Abstract
The frequent exposure of plants to several environmental constraints (abiotic and biotic stress) limit plant growth and yield globally. These constraints are managed by plants through morphological, physiological and molecular alteration to resume the loss underway. Amongst, the priming with chemicals including GABA is a promising approach. GABA (γ-aminobutyric acid) is a non-protein amino acid produced through GABA-shunt, and usually accumulates in several plant species when subjected to any environmental stress (salt, drought, heat, etc.). Its accumulation or exogenous application leads to adjustments at many levels under stress conditions, to maintain the usual plant growth and development and therefore may provide better outcome in terms of productivity. The present chapter offers understanding of GABA metabolism and its significance towards promoting abiotic stress tolerance in plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
The plants being sessile are frequently exposed to various environmental constraints both naturally or under agricultural set-up. These limitations are majorly unpredictable and lead to significant loss in crop productivity. Amongst such constraints, the abiotic factors/stress (salinity, drought, flooding, heat, cold, freezing, excess light, UV radiation, and heavy metal toxicity) play higher participation and majorly limit the global crop yields (Wang et al. 2003; Jain 2013). This majorly involves oxidative stress mediated by ROS, which may impact the plant by regulating several developmental features (Mishra et al. 2017). In certain instances, abiotic stress also provides ways to invite several biotic stress (Vijayakumari et al. 2016), thereby further promotes crop losses. To cope with these limitations, plants have developed methods that mainly involve participation of stress-related genes. Such genes remain silent during the normal course of plants’ life and get up-regulated during stress conditions (Gao et al. 2007, Atkinson and Urwin 2012). Despite their direct relevance in stress tolerance, the transgenic for such genes many times exhibited compromise of desired traits; therefore, the development of methodologies that can better address the plant stress tolerance along with maintaining plant productivity is essentially required.
Plant priming (sensitization or hardening) is considered as one of the effective strategies which activate faster and/or stronger protective responses, when stress (biotic or abiotic) pressure is encountered. The strategy is originally devised for biotic stress, where a non-pathogenic microbe can boost plant immunity, thereby providing a strong defence, when the plant encounters any pathogenic agent in subsequent life. In recent past, the exogenous application of chemicals that can mitigate stress conditions (chemical priming) was found effective to better manage plants encountered to stress. In the ‘primed’ state, plants attain a unique physiological condition, which generates mild stress, and thus let plants to withstand further strong stress conditions (Savvides et al. 2016). Some of the important priming agents are sodium nitroprusside, hydrogen peroxide, sodium hydrosulfide, melatonin, polyamines, GABA, BABA, etc. (Vijayakumari et al. 2016; Savvides et al. 2016).
The γ-aminobutyric acid (4-aminobutyric acid, GABA), is a non-protein amino acid, which possesses an amino group at γ-carbon rather than α-carbon, therefore does not integrate into proteins. It contributes significantly to the free amino acid pool and exists in unbound form (Shelp et al. 1999). It exhibits zwitterionic features at physiological pH, high solubility in water and can attain many conformations in solution, including cyclic structure like proline (Christensen et al. 1994). The metabolism behind GABA is known as GABA shunt which bypasses two steps of the tricarboxylic acid cycle (Bouche and Fromm 2004). GABA has a wide distribution in prokaryotes and eukaryotes. The discovery of GABA in plants was reported by Steward et al. (1949); however, its occurrence is conserved from bacteria to plants and to vertebrates (Bouche and Fromm 2004). In animals, the study mainly focuses GABA as a signalling molecule and found to be accumulated in brain and functions in neurotransmission (Kinnersley and Turano 2000; Bouche and Fromm 2004). In crayfish stretch receptor neurons, it was reported to suppress impulses (Awapara et al. 1950; Elliott and Jasper 1959).
Though the contribution of GABA as a signalling molecule was considered quite early in animals, in plants, until recently, it was generally considered as a carbon-nitrogen metabolite (Shelp et al. 1999; Bouche et al. 2003; Bouche and Fromm 2004). In plants, the recent outlook suggested it as an endogenous signalling molecule for plant growth regulation and plant development (Carillo 2018). In plants, the accumulation of GABA is noticed under environmental stress conditions which facilitate plant chemical response to mitigate stress. Apart, it also has multiple functions under non-stressed conditions. It serves as a key metabolite of nitrogen metabolism and involves both primary and secondary metabolic pathways. Besides, it also provides source of carbon skeleton through GABA shunt for further down-stream metabolism (Ramos-Ruiz et al. 2019). Two mechanisms have been noticed for stress-induced GABA accumulation in plants viz. cytosolic acidification and Ca2+ rise. It has been noticed that stress resulted in cytosolic acidification which induces low pH-dependent activation on glutamate decarboxylase and therefore GABA biosynthesis and accumulation. Such acidic conditions are predominantly found under oxygen-deprived conditions as generated during flood stress. Further, other stresses (viz, cold, heat, salt, etc.) subsequently increase Ca2+ level in the cellular environment. These high cytosolic Ca2+ levels activate calmodulin-dependent glutamate decarboxylase activity and GABA biosynthesis (Kinnersley and Turano 2000).
The early decades of GABA research mostly considered it as metabolite, though argue exist regarding its functioning as signalling molecules (Bouche and Fromm 2004; Bown and Shelp 2016). However, there are certain observations that backed the signalling feature of GABA as highlighted in a review (Ramesh et al. 2015), which include features like rapid increase in response to biotic and abiotic stress, gradient of GABA concentration across plant tissues, inter- and intracellular compartmentation of GABA metabolism, discovery of GABA-binding site in plant cell membrane, and GABA-regulated ion channels in plants. Under several abiotic (hypoxia, heat, cold, drought, mechanical wounding) or biotic stress (wounding due to herbivory and infection), the quick accumulation of GABA was noticed (Shelp et al. 1999; Shelp et al. 2012). The accumulation promotes plant growth and can alleviate stress by up-regulating the anti-oxidant defence systems. Its metabolism is also involved in the nitrogen recycling and reallocation during leaf senescence in response to abiotic stress (Jalil et al. 2017; Jalil and Ansari 2020). Considering the diverse aspects of GABA in plant stress management, present chapter will provide judicial compilation on the representative studies of GABA as a stress alleviator and promises to combat the adverse situation.
7.2 GABA Shunt and GABA Metabolism
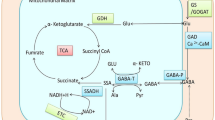
The biogenesis or metabolism of GABA involves a short pathway referred as GABA shunt which bypasses two steps of tricarboxylic (citric) acid cycle as mentioned in Fig. 7.1. Compared to TCA cycle, the shunt provides less energy as it directly converts α-ketoglutarate to succinate, and therefore does not produce high-energy molecule viz., reduced nicotinamide adenine dinucleotide phosphate (NADH) molecule and guanosine triphosphate (GTP) molecule (Singh and Roychoudhary 2020). The precursor molecule of this shunt is α-ketoglutarate, which in mitochondria undergoes oxidative deamination and is converted to glutamate by glutamate dehydrogenase (GDH). Glutamate is then transported to cytosol and subsequently processed by GABA shunt enzymes.
Schematic representation of the metabolism of GABA via GABA-shunt and citric acid cycle regulation; enzymes involved are indicated in green bold, reversible reactions in arrow with both heads. Other abbreviations include GDH, glutamate dehydrogenase; GAD, glutamate decarboxylase; GABA-TP, GABA: pyruvate transaminase; GABA-TK, GABA: α-ketoglutarate transaminase; SSADH, succinic semi-aldehyde dehydrogenase
The metabolism further involves participation of enzymes, comprising of one cytosolic (Glutamate decarboxylase, GAD) and two mitochondrial (GABA transaminase, GABA-T and Succinic semi-aldehyde dehydrogenase, SSADH) enzymes. The first enzyme (GAD) converts glutamate to GABA, along with a co-product carbon dioxide. In Arabidopsis, it was reported that GAD isoforms are expressed in tissue-dependent manner and their activity is modulated by Ca2+–CaM (Zik et al. 1998a, b). However, this feature of Ca2+–CaM is not mandatory as in rice, here GAD isoform lacks CaM-binding domain (Akama et al. 2001).
The GABA is then transported (through mediation of gamma-aminobutyric acid permease, GABAP; Michaeli et al. 2011) and metabolized in mitochondria to produce succinic semi-aldehyde (SSA). The GABA-T (GABA transaminase) facilitates this conversion, using either α-ketoglutarate (GABA-TK) or pyruvate (GABA-TP) as amino acid acceptors in plants, producing glutamate or alanine, respectively (Shelp et al. 1999). The succinic semi-aldehyde is then converted to succinic acid by the activity of succinic semi-aldehyde dehydrogenase (SSADH) (Ham et al. 2012). The association of GABA shunt with tricarboxylic acid cycle links carbon and nitrogen metabolism in plants. Beside this, SSA can also be transported to cytosol, where it produces γ-hydroxybutyric acid (GHB), through the activity of succinic semi-aldehyde reductase (SSR) (Singh and Roychoudhary 2020). Addition to this polyamine (spermidine and putresscine) catabolism and proline under oxidative stress can also produce GABA (Fait et al. 2008; Signorelli et al. 2015).
7.3 GABA: Significance Under Abiotic Environmental Constraints
During environment stress such as drought (Hanower and Brzozowsak 1975), heat (Mayer et al. 1990), cold (Wallace et al. 1984), there is high production of GABA; the intensity of increase is so high, as the cellular level of this non-protein amino acid exceeds the amino acid involved in protein synthesis (Shelp et al. 1999; Kinnersley and Turano 2000). It was observed that large amount of GABA is produced during biotic and abiotic stress and its metabolism was found connected with many features viz., C:N balance, regulation of cytosolic pH, osmoregulation, defence against oxidative stress, etc. (Bouche and Fromm 2004; Ham et al. 2012).
The endogenous production of GABA or its exogenous application has very high implication on plant stress mitigation. During this, the GABA is proposed to function as signalling molecule and found associated with nitrogen metabolism, cytosolic pH regulation, and offer protection against oxidative damage generated in response to several abiotic stresses (AL-Quraan et al. 2013). The priming using different chemicals has been observed as an effective strategy for the enhancement of abiotic stress tolerance. Vijayakumari et al. (2016) has recently reviewed the priming potential for non-protein amino acid-GABA along with BABA (β-amino butyric acid). Rapid γ-Aminobutyric acid (GABA) accumulation was observed, when plants are exposed to stress, which either exhibit the metabolism regulation in response to stress or an adaptive response for stress mitigation (Bown and Shelp 2016).
Further, the exogenous GABA application bestows stress tolerance by modulation of the genes associated with plant signalling, transcriptional regulation, hormone biosynthesis, reactive oxygen species production, and polyamine metabolism (Podlešáková et al. 2019). The accumulation and metabolism of GABA itself are regulated by several other biological molecules. Many evidence has also suggested the cross-talk between GABA and polyamines/ hormones (viz. abscisic acid, cytokinins, auxins, gibberellins and ethylene), where one affects the signalling pathway and metabolism of others (Podlešáková et al. 2019). Recently, the contribution of GABA in alleviation of salts stress, salinity-alkalinity stress, drought stress, heat stress, chromium stress etc., has been reported in a number of studies using different plants (Table 7.1). Few of the relevant stress studies are as follows:
7.3.1 Salt Stress
The salinity in soil has been reported to severely devastate plant growth, thereby crop productivity, due to its negative impact on several metabolic and physiological processes. This impact mostly includes photosynthesis inhibition, ROS, and other metabolism. It has been reported that in salt-sensitive variety, there is inhibition of growth and development, reduction in respiration, photosynthesis, and protein synthesis (Parida and Das 2005; Munns and Tester 2008; Hussain et al. 2013). In addition to the above effects, the salt stress also causes generation of reactive oxygen species in chloroplast and mitochondria that lead to oxidative damage in plants (Masood et al. 2006). To deal with the stress, plant produces various metabolites that offer stress tolerance including GABA. Like other stresses, the GABA accumulates during salt stress, which maintains the pH and carbon/nitrogen metabolism for Kreb’s cycle and scavenges free radicals through anti-oxidant systems (Jalil and Ansari 2020). Jalil and Ansari (2020) have described the salt tolerance mechanism by GABA, which includes promotion of photosynthesis and chlorophyll fluorescence, regulation of oxidative stress, and up-regulation of anti-oxidant activity and osmotic regulation through osmolyte accumulation.
The exogenous GABA application exhibited improvement in photosynthetic capacity and anti-oxidant enzyme activities and decreased MDA content and electrolyte conductivity, thereby mitigating the impact of salinity on the wheat seedlings (Li et al. 2016). The exogenous root-drenching-based GABA application to salt-stressed (moderate-150 mM to severe- 300 mM) maize seedlings exhibited an increase in endogenous GABA concentration, improves seedling growth, and demonstrates reduced GAD activity (Wang et al. 2017a, b). The exogenous applications offered attributes such as alleviation of membrane damage, accumulation of proline and soluble sugars in leaves, reduction in water loss, along with reduced oxidative damage (particularly of superoxide anion (O2·−) and malondialdehyde content) and increased anti-oxidant enzyme activity. Thus, the exogenous application of GABA enhanced the salt tolerance of maize seedlings (Wang et al. 2017a, b). Under saline condition, GABA improves seed germination and plant growth in lettuce, by reducing the effects of salt stress on photosynthetic performance and salt stress–induced oxidative stress (Kalhor et al. 2018). In Triticum aestivum and Hordeum vulgare, the abundance of GABA shunt metabolites (GABA, glutamate, and alanine) was observed along with the increasing concentration of salt (Al-Quraan et al. 2019) which signify its importance during stress.
Many times, salt along with other stresses were also studied and this part of the chapter also describes some of few such instances. The NaCl treatment in Arabidopsis up-regulates GABA metabolism. Precisely, GABA-T (first step of its catabolism) was found to play a crucial function in salt tolerance, as the loss-of-function of its corresponding gene mutant (pop2–1 mutant) was oversensitive to ionic stress but not to osmotic stress (Renault et al. 2010). In a study, Al-Quraan et al. (2013) have reported a significant increase in GABA, MDA, and GAD mRNA levels under salt and osmotic stress in the five wheat cultivars (Hurani 75, Sham I, Acsad 65, Um Qayes and Nodsieh). The study suggested key contribution of GABA shunt, which permits salt stress management in wheat. Sheteiwy et al. (2019) have demonstrated that the high tolerance to OS+S (PEG + NaCl) involves the GABA potentiality to regulate ROS level, secondary metabolism, and transcription. Jin et al. (2019), while working on salinity-alkalinity stress in muskmelon, have observed that the stress increased MDA content, relative electrical conductivity, and the activities of many anti-oxidant enzymes including SOD, APX, and DHAR. Along with that, it decreases shoot weight (dry and fresh), leaf area, and antioxidant metabolites. The pre-treatment with GABA, H2O2, GSH, or AsA was found effective and reduces the salinity-alkalinity-induced effects. Further, the mitigation effect of GABA involves the surplus accumulation of chl and its precursors to evade photo-oxidation injury. In this study, it was also demonstrated that GABA stimulates the free polyamine contents (PAs), which in turn reduces Na+/K+ ratio and alleviates membrane lipid peroxidation, under salinity-alkalinity stress in muskmelon (Xu et al. 2019).
7.3.2 Drought Stress
In nature, the combined interplay of less rainfall, reduced groundwater table, and insufficient water availability lead to drought stress (Singh and Laxmi 2015; Singh et al. 2015). The drought stress affects different physiological and metabolic processes of plant and significantly reduced growth, water content, chlorophyll content, and change in different fluorescence parameters (Levitt 1980; Ahmed et al. 2017). It also results in the production of reactive oxygen species, which in turn causes damage to cellular constituent such as lipids, nucleic acids, carbohydrates, and proteins (Waraich et al. 2011). Besides, the drought stresses also regulate genes associated with stress response (Mishra et al. 2015).
The possible contribution of hypoxia and drought stress on GAD in vivo activity was reported by Serraj et al. (1998). The analysis of GABA suggested its six-fold accumulation after 6 h hypoxia exposure to soybean nodules and no changes after PEG treatment. The GABA application in drought-stressed perennial ryegrass (Lolium perenne) demonstrated higher relative water content, turf quality , and peroxidise activity. However, lowering of wilt rating, canopy temperature depression, electrolyte leakage, and lipid peroxidation were also observed as compared to untreated plants. No significant impact of GABA application was observed in the activity of superoxide dismutase and catalase in this study (Krishnan et al. 2013). Vijayakumari and Puthur (2016) have reported the contribution of GABA priming for the enhancement of osmotic stress tolerance in Piper nigrum Linn, stressed with PEG. In leaves of white clover (Trifolium repens), the drought (15% PEG)-induced damage can be effectively managed by exogenous application of GABA. The physiological features include behind this alleviation are higher relative water content, lower electrolyte leakage, lipid peroxidation, and leaf wilting. This improvement of GABA-mediated drought tolerance was observed in white clover, which involves participation of GABA-shunt, polyamines, and proline metabolism (Yong et al. 2017). In order to further understand the specificity and GABA functioning, Mekonnen et al. (2016) have investigated the gad1/2 (with GABA depletion) to drought stress. The GAD (glutamate decarboxylase) performs primary function of GABA biosynthesis and decarboxylate glutamate. In A. thaliana, five copies of GAD are available in genome. Amongst these, GAD1 and GAD2 are highly expressed and responsible for significant reduction of GABA content in knockouts. The gad1/2 mutant phenotypically exhibited reduced shoot growth and early wilting after prolonged drought stress, mainly because of defect in stomata functioning (closure). This over-sensitivity can be reversed by functional complementation, which increases GABA content in leaves (Mekonnen et al. 2016).
7.3.3 Temperature Stress
Any variation to plants’ optima temperature is considered as ‘Temperature stress’, which can be of two types: heat stress (above optima) and chilling/cold (Below optima) stress. The application of GABA in mitigation of temperature has been demonstrated in both cold and heat stress (Nayyar et al. 2014; Li et al. 2016a; Wang et al. 2016). High temperature or heat stress (HT) is one of the major environmental stresses that limit the growth, development, and productivity of plant globally (Hasanuzzaman et al. 2013). Plant responses to HT vary with type of plant, duration, and degree of high temperature. Plants are generally susceptible to high temperature in almost every development stage, but reproductive stage is most sensitive stage (Hasanuzzaman et al. 2013). Heat-triggered morphological, physiological, biochemical, and molecular changes in plants limit plant growth and development. HT inhibits seed germination, scoring of stems and leaves, root and shoot growth inhibition, senescence, and abscission of leaf (Vollenweider and Gunthardt-Goerg 2005; Wassie et al., 2019). It has also been reported that HT reduces relative water content, impaired photosynthesis, and increases accumulation of reactive oxygen species which in turn results in oxidative stress. It is responsible for lipid peroxidation, disturbing the stability of membrane and enzymes and nucleic acid denaturation (Hasanuzzaman et al. 2013; Wassie et al. 2019). The heat stress was also demonstrated to regulate the transcript of stress-related genes including heat shock transcription factors and heat shock proteins, specifically characterised for heat stress, though they may also have some other applications (Mishra et al. 2019). Further, it has been reported that application of exogenous osmolytes, signalling molecules, polyamines, and phytohormones, can alleviate heat stress (Mishra et al. 2020).
In rice seedlings, with the rise in temperature the length of roots and shoots were found inhibited and caused decrease in survival, especially at 42/37 °C (Nayyar et al. 2014). The contrasting fluctuation in endogenous GABA content was observed under moderately (35/25 °C) and severe stress (42/37 °C) conditions, with more than two-fold increase and seven-fold decrease as compared to moderately stressed plants (possible reason for reduction in growth and survival), respectively. With exogenous GABA application, the growth of survival of heat-stressed plants improved, which found associated with lowering of membrane damage, better cellular reducing feature, chlorophyll content, and shoots photochemical efficiency. Further, improvement in relative water content and stomatal content was also noticed, which was found associated with increase in osmolyte (proline and trehalose) accumulation. Moreover, the damage caused in anti-oxidant system (enzymatic: superoxide dismutase, catalase, ascorbate peroxidase, and glutathione reductase and metabolite /non-enzymatic: ascorbate and glutamate) under heat stressed condition was also improved with GABA application in rice (Nayyar et al. 2014).
In another study, Li et al. (2016) have observed that pre-treatment of GABA (0.5 mM), before heat stress, improved heat tolerance in creeping bentgrass (Agrostis stolonifera). The metabolic analysis suggested that GABA application leads to higher accumulation of amino acids (glutamic acid, aspartic acid, alanine, threonine, serine, and valine), organic acids (aconitic acid, malic acid, succinic acid, oxalic acid, and threonic acid), sugars (sucrose, fructose, glucose, galactose, and maltose), and sugar alcohols (mannitol and myo-inositol). The study suggested the involvement of photosynthesis and ascorbate-glutathione cycle enhancement, osmotic adjustment, and GABA shunt in GABA-induced heat tolerance. Further studies suggested that during heat stress, the exogenous GABA application enhances expression of stress genes viz., ABF3, POD, APX, HSP90, DHN3, and MT1, which could be the possible reason to offer heat tolerance (Li et al. 2018). The global investigation of mRNAs and miRNAs suggested about the important adaptive responses, triggered by GABA, which includes HSFs pathway regulation, increased carbon metabolism, and biosynthesis of amino acids and plant hormone signalling (Li et al. 2019a, b). This study also reported the close association of heat-survival with significant changes of miR398s, cca-miR156b, aly-miR159c-3p, and ata-miR408-3p. Along with the involvement of vvi-miR845c, ama-miR156, and other novel miRNAs such as novel-24,223, novel-2964, and novel-10,098 was also observed in GABA-regulated heat tolerance. Over all, the GABA-mediated heat tolerance in creeping bentgrass involved physiological, transcriptional, and post-transcriptional regulation.
Besides, the contribution of GABA towards cold-stress, another variant of temperature stress was also observed, which also has many negative impacts on plant growth and development. The nitric oxide–mediated chilling tolerance in cold-stored banana involves GABA metabolism and increased glutamate decarboxylase and decreased GABA transaminase activity, resulting into GABA accumulation (Wang et al. 2016). The investigation of amino acid homeostasis in frost-resistant and sensitive barley and wheat seedling under cold or freezing stress conditions revealed the significant conversion of glutamate to GABA, which was proportional to stress severity. Cold acclimation resulted into amino acid pool and GABA shunt gene induction, suggesting their contribution in frost tolerance (Mazzucotelli et al. 2006). The Isobaric tags for relative and absolute quantification (iTRAQ) based analysis suggested the association of the GABA-induced physiological effects with cold tolerance in tea plants. Further protein-protein interaction studies suggested the endogenous GABA alteration and stress response factors during improvement of cold tolerance and were linked with stimulated interactions among photosynthesis, amino acid biosynthesis, and carbon and nitrogen metabolism (Zhu et al. 2019).
7.3.4 Heavy Metal Stress
From a long time, heavy metals such as Mn, Fe, Cu, Cd, Zn, Hg, As, and Ni have been accumulated in the soil through industrial waste and sewage disposal. Though, some of these metals are essential micronutrient for growth and development of plant, however, their excess concentrations in the soil have detrimental effect on growth and development of plant (Ghori et al. 2019). Heavy metal stress cause denaturation or inactivation of enzymes which are important for many basic plant metabolic reaction associated with homeostasis, respiration, and photosynthesis (Hossain et al. 2012). It also triggers the production of reactive oxygen species such as hydrogen peroxide, superoxide radical, and hydroxyl radical which in turn cause oxidative damage to plant (Ahmad et al. 2012).
The contribution of GABA in reversing the negative effect of heavy metals (Cd, Cadmium; Cr, Chromium; As, Arsenic) on plant growth and physiological parameters has been shown (Mahmud et al. 2017; Kumar et al. 2017, 2019; Maryam et al. 2020). The improvement of physiological response in Cr-treated mustard seedlings was conferred by GABA (Mahmud et al. 2017). The supplementation of GABA on Cr-treated seedlings reversed most of the effect, which involves reduction of Cr uptake and reduction of oxidative damage by up-regulating non-enzymatic (ascorbate, AsA; glutathione, GSH) and enzymatic (ascorbate peroxidise, APX; monodehydroascorbate reductase, MDHAR; dehydroascorbate reductase, DHAR; glutathione reductase, GR etc.), anti-oxidant systems. Further, the application of GABA also increased leaf RWC and chl content and decreased proline and phytochelatin content. The GABA accumulation lowered the expression of Lsi-1 and Lsi-2 transporter expression, and hence reduced the As accumulation, along with reduction of oxidative stress marker TBARS and H2O2 with GABA accumulation. The GABA-mediated modulation of physiological responses was noted, which exhibited better tolerance of Oryza sativa for As stress (Kumar et al. 2017). Kumar et al. (2019) have observed that the exogenous application of GABA (0.5 mM) against As(III) stressed rice seedlings, reduced the levels of H2O2 and TBARS, and improved the growth parameters. Further, its application along with As(III) enhanced level of unsaturated fatty acids (USFAs, particularly linolenic acid) as compared to saturated fatty acids (SFAs). The similar enhancement was also observed in case of amino acids (AA, proline, methionine, glutamic acid, and cysteine) and expression of genes associated with polyamine (PA) biosynthesis (arginine decarboxylase, spermine and spermidine synthase). This study cumulatively demonstrated that GABA at low concentrations provides solution for managing As(III) tolerance in rice, due to its positive effect on biosynthesis of USFA, AA, and PA (mainly putrescine) and negative effect on TBARS and H2O2. The application of GABA on Cd-treated maize plants demonstrated improvement of growth parameters. Further, its application conferred reduction in Cd uptake, increased activities of antioxidant enzymes (catalase and superoxide dismutase), along with induction of polyamine biosynthesis-responsive genes (ornithine decarboxylase and spermidine synthase), and suppression of polyamine oxidase, responsible for polyamine catabolism (Maryam et al. 2020). The contribution of GABA has also been observed during Cd stress in Monoraphidium sp. QLY-1, which resulted in higher protein , glutathione content, up-regulation of α-amylase activity, and reduction of starch, ROS, and Cd2+. Further, GABA under Cd stress positively increases lipid production in this alga (Zhao et al. 2020).
7.4 Conclusion and Prospects
The present chapter addresses the significance of GABA in various types of environmental constraints mainly salt, drought, heat, cold, and heavy metals. The endogenous (one of the survival strategy) or exogenous GABA attributes advantages viz., reduction of photosynthesis inhibition, polyamine accumulation, reduction of ROS etc., and assists plant to maintain their normal growth and development. Though the investigations suggested its contribution in offering stress tolerance feature in plants, still the knowledge of its association in the management of several stress-related traits is not well understood at molecular level. Along with the transcriptional regulation of GABA-shunt genes under diverse conditions (developmental, hormonal, pathogenic, etc.) also would be an interesting area to work on. Further, their relations with transcriptionally modulated genes during stress, particularly transcription factors, also need attention, which will give better understanding to address combinatorial methods for stress management in crop plants to meet desired production.
References
Abid G, Ouertani RN, Jebara SH, Boubakri H, Muhovski Y, Ghouili E, Abdelkarim S, Chaie O, Hidri Y, Kadri S, El Ayed M (2020) Alleviation of drought stress in faba bean (Vicia faba L.) by exogenous application of β-aminobutyric acid (BABA). Physiol Mol Biol Plants 26(6):1173–1186
Ahmad P, Ozturk M, Gucel S (2012) Oxidative damage and antioxidants induced by heavy metal stress in two cultivars of mustard (Brassica juncea L.) plants. Fresenius Environ Bull 21(10):2953–2961
Akama K, Akihiro T, Kitagawa M, Takaiwa F (2001) Rice (Oryza sativa) contains a novel isoform of glutamate decarboxylase that lacks an authentic calmodulin-binding domain at the C-terminus. Biochimica et Biophysica Acta (BBA)-Gene Structure Exp 1522(3):143–150
Al-Quraan NA, Sartawe FAB, Qaryouti MM (2013) Characterization of γ-aminobutyric acid metabolism and oxidative damage in wheat (Triticum aestivum L.) seedlings under salt and osmotic stress. J Plant Physiol 170(11):1003–1009
Al-Quraan NA, Al-Ajlouni ZI, Obedat DI (2019) The GABA shunt pathway in germinating seeds of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) under salt stress. Seed Sci Res 29(4):250–260
Atkinson NJ, Urwin PE (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot 63(10):3523–3543
Awapara J, Landua AJ, Fuerst R, Seale B (1950) Free γ-aminobutyric acid in brain. J Biol Chem 187:35–39
Bouche N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9(3):110–115
Bouche N, Fait A, Bouchez D, Møller SG, Fromm H (2003) Mitochondrial succinic-semialdehyde dehydrogenase of the caminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc Natl Acad Sci U S A 100(11):6843–6848
Bown AW, Shelp BJ (2016) Plant GABA: not just a metabolite. Trends Plant Sci 21(10):811–813
Carillo P (2018) GABA shunt in durum wheat. Front Plant Sci 9:100. https://doi.org/10.3389/fpls.2018.00100
Chen H, Liu T, Xiang L, Hu L, Hu X (2018) GABA enhances muskmelon chloroplast antioxidants to defense salinity-alkalinity stress. Russ J Plant Physiol 65(5):674–679
Cheng B, Li Z, Liang L, Cao Y, Zeng W, Zhang X, Ma X (2018) The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int J Mol Sci 19(9):2520
Christensen HN et al (1994) Special transport and neurological significance of two amino acids in a configuration conventionally designated as D. J Exp Biol 196:297–305
Elliott K, Jasper HH (1959) Gamma-aminobutyric acid. Physiol Rev 39(2):383–406
Fait A, Fromm H, Walter D, Galili G, Fernie AR (2008) Highway or byway: the metabolic role of the GABA shunt in plants. Trends Plant Sci 13(1):14–19
Gao JP, Chao DY, Lin HX (2007) Understanding abiotic stress tolerance mechanisms: recent studies on stress response in rice. J Integr Plant Biol 49(6):742–750
Ghori NH, Ghori T, Hayat MQ, Imadi SR, Gul A, Altay V, Ozturk M (2019) Heavy metal stress and responses in plants. Int J Environ Sci Technol 16(3):1807–1828
Ham TH, Chu SH, Han SJ, Ryu SN (2012) γ-Aminobutyric acid metabolism in plant under environment stressses. Korean J Crop Sci 57(2):144–150
Hanower P, Brzozowska J (1975) Influence d'un choc osmotique sur la composition des feuilles de cotonnier en acides amines libres. Phytochemistry 14(8):1691–1694
Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14(5):9643–9684
Hossain MA, Piyatida P, Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot 2012:1–37
Hu X, Xu Z, Xu W, Li J, Zhao N, Zhou Y (2015) Application of γ-aminobutyric acid demonstrates a protective role of polyamine and GABA metabolism in muskmelon seedlings under Ca (NO3) 2 stress. Plant Physiol Biochem 92:1–10
Hussain S, Khaliq A, Matloob A, Wahid MA, Afzal I (2013) Germination and growth response of three wheat cultivars to NaCl salinity. Soil Environ 32:36–43
Jain M (2013) Emerging role of metabolic pathways in abiotic stress tolerance. J Plant Biochem Physiol 1(108):10–4172
Jalil SU, Ansari MI (2020) Physiological role of gamma-aminobutyric acid in salt stress tolerance. In: Salt and drought stress tolerance in plants. Springer, Cham, pp 337–350
Jalil SU, Ahmad I, Ansari MI (2017) Functional loss of GABA transaminase (GABA-T) expressed early leaf senescence under various stress conditions in Arabidopsis thaliana. Curr Plant Biol 9:11–22
Jin X, Liu T, Xu J, Gao Z, Hu X (2019) Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biol 19(1):1–15
Kalhor MS, Aliniaeifard S, Seif M, Asayesh EJ, Bernard F, Hassani B, Li T (2018) Enhanced salt tolerance and photosynthetic performance: implication of ɤ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol Biochem 130:157–172
Kinnersley AM, Turano FJ (2000) Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci 19(6):479–509
Krishnan S, Laskowski K, Shukla V, Merewitz EB (2013) Mitigation of drought stress damage by exogenous application of a non-protein amino acid γ–aminobutyric acid on perennial ryegrass. J Am Soc Hortic Sci 138(5):358–366
Kumar N, Dubey AK, Upadhyay AK, Gautam A, Ranjan R, Srikishna S, Sahu N, Behera SK, Mallick S (2017) GABA accretion reduces Lsi-1 and Lsi-2 gene expressions and modulates physiological responses in Oryza sativa to provide tolerance towards arsenic. Sci Rep 7(1):1–11
Kumar N, Gautam A, Dubey AK, Ranjan R, Pandey A, Kumari B, Singh G, Mandotra S, Chauhan PS, Srikrishna S, Dutta V (2019) GABA mediated reduction of arsenite toxicity in rice seedling through modulation of fatty acids, stress responsive amino acids and polyamines biosynthesis. Ecotoxicol Environ Saf 173:15–27
Levitt J (1980) Responses of plants to environmental stresses, water, radiation, salt and other stresses, 2nd edn. Academic Press, New York
Li MF, Guo SJ, Yang XH, Meng QW, Wei XJ (2016) Exogenous gamma-aminobutyric acid increases salt tolerance of wheat by improving photosynthesis and enhancing activities of antioxidant enzymes. Biol Plant 60(11):123–131
Li Z, Yu J, Peng Y, Huang B (2016a) Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci Rep 6(1):1–16
Li Z, Peng Y, Huang B (2016b) Physiological effects of γ-aminobutyric acid application on improving heat and drought tolerance in creeping bentgrass. J Am Soc Hortic Sci 141(1):76–84
Li MF, Guo SJ, Yang XH, Men QW, Wei XJ (2016c) Exogenous gamma-aminobutyric acid increases salt tolerance of wheat by improving photosynthesis and enhancing activities of antioxidant enzymes. Biol Plant 60(1):123–131
Li Z, Peng Y, Huang B (2018) Alteration of transcripts of stress-protective genes and transcriptional factors by γ-aminobutyric acid (GABA) associated with improved heat and drought tolerance in creeping bentgrass (Agrostis stolonifera). Int J Mol Sci 19(6):1623
Li Z, Cheng B, Zeng W, Liu Z, Peng Y (2019a) The transcriptional and post-transcriptional regulation in perennial creeping bentgrass in response to γ-aminobutyric acid (GABA) and heat stress. Environ Exp Bot 162:515–524
Li Z, Huang T, Tang M, Cheng B, Peng Y, Zhang X (2019b) iTRAQ-based proteomics reveals key role of γ-aminobutyric acid (GABA) in regulating drought tolerance in perennial creeping bentgrass (Agrostis stolonifera). Plant Physiol Biochem 145:216–226
Liu T, Liu Z, Li Z, Peng Y, Zhang X, Ma X, Huang L, Liu W, Nie G, He L (2019) Regulation of heat shock factor pathways by γ-aminobutyric acid (GABA) associated with thermotolerance of creeping bentgrass. Int J Mol Sci 20(19):4713
Ma Y, Wang P, Chen Z, Gu Z, Yang R (2018) GABA enhances physio-biochemical metabolism and antioxidant capacity of germinated hulless barley under NaCl stress. J Plant Physiol 231:192–201
Mahmud JA, Hasanuzzaman M, Nahar K, Rahman A, Hossain MS, Fujita M (2017) γ-Aminobutyric acid (GABA) confers chromium stress tolerance in Brassica juncea L. by modulating the antioxidant defense and glyoxalase systems. Ecotoxicology 26(5):675–690
Maryam S, Sasan A, Bernard F, Mehdi S, Mojgan L, Batool H, Fardad D, Massimo B, Hassan R, Li T (2020) γ-Aminobutyric acid confers cadmium tolerance in maize plants by concerted regulation of polyamine metabolism and antioxidant defense systems. Sci Rep (Nature Publisher Group) 10(1)
Masood A, Shah NA, Zeeshan M, Abraham G (2006) Differential response of antioxidant enzymes to salinity stress in two varieties of Azolla (Azolla pinnata and Azolla filiculoides). Environ Exp Bot 58:216–222
Mayer RR, Cherry JH, Rhodes D (1990) Effects of Heat Shock on Amino Acid Metabolism of Cowpea Cells. Plant Physiol 94(2):796–810
Mazzucotelli E, Tartari A, Cattivelli L, Forlani G (2006) Metabolism of γ-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J Exp Bot 57(14):3755–3766
Mekonnen DW, Flügge UI, Ludewig F (2016) Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci 245:25–34
Michaeli S, Fait A, Lagor K, Nunes-Nesi A, Grillich N, Yellin A, Bar D, Khan M, Fernie AR, Turano FJ, Fromm H (2011) A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J 67(3):485–498
Mishra S, Phukan UJ, Tripathi V, Singh DK, Luqman S, Shukla RK (2015) PsAP2 an AP2/ERF family transcription factor from Papaver somniferum enhances abiotic and biotic stress tolerance in transgenic tobacco. Plant Mol Biol 89(1–2):173–186
Mishra S, Srivastava V, Mehrotra S, Quadri S.N (2017) The Regulation of Plant Development: Cross-talk of Reactive Oxygen Species and Plant Hormones. Revisiting the role of reactive oxygen species (ros) in plants: ros boon or bane for plants
Mishra S, Chowdhary AA, Mehrotra S, Srivastava V (2019) Function of plant heat shock transcription factors in abiotic stress. In: Molecular approaches in plant biology and environmental challenges. Springer, Singapore, pp 113–126
Mishra S, Bhardwaj M, Mehrotra S, Chowdhary A.A, Srivastava V (2020) The contribution of Phytohormones in plant Thermotolerance. Heat Stress Tolerance in Plants: Physiological, Molecular and Genetic Perspectives:213–238
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Physiol 59:651–681
Nayyar H, Kaur R, Kaur S, Singh R (2014) γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J Plant Growth Regul 33(2):408–419
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60(3):324–349
Podlešáková K, Ugena L, Spíchal L, Doležal K, De Diego N (2019) Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol 48:53–65
Priya M, Sharma L, Kaur R, Bindumadhava H, Nair RM, Siddique KHM, Nayyar H (2019) GABA (γ-aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci Rep 9(1):1–14
Ramesh SA, Tyerman SD, Xu B, Bose J, Kaur S, Conn V, Domingos P, Ullah S, Wege S, Shabala S, Feijó JA (2015) GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun 6:7879
Ramos-Ruiz R, Martinez F, Knauf-Beiter G (2019) The effects of GABA in plants. Cogent Food Agric 5(1):1670553
Renault H, Roussel V, El Amrani A, Arzel M, Renault D, Bouchereau A, Deleu C (2010) The Arabidopsis pop2-1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol 10(1):1–16
Rezaei-Chiyaneh E, Seyyedi SM, Ebrahimian E, Moghaddam SS, Damalas CA (2018) Exogenous application of gamma-aminobutyric acid (GABA) alleviates the effect of water deficit stress in black cumin (Nigella sativa L.). Ind Crop Prod 112:741–748
Savvides A, Ali S, Tester M, Fotopoulos V (2016) Chemical priming of plants against multiple abiotic stresses: mission possible? Trends Plant Sci 21(4):329–340
Seifikalhor M, Aliniaeifard S, Bernard F, Seif M, Latifi M, Hassani B, Didaran F, Bosacchi M, Rezadoost H, Li T (2020) γ-Aminobutyric acid confers cadmium tolerance in maize plants by concerted regulation of polyamine metabolism and antioxidant defense systems. Sci Rep 10(1):1–18
Serraj R, Shelp BJ, Sinclair TR (1998) Accumulation of γ-aminobutyric acid in nodulated soybean in response to drought stress. Physiol Plant 102(1):79–86
Shelp BJ, Bown AW, McLean MD (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4(11):446–452
Shelp BJ, Bozzo GG, Zarei A, Simpson JP, Trobacher CP, Allan WL (2012) Strategies and tools for studying the metabolism and function of γ-aminobutyrate in plants. II Integrated analysis. Botany 90(9):781–793
Sheteiwy MS, Shao H, Qi W, Hamoud YA, Shaghaleh H, Khan NU, Yang R, Tang B (2019) GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int J Mol Sci 20(22):5709
Signorelli S, Dans PD, Coitiño EL, Borsani O, Monza J (2015) Connecting proline and γ-aminobutyric acid in stressed plants through non-enzymatic reactions. PLoS One 10(3):0115349
Singh D, Laxmi A (2015) Transcriptional regulation of drought response: a tortuous network of transcriptional factors. Front Plant Sci 6:895
Singh A, Roychoudhury A (2020) Role of γ-aminobutyric acid in the mitigation of abiotic stress in plants. Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives 413
Singh B, Bohra A, Mishra S, Joshi R, Pandey S (2015) Embracing new-generation ‘omics’ tools to improve drought tolerance in cereal and food-legume crops. Biol Plant 59(3):413–428
Steward GR et al (1949) γ -Aminobutyric acid: a constituent of the potato tuber? Science 110:439–440
Vijayakumari K, Puthur J.T (2016) γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. Plants subjected to PEG-induced stress. Plant Growth Regul, 78(1):57–67
Vijayakumari K, Jisha KC, Puthur JT (2016) GABA/BABA priming: a means for enhancing abiotic stress tolerance potential of plants with less energy investments on defence cache. Acta Physiologiae Plantarum 38(9):230
Vollenweider P, Günthardt-Goerg MS (2005) Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage. Environ Pollut 137:455–465
Wallace W, Secor J, Schrader LE (1984) Rapid accumulation of γ-aminobutyric acid and alanine in soybean leaves in response to an abrupt transfer to lower temperature, darkness, or mechanical manipulation. Plant Physiol 75(1):170–175
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218(1):1–14
Wang Y, Luo Z, Mao L, Ying T (2016) Contribution of polyamines metabolism and GABA shunt to chilling tolerance induced by nitric oxide in cold-stored banana fruit. Food Chem 197:333–339
Wang X, Dong H, Hou P, Zhou H, He L, Wang C (2017a) August. Effects of exogenous gamma-aminobutyric acid on absorption and regulation of ion in wheat under salinity stress. In: International conference on computer and computing technologies in agriculture. Springer, Cham, pp 347–357
Wang Y, Gu W, Meng Y, Xie T, Li L, Li J, Wei S (2017b) γ-Aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci Rep 7:43609
Waraich EA, Ahmad R, Saifullah MYA, Ahmad M (2011) Improving agricultural water use efficiency by nutrient management. Acta Agric Scandinavica, Section B- Soil Plant Sci 61(4):291–304
Wassie M, Zhang W, Zhang Q, Ji K, Chen L (2019) Effect of heat stress on growth and physiological traits of alfalfa (Medicago sativa L.) and a comprehensive evaluation for heat tolerance. Agronomy 9(10):597
Wu X, Jia Q, Ji S, Gong B, Li J, Lv G, Gao H ( 2020) Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism
Xiang L, Hu L, Xu W, Zhen A, Zhang L, Hu X (2016) Exogenous γ-aminobutyric acid improves the structure and function of photosystem II in muskmelon seedlings exposed to salinity-alkalinity stress. PLoS One 11(10)
Xu J, Liu T, Yang S, Jin X, Qu F, Huang N, Hu X (2019) Polyamines are involved in GABA-regulated salinity-alkalinity stress tolerance in muskmelon. Environ Exp Bot 164:181–189
Yong B, Xie H, Li Z, Li YP, Zhang Y, Nie G, Zhang XQ, Ma X, Huang LK, Yan YH, Peng Y (2017) Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-shunt, polyamines, and proline metabolism in white clover. Front Physiol 8:1107
Zhao Y, Song X, Zhong DB, Yu L, Yu X (2020) γ-Aminobutyric acid (GABA) regulates lipid production and cadmium uptake by Monoraphidium sp. QLY-1 under cadmium stress. Bioresource Technol 297:122500
Zhu X, Liao J, Xia X, Xiong F, Li Y, Shen J, Wen B, Ma Y, Wang Y, Fang W (2019a) Physiological and iTRAQ-based proteomic analyses reveal the function of exogenous γ-aminobutyric acid (GABA) in improving tea plant (Camellia sinensis L.) tolerance at cold temperature. BMC Plant Biol 19(1):1–20
Zhu X, Liao J, Xia X, Xiong F, Li Y, Shen J, Wen B, Ma Y, Wang Y, Fang W (2019b) Physiological and iTRAQ-based proteomic analyses reveal the function of exogenous γ-aminobutyric acid (GABA) in improving tea plant (Camellia sinensis L.) tolerance at cold temperature. BMC Plant Biol 19(1):43
Zik M, Arazi T, Snedden W.A, Fromm H (1998a) Two isoforms of glutamate decarboxylase in Arabidopsis are regulated by calcium/calmodulin and differ in organ distribution. Plant Mol Biol 37(6):967–975
Zik M et al (1998b) Two isoforms of glutamate decarboxylase in Arabidopsis are regulated by calcium/calmodulin and differ in organ distribution. Plant Mol Biol 37:967–975
Acknowledgement
VS, AC, and SL acknowledge Central University of Jammu for providing work facility.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Srivastava, V., Mishra, S., Chowdhary, A.A., Lhamo, S., Mehrotra, S. (2021). The γ-Aminobutyric Acid (GABA) Towards Abiotic Stress Tolerance. In: Wani, S.H., Gangola, M.P., Ramadoss, B.R. (eds) Compatible Solutes Engineering for Crop Plants Facing Climate Change. Springer, Cham. https://doi.org/10.1007/978-3-030-80674-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-80674-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80673-6
Online ISBN: 978-3-030-80674-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)