Abstract
Magnetic nanoparticles (MNPs) have been used in engineering applications for different purposes in the last few decades, increasing their relevance recently on biomedical studies, with alternative treatments to most complex diseases, and microelectronic fields, as an excellent way to improve aspects such as thermal and electric conductivity. The use of nanomagnetic ferrite particles in cancer therapy and to control antibacterial agents is also noteworthy, because of their advantages in terms of resistance to temperature variations, chemical stability, and long-term durability. One of the promising applications of these nanoparticles includes water-purifying systems. In this chapter, those outstanding aspects of nanoparticle ferrites were treated since their very applicable point-of-view. In this sense, the structure properties of this class of materials are a very important matter to discuss, investigating how their unique ferrimagnetic face centered behavior could directly influence their potential in technological fields and innovative medical treatments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nanomagnetic ferrites
- Synthesis
- Surface functionalization
- Biomedical & bioengineering
- Technological and physical field
1 Introduction
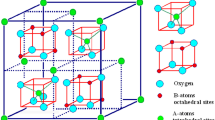
Ferrites are usually ferrimagnetic ceramic compounds, a metallic oxide mixture with 70% of this composition constituted with hematite (Fe2O3) and the remains 30% filled with other bivalent transition metals. Those nanoparticles show structural formula MFe2O4, which M represents an bivalent ion (as Ni2+, Mn2+, Cu2+ etc.), and the crystal structure is classically composed by divalent and trivalent ions on an atomic packing with face-centered cubic symmetry where the oxygen ions occupies positions in tetrahedral and octahedral symmetry sites [1].
Spinel ferrites XFe2O4 (where X=Al, Co, Mg, Mn, Fe, etc.) have cubic crystal structure. Spinel ferrites have two interstitial sites that are entitled as tetrahedral and octahedral sites. Tetrahedral sites are predominantly filled with divalent ions while octahedral sites occupied with trivalent ions [2]. Unit cell is equanimous of 64 tetrahedral and 32 octahedral sites, from which un-occupied sites are 56 tetrahedral and 16 octahedral sites [3]. Spinel ferrites are diversified into normal spinel-structure and inverse spinel-structure to mixed (partial) spinel structures. Structural formula of normal spinel is Me2+[Fe23+]O42−. In normal spinel structures, tetrahedral and octahedral sites is occupied by divalent and trivalent cations respectively. Structural formula of inverse ferrites are Fe3+[Me2+Fe3+]O42−. Magnetic moments of inverse spinels are remunerated mutually leads resulting moments because of cations magnetic moments. In inverse spinel structures, octahedral site is consists upon divalent cations while trivalent cations are distributed indiscriminately on octahedral and tetrahedral sites. Mixed spinels have structural formula Me1–δ2+Feδ3+[Meδ2+Fe2–δ3+]O42− (here δ represents degree of freedom). In Mixed (partial) spinel ferrites, divalent and trivalent cations are distributed arbitrarily [3,4,5]. Fe2+ cations are present on 1/8 tetrahedral sites while ½ octahedral sites are filled by Al3+ cations. This degree of inversion can be varied by varying synthesis techniques.
With wide possibilities of structural symmetry, ferrites can be natural and found in certain minerals or artificially produced in research laboratories. These materials ratify their extreme importance in various science fields due to the uniqueness of their optical, magnetic, and electric properties [6]. Although ferrites are composed of a mixture of oxides, their electrical and magnetic characteristics are derived from the metals that compose it. In general, they present great permeability, high magnetization saturation, and high magnetocrystalline anisotropy [6, 7]. Among the alternatives of magnetic materials, spinel ferrites are usually particularly attractive for physical and biological applications due to their unique properties, such as chemical and thermal stability, guaranteed by their cubic crystalline structure [8]. Several promising applications such as catalysis, sensors, storage systems, thin films, magnetic fluids, and medical treatments are already in use, with new solutions in growing development emerging every day [9].
Saturation magnetization values of magnetic ferrites are smaller as compared to ferromagnetic alloys. Properties of ferrites such as higher corrosion resistance and greater heat resistance make them promising candidates towards many applications [7, 10]. In recent years, spinel ferrite NPs have been of great interest in biomedical applications, need precise control on morphology, dispersion, and particle size, as well as of other prompting factors that influence these types of properties.
2 Synthesis and Fabrication of Nanomagnetic Ferrites
On nano scale, materials having low dimensional structures with advanced morphological features are extensively attracted towards potential applications due to their distinctive properties in magnetic, optical, and electronic devices. Hybrid nanomaterials exhibiting additive effects and facilitate as multifunctional have been fabricated through different techniques [11]. Several techniques are reported for synthesizing spinel ferrite nanoparticles including solid-state reaction, microemulsion, combustion, sol–gel, mechanical milling, chemical coprecipitation, and microwave synthesis (Fig. 1) [12]. Each one of that processes exhibits excellent results in terms of physical and chemical properties, and some of that synthesis techniques offers greater control over homogeneity, powder morphology and elemental composition.

Reprinted with permission from [13]
Synthesis protocols for nanomagnetic ferrites.
2.1 Solid-State Reaction Method
This method is a relatively novel progress and possesses great advantages likewise their extreme simplicity and the fact that give a high yield of products. In addition, this method involves less solvent, which is preponderant to reduce contamination under the products. Several authors [12, 14] reported Ni ferrite nanoparticles produced through this method, annealing Ni and Fe nanoparticles in ambient conditions [12] or using NaCl as dispersant in an ambient with calculated proportions of sodium hydroxide, ferrous and nickel sulfate [15]. Superparamagnetic Zn [16] and Mn–Zn [17] ferrites are reported to be synthesized by a low-temperature solid-state reaction (LTSSR) method. In each case, a simple stoichiometric mixture was applied using simple reagents such as ferric chloride, sodium hydroxide (NaOH), manganese chloride and zinc chloride.
2.2 Microemulsion Method
The microemulsion involves the thermodynamically stable dispersions of relatively immiscible two liquids stabilized by surfactant methods [9]. Some main recompences of this method include the variety of synthesis paths only varying the combination of surfactants, co-surfactant, and oil–water ratio, which leads to an effective particle size control. This method is also one of the most cost-effective routes to obtain ferrite nanoparticles with a refined control over impurities [9]. This reaction is favorable and eco-friendly, which can be perform even at low temperatures. This type of synthesis protocol allows reuse of surfactants for many times during preparation method, which lead towards their use in commercial products [8]. This leads striking aspect about microemulsion is that stable nanosized ferrites can be produced, and the fact that method permits for the reuse and recovery of oil and surfactants oil for NPs synthesis cycles [9]. However, the particles obtained sometimes exhibits a poor crystalline nature due to prerequisite of large amounts of solvents involved and are more polydisperse due to the slow nucleation proportion at low temperatures [17]. There are two major categories of this method: the reverse water-in-oil and normal oil-in-water and monodisperse nanometric droplets are shown in the dispersed phase, which offers a curbed environment for the preparation of nano-scale particles. Some reported nanoparticles synthesized by the microemulsion method involve Fe2Mn0.5Zn0.5O4 [18, 19], and Fe3O4 [20].
2.3 Combustion Solution Method
The solution combustion synthesis (SCS) drives other methods for the synthesis of nanoparticles maintaining its owing characteristics. This technique is very known for oxide materials; however, it is no very common to produce superparamagnetic materials which were reported recently for the first time [21]. This technique can synthesize homogeneously form particles through atomization of the liquid before the explosive flora of the reaction itself. Firstly, a homogeneity must be achieved in the system between the hydrate nitrates precursors and the complexing agent (commonly citric acid, glycine, or urea), which is achieved by stoichiometric balance. Given that the system is mixed on an atomic scale in solution leading to a diffusion this process is restricted to the size of the liquid drop before drying [22]. A chemical pyrolization occurs in the fluid compound powder, usually a mixture of metal oxides as the eventual result of the method. The triumph of the process is because of an excellent blending amid the constituents using an appropriate fuel through an exothermic redox reaction of complexing agent in an aqueous medium between oxidizer Magnetite (Fe3O4), Maghemite (α-Fe2O3) and fuel were reported to be produced by the SCS method with dissimilar fuel-to-oxidant molar ratios with urea as a fuel and ferric nitrate as oxidizer [21]. Copper-silver ferrites (Cu1-xAgxFe2O4) were synthesized for the first time by a modification of the SCS using glycine (C2H5NO2) as reducing agent [22]. It is noticed that a major effect in regulating particle size and microstructure of the artifact for combustion depends on different fuel-to-oxidant ratios. One of the most advantages in using the combustion solution synthesis is that be very economical, fast, and practical. The self-sustained combustion process occurs on the entire volume and normally taken no more than a few minutes to be reached, lead towards high-volume powder with dark precipitates distinct by the amount of oxides present in the samples.
2.4 Sol–gel
The sol–gel synthesis method is an attractive and widely used technique for the preparation of metal oxide nanosized particles. This technique normally includes the use of metal alkoxide solutions and exhibits some interesting advantages such as great stoichiometric control and the manufacturing of ultrafine particles with a slight size distribution in a comparatively short processing time at lesser temperatures [17, 23]. In this method, the metal solution undergoes condensation polymerization reactions and hydrolysis to form a gel at room temperature [4]. The reaction temperature in sol–gel varies between 25 and 200 °C, which is very interesting due to the possibilities to synthesize NPs with narrow size distribution and controlled shape. In order to obtain the final crystalline state, any volatile impurities that may appear during synthesis could be removed further by heat treatment after the combination reaction [23]. This lack of purity of the final product could be understood as one of the major limitations of the sol-gel technique, and thus heat treatment is needed after the production to attain the high purity state [24]. Other advantages of the sol-gel synthesis method include the fact that it functions under low temperature, cost-effective and no special instruments are needed to carry it on. The ease in terms of synthesis procedures merged with these advantages makes the sol-gel method a very attractive route for synthesize nanocomposites. Normally citric acid is used as conventional chelating agent, but other reagents like polyacrylic acid (PAA) which is reported to being additional carboxylic acid groups to formulize chelates with mixed cations could be used to produce superparamagnetic ferrites such as NiFe2O4 [23] and CoFe2O4 [25, 26]. Because of these outstanding benefits, sol–gel is one of the preferred synthesis methods to achieve homogeneity, composition control, microstructure, particle size, and particle distribution of NP, by regulating different limitations such as sol concentration, stirring rate, and annealing temperature [9, 16].
2.5 Mechanical Milling
The mechanical milling technique, as reported by its name, is a recurrent method used for the preparation of nanoparticles and involves a top-down approach. This method is a low-cost alternative for a rapid and simple synthesis route and offers the possibility to obtain a large-scale quantity of materials for various applications. Some ferrimagnetic nanoparticles synthesized by this method include CuFe2O4 [27,28,29] and nickel-substitute manganese ferrite (Ni1-xMnxFe2O4) [23, 29]. One of the disadvantages of this technique is frequent contamination of nanoparticles during longtime milling, which in turn will change the stoichiometry of the obtained NPs. Also, some studies show that increasing the calcination temperature the saturation magnetization Ms from almost 10 emu g−1 and a decrease in coercivity from above 25 Oe, which changes the average size to about 15 nm. These results demonstrate the impact of synthesis method on the magnetic properties and shows that refining the synthesis technique is one of the most required conditions to get ferrites nanoparticles with higher crystallinities and outstanding superparamagnetic properties [27].
2.6 Coprecipitation
The chemical coprecipitation method is one of the most frequently applied techniques to synthesize magnetic oxides, due to its simplicity, good control of grain size, and uniformity of NPs produced. However, some reports [30] observed that some undesirable intermediate phases could be found among the other metallic phases, which led to poor magnetic properties and irregular shape for the derived ferrite particles. This method employs a solution mixture of divalent to trivalent transition metals soluble salts, bonded in 1:2 molar ratio commonly adjustable in an alkaline medium. It is notable that this synthesis method needs a controlled monitoring of pH solution, which is usually attuned through ammonium solution or sodium hydroxide solution [8]. The coprecipitation method involves synthesize of mixed FNPs with different magnetic properties, such as by doping rare earth metals (Nd, Eu, and Gd) into CoFe2O4 [12, 30]. The major disadvantage of this method is the low crystallinity of prepared superparamagnetic ferrites and hence following heat treatment is essential in order to attain better crystallinity [31].
3 Surface Functionalization of Magnetic Nano Ferrites
One of the crucial steps involved in the fabrication of nanodevices is surface functionalization of synthesized nanomagnetic ferrites, as functionalization controls many physio-chemical processes which tune electrical, optical, and magnetic properties according to the requirement. However, in situ functionalizations of prepared ferrite NPs can be done through many synthetic methods. Many environmental and biomedical applications required different types of hydrophilic coatings possessing definite chemical groups [32, 33]. one of the important features for surface functionalization is accessibility of superficial transition metals with d orbitals working ass Lewis acids in existence of donor ligands. Spinel ferrite surface behave reactive towards different chemical groups, which provide a chamber for multiple combinations. Ligands provide many high and low-weight compounds. For surface complexation, many functional groups are available including amides, hydroxamic acids, carboxylic acids, phosphonic, and hydroxyl [34, 35].
Three major approaches are involved to make NPs hydrophilic in nature: (1) polymer coatings, (2) silica coatings, and (3) ligand exchange reaction. Ligand exchange reactions efficiently transfer many hydrophobic particles to the aqueous medium by replacing hydrophobic ligands without changing magnetic core considerably with different hydrophilic ligands. Silica coatings provide an excellent chemical stability and also prevent magnetic interactions, which denigrated the colloidal stability. Stober established the hydrolysis-condensation method to attain silica shells with meticulous thickness by adding tetraethyl orthosilicate to the dispersion of NPs without appearing single silica particles for enhancing the magnetic interactions. Silica coatings can be used with many organosilanes consisting groups such as -NH and -SH [36, 37]. Two major possibilities for polymer coatings of NPs are: (i) NPs surface functionalization with a molecule which acts as initiator for controlled-interfacial polymerization and (ii) preparation of polymer following the first step by surface anchoring. Second route is more simpler and allows variety of macromolecules for surface binding with suitable functional groups. Former route is more laborious and possess the advantage of controlling surface density by monitoring the length of chains and grafting the polymer [38].
Zhao et al., synthesized hydroamic acide coated Fe3O4/poly(acrylaide) nanocomposites (PAM). Authors treated amide bonds with the hydroxylamine solution [39]. While, Zhao et al., familiarized amine groups by ethlenediamine with epoxy moieties consisting polymer attached with the Fe3O4 NPs [15]. A similar method was also reported with the addition of NaSH into episulfide moieties. Ester and amide formations are good strategies to achieve better conjugation of NPs; carbodiimides are used as a coupling agent in these reactions. Ren et al., fused EDTA ligands with Fe3O4@silica@chitosan particles through amide bonding between NH2 groups and EDTA-COO− moieties in chitosan shells (Fig. 2) [40]. Ge et al., introduced polycarboxylic acid in amine coated magnetite [41].

Reprinted with permission from [40]
Synthesis protocol followed for EDTA-consisting nano ferrites by amidation of chitosan.
4 Applications of Magnetic Ferrites
Magnetic semiconductors, heusler alloys, and metal oxides are amidst materials that are considered auspicious candidates for wide applications in spintronics (Fig. 3). Exceedingly essential requirements for appropriate and multi-functioning of these devices are high curie temperature, low eddy currents, and high spin polarization. Ferrites are not regarded conventionally as magnetic materials but they are considered as magnetic in nature due to their advanced magnetic/structural properties, high degree of freedom, flexibility, high resistivity ranges, low leakage inductances, less eddy current losses, and multiple functionalities that motivate scientists to search for such type of materials [2, 3, 42, 43].
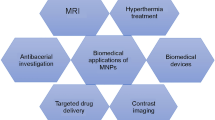
Modern applications of nanoferrites [1]
4.1 Applications in Biomedical and Bioengineering Fields
Magnetic nanoparticles show tremendous potential in several applications owing to their unique material properties. The use of magnetic nanoparticles for clinical and biological applications is one of the most thought-provoking research areas in the field of nanomagnetism [17, 44]. The ability to precisely control the behavior of the material using an externally applied magnetic field has made MNPs a promising nano agent in biosensors, magnetic separation, antibacterial control, targeted drug delivery, magnetic hyperthermia, magnetic resonance imaging (MRI) (Fig. 4) [45, 46]. In that way, the use of MNPs for drug delivery in cancer therapy has gained recent attention as a more effective tool than the usual chemotherapy and radiotherapy treatments [47].
Due to heat activity of ferrimagnetic nanoferrites have been used in gene therapy, drug carrier, and cancer treatment. Magnetic NPs also utilized as contrast agents in magnetic resonance imaging and magnetic particle imaging [55]
Advancements in technology provide many targeted approaches in NPs domain uptake in tissues, assembling the complexes to target specific tissues [48]. This research area is considered as a novel, with the increasing attention of different research groups around the world that have been exploring the role of different NPs in the treatment of cancer. Iron oxide nanoparticles such as Fe3O4 and γ-Fe2O3 show great therapeutic potential, which make them a good candidate for magnetic hyperthermia due to excellent superparamagnetic properties, nontoxicity, and higher biocompatibility [48]. Different types of agents are administrated during chemotherapy, which affects transcription phenomenon and DNA replication in dividing cells [46, 48].less side effects have been generated during targeted delivery systems, which enhances the capability of administrated therapeutic agents linked with their ligands. These NPs are functionalized with different types of cancer-specific antibodies/folic acid [49]. While drug delivery efficiency can be enhanced, NPs synthesis is required with responsive pH, rendering to different types of body tissues. These nanomaterials have many advantages which make them attractive candidate in drug carrier materials, which accumulates in tumor tissues, easy penetration by cellular membranes in different types of intracellular environments with higher specific surface area, which result in efficient drug loading [47].
Hyperthermia is a treatment performed through heat generation at a specific site and focused on regional, local, and whole body. Among different types of hyperthermia, local hyperthermia is gaining much attention in which tumor cells are treated between 42 and 46 °C heat produced through spinel ferrite NPs. Tumor cells are sensitive towards hyperthermic effects as compared to the normal healthy cells because of higher metabolic rates [50,51,52,53,54].
4.2 Applications in Technological and Physical Field
Spinel ferrites were focused from the last few years for technical applications such as remote monitoring, computers, industrial automation, and communication technologies. Ferrites are considered as excellent materials for their use in electrochemical biosensing due to their greater sensing accuracy, quick analysis, and low detection levels of different analytes (Fig. 5). For optimization in electronic devices, nanomaterials specially ferrites are used widely due to higher permeability, saturation magnetization, and greater magnetic relaxation frequency. These types of ferrites are used in electrical and electronic industries especially magnetic recording heads, transducers, magnetic memory chips and transformers, etc. [56].

Reprinted with permission from [57]
Zinc ferrite nanorods as gas sensors for ethanol and acetone detection: a–e TEM images of nanorods. f Response of zinc ferrite nanorods as gas sensor for different acetone and ethanol concentration. g Response curve for acetone and ethanol for different times at 260 °C.
Ferrites also play an important role as multilayer chip inductor and wire-wound chip inductor magnetic materials, used primarily for microwave gyromagnetic devices, capacitors, transformers, and components in electronic products like cell phones, wireless communication systems, and laptops [58]. Additionally many other metal ferrites composites, like CoFe2O4 and SnFe2O4 among others, have been largely used in supercapacitors applications, such charge storage applications, due to their outstanding electrochemical activity [58]. Other applications of ferrite materials include electromagnetic microwave absorption [58] and photocatalysis studies [59] and storage devices [60].
Removal of Organic and Inorganic Contaminants by Using Different Adsorption Techniques
For removal of toxic elements from waste/drinking water, majorly due to high efficiency and simplicity; basic advantage is sorbent separation during adsorption process, which is more energy consuming and tedious. For adsorption, use of magnetic materials make this task easier through magnetic decantation with the help of a permanent magnet. At room temperature, higher surface area of ferrites-based NPs enhances the binding specificity and superparamagnetic properties for strong binding of functional groups at the surfaces of NPs, which makes them the ideal candidates for the development of many innovative adsorption techniques [61].
Heavy Metal Cations
In waste/natural water, heavy metal cations are present due to many industrial activities, consist of many toxic substances with high impact on humans. Here we focus on those studies which concentrate on two aspects: (1) on adsorption mechanism, shedding light at atomic and molecular level (2) improving selectivity and adsorption capacity towards certain contaminant through surface functionalized ferrite NPs [62]. Ren et al., noticed the adsorption capacity of metal-EDTA complex stability: Cu(II) > Pb(II) > Cd(II). Authors verified the metal-carboxl.coated NPs through Fourier transform infrared spectroscopic measurements. Carboxyl-coated NPs from crotonic and acrylic acid copolymer were tested for Pb(II), Cd(II), Cu(II), and Zn(II). However, authors did not provide the spectroscopic evidences based on metal-carboxylated interactions, adsorption capacity increases with the increment in hardness of Lewis acid. Mahdavian et al., discussed adsorption properties of Cd(II), Cu(II), Ni(II), and Pb(II) with a nanoplatform containing chains of polyacrylic acid grown on magnetite NPs surface. pH increased with metal uptake, suggested the formation of chelates [36, 40].
Oxidation Technology
Oxidation technology comprises upon assisted degradation mechanism of pollutant through the use of highly transient oxidizing species. These species can be activated through substances which can act as catalyst. Since toxicity, removal, and reuse of catalysts are the main concerns, studies have been reported on the synthesis of heterogeneous magnetic materials which can efficiently active the oxidizing mechanism of pollutants and as well minimize some contamination events. Because of these major reasons, ferrite NPs are becoming potential candidates towards many applications: (1) onset superparamagnetic properties activate the facile removal of catalysts; (2) catalytic activity enhanced due to large surface to volume ratio; (3) chemically stable ferrite structure avoids metal leakage in the environment and (4) ferrite compositions versatility enhances photodegradation mechanisms. Improved catalytic properties can be achieved using rGo and multiwall carbon nanotubes. This synergic effect is the result of large surface-to-volume ratio of the synthesized composites with enhanced electronic properties as evidenced in the figure below. Fu et al., reported CoFe2O4/rGo nanocomposites with concentration greater than 40% of GO, no H2O2 was needed to achieve catalytic degradation. For dye degradation mechanism, CoFe2O4TiO2 nanocomposites were required.
Inductors
magnetic ferrites are promisingly sued in inductors as inductive components in different electronic circuits such as voltage-controlled oscillators, low noise amplifiers, impedance matching networks, and filters. Their applications in inductors possess general trend in integration and miniaturization for multi-functional electronic devices. This multilayer technology has become important for the mass production of multilayered integrated devices, which allow high degree of integration density. Multilayered capacitors are introduced few decades ago in the market, while inductors penetrated in 1980s [63]. Basic components such as metallic coils and soft ferrites are used to produce inductance. Ferrites-based thin films are used to produce higher permeability at the given operational frequency. Such type of films can be produced through sputtering, but composition and accuracy is difficult to control. Pulsed laser deposition provide high-quality thin films, while method involving the synthesis protocol for ferrite films by a combination of spin coating and sol-gel seems cost-effective and easier [64].
Electromagnetic Interference Suppression (EMI)
Many electronic equipments such as digital camera, digital high-speed interface based notebooks, scanners, and computers in small areas has enhanced technology by electromagnetic interference. Fast development in the field of wireless communications led towards the interference produced by magnetic and electric fields. Electromagnetic interference is the degradation in electronic systems produced by the electromagnetic disturbance. Noise produced by the electric devices produced at higher frequency than circuit signals. To reduce/avoid the EMI, different types of suppressors worked at low-pass filters in circuits to block higher frequency signals [65, 66].
5 Conclusions
In this chapter, ferrite nanoparticles were presented as one of the most interesting materials applicable in several applications, for very distinguished areas of science and technology. In this way, magnetic spinel nanoparticles attract attention due to their possibility to be manipulated under application of an external magnetic field. These properties lead spinel ferrites to be utilized on electronic devices, gas sensors, bacterial inactivation, smart drug delivery, photocatalysts, and others. The crystal structure, magnetic and optical properties of spinel ferrites depend on the preparation method, and some usual synthesis methods (microemulsion, sol-gel, combustion solution, coprecipitation, etc.) could be employed depending on the refinement or property optimization required. The efficiency of spinel ferrites in different kinds of science fields shows that those materials are highly versatile products, which makes the ferrite nanoparticles suitable for many biomedical and technological applications.
References
Heinz H et al (2017) Nanoparticle decoration with surfactants: molecular interactions, assembly, and applications. Surf Sci Rep 72(1):1–58
Goldman A (2012) Handbook of modern ferromagnetic materials. Springer, US
Riaz S et al (2014) Magnetic and magnetization properties of iron aluminum oxide thin films prepared by sol-gel. IEEE Trans Magn 50(8):1–4
Cullity BD, Graham CD (2011) Introduction to magnetic materials. Wiley
Naseri MG, Saion EB (2012) Crystalization in spinel ferrite nanoparticles. In: Advances in crystallization processes, pp 349–380
Kumar GR, Kumar KV, Venudhar YC (2012) Synthesis, structural and magnetic properties of copper substituted nickel ferrites by sol-gel method
Murthy Y, Rao TK, Singh R (2010) Synthesis and characterization of nano silver ferrite composite. J Magn Magn Mater 322(14):2071–2074
Tatarchuk T et al (2016) Spinel ferrite nanoparticles: synthesis, crystal structure, properties, and perspective applications. In: International conference on nanotechnology and nanomaterials. Springer
Kefeni KK, Msagati TA, Mamba BB (2017) Ferrite nanoparticles: synthesis, characterisation and applications in electronic device. Mater Sci Eng B 215:37–55
Hanini A et al (2018) Ferrite nanoparticles for cancer hyperthermia therapy. Handbook of nanomaterials for industrial applications. Elsevier, pp 638–661
Shi D et al (2012) Controlled nanostructuring of multiphase core-shell nanowires by a template-assisted electrodeposition approach. Nanotechnology 23(30):305601
Sanpo N et al (2013) Transition metal-substituted cobalt ferrite nanoparticles for biomedical applications. Acta Biomater 9(3):5830–5837
Goutam SP et al (2020) Green synthesis of nanoparticles and their applications in water and wastewater treatment. Bioremediation of industrial waste for environmental safety. Springer, pp 349–379
Low C, Wills R, Walsh F (2006) Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf Coat Technol 201(1–2):371–383
Zhao Y-G et al (2010) Synthesis, characterization and properties of ethylenediamine-functionalized Fe3O4 magnetic polymers for removal of Cr (VI) in wastewater. J Hazard Mater 182(1–3):295–302
Li F et al (2007) Magnetic properties of ZnFe2O4 nanoparticles produced by a low-temperature solid-state reaction method. J Magn Magn Mater 309(2):295–299
Amiri GR et al (2011) Magnetic properties and microwave absorption in Ni–Zn and Mn–Zn ferrite nanoparticles synthesized by low-temperature solid-state reaction. J Magn Magn Mater 323(6):730–734
Pemartin K et al (2014) Synthesis of Mn–Zn ferrite nanoparticles by the oil-in-water microemulsion reaction method. Colloids Surf A 451:161–171
Zeng S et al (2014) Magnetically separable Ni0.6Fe2.4O4 nanoparticles as an effective adsorbent for dye removal: synthesis and study on the kinetic and thermodynamic behaviors for dye adsorption. Chem Eng J 258:218–228
Ai Z et al (2010) Facile microwave-assisted synthesis and magnetic and gas sensing properties of Fe3O4 nanoroses. J Phys Chem C 114(14):6237–6242
Toniolo J et al (2007) Synthesis by the solution combustion process and magnetic properties of iron oxide (Fe3O4 and α-Fe2O3) particles. J Mater Sci 42(13):4785–4791
Gomes GA, da Costa GL, da Silva Figueiredo AB-H (2018) Synthesis of ferrite nanoparticles Cu1−xAgxFe2O4 and evaluation of potential antibacterial activity. J Mater Res Technol 7(3):381–386
Chen D-H, He X-R (2001) Synthesis of nickel ferrite nanoparticles by sol-gel method. Mater Res Bull 36(7–8):1369–1377
BV, D.C. and D. HYDRAULICS (1962). Absorption spectroscopy
Meron T et al (2005) Synthesis and assembly of high-quality cobalt ferrite nanocrystals prepared by a modified sol-gel technique. J Magn Magn Mater 292:11–16
Avazpour L, Toroghinejad M, Shokrollahi H (2015) Synthesis of single-phase cobalt ferrite nanoparticles via a novel EDTA/EG precursor-based route and their magnetic properties. J Alloy Compd 637:497–503
Marinca TF, Chicinaş I, Isnard O (2013) Structural and magnetic properties of the copper ferrite obtained by reactive milling and heat treatment. Ceram Int 39(4):4179–4186
Manova E et al (2004) Mechano-synthesis, characterization, and magnetic properties of nanoparticles of cobalt ferrite, CoFe2O4. Chem Mater 16(26):5689–5696
Zhang Z et al (2013) Synthesis and Characterization of NiFe2O4 Nanoparticles via Solid-State Reaction. Int J Appl Ceram Technol 10(1):142–149
Zi Z et al (2009) Synthesis and magnetic properties of CoFe2O4 ferrite nanoparticles. J Magn Magn Mater 321(9):1251–1255
Akhtar K et al (2020) Functionalized cobalt ferrite cubes: toxicity, interactions and mineralization into ferritin proteins. Appl Nanosci 10(9):3659–3674
Baldi G et al (2007) Synthesis and coating of cobalt ferrite nanoparticles: a first step toward the obtainment of new magnetic nanocarriers. Langmuir 23(7):4026–4028
Dai Q et al (2010) Monodisperse cobalt ferrite nanomagnets with uniform silica coatings. Langmuir 26(22):17546–17551
Deligöz H et al (2012) Synthesis, structural and electrical properties of triethylene glycol (TREG) stabilized Mn0.2Co0.8Fe2O4 NPs. Mater Res Bull 47(3):537–543
Duan H et al (2008) Reexamining the effects of particle size and surface chemistry on the magnetic properties of iron oxide nanocrystals: new insights into spin disorder and proton relaxivity. J Phys Chem C 112(22):8127–8131
Odio OF, Reguera E (2017) Nanostructured spinel ferrites: Synthesis, functionalization, nanomagnetism and environmental applications. In: Magnetic spinels-synthesis, properties and applications, pp 185–216
Ge J et al (2007) One‐step synthesis of highly water‐soluble magnetite colloidal nanocrystals. Chem Euro J 13(25):7153–7161
Costa FBT et al (2019) Highly magnetizable crosslinked chloromethylated polystyrene-based nanocomposite beads for selective molecular separation of 4-aminobenzoic acid. ACS Omega 4(3):5640–5649
Zhao F et al (2015) Green synthesis of magnetic EDTA-and/or DTPA-cross-linked chitosan adsorbents for highly efficient removal of metals. Ind Eng Chem Res 54(4):1271–1281
Ren Y et al (2013) Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: preparation, characterization, and application in heavy metal adsorption. Chem Eng J 226:300–311
Ge F et al (2012) Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J Hazard Mater 211:366–372
Bakshi UA, Bakshi VU (2009) Electrical circuits and machines. Technical Publications
Pervaiz E, Gul I, Virk MS (2014) Magnetic and RF-electromagnetic absorbing study of aluminum doped nickel ferrites prepared by two techniques. Mater Res Exp 1(1):016104
Hussain MI et al (2020) Ferrite nanoparticles for biomedical applications. Magnetic nanoheterostructures. Springer, pp 243–265
Javed Y et al (2017) MRI based on iron oxide nanoparticles contrast agents: effect of oxidation state and architecture. J Nanopart Res 19(11):1–25
Srinivasan SY et al (2018) Applications of cobalt ferrite nanoparticles in biomedical nanotechnology. Nanomedicine 13(10):1221–1238
Hajba L, Guttman A (2016) The use of magnetic nanoparticles in cancer theranostics: toward handheld diagnostic devices. Biotechnol Adv 34(4):354–361
Karponis D, Azzawi M, Seifalian A (2016) An arsenal of magnetic nanoparticles; perspectives in the treatment of cancer. Nanomedicine 11(16):2215–2232
Huang HS, Hainfeld JF (2013) Intravenous magnetic nanoparticle cancer hyperthermia. Int J Nanomed 8:2521
Organization WH (2014) Antimicrobial resistance: global report on surveillance. World Health Organization
Raffi M et al (2010) Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Annals of microbiology 60(1):75–80
Hundáková M, Dědková K, Martynková GS (2017) Decoration of inorganic substrates with metallic nanoparticles and their application as antimicrobial agents. Metal nanoparticles in pharma. Springer, pp 295–336
Lin L et al (2013) Ag–CuFe2O4 magnetic hollow fibers for recyclable antibacterial materials. J Mater Chem B 1(21):2719–2723
Gong P et al (2007) Preparation and antibacterial activity of Fe3O4@ Ag nanoparticles. Nanotechnology 18(28):285604
Mohapatra J, Xing M, Liu JP (2019) Inductive thermal effect of ferrite magnetic nanoparticles. Materials 12(19):3208
Praveena K et al (2016) Enhanced magnetic domain relaxation frequency and low power losses in Zn2+ substituted manganese ferrites potential for high frequency applications. J Magn Magn Mater 420:129–142
Li L et al (2017) Porous ZnFe2O4 nanorods with net-worked nanostructure for highly sensor response and fast response acetone gas sensor. Sens Actuat B Chem 248:85–91
Stergiou CA, Litsardakis G (2016) Y-type hexagonal ferrites for microwave absorber and antenna applications. J Magn Magn Mater 405:54–61
Casbeer E, Sharma VK, Li X-Z (2012) Synthesis and photocatalytic activity of ferrites under visible light: a review. Sep Purif Technol 87:1–14
Shad NA et al (2019) Photocatalytic degradation performance of cadmium tungstate (CdWO4) nanosheets-assembly and their hydrogen storage features. Ceram Int 45(15):19015–19021
Gómez-Pastora J, Bringas E, Ortiz I (2014) Recent progress and future challenges on the use of high performance magnetic nano-adsorbents in environmental applications. Chem Eng J 256:187–20s4
Hua M et al (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211:317–331
Ahmed MAA (2017) A review on the properties and uses of ferrite magnet. Sudan University of Science and Technology
Yang C et al (2006) Fully integrated ferrite-based inductors for RF ICs. Sens Actuators A 130:365–370
Stojanovic G et al (2006) High-performance zig-zag and meander inductors embedded in ferrite material. J Magn Magn Mater 297(2):76–83
Feng Y-B et al (2006) Electromagnetic and absorption properties of carbonyl iron/rubber radar absorbing materials. IEEE Trans Magn 42(3):363–368
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gomes, G.A., Akhtar, K., da Costa, G.L., Javed, Y., Sharma, S.K. (2021). Modern Applications of Ferrites: An Important Class of Ferrimagnetic System. In: Sharma, S.K. (eds) Spinel Nanoferrites. Topics in Mining, Metallurgy and Materials Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-79960-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-79960-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-79959-5
Online ISBN: 978-3-030-79960-1
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)