Addiction. The American Psychologist, 71(8), 670–679. https://doi.org/10.1037/amp0000059.
Bikson, M., Datta, A., Rahman, A., & Scaturro, J. (2010). Electrode montages for tDCS and weak transcranial electrical stimulation: Role of “return” electrode’s position and size. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 121(12), 1976–1978. https://doi.org/10.1016/j.clinph.2010.05.020
Bikson, M., Name, A., & Rahman, A. (2013). Origins of specificity during tDCS: Anatomical, activity-selective, and input-bias mechanisms. Frontiers in Human Neuroscience, 7, 688. https://doi.org/10.3389/fnhum.2013.00688
Bikson, M., Esmaeilpour, Z., Adair, D., Kronberg, G., Tyler, W. J., Antal, A., Datta, A., Sabel, B. A., Nitsche, M. A., Loo, C., Edwards, D., Ekhtiari, H., Knotkova, H., Woods, A. J., Hampstead, B. M., Badran, B. W., & Peterchev, A. V. (2019). Transcranial electrical stimulation nomenclature. Brain Stimulation, 12(6), 1349–1366. https://doi.org/10.1016/j.brs.2019.07.010
Boffo, M., Zerhouni, O., Gronau, Q. F., van Beek, R. J. J., Nikolaou, K., Marsman, M., & Wiers, R. W. (2019). Cognitive bias modification for behavior change in alcohol and smoking addiction: Bayesian meta-analysis of individual participant data. Neuropsychology Review, 29(1), 52–78. https://doi.org/10.1007/s11065-018-9386-4
Boggio, P. S., Nunes, A., Rigonatti, S. P., Nitsche, M. A., Pascual-Leone, A., & Fregni, F. (2007). Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restorative Neurology and Neuroscience, 25(2), 123–129.
Bollen, Z., Dormal, V., & Maurage, P. (2021). How should transcranial direct current stimulation be used in populations with severe alcohol use disorder? A clinically oriented systematic review. Clinical EEG and Neuroscience, 15500594211001212. https://doi.org/10.1177/15500594211001212
Bonson, K. (2002). Neural systems and cue-induced cocaine craving. Neuropsychopharmacology, 26(3), 376–386. https://doi.org/10.1016/S0893-133X(01)00371-2
Bracht, T., Soravia, L., Moggi, F., Stein, M., Grieder, M., Federspiel, A., Tschümperlin, R., Batschelet, H. M., Wiest, R., & Denier, N. (2021). The role of the orbitofrontal cortex and the nucleus accumbens for craving in alcohol use disorder. Translational Psychiatry, 11(1), 267. https://doi.org/10.1038/s41398-021-01384-w
Braver, T. S. (2012). The variable nature of cognitive control: A dual-mechanisms framework. Trends in Cognitive Sciences, 16(2), 106–113. https://doi.org/10.1016/j.tics.2011.12.010
Brunoni, A. R., Nitsche, M. A., Bolognini, N., Bikson, M., Wagner, T., Merabet, L., Edwards, D. J., Valero-Cabre, A., Rotenberg, A., Pascual-Leone, A., Ferrucci, R., Priori, A., Boggio, P. S., & Fregni, F. (2012). Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimulation, 5(3), 175–195. https://doi.org/10.1016/j.brs.2011.03.002
Campanella, S., Pogarell, O., & Boutros, N. (2014). Event-related potentials in substance use disorders: A narrative review based on articles from 1984 to 2012. Clinical EEG and Neuroscience, 45(2), 67–76.
Carter, A., & Hall, W. (2011). Proposals to trial deep brain stimulation to treat addiction are premature: Editorial. Addiction, 106(2), 235–237. https://doi.org/10.1111/j.1360-0443.2010.03245.x
Caton, R. (1875). Electrical currents of the brain. The Journal of Nervous and Mental Disease, 2(4), 610.
Chen, R., Classen, J., Gerloff, C., Celnik, P., Wassermann, E. M., Hallett, M., & Cohen, L. G. (1997). Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology, 48(5), 1398–1403.
Chen, J., Qin, J., He, Q., & Zou, Z. (2020a). A meta-analysis of transcranial direct current stimulation on substance and food craving: What effect do modulators have? Frontiers in Psychiatry, 11, 598. https://doi.org/10.3389/fpsyt.2020.00598
Chen, T., Su, H., Li, R., Jiang, H., Li, X., Wu, Q., Tan, H., Zhang, J., Zhong, N., Du, J., Gu, H., & Zhao, M. (2020b). The exploration of optimized protocol for repetitive transcranial magnetic stimulation in the treatment of methamphetamine use disorder: A randomized sham-controlled study. eBioMedicine, 60, 103027. https://doi.org/10.1016/j.ebiom.2020.103027
Coles, A. S., Kozak, K., & George, T. P. (2018). A review of brain stimulation methods to treat substance use disorders. The American Journal on Addictions, 27(2), 71–91. https://doi.org/10.1111/ajad.12674
Cooper, P. S., Wong, A. S. W., Fulham, W. R., Thienel, R., Mansfield, E., Michie, P. T., & Karayanidis, F. (2015). Theta frontoparietal connectivity associated with proactive and reactive cognitive control processes. NeuroImage, 108, 354–363. https://doi.org/10.1016/j.neuroimage.2014.12.028
Cox, W. M., Subramanian, L., Linden, D. E. J., Lührs, M., McNamara, R., Playle, R., Hood, K., Watson, G., Whittaker, J. R., Sakhuja, R., & Ihssen, N. (2016). Neurofeedback training for alcohol dependence versus treatment as usual: Study protocol for a randomized controlled trial. Trials, 17(1), 480. https://doi.org/10.1186/s13063-016-1607-7
Crivelli, D., Fronda, G., & Balconi, M. (2019). Neurocognitive enhancement effects of combined mindfulness-neurofeedback training in sport. Neuroscience, 412, 83–93. https://doi.org/10.1016/j.neuroscience.2019.05.066
Dalkner, N., Unterrainer, H. F., Wood, G., Skliris, D., Holasek, S. J., Gruzelier, J. H., & Neuper, C. (2017). Short-term beneficial effects of 12 sessions of neurofeedback on avoidant personality accentuation in the treatment of alcohol use disorder. Frontiers in Psychology, 8, 1688. https://doi.org/10.3389/fpsyg.2017.01688
Dedoncker, J., Brunoni, A. R., Baeken, C., & Vanderhasselt, M. A. (2016). A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: Influence of stimulation parameters. Brain Stimulation, 9(4), 501–517. https://doi.org/10.1016/j.brs.2016.04.006
Dedoncker, J., Baeken, C., De Raedt, R., & Vanderhasselt, M. A. (2021). Combined transcranial direct current stimulation and psychological interventions: State of the art and promising perspectives for clinical psychology. Biological Psychology, 158, 107991. https://doi.org/10.1016/j.biopsycho.2020.107991
Dickerson, K. C. (2018). Upregulating brain activity using non-drug reward imagery and real-time fMRI neurofeedback-A new treatment approach for addiction? eBioMedicine, 38, 21–22. https://doi.org/10.1016/j.ebiom.2018.11.021
Dinur-Klein, L., Dannon, P., Hadar, A., Rosenberg, O., Roth, Y., Kotler, M., & Zangen, A. (2014). Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: A prospective, randomized controlled trial. Biological Psychiatry, 76(9), 742–749. https://doi.org/10.1016/j.biopsych.2014.05.020
Dousset, C., Kajosch, H., Ingels, A., Schröder, E., Kornreich, C., & Campanella, S. (2020). Preventing relapse in alcohol disorder with EEG-neurofeedback as a neuromodulation technique: A review and new insights regarding its application. Addictive Behaviors, 106, 106391. https://doi.org/10.1016/j.addbeh.2020.106391
Ekhtiari, H., Tavakoli, H., Addolorato, G., Baeken, C., Bonci, A., Campanella, S., Castelo-Branco, L., Challet-Bouju, G., Clark, V. P., Claus, E., Dannon, P. N., Del Felice, A., den Uyl, T., Diana, M., di Giannantonio, M., Fedota, J. R., Fitzgerald, P., Gallimberti, L., Grall-Bronnec, M., … Hanlon, C. A. (2019). Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: A consensus paper on the present state of the science and the road ahead. Neuroscience and Biobehavioral Reviews, 104, 118–140. https://doi.org/10.1016/j.neubiorev.2019.06.007
Elmasry, J., Loo, C., & Martin, D. (2015). A systematic review of transcranial electrical stimulation combined with cognitive training. Restorative Neurology and Neuroscience, 33(3), 263–278. https://doi.org/10.3233/RNN-140473
Emmert, K., Kopel, R., Sulzer, J., Brühl, A. B., Berman, B. D., Linden, D. E. J., Horovitz, S. G., Breimhorst, M., Caria, A., Frank, S., Johnston, S., Long, Z., Paret, C., Robineau, F., Veit, R., Bartsch, A., Beckmann, C. F., Van De Ville, D., & Haller, S. (2016). Meta-analysis of real-time fMRI neurofeedback studies using individual participant data: How is brain regulation mediated? NeuroImage, 124(A), 806–812. https://doi.org/10.1016/j.neuroimage.2015.09.042
Enokibara, M., Trevizol, A., Shiozawa, P., & Cordeiro, Q. (2016). Establishing an effective TMS protocol for craving in substance addiction: Is it possible? The American Journal on Addictions, 25(1), 28–30. https://doi.org/10.1111/ajad.12309
Euston, D. R., Gruber, A. J., & McNaughton, B. L. (2012). The role of medial prefrontal cortex in memory and decision making. Neuron, 76(6), 1057–1070. https://doi.org/10.1016/j.neuron.2012.12.002
Fertonani, A., & Miniussi, C. (2017). Transcranial electrical stimulation: What we know and do not know about mechanisms. The Neuroscientist, 23(2), 109–123. https://doi.org/10.1177/1073858416631966
Fovet, T., Jardri, R., & Linden, D. (2015). Current issues in the use of fMRI-based neurofeedback to relieve psychiatric symptoms. Current Pharmaceutical Design, 21(23), 3384–3394. https://doi.org/10.2174/1381612821666150619092540
Fregni, F., El-Hagrassy, M. M., Pacheco-Barrios, K., Carvalho, S., Leite, J., Simis, M., Brunelin, J., Nakamura-Palacios, E. M., Marangolo, P., Venkatasubramanian, G., San-Juan, D., Caumo, W., Bikson, M., Brunoni, A. R., & Neuromodulation Center Working Group. (2020). Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation (tDCS) in neurological and psychiatric disorders. The International Journal of Neuropsychopharmacology, 24(4), 256–313. https://doi.org/10.1093/ijnp/pyaa051
Fried, P. J., Santarnecchi, E., Antal, A., Bartres-Faz, D., Bestmann, S., Carpenter, L. L., Celnik, P., Edwards, D., Farzan, F., Fecteau, S., George, M. S., He, B., Kim, Y. H., Leocani, L., Lisanby, S. H., Loo, C., Luber, B., Nitsche, M. A., Paulus, W., … Pascual-Leone, A. (2021). Training in the practice of noninvasive brain stimulation: Recommendations from an IFCN committee. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 132(3), 819–837. https://doi.org/10.1016/j.clinph.2020.11.018
Garcia, J. O., Brooks, J., Kerick, S., Johnson, T., Mullen, T. R., & Vettel, J. M. (2017). Estimating direction in brain-behavior interactions: Proactive and reactive brain states in driving. NeuroImage, 150, 239–249. https://doi.org/10.1016/j.neuroimage.2017.02.057
Goldstein, R. Z., & Volkow, N. D. (2002). Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. The American Journal of Psychiatry, 159(10), 1642–1652. https://doi.org/10.1176/appi.ajp.159.10.1642
Goldstein, R. Z., & Volkow, N. D. (2011). Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews Neuroscience, 12(11), 652–669. https://doi.org/10.1038/nrn3119
Gonçalves-Ferreira, A., do Couto, F. S., Rainha Campos, A., Lucas Neto, L. P., Gonçalves-Ferreira, D., & Teixeira, J. (2016). Deep brain stimulation for refractory cocaine dependence. Biological Psychiatry, 79(11), e87–e89. https://doi.org/10.1016/j.biopsych.2015.06.023
Grall-Bronnec, M., Bulteau, S., Vanelle, J. M., & Sauvaget, A. (2014). tDCS et addictions comportementales: Le coup de foudre! European Psychiatry, 29(8), 532. https://doi.org/10.1016/j.eurpsy.2014.09.391
Gunkelman, J. D., & Johnstone, J. (2005). Neurofeedback and the brain. Journal of Adult Development, 12(2–3), 93–98. https://doi.org/10.1007/s10804-005-7024-x
Hanlon, C. A., Hartwell, K. J., Canterberry, M., Li, X., Owens, M., LeMatty, T., Prisciandaro, J. J., Borckardt, J., Brady, K. T., & George, M. S. (2013). Reduction of cue-induced craving through realtime neurofeedback in nicotine users: The role of region of interest selection and multiple visits. Psychiatry Research, 213(1), 79–81. https://doi.org/10.1016/j.pscychresns.2013.03.003
Hartwell, K. J., Hanlon, C. A., Li, X., Borckardt, J. J., Canterberry, M., Prisciandaro, J. J., Moran-Santa Maria, M. M. M.-S., LeMatty, T., George, M. S., & Brady, K. T. (2016). Individualized real-time fMRI neurofeedback to attenuate craving in nicotine-dependent smokers. Journal of Psychiatry and Neuroscience, 41(1), 48–55. https://doi.org/10.1503/jpn.140200
Herrington, T. M., Cheng, J. J., & Eskandar, E. N. (2016). Mechanisms of deep brain stimulation. Journal of Neurophysiology, 115(1), 19–38. https://doi.org/10.1152/jn.00281.2015
Higgins, E. S., & George, M. S. (2019). Brain stimulation therapies for clinicians. American Psychiatric Publishing.
Hone-Blanchet, A., Ciraulo, D. A., Pascual-Leone, A., & Fecteau, S. (2015). Noninvasive brain stimulation to suppress craving in substance use disorders: Review of human evidence and methodological considerations for future work. Neuroscience and Biobehavioral Reviews, 59, 184–200. https://doi.org/10.1016/j.neubiorev.2015.10.001
Horrell, T., El-Baz, A., Baruth, J., Tasman, A., Sokhadze, G., Stewart, C., & Sokhadze, E. (2010). Neurofeedback effects on evoked and induced EEG gamma band reactivity to drug-related cues in cocaine addiction. Journal of Neurotherapy, 14(3), 195–216. https://doi.org/10.1080/10874208.2010.501498
Ibrahim, C., Rubin-Kahana, D. S., Pushparaj, A., Musiol, M., Blumberger, D. M., Daskalakis, Z. J., Zangen, A., & Le Foll, B. (2019). The insula: A brain stimulation target for the treatment of addiction. Frontiers in Pharmacology, 10, 720. https://doi.org/10.3389/fphar.2019.00720
Jaberzadeh, S., Bastani, A., & Zoghi, M. (2014). Anodal transcranial pulsed current stimulation: A novel technique to enhance corticospinal excitability. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 125(2), 344–351. https://doi.org/10.1016/j.clinph.2013.08.025
Jakobs, M., Fomenko, A., Lozano, A. M., & Kiening, K. L. (2019). Cellular, molecular, and clinical mechanisms of action of deep brain stimulation - A systematic review on established indications anfd outlook on future developments. EMBO Molecular Medicine, 11(4), e9575. https://doi.org/10.15252/emmm.201809575
Jansen, J. M., Daams, J. G., Koeter, M. W. J., Veltman, D. J., van den Brink, W., & Goudriaan, A. E. (2013). Effects of non-invasive neurostimulation on craving: A meta-analysis. Neuroscience and Biobehavioral Reviews, 37(10), 2472–2480. https://doi.org/10.1016/j.neubiorev.2013.07.009
Jones, K. A., Porjesz, B., Chorlian, D., Rangaswamy, M., Kamarajan, C., Padmanabhapillai, A., Stimus, A., & Begleiter, H. (2006). S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clinical Neurophysiology, 117(10), 2128–2143. https://doi.org/10.1016/j.clinph.2006.02.028
Jones, A., Di Lemma, L. C. G., Robinson, E., Christiansen, P., Nolan, S., Tudur-Smith, C., & Field, M. (2016). Inhibitory control training for appetitive behaviour change: A meta-analytic investigation of mechanisms of action and moderators of effectiveness. Appetite, 97, 16–28. https://doi.org/10.1016/j.appet.2015.11.013
Kalivas, P. W., & Volkow, N. D. (2005). The neural basis of addiction: A pathology of motivation and choice. The American Journal of Psychiatry, 162(8), 1403–1413. https://doi.org/10.1176/appi.ajp.162.8.1403
Kamarajan, C., Porjesz, B., Jones, K. A., Choi, K., Chorlian, D. B., Padmanabhapillai, A., Rangaswamy, M., Stimus, A. T., & Begleiter, H. (2004). The role of brain oscillations as functional correlates of cognitive systems: A study of frontal inhibitory control in alcoholism. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 51(2), 155–180. https://doi.org/10.1016/j.ijpsycho.2003.09.004
Kang, N., Kim, R. K., & Kim, H. J. (2019). Effects of transcranial direct current stimulation on symptoms of nicotine dependence: A systematic review and meta-analysis. Addictive Behaviors, 96, 133–139. https://doi.org/10.1016/j.addbeh.2019.05.006
Karch, S., Paolini, M., Gschwendtner, S., Jeanty, H., Reckenfelderbäumer, A., Yaseen, O., Maywald, M., Fuchs, C., Rauchmann, B. S., Chrobok, A., Rabenstein, A., Ertl-Wagner, B., Pogarell, O., Keeser, D., & Rüther, T. (2019). Real-time fMRI neurofeedback in patients with tobacco use disorder during Smoking Cessation: Functional differences and implications of the first training session in regard to future abstinence or relapse. Frontiers in Human Neuroscience, 13, 65. https://doi.org/10.3389/fnhum.2019.00065
Kekic, M., Boysen, E., Campbell, I. C., & Schmidt, U. (2016). A systematic review of the clinical efficacy of transcranial direct current stimulation (tDCS) in psychiatric disorders. Journal of Psychiatric Research, 74, 70–86. https://doi.org/10.1016/j.jpsychires.2015.12.018
Kirsch, M., Gruber, I., Ruf, M., Kiefer, F., & Kirsch, P. (2016). Real-time functional magnetic resonance imaging neurofeedback can reduce striatal cue-reactivity to alcohol stimuli. Addiction Biology, 21(4), 982–992. https://doi.org/10.1111/adb.12278
Klauss, J., Penido Pinheiro, L. C., Silva Merlo, B. L., Correia Santos, G. A., Fregni, F., Nitsche, M. A., & Miyuki Nakamura-Palacios, E. (2014). A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. The International Journal of Neuropsychopharmacology, 17(11), 1793–1803. https://doi.org/10.1017/S1461145714000984
Klauss, J., Anders, Q. S., Felippe, L. V., Ferreira, L. V. B., Cruz, M. A., Nitsche, M. A., & Nakamura-Palacios, E. M. (2018a). Lack of effects of extended sessions of transcranial direct current stimulation (tDCS) over dorsolateral prefrontal cortex on craving and relapses in crack-cocaine users. Frontiers in Pharmacology, 9, 1198. https://doi.org/10.3389/fphar.2018.01198
Klauss, J., Anders, Q. S., Felippe, L. V., Nitsche, M. A., & Nakamura-Palacios, E. M. (2018b). Multiple sessions of transcranial direct current stimulation (tDCS) reduced craving and relapses for alcohol use: A randomized placebo-controlled trial in alcohol use disorder. Frontiers in Pharmacology, 9, 716. https://doi.org/10.3389/fphar.2018.00716
Koechlin, E., & Hyafil, A. (2007). Anterior prefrontal function and the limits of human decision-making. Science (New York, N.Y.), 318(5850), 594–598. https://doi.org/10.1126/science.1142995
Koob, G. F., & Volkow, N. D. (2016). Neurobiology of addiction: A neurocircuitry analysis. The Lancet Psychiatry, 3(8), 760–773. https://doi.org/10.1016/S2215-0366(16)00104-8
Krishnan, C., Santos, L., Peterson, M. D., & Ehinger, M. (2015). Safety of noninvasive brain stimulation in children and adolescents. Brain Stimulation, 8(1), 76–87. https://doi.org/10.1016/j.brs.2014.10.012
Kubben, P. L. (2012). Brain Mapping: From Neural Basis of Cognition to Surgical Applications. Surgical Neurology International, 3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3424681/
Kuhn, J., Lenartz, D., Huff, W., Lee, S., Koulousakis, A., Klosterkoetter, J., & Sturm, V. (2007). Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: Valuable therapeutic implications? Journal of Neurology, Neurosurgery, and Psychiatry, 78(10), 1152–1153. https://doi.org/10.1136/jnnp.2006.113092
Kuhn, J., Bauer, R., Pohl, S., Lenartz, D., Huff, W., Kim, E. H., Klosterkoetter, J., & Sturm, V. (2009). Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens. European Addiction Research, 15(4), 196–201. https://doi.org/10.1159/000228930
Kuhn, J., Gründler, T. O. J., Bauer, R., Huff, W., Fischer, A. G., Lenartz, D., Maarouf, M., Bührle, C., Klosterkötter, J., Ullsperger, M., & Sturm, V. (2011). Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addiction Biology, 16(4), 620–623. https://doi.org/10.1111/j.1369-1600.2011.00337.x
Kuhn, J., Möller, M., Treppmann, J. F., Bartsch, C., Lenartz, D., Gruendler, T. O. J., Maarouf, M., Brosig, A., Barnikol, U. B., Klosterkötter, J., & Sturm, V. (2014). Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Molecular Psychiatry, 19(2), 145–146. https://doi.org/10.1038/mp.2012.196
Lapenta, O. M., Sierve, K. D., de Macedo, E. C., Fregni, F., & Boggio, P. S. (2014). Transcranial direct current stimulation modulates ERP-indexed inhibitory control and reduces food consumption. Appetite, 83, 42–48. https://doi.org/10.1016/j.appet.2014.08.005
Lefaucheur, J. P., Antal, A., Ayache, S. S., Benninger, D. H., Brunelin, J., Cogiamanian, F., Cotelli, M., De Ridder, D., Ferrucci, R., Langguth, B., Marangolo, P., Mylius, V., Nitsche, M. A., Padberg, F., Palm, U., Poulet, E., Priori, A., Rossi, S., Schecklmann, M., … Paulus, W. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology, 128(1), 56–92. https://doi.org/10.1016/j.clinph.2016.10.087
Leshner, A. I. (1997). Addiction is a brain disease, and it matters. Science (New York, N.Y.), 278(5335), 45–47. https://doi.org/10.1126/science.278.5335.45
Li, X., Hartwell, K. J., Borckardt, J., Prisciandaro, J. J., Saladin, M. E., Morgan, P. S., Johnson, K. A., Lematty, T., Brady, K. T., & George, M. S. (2013). Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: A preliminary real-time fMRI study. Addiction Biology, 18(4), 739–748. https://doi.org/10.1111/j.1369-1600.2012.00449.x
Li, L. M., Uehara, K., & Hanakawa, T. (2015). The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Frontiers in Cellular Neuroscience, 9, 181. https://doi.org/10.3389/fncel.2015.00181
Li, X., Hartwell, K. J., Henderson, S., Badran, B. W., Brady, K. T., & George, M. S. (2020). Two weeks of image-guided left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation improves smoking cessation: A double-blind, sham-controlled, randomized clinical trial. Brain Stimulation, 13(5), 1271–1279. https://doi.org/10.1016/j.brs.2020.06.007
Lindgren, K. P., Hendershot, C. S., Ramirez, J. J., Bernat, E., Rangel-Gomez, M., Peterson, K. P., & Murphy, J. G. (2019). A dual process perspective on advances in cognitive science and alcohol use disorder. Clinical Psychology Review, 69, 83–96. https://doi.org/10.1016/j.cpr.2018.04.002
Liu, T., Li, Y., Shen, Y., Liu, X., & Yuan, T.-F. (2019). Gender does not matter: Add-on repetitive transcranial magnetic stimulation treatment for female methamphetamine dependents. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 92, 70–75. https://doi.org/10.1016/j.pnpbp.2018.12.018
Liu, X., Zhao, X., Liu, T., Liu, Q., Tang, L., Zhang, H., Luo, W., Daskalakis, Z. J., & Yuan, T. F. (2020). The effects of repetitive transcranial magnetic stimulation on cue-induced craving in male patients with heroin use disorder. eBioMedicine, 56, 102809. https://doi.org/10.1016/j.ebiom.2020.102809
Loijen, A., Vrijsen, J. N., Egger, J. I. M., Becker, E. S., & Rinck, M. (2020). Biased approach-avoidance tendencies in psychopathology: A systematic review of their assessment and modification. Clinical Psychology Review, 77, 101825. https://doi.org/10.1016/j.cpr.2020.101825
Luigjes, J., van den Brink, W., Feenstra, M., van den Munckhof, P., Schuurman, P. R., Schippers, R., Mazaheri, A., De Vries, T. J., & Denys, D. (2012). Deep brain stimulation in addiction: A review of potential brain targets. Molecular Psychiatry, 17(6), 572–583. https://doi.org/10.1038/mp.2011.114
Luigjes, J., Breteler, R., Vanneste, S., & de Ridder, D. (2013). Neuromodulation as an intervention for addiction: Overview and future prospects. Tijdschrift voor Psychiatrie, 55(11), 841–852.
Luigjes, J., Segrave, R., de Joode, N., Figee, M., & Denys, D. (2019). Efficacy of invasive and non-invasive brain modulation interventions for addiction. Neuropsychology Review, 29(1), 116–138. https://doi.org/10.1007/s11065-018-9393-5
Luijten, M., Machielsen, M. W., Veltman, D. J., Hester, R., de Haan, L., & Franken, I. H. (2014). Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people. Journal of Psychiatry and Neuroscience, 39(3), 149–169. https://doi.org/10.1503/jpn.130052
Lupi, M., Martinotti, G., Santacroce, R., Cinosi, E., Carlucci, M., Marini, S., Acciavatti, T., & di Giannantonio, M. (2017). Transcranial direct current stimulation in substance use disorders: A systematic review of scientific literature. The Journal of ECT, 33(3), 203–209. https://doi.org/10.1097/YCT.0000000000000401
Lüscher, C., Robbins, T. W., & Everitt, B. J. (2020). The transition to compulsion in addiction. Nature Reviews Neuroscience, 21(5), 247–263. https://doi.org/10.1038/s41583-020-0289-z
Maiti, R., Mishra, B. R., & Hota, D. (2017). Effect of high-frequency transcranial magnetic stimulation on craving in substance use disorder: A meta-analysis. The Journal of Neuropsychiatry and Clinical Neurosciences, 29(2), 160–171. https://doi.org/10.1176/appi.neuropsych.16040065
Mann, C., Frieden, T., Hyde, P. S., Volkow, N. D., & Koob, GF. (2014). Informational bulletin: Medication assisted treatment for substance use disorders. https://www.medicaid.gov/federal-policy-guidance/downloads/cib-07-11-2014.pdf
Manning, V., Garfield, J. B. B., Staiger, P. K., Lubman, D. I., Lum, J. A. G., Reynolds, J., Hall, K., Bonomo, Y., Lloyd-Jones, M., Wiers, R. W., Piercy, H., Jacka, D., & Verdejo-Garcia, A. (2021). Effect of cognitive bias modification on early relapse among adults undergoing inpatient alcohol withdrawal treatment: A randomized clinical trial. JAMA Psychiatry, 78, 133. https://doi.org/10.1001/jamapsychiatry.2020.3446
Marzbani, H., Marateb, H. R., & Mansourian, M. (2016). Neurofeedback: A comprehensive review on system design, methodology and clinical applications. Basic and Clinical Neuroscience, 7(2), 143–158. https://doi.org/10.15412/J.BCN.03070208
Mayberg, H. S., Lozano, A. M., Voon, V., McNeely, H. E., Seminowicz, D., Hamani, C., Schwalb, J. M., & Kennedy, S. H. (2005). Deep brain stimulation for treatment-resistant depression. Neuron, 45(5), 651–660. https://doi.org/10.1016/j.neuron.2005.02.014
McClure, S. M., & Bickel, W. K. (2014). A dual-systems perspective on addiction: Contributions from neuroimaging and cognitive training. Annals of the New York Academy of Sciences, 1327, 62–78. https://doi.org/10.1111/nyas.12561
Micoulaud-Franchi, J. A., Quiles, C., & Vion-Dury, J. (2013). Éléments pour une histoire de l’électricité et du cerveau en psychiatrie. Applications thérapeutiques de la stimulation externe et l’enregistrement électrique en psychiatrie (Partie II). Annales Médico-Psychologiques, Revue Psychiatrique, 171(5), 323–328.
Micoulaud-Franchi, J.-A., Fovet, T., Thibault, R. T., & Raz, A. (2018). Two part correspondence: A framework for disentangling the hyperbolic truth of neurofeedback; A consensus framework for neurofeedback research. ArXiv. https://doi.org/10.31234/osf.io/apzs7
Montgomery, E. B., & Gale, J. T. (2008). Mechanisms of action of deep brain stimulation (DBS). Neuroscience and Biobehavioral Reviews, 32(3), 388–407. https://doi.org/10.1016/j.neubiorev.2007.06.003
Müller, U. J., Sturm, V., Voges, J., Heinze, H. J., Galazky, I., Heldmann, M., Scheich, H., & Bogerts, B. (2009). Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: First experience with three cases. Pharmacopsychiatry, 42(6), 288–291. https://doi.org/10.1055/s-0029-1233489
Müller, U. J., Sturm, V., Voges, J., Heinze, H. J., Galazky, I., Büntjen, L., Heldmann, M., Frodl, T., Steiner, J., & Bogerts, B. (2016). Nucleus accumbens deep brain stimulation for alcohol addiction - Safety and clinical long-term results of a pilot trial. Pharmacopsychiatry, 49(4), 170–173. https://doi.org/10.1055/s-0042-104507
Naqvi, N. H., & Bechara, A. (2010). The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Structure and Function, 214(5–6), 435–450. https://doi.org/10.1007/s00429-010-0268-7
Naqvi, N. H., Rudrauf, D., Damasio, H., & Bechara, A. (2007). Damage to the insula disrupts addiction to cigarette smoking. Science (New York, N.Y.), 315(5811), 531–534. https://doi.org/10.1126/science.1135926
Nitsche, M. A., & Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology, 527(3), 633–639.
Nitsche, M. A., & Paulus, W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57(10), 1899–1901.
Nitsche, M. A., Liebetanz, D., Lang, N., Antal, A., Tergau, F., & Paulus, W. (2003). Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 114(11), 2220–2222. author reply 2222.
Noël, X., Brevers, D., & Bechara, A. (2013). A neurocognitive approach to understanding the neurobiology of addiction. Current Opinion in Neurobiology, 23(4), 632–638. https://doi.org/10.1016/j.conb.2013.01.018
Noël, X., Bechara, A., Saeremans, M., Kornreich, C., Dousset, C., Campanella, S., Chatard, A., Jaafari, N., & Dubuson, M. (2019). Addiction: Brain and cognitive stimulation for better cognitive control and far beyond. In Inhibitory control training - A multidisciplinary approach. IntechOpen. https://doi.org/10.5772/intechopen.88869
Nuttin, B., Cosyns, P., Demeulemeester, H., Gybels, J., & Meyerson, B. (1999). Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. The Lancet, 354(9189), 1526. https://doi.org/10.1016/S0140-6736(99)02376-4
Orndorff-Plunkett, F., Singh, F., Aragón, O. R., & Pineda, J. A. (2017). Assessing the effectiveness of neurofeedback training in the context of clinical and social neuroscience. Brain Sciences, 7(8), 95. https://doi.org/10.3390/brainsci7080095
Pandey, A. K., Kamarajan, C., Rangaswamy, M., & Porjesz, B. (2012). Event-related oscillations in alcoholism research: A review. Journal of Addiction Research and Therapy, 7(1), 3844. https://doi.org/10.4172/2155-6105.S7-001
Parvaz, M. A., Alia-Klein, N., Woicik, P. A., Volkow, N. D., & Goldstein, R. Z. (2011). Neuroimaging for drug addiction and related behaviors. Reviews in the Neurosciences, 22(6), 609–624. https://doi.org/10.1515/RNS.2011.055
Peniston, E. G., & Kulkosky, P. J. (1989). Alpha-theta brainwave training and beta-endorphin levels in alcoholics. Alcoholism: Clinical and Experimental Research, 13(2), 271–279. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x
Perna, G., Grassi, M., Caldirola, D., & Nemeroff, C. B. (2018). The revolution of personalized psychiatry: Will technology make it happen sooner? Psychological Medicine, 48(5), 705–713.
Petit, G., Cimochowska, A., Kornreich, C., Hanak, C., Verbanck, P., & Campanella, S. (2014). Neurophysiological correlates of response inhibition predict relapse in detoxified alcoholic patients: Some preliminary evidence from event-related potentials. Neuropsychiatric Disease and Treatment, 10, 1025–1037. https://doi.org/10.2147/NDT.S61475
Philip, N. S., Nelson, B. G., Frohlich, F., Lim, K. O., Widge, A. S., & Carpenter, L. L. (2017). Low-intensity transcranial current stimulation in psychiatry. American Journal of Psychiatry, 174(7), 628–639. https://doi.org/10.1176/appi.ajp.2017.16090996
Pierce, R. C., & Vassoler, F. M. (2013). Deep brain stimulation for the treatment of addiction: Basic and clinical studies and potential mechanisms of action. Psychopharmacology, 229(3), 487–491. https://doi.org/10.1007/s00213-013-3214-6
Reiter, K., Andersen, S. B., & Carlsson, J. (2016). Neurofeedback treatment and posttraumatic stress disorder: Effectiveness of neurofeedback on posttraumatic stress disorder and the optimal choice of protocol. Journal of Nervous and Mental Disease, 204(2), 69–77. https://doi.org/10.1097/NMD.0000000000000418
Rieger, K., Rarra, M. H., Diaz Hernandez, L., Hubl, D., & Koenig, T. (2018). Neurofeedback-based enhancement of single-trial auditory evoked potentials: Treatment of auditory verbal hallucinations in schizophrenia. Clinical EEG and Neuroscience, 49(6), 367–378. https://doi.org/10.1177/1550059418765810
Robinson, T. E., & Berridge, K. C. (1993). The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research. Brain Research Reviews, 18(3), 247–291. https://doi.org/10.1016/0165-0173(93)90013-p
Robbins, T. W., & Everitt, B. J. (1996). Neurobehavioural mechanisms of reward and motivation. Current Opinion in Neurobiology, 6(2), 228–236. https://doi.org/10.1016/s0959-4388(96)80077-8
Robinson, T. E., & Berridge, K. C. (2008). The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society, B: Biological Sciences, 363(1507), 3137–3146. https://doi.org/10.1098/rstb.2008.0093
Ros, T., Enriquez-Geppert, S., Zotev, V., Young, K. D., Wood, G., Whitfield-Gabrieli, S., Wan, F., Vuilleumier, P., Vialatte, F., Van De Ville, D., Todder, D., Surmeli, T., Sulzer, J. S., Strehl, U., Sterman, M. B., Steiner, N. J., Sorger, B., Soekadar, S. R., Sitaram, R., … Thibault, R. T. (2020). Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist). Brain: A Journal of Neurology, 143(6), 1674–1685. https://doi.org/10.1093/brain/awaa009
Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A., & Safety of TMS Consensus Group. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 120(12), 2008–2039. https://doi.org/10.1016/j.clinph.2009.08.016
Rossi, S., Antal, A., Bestmann, S., Bikson, M., Brewer, C., Brockmöller, J., Carpenter, L. L., Cincotta, M., Chen, R., Daskalakis, J. D., Di Lazzaro, V., Fox, M. D., George, M. S., Gilbert, D., Kimiskidis, V. K., Koch, G., Ilmoniemi, R. J., Lefaucheur, J. P., Leocani, L., … Hallett, M. (2020). Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 132(1), 269–306. https://doi.org/10.1016/j.clinph.2020.10.003
Rossini, P. M., Barker, A. T., Berardelli, A., Caramia, M. D., Caruso, G., Cracco, R. Q., Dimitrijević, M. R., Hallett, M., Katayama, Y., & Lücking, C. H. (1994). Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology, 91(2), 79–92. https://doi.org/10.1016/0013-4694(94)90029-9
Sauvaget, A., Trojak, B., Bulteau, S., Jiménez-Murcia, S., Fernández-Aranda, F., Wolz, I., Menchón, J. M., Achab, S., Vanelle, J. M., & Grall-Bronnec, M. (2015). Transcranial direct current stimulation (tDCS) in behavioral and food addiction: A systematic review of efficacy, technical, and methodological issues. Frontiers in Neuroscience, 9, 349. https://doi.org/10.3389/fnins.2015.00349
Saxby, E., & Peniston, E. G. (1995). Alpha-theta brainwave neurofeedback training: An effective treatment for male and female alcoholics with depressive symptoms. Journal of Clinical Psychology, 51(5), 685–693.
Schluter, R. S., Daams, J. G., van Holst, R. J., & Goudriaan, A. E. (2018). Effects of non-invasive neuromodulation on executive and other cognitive functions in addictive disorders: A systematic review. Frontiers in Neuroscience, 12, 642. https://doi.org/10.3389/fnins.2018.00642
Scott, W. C., Kaiser, D., Othmer, S., & Sideroff, S. I. (2005). Effects of an EEG biofeedback protocol on a mixed substance abusing population. The American Journal of Drug and Alcohol Abuse, 31(3), 455–469. https://doi.org/10.1081/ADA-200056807
Siebner, H. R., Peller, M., Willoch, F., Minoshima, S., Boecker, H., Auer, C., Drzezga, A., Conrad, B., & Bartenstein, P. (2000). Lasting cortical activation after repetitive TMS of the motor cortex: A glucose metabolic study. Neurology, 54(4), 956–963.
Sitaram, R., Ros, T., Stoeckel, L., Haller, S., Scharnowski, F., Lewis-Peacock, J., Weiskopf, N., Blefari, M. L., Rana, M., Oblak, E., Birbaumer, N., & Sulzer, J. (2017). Closed-loop brain training: The science of neurofeedback. Nature Reviews. Neuroscience, 18(2), 86–100. https://doi.org/10.1038/nrn.2016.164
Sokhadze, T. M., Cannon, R. L., & Trudeau, D. L. (2008). EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy, and recommendations for further research. Applied Psychophysiology and Biofeedback, 33(1), 1–28. https://doi.org/10.1007/s10484-007-9047-5
Song, S., Zilverstand, A., Gui, W., Li, H. J., & Zhou, X. (2019). Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: A meta-analysis. Brain Stimulation, 12(3), 606–618. https://doi.org/10.1016/j.brs.2018.12.975
Spagnolo, P. A., & Goldman, D. (2017). Neuromodulation interventions for addictive disorders: Challenges, promise, and roadmap for future research. Brain, 140(5), 1183–1203. https://doi.org/10.1093/brain/aww284
Stagg, C. J., & Nitsche, M. A. (2011). Physiological basis of transcranial direct current stimulation. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 17(1), 37–53. https://doi.org/10.1177/1073858410386614
Stein, E. R., Gibson, B. C., Votaw, V. R., Wilson, A. D., Clark, V. P., & Witkiewitz, K. (2019). Non-invasive brain stimulation in substance use disorders: Implications for dissemination to clinical settings. Current Opinion in Psychology, 30, 6–10. https://doi.org/10.1016/j.copsyc.2018.12.009
Stephen, J. H., Halpern, C. H., Barrios, C. J., Balmuri, U., Pisapia, J. M., Wolf, J. A., Kampman, K. M., Baltuch, G. H., Caplan, A. L., & Stein, S. C. (2012). Deep brain stimulation compared with methadone maintenance for the treatment of heroin dependence: A threshold and cost-effectiveness analysis. Addiction (Abingdon, England), 107(3), 624–634. https://doi.org/10.1111/j.1360-0443.2011.03656.x
Sterman, M. B. (1996). Physiological origins and functional correlates of EEG rhythmic activities: Implications for self-regulation. Biofeedback and Self-Regulation, 21(1), 3–33. https://doi.org/10.1007/BF02214147
Strube, W., Bunse, T., Nitsche, M. A., Nikolaeva, A., Palm, U., Padberg, F., Falkai, P., & Hasan, A. (2016). Bidirectional variability in motor cortex excitability modulation following 1 mA transcranial direct current stimulation in healthy participants. Physiological Reports, 4(15), e12884. https://doi.org/10.14814/phy2.12884
Su, H., Zhong, N., Gan, H., Wang, J., Han, H., Chen, T., Li, X., Ruan, X., Zhu, Y., Jiang, H., & Zhao, M. (2017). High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: A randomised clinical trial. Drug and Alcohol Dependence, 175, 84–91. https://doi.org/10.1016/j.drugalcdep.2017.01.037
Su, H., Chen, T., Jiang, H., Zhong, N., Du, J., Xiao, K., Xu, D., Song, W., & Zhao, M. (2020). Intermittent theta burst transcranial magnetic stimulation for methamphetamine addiction: A randomized clinical trial. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 31, 158–161. https://doi.org/10.1016/j.euroneuro.2019.12.114
Terney, D., Chaieb, L., Moliadze, V., Antal, A., & Paulus, W. (2008). Increasing human brain excitability by transcranial high-frequency random noise stimulation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(52), 14147–14155. https://doi.org/10.1523/JNEUROSCI.4248-08.2008
Thibault, R. T., & Raz, A. (2018). A consensus framework for neurofeedback research (and the perils of unfounded neuroreductionism): Reply to Micoulaud-Franchi and Fovet (2018). American Psychologist, 73(7), 936–937. https://doi.org/10.1037/amp0000366
Thibault, R. T., Lifshitz, M., & Raz, A. (2016). The self-regulating brain and neurofeedback: Experimental science and clinical promise. Cortex, 74, 247–261. https://doi.org/10.1016/j.cortex.2015.10.024
Thibault, R. T., Lifshitz, M., & Raz, A. (2017). Neurofeedback or neuroplacebo? Brain, 140(4), 856–857. https://doi.org/10.1093/brain/awx033
Thompson, M., & Thompson, L. (2015). The neurofeedback book (2nd ed.). The Association for Applied Psychophysiology & Biofeedback.
Tortella, G. (2015). Transcranial direct current stimulation in psychiatric disorders. World Journal of Psychiatry, 5(1), 88. https://doi.org/10.5498/wjp.v5.i1.88
Trojak, B., Meille, V., Achab, S., Lalanne, L., Poquet, H., Ponavoy, E., Blaise, E., Bonin, B., & Chauvet-Gelinier, J. C. (2015). Transcranial magnetic stimulation combined with nicotine replacement therapy for Smoking Cessation: A randomized controlled trial. Brain Stimulation, 8(6), 1168–1174. https://doi.org/10.1016/j.brs.2015.06.004
den Uyl, T. E., Gladwin, T. E., & Wiers, R. W. (2016). Electrophysiological and behavioral effects of combined transcranial direct current stimulation and alcohol approach bias retraining in hazardous drinkers. Alcoholism: Clinical and Experimental Research, 40(10), 2124–2133. https://doi.org/10.1111/acer.13171
den Uyl, T. E., Gladwin, T. E., Rinck, M., Lindenmeyer, J., & Wiers, R. W. (2017). A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining: Clinical trial tDCS and CBM. Addiction Biology, 22(6), 1632–1640. https://doi.org/10.1111/adb.12463
den Uyl, T. E., Gladwin, T. E., Lindenmeyer, J., & Wiers, R. W. (2018). A clinical trial with combined transcranial direct current stimulation and attentional bias modification in alcohol-dependent patients. Alcoholism: Clinical and Experimental Research, 42(10), 1961–1969. https://doi.org/10.1111/acer.13841
Valencia-Alfonso, C. E., Luigjes, J., Smolders, R., Cohen, M. X., Levar, N., Mazaheri, A., van den Munckhof, P., Schuurman, P. R., van den Brink, W., & Denys, D. (2012). Effective deep brain stimulation in heroin addiction: A case report with complementary intracranial electroencephalogram. Biological Psychiatry, 71(8), e35–e37. https://doi.org/10.1016/j.biopsych.2011.12.013
Verveer, I., van der Veen, F. M., Shahbabaie, A., Remmerswaal, D., & Franken, I. H. A. (2020). Multi-session electrical neuromodulation effects on craving, relapse and cognitive functions in cocaine use disorder: A randomized, sham-controlled tDCS study. Drug and Alcohol Dependence, 217, 108429. https://doi.org/10.1016/j.drugalcdep.2020.108429
Voges, J., Waerzeggers, Y., Maarouf, M., Lehrke, R., Koulousakis, A., Lenartz, D., & Sturm, V. (2006). Deep-brain stimulation: Long-term analysis of complications caused by hardware and surgery - Experiences from a single centre. Journal of Neurology, Neurosurgery, and Psychiatry, 77(7), 868–872. https://doi.org/10.1136/jnnp.2005.081232
Wiers, R. W., Bartholow, B. D., van den Wildenberg, E., Thush, C., Engels, R. C. M. E., Sher, K. J., Grenard, J., Ames, S. L., & Stacy, A. W. (2007). Automatic and controlled processes and the development of addictive behaviors in adolescents: A review and a model. Pharmacology Biochemistry and Behavior, 86(2), 263–283. https://doi.org/10.1016/j.pbb.2006.09.021
Wiers, R. W., Rinck, M., Dictus, M., & van den Wildenberg, E. (2009). Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes, Brain, and Behavior, 8(1), 101–106. https://doi.org/10.1111/j.1601-183X.2008.00454.x
Wilson, S. J., Sayette, M. A., & Fiez, J. A. (2004). Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience, 7(3), 211–214. https://doi.org/10.1038/nn1200
Zhang, J. J. Q., Fong, K. N. K., Ouyang, R. G., Siu, A. M. H., & Kranz, G. S. (2019a). Effects of repetitive transcranial magnetic stimulation (rTMS) on craving and substance consumption in patients with substance dependence: A systematic review and meta-analysis. Addiction (Abingdon, England), 114(12), 2137–2149. https://doi.org/10.1111/add.14753
Zhang, Y., Song, H., Chen, Y., Zuo, L., Xia, X., & Zhang, X. (2019b). Thinking on transcranial direct current stimulation (tDCS) in reading interventions: Recommendations for future research directions. Frontiers in Human Neuroscience, 13, 157. https://doi.org/10.3389/fnhum.2019.00157
Zhao, H., Qiao, L., Fan, D., Zhang, S., Turel, O., Li, Y., Li, J., Xue, G., Chen, A., & He, Q. (2017). Modulation of brain activity with noninvasive transcranial direct current stimulation (tDCS): Clinical applications and safety concerns. Frontiers in Psychology, 8, 685. https://doi.org/10.3389/fpsyg.2017.00685
Zhou, H., Xu, J., & Jiang, J. (2011). Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: A case report. Biological Psychiatry, 69(11), e41–e42. https://doi.org/10.1016/j.biopsych.2011.02.012
Zilverstand, A., Huang, A. S., Alia-Klein, N., & Goldstein, R. Z. (2018). Neuroimaging impaired response inhibition and salience attribution in human drug addiction. A systematic review. Neuron, 98(5), 886–903. https://doi.org/10.1016/j.neuron.2018.03.048
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dubuson, M., Dousset, C., Noël, X., Campanella, S. (2021). Neuromodulation Techniques in the Treatment of Addictions. In: Balconi, M., Campanella, S. (eds) Advances in Substance and Behavioral Addiction . Advances in Mental Health and Addiction. Springer, Cham. https://doi.org/10.1007/978-3-030-82408-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-82408-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-82407-5
Online ISBN: 978-3-030-82408-2
eBook Packages: Behavioral Science and PsychologyBehavioral Science and Psychology (R0)