Abstract
Industrial wastes are widely involved in the building ceramic production. Sewage sludge ashes (SSA) are promising secondary sources for building ceramics production. According to modern concepts, formation processes of ceramic structure can be controlled by adjusting ceramic mass compositions by additives of nanosized particles. In this study, the filtrate of drinking water treatment sludge obtained at the water treatment facilities of Novosibirsk, which is a silicate sol with nanodispersed particles, was used as an additive. Purpose of the study was to assess the effect of silicate sol from drinking water treatment sludge on sewage sludge ash ceramics phase composition and microstructure. Using the thermal analysis, X-ray phase analysis and electron microscopy, the drinking water treatment sludge filtrate additive effect on SSA-clay samples phase composition and microstructure was established. It was found that ceramic obtained by sintering SSA with drinking water treatment sludge filtrate additive is characterized by a matrix microstructure.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
More and more industrial wastes are involved in the building ceramic production. Sewage sludge ashes (SSA) are promising secondary sources for building ceramics production.
SSA properties were studied in a number of works [1,2,3], but the results were not widely used in building ceramic industry, because SSA ceramic products not always have the necessary strength characteristics. It is due to the SSA chemical composition, which affects to the phase composition formation of ceramic.
Ceramics phase composition can be controlled by adjusting the ceramic mass composition using special additives. The most suitable modifiers for clay minerals structures are nanosized oxides of silicon and aluminum, which are realized in hydrosols [4].
Earlier in [5], it was shown that the drinking water treatment sludge filtrate, obtained after high-speed filters in the Novosibirsk water utility filtering station, is a sol of a silicate composition with a nanoscale particle size.
The purpose of study was to assess the drinking water treatment sludge filtrate effect on the SSA-clay ceramics phase composition and structure formation.
2 Materials and Methods of Research
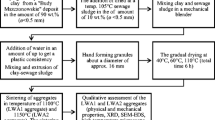
Kamenskoye deposit (Novosibirsk region) montmorillonite-hydromica clay was used in this study.
Sewage sludge was obtained from Novosibirsk wastewater treatment plant (map No. 39). The sludge was burned in laboratory furnace at temperatures of 850 °C. Drinking water treatment sludge filtrate, obtained after high-speed filters in the Novosibirsk water utility filtering station and separated from the coarse fraction, was used as a modifying additive in the study.
Ceramic mixtures compositions are presented in Table 1.
Weighed portions of the mixtures with different additives amounts were mixed with water or drinking water treatment sludge filtrate. Samples were dried at 110 °C. Then the SSA-clay samples were sintered at 1100 °C with holding at a maximum temperature for 1 h.
Differential thermal analysis (DTA) was performed to identify the phase formation features of the SSA-clay samples. X-ray phase analysis (XPA), which was performed on a Bruker D8 Advance diffractometer using Cu-Kα radiation, was used to determine the phase composition of SSA-clay ceramic samples. The PDF2 database with the Search-Match shell was used to identify the obtained diffraction patterns.
Element maps and microstructure images of SSA-clay ceramic samples were obtained using Hitachi TM 3000 electron microscope with an energy dispersive analyzer.
3 Results and Discussion
The endothermic effect at 50–150 °C temperature range is due to the sorbed water loss by clay minerals. In this case, there is a loss of mass—4.5%. The weight loss occurs at a much lower rate with further mixture heating. Heat absorption is caused by the release of chemically bound water by clay minerals in the 300–500 °C range. Kaolinite is initially dehydrate, turning into metakaolinite, and then decompose into oxides at 750–900 °C [6] (Fig. 1).
The endothermic effect at 700 °C is associated with the montmorillonite crystal structure destruction. According [7], calcium carbonate dissociates in the 770–1010 °C temperature range.
Exothermic effects on the heating curve are due to new crystalline phases formation. The total weight loss for the test sample was 10.8%.
According to the XPA data (Fig. 2), for all SSA-clay samples after sintering at 1100 °C, the main identified phases are anorthite (3.10; 4.05 A0), quartz (3.35 A0) and hematite (2.7 A0).
XPA diffraction patterns of ceramic samples: a—50% SSA: 50% clay: 0% drinking water treatment sludge filtrate; b—50% SSA: 50% clay: 0.05% drinking water treatment sludge filtrate; c—50% SSA: 50% clay: 0.1% drinking water treatment sludge filtrate; d—50% SSA: 50% clay: 0.25% drinking water treatment sludge filtrate
The anorthite (CaO · Al2O3 · SiO2) formation is due to solid-phase reaction between amorphous γAl2O3 formed in SSA from aluminum oxychloride and calcium silicate.
Another possible anorthite formation mechanism may be associated with the reaction between amorphous silica and calcium oxide. According study [8], kaolinite dehydration calcium carbonate dissociation are promoted by the anorthite crystallization in clay. The reaction equation has the form (Eq. 1):
The phase composition results (Table 2) indicate to increase anorthite content with adding drinking water treatment sludge filtrate.
The microstructure of all samples is characterized by uniform pores and crystalline quartz compounds distribution. At the same time, SSA-clay samples with drinking water treatment sludge filtrate addition are still noticeably different in relative position of crystalline and amorphous phases.
The SSA-clay with drinking water treatment sludge filtrate additive samples microstructure feature is matrix microstructure. The spatially arranged matrix microstructure has phase composition differences between filler and binder, which confirmed by the electron microscopy data (Fig. 3d, f). Matrix formed from clay particles is a “binder”. “Filler” formed from quartz grains contained in sewage sludge ash. It is important to note that matrix include amorphous and crystalline anorthite phase. Anorthite crystals reinforce matrix SSA-clay samples microstructure, which provides higher strength and frost resistance.
SSA-clay samples with drinking water treatment sludge filtrate additive are characterized by increased density from 2.15 to 2.21 g/cm3 and compressive strength from 27.60 to 37.15 MPa (Table 3), which may be due to their matrix microstructure and increased anorthite phase content (from 64 to 68%).
4 Conclusions
DTA, XPA and electron microscopy results allow us to conclude that:
-
1.
SSA-clay samples phase composition formation is determined by calcium ions diffusion into the metakaolinite structure and anorthite crystallization from melt enriched with aluminum, calcium and silicon oxides.
-
2.
SSA-clay samples with drinking water treatment sludge filtrate additive are characterized by increased density and compressive strength, which may be due to their matrix microstructure and increased anorthite phase content.
References
Turovskij IS (1988) Sewage sludge treatment. Construction publishing
Hakimov FI (1999) Recommendations for the disposal of urban treatment facilities sludge. The Russian State Committee of Ecology
Shakhov SA, Klyuchnikova NS, Kozhemyachenko AS (2014) Composition and technological properties of sewage sludge and sewage sludge ashes. Univ News Constr 11(671):103–113
Zhenzhurist IA, Zaripova VM, Mubarakshina LF, Hozin VG (2010) The effect of silicon and aluminum oxides hydrosols nanosized particles on the structure formation of clay minerals in an aqueous medium. Glass Ceram 7:28–32
Shahov SA, Nikolaev NYu, Rudaya TL (2018) Potential of drinking water treatment sludge as a raw material component of building ceramics. Siberian Transp Univ Bull 1:61–67
Kanygina ON, Chetverikova AG, Lazarev DA, Sal’nikov EV (2010) High-temperature phase transformations in iron-bearing clays of the Orenburg region. Bull OSU 6(112):113–118
Buruchenko AE, Haruk GN, Musharapova SI, Sergeev AA (2018) The calcium carbonate influence on the phase composition formation of ceramics based on low-melting and refractory clays during firing. Univ News Constr 2:21–28
Levickij IA, Klimov YuA (2005) Structuring of densely sintered ceramic for domestic use. Glass Ceram 6:32–36
Acknowledgements
The reported study was funded by RFBR, project number 20-33-90197.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Shakhov, S.A., Nikolaev, N.Y. (2022). Phase Composition of Sewage Sludge Ash Ceramics Modified by Drinking Water Treatment Sludge Filtrate. In: Klyuev, S.V. (eds) Digital Technologies in Construction Engineering. Lecture Notes in Civil Engineering, vol 173. Springer, Cham. https://doi.org/10.1007/978-3-030-81289-8_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-81289-8_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-81288-1
Online ISBN: 978-3-030-81289-8
eBook Packages: EngineeringEngineering (R0)