Abstract
Overfishing for bleeding has been identified as the major attribute to the substantial decline of Asian horseshoe crab populations. Owing to the low willingness of Tachypleus amebocyte lysate (TAL) firms to return bled horseshoe crabs to the sea, the pond culture practice has been proposed as the alternative to keep wild-caught adults or subadults in outdoor ponds for years for repeated bleeding. The potential effects of the prolonged culture on hemolymph quality of horseshoe crabs are questioned since their general health status has an impact on the TAL production quality and the resource sustainability for bleeding. However, the baseline information regarding immune-related parameters of horseshoe crabs, particularly the Asian species, is very limited. A lacking of standardized classification scheme of horseshoe crab hemocytes makes the health indicator measurement, such as differential hemocyte counts, and the comparison among studies more challenging. In this study, hemocyte morphology and immune-related enzymatic activity in T. tridentatus and Carcinoscorpius rotundicauda were determined, in which the individuals had been kept in outdoor ponds for one year. Wright–Giemsa staining result demonstrated that three hemocyte types, namely granulocyte, non-granulocyte, and macrophage, were found. Granulocyte was the major cell type in both species, and the other two hemocyte types were noted in very small proportions surrounded by the basophil granulocytes. The cytoplasmic granules in granulocytes were stained in either blue (basophil) or purple–red (eosinophil), but the macrophages contain a mixture of blue- and red-stained granules. For alkaline phosphatase (AKP) and glutathione peroxidase (GSH-Px) activities, there were no significant differences either between species or among digestive tract tissues, except that a statistical higher AKP activity level was noted in T. tridentatus serum than in Carcinoscorpius rotundicauda. The AKP and GSH-Px activity levels, in general, were higher compared to those reported in smaller-sized aquatic invertebrates. The findings may be useful as the baseline data in further immunological studies of Asian horseshoe crabs, especially for developing health monitoring system and improving sustainable bleeding practices.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Horseshoe crabs belong to the Phylum Arthropoda, Class Merostomata, Order Xiphosura, and Family Limulidae. There are only four extant species of horseshoe crab: the Atlantic horseshoe crab Limulus polyphemus found on the eastern coast of North America and Gulf of Mexico; the tri-spine horseshoe crab Tachypleus tridentatus, the coastal horseshoe crab T. gigas, and the mangrove horseshoe crab Carcinoscorpius rotundicauda occur in the Indo-Pacific region, ranging from southern Japan to the Bay of Bengal (Smith et al. 2017; John et al. 2018). Horseshoe crabs are among the few animals to be characterized as “living fossils,” as their oldest fossils can be dated to the Ordovician period, 445 million years ago (Rudkin and Young 2009). Horseshoe crabs are important benthic predators in coastal and estuarine ecosystems (Botton 2009), and as the potential indicator species (Chen et al. 2015) or sentinel species (Kwan et al. 2018) to reflect the general health of the ecosystems.
The biomedical use of horseshoe crabs was discovered by Bang (1956), and further discussed in Levin and Bang (1964), which described the hemocytes present in horseshoe crab hemolymph and their endotoxin-induced coagulation by releasing an enzyme coagulation cascade. This discovery has led to the development of Limulus amoebocyte lysate (LAL), a widely used reagent in the detection of bacterial endotoxin in medical diagnosis and in drug assays. Horseshoe crab hemolymph was then demonstrated to contain a variety of active substances such as horseshoe crab factors, antibacterial and antiviral compounds that have great potential for medical development (Iwanaga 2002). The use of LAL reagent as an official test for pyrogen was approved by the Food and Drug Administration, USA, in 1987, and led to the substantial development in LAL industry to meet the growing market demands. The number of L. polyphemus individuals harvested for LAL production increased from approximately 32,000 individuals in 2005 to recently circa 60,000 individuals per annum, with an estimated biomedical mortality at 15% (Gauvry 2015). The Asian pharmaceutical market is growing rapidly, and in China, Tachypleus tridentatus individuals used for TAL production was estimated to reach 1.5 million per annum to fulfill the demand for testing more than 300 injectable pharmaceuticals listed in Chinese Pharmacopoeia (Li et al. 2018; Yan et al. 2018). Due to the different bleeding practices in China and possibly other Asian places, T. tridentatus bleeding can cause 100% mortality, and the individuals may be then diverted to the food market. Consequently, overfishing for bleeding has been identified as one of the main attributes to the substantial decline of Asian horseshoe crab populations (John et al. 2018; Liao et al. 2019; Zhu et al. 2020). Tachypleus tridentatus is listed as Endangered in 2019 on the IUCN Red List (Laurie et al. 2019).
The decreasing T. tridentatus population number along the Chinese coast has resulted in the refusal of TAL firms to return the bled horseshoe crabs to sea under the absence of local regulatory policy. The pond culture practice has been proposed as the alternative to facilitate sustainable bleeding of Asian horseshoe crabs, in which the wild-caught subadults and adults are kept in outdoor ponds in coastal area for generally more than a year. The individuals will be transported to bleed in nearby facilities and returned to the ponds repeatedly within a fixed time interval (Xie X. 2018, pers. comm.). Concerns are raised about whether hemolymph quality of captive horseshoe crabs can be maintained under prolonged culture, in which the issue is primary to TAL production and quality assurance. Previous studies have demonstrated that plasma protein level, hemocyte viability, and their morphological state can be sensitive to environmental conditions, such as the presence of captivity stress, heavy metals, and excessive nutrients (Kwan et al. 2014, 2015, 2018). James-Pirri et al. (2012) compared hemolymph constituents of wild-caught, captive control, and captive bled adult L. polyphemus under current or best management practices. The study found that captive bled horseshoe crabs had lower plasma protein concentration compared to other treatment groups, while the wild individuals had higher concentrations in hemolymph glucose, potassium, and creatinine. However, little is known about the effect of prolonged captivity or bleeding on hemolymph quality of Asian species.
In this study, hemocyte morphology and immune-related enzymatic activities in both serum and digestive tracts of adult T. tridentatus and C. rotundicauda under prolonged pond culture was examined. Alkaline phosphatase (AKP) and glutathione peroxidase (GSH-Px) activities were selected to reflect the general health status of captive horseshoe crabs. AKP plays important roles in nutrient metabolite transport and ion secretion (Lallès 2010; Cheng et al. 2017), which is sensitive to changes in nutritional conditions. GSH-Px activity indicates the organism’s antioxidant capacity of which inflammation, hormone level, and other developmental differentiation can affect the enzymatic activity (Sugino et al. 1998; Rogers et al. 2000; Wei and Lee 2002). This preliminary study attempts to provide baseline information on hemocyte morphology and immunity of Asian horseshoe crabs kept in prolonged captivity for bleeding. Carcinoscorpius rotundicauda has not been utilized to serve current bleeding practices due to its smaller body size (prosomal width, PW: 11–13 cm, Fauziyah et al. 2019), but the species is still vulnerable to bleeding and exploitation when other Asian horseshoe crab populations are diminishing. The findings may be useful in developing a health monitoring system in the sustainable bleeding process and other relevant immunological studies.
2 Materials and Methods
2.1 Collection and Culture of Horseshoe Crabs
The pond culture of horseshoe crabs was initiated on November 5, 2016 on the western part of Yangjiang, a prefecture-level city in Guangdong province, China. A total of 1062 individuals of adult T. tridentatus (average PW: 23.5 cm; wet weight, WW: 311 g) and C. rotundicauda (average PW: 12.1 cm; WW: 213 g) were kept in a circa 1300 m2 outdoor pond with 2.5 m depth. Individuals from both species were obtained from coastal waters in Zhanjiang, Guangdong province. The environmental conditions were maintained as follows: water temperature 16.5–31.6°, salinity 16–30 ppt, and pH 7.2–8.4. The salinity level is higher in winter compared to summer. At the bottom of the pond, a 0.3 m sediment layer (25–55% sand particles) was provided to enhance the survival of horseshoe crabs (Hong et al. 2009). The sediment grain size is relatively larger along the edges of the pond, whereas the middle of the pond contains finer particles. A waterwheel aerator was set up to increase dissolved oxygen and lower ammonia-nitrogen levels in the pond. Half of the total volume of pond water was renewed weekly during the rising/falling tides. The horseshoe crabs were fed with frozen mussel meat and small fish twice a week.
Two individuals of T. tridentatus and three individuals of C. rotundicauda were collected from the pond after one year of culture on November 29–30, 2017. The horseshoe crabs were transported back to the laboratory and held in a 1000-L aquarium tank for three days before the bleeding to simulate the standard bleeding procedure in TAL-producing facilities. The water temperature was kept at 29–30 °C, salinity 16–30 ppt and dissolved oxygen >6 mg/L. The sediment (25–55% sand) at the bottom of the tank was disinfected by ultraviolet-C light before the experiment. The horseshoe crabs were provided clam meat daily at 16:00, and one-third of the water volume was renewed prior to the feeding.
2.2 Hemolymph Sampling and Preparation
Needles and syringes were pre-disinfected by ultraviolet-C light during the needle/syringe production, tweezers were autoclaved, and the coverslips for the blood smear were cleaned and disinfected using 75% ethanol. Approximately 5 mL of hemolymph was extracted from each individual using a 22-gauge needle from the hinge between prosoma and opisthosoma of horseshoe crabs. The hinge part was wiped with 75% ethanol followed by distilled water before and after the bleeding. The sampled hemolymph was stored on ice to reduce spontaneous cellular aggregation. Half portion of the hemolymph was used for blood smear preparation, whereas another half was centrifuged at 4 °C and 500 rpm for 3 min. The supernatant after centrifugation was used for enzymatic activity measurements. At the same time, the digestive tract was dissected and separated into esophagus, stomach, pylorus, midgut, and hindgut. The enzymatic activity of these tissues was examined immediately after the collection.
To prepare a blood smear, in the sterile room, a drop (circa 50 μL) of the collected hemolymph was placed on a slide, and a thin glass cover was added on top to form a thin hemolymph film. The blood smear was allowed to be air-dried before the staining. The Wright–Giemsa Stain Solution (Servicebio Co., Ltd., Wuhan, China) was applied following the experimental procedures described in the product protocol (http://www.servicebio.cn/html/all/cp/bl/rs/1757.html). The Wright–Giemsa stain is widely used in hematological studies to facilitate the differentiation of cell types, which is essential for accurate interpretation of their morphological characteristics and detection of abnormal cellular components for disease diagnosis (Dunning and Safo 2011). The hemolymph samples were allowed to stain for 5 min, washed with distilled water, air-dried, sealed with glycerin, and photographed using an optical microscope (AxioScope A1, ZEISS, Jena, Germany).
2.3 Determination of Enzymatic Activity
The AKP and GSH-Px activities in hemolymph and different tissues of digestive tract (i.e., esophagus, stomach, pylorus, midgut, and hindgut) of the two horseshoe crab species was determined using the AKP and GSH-Px assay kits developed from the Nanjing Jiancheng Bioengineering Institute, Wuhan, China (http://www.njjcbio.com/). The AKP activity was determined using the phenyldiphenyl phosphate method, in which a unit of enzymatic activity was defined as the amount required for either 100 ml of serum (U/100 mL) or 1 gram of tissue protein (U/g protein) to produce 1 mg of phenol following application to the substrate at 37 °C for 15 min. Double-distilled water and phenol was used as the blank and standard, respectively, for the determination. For GSH-Px activity, it was quantified based on the colorimetric method that a unit of enzymatic activity was defined as the amount required to decrease the concentration of reduced glutathione in the reaction system by 1 μmol/L at 37 °C after 5-min reaction per 0.1 ml of serum (U/0.1 ml) or per milligram of protein (U/mg protein), and after the effect of non-enzymatic reaction was subtracted. Blank and standard solutions were provided together with the testing kit. The protein content from each assay group was determined using the Coomassie Brilliant Blue method (Bradford 1976).
2.4 Statistical Analysis
Data were first checked for normality and homogeneity of variance using Shapiro–Wilk and Levene’s tests, respectively. The differences in serum AKP and GSH-Px levels between species were examined by unpaired sample t-test, whereas their AKP and GSH-Px activity level differences among different digestive tract tissues and between species were compared using two-way analysis of variance (ANOVA). All the above analyses were performed using SPSS software version 22.0 (IBM, New York, USA). A significance level of p < 0.05 was considered in all statistical procedures.
3 Results
3.1 Morphological Characteristics of Hemocytes
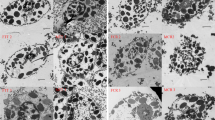
Granulocytes, non-granulocytes, and macrophages were observed in hemolymph from both T. tridentatus and C. rotundicauda (Fig. 1). The majority of hemocytes were round or oval in shape. Granulocytes accounted for the vast majority of hemocytes, in which the number of basophil granulocytes (i.e., basophils) was considerably higher than eosinophil granulocytes (i.e., eosinophils). It is noted that the cytoplasmic granules of basophils showed different levels of stain, including darker blue and lighter blue stain. The darker-blue basophils contained a central nucleus and higher number of granules in the cell, while the lighter-blue basophils consisted of a less evident nucleus and fewer granules. Light-blue basophiles were the major cell types among the granulocytes. A similar situation was observed in eosinophils, which included purple–red cells with evident intracellular granules and less evident nucleus, and light-red cells with a lower intracellular granule number and the nucleus located on one edge of the cytoplasm.
The morphology of hemocytes from T. tridentatus (a–c) and C. rotundicauda (d–f) under a light microscope. Three types of hemocytes were indicated by arrows in different colors: granulocytes (basophils: black, eosinophils: blue), non-granulocytes (white) and macrophages (red). Basophiles were stained in purple–red or light-red, whereas basophils were stained in dark- or light-blue
The number of non-granulocytes and macrophages were very few, and mainly surrounded by the basophiles (Fig. 1). The non-granulocyte had similar size to granulocyte with a central, clearly defined nucleus. The macrophages were larger in size with the nucleus located at the upper middle portion of the cell. A mixture of blue and red-stained intracellular granules was clearly observed within the macrophages (Fig. 1). No apparent inter-specific differences in staining or morphology of the hemocyte types were observed.
3.2 Enzymatic Activity Determination
The horseshoe crab AKP activity level in serum ranged 0.14–0.15 U/mL (Fig. 2), and that in different digestive tract, tissues ranged 10.89–19.37 U/g protein (Fig. 3). The AKP level was varied among individuals and tissues. The highest mean AKP activity level was recorded in the T. tridentatus hindgut tissue (16.13 U/g protein), whereas the lowest mean was observed in the C. rotundicauda midgut tissue (12.51 U/g protein). While there was no significant difference in AKP level among different digestive tract tissues and between species (species: F = 0.411, p = 0.910; tissue: F = −0.089, p = 0.985; species × tissue: F = 0.809, p = 0.539), a significantly higher in serum AKP level was observed in T. tridentatus compared to that of C. rotundicauda (t = 4.871, p = 0.017).
For the GSH-Px activity, the serum level was in the range of 29.01–36.88 U/mL (Fig. 2), whereas the digestive tract level was in the range of 22.21–49.13 U/mg protein (Fig. 4). Similar to the AKP activity, the highest and lowest mean GSH-Px level was found in the hindgut (41.40 U/mg protein) and midgut tissues (27.31 U/mg protein) of T. tridentatus, respectively. The AKP level difference in serum between species was insignificant (t = −1.500, p = 0.231). No significant differences in GSH-Px activity levels were observed between species and among digestive tract tissues (species: F = 0.035, p = 0.854; tissue: F = 0.188, p = 0.941; species × tissue: F = 1.527, p = 0.245).
4 Discussion
4.1 Classification of Horseshoe Crab Hemocytes
Owing to the very limited studies on their circulatory system and cellular components, at present, there is no standardized classification scheme for horseshoe crab hemocytes. Armstrong (1979) examined the hemocytes in a freshly excised gill leaflet and suggests that, L. polyphemus hemolymph contained solely a single cell type, the granular amebocyte (also named as granulocyte) that showing three morphological states in vitro, including the contracted, flattened granular and flattened degranulated forms. Suhr-Jessen et al. (1989) found that, in addition to granulocytes, there was another distinct cell, the plasmatocyte, which comprised 1–3% of the hemocytes in L. polyphemus hemolymph. Compared to granulocytes, the plasmatocytes had an euchromatic nucleus, flattened and well-developed rough endoplasmic reticulum, a greater number of free ribosomes and mitochondria, but fewer, if any, large secretory granules in the cell (Suhr-Jessen et al. 1989). Coates et al. (2012) followed the hemocyte classification described in Armstrong (1979), and modified the morphological classification into granular-spherical, granular-flat, and dendritic-like forms.
For Asian species, Toh et al. (1991) examined the hemocytes in freshly prepared hemolymph from T. tridentatus using an electron microscopy and classified the hemocytes into two types, including granular and non-granular hemocytes, according to the number of granules present in the cells. The population density ratio of granular to non-granular hemocytes was 100: 1, in which the ratio was consistent to that reported in Suhr-Jessen et al. (1989). Toh et al. (1991) identified two types of granules in the granulocytes, L-granules and D-granules, with differences in their maximal diameter, cross-sectioned area, identified contents, electron density, and matrix characteristics. Kwan et al. (2014, 2015, 2018) followed the granulocyte classification scheme by Coates et al. (2012) and ignored the very low occurrence of non-granulocytes present in juvenile T. tridentatus hemolymph. Wu et al. (2015) classified the hemocytes in both T. tridentatus and C. rotundicauda species using light-scanning electron microscopy and particle size analyzer based on their cell size, morphology, cytoplasmic granule size and quantity, and nuclear-cytoplasmic ratio. Three types of hemocytes were identified, the significantly larger-sized granular cells with abundant granules, the semi-granular cells with lesser granules, and the significantly smaller-sized hyaline cells containing few granules but a significantly greater nuclear-cytoplasmic ratio. Their morphology was indistinguishable between species and sexes, but a significantly higher hemocyte count in females of both species. The granular cell was the major cell type in both sexes of the two species. Wu et al. (2019) re-examined the results using a transmission electron microscopy and found the consistently three cell types as described in Wu et al. (2015). However, the quantification of hemocytes by flow cytometry demonstrated that the hyaline cell was dominant in numbers that might be caused by the handling process.
In this study, the hemocytes of T. tridentatus and C. rotundicauda in hemolymph were stained by the Wright–Giemsa method and examined under a light microscopy. Consistent with most relevant reports in horseshoe crabs, the granulocyte was the major cell type for captive horseshoe crabs, in which their intracellular granules were in two different staining colors, the basophil granulocyte in blue and the eosinophil granulocyte in purple–red. Similar to the previous studies, we differentiated the granulocyte from non-granulocyte according to the number of cytoplasmic granules present in the hemocytes. The third identified hemocyte type was macrophage with a mixture of blue and red-stained intracellular granules. While the classification scheme and naming of horseshoe crab hemocytes varied among studies, the three cell types mentioned in Wu et al. (2015, 2019), according to our justifications, can be considered as the granulocytes (or amebocytes) under different morphological states (Coates et al. 2012; Kwan et al. 2014). It is also important to note that the hemocyte morphology can be heavily affected by the degranulation process. The degranulation in horseshoe crab hemocytes can be induced from increased temperature (Coates et al. 2012), captivity stress (Kwan et al. 2014), deteriorated water quality (Kwan et al. 2015, 2018), and anticoagulant formulation for bleeding (Sheikh et al. 2021).
Hemocytes play a primary role in the immune defenses of varying aquatic invertebrates (Buchmann 2014; Huang and Ren 2020). A spherical-granular state appears to be the optimum, most viable cell morphology in horseshoe crabs (Armstrong 1979; Hurton et al. 2005). Wu et al. (2019) also found that granular cells contained higher reactive oxygen species, phagocytosis, and non-specific esterase. Therefore, the total and differential hemocyte counts in horseshoe crabs are fundamental in assessing their physiological state and health status, which in turn, affects the resource sustainability for bleeding and the TAL/LAL production quality. Despite the fact that the total number of different hemocyte types was not quantified in this study, it was apparent that only a very low proportion of non-granulocytes and macrophages was observed surrounded by the basophil granulocytes. Based on the preliminary morphological observations of hemocytes in this study, it seems that there was no observable difference in our captive adult horseshoe crabs compared to that reported in wild-caught horseshoe crabs in the literature. The antimicrobial and clotting substances available in basophils and eosinophils of horseshoe crabs, as well as their implication to health status indication require further investigations.
4.2 Characterization of Enzymatic Activity in Different Tissues
The AKP and GSH-Px activities have been widely utilized to reflect non-specific immunological performance in aquatic invertebrates (Javahery et al. 2019; Qi et al. 2019; Amoah et al. 2020; Xu et al. 2020). AKP is an intrinsic plasma membrane enzyme available in the cell membranes of organisms (except some plants), which plays a key role in the degradation and transport of exogenous substances, such as proteins, carbohydrates, and lipids (Blasco et al. 1993). For marine invertebrates, AKP involves in the absorption of phosphorus and calcium in seawater, and also the formation of calcium phosphate and chitin (Xu et al. 2020). Dietary supplementations with immunostimulants and essential dietary elements (Javahery et al. 2019; Qi et al. 2019) as well as changes in environmental conditions (Pinoni et al. 2005; Li et al. 2008) were demonstrated to affect the AKP activities in aquatic invertebrates. For example, arginine was demonstrated to increase the AKP activities in juvenile Chinese mitten crab, Eriocheir sinensis. Similar observation was reported in Xu et al. (2020) that the addition of copper supplement to T. tridentatus diets for two weeks had significantly increased their plasma AKP activity level. Compared with the present findings, plasma AKP activity level in T. tridentatus under prolonged captivity (one year) was approximately four times higher than that reported in Xu et al. (2020), which is maintained in laboratory for totally two months without copper supplementation. Significantly higher plasma AKP level was also noted in T. tridentatus compared to C. rotundicauda in this study, which may be attributed to the considerable differences in their body size. However, the AKP levels in different digestive tissues were statistically similar in both Asian species.
The GSH-Px is a primary component in animal’s detoxification system by catalyzing hydrogen peroxide into water and inhibiting active oxygen and hydroxyl free radical productions (Moreira et al. 2016; Sui et al. 2017). Therefore, the GSH-Px activity can be regarded as an important indicator to reflect an organism’s antioxidant capacity. A variety of factors have been showed to influence the GSH-Px activity such as inflammation (Jones et al. 1997; Rogers et al. 2000), developmental differentiation Hayashibe et al. 1990; Bravard et al. 1999), and hormonal regulation (Dougall and Nick 1991; Sampath and Perez-Polo 1997; Sugino et al. 1998). Similar to the AKP level, plasma GSH-Px activity levels in T. tridentatus in this study were about five times greater than that described in Xu et al. (2020) under laboratory culture for two months. The difference may be resulted from culture conditions (Capparelli et al. 2019; Schvezov et al. 2019) and diets (Wang et al. 2019; Yang et al. 2019; Liu et al. 2020). For digestive tract tissues, in this study, the GSH-Px activity levels maintained in the range of 27–41 U/mg protein regardless of species and tissue types. The GSH-Px activity levels in horseshoe crabs, in general, were higher than that of intestine tissues from other smaller-sized aquatic invertebrates, such as whiteleg shrimp, Litopenaeus vannamei (7–8 U/mg protein, Duan et al. 2018), and juvenile Chinese mitten crab, Eriocheir sinensis (8–15 U/mg protein, Lu et al. 2019). While the horseshoe crab baseline data regarding the non-specific immunological parameters are very limited, the present findings may be useful in further immunological studies for developing health monitoring system and improving sustainable bleeding practices.
References
K. Amoah, Q.C. Huang, X.H. Dong, B.P. Tan, S. Zhang, S.Y. Chi, Q.H. Yang, H.Y. Liu, Y.Z. Yang, Paenibacillus polymyxa improves the growth, immune and antioxidant activity, intestinal health, and disease resistance in Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Aquaculture 518, 734563 (2020)
P. B. Armstrong, Motility of the Limulus blood cell. J. Cell Sci. 37(1), 169–180 (1979)
F.C. Bang, A bacterial disease of Limulus polyphemus. Bull. Johns Hopkins Hosp. 98, 325–351 (1956)
J. Blasco, J. Puppo, M.C. Sarasquete, Acid and alkaline phosphatase activities in the clam Ruditapes philippinarum. Mar. Biol. 115(1), 113–118 (1993)
M.L. Botton, The ecological importance of horseshoe crabs in estuarine and coastal communities: A review and speculative summary, in Biology and Conservation of Horseshoe Crabs, ed. by J. T. Tanacredi, M. L. Botton, D. R. Smith, (Springer, New York, 2009), pp. 45–63.
M.M. Bradford, Rapid and sensitive method for quantification of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976)
A. Bravard, F. Petridis, C. Luccioni, Modulation of antioxidant enzymes p21WAF1 and p53 expression during proliferation and differentiation of human melanoma cell lines. Free Radic. Biol. Med. 26, 1027–1033 (1999)
K. Buchmann, Evolution of innate immunity: Clues from invertebrates via fish to mammals. Front. Immunol. 5, 459 (2014)
M.V. Capparelli, P.K. Gusso-Choueri, D.M. de Souza Abessa, J.C. McNamara, Seasonal environmental parameters influence biochemical responses of the fiddler crab Minuca rapax to contamination in situ. Comp. Biochem. Physiol. C 216, 93–100 (2019)
C.P. Chen, H.Q. Fan, Y.Y. Liao, G.L. Qiu, H.L. Hsieh, W.Y. Lin, Horseshoe crab, the living fossil animal facing threats to survival. Science 67(3), 60–62 [in Chinese] (2015)
L. Cheng, Y. Chen, Y.Y. Zheng, Y. Zhan, H. Zhao, J.L. Zhou, Bioaccumulation of sulfadiazine and subsequent enzymatic activities in Chinese mitten crab (Eriocheir sinensis). Mar. Pollut. Bull. 121(1–2), 176–182 (2017)
C.J. Coates, E. Bradford, C.A. Krome, J. Nairn, Effect of temperature on biochemical and cellular properties of captive Limulus polyphemus. Aquaculture 334–337, 30–38 (2012)
W.C. Dougall, H.S. Nick, Manganese superoxide dismutase: A hepatic acute phase protein regulated by interleukin-6 and glucocorticoids. Endocrinology 129, 2376–2384 (1991)
Y. Duan, Y. Wang, J. Zhang, D. Xiong, Elevated temperature disrupts the mucosal structure and induces an immune response in the intestine of whiteleg shrimp Litopenaeus vannamei (Boone, 1931) (Decapoda: Dendrobranchiata: Penaeidae). J Crustacean Biol 38(5), 635–640 (2018)
K. Dunning, A.O. Safo, The ultimate Wright-Giemsa stain: 60 years in the making. Biotech. Histochem. 86(2), 69–75 (2011)
G. Gauvry, Current horseshoe crab harvesting practices cannot support global demand for TAL/LAL: The pharmaceutical and medical device industries’ role in the sustainability of horseshoe crabs, in Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management, ed. by R. H. Carmichael, M. L. Botton, P. K. S. Shin, S. G. Cheung, (Springer, Cham, 2015), pp. 475–482
H. Hayashibe, K. Asayama, K. Dobashi, K. Kato, Prenatal development of antioxidant enzymes in rat lung, kidney, and heart: Marked increase in immunoreactive superoxide dismutases, glutathione peroxidase and catalase in the kidney. Pediatr. Res. 27, 472–475 (1990)
S. Hong, X. Zhang, Y. Zhao, Y. Xie, Y. Zhang, H. Xu, Effect of sediment type on growth and survival of juvenile horseshoe crabs (Tachypleus tridentatus), ed. by J. T. Tanacredi, M. L. Botton, D. R. Smith, pp. 535–540 (Springer, New York, 2009)
Y. Huang, Q. Ren, Research progress in innate immunity of freshwater crustaceans. Dev. Comp. Immunol. 104, 103569 (2020)
L.V. Hurton, J.M. Berkson, S.A. Smith, Selection of a standard culture medium for primary culture of Limulus polyphemus amebocytes. Vitro Cell Dev. Biol-Anim. 41(10), 325–329 (2005)
S. Iwanaga, The molecular basis of innate immunity in the horseshoe crab. Curr. Opin. Immunol. 14(1), 87–95 (2002)
M.J. James-Pirri, P.A. Veillette, A.S. Leschen, Selected hemolymph constituents of captive, biomedically bled, and wild caught adult female American horseshoe crabs (Limulus polyphemus). Mar. Freshw. Behav. Phy. 45(4), 281–289 (2012)
S. Javahery, A. Noori, S.H. Hoseinifar, Growth performance, immune response, and digestive enzyme activity in Pacific white shrimp, Penaeus vannamei Boone, 1931, fed dietary microbial lysozyme. Fish Shellfish Immunol. 92, 528–535 (2019)
B.A. John, B.R. Nelson, H.I. Sheikh, S.G. Cheung, Y. Wardiatno, B.P. Dash, K. Tsuchiya, Y. Iwasaki, S. Pati, A review on fisheries and conservation status of Asian horseshoe crabs. Biodivers. Conserv. 27(14), 3573–3598 (2018)
P.L. Jones, D. Ping, J.M. Boss, Tumor necrosis factor alpha and interleukin-1b regulate the murine manganese superoxide dismutase gene through a complex intronic enhancer involving C/EBP-b and NF-jB. Mol. Cell. Biol. 17, 6970–6981 (1997)
B.K.Y. Kwan, A.K.Y. Chan, S.G. Cheung, P.K.S. Shin, Responses of growth and hemolymph quality in juvenile Chinese horseshoe crab Tachypleus tridentatus (Xiphosura) to sublethal tributyltin and cadmium. Ecotoxicology 24, 1880–1896 (2015)
B.K.Y. Kwan, A.K.Y. Chan, S.G. Cheung, P.K.S. Shin, Hemolymph quality as indicator of health status in juvenile Chinese horseshoe crab Tachypleus tridentatus (Xiphosura) under laboratory culture. J. Exp. Mar. Biol. Ecol. 457, 135–142 (2014)
B.K.Y. Kwan, V.K.Y. Un, S.G. Cheung, P.K.S. Shin, Horseshoe crabs as potential sentinel species for coastal health: Juvenile haemolymph quality and relationship to habitat conditions. Mar. Freshw. Res. 69(6), 894–905 (2018)
J.P. Lallès, Intestinal alkaline phosphatase: Multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr. Rev. 68, 323–332 (2010)
K. Laurie, C.P. Chen, S.G. Cheung, V. Do, H.L. Hsieh, A. John, F. Mohamad, S. Seino, S. Nishida, P. Shin, M.C. Yang, Tachypleus tridentatus. The IUCN Red List of Threatened Species 2019: E.T21309A133299524 (9 May 2019). https://doi.org/10.2305/IUCN.UK.2019-1.RLTS.T21309A133299524.en
J. Levin, F.B. Bang, The role of endotoxin in the extracellular coagulation of limulus blood. Bull. Johns Hopkins Hosp. 115, 265–274 (1964)
N. Li, Y. Zhao, J. Yang, Effects of water-borne copper on digestive and metabolic enzymes of the giant freshwater prawn Macrobrachium rosenbergii. Arch. Environ. Contam. Toxicol. 55(1), 86–93 (2008)
Y.H. Li, X.Y. Xie, K.Y. Kwan, Endangered status and declaration on conservation of the “living fossil” Tachypleus tridentatus. Wetl. Sci. 16(5), 690–692 (2018) [in Chinese with English abstract]
Y.Y. Liao, H.L. Hsieh, S.Q. Xu, Q.P. Zhong, J. Lei, M.Z. Liang, H.Y. Fang, L.L. Xu, W.Y. Lin, X.B. Xiao, C.P. Chen, Wisdom of Crowds reveals decline of Asian horseshoe crabs in Beibu Gulf, China. Oryx 53(2), 222–229 (2019)
J.D. Liu, W.B. Liu, D.D. Zhang, C.Y. Xu, C.Y. Zhang, X.C. Zheng, C. Chi, Dietary reduced glutathione supplementation can improve growth, antioxidant capacity, and immunity on Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 100, 300–308 (2020)
J. Lu, C. Qi, S.M. Limbu, F. Han, L. Yang, X. Wang, J.G. Qin, L. Chen, Dietary mannan oligosaccharide (MOS) improves growth performance, antioxidant capacity, non-specific immunity and intestinal histology of juvenile Chinese mitten crabs (Eriocheir sinensis). Aquaculture 510, 337–346 (2019)
A. Moreira, E. Figueira, A.M.V.M. Soares, R. Freitas, The effects of arsenic and seawater acidification on antioxidant and biomineralization responses in two closely related Crassostrea species. Sci. Total Environ. 545, 569–581 (2016)
S.A. Pinoni, A.L. Goldemberg, A.L. Mañanes, Alkaline phosphatase activities in muscle of the euryhaline crab Chasmagnathus granulatus: Response to environmental salinity. J. Exp. Mar. Biol. Ecol. 326(2), 217–226 (2005)
Fauziyah, A.I. Purwiyanto, W.A. Putri, F. Agustriani, A.Z. Mustopa, The first investigation record of threatened horseshoe crabs in the Banyuasin estuarine, South Sumatra, Indonesia. Ecol. Montenegrina 24, 17–24 (2019)
C. Qi, X. Wang, F. Han, Y. Jia, Z. Lin, C. Wang, J. Lu, L. Yang, X. Wang, E. Li, J.G. Qin, Arginine supplementation improves growth, antioxidant capacity, immunity and disease resistance of juvenile Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 93, 463–473 (2019)
R.J. Rogers, S.E. Chesrown, S. Kuo, J.M. Monnier, H.S. Nick, Cytokine-inducible enhancer with promoter activity in both the rat and human manganese-superoxide dismutase genes. Biochem. J. 347, 233–242 (2000)
D.M. Rudkin, G.A. Young, Horseshoe crabs–an ancient ancestry revealed, in Biology and Conservation of Horseshoe Crabs, ed. by J. T. Tanacredi, M. L. Botton, D. R. Smith, pp. 25–44 (Springer, New York, 2009)
D. Sampath, R. Perez-Polo, Regulation of antioxidant enzyme expression by NGF. Neurochem. Res. 22, 351–362 (1997)
N. Schvezov, G.A. Lovrich, F. Tapella, M. Gowland-Sainz, M.C. Romero, Effect of the temperature of air exposure on the oxidative stress status of commercial male southern king crab Lithodes santolla. Fish. Res. 212, 188–195 (2019)
H.I. Sheikh, B.A. John, S.J. Ichwan, B.Y. Kamaruzzaaman, Effect of prolonged captivity on the hemolymph profile of Tachypleus gigas using the various anticoagulant formulations. Aquacult. Rep. 20, 100760 (2021)
D.R. Smith, H.J. Brockmann, M.A. Beekey, T.L. King, M.J. Millard, J. Zaldívar-Rae, Conservation status of the American horseshoe crab, (Limulus polyphemus): A regional assessment. Rev. Fish. Biol. Fisher. 27(1), 135–175 (2017)
N. Sugino, M. Hirosawa-Takamori, L. Zhong, C.M. Telleria, K. Shiota, G. Gibori, Hormonal regulation of copper-zinc superoxide dismutase and manganese superoxide dismutase messenger ribonucleic acid in the rat corpus luteum: Induction by prolactin and placental lactogens. Biol. Reprod. 59, 599–605 (1998)
P. Suhr-Jessen, L. Baek, P.P. Jakobsen, Microscopical, biochemical, and immunological studies of the immune defense system of the horseshoe crab, Limulus polyphemus. Biol. Bull. 176(3), 290–300 (1989)
Y.M. Sui, M.H. Hu, Y.Y. Shang, F.L. Wu, X.Z. Hu, S. Dupont, Y.J. Wang, Antioxidant response of the hard-shelled mussel Mytilus coruscus exposed to reduced pH and oxygen concentration. Ecotoxicol. Environ. Saf. 137, 94–102 (2017)
Y. Toh, A. Mizutani, F. Tokunaga, T. Muta, S. Iwanaga, Morphology of the granular hemocytes of the Japanese horseshoe crab Tachypleus tridentatus and immunocytochemical localization of clotting factors and antimicrobial substances. Cell Tissue Res. 266, 137–147 (1991)
X. Wang, Z. Shen, C. Wang, E. Li, J.G. Qin, L. Chen, Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir sinensis under nitrite stress. Fish Shellfish Immunol. 87, 22–31 (2019)
Y.H. Wei, H.C. Lee, Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp. Biol. Med. 227, 671–682 (2002)
F.L. Wu, X.Z. Huang, Q.Z. Li, M.H. Hu, W.Q. Lu, Y.J. Wang, Classification and characterization of hemocytes between Tachypleus tridentatus and Carcinoscorpius rotundicauda. Acta Hydrobiol. Sin. 39(6), 1169–1176 (2015) [in Chinese with English abstract]
F.L. Wu, Z. Xie, M.Y. Yan, Q.Z. Li, J. Song, M.H. Hu, Y.J. Wang, Classification and characterization of hemocytes from two Asian horseshoe crab species Tachypleus tridentatus and Carcinoscorpius rotundicauda. Sci. Rep. 9(1), 7095 (2019)
Z. Xu, Y. Wang, Y. Gul, Q. Li, J. Song, M. Hu, Effects of copper supplement on the immune function and blood-chemistry in adult Chinese horseshoe crab Tachypleus tridentatus. Aquaculture 515, 734576 (2020)
M.Y. Yan, W. Yu, H.X. Gu, Y.J. Wang, Q.Z. Li, Z.F. Fan, B. Sun, M.H. Hu, Current situation of Tachypleus amebocyte lysate industry in China and relevant suggestions. J. Biol. 35(2), 88–91 (2018) [in Chinese with English abstract]
C. Yang, R. Hao, X. Du, Q. Wang, Y. Deng, R. Sun, Response to different dietary carbohydrate and protein levels of pearl oysters (Pinctada fucata martensii) as revealed by GC–TOF/MS-based metabolomics. Sci. Total Environ. 650, 2614–2623 (2019)
J.H. Zhu, Z. Wu, B.B. Feng, S.S. Deng, W.Q. Zhen, Y.Y. Liao, X.Y. Xie, K.Y. Kwan, Global conservation status of Tachypleus tridentatus and its introspection. Biodivers. Sci. 28(5), 621–629 (2020) [in Chinese with English abstract]
Acknowledgements
This work was supported by “Special Fund for Basic Scientific Research of Central Public Research Institutes” of the South China Sea Fisheries Research Institute, Chinese Academy of Fishery (2019TS21), Guangxi BaGui Youth Scholars Programme, and Guangxi Recruitment Program of 100 Global Experts.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Xie, X., Kwan, K.Y., Zhong, J., Xie, M., Ye, G., Bao, Y. (2022). Preliminary Characterization of Hemocyte and Immunity of Asian Horseshoe Crabs, Tachypleus tridentatus, and Carcinoscorpius rotundicauda in Captivity. In: Tanacredi, J.T., et al. International Horseshoe Crab Conservation and Research Efforts: 2007- 2020. Springer, Cham. https://doi.org/10.1007/978-3-030-82315-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-82315-3_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-82314-6
Online ISBN: 978-3-030-82315-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)